Abstract
Objectives
To assess the safety of live attenuated herpes zoster vaccine live (ZVL) through cumulative analysis of near real-time, participant-based active surveillance from Australia’s AusVaxSafety system.
Design and setting
ZVL was funded in Australia for adults aged 70 years from November 2016, with a time-limited catch up programme for those up to 79 years. This cohort study monitored safety in the first two programme years through active surveillance at 246 sentinel surveillance immunisation sites.
Participants
Adults aged 70–79 years vaccinated with ZVL who responded to an opt-out survey sent via automated short message service (SMS) 3 days following vaccination (n=17 458) or contributed supplementary data through a separate, opt-in online survey at 16 and 24 days following vaccination (n=346).
Primary and secondary outcome measures
Rates of overall and prespecified adverse events following immunisation (AEFI) by sex, concomitant vaccination and underlying medical condition. Signal detection methods (fast initial response cumulative summation and Bayesian updating analyses) were applied to reports of medical attendance.
Results
The median age of participants was 72 years; 53% were female. The response rate following automated SMS was high (73% within 7 days of vaccination). Females were more likely than males to report any adverse event within 7 days of vaccination (RR 2.07, 95% CI 1.86 to 2.31); injection site reaction was the most commonly reported (2.3%, n=377). Concomitant vaccination was not associated with higher adverse event rates (RR 1.05, 95% CI 0.93 to 1.18). Rates of medical attendance were low (0.3%) with no safety signals identified. Supplementary opt-in survey data on later onset adverse events did not identify any difference in AEFI rates between those with and without underlying medical conditions.
Conclusions
ZVL has a very good safety profile in the first week after vaccination in older adults. Active, participant-based surveillance in this primary care cohort is an effective method to monitor vaccine safety among older adults and will be used as a key component of COVID-19 vaccine safety surveillance in Australia.
Keywords: public health, adverse events, primary care, preventive medicine
Strengths and limitations of this study.
High participation rates among older adults in an active, short message service-based, near-real-time vaccine safety surveillance system.
Participant data enabled analyses of adverse events reported up to 7 days post-vaccination by sex and concomitant vaccination.
Near real-time monitoring and signal detection will be used as a key component of COVID-19 vaccine safety surveillance in Australia.
Only a small study group with underlying medical conditions were followed out to 24 days post-vaccination, limiting our ability to capture any late-onset adverse events.
Introduction
Herpes zoster (HZ) is a painful rash associated with significant morbidity, including postherpetic neuralgia in approximately 20% of those with HZ.1 Live attenuated herpes zoster vaccine live (ZVL; Zostavax) is recommended to prevent HZ infection in older adults.2 In Australia, a single dose of ZVL has been funded under the National Immunisation Programme (NIP) since November 2016 for adults aged 70 years, with catch-up until October 2021 for those aged 71–79 years. This is the first time a live attenuated vaccine has been routinely used in older adults in Australia, with guidance providing detailed information on contraindications in those with severe immunocompromise.3
Prior to inclusion of ZVL in the Australian NIP, data on ZVL safety in immunocompetent adults were predominantly available from clinical trials.4–7 These studies identified a risk of localised injection site reactions (ISR) (48% in vaccine recipients compared with 16% of placebo recipients in the Shingles Prevention Study)7 and no evidence of an increased risk of serious adverse events, hospitalisation or death.2 4–9 Higher rates of ISR were reported when ZVL was administered concomitantly with influenza vaccine (42.9% compared with 35.4% within 5 days)10 and pneumococcal polysaccharide vaccine (23vPPV) (43.8% compared with 35.9% within 5 days)11; the rate of systemic adverse events was similar. One vaccine-related death was reported during post-marketing use of ZVL in an immunocompromised individual in the UK, contraindicated to receive the vaccine.12 Shortly after commencement of vaccination under the NIP, a death in a vaccine contraindicated individual was also reported from Australia.13
Passive (spontaneous) postmarketing surveillance is used routinely in Australia to monitor safety following the introduction of a new vaccine. While this is an important tool to identify rare or population-specific adverse events following immunisation (AEFI) and has the advantages of being relatively low cost and open to reporting from the whole population, it is limited by the potential for under-reporting and biased reporting, lack of contemporary vaccinated population denominator data and, for ZVL, is confounded by the higher prevalence of chronic disease in the older target population.8 14 In addition, lack of denominators (vaccine doses administered) and fluctuations in reporting numbers over time hinder analysis of data and signal detection. The addition of active surveillance of AEFI is increasingly recognised as an important component of postmarketing safety monitoring and can be undertaken using a range of different approaches.15
AusVaxSafety is an Australian Government Department of Health funded system that undertakes regular monitoring of AEFI through collection of survey data from individuals following routinely administered vaccines at sentinel sites across Australia.16 This active, participant-based surveillance system uses two monitoring platforms (SmartVax and Vaxtracker),17–19 and near-real-time surveillance data are analysed using signal detection methods. To coincide with introduction of the funded programme, active safety surveillance for ZVL, including fortnightly to monthly detailed analysis and reporting, was conducted for 2 years through AusVaxSafety.20 This was the first time AusVaxSafety had been used for a live vaccine in an older adult population; however, this cohort is also included in surveillance of influenza16 21 and pneumococcal vaccine safety.20
We aimed to cumulatively analyse prospectively collected AusVaxSafety data to provide a detailed assessment of the rates of specific early-onset AEFI following administration of ZVL and any concomitant vaccines (particularly influenza and 23vPPV vaccines) in adults aged 70–79 years from November 2016 to November 2018. In a subset with underlying medical conditions, we aimed to identify both early and later onset AEFI through the Vaxtracker monitoring platform.
Methods
Study design
This was an observational cohort study conducted in 246 Australian sentinel primary care surveillance sites. Data were collected prospectively through AusVaxSafety active surveillance with AEFI rates and signal detection data reported in near real time. This study assessed cumulative data for the entire surveillance period and summarised near-real-time signal detection analyses.
Data sources
AusVaxSafety undertakes regular monitoring through collection of data from patients attending sentinel, primary care immunisation surveillance sites (general practices and hospital-based clinics); the system has been described previously.16 21 22 AusVaxSafety was originally established to monitor influenza vaccine safety and had therefore focused on automated, short-term AEFI monitoring. For ZVL, the SmartVax monitoring platform was the primary data collection tool, focusing on early-onset AEFI from November 2016 to November 2018. In addition, supplementary data were collected from a separate patient cohort using an opt-in, online survey administered via the Vaxtracker platform up to 24 days following vaccination. These data were collected to allow for identification of later-onset AEFI and underlying medical conditions in this additional cohort.
SmartVax is an opt-out programme using an automated tool that integrates with immunisation provider software. Patients are automatically enrolled by their clinic and receive a communication via short message service (SMS) 3 days after vaccination asking whether they experienced any ‘reactions’ to the vaccine/s administered (as SMS are not sent on weekends, some may be sent up to 5 days postvaccination). For those who respond ‘yes’ (ie, report an AEFI), a second SMS is sent seeking information on whether medical attention was sought and a simultaneous SMS links to an online survey requesting further details (online supplemental appendix 1). During the period of this study, Vaxtracker was an opt-in programme, employing an initial manual step which required patients to be explicitly consented and enrolled by clinic staff following vaccination.19 For ZVL, Vaxtracker sent a welcome message 3 days after vaccination by SMS or email (according to participant preference), confirming enrolment and advising to expect the survey at a later date. An initial online survey link was then sent 16 days after vaccination; for those who responded, a final survey link was sent 24 days following vaccination (online supplemental appendix 1).
bmjopen-2020-043880supp001.pdf (61.8KB, pdf)
The SmartVax SMS/survey and Vaxtracker day 16 survey collected data on any post-vaccination adverse event or symptom and on medical attendance; the day 24 Vaxtracker survey asked participants if they experienced specific adverse events (a chickenpox-like rash or influenza-like symptoms) or if they had been hospitalised since vaccination for any reason, which could potentially indicate later-onset vaccine associated AEFI, in particular, disseminated varicella zoster virus (VZV) infection (online supplemental appendix 1). For SmartVax, demographic information was automatically extracted from the practice management software. For Vaxtracker, demographic information (including Indigenous status and sex) was self-reported in the day 16 survey; age was collected at enrolment because the vaccine was restricted by age. Only Vaxtracker collected self-reported information on participants’ underlying medical conditions. For both platforms, reports of medically attended events triggered clinical follow-up by immunisation providers and/or public health authorities in each respective state or territory.
Study population
The primary cohort was adults aged 70–79 years vaccinated with ZVL and enrolled via the Smartvax platform. Individuals were included in the primary, short-term AEFI analysis if they responded to the Smartvax SMS/survey within 7 days of the vaccination, in order to minimise the risk of recall bias. The supplementary cohort was adults aged 70–79 years vaccinated with ZVL enrolled via the Vaxtracker platform. Individuals were included in analysis of the initial Vaxtracker survey data if they responded within 7 days of receipt of the initial (day 16) survey. In order to explore the usefulness of the final (day 24) Vaxtracker survey, which was designed to assess later-onset AEFI, participants were included regardless of the timeliness of their response (online supplemental appendix 1).
Data analysis
Data from the two platforms were analysed separately due to the different reporting timeframes and data collection processes. The median age of respondents and non-respondents, and of those responding within and following the 7-day period, were examined for both cohorts; sex differences were examined in the primary cohort only as sex of non-respondents was unknown for the supplementary cohort.
Rates of overall and specific AEFI, including 95% CIs, were calculated by sex and receipt of concomitant vaccination; where sex was missing, individuals were still included in overall rates. Supplementary analysis of later-onset AEFI also examined rates by concomitant vaccination and self-reported underlying medical condition. Analyses were conducted using R v3.5.1.23 Where medical attendance was documented, further information was obtained, where available, from the healthcare provider and/or public health authorities involved in follow-up.
Signal detection
During the active surveillance period, rates of participant-reported medical attendance were analysed using signal detection and descriptive methods. Results were reported fortnightly to all relevant health authorities and made available publicly at ausvaxsafety.org.au from November 2016 to November 2017, and then monthly to November 2018. Fast initial response cumulative summation (FIR CUSUM) control charts monitored log-likelihood ratios of medical attendance being at a maximum acceptable level vs an expected level. The maximum acceptable (3%) and expected (2%) medical attendance rates were based on AEFI data from clinical trials and post-marketing surveillance.6 7 24 A safety signal was ‘detected’ if the log-likelihood ratios exceeded a predetermined threshold log-likelihood ratio. Using simulated vaccination data, the threshold log-likelihood ratio was selected such that there was ≥80% probability of signal generation within 3 weeks if the event rate was at the maximum acceptable level, and an overall ≤2% probability of (false) signal generation when the event rate was at the expected level.
Bayesian updating analyses were conducted for robust estimates of the 95% credible interval (calculated from the posterior beta distribution) for true cumulative medical attendance rates. Data from the literature were used to establish the mean of the beta distribution (initial prior probability) for medical attendance at the commencement of the surveillance period.6 7 24 Priors were updated with each fortnight or month’s observed data throughout the surveillance period.
During real-time enhanced surveillance, both analyses included all participants (not limited to those responding within 7 days). All data on signal detection presented here thus reflects the cumulative result of real-time analyses that were conducted during the active surveillance period.
Patient and public involvement
The AusVaxSafety data monitoring platforms were piloted and developed with feedback from users. The AusVaxSafety surveillance system Advisory Committee includes a consumer representative. Surveillance results are uploaded to the AusVaxSafety website, www.ausvaxsafety.org.au, and available to the public.
Results
Participation
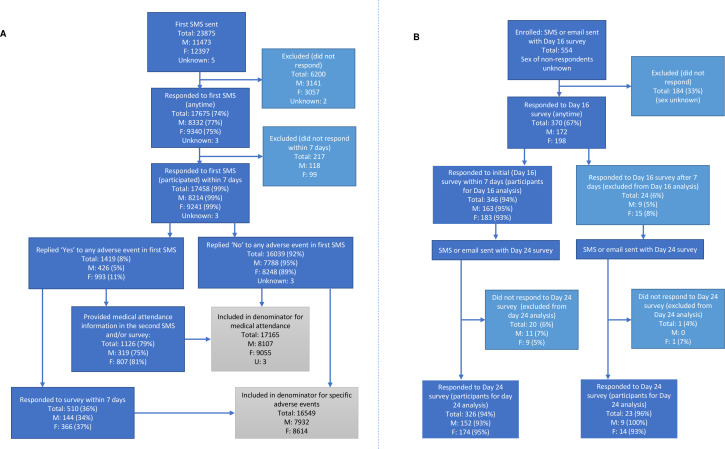
Between 1 November 2016 and 4 November 2018, 23 875 individuals who received ZVL were enrolled in SmartVax; 74% responded to the first SMS (n=17 675) (figure 1A). Those who did not respond were similar in age to those who did (median 74 vs 72 years). There was little difference in the proportion of females and males who responded (75% vs 73%, respectively). Of those who responded to the first SMS, 99% (n=17 458) responded within 7 days of vaccination and were considered participants for the remainder of the primary analysis of short-term AEFI (figure 1A). The median age (72 vs 73 years) and proportion of males and females was similar among those who responded within 7 days compared with those who took longer to respond.
Figure 1.
Number of individuals responding to and participating in sentinel, active participant-based surveillance platforms contributing to AusVaxSafety surveillance of live attenuated herpes zoster vaccine. (A) Short-term AEFI monitoring platform, SmartVax primary cohort (1 November 2016 to 4 November 2018). (B) Later-onset AEFI monitoring platform, Vaxtracker supplementary cohort (13 December 2016 to 10 May 2018). AEFI, adverse events following immunisation; SMS, short message service.
Between 13 December 2016 and 10 May 2018, 554 individuals were enrolled and invited to respond to the Vaxtracker survey; 67% (n=370) responded to the initial (day 16) survey. The age of those who responded was similar to the age of those that did not respond (median 73 vs 74 years). Of those who responded, 94% (n=346) responded within 7 days of receipt of the initial survey and were considered participants for the analysis of initial survey data (figure 1B). The median age (73 vs 75 years) and proportion of males and females was similar among those who responded within 7 days compared with those who responded later. Most participants in the initial survey also responded to final (day 24) survey (n=326, 94%); 23 individuals who responded to the initial survey after 7 days and responded to the final survey and were included in analysis of the final survey data (figure 1B).
Participant demographics
The median age of participants was 72 years and 47% were male; demographics were similar between participants using the two surveillance platforms (table 1). Concomitant vaccines were received slightly more frequently among participants in the primary cohort than in the supplementary cohort. Underlying medical conditions were reported by 41% of respondents in the supplementary cohort (table 1); the most common conditions were arthritis, diabetes, heart disease and respiratory disease.
Table 1.
Demographic characteristics of AusVaxSafety participants who received live attenuated herpes zoster vaccine*
| Variable | Category | Primary cohort (SmartVax) N=17 458† |
Supplementary cohort (Vaxtracker) N=346 |
Overall N=17 804‡ |
| Median age (IQR) | 72 years (70–75) | 73 years (71–76) | 72 years (70–75) | |
| Sex n (%) |
Male | 8214 (47.1) | 163 (47.1) | 8377 (47.1) |
| Female | 9241 (52.9) | 183 (52.9) | 9424 (52.9) | |
| Indigenous status n (%) |
Aboriginal | 59 (0.4) | 2 (0.6) | 61 (0.4) |
| Torres Strait Islander | 8 (0.06) | 0 | 8 (0.05) | |
| Both | 5 (0.03) | 0 | 5 (0.03) | |
| Total | 72 (0.5) | 2 (0.6) | 74 (0.5) | |
| Concomitant vaccination§ n (%) |
At least one concomitant vaccine | 3993 (22.9) | 57 (16.5) | 4050 (22.8) |
| 23vPPV | 657 (3.8) | 11 (3.2) | 668 (3.8) | |
| Influenza vaccine | 3218 (18.4) | 45 (13.0) | 3263 (18.3) | |
| Diphtheria/tetanus vaccine | 235 (1.3) | 3 (0.9) | 238 (1.3) | |
| Underlying medical condition n (%) |
Yes | NA | 143 (41.3) | NA |
| No | NA | 203 (58.7) | NA |
*SmartVax participants responding to an opt-out SMS within 7 days of vaccination between November 2016 and November 2018; Vaxtracker participants responding to an opt-in survey via SMS or email within 7 days of survey receipt following vaccination between December 2016 and May 2018.
†Denominator 17 455 for sex which was missing in three reports; denominator 14 342 for Indigenous status, which was missing in 3116 reports.
‡Denominator 17 801 for sex which was missing in three reports; denominator 14 688 for indigenous status, which was missing in 3116 reports.
§Some participants received more than one concomitant vaccine.
NA, not available; SMS, short message service; 23vPPV, 23-valent pneumococcal vaccine.
Short-term AEFI (primary analysis)
Of the 17 458 participants, 8.1% reported any AEFI (n=1419); females were significantly more likely than males to report AEFI (table 2). Thirty-six per cent of those who reported an AEFI responded to the online survey and provided additional details (figure 1A); injection site reaction was the most commonly reported specific AEFI (2.3%, n=377) (table 2). Of participants who reported fever, 72.3% (60 of 83) reported the use of antipyretics or analgesics. Participants who had received one or more concomitant vaccine (22.9%) were no more likely to report any AEFI than if those who received ZVL alone; however, they were less likely to report a rash and more likely to report fever (table 2). Of those receiving only influenza vaccine concomitantly, 7.6% (n=230 of 3032) reported any AEFI, compared with 11.6% (n=57 of 492) of those receiving only 23vPPV with ZVL; 19.4% (n=28 of 144) of those who received both influenza and 23vPPV concomitantly (with no other vaccines) reported any AEFI.
Table 2.
Short-term AEFI reported by AusVaxSafety participants following live attenuated herpes zoster vaccine live (ZVL) by sex and concomitant vaccination
| Males n (%) |
Females n (%) |
RR (95% CI) (female vs male) |
ZVL alone n (%) |
ZVL+concomitant vaccine/s n (%) |
RR (95% CI) (concomitant vs ZVL alone) |
|
| Any AEFI * | 426 (5.2) | 993 (10.7) | 2.07 (1.86 to 2.31) | 1082 (8.0%) | 337 (8.4%) | 1.05 (0.93 to 1.18) |
| Injection site reaction † | 86 (1.1) | 291 (3.4) | 3.12 (2.45 to 3.96) | 304 (2.4%) | 73 (1.9%) | 0.81 (0.63 to 1.05) |
| Fever † | 23 (0.3) | 60 (0.7) | 2.40 (1.49 to 3.88) | 56 (0.4%) | 27 (0.7%) | 1.63 (1.03 to 2.58) |
| Rash † | 12 (0.2) | 54 (0.6) | 4.14 (2.22 to 7.74) | 59 (0.5%) | 7 (0.2%) | 0.40 (0.18 to 0.88) |
| Medical attendance ‡ | 16 (0.2) | 33 (0.4) | 1.85 (1.02 to 3.35) | 38 (0.3%) | 11 (0.3%) | 0.98 (0.50 to 1.91) |
*Denominator includes SmartVax participants responding to an opt-out SMS within 7 days of vaccination (M=8214, F=9241, total: 17 458, sex missing in 3). ZVL alone was received by 13 465 participants and concomitant vaccine/s by 3993 participants.
†Denominator includes SmartVax participants who reported any AEFI within 7 days of vaccination and then also responded to a survey within 7 days of vaccination, and SmartVax participants who reported no AEFI within 7 days of vaccination (M: 7932, F: 8614, total: 16 549, sex missing in n=3). In this subset, ZVL alone was received by 12 778 participants and concomitant vaccines were received by 3771 participants.
‡Denominator includes SmartVax participants who reported any AEFI within 7 days of vaccination and then also provided medical attendance information within 7 days via SMS and/or the online survey, and SmartVax participants who or reported no AEFI within 7 days of vaccination (M=8107, F=9055, Total: 17 165, sex missing in 3). In this subset, ZVL alone was received by 13 246 participants and concomitant vaccines were received by 3919 participants.
AEFI, adverse events following immunisation; SMS, short message service.
Medical attendance within a week following vaccination was reported by 0.3% (n=49) of the participants who provided a response regarding medical attendance (figure 1 and table 2). Of those who provided more detailed information (n=13), most attended a primary care provider (n=11) and two attended a hospital emergency department. Detailed data were provided by jurisdictions for seven of these reports. Three involved a reaction at the injection site, one of which also reported rash. One report described systemic symptoms including fever, headache, fatigue and weakness 8 hours following vaccination. One report was for hyperglycaemia in a known diabetic and one was an unrelated surgical admission. All were resolved or resolving on follow-up.
Later-onset AEFI (supplementary analysis)
Of 346 participants providing supplementary data through the initial Vaxtracker survey, 15.0% (n=52) reported any AEFI and ISR was the most common specific event (6.6%, n=23). Females were no more likely than males to report an AEFI, apart from ISR (table 3), and concomitant vaccination was not associated with a change in reported AEFI. Those with a self-reported underlying medical condition (41.3%, n=143) were no more likely to report an adverse event than those without (table 3).
Table 3.
Later-onset AEFI reported by AusVaxSafety participants following live attenuated herpes zoster vaccine live (ZVL) by sex, concomitant vaccination and underlying medical condition (initial survey)*
| Males n (%) |
Females n (%) |
RR (95% CI) (female vs male) | ZVL alone n (%) |
ZVL +concomitant vaccine/s n (%) | RR (95% CI) (concomitant vs ZVL alone) | No medical condition n (%) |
Medical condition n (%) |
RR (95% CI) (medical condition vs no condition) | |
| Any AEFI | 19 (11.7) | 33 (18.0) | 1.55 (0.92 to 2.61) | 46 (15.9) | 6 (10.5) | 0.66 (0.30 to 1.48) | 28 (13.8) | 24 (16.8) | 1.22 (0.74 to 2.01) |
| Injection site reaction | 6 (3.7) | 17 (9.3) | 2.52 (1.02 to 6.25) | 19 (6.6) | 4 (7.0) | 1.07 (0.38 to 3.02) | 13 (6.4) | 10 (7.0) | 1.09 (0.49 to 2.42) |
| Fever | 2 (1.2) | 6 (3.3) | 2.67 (0.55 to 13.1) | 8 (2.8) | 0 (0.0) | NA | 2 (1.0) | 6 (4.2) | 4.26 (0.87 to 20.8) |
| Rash | 3 (1.8) | 5 (2.7) | 1.48 (0.36 to 6.12) | 7 (2.4) | 1 (1.8) | 0.72 (0.09 to 5.77) | 5 (2.5) | 3 (2.1) | 0.85 (0.21 to 3.51) |
| Medical attendance † | 2 (1.2) | 4 (2.2) | 1.78 (0.33 to 9.60) | 5 (1.7) | 1 (1.8) | 1.01 (0.12 to 8.52) | 4 (2.0) | 2 (1.4)‡ | 0.71 (0.13 to 3.82) |
*Denominator includes Vaxtracker participants responding within 7 days to an initial opt-in survey by SMS or email sent 16 days following vaccination (M: 163, F: 183, total: 346). Of these, 57 had received a concomitant vaccine and 143 had an underlying medical condition.
†All those reporting medical attendance reported visiting a primary care provider.
‡One participant had arthritis and one had diabetes.
AEFI, adverse events following immunisation; NA, not available; SMS, short message service.
Medical attendance was reported by 1.7% of participants (n=6) in the initial survey; all six participants visited a primary care provider. These included three reports of influenza-like illness within 2 days of vaccination; one report also described leg pain. There were two reports of rash including one report of hives (timing after vaccination unknown) and one reported diagnosis of eczema at day 14. There was one report of ISR on the day of vaccination, which resolved.
Of those completing the final survey (n=349), 151 (43%) had an underlying medical condition. Those with a medical condition were no more likely to report influenza-like illness or chickenpox-like rash than those without (table 4). Of participants reporting influenza-like illness, most (84%) had received ZVL alone. Two participants reported hospitalisation for allergic reaction (one following a dental procedure and one following consumption of shellfish).
Table 4.
Later-onset AEFI reported by AusVaxSafety participants following live attenuated herpes zoster vaccine by sex and underlying medical condition (final survey)*
| Males n (%) |
Females n (%) |
RR (95% CI) (females vs males) | No medical condition n (%) |
Medical condition n (%) |
RR (95% CI) (medical condition vs no condition) | |
| Influenza-like Illness | 10 (6.2) | 22 (11.7) | 1.88 (0.92 to 3.86) | 17 (8.6) | 15 (9.9) | 1.16 (0.60 to 2.24) |
| Chickenpox-like rash | 1 (0.6) | 7 (3.7) | 5.99 (0.75 to 48.2) | 4 (2.0) | 4 (2.6) | 1.31 (0.33 to 5.16) |
| Hospitalisation † | 1 (0.6) | 1 (0.5) | 0.86 (0.05 to 13.64) | 2 (1.0) | 0 (0.0) | NA |
*Denominator includes Vaxtracker participants responding to a final opt-in survey by SMS or email sent 24 days following vaccination (M: 161, F: 188, total: 349). Of these, 151 had an underlying medical condition.
†Hospitalisation for allergic reaction (one following a dental procedure and one following consumption of shellfish).
AEFI, adverse events following immunisation; NA, not available; SMS, short message service.
Cumulative event rates and signal detection
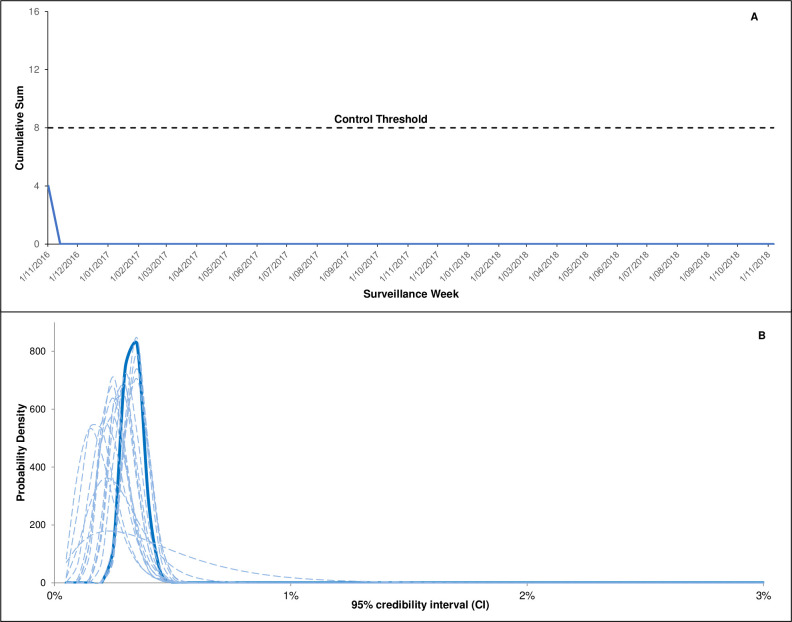
Overlay of bimonthly Bayesian analyses conducted during near real-time surveillance demonstrated increased precision of the rate estimates with data accumulation (figure 2); rates of participant-reported medical attendance remained below the prespecified maximum threshold rate. The FIR CUSUM control charts for the entire surveillance period found no evidence that the event rate for medical attendance was closer to the maximum threshold than the expected rate (figure 2).
Figure 2.
Cumulative signal detection analyses and cumulative event rates following live attenuated herpes zoster vaccine for respondents using the SmartVax platform (regardless of timeliness of response). (A) Fast initial response cumulative sum (FIR CUSUM) safety signal detection chart for medical attendance following live attenuated herpes zoster vaccine during the surveillance period (FIR CUSUM tracks the relative log-likelihood ratio of the event rate being at the maximum acceptable rate (set at 3%) vs expected rate (set at 2%) given the accumulated data). (B) Overlayed bimonthly Bayesian analyses showing the probability density curve of medical attendance (dotted lines indicate bimonthly posterior density curves throughout the surveillance period; Solid line is the final posterior density curve). FIR CUSUM, fast initial response cumulative summation.
Discussion
Using this unique, active postmarketing vaccine safety surveillance programme in Australia, AusVaxSafety, we found ZVL to have a very good safety profile in the first week after vaccination in older adults. Participation rates among the primary cohort were high using an opt-out surveillance platform (SmartVax), which provided 98% of all data (n=17 458 participants). As most participants responded quickly, data was provided in near real-time, enabling AusVaxSafety to efficiently monitor the introduction of a new immunisation programme, including through signal detection methods. This active surveillance complemented existing passive surveillance and did not identify any safety signals for ZVL; however, the rare reports of vaccine associated death due to disseminated VZV infection with onset weeks after vaccination, in immunocompromised individuals, remains an issue of concern which is being closely examined.25 26 Active vaccine safety surveillance with SMS-based technology in older adults has also been effective in monitoring influenza vaccine safety (response rate 69.6%)21 and 23vPPV20 and will be used for surveillance of COVID-19 vaccine safety in Australia. In the USA, a similar system (V-Safe) has been introduced to support safety monitoring for COVID-19 vaccine.27
Self-reported AEFI rates in our study were low and similar to those reported by AusVaxSafety following various inactivated influenza vaccines in adults over 65 years (4.8%–8.9%).16 21 Rates of medical attendance (as a proxy for serious adverse events) were also low, consistent with other studies that have not identified an increased risk of serious adverse events following administration of ZVL.8 28–30 ISR was the most commonly reported specific AEFI in our study, which has also been observed via passive surveillance in Australia, the USA and globally.2 8 25 A recent Australian study using a large general practice dataset similarly demonstrated an increased risk of ISR following vaccination with ZVL using the self-controlled case series method.29 Our study did not identify an increased risk of ISR with concomitant vaccination, consistent with the findings of a more recent randomised controlled trial comparing ZVL administered alone or concomitantly with quadrivalent influenza vaccine.31 While AusVaxSafety has previously demonstrated significantly higher rates of AEFI for individuals receiving 23vPPV concomitantly with influenza vaccine, this has not been shown for ZVL administered with influenza vaccine, compared with influenza vaccine alone.21 Interestingly, AEFI were reported more commonly by females than males, as has been observed in other vaccine safety surveillance in Australia (for various inactivated influenza vaccines using AusVaxSafety data: 8.7% of women reported AEFI vs 5.8% of men)21 32 and internationally.33 Biological differences in immune function34 and behavioural differences that may have influenced reporting rates are potential factors underpinning these observed differences.33
This study included supplementary analysis of longer-term data in a small group of participants, in view of the potential for late-onset AEFI. Rates of AEFI were higher over the longer follow-up period (15% from the initial survey, sent at day 16, compared with 8.1% in the first week for any AEFI), which may relate to the potential to capture more AEFI over a longer time period, and to the intrinsic differences in the way in which participant responses were solicited via this opt-in survey. Despite this, our analysis did not signal any vaccine safety concerns; the rate of medical attendance (1.7%) which, based on patient descriptors, sometimes included routine attendance for unrelated matters, was similar to the rate of serious adverse events reported in clinical trials (1.4% in the Shingles Prevention Study).7 Similarly, no increased risk of late-onset (up to 42 days postvaccination) AEFI, including cardiovascular and cerebrovascular events, was demonstrated in two large studies of older adults using data from the sites participating in the US Vaccine Safety Datalink project,28 30 or in a self-controlled case series analysis of Australian general practice data from 150 054 older adults.29
Use of ZVL in immunocompromised patients has been associated with vaccine strain disseminated VZV disease occurring up to 7 weeks following ZVL vaccination,8 12 13 with fatal outcomes reported in immunocompromised individuals from the UK12 and Australia,12 13 including a case reported shortly after programme commencement13 and two additional individuals following completion of this study.26 Australian guidance provides detailed information on contraindications in immunocompromised patients.3 In our study, those with underlying medical conditions were no more likely to report an AEFI or medical attendance than those without, and there was no increased risk of any of the AEFI prespecified in the final survey. Similarly, a recent prospective cohort study of 1500 patients in Japan did not identify an increased risk of AEFI following ZVL among those with underlying conditions such as malignancy, diabetes mellitus, autoimmune diseases and renal diseases,35 and an analysis of UK primary care data identified only two cases of VZV disease among 1742 individuals who were inadvertently vaccinated while immunosuppressed; neither were hospitalised.36
Recorded coverage of ZVL in Australia was 33.9% in 70-year-old adults from commencement of the NIP programme in November 2016 until 31 March 2018 (noting that underreporting is likely given that only 489 605 of 1 370 395 doses distributed were recorded as being administered).25 The recombinant VZV vaccine is registered in Australia but not currently available; in future, this vaccine may provide an alternative option for immunocompromised individuals.37
This study has a number of limitations. The supplementary cohort was small and our assessment of later-onset AEFI was likely limited by the potential for recall bias; larger studies are required to assess the risk of later-onset AEFI, including in individuals with underlying medical conditions. The opt-out approach for the primary cohort resulted in a high initial response to the SMS on the presence of absence of AEFI (74%) but a lower response to the more detailed survey (36%). A similar trend has been observed through active surveillance for other vaccines, including influenza and 23vPPV vaccination in older adults.20 While survey completion rates were higher using an opt-in approach for the supplementary cohort, consistent with previous studies,38 this is more resource intensive and difficult to implement for a large cohort. In use of this methodology for COVID-19 vaccine safety surveillance, AusVaxSafety has now combined the initial SMS contact and detailed survey into one message with the aim of increasing response rates to all study questions; data will also be collected several weeks following vaccination for COVID-19. As for all observed AEFI, a causal relationship between the reported events and vaccination cannot be assumed; AEFI event rates reported here are comparable to those reported for ZVL and other vaccines in post-marketing surveillance of this age group.16 21 29
Conclusion
AusVaxSafety’s active, participant-based surveillance system contributed timely safety data, particularly on short-term AEFI, following implementation of a funded ZVL programme in an older Australian population, confirming the known low risk of ISR, and with no safety signals identified. This system is an efficient, automated addition to Australia’s established passive vaccine safety surveillance. However, limitations remain in utilising individual reporting systems alone; the ability to routinely link this vaccine safety surveillance data (both active and passive AEFI reports), denominator data from the Australian Immunisation Register,39 and data sources that include medical presentations for adverse events, such as primary care data, hospitalisation and mortality data would further assist the assessment of serious or late-onset AEFI.29 With the implementation of COVID-19 immunisation programmes, targeted at older adults and people with underlying medical conditions that may increase the risk of AEFI, expansion of effective real-world vaccine safety surveillance systems, particularly those that can detect rare, novel, or late onset AEFI, is paramount and is already occurring in Australia.
Supplementary Material
Acknowledgments
This manuscript was written on behalf of the AusVaxSafety Expert Leadership Group. We thank the AusVaxSafety Expert Leadership Group for their oversight of this system. We also acknowledge the participants and staff at surveillance sites. We acknowledge jurisdictional health departments and thank the SAEFVIC and WAVSS teams for their input. We acknowledge the contribution of both the SmartVax and Vaxtracker platforms.
Footnotes
Contributors: AP, CG, TLS, NC and KM made substantial contributions to the conception or design of the manuscript. AP and CG were responsible for drafting the manuscript and conducting all data analyses, with the exception of signal detection analyses, which were performed by PF. TLS and PF were responsible for the conceptualisation and execution of the weekly safety signal detection analyses. AL, as codeveloper of the SmartVax system, served as the system operator and advisor regarding SmartVax data. PC and DD served as system operator and advisors regarding Vaxtracker data. PC, AL, TLS, DD and KM were integral to the design and development of the AusVaxSafety vaccine safety surveillance system and served as key vaccine safety experts. All authors made substantial contributions to the interpretation of data for the work and revised the manuscript critically for important intellectual content. All authors had final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: AusVaxSafety surveillance was funded under a contract with the Australian Government Department of Health (grant number NA). AP receives PhD stipend funding from the National Health and Medical Research Council (NHMRC) and the Royal Australasian College of Physicians (RACP) (grant number NA). TLS is supported by a Career Development Fellowship from the National Health and Medical Research Council (GNT1111657).
Competing interests: All authors except AP are either located at organisations that hold the AusVaxSafety contract from the Australian Government Department of Health or are subcontract holders.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the Sydney Children’s Hospitals Network Human Research Ethics Committee (HREC/16/SCHN/19). AusVaxSafety operates under this approval as well as approval obtained from the Royal Australian College of General Practitioners National Research and Evaluation Ethics Committee (NREEC15-007).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data may be obtained from a third party and are not publicly available. AusVaxSafety compiles ongoing, deidentified surveillance data of patient-reported adverse events for specific vaccines as contracted by the Australian Government Department of Health. Summarised results are publicly available on the AusVaxSafety website (www.ausvaxsafety.org.au) but AusVaxSafetydata sets are not publicly available.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Yawn BP, Saddier P, Wollan PC, et al. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007;82:1341–9. 10.4065/82.11.1341 [DOI] [PubMed] [Google Scholar]
- 2.Willis ED, Woodward M, Brown E, et al. Herpes zoster vaccine live: A 10 year review of post-marketing safety experience. Vaccine 2017;35:7231–9. 10.1016/j.vaccine.2017.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Australian Technical Advisory Group on Immunisation (ATAGI) . Australian immunisation Handbook Canberra: Australian government department of health, 2019. Available: https://immunisationhandbook.health.gov.au [Accessed 2 Dec 2019].
- 4.Gagliardi AMZ, Andriolo BNG, Torloni MR, et al. Vaccines for preventing herpes zoster in older adults. Cochrane Database Syst Rev 2016;3:CD008858. 10.1002/14651858.CD008858.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005;352:2271–84. 10.1056/NEJMoa051016 [DOI] [PubMed] [Google Scholar]
- 6.Schmader KE, Levin MJ, Gnann JW, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis 2012;54:922–8. 10.1093/cid/cir970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simberkoff MS, et al. Safety of herpes zoster vaccine in the shingles prevention study. Ann Intern Med 2010;152:545–54. 10.7326/0003-4819-152-9-201005040-00004 [DOI] [PubMed] [Google Scholar]
- 8.Miller ER, Lewis P, Shimabukuro TT, et al. Post-licensure safety surveillance of zoster vaccine live (Zostavax®) in the United States, vaccine adverse event reporting system (VAERS), 2006-2015. Hum Vaccin Immunother 2018;14:1963–9. 10.1080/21645515.2018.1456598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray AV, Reisinger KS, Kerzner B, et al. Safety and tolerability of zoster vaccine in adults ≥60 years old. Hum Vaccin 2011;7:1130–6. 10.4161/hv.7.11.17982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerzner B, Murray AV, Cheng E, et al. Safety and immunogenicity profile of the concomitant administration of ZOSTAVAX and inactivated influenza vaccine in adults aged 50 and older. J Am Geriatr Soc 2007;55:1499–507. 10.1111/j.1532-5415.2007.01397.x [DOI] [PubMed] [Google Scholar]
- 11.MacIntyre CR, Egerton T, McCaughey M, et al. Concomitant administration of zoster and pneumococcal vaccines in adults ≥60 years old. Hum Vaccin 2010;6:894–902. 10.4161/hv.6.11.12852 [DOI] [PubMed] [Google Scholar]
- 12.Costa E, Buxton J, Brown J, et al. Fatal disseminated varicella zoster infection following zoster vaccination in an immunocompromised patient. BMJ Case Rep 2016;2016:bcr2015212688. 10.1136/bcr-2015-212688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander KE, Tong PL, Macartney K, et al. Live zoster vaccination in an immunocompromised patient leading to death secondary to disseminated varicella zoster virus infection. Vaccine 2018;36:3890–3. 10.1016/j.vaccine.2018.05.078 [DOI] [PubMed] [Google Scholar]
- 14.Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the vaccine adverse event reporting system (VAERS). Vaccine 2015;33:4398–405. 10.1016/j.vaccine.2015.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crawford NW, Clothier H, Hodgson K, et al. Active surveillance for adverse events following immunization. Expert Rev Vaccines 2014;13:265–76. 10.1586/14760584.2014.866895 [DOI] [PubMed] [Google Scholar]
- 16.Pillsbury AJ, Glover C, Jacoby P, et al. Active surveillance of 2017 seasonal influenza vaccine safety: an observational cohort study of individuals aged 6 months and older in Australia. BMJ Open 2018;8:e023263. 10.1136/bmjopen-2018-023263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cashman P, Macartney K, Khandaker G, et al. Participant-centred active surveillance of adverse events following immunisation: a narrative review. Int Health 2017;9:164–76. 10.1093/inthealth/ihx019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leeb A, Regan AK, Peters IJ, et al. Using automated text messages to monitor adverse events following immunisation in general practice. Med J Aust 2014;200:416–8. 10.5694/mja13.11166 [DOI] [PubMed] [Google Scholar]
- 19.Cashman P, Moberley S, Dalton C, et al. Vaxtracker: active on-line surveillance for adverse events following inactivated influenza vaccine in children. Vaccine 2014;32:5503–8. 10.1016/j.vaccine.2014.07.061 [DOI] [PubMed] [Google Scholar]
- 20.National Centre for Immunisation Research and Surveillance . AusVaxSafety: an NCIRS led collaboration Sydney: NCIRS, 2019. Available: http://www.ausvaxsafety.org.au/about-us [Accessed 2 Dec 2019].
- 21.Pillsbury AJ, Fathima P, Quinn HE, et al. Comparative Postmarket safety profile of adjuvanted and high-dose influenza vaccines in individuals 65 years or older. JAMA Netw Open 2020;3:e204079. 10.1001/jamanetworkopen.2020.4079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacoby P, Glover C, Damon C, et al. Timeliness of signal detection for adverse events following influenza vaccination in young children: a simulation case study. BMJ Open 2020;10:e031851. 10.1136/bmjopen-2019-031851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2019. [Google Scholar]
- 24.Gabutti G, Valente N, Kuhdari P, et al. Prevention of herpes zoster and its complications: from the clinic to the real-life experience with the vaccine. J Med Microbiol 2016;65:1363–9. 10.1099/jmm.0.000386 [DOI] [PubMed] [Google Scholar]
- 25.National Centre for Immunisation Research and Surveillance . Evaluation of the National shingles vaccination program process and early impact evaluation. Sydney: NCIRS, 2018. Available: http://www.ncirs.org.au/our-work/program-evaluation
- 26.Therapeutic Goods Administration . Safety advisory - not to be used in people with compromised immune function. Canberra: Department of Health, 2020. Available: https://www.tga.gov.au/alert/zostavax-vaccine-0 [Accessed 18 Jan 2021].
- 27.Centres for Disease Control . Ensuring the safety of COVID-19 vaccines in the United States. Atlanta: CDC, 2020. Available: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety.html [Accessed Dec 2020].
- 28.Baxter R, Tran TN, Hansen J, et al. Safety of Zostavax™--a cohort study in a managed care organization. Vaccine 2012;30:6636–41. 10.1016/j.vaccine.2012.08.070 [DOI] [PubMed] [Google Scholar]
- 29.Totterdell J, Phillips A, Glover C, et al. Safety of live attenuated herpes zoster vaccine in adults 70-79 years: A self-controlled case series analysis using primary care data from Australia's MedicineInsight program. Vaccine 2020;38:3968–79. 10.1016/j.vaccine.2020.03.054 [DOI] [PubMed] [Google Scholar]
- 30.Tseng HF, Liu A, Sy L, et al. Safety of zoster vaccine in adults from a large managed-care cohort: a vaccine safety Datalink study. J Intern Med 2012;271:510–20. 10.1111/j.1365-2796.2011.02474.x [DOI] [PubMed] [Google Scholar]
- 31.Levin MJ, Buchwald UK, Gardner J, et al. Immunogenicity and safety of zoster vaccine live administered with quadrivalent influenza virus vaccine. Vaccine 2018;36:179–85. 10.1016/j.vaccine.2017.08.029 [DOI] [PubMed] [Google Scholar]
- 32.Dey A, Wang H, Quinn H, et al. Surveillance of adverse events following immunisation in Australia: annual report, 2018. Commun Dis Intell 2020;44. 10.33321/cdi.2020.44.12. [Epub ahead of print: 16 Mar 2020]. [DOI] [PubMed] [Google Scholar]
- 33.Harris T, Nair J, Fediurek J, et al. Assessment of sex-specific differences in adverse events following immunization reporting in Ontario, 2012-15. Vaccine 2017;35:2600–4. 10.1016/j.vaccine.2017.03.035 [DOI] [PubMed] [Google Scholar]
- 34.Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg 2015;109:9–15. 10.1093/trstmh/tru167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohfuji S, Ito K, Inoue M, et al. Safety of live attenuated varicella-zoster vaccine in patients with underlying illnesses compared with healthy adults: a prospective cohort study. BMC Infect Dis 2019;19:95. 10.1186/s12879-019-3719-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grint DJ, McDonald HI, Walker JL, et al. Safety of inadvertent administration of live zoster vaccine to immunosuppressed individuals in a UK-based observational cohort analysis. BMJ Open 2020;10:e034886. 10.1136/bmjopen-2019-034886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jayasinghe S, Sheridan S, Macartney K. Herpes zoster vaccination in Australia: what's available and who benefits? Aust Prescr 2020;43:2–6. 10.18773/austprescr.2020.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munnoch S-A, Cashman P, Peel R, et al. Participant-Centered online active surveillance for adverse events following vaccination in a large clinical trial: feasibility and usability study. J Med Internet Res 2019;21:e14791. 10.2196/14791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Australian Government . Australian immunisation register. Canberra: Australian Government, 2020. Available: https://www.servicesaustralia.gov.au/individuals/services/medicare/australian-immunisation-register [Accessed 23 Jul 2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-043880supp001.pdf (61.8KB, pdf)