Abstract
Context:
CT scan is a quick and effective method to triage patients in the Covid-19 pandemic to prevent the heathcare facilities from getting overwhelmed.
Aims:
To find whether an initial HRCT chest can help triage patient by determining their oxygen requirement, place of treatment, laboratory parameters and risk of mortality and to compare 3 CT scoring systems (0-20, 0-25 and percentage of involved lung models) to find if one is a better predictor of prognosis than the other.
Settings and Design:
This was a prospective observational study conducted at a Tertiary care hospital in Mumbai, Patients undergoing CT scan were included by complete enumeration method.
Methods and Material:
Data collected included demographics, days from swab positivity to CT scan, comorbidities, place of treatment, laboratory parameters, oxygen requirement and mortality. We divided the patients into mild, moderate and severe based on 3 criteria - 20 point CT score (OS1), 25 point CT score (OS2) and opacity percentage (OP). CT scans were analysed using CT pneumonia analysis prototype software (Siemens Healthcare version 2.5.2, Erlangen, Germany).
Statistical Analysis:
ROC curve and Youden's index were used to determine cut off points. Multinomial logistic regression used to study the relations with oxygen requirement and place of admission. Hosmer-Lemeshow test was done to test the goodness of fit of our models.
Results:
A total of 740 patients were included in our study. All the 3 scoring systems showed a significant positive correlation with oxygen requirement, place of admission and death. Based on ROC analysis a score of 4 for OS1, 9 for OS2 and 12.7% for OP was determined as the cut off for oxygen requirement.
Conclusions:
CT severity scoring using an automated deep learning software programme is a boon for determining oxygen requirement and triage. As the score increases, the chances of requirement of higher oxygen and intubation increase. All the three scoring systems are predictive of oxygen requirement.
Keywords: Covid-19, HRCT chest, oxygen requirement
Introduction
SARS CoV 2 infection, which initially began in Wuhan, China in December 2019 has been declared as a pandemic by WHO.[1] The main concern of a pandemic is the overwhelming of medical facilities due to the sheer number of patients presenting at the same time.[2] This may also trigger panic in the general population leading to societal issues. Lockdowns are primarily to slow the number of patients presenting at the same time, allowing medical facilities to try and cope. An important component of alleviating the pressure on medical establishments is to triage patients.[3] COVID-19 pneumonia has a wide spectrum of presentations and outcomes, ranging from asymptomatic to severe hypoxia which may result in death.[4] Triage is needed to decide the order of treatment when the number of patients is large, outweighing the capacity of the available facilities. As the clinical presentation, management, and outcomes of COVID-19 is so varied, triaging patients into those who require management at home, covid care facilities or those who require hospitalization is essential. Additionally, for the patients in hospital, whether they will require admission in a ward or ICU, whether the oxygen requirement would be low flow, high flow oxygen, or whether they would require non-invasive ventilation or mechanical ventilation via intubation is also important. The role of CT for triaging patients has been emerging.
COVID-19 pneumonia pathologically is a diffuse alveolar damage, which progresses in a temporal manner.[5] Initially patients may be mildly symptomatic but can rapidly progress to severe hypoxia requiring oxygen support and hospitilisation. The extent of lung involvement in the second phase of diffuse alveolar damage approximately between day 7 and 10 may be a good surrogate to decide disease burden.[6] A low percentage of aerated lung tissue corelates with a poor prognosis. For this purpose, a CT severity score has been proposed to determine the extent of lung involvement based on extent of involvement on CT scan.[7]
To test this hypothesis, we undertook a study to determine whether extent of lung involvement on a CT scan can help triage. The main components of which include the requirement for oxygen, type of oxygen support required as well as location of admission in the health care facility.
Subjects and Methods
This was a prospective observational study conducted at Breach Candy Hospital Trust, which is a tertiary care hospital at Mumbai. Laboratory, clinical, and radiological data of all patients who underwent Ct scan for covid between April and September 2020 was collected. Ethical committee waived the need for ethical clearance and informed consent for this non interventional study.
All patients with RT PCR positive for SARS COV2 who underwent Ct scan in our hospital were included. All CT scans were done within one week of disease onset. We excluded patients with 2 consecutive negative RT PCR, patients lost to follow up, and patients with underlying chronic lung disease like ILD, organising pneumonias, carcinoma lung and Tb sequelae.
We collected the baseline demographics – age, gender and comorbidities. Laboratory data collected included IL6, D-dimer, CRP, procalcitonin and ferritin levels. The blood tests were done within 48 hours of CT scan. We also noted the need for hospitalisation, need for ICU admission, medical therapy administered and mortality. Of note, we differentiated between the oxygen requirement of patients as those on room air, with low oxygen requirement (up to 6 L/min), high oxygen requirement (7-15 L/min), NIV or HFNC, and need for intubation and mechanical ventilation. All patients undergoing CT scan from August 2020 to October 2020 were included by complete enumeration method and were followed up for as long as they were admitted in the hospital, resulting either in death or discharge.
We evaluated all the patients based on 3 criteria- 20 point CT score (OS1), 25 point CT score (OS2) and opacity percentage (OP). For each of the 3 criteria, patients were divided into mild moderate and severe group based on the cut offs as shown in Table 1.
Table 1.
Group cut-offs
| OS1 (0-20) | OS2 (0-25) | OP | |
|---|---|---|---|
| Mild | 0-4 | 0 to 7 | 0-10% |
| Moderate | 5-10 | 8-14 | 10-30% |
| Severe | 10 and above | 15 and above | 30% and above |
CT analysis
All chest CT scans were performed using a 16 slice CT scanner (Siemens Biograph Horizon) with the following parameters: 130 kV, tube voltage 100-200 mAs, rotation time 0·6 s, pitch 1·35. 1 mm slice thickness, sharp convolution kernel reconstructions with a window width of 1200 HU and a window length -600 HU was performed. The scanning range was from the apex of the lung to the costophrenic angle. No IV contrast was administered. The scan was captured in the end-inspiratory phase, whenever it was possible for the patient to hold the breath adequately. Using CT pneumonia analysis prototype software (Siemens Healthcare version 2·5·2, Erlangen, Germany), an AI algorithm based on three-dimensional segmentation automatically. The software automatically detected and quantified abnormal tomographic patterns (ground-glass opacities and consolidations) in each and both lung parenchyma based on deep learning and deep reinforcement learning.
The CT was assessed for features of COVID-19 infection, like ground-glass opacities, consolidation, crazy paving pattern, etc., by 2 radiologists with more than 10 years of experience. All CT scans were evaluated by CT pneumonia analysis software. The result provided the percentage of opacity in the lungs and a severity score based on the percentage of opacities in individual lung lobes. The AI software, automatically processed CT-SS, volume and percentage of opacity. Opacity score was calculated by dividing the lung parenchyma into five anatomical lobes and assigning scores by adding percentage of opacity within the lobes. If the parenchymal involvement was 0, <25%, 25-50%, 50-75% and >75% they were assigned a score of 0, 1, 2, 3 and 4 respectively for OS1 and involvement was 0-5%, 5-25%, 25-50%, 50-75% and >75% they were assigned a score of 1, 2, 3, 4 and 5 respectively for OS2. The scores for each lobe were added to provide the final CT severity score. The software also provided a total percentage of lung involvement representing opacity percentage of lung involvement.
Based on this result patients were divided into three groups – mild, moderate and severe disease.
Statistical analysis
All clinical and imaging data items were entered into Microsoft Office Excel and all statistical analyses were performed using STATA statistical software. Categorical variables are presented as frequency and percentages, and continuous variables as the median. ROC curve and Youden's index were used to determine the cut off points for OS1, OS2 and OP. Multinomial logistic regression was used to study the relations of OS1, OS2 and OP with oxygen requirement and place of admission. Hosmer-Lemeshow test was done to test the goodness of fit of our models. A two-sided P value of less than 0.05 was considered to be statistically significant. Details of variables considered are mentioned in Table 2.
Table 2.
Variable Used
| Name | Details |
|---|---|
| Oxygen Requirement | 0 - Room air |
| 1 - Low Oxygen | |
| 2 - High Oxygen | |
| 3 - HVNC/NIV | |
| 4 - Intubated | |
| Place of Admission | 0 - Opd |
| 1 - Ward | |
| 2 - ICU |
The patient enrolment flowchart As shown in Figure 1.
Figure 1.
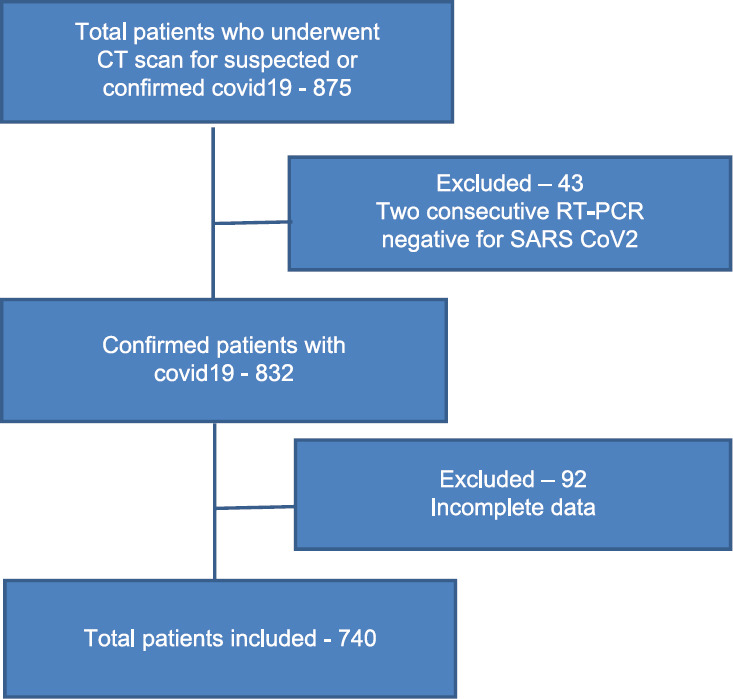
Flowchart
Results
After exclusion, 740 patients were included in our study. Majority of them belonged to the mild group (64%, 69% and 73% based on OS1, OS2 and OP, respectively). This was followed by moderate group and the least number of patients were in the severe group (based on OS1 27% and 9%, OS2 22% and 9% and on OP 16% and 11% belonged to moderate and severe group respectively). Thus, the distribution of the patients was not equal between the 3 groups and the patient characteristics between the 3 categories also varied.
The mean age of the patients was 59 years and there were 65% males (n = 483). The mean age was lesser in the mild group as compared to moderate and severe. The mean duration between swab positivity and CT was 2 days. 34·18% of the patients had no comorbidities. Of the patients with comorbidities, the most common ones were diabetes mellitus in 41·58%, hypertension in 44·6% and ischemic heart disease in 15·4%. The remaining comorbidities along with their distribution between the 3 groups and the treatment given are as shown in Table 3.
Table 3.
Baseline Demographics and Comorbidities
| Total | OS1 | OP | OS2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Mild | Moderate | Severe | Mild | Moderate | Severe | ||
| Total number | 740 | 473 | 201 | 66 | 541 | 120 | 79 | 511 | 165 | 64 |
| Age (in years) | 59 | 55 | 61 | 62 | 56 | 61 | 62 | 55 | 63 | 63 |
| Male | 483 (65%) | 292 (62%) | 144 (72%) | 47 (71%) | 338 (62%) | 91 (76%) | 54 (68%) | 312 (61%) | 124 (75%) | 47 (73%) |
| Comorbidities- Diabetes Mellitus | 308 (42%) | 189 (40%) | 86 (43%) | 33 (50%) | 216 (40%) | 54 (45%) | 38 (48%) | 201 (40%) | 76 (46%) | 31 (48%) |
| Hypertension | 331 (45%) | 194 (41%) | 100 (50%) | 37 (56%) | 222 (41%) | 65 (54%) | 44 (55%) | 208 (41%) | 87 (53%) | 36 (56%) |
| Ischemic Heart Disease | 113 (15%) | 66 (14%) | 34 (17%) | 13 (20%) | 75 (14%) | 23 (19%) | 15 (19%) | 69 (13%) | 31 (19%) | 13 (20%) |
| Obstructive airway disease | 32 (4%) | 18 (4%) | 10 (5%) | 4 (6%) | 24 (4%) | 4 (3%) | 4 (5%) | 21 (4%) | 7 (4%) | 4 (6%) |
| Hypothyroid | 48 (6%) | 34 (7%) | 10 (0.5%) | 4 (6%) | 37 (7%) | 6 (5%) | 5 (6%) | 33 (6%) | 12 (7%) | 3 (5%) |
| Chronic kidney disease | 21 (3%) | 7 (1·5%) | 10 (5%) | 4 (6%) | 14 (3%) | 4 (3%) | 3 (4%) | 10 (2%) | 7 (4%) | 4 (6%) |
| Parkinson's disease | 6 (1%) | 6 (1.3%) | 0 | 0 | 4 (1%) | 2 (2%) | 0 | 5 (1%) | 1 | 0 |
| Liver cirrhosis | 2 (0.27%) | 2 (0.4%) | 0 | 0 | 2 (0.4%) | 0 | 0 | 2 (0·4%) | 0 | 0 |
| Others | 7 (9%) | 4 (8%) | 1 (0·5%) | 2 (3%) | 5 (9%) | 0 | 2 (2·5%) | 4 (0.8%) | 0 | 3 (5%) |
| Time between swab and CT (in days) | 3 | 3 | 3 | 4 | 3 | 4 | 5 | 3 | 3 | 4 |
| Treatment- Antiviral (Remdesivir, Favipiravir) | 479 (65%) | 295 (62%) | 120 (60%) | 64 (97%) | 337 (62%) | 70 (59%) | 72 (91%) | 317 (62%) | 102 (62%) | 60 (94%) |
| Steroid (Dexamethasone, Methylprednisolone) | 556 (75%) | 320 (68%) | 170 (85%) | 66 (100%) | 387 (71%) | 90 (75%) | 79 (100%) | 373 (73%) | 119 (72%) | 64 (100%) |
| Plasma therapy | 17 (2%) | 0 | 2 (0.1%) | 15 (23%) | 0 | 0 | 17 (26%) | 0 | 2 (1%) | 15 (23%) |
| Hydrochloroquine | 517 (70%) | 392 (83%) | 100 (50%) | 25 (38%) | 447 (82%) | 40 (33%) | 30 (38%) | 395 (77%) | 97 (59%) | 25 (39%) |
| Others (Doxycycline, Azithromycin) | 590 (80%) | 365 (77%) | 176 (87%) | 49 (74%) | 429 (80%) | 103 (86%) | 58 (73%) | 406 (80%) | 139 (84%) | 45 (70%) |
| Tocilizumab/Itolizumab | 84 (11%) | 0 | 50 (25%) | 34 (51%) | 0 | 32 (26%) | 52 (66%) | 0 | 42 (25%) | 42 (65%) |
Relation with oxygen requirement
80·75% of the patients were on room air. Majority of them belonged to the mild group (77%, 81% and 85% based on OS1, OS2 and OP respectively). Only 0·67% from OS, 0·67% from OS2 and 1·5% from OP patients from severe group were on room air. 10% of the patients were on low oxygen (oxygen requirement 1-6 L/min). Majority of them belonged to mild group followed by moderate group. Only 22% (based on OS1), 21% (based on OS2) and 29% (based on OP) of the patients were from severe group. A clear majority of patients who were on high oxygen, HFNC/NIV and intubated belonged to the severe group as shown in Table 4.
Table 4.
Relation with Oxygen Requirement
| Oxygen Requirement | OS1 | OP | OS2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Mild | Moderate | Severe | Mild | Moderate | Severe | |
| RA | |||||||||
| Total Number | 458 | 132 | 4 | 509 | 76 | 9 | 486 | 104 | 4 |
| % within category | 96·82% | 65·67% | 6·06% | 94·08% | 63·33% | 11·39% | 95·10% | 63·03% | 6·25% |
| % of total | 77·10% | 22·22% | 0·67% | 85·69% | 12·79% | 1·51% | 81·81% | 17·63% | 0·67% |
| Low oxygen | |||||||||
| Total Number | 9 | 49 | 17 | 21 | 32 | 22 | 17 | 42 | 16 |
| % within category | 4·47% | 24·37% | 25·75% | 3·88% | 26·66% | 27·84% | 3·32% | 25·45% | 25% |
| % of total | 12% | 65·33% | 22·66% | 28% | 42·66% | 29·33% | 22·66% | 56% | 21·33% |
| High oxygen | |||||||||
| Total Number | 2 | 12 | 14 | 7 | 5 | 16 | 6 | 8 | 14 |
| % within category | 0·42% | 5·97% | 21·21% | 1·29% | 4·16% | 20·25% | 1·17% | 4·84% | 21·87% |
| % of total | 7.14% | 42.85% | 50% | 25% | 17·85% | 57·14% | 21·42% | 28·57% | 50% |
| HFNC/NIV | |||||||||
| Total Number | 2 | 3 | 12 | 1 | 4 | 12 | 1 | 5 | 11 |
| % within category | 0·42% | 1·49% | 18·18% | 0·18% | 3·33% | 15·18% | 0·19% | 3·03% | 17·18% |
| % of total | 11·76% | 17·64% | 70·58% | 5·88% | 23·52% | 70·58% | 5·88% | 29·41% | 64·70% |
| Intubated | |||||||||
| Total number | 2 | 5 | 19 | 3 | 3 | 20 | 1 | 6 | 19 |
| % within category | 0·42% | 2·48% | 28·78% | 0·55% | 2·5% | 25·31% | 0·19% | 3·63% | 29·68% |
| % of total | 7·69% | 19·23% | 73·07% | 11·53% | 11·53% | 76·92% | 3·84% | 23·07% | 73·07% |
We ran a multinomial logistic regression to study the relation of OS1, OS2 and OP with oxygen requirement. The regression shows a significant positive relation between the scores and oxygen requirement for all 3 categories (p-value = 0·000). The models show that OS1 and OS2 were slightly better than OP to predict the oxygen requirement. The adjusted prediction graphs, Figures 2–4, show how the probability of a patient in room air (Outcome = 0) decreases as OS1, OS2 and OP increase, while the probability of a patient being intubated (Outcome = 4) increases as OS1, OS2 and OP increase.
Figure 2.
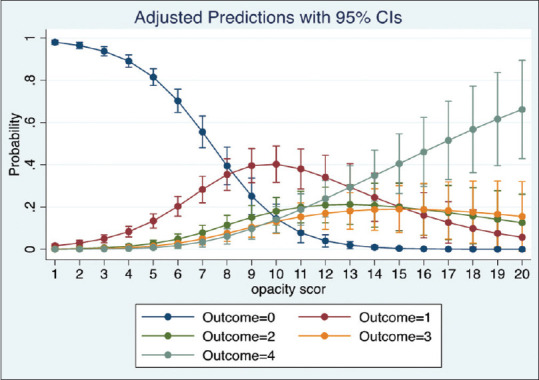
Prediction of oxygen requirement by OS1
Figure 4.
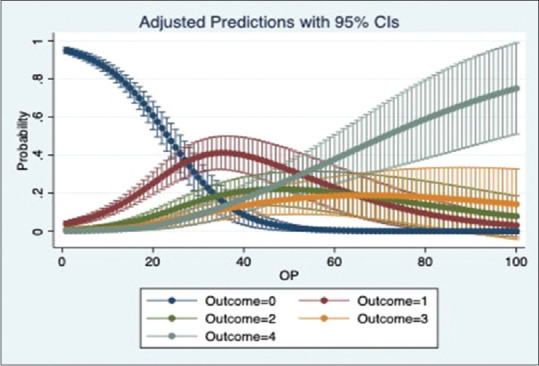
Prediction of oxygen requirement by OP
Figure 3.
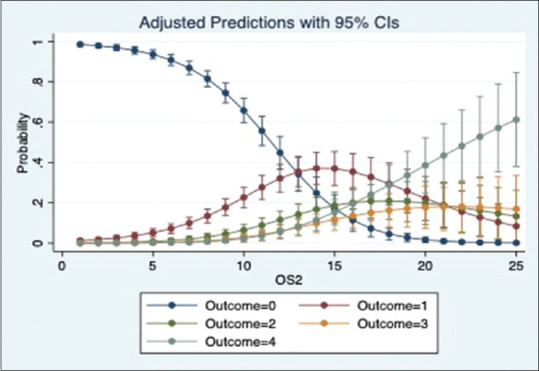
Prediction of oxygen requirement by OS2
ROC curve analysis showed a score of 4 for OS1 (AUC-0·9230 Youden Index – 0·668304 Sensitivity – 61% specificity – 98%), 9 for OS2 (AUC-0·9197 Youden index-0·6711637 sensitivity- 58% specificity – 97%) and 12·7% according to OP (AUC- 0·9135 Youden Index- 0·6644 sensitivity – 58% specificity – 97%) as the cut off for oxygen requirement.
Relation with place of admission
Majority of the patients were admitted in ward (51%) followed by OPD (44%). Almost all the patients treated on OPD basis belonged to the mild group. Only 4%, 2% and 05% patients from moderate group in OS1 OS2 and OP category were treated as OPD patients. Majority of the patients from severe category were admitted in the ICU (74% in OS1, 76% in OS2 and 83% in OP group) and the remaining were admitted in ward. Majority of patients with moderate disease were admitted in ward (73% in OS1, 72% in OS2 and 60% in OP group). The distribution of patients is as shown in Table 5.
Table 5.
Relation with Place of admission
| Place of treatment | Total | OS1 | OP | OS2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Mild | Moderate | Severe | Mild | Moderate | Severe | ||
| OPD | 251 (44%) | 239 (95·21%) | 12 (4·78%) | 0 | 245 (97·62%) | 5 (1·99%) | 1 (0·39%) | 244 (97·21%) | 7 (2·79%) | 0 |
| Hospital ward | 381 (51%) | 217 (56·85%) | 147 (38·58%) | 17 (4·46%) | 270 (70·86%) | 88 (23·09%) | 23 (6·03%) | 246 (64·56%) | 120 (31·49%) | 15 (3·83%) |
| ICU | 108 (15%) | 17 (15·74%) | 42 (38·88%) | 49 (45·37%) | 26 (24·07%) | 27 (25%) | 55 (50·92%) | 21 (19·44%) | 38 (35·18%) | 49 (45·37%) |
We ran a multinomial logistic regression to study the relation of OS1, OS2 and OP with place of admission. The regression showed that the odds of a patient being admitted to Ward and ICU significantly increases with an increase in OS1, OS2 and OP (p value = 0·000). Based on the pseudo r-squared values, model using OS1 performed marginally better than the models using OS2 and OP. The adjusted prediction graphs, Figures 5–7, show the probability of a patient being admitted to OPD decreases and ICU increases as the scores increase.
Figure 5.
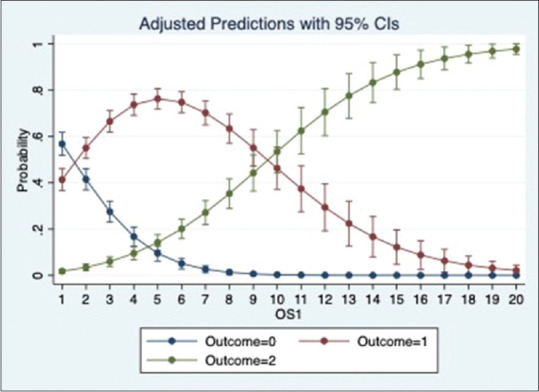
Prediction of place of admission by OS1
Figure 7.
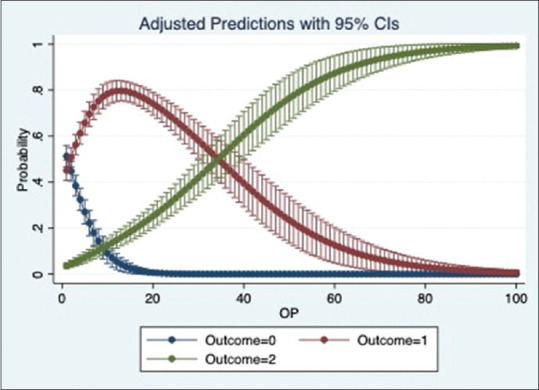
Prediction of place of admission by OP
Figure 6.
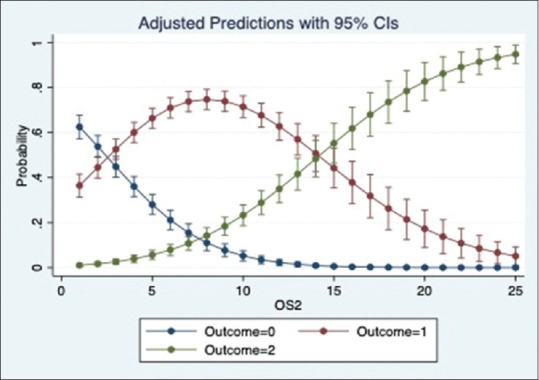
Prediction of place of admission by OS2
Relation with laboratory parameters
The laboratory parameters assessed included D-dimer, Ferritin, Interleukin 6, Procalcitonin and CRP. The values were available for only 390, 285, 268, 54 and 188 patients respectively as the lab tests were done depending upon the clinical condition of the patient and on discretion of the treating consultant. The median values of the lab parameters for each group are mentioned in Table 6. Even though the values are higher as the severity increases, no statistically significant correlation could be found between lab values and severity scores. The correlation matrix between laboratory values and Ct scores is shown in Figure 8.
Table 6.
Relation with laboratory parameters
| LAB parameters | OS1 | OP | OS2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Mild | Moderate | Severe | Mild | Moderate | Severe | |
| D-dimer | 344 | 499 | 1135 | 360 | 547·5 | 1092 | 344 | 539 | 1185 |
| CRP | 11 | 43 | 53 | 18 | 57 | 57·85 | 12·8 | 57 | 53 |
| Ferritin | 124 | 316 | 670 | 156 | 408 | 512 | 151 | 337 | 700 |
| Procalcitonin | 0·08 | 0·09 | 0·545 | 0·08 | 0·09 | 0·52 | 0·073 | 0·09 | 0·54 |
| IL6 | 32 | 46 | 81 | 32·28 | 50 | 76 | 29 | 49 | 84 |
Figure 8.
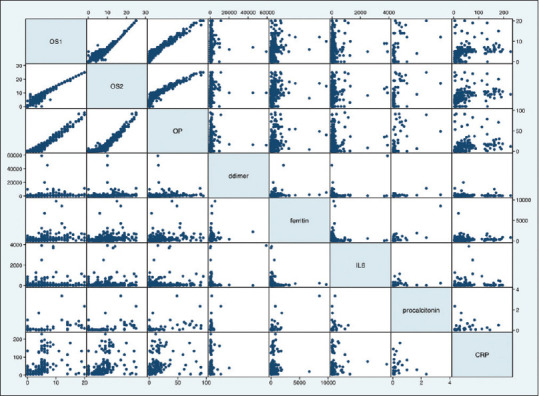
Scatterplot matrix of laboratory parameters and scores
Relation with mortality
There were 23 deaths in our study group. The distribution is as shown in the bar graph [Figure 9]. Majority of deaths were in the severe group. The relationship between severity score and mortality was found to be statistically significant in all the 3 groups (P value- 0·000). The correlation between mortality and severity scores is as shown in Figures 10–12.
Figure 9.
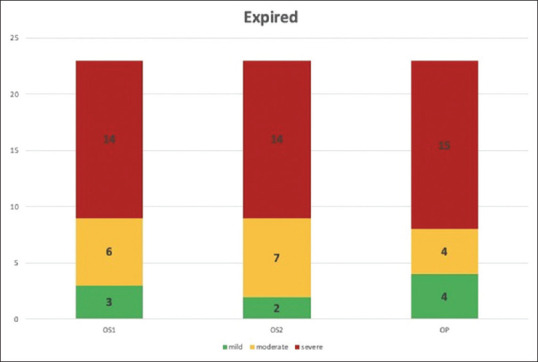
Deaths by group
Figure 10.
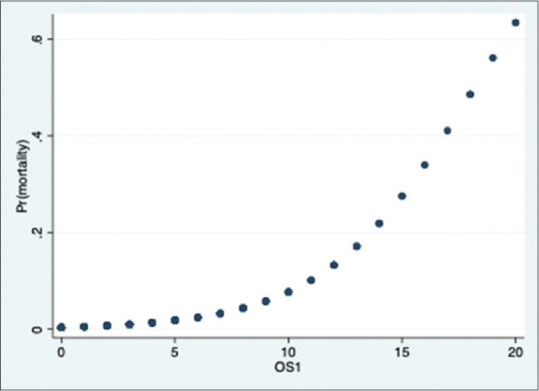
Probability of death & OS1
Figure 12.
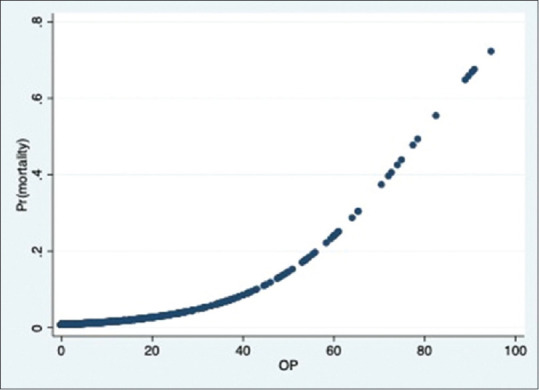
Probability of death & OP
Figure 11.
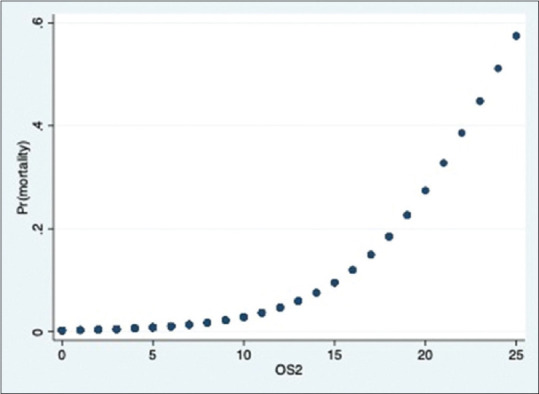
Probability of death & OS2
Discussion
A number of CT severity scoring systems have been proposed. There are 20, 25, 40 and 72 point scales which evaluate extent of lung involvement depending upon the involvement of each lobe, expressed as percentage, which is finally summed up to provide the final score.[8,9,10,11] This may be done subjectively with visual interpretation of the scans or by an automated deep learning software programme.[12,13] The subjective visual method is fraught with numerous inter and intra observer errors as it is a visual assumption, resulting in significant under and over estimation. This process is manual; thus, it is extremely time consuming which does not help in a pandemic where accurate and timely information is required for triage. In view of this we utilized an automated deep learning software - Siemens Healthcare version 2·5·2, Erlangen, Germany.
The extent of lung involvement was correlated with oxygen requirement, laboratory parameters, place of admission and mortality.
We found a statistically significant relation between the severity scores and oxygen requirement, need for hospital and ICU admission and mortality. There was no significant relation between the scores and laboratory parameters, which could probably be because laboratory parameters were available for a limited number of patients in our study. Since only the early CT scans, done within one week of disease, were taken into consideration, these findings can help in early triaging of the patients who are likely to deteriorate and administer early medical therapy even if the patients clinically have mild symptom.
Since a number of scoring systems are available, we compared the 20, 25 point scales and percentage of lung involvement to determine whether one predictor is better than other. Our study found that all three scoring systems related well with the oxygen requirement, though the performance of 0-20 scale model was slightly better. Thus, any of the scoring systems are equally predictive of patient outcome and can be used for assessing the prognosis of patients. On ROC curve analysis we found a cut off of 4 for OS1, 9 for OS2 and 12·5% for OP to be a predictor of oxygen requirement.
Majority of the patients in the mild group were on room air and were treated on an OPD basis whereas majority of the patients in severe group were admitted in ICU and were either on NIV/HFNC or were intubated. This finding was consistent for all the 3 scoring systems. Mortality was highest in the severe group. Unlike the findings of Zhang et al., we could not find a correlation between the scoring system and the laboratory parameters.[14]
The review of relevant literature is as shown in Table 7.
Table 7.
Literature review
| Author | SCORING SYSTEM USED | No of patients | Result |
|---|---|---|---|
| Lanza et al.[15] | Percentage of compromised lung | 222 | Compromised lung volume was the most accurate outcome predictor (logistic regression, P<0·001) Compromised lung volume values in the 6-23% range increased risk of oxygenation support; values above 23% were at risk for intubation. |
| Colombi et al.[16] | Percentage of well aerated lung | 236 | A percentage of well aerated lung less than 73% was a predictor of ICU admission or death |
| Leonardi et al.[17] | Percentage of compromised lung | 189 | A cut-off of 23% of lung involvement showed distinguished critically ill patients from patients with less severe disease. |
| Sandoval et al.[18] | AI based software- percentage involvement of lung | 166 | Threshold for 51% for mortality and 25% for mechanical ventilation |
| Jiayi Liu et al.[8] | 0-20 | 24 | As the severity increased, the number of lobe involved and CT severity score increased from 4 to 5 and 6 to 12 respectively. A cut off of 5 helped to identify cases with severe pneumonia (i.e. SpO2 less than 93% on room air and P/F<300) |
| Tabatabei et al.[19] | 0-20 | 90 non-elderly patients. 30 who expired were in case group and 60 who were discharged were in control group | CT severity score is the only statistically significant CT predictor of mortality. A score of 7.5 was cut-off point of CT severity score with the highest sensitivity (0·83) and specificity for predicting mortality. |
| Lyu et al.[20] | 0-20. used both qualitative and quantitative indicators | 51 | Cut off >10 to differentiate severe cases from mild and moderate. |
| Li et al.[12] | 0-20 | 78 | Cut off of 7.5 to diagnose severe- critical cases (SpO2 less than 93% on room air) |
| Fancone et al.[9] | 0-25 | 130 | CT score was significantly higher in critical and severe than in mild stage. A CT score of ≥18 was associated with an increased mortality risk and was found to be predictive of death. |
| Saeed et al.[21] | 0-25 | 902 | The 25-point CT severity score correlates well with the Covid-19 clinical severity. |
| Mahdjoub et al.[22] | 0-25 | 142 | CT score ≥13 was related to poor 5-day outcome |
| Zhou et al.[23] | 0-25 | 134 | The cut-off value of total CT scores was determined to be 16·5 for predicting poor prognosis in patients with Covid-19. |
| Feng et al.[24] | 0-25 | 298 | CT severity score is an independent predictor for progression to severe Covid-19 pneumonia |
| Abbasi et al.[25] | 0-24 | 262 | Optimal CT severity score threshold for identifying deceased patients was 10. The mean score of survivors was 7 and deceased patients was 14. |
This was a prospective study conducted with an appropriate sample size. This study demonstrated a direct relationship between the CT severity scores and oxygen requirement and need for intubation, independent of the laboratory values and comorbidities of the patients. To the best of our knowledge, no study has provided a comparison between the different scoring systems for CT severity. This study showed that all 3 scoring systems were predictive and none was significantly superior to the other. While most of the studies provided a cut off for predicting mechanical ventilation and oxygen requirement based on opacity scores, this study provides a cut off for predicting oxygen requirement.
However, our study has certain limitations. Studies were not controlled by number of days since start of symptoms, which could have potential limitations for interpretation of CT severity score. The first scan performed was utilized, it is known severe disease may have a longer interval between beginning of symptoms and height of disease. Laboratory values were available only for a limited number of patients in our study and hence we could not find any correlation. Factors like age, comorbidities, inflammatory markers can also affect the outcome and oxygen requirement. We did not independently analyse the effect of these parameters in our study. It was a single centre study and hence representative of data in a particular community. The results may vary for different communities. Even with these limitations there was an excellent correlation over a large cohort of patients.
In conclusion, an early CT scan in patients affected with Covid-19 is predictive of the oxygen requirement of the patient. As severity scores increase the chances of requirement of higher oxygen and intubation increase. The severity scoring system can be based on lobar involvement and scored 0-20 or 0-25 or based on percentage of lung involved, as they are all predictive of oxygen requirement. CT severity scoring using an automated deep learning software programme is a great boon for determining oxygen requirement and triage.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Would like to acknowledge the contribution of Sanya Jha for data analysis and interpretation in the study.
References
- 1. [Last accessed on 2020 Dec 22]. Available from: https://www.who.int .
- 2.Cavallo J, Donoho D, Forman H. Hospital capacity and operations in the coronavirus disease 2019 (COVID-19) pandemic—Planning for the Nth Patient. JAMA Health Forum. 2020 doi: 10.1001/jamahealthforum.2020.0345. doi: 10.1001/jamahealthforum.2020.0345. [DOI] [PubMed] [Google Scholar]
- 3.Udugama B, Kadhiresan P, Kozlowski H, Malekjahani A, Osborne M, Li V, et al. Diagnosing COVID-19: The disease and tools for detection. ACS Nano. 2020;14:3822–35. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- 4.Guan W, Ni Z, Liang W, Ou C, He J, Liu L, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borczuk A, Salvatore S, Surya S, Patel S, Bussel J, Mostyka M, et al. COVID-19 pulmonary pathology: A multi-institutional autopsy cohort from Italy and New York City. Mod Pathol. 2020;33:2156–68. doi: 10.1038/s41379-020-00661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan F, Ye T, Gui S, Sun P, Liang B, Zheng D, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295:715–21. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wasilewski P, Mruk B, Mazur S, Szymczak G, Sklinda K, Walecki J. COVID-19 severity scoring systems in radiological imaging-A review. Pol J Radiol. 2020;85:e361–8. doi: 10.5114/pjr.2020.98009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Chen T, Yang H, Cai Y, Yu Q, Chen J, et al. Clinical and radiological changes of hospitalised patients with COVID-19 pneumonia from disease onset to acute exacerbation: A multicenter paired cohort study. Eur Radiol. 2020;30:5702–8. doi: 10.1007/s00330-020-06916-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francone M, Lafrate F, Masci G, Coco S, Cilia F, Manganaro L, et al. Chest CT score in COVID-19 patients: Correlation with disease severity and short-term prognosis. Eur Radiol. 2020;30:6808–17. doi: 10.1007/s00330-020-07033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang R, Li X, Liu H, Zhen Y, Zhang X, Xiong Q, et al. Chest CT severity score: An imaging tool for assessing severe COVID-19. Radiol Cardiothoracic Imaging. 2020;2:e200047. doi: 10.1148/ryct.2020200047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan M, Yin W, Tao Z, Tan W, Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS One. 2020;15:e0230548. doi: 10.1371/journal.pone.0230548. doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li K, Fang Y, Li W, Pan C, Qin P, Zhong Y, et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020;30:4407–16. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang L, Han R, Ai T, Yu P, Kang H, Tao Q, et al. Serial quantitative chest CT assessment of COVID-19: A deep learning approach? Radiol Cardiothorac Imaging. 2020;2:e200075. doi: 10.1148/ryct.2020200075. doi: 10.1148/ryct.2020200075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Meng G, Li W, Shi B, Dong H, Su Z, et al. Relationship of chest CT score with clinical characteristics of 108 patients hospitalized with COVID-19 in Wuhan, China. Respir Res. 2020;21:180. doi: 10.1186/s12931-020-01440-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanza E, Muglia R, Bolengo I, Santonocito O, Lisi C, Angelotti G, et al. Quantitative chest CT analysis in COVID-19 to predict the need for oxygenation support and intubation. Eur Radiol. 2020;30:6770–8. doi: 10.1007/s00330-020-07013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colombi D, Bodini F, Petrini M, Maffi G, Morelli N, Milanese G, et al. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology. 2020;296:E86–7. doi: 10.1148/radiol.2020201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonardi A, Scipione R, Alfieri G, Petrillo R, Dolciami M, Ciccarelli F, et al. Role of computed tomography in predicting critical disease in patients with covid-19 pneumonia: A retrospective study using a semiautomatic quantitative method. Eur J Radiol. 2020;130:109202. doi: 10.1016/j.ejrad.2020.109202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimura-Sandoval Y, Arévalo-Molina ME, Cristancho-Rojas CN, Kimura-Sandoval Y, Rebollo-Hurtado V, Licano-Zubiate M, et al. Validation of chest computed tomography artificial intelligence to determine the requirement for mechanical ventilation and risk of mortality in hospitalized coronavirus disease-19 patients in a tertiary care center In Mexico City. Rev Invest Clın. 2020 doi: 10.24875/RIC.20000451. doi: 10.24875/RIC.20000451. [DOI] [PubMed] [Google Scholar]
- 19.Tabatabaei S, Rajebi H, Moghaddas F, Ghasemiadl M, Talari H. Chest CT in COVID-19 pneumonia: What are the findings in mid-term follow-up? Emerg Radiol. 2020;27:711–9. doi: 10.1007/s10140-020-01869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyu P, Liu X, Zhang R, Shi L, Gao J. The performance of chest CT in evaluating the clinical severity of COVID-19 pneumonia: Identifying critical cases based on CT characteristics. Invest Radiol. 2020;55:412–21. doi: 10.1097/RLI.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saeed G, Gaba W, Shah A, Helali A, Raidullah E, Ali A, et al. Correlation between Chest CT severity scores and the clinical parameters of adult patients with COVID-19 pneumonia. medRxiv. 2020 doi: 10.1155/2021/6697677. doi: 10.1101/2020.10.15.20213058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahdjoub E, Mohammad W, Lefevre, Debray MP, Khalil A Study Group§. Admission chest CT score predicts 5-day outcome in patients with COVID-19. Intensive Care Med. 2020;46:1648–50. doi: 10.1007/s00134-020-06118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou S, Chengyang C, Hu Y, Lv W, Ai T, Xia L. Chest CT imaging features and severity scores as biomarkers for prognostic prediction in patients with COVID-19? Ann Transl Med. 2020;8:1449. doi: 10.21037/atm-20-3421. doi: 10.21037/atm-20-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng Z, Yu Q, Yao S, Luo L, Duan J, Yan Z, et al. Early prediction of disease progression in COVID-19 pneumonia patients with chest CT and clinical characteristics. Nat Commun. 2020;11:4968. doi: 10.1038/s41467-020-18786-x. doi: 10.1038/s41467-020-18786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbasi B, Akhavan R, Ghamari Khameneh A, Zandi B, Farrokh D, Pezeshki Rad M, et al. Evaluation of the relationship between inpatient COVID-19 mortality and chest CT severity score. Am J Emerg Med. 2020;20:S0735–6757. 30851–2. doi: 10.1016/j.ajem.2020.09.056. doi: 10.1016/j.ajem.2020.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]


