Abstract
Pharmacological interactions limit treatment options for children living with human immunodeficiency virus (HIV) and tuberculosis (TB). We found that 12 mg/kg twice daily raltegravir chewable tablets (administered after crushing) safely achieved pharmacokinetic targets in children living with HIV aged 4 weeks to <2 years receiving concurrent rifampin to treat TB.
Clinical Trials Registration
Keywords: antiretroviral therapy, drug interactions, pediatrics, rifampin, tuberculosis
Active tuberculosis (TB) and human immunodeficiency virus (HIV) are often diagnosed simultaneously in high-incidence countries. The World Health Organization recommends near simultaneous initiation of anti-tuberculous and combination antiretroviral therapy [1] (cART). Rifampin (RIF), an essential component of first-line antibacterial regimens for TB [2], is a strong inducer of enzymes involved in the metabolism of many antiretroviral drugs (ARVs) [3–5]. This complicates the construction of effective and tolerable cART regimens for young children [6]. Lopinavir/ritonavir, with additional ritonavir (“superboosting”), can promote adequate lopinavir drug exposures but may not be tolerated due to poor palatability, toxicity, and extra dosing volumes [7, 8].
Raltegravir (RAL) and other integrase strand transfer inhibitors (INSTIs) are well-tolerated ARVs and have been proposed as alternatives for use during TB treatment. We recently reported that 12 mg/kg doses of RAL (twice the usual dose) resulted in pharmacokinetic (PK) exposures that were well tolerated in children aged 2 to <12 years living with both TB and HIV [9]. Here, we report PK results from children aged 4 weeks to <2 years using commercially available chewable RAL tablets dispersed in liquid.
METHODS
Study Design
IMPAACT P1101 is a phase 1/2 open-label study conducted at 4 South African sites [9]. Infants and children aged 4 weeks to <2 years were eligible to participate if they were receiving RIF treatment for pulmonary TB or tuberculous adenitis combined with at least 1 other antimycobacterial medication for at least 1 week and not more than 20 weeks. Children were excluded if they had protein-calorie malnutrition, were known or suspected to have multidrug- or extremely drug-resistant TB, or had acute serious infections other than TB that required active treatment (eg, Pneumocystis jirovecii) or any grade 4 or higher clinical or laboratory event at entry per the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events [10]. Participants were also excluded if they were receiving other INSTIs or drugs likely to alter RAL pharmacokinetics.
A minicohort of 6 participants was initially enrolled. Participants were evaluated at entry; weeks 1, 2, 4, and 8 weeks after ART initiation; and then every 4 weeks while on therapy, again at discontinuation of RAL and TB treatment, and then at 4 and 12 weeks off RAL treatment. Clinical assessment, HIV plasma viral load, CD4 counts, and routine blood counts and biochemical tests were regularly conducted, as described previously [9]). Participants began cART with 2 nucleoside reverse transcriptase inhibitors (NRTIs) and the chewable tablet formulation of RAL at 12 mg/kg per dose (twice the approved dose) twice daily. The RAL chewable tablets were initially moistened with water, apple juice, breast milk, or formula and then crushed and stirred until evenly dispersed. Intensive RAL PK sampling was performed 5–8 days after ART initiation. On the day of the PK sampling, a fourth ARV agent (selected according to local guidelines) was initiated to ensure that a minimum of 3 ARVs were present at effective concentrations. After completion of TB treatment and RAL, the fourth drug was continued per local guidelines for HIV in this age group. RAL pharmacokinetic targets were the geometric mean (GM) area under the curve (AUC)0-12h of 14–45 µM-h and GM C12h (Concentration 12 hours after the last dose) ≥75 nM (33 ng/mL)[9]. Initially, RAL was also stopped if PK analysis revealed an AUC0-12 h >63 µM-h. The protocol was later amended to allow the protocol team to permit continued RAL treatment after reviewing all clinical and laboratory data.
The dose was considered safe for the minicohort based on data through week 4 if none of the first 6 participants had died, experienced a life-threatening grade 4 adverse event (AE) deemed at least possibly related to RAL, or experienced any grade 4 AE probably or definitely attributable to RAL and if no more than 2 of these 6 participants had permanently discontinued RAL due to a grade 3 or grade 4 AE deemed at least possibly related to RAL. After the minicohort passed both safety and PK guidelines, additional participants were enrolled to achieve a full cohort of 12 evaluable patients. The full cohort was evaluated based on week 4 safety data and PK results following similar guidelines as above with a modified safety criterion: no more than 33% of these participants had permanently discontinued RAL due to a grade 3 or a grade 4 AE that was deemed at least possibly treatment related.
The primary definition of a virologic response was achieving ≥1-log10 reduction in HIV-1 RNA copies/mL from baseline or HIV-1 RNA ≤400 copies/mL at week 8. A secondary, more stringent definition of virologic response was achieving HIV RNA ≤50 copies/mL. Treatment failure was defined as failure to respond at week 8 with a reduction in viral load (HIV RNA (copies/mL) to <400 copies/mL or <1-log10 drop from baseline viral load.
Sample and Data Analyses
RAL concentrations were measured and PK parameters were calculated from RAL concentration-time data as recently described [9]. The safety analysis included all participants exposed to RAL at the final recommended dose. Virologic and immunologic responses were analyzed using an “as-treated” analysis such that only participants who remained on RAL and with evaluable data were included. SAS version 9.4 (Cary, NC) was used for analysis.
RESULTS
Participants
The 13 participants (all black and non-Hispanic) had a median age at baseline of 12.3 months (range, 4.1–20.4; Table 1) and all had pulmonary TB. The median baseline log10 HIV plasma viral load (copies/mL) was 5.13 (interquartile range [IQR], 5.01 to 5.60). The median CD4 cells/μL was 1513 (IQR, 1337 to 2008) with a median CD4 percent of 16.8% (IQR, 15.4% to 19.1%). All received abacavir and lamivudine as NRTIs; the fourth drug added after PK was completed was ritonavir-boosted lopinavir in all cases. Three participants were known to have received nevirapine and zidovudine for prevention of mother-to-child transmission and the rest were treatment naive. One of the 3 participants was removed from the study due to the use of a disallowed medication. The other 2 were virological successes (see below).
Table 1.
Baseline Characteristics and Pharmacokinetic Parameters
| Number of participants | 13 |
| Median age, mo (IQR)a | 12.3 (10.9–18.0) |
| Gender (male/female) | 8/5 |
| Median CD4, % (IQR) | 16.8 (15.4–19.1) |
| Median CD4, cells/µL (IQR) | 1513 (1337–2008) |
| Median human immunodeficiency virus type 1, log10 copies/mL (IQR) | 5.13 (5.01–5.60) |
| Area under the curve0-12H μM-h GM (CV) | 32.7 (49%) |
| C12h nM GM (CV) | 106.5(57%) |
Abbreviations: CV, coefficient of variation; IQR, interquartile range.
aMinimum age, 4.1months; maximum age, 20.4 months.
Pharmacokinetic and Safety Data
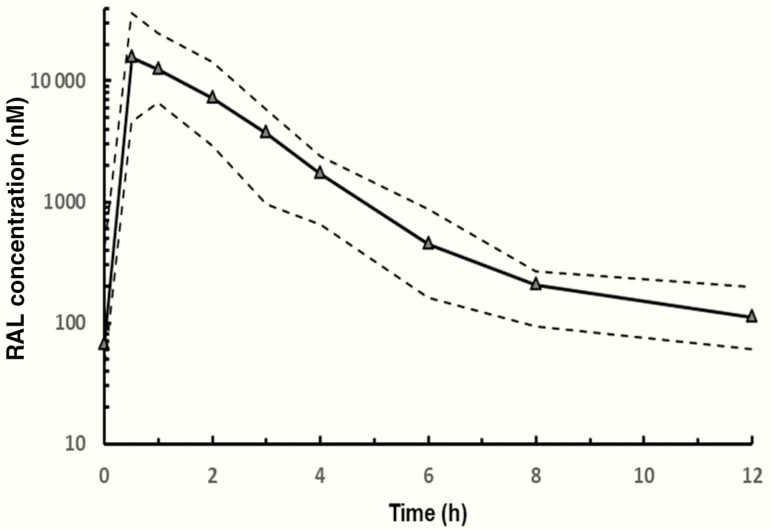
PK analysis was performed in all 13 participants enrolled, including 2 who were twins. Serum RAL concentrations generally reached peak levels by 1 hour after dosing and then declined with a GM half-life of 2.9 hours (Figure 1). PK data from these 13 children revealed a GM AUC0-12h (% coefficient of variation) of 32.7 μM-h (49%) and a GM C12h of 106.5 nM (57%).
Figure 1.
Pharmacokinetic profiles of RAL in children receiving RAL concurrently with rifampin-based therapy for tuberculosis. The solid line represents the median value at each time point; the dashed lines display the 10th and 90th percentiles. Abbreviation: RAL, raltegravir.
Cohort PK targets for C12h and AUC0-12h were also achieved when either twin was excluded from consideration. Two participants had drug exposures that exceeded the protocol limit of 63 μM-h. Neither was symptomatic, but RAL was discontinued for 1 as specified by the study protocol at the time; RAL was not stopped in the second child, as permitted by a later study amendment. Twelve of the 13 participants had evaluable efficacy data at week 8 (1/13 discontinued RAL prior to the week 8 visit, but after PK collection, due to disallowed medications). None of the 13 participants who received RAL experienced any adverse events deemed at least possibly related to RAL.
RAL was permanently discontinued in 6/13 participants: 1 had AUC0-12h >63 μM-h (as mentioned previously); 1 received a disallowed medication (a corticosteroid medication for possible HIV-related nephritis); 1 had grade 4 neutropenia, deemed likely related to TB medication); and 3 due to virologic failure.
Virologic and Immunologic Responses
Protocol-defined virologic responses occurred in 11/12 (92%; 95% confidence interval [CI], 62% to 100%) participants by week 8, with a median change in HIV viral load of –3.05 log10 copies/mL (IQR, –3.45 to –2.63). The 1 participant who did not meet the virological response criterion at week 8 had PK values during the prior intensive PK study that were within the range of target values but was ill at the time of virological failure. Malabsorption was suspected but not proven.
However, only 2/12 (17%; 95% CI, 2% to 48%) had a documented HIV viral load of <50 copies/mL at week 8 (7/12 had >50 copies/mL and 3/12 could not be determined for technical reasons).Three children experienced virologic failure at or after 8 weeks. One child failed to meet virologic success criteria at week 8 and discontinued RAL at week 10. Two others had virologic failure at week 12. One was thought to have resulted from poor adherence to the ART regimen and stopped RAL at week 12. The other had unexplained virologic failure, and RAL was permanently discontinued at week 16.
A prompt rise in CD4 count was seen by 8 weeks of treatment in the 12 children with evaluable data, with a median change from baseline of 105.5 cells/μL (IQR, –191.5 to 755.5) and a CD4 percent change of 4.9% (IQR, 2.80 to 6.05).
DISCUSSION
Infants and children aged 4 weeks to <2 years who were simultaneously receiving RIF safely achieved protocol-defined PK targets for RAL while receiving doses of 12 mg/kg twice daily. Despite active TB and CD4 T-cell values indicative of moderate to severe immunodeficiency [11], most participants also achieved protocol-defined suppression of HIV at week 8 (≥1-log10 reduction in HIV-1 RNA copies/mL from baseline or HIV-1 RNA ≤400 copies/mL). Additionally, the successful use of the chewable formulation of raltegravir in this young age group offers potential for use in all infants living with HIV, including those without TB.
Further experience with RAL and other integrase inhibitors during treatment with RIF is needed in children in this age range to better define the potency of such regimens. We were unable to fully assess the potency, durability, and antiretroviral activity of RAL and 2 nucleoside analogue agents during TB therapy since we added a fourth ARV agent after completion of the intensive PK studies. Addition of the fourth drug reflected concern that children might be exposed to subtherapeutic RAL concentrations. Additional studies are required to determine if RAL or other INSTIs are sufficiently potent to promote control of HIV replication in all children living with both HIV and TB. An additional limitation of this study is the exclusion of children with complicated TB, such as tuberculous meningitis and abdominal TB, in whom drug–drug interactions and drug toxicity may be more likely since additional agents are used to treat their TB.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Financial support. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health, all components of the National Institutes of Health (NIH), under awards UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC), and UM1AI106716 (IMPAACT LC) and by NICHD contract HHSN275201800001I.
Potential conflicts of interest. H. T. is employed by Merck & Co, Inc, Palo Alto, California. All remaining authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global tuberculosis report 2018. World Health Organization. 2018. Available at: https://apps.who.int/iris/handle/10665/274453. Accessed November 20, 2019. [Google Scholar]
- 2. World Health Organization. Guidelines for treatment of drug-susceptible tuberculosis and patient care, 2017 update. 2017. Available at: https://apps.who.int/iris/bitstream/handle/10665/255052/9789241550000-eng.pdf?sequence=1. Accessed November 20, 2019.
- 3. Bhatt NB, Baudin E, Meggi B, et al. ; ANRS 12146/12214-CARINEMO Study Group . Nevirapine or efavirenz for tuberculosis and HIV coinfected patients: exposure and virological failure relationship. J Antimicrob Chemother 2015; 70:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ren Y, Nuttall JJ, Eley BS, et al. . Effect of rifampicin on efavirenz pharmacokinetics in HIV-infected children with tuberculosis. J Acquir Immune Defic Syndr 2009; 50:439–43. [DOI] [PubMed] [Google Scholar]
- 5. Wenning LA, Hanley WD, Brainard DM, et al. . Effect of rifampin, a potent inducer of drug-metabolizing enzymes, on the pharmacokinetics of raltegravir. Antimicrob Agents Chemother 2009; 53:2852–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization. WHO Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. 2nd ed. 2016. Available at: https://apps.who.int/iris/bitstream/handle/10665/208825/9789241549684_eng.pdf?sequence=1. Accessed November 20, 2019. [PubMed] [Google Scholar]
- 7. Turkova A, Bamford A. Treating young children co-infected with tuberculosis and HIV. Lancet HIV 2019; 6:E4–6. [DOI] [PubMed] [Google Scholar]
- 8. Rabie H, Denti P, Lee A, et al. . Lopinavir-ritonavir super-boosting in young HIV-infected children on rifampicin-based tuberculosis therapy compared with lopinavir-ritonavir without rifampicin: a pharmacokinetic modelling and clinical study. Lancet HIV 2019; 6:E32–42. [DOI] [PubMed] [Google Scholar]
- 9. Meyers T, Samson P, Acosta EP, et al. ; IMPAACT P1101 Team . Pharmacokinetics and safety of a raltegravir-containing regimen in HIV-infected children aged 2-12 years on rifampicin for tuberculosis. AIDS 2019; 33:2197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. US Department of Health and Human Services, NIH, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS). Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 2.0. [November 2014]. 2014. Available at: https://rsc.niaid.nih.gov/clinical-research-sites/daids-adverse-event-grading-tables. Accessed May 6, 2020. [Google Scholar]
- 11. Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the use of antiretroviral agents in pediatric HIV infection. Available at: https://aidsinfo.nih.gov/guidelines. Accessed October 30, 2019.