Abstract
Patients with antiretroviral therapy interruption have a high risk of virological failure when re-initiating antiretroviral therapy (ART), especially those with HIV drug resistance. Next-generation sequencing may provide close scrutiny on their minority drug resistance variant. A cross-sectional study was conducted in patients with ART interruption in five regions in China in 2016. Through Sanger and next-generation sequencing in parallel, HIV drug resistance was genotyped on their plasma samples. Rates of HIV drug resistance were compared by the McNemar tests. In total, 174 patients were included in this study, with a median 12 (interquartile range (IQR), 6–24) months of ART interruption. Most (86.2%) of them had received efavirenz (EFV)/nevirapine (NVP)-based first-line therapy for a median 16 (IQR, 7–26) months before ART interruption. Sixty-one (35.1%) patients had CRF07_BC HIV-1 strains, 58 (33.3%) CRF08_BC and 35 (20.1%) CRF01_AE. Thirty-four (19.5%) of the 174 patients were detected to harbor HIV drug-resistant variants on Sanger sequencing. Thirty-six (20.7%), 37 (21.3%), 42 (24.1%), 79 (45.4%) and 139 (79.9) patients were identified to have HIV drug resistance by next-generation sequencing at 20% (v.s. Sanger, p = 0.317), 10% (v.s. Sanger, p = 0.180), 5% (v.s. Sanger, p = 0.011), 2% (v.s. Sanger, p < 0.001) and 1% (v.s. Sanger, p < 0.001) of detection thresholds, respectively. K65R was the most common minority mutation, of 95.1% (58/61) and 93.1% (54/58) in CRF07_BC and CRF08_BC, respectively, when compared with 5.7% (2/35) in CRF01_AE (p < 0.001). In 49 patients that followed-up a median 10 months later, HIV drug resistance mutations at >20% frequency such as K103N, M184VI and P225H still existed, but with decreased frequencies. The prevalence of HIV drug resistance in ART interruption was higher than 15% in the survey. Next-generation sequencing was able to detect more minority drug resistance variants than Sanger. There was a sharp increase in minority drug resistance variants when the detection threshold was below 5%.
Keywords: HIV drug resistance, sanger sequencing, next-generation sequencing, interrupted antiretroviral therapy
1. Introduction
The scale-up of antiretroviral therapy (ART) has reduced HIV-related deaths and prevented new HIV infections [1]. By the end of 2019, 25.4 million people globally had received ART, with an increase of 19 million when compared with 2009 [2]. However, while the number of patients with ART increases, so does the number of patients with treatment interruption. The rate of ART discontinuation ranges from 10% to 78% under different settings [3,4,5,6] and keeps rising with ART prolonged [7]. Patients with ART interruption have decreased CD4+ T cell counts [8], a higher risk of AIDS or death [9] and are a potential source of HIV transmission.
The emergence of HIV drug resistance (HIVDR) results from the low fidelity of HIV reverse transcriptase, the rapid replication of the virus and the selective pressure of antiretroviral drugs [10]. It will compromise the efficacy of ART, lead to virological failure and hamper the progress of HIV/AIDS treatment and prevention [11]. Under ART interruption, HIVDR variants may persist, or revert to wild-type strains or to a resistant revertant like T215rev [12,13]. In addition, new HIVDR variants may be selected by residual drugs with longer half-lives in combined antiretroviral regimens [14].
HIVDR assays are usually carried out using Sanger sequencing (SS), which can detect minority variants at a 15%–20% frequency in HIV viral populations (quasi species) within patients [15]. Next-generation sequencing (NGS) has been increasingly valued in recent years, having the ability to detect lower-frequency mutants and thus more HIVDR variants [16], with increased throughput and higher cost-efficiency [17]. The HIVDR mutation frequencies detected by NGS, but not SS, concentrate between 1.1% and 21.3% [18]. NGS could identify HIV drug-resistant variants at a frequency as low as 0.4% [19]. When patients with low-frequency HIVDR mutations receive ART again, the minority drug-resistant strains may return as predominant ones under the selective pressure of the drug [20]. In addition, multiple studies have shown that the presence of low-frequency HIVDR mutations is often related to treatment failure [21,22].
In this study, we conducted a cross-sectional survey among patients under ART interruption, and compared the mutation detection between NGS and SS using plasma samples from patients with ART interruption, to provide more information about detecting low-frequency drug resistance mutations and further assistance in implementing ultrasensitive HIVDR surveillance in routine assays and to guide the choice of treatment regimen.
2. Results
2.1. Characteristics of the Study Population
A total of 174 patients were included in the survey in 2016 (Table 1). Only 60 (34.5%) patients were followed up a median 10 months later, with 49 still in ART interruption and 11 re-initiating ART. Among the 174 patients, 88.5% (154/174) were aged 30 and above; 66.1% (115/174) were male; 65.5% (114/174) were illiterate or had a primary school education; 67.2% (117/174) were married or living with a partner; 61.5% (107/174) and 4.0% (7/174) of the patients were infected through heterosexual and homosexual contacting, respectively and 27.6% (48/174) were infected through injection drug using. The numbers of patients with CRF01_AE, CRF07_BC and CRF08_BC HIV-1 strains were 35, 61 and 58 (20.1%, 35.1% and 33.3%), respectively. Seventy (40.2%) patients had a CD4+ T cell count of <200 cells/mm3 at investigation. A total of 150 (86.2%) patients had received non-nucleotide reverse transcriptase inhibitor (NNRTI)-based first-line antiretroviral regimens (stavudine (d4T)/azidothymidine (AZT)/tenofovir (TDF) + lamivudine (3TC) + efavirenz (EFV)/nevirapine (NVP)) before ART interruption. The median duration of treatment before ART interruption was 16 (interquartile range (IQR): 7–26) months, while the median duration of ART interruption was 12 (IQR: 6–24) months at survey.
Table 1.
Characteristics of participants with antiretroviral therapy (ART) interruption.
| Characteristics | Number (%) |
|---|---|
| Total | 174 |
| Age | |
| 18–30 | 20 (11.5) |
| 30–50 | 78 (44.8) |
| ≥50 | 76 (43.7) |
| Gender | |
| Male | 115 (66.1) |
| Female | 59 (33.9) |
| Education | |
| Illiterate and primary school | 114 (65.5) |
| Secondary school and above | 60 (34.5) |
| Marital status | |
| Married or living with partner | 117 (67.2) |
| Single | 32 (18.4) |
| Other | 25 (14.4) |
| Route of HIV infection | |
| Heterosexual | 107 (61.5) |
| Homosexual | 7 (4.0) |
| Injecting drug using | 48 (27.6) |
| Other | 12 (6.9) |
| Subtype | |
| CRF01_AE | 35 (20.1) |
| CRF07_BC | 61 (35.1) |
| CRF08_BC | 58 (33.3) |
| B′ | 16 (9.2) |
| Other a | 4 (2.3) |
| CD4+ T cell count at the time of investigation(per uL) | |
| <200 | 70 (40.2) |
| 200–350 | 62 (35.6) |
| ≥350 | 42 (24.2) |
| Antiretroviral regimen before discontinuation | |
| d4T+3TC+EFV/NVP | 1 (0.6) |
| AZT+3TC+EFV/NVP | 66 (37.9) |
| TDF+3TC+EFV/NVP | 83 (47.7) |
| AZT/TDF+3TC+LPV/r | 24 (13.8) |
| Duration of treatment before ART interruption(median, (IQR), months) | 16 (7–26) |
| Duration of ART interruption at survey (median, (IQR), months) | 12 (6–24) |
a includes CRF 55_01B (2, 1.1%), CRF 62_BC (1, 0.6%), Unknown (1, 0.6%). HIV, Human immunodeficiency virus; ART, antiretroviral therapy; IQR, interquartile range; d4T, Stavudine; 3TC, Lamivudine; AZT, Azidothymidine; TDF, Tenofovir; EFV, Efavirenz; NVP, Nevirapine; LPV/r, Lopinavir/r.
2.2. Detected HIV Drug Resistance Mutations
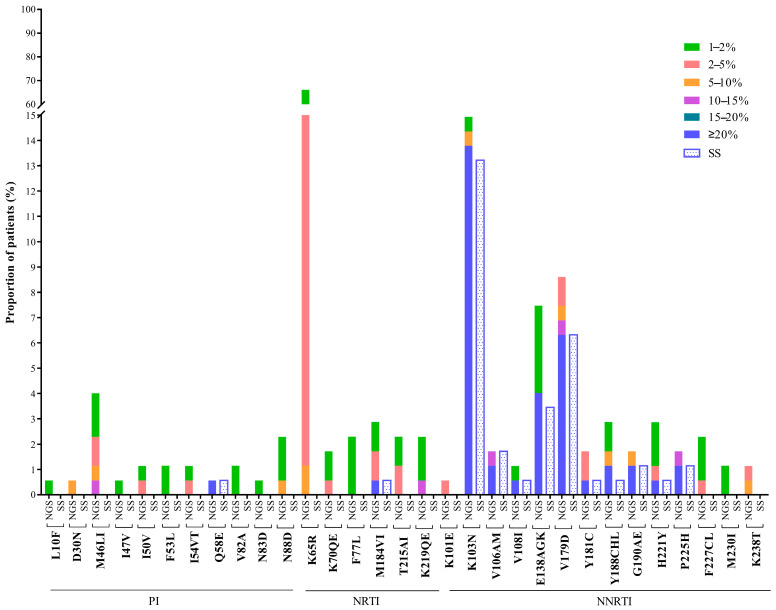
Drug resistance mutations (DRMs) were detected at 12 positions in the partial pol region by Sanger sequencing (Figure 1), including one in the protease (PR) region, an accessory protease inhibitor (PI)-related resistance mutation (Q58E), and eleven in the reverse transcriptase (RT) region, including one nucleotide reverse transcriptase inhibitor (NRTI)-related (M184V), and ten others were NNRTI-related. The most common drug resistance mutation was K103N (13.2%), followed by V179D (6.3%) and E138AGK (3.5%). At the 20% detection threshold, NGS detected all the mutations identified by SS except a V106M mutation in one patient, as there were mixtures in the first (G to R) and third nucleotides (A to R) in the codon at Sanger sequencing. In addition, NGS detected three more mutations in three patients: Y188C, E138A and K103N, at frequencies of 22.96%, 27.38% and 43.68%, respectively. There were no additional DRMs detected at the frequencies between 15% and 20%. Low-frequency DRMs (<15% frequency) were only detected by NGS, but not by SS. K65R was the most common low-frequency DRM with frequencies between 1% and 9%, concentrated at frequencies from 2% to 5%. It is interesting that this low-frequency K65R mutation is significantly unevenly distributed among subtypes; 5.7% (2/35) in CRF01_AE, when compared with 95.1% (58/61) in CRF07_BC and 93.1% (54/58) in CRF08_BC (p < 0.001). Other low-frequency NRTI-related mutations were K70QE, F77L, T215AI and K219QE. M46LI was the common low-frequency PI-related mutation, with frequencies ranging from 1% to 13%. Other PI-related mutations such as L10F, I47V, I50V, F53L, I54VT and N83D have the mutation frequency of about 2%.
Figure 1.
Frequency and pattern of HIV drug resistance (HIVDR) mutations detected by Sanger sequencing (SS) and next-generation sequencing (NGS) at different detection thresholds. Note: HIVDR, HIV drug resistance; SS, Sanger sequencing; NGS, Next-generation sequencing; PI, Protease inhibitor; NRTI, Nucleoside reverse-transcriptase inhibitor; NNRTI, non-nucleoside reverse-transcriptase inhibitor.
2.3. HIV Drug Resistance Interpretations
Based on Sanger sequencing, 19.5% (34/174) of the patients were shown to have drug resistance variants. With NGS, the rate of resistance was the same; 20.7% (36/174) at the detection thresholds 20% and 15%. It climbed up to 21.3% (37/174), 24.1% (42/174), 45.4% (79/174) and 79.9% (139/174) at thresholds 10%, 5%, 2% and 1%, respectively. Compared with SS, NGS got significantly higher rates of drug resistance at the 1%, 2% and 5% thresholds (p < 0.05). For PI and NRTI, the prevalence of HIVDR was the same at 0.6%, identified by SS and NGS at the 15% detection threshold. However, NGS at the 1% and 2% thresholds identified more NRTI-related drug resistance variants (69.0% and 24.7%, respectively) than SS. Compared with NGS, a slightly lower percentage of HIVDR was found by SS for NNRTI-related drug resistance (19.0%, Table 2). For the efavirenz (EFV) or nevirapine (NVP) in first-line NNRTI, the difference between NGS and SS in the identification of drug resistance levels was relatively small. EFV- or NVP-related resistance rates were identified in 15.5% (27/174) and 16.1% (28/174) of patients by SS and NGS (>15% frequencies), respectively.
Table 2.
Prevalence of HIV drug resistance detected by SS and NGS.
| SS | 20% NGS a | 15% NGS a | 10% NGS a | 5% NGS a | 2% NGS a | 1% NGS a | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SS or NGS at Various Thresholds | N (%) | N (%) | p Value | N (%) | p Value | N (%) | p Value | N (%) | p Value | N (%) | p Value | N (%) | p Value |
| Any classes | 34 (19.5) | 36 (20.7) | 0.317 | 36 (20.7) | 0.317 | 37 (21.3) | 0.180 | 42 (24.1) | 0.011 | 79 (45.4) | <0.001 | 139 (79.9) | <0.001 |
| PI-related | 1 (0.6) | 1 (0.6) | 1.000 | 1 (0.6) | 1.000 | 2 (1.2) | 0.317 | 5 (2.9) | 0.046 | 9 (5.2) | 0.005 | 23 (13.2) | <0.001 |
| NRTI-related | 1 (0.6) | 1 (0.6) | 1.000 | 1 (0.6) | 1.000 | 1 (0.6) | 1.000 | 3 (1.7) | 0.157 | 43 (24.7) | <0.001 | 120 (69.0) | <0.001 |
| NNRTI-related | 33 (19.0) | 35 (20.1) | 0.317 | 35 (20.1) | 0.317 | 35 (20.1) | 0.317 | 36 (20.7) | 0.180 | 43 (24.7) | 0.004 | 51 (29.3) | <0.001 |
| EFV/NVP | 27 (15.5) | 28 (16.1) | 0.564 | 28 (16.1) | 0.564 | 29 (16.7) | 0.317 | 31 (17.8) | 0.103 | 37 (21.3) | 0.004 | 46 (26.4) | <0.001 |
SS, Sanger sequencing; a NGS, next-generation sequencing at 1%, 2%, 5%, 10%, 15%, or 20% detection thresholds; PI, Protease inhibitor; NRTI, Nucleoside reverse-transcriptase inhibitor; NNRTI, non-nucleoside reverse-transcriptase inhibitor.
2.4. Relationship between CD4+ T Cell Count, Viral Load and HIVDR Mutation Frequency
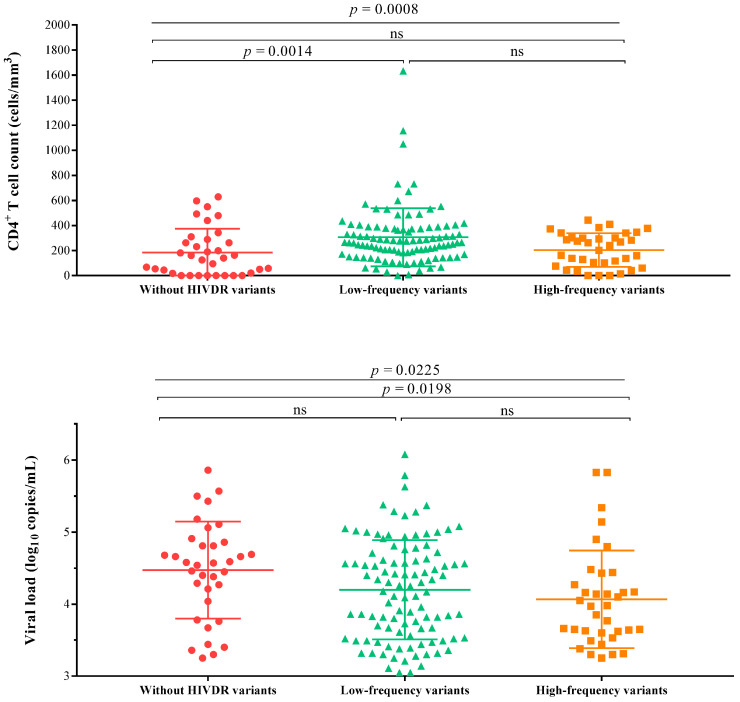
The patients were divided into three groups across the mutation frequencies detected by NGS: 35 patients without HIVDR variants, 103 patients with mutation frequencies lower than 15% and 36 patients with mutation frequencies higher than 15%. Their median CD4+ T cell counts were 140 cell/mm3 (18–289), 265 cell/mm3 (IQR: 172–378) and 222 cell/mm3 (IQR: 82–302), respectively (p < 0.05). In addition, their median viral loads were 4.6 log10 copies/mL (4.0–4.9), 4.3 log10 copies/mL (IQR: 3.6–4.7) and 4.0 log10 copies/mL (IQR: 3.6–4.4), respectively (p < 0.05). In addition, there was a statistically significant difference in the viral loads between patients with high-frequency variants and patients without variants (p = 0.0198, Figure 2).
Figure 2.
The relationship between the CD4+ T cell count, viral load and HIV drug resistance mutation frequency. Note: The patients were divided into three groups according to the mutation frequency detected by next-generation sequencing. High-frequency variants mean that their mutation frequencies were higher than 15% and low-frequency variants mean that their mutation frequencies were less than 15%. HIVDR, HIV drug resistance; ns, no significance.
2.5. Changes of HIVDR Mutations at Follow-Up
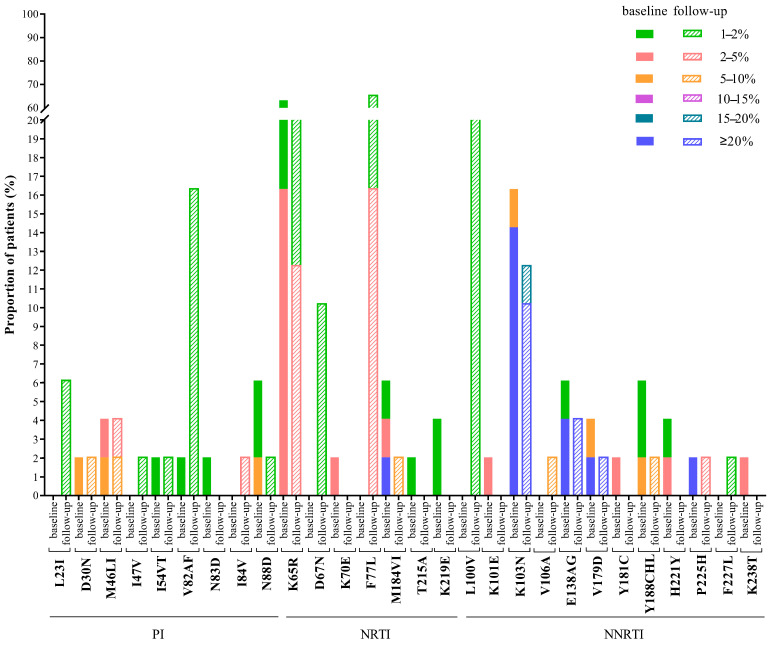
Forty-nine patients were still with ART interruption and were available for follow-up after the median of ten months (IQR: 8–11). At baseline, mutations with a frequency of 20% and above were NRTI-related, such as M184VI (2.0%, 1/49), and NNRTI-related like K103N (14.3%, 7/49), E138AG (4.1%, 2/49), V179D (2.0%, 1/49) and P225H (2.0%, 1/49). Although these variants still existed at follow-up, the frequencies of the mutations M184VI, K103N and P225H decreased over time, and most of them remained at frequencies of more than 20%. However, the frequency of K103N in one patient (GX064) had dropped from 43.7% to 15.3%, and the mutation K103N in another patient (CQ046) with a frequency of 36.9% disappeared (Supplementary Materials). Within a year, some minority DRMs at frequencies 1%–10% remained unchanged, including: PI-related D30N, M46LI, I54VT and N88D, NRTI-related K65R and NNRTI-related Y188CHL. Moreover, K65R was still the most common low-frequency mutation at the follow-up. However, some minority DRMs at frequencies of 1%–5% disappeared, including N83D with PI-related, K70E, T215A, and K219E with NRTI-related and K101E, Y181C, H221Y and K238T with NNRTI-related, while others emerged, such as NNRTI-related V106A in patient GX088 at a frequency of 8.7%, and L23I, I47V and I84V with PI-related, D67N and F77L with NRTI-related and L100V and F227L with NNRTI-related, which appeared at frequencies of 1%–5% (Figure 3).
Figure 3.
Changes of HIV drug resistance mutations during baseline and follow-up. Note: 49 of the 174 patients were followed up nearly a year later. Mutations and mutation frequency were detected by next-generation sequencing. PI, Protease inhibitor; NRTI, Nucleoside reverse-transcriptase inhibitor; NNRTI, non-nucleoside reverse-transcriptase inhibitor.
3. Discussion
In this study, a cross-sectional survey was conducted among 174 patients with ART interruption in five areas more heavily affected by HIV/AIDS in China. The prevalence of HIVDR was 19.5% at Sanger sequencing, which was obviously higher than a pretreatment HIVDR survey conducted among ART naïve patients in 2017 [23]. A cross-sectional survey of patients with a median of 13.9 months on antiretroviral therapy in eight provinces in China showed that the prevalence of HIVDR in patients receiving ART was 4.3% [24]. Other studies have also suggested that the prevalence of drug resistance in patients receiving ART in some areas of China during the same period is less than 5% [25,26,27]. Compared with patients who have been receiving antiretroviral therapy, patients whose ART has been interrupted have a higher HIVDR prevalence. Furthermore, the rate of resistance to EFV and/or NVP (15.5%) was more than three times that (4.6%) in those patients. These findings were consistent with those in other countries [28], providing more evidence that patients with ART interruption should consider substituting EFV/NVP for PI or integrase inhibitors when re-initiating antiretroviral treatment.
It was unexpected to get higher rates of HIVDR at NGS than through Sanger sequencing. However, the increase in HIVDR was not statistically significant until the detection threshold was lowered to 5%. Moreover, DRMs detected in protease would have limited impacts on such PIs as Lopinavir/r (LPV/r) in second-line regimens. Four DRMs including D30N (1), M46I (2) and N88D (1) were detected in protease in four participants at a 5%–15% frequency, in addition to one Q58E at a frequency above 90%. The former two confer high or intermediate resistance to Nelfinavir, which is seldomly used at present. N88D and Q58E are PI accessary resistance mutations. There were slight increases in NRTI and NNRTI resistance at the threshold of 5%, when compared with the threshold of 20% or SS, which was consistent with other previous studies [29,30].
Despite the same primer sets for reverse transcription and PCR amplification, there were three NNRTI DRMs in three patients with frequencies between 23% and 44% detected by NGS, not SS. These discrepancies were more likely to be PCR resampling produced at the first several rounds of amplification, and less likely to be the artifacts during NGS sequencing, as NGS artifacts had a <1% frequency and were randomly distributed. Compared with that at the threshold of 5%, the rates of HIVDR were almost doubled when the threshold decreased to 2%, and tripled when down to 1%, as very-low-frequency DRMs were dramatically increased between 1% and 5%. The minority mutations detected by NGS may have resulted from apolipoprotein B mRNA editing catalytic polypeptide (APOBEC)-mediated G-to-A hypermutation such as E138K, M184I and G190E in RT or spontaneous mutation during viral replication, or from PCR error, which was introduced by error-prone reverse transcriptase or PCR enzymes [31]. The sharp increase in low-frequency DRMs agrees with the 5% detection threshold mentioned in other studies [32,33]. At present, the optimal threshold for the NGS detection of low-frequency mutation sites is still inconclusive. Studies have shown that a 5% threshold can provide a reproducible clinically relevant treatment result [34], suggested using a detection threshold of 5% to minimize technical artifacts [31,35]. Using a 5% threshold to report low-frequency variation allows NGS to achieve a moderately increased sensitivity to detect low-frequency DRMs, without compromising sequence accuracy [36,37].
The most common NNRTI DRM above a 5% frequency was K103N (14.4%) in RT, which is consistent with the ART history of EFV/NVP-based first-line therapy. In NRTI DRMs, K65R was the highest (1.2%) at a >5% frequency, which was similar to other research results [29,38]. In addition, this mutation was dramatically increased to 20.7% at 2%–5% frequency. We also found that the distribution of the K65R mutation was related to the HIV subtypes CRF07_BC and CRF08_BC. The K65R mutation causes high-level resistance to tenofovir and intermediate resistance to lamivudine, which are the backbone of first- and second-line regimens. The minority K65R mutations may become major mutations when re-initiating ART, giving rise to virological failure. The subtype-specific differences in the K65R mutation distribution are mainly due to differences in codon usage [39], as it is subtype C in this region in the CRF07_BC and CRF08_BC strains.
The disease progression in patients with HIV is usually monitored by measuring their CD4+ T cell counts. After the interruption of antiretroviral therapy, the CD4+ T cell count drops rapidly in the first few months, then declines slowly [40,41]. A previous study did not find a significant difference in the CD4+ T cell count between patients with and without HIVDR variants [42]. In this study, the patients were divided into three groups across mutation frequencies detected by NGS at a 15% threshold: patients without variants, patients with low-frequency variants and patients with high-frequency variants. Interestingly, the Kruskal–Wallis test found significant differences in the CD4+ T cell counts among the three groups. After the interruption of ART, the viral load level increased exponentially, reaching a peak of 106 copies/mL. Then, the viral load usually drops to 103–105 copies/mL within a few weeks, which can remain relatively stable for many years [43]. In this study, the viral load of patients under ART interruption was above 103 copies/mL, and there were statistical differences in the viral loads among the three groups.
Generally speaking, the drug resistance rate will decrease over time in patients after ART interruption. This trend is mainly due to the fact that, in the absence of drug selection pressure, wild-type strains with high viral fitness replace resistant strains to become the main strains in patients [44]. Compared with the baseline, the HIVDR mutations, such as N88D, K65R, M184VI, K103N, E138AG, V179D and P225H, still existed but the frequency gradually decreased, consistent with earlier studies [18,42]. Nevertheless, we found new low-frequency mutations such as I84V, F77L and V106A at follow-ups in this study. A study also showed that new mutations emerge in the absence of drug selection pressure [18]. The emergence of new mutations may be due to the reason that, without the drug selection pressure, virus strains carrying resistance-related mutations may have reduced replication fitness, so they cannot become dominant strains. The results of this study show that new mutations emerge in the form of low frequency, but whether they will continue to exist as the main drug-resistant strains over time is still unknown, and further research is needed.
There are limitations to this study. Firstly, the number of participants was limited. The findings could not be simply extrapolated to other patients with ART interruption. However, the fall or rise in DRMs would be similar to the other settings without ART. The minority DRMs detected by NGS were consistent with other pre-treatment surveys. Secondly, the participants in this study mainly used NNRTI-based first-line regimens before ART interruption. Thus, the findings were not able to reflect the situations with the interruption of ART on PI or integrase inhibitors. Thirdly, the rate of follow-up was low, as patients with ART interruption also dropped out of the HIV care program and were reluctant to keep the follow-up appointments.
In conclusion, patients with ART interruption had higher HIVDR, especially to EFV/NVP. NGS is able to detect more DRMs in such patients than SS, but largely at frequencies of less than 5%. Studies have shown that the drug resistance reports based on a 5% threshold can predict the failure of ART [21,45]. Due to the limitations of this study, the optimal NGS threshold for predicting the failure of antiviral therapy has not yet been determined. Further studies are needed to define the NGS detection threshold for providing a predicative of ART failure.
4. Materials and Methods
4.1. Study Design and Study Participants
We conducted a cross-sectional study among adult patients with ART interruption between 1 January and 31 December 2016, in four provinces and one municipality of China, including Guangxi, Henan, Sichuan, Yunnan and Chongqing, where in China the HIV epidemic was more concentrated, as described previously [46]. Inclusion criteria were: patients aged eighteen years or older, had discontinued ART for at least one month at survey, had received ART for at least three months before ART interruption and had agreed to provide written informed consent. These patients were followed up in 2017. Plasma samples were handled locally and transported in dry ice to the laboratory at the National Center for AIDS/STD Control and Prevention (NCAIDS), Chinese Center for Disease Control and Prevention (China CDC), where the HIV viral load was measured, and HIV drug resistance was genotyped on samples with a viral load ≥1000 copies/mL.
4.2. HIV RNA Extraction and Sample Amplification
Viral RNA was extracted using the QIAamp viral RNA mini kit (Qiagen, Hilton, Germany), following the manufacturer’s protocol. Extracted RNA was used for amplification of the PR region (4–99 amino acids) and the partial RT region (1–251 amino acids) of the HIV-1 pol gene region using an in-house method [47]. Briefly, the first-round PCR was performed in 25 µL volume reactions using the one-step RT-PCR kit (Promega, Madison, WI, USA) with the primers PRTM-F1 and RT-R1. The second-round PCR was performed in 50 µL volume reactions using the primers PRT-F2 and RT-R2.
4.3. Sanger Sequencing and Drug Resistance Analysis
The amplified products were sequenced using Sanger sequencer ABI 3730 (Applied Biosystems, Carlsbad, CA, USA). Obtained raw sequences were edited and assembled using Sequencher (version 4.10.1, Gene Codes Corporation, Ann Arbor, MI). The secondary peak threshold was set to 20%. The edited sequence was aligned with the HXB2 reference sequence through BioEdit (version 7.0.9, Informer Technologies Inc.). Sample contamination and other sequence quality controls were monitored using the WHO HIVDR QC tool (https://recall.bccfe.ca/who_qc/, accessed on 23 September 2019) and through constructing Neighbor joining phylogenetic trees using MEGA (version 6.06). HIV-1 subtypes were determined using reference sequences from HIV Databases (https://www.hiv.lanl.gov/content/index, accessed on 8 October 2019) through phylogenetic trees. The drug resistance mutations were identified and interpreted using the HIVdb version 8.9-1 (https://hivdb.stanford.edu/hivdb/, accessed on 10 October 2019).
4.4. Next-Generation Sequencing and Drug Resistance Analysis
Second-round amplicons were cleaned using KAPA PureBeads (Roche, Basel, Switzerland) and quantified using the Qubit dsDNA HS assay kit (Thermo Fisher Scientific, Carlsbad, CA, USA). Sequencing libraries were prepared using the 96-sample KAPA HyperPlus Kit (Roche, Basel, Switzerland). The samples were pooled in each run with a 40% Phix control library (v3, Illumina, San Diego, CA, USA) to increase the diversity of libraries and then sequenced on the Illumina Miseq system [48] using a v3 600-cycle reagent kit (Illumina, San Diego, CA, USA). The raw data were analyzed using the HyDRA Web tool (http://hydra.canada.ca/, accessed on 20 January 2020) according to HyDRA Web User Guide [49], producing lists and frequencies of drug resistance mutations, and then interpreted by using the HIVdb algorithm.
4.5. Statistical Analysis
DRMs were identified according to the 2019 International Antiviral Society (IAS) list. HIV drug resistance was defined as low-, intermediate- or high-levels of drug resistance according to the HIVdb algorithm 8.9-1. HIV drug resistance was determined based on NGS with detection thresholds of 20%, 15%, 10%, 5%, 2% and 1%, and was compared with that detected based on Sanger sequencing by the McNemar test, respectively. Quantitative data are presented as medians and were analyzed by the Kruskal–Wallis test. All data were analyzed using the Statistical Analysis System version 9.4 (SAS Institute Inc., Cary, NC, USA). Two-sided p values of <0.05 were considered statistically significant.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/10/3/264/s1, Table S1: The HIV drug resistance mutations of each sample (n = 174) were detected by Sanger sequencing and the next-generation sequencing; Table S2: The drug resistance mutations of each sample (n = 49) at baseline and follow-up detected by the next-generation sequencing, and the value in brackets is the mutation frequencies.
Author Contributions
M.L., H.X., Y.R., Y.S. and L.L. (Lingjie Liao). were responsible for study design and planning. S.L. (Shujia Liang), C.Z., M.C., S.L. (Shu Liang) and C.L. contributed to data collection. M.L., L.L. (Lei Liu), Z.Z., Y.F., C.S., H.X., Y.R., Y.S. and L.L. (Lingjie Liao) contributed to data analysis and interpretation. M.L., H.X., Y.R., Y.S. and L.L. (Lingjie Liao) contributed to writing the manuscript. All authors read and approved the final version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (11971479), the Ministry of Science and Technology of China (grant 2017ZX10201101, 2018ZX10721102-006), the State Key Laboratory of Infectious Disease Prevention and Control, the Department of Science and Technology of Guangxi Zhuang Autonomous Region (AB16380213), the Key technical posts for HIV/AIDS prevention and control of Guangxi Bagui scholars, and special funds for AIDS prevention and control. The fund providers had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Institutional review board approval was granted by the National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Poorolajal J., Hooshmand E., Mahjub H., Esmailnasab N., Jenabi E. Survival rate of AIDS disease and mortality in HIV-infected patients: A meta-analysis. Public Health. 2016;139:3–12. doi: 10.1016/j.puhe.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS Latest Global and Regional Statistics on the Status of the AIDS Epidemic. [(accessed on 27 September 2020)];2020 Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf.
- 3.Touloumi G., Pantazis N., Antoniou A., Stirnadel H.A., Walker S.A., Porter K. Highly Active Antiretroviral Therapy Interruption. JAIDS J. Acquir. Immune Defic. Syndr. 2006;42:554–561. doi: 10.1097/01.qai.0000230321.85911.db. [DOI] [PubMed] [Google Scholar]
- 4.Olsen C.H., Mocroft A., Kirk O., Vella S., Blaxhult A., Clumeck N., Fisher M., Katlama C., Phillips A., Lundgren J., et al. Interruption of combination antiretroviral therapy and risk of clinical disease progression to AIDS or death. HIV Med. 2007;8:96–104. doi: 10.1111/j.1468-1293.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 5.Kavasery R., Galai N., Astemborski J., Lucas G.M., Celentano D.D., Kirk G.D., Mehta S.H. Nonstructured Treatment Interruptions Among Injection Drug Users in Baltimore, MD. JAIDS J. Acquir. Immune Defic. Syndr. 2009;50:360–366. doi: 10.1097/QAI.0b013e318198a800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu H., Napravnik S., Eron J., Cole S., Ma Y., Wohl D., Dou Z., Zhang Y., Liu Z., Zhao D., et al. Attrition among Human Immunodeficiency Virus (HIV)- Infected Patients Initiating Antiretroviral Therapy in China, 2003–2010. PLoS ONE. 2012;7:e39414. doi: 10.1371/journal.pone.0039414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvarez-Uria G., Naik P.K., Pakam R., Midde M. Factors associated with attrition, mortality, and loss to follow up after antiretroviral therapy initiation: Data from an HIV cohort study in India. Glob. Health Action. 2013;6:21682. doi: 10.3402/gha.v6i0.21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ananworanich J., Gayet-Ageron A., Le Braz M., Prasithsirikul W., Chetchotisakd P., Kiertiburanakul S., Munsakul W., Raksakulkarn P., Tansuphasawasdikul S., Sirivichayakul S., et al. CD4-guided scheduled treatment interruptions compared with continuous therapy for patients infected with HIV-1: Results of the Staccato randomised trial. Lancet. 2006;368:459–465. doi: 10.1016/S0140-6736(06)69153-8. [DOI] [PubMed] [Google Scholar]
- 9.Hogg R.S., Heath K., Bangsberg D., Yip B., Press N., O’Shaughnessy M.V., Montaner J.S.G. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002;16:1051–1058. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- 10.Pereira-Vaz J., Duque V., Trindade L., Saraiva-Da-Cunha J., Meliço-Silvestre A. Detection of the protease codon 35 amino acid insertion in sequences from treatment-naïve HIV-1 subtype C infected individuals in the Central Region of Portugal. J. Clin. Virol. 2009;46:169–172. doi: 10.1016/j.jcv.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization Global Action Plan on HIV Drug Resistance 2017–2021. [(accessed on 15 September 2020)]; Available online: https://www.who.int/hiv/pub/drugresistance/hivdr-action-plan-2017-2021/en/
- 12.Devereux H.L., Youle M., Johnson M.A., Loveday C. Rapid decline in detectability of HIV-1 drug resistance mutations after stopping therapy. Aids. 1999;13:F123–F127. doi: 10.1097/00002030-199912240-00001. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence J., Mayers D.L., Hullsiek K.H., Collins G., Abrams D.I., Reisler R.B., Crane L.R., Schmetter B.S., Dionne T.J., Saldanha J.M., et al. Structured Treatment Interruption in Patients with Multidrug-Resistant Human Immunodeficiency Virus. N. Engl. J. Med. 2003;349:837–846. doi: 10.1056/NEJMoa035103. [DOI] [PubMed] [Google Scholar]
- 14.Taylor S., Jayasuriya A., Fisher M., Allan S., Wilkins E., Gilleran G., Heald L., Fidler S., Owen A., Back D., et al. Lopinavir/ritonavir single agent therapy as a universal combination antiretroviral therapy stopping strategy: Results from the STOP 1 and STOP 2 studies. J. Antimicrob. Chemother. 2011;67:675–680. doi: 10.1093/jac/dkr491. [DOI] [PubMed] [Google Scholar]
- 15.Casadellà M., Paredes R. Deep sequencing for HIV-1 clinical management. Virus Res. 2017;239:69–81. doi: 10.1016/j.virusres.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Arias A., López P., Sánchez R., Yamamura Y., Rivera-Amill V. Sanger and Next Generation Sequencing Approaches to Evaluate HIV-1 Virus in Blood Compartments. Int. J. Environ. Res. Public Health. 2018;15:1697. doi: 10.3390/ijerph15081697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John E.P.S., Simen B.B., Turenchalk G.S., Braverman M.S., Abbate I., Aerssens J., Bouchez O., Gabriel C., Izopet J., Meixenberger K., et al. A Follow-Up of the Multicenter Collaborative Study on HIV-1 Drug Resistance and Tropism Testing Using 454 Ultra Deep Pyrosequencing. PLoS ONE. 2016;11:e0146687. doi: 10.1371/journal.pone.0146687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nouchi A., Nguyen T., Valantin M.A., Simon A., Sayon S., Agher R., Calvez V., Katlama C., Marcelin A.G., Soulie C. Dynamics of drug resistance-associated mutations in HIV-1 DNA reverse tran-scriptase sequence during effective ART. J. Antimicrob. Chemother. 2018;73:2141–2146. doi: 10.1093/jac/dky130. [DOI] [PubMed] [Google Scholar]
- 19.Kozal M.J., Chiarella J., John E.P.S., Moreno E.A., Simen B.B., Arnold T.E., Lataillade M. Prevalence of low-level HIV-1 variants with reverse transcriptase mutation K65R and the effect of antiretroviral drug exposure on variant levels. Antivir. Ther. 2011;16:925–929. doi: 10.3851/IMP1851. [DOI] [PubMed] [Google Scholar]
- 20.Metzner K.J., Giulieri S.G., Knoepfel S.A., Rauch P., Burgisser P., Yerly S., Gunthard H.F., Cavassini M. Minority Quasispecies of Drug-Resistant HIV-1 That Lead to Early Therapy Failure in Treatment-Naive and -Adherent Patients. Clin. Infect. Dis. 2009;48:239–247. doi: 10.1086/595703. [DOI] [PubMed] [Google Scholar]
- 21.Mbunkah A.H., Bertagnolio S., Hamers R.L., Hunt G., Inzaule S., De Wit T.F.R., Paredes R., Parkin N.T., Jordan M.R., Metzner K.J., et al. Low-Abundance Drug-Resistant HIV-1 Variants in Antiretroviral Drug-Naive Individuals: A Systematic Review of Detection Methods, Prevalence, and Clinical Impact. J. Infect. Dis. 2019;221:1584–1597. doi: 10.1093/infdis/jiz650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inzaule S.C., Hamers R.L., Noguera-Julian M., Casadellà M., Parera M., Kityo C., Steegen K., Naniche D., Clotet B., De Wit T.F.R., et al. Clinically relevant thresholds for ultrasensitive HIV drug resistance testing: A multi-country nested case-control study. Lancet HIV. 2018;5:e638–e646. doi: 10.1016/S2352-3018(18)30177-2. [DOI] [PubMed] [Google Scholar]
- 23.Kang R.-H., Liang S.-J., Ma Y.-L., Liang S., Xiao L., Zhang X.-H., Lu H.-Y., Xu X.-Q., Luo S.-B., Sun X.-G., et al. Pretreatment HIV drug resistance in adults initiating antiretroviral therapy in China, 2017. Infect. Dis. Poverty. 2020;9:1–9. doi: 10.1186/s40249-020-00668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuo Z., Liang S., Sun X., Bussell S., Yan J., Kan W., Leng X., Liao L., Ruan Y., Shao Y., et al. Drug Resistance and Virological Failure among HIV-Infected Patients after a Decade of Antiretroviral Treatment Expansion in Eight Provinces of China. PLoS ONE. 2016;11:e0166661. doi: 10.1371/journal.pone.0166661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin B., Sun X., Su S., Lv C., Zhang X., Lin L., Wang R., Fu J., Kang D. HIV drug resistance in HIV positive individuals under antiretroviral treatment in Shandong Province, China. PLoS ONE. 2017;12:e0181997. doi: 10.1371/journal.pone.0181997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J., He C., Hsi J.H., Xu X., Liu Y., He J., Ling H., Ding P., Tong Y., Zou X., et al. Virological Outcomes and Drug Resistance in Chinese Patients after 12 Months of 3TC-Based First-Line Antiretroviral Treatment, 2011–2012. PLoS ONE. 2014;9:e88305. doi: 10.1371/journal.pone.0088305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xing H., Wang X., Liao L., Ma Y., Su B., Fu J., He J., Chen L., Pan X., Dong Y., et al. Incidence and Associated Factors of HIV Drug Resistance in Chinese HIV-Infected Patients Receiving Antiretroviral Treatment. PLoS ONE. 2013;8:e62408. doi: 10.1371/journal.pone.0062408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization HIV Drug Resistance Report 2019. [(accessed on 28 August 2020)]; Available online: https://www.who.int/hiv/pub/drugresistance/hivdr-report-2019/en/
- 29.Moscona R., Ram D., Wax M., Bucris E., Levy I., Mendelson E., Mor O. Comparison between next-generation and Sanger-based sequencing for the detection of transmitted drug-resistance mutations among recently infected HIV-1 patients in Israel, 2000–2014. J. Int. AIDS Soc. 2017;20:21846. doi: 10.7448/IAS.20.1.21846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor T., Lee E.R., Nykoluk M., Enns E., Liang B., Capina R., Gauthier M.-K., Van Domselaar G., Sandstrom P., Brooks J., et al. A MiSeq-HyDRA platform for enhanced HIV drug resistance genotyping and surveillance. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-019-45328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tzou P.L., Ariyaratne P., Varghese V., Lee C., Rakhmanaliev E., Villy C., Yee M., Tan K., Michel G., Pinsky B.A., et al. Comparison of anIn VitroDiagnostic Next-Generation Sequencing Assay with Sanger Sequencing for HIV-1 Genotypic Resistance Testing. J. Clin. Microbiol. 2018;56:e00105-18. doi: 10.1128/JCM.00105-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stelzl E., Pröll J., Bizon B., Niklas N., Danzer M., Hackl C., Stabentheiner S., Gabriel C., Kessler H.H. Human immunodeficiency virus type 1 drug resistance testing: Evaluation of a new ultra-deep sequencing-based protocol and comparison with the TRUGENE HIV-1 Genotyping Kit. J. Virol. Methods. 2011;178:94–97. doi: 10.1016/j.jviromet.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 33.Chen N.-Y., Kao S.-W., Liu Z.-H., Wu T.-S., Tsai C.-L., Lin H.-H., Wong W.-W., Chang Y.-Y., Chen S.-S., Ku S.W.-W. Shall I trust the report? Variable performance of Sanger sequencing revealed by deep sequencing on HIV drug resistance mutation detection. Int. J. Infect. Dis. 2020;93:182–191. doi: 10.1016/j.ijid.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Ávila-Ríos S., Parkin N., Swanstrom R., Paredes R., Shafer R., Ji H., Kantor R. Next-Generation Sequencing for HIV Drug Resistance Testing: Laboratory, Clinical, and Implementation Considerations. Viruses. 2020;12:617. doi: 10.3390/v12060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trabaud M.-A., Icard V., Ramière C., Tardy J.-C., Scholtes C., André P. Comparison of HIV-1 drug-resistance genotyping by ultra-deep sequencing and sanger sequencing using clinical samples. J. Med. Virol. 2017;89:1912–1919. doi: 10.1002/jmv.24872. [DOI] [PubMed] [Google Scholar]
- 36.Fogel J.M., Bonsall D., Cummings V., Bowden R., Golubchik T., De Cesare M., Wilson E.A., Gamble T., Del Rio C., Batey D.S., et al. Performance of a high-throughput next-generation sequencing method for analysis of HIV drug resistance and viral load. J. Antimicrob. Chemother. 2020;75:3510–3516. doi: 10.1093/jac/dkaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dessilly G., Goeminne L., Vandenbroucke A.-T., Dufrasne F.E., Martin A., Kabamba-Mukabi B. First evaluation of the Next-Generation Sequencing platform for the detection of HIV-1 drug resistance mutations in Belgium. PLoS ONE. 2018;13:e0209561. doi: 10.1371/journal.pone.0209561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu T.F., Shafer R.W. Web Resources for HIV Type 1 Genotypic-Resistance Test Interpretation. Clin. Infect. Dis. 2006;42:1608–1618. doi: 10.1086/503914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clutter D.S., Jordan M.R., Bertagnolio S., Shafer R.W. HIV-1 drug resistance and resistance testing. Infect. Genet. Evol. 2016;46:292–307. doi: 10.1016/j.meegid.2016.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mocroft A., Phillips A.N., Gatell J., Ledergerber B., Fisher M., Clumeck N., Losso M., Lazzarin A., Fätkenheuer G., Lundgren J.D. Normalisation of CD4 counts in patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: An observational cohort study. Lancet. 2007;370:407–413. doi: 10.1016/S0140-6736(07)60948-9. [DOI] [PubMed] [Google Scholar]
- 41.El-Sadr W., Lundgren J.D., Neaton J.D., Gordin F., Abrams D., Arduino R.C., Babiker A., Burman W.J., Clumeck N., Cohen C., et al. CD4+ Count–Guided Interruption of Antiretroviral Treatment. N. Engl. J. Med. 2006;355:2283–2296. doi: 10.1056/nejmoa062360. [DOI] [PubMed] [Google Scholar]
- 42.E Iarikov D., Irizarry-Acosta M., Martorell C., A Rauch C., Hoffman R.P., Skiest D.J. Use of HIV Resistance Testing After Prolonged Treatment Interruption. JAIDS J. Acquir. Immune Defic. Syndr. 2010;53:333–337. doi: 10.1097/QAI.0b013e3181c79ab0. [DOI] [PubMed] [Google Scholar]
- 43.Hill A.L., Rosenbloom D.I.S., Nowak M.A., Siliciano R.F. Insight into treatment of HIV infection from viral dynamics models. Immunol. Rev. 2018;285:9–25. doi: 10.1111/imr.12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paredes R., Sagar M., Marconi V.C., Hoh R., Martin J.N., Parkin N.T., Petropoulos C.J., Deeks S.G., Kuritzkes D.R. In Vivo Fitness Cost of the M184V Mutation in Multidrug-Resistant Human Immunodeficiency Virus Type 1 in the Absence of Lamivudine. J. Virol. 2008;83:2038–2043. doi: 10.1128/JVI.02154-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohamed S., Ravet S., Camus C., Khiri H., Olive D., Halfon P. Clinical and analytical relevance of NNRTIs minority mutations on viral failure in HIV-1 infected patients. J. Med. Virol. 2014;86:394–403. doi: 10.1002/jmv.23853. [DOI] [PubMed] [Google Scholar]
- 46.Lei L., Zhong-Bao Z., Ling-Jie L., Shu-Jia L., Yan-Ling M., Guo-Hui W., Shu L., Sui-An T., Jian-Mei H., Yi-Ming S., et al. The drug resistance in HIV/AIDS patients who had stopped ART in 2016. China Trop. Med. 2018;18:1613–1618. [Google Scholar]
- 47.Inzaule S., Yang C., Kasembeli A., Nafisa L., Okonji J., Oyaro B., Lando R., Mills L.A., Laserson K., Thomas T., et al. Field Evaluation of a Broadly Sensitive HIV-1 In-House Genotyping Assay for Use with both Plasma and Dried Blood Spot Specimens in a Resource-Limited Country. J. Clin. Microbiol. 2012;51:529–539. doi: 10.1128/JCM.02347-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ravi R.K., Walton K., Khosroheidari M. Methods in Molecular Biology. Volume 1706. Springer Science and Business Media LLC; Berlin/Heidelberg, Germany: 2018. MiSeq: A Next Generation Sequencing Platform for Genomic Analysis; pp. 223–232. [DOI] [PubMed] [Google Scholar]
- 49.Nykoluk M., Taylor T., Enns E., Ji H. HyDRA Web User Guide. [(accessed on 20 August 2019)];2016 Available online: https://hydra.canada.ca/pages/about?lang=en-CA.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article.