Abstract
Cannabis sativa (Cannabis) is one of the world’s most well-known, yet maligned plant species. However, significant recent research is starting to unveil the potential of Cannabis to produce secondary compounds that may offer a suite of medical benefits, elevating this unique plant species from its illicit narcotic status into a genuine biopharmaceutical. This review summarises the lengthy history of Cannabis and details the molecular pathways that underpin the production of key secondary metabolites that may confer medical efficacy. We also provide an up-to-date summary of the molecular targets and potential of the relatively unknown minor compounds offered by the Cannabis plant. Furthermore, we detail the recent advances in plant science, as well as synthetic biology, and the pharmacology surrounding Cannabis. Given the relative infancy of Cannabis research, we go on to highlight the parallels to previous research conducted in another medically relevant and versatile plant, Papaver somniferum (opium poppy), as an indicator of the possible future direction of Cannabis plant biology. Overall, this review highlights the future directions of cannabis research outside of the medical biology aspects of its well-characterised constituents and explores additional avenues for the potential improvement of the medical potential of the Cannabis plant.
Keywords: Cannabis sativa (Cannabis), cannabinoids, tetrahydrocannabinol (THC), cannabidiol (CBD), cannabinoid receptors (CB1 and CB2), Papaver somniferum (opium poppy), secondary metabolites
1. Introduction
Cannabis sativa (Cannabis) is arguably one of the world’s most versatile crops. While the genetic origin and evolution of Cannabis is a long-standing and heavily debated topic [1,2,3,4], in broad terms, today, Cannabis can be separated into two distinct categories, specifically ‘hemp’ and ‘marijuana’. Much like other agricultural crop commodities, Cannabis has been domesticated and bred for thousands of years to produce phenotypic and/or chemotypic traits of value to humans [2,3,4,5]. The chemotypic distinction between hemp and marijuana predominantly stems from the abundance of the principal psychoactive cannabinoid, Δ9-tetrahydrocannabinol (THC), present in the plant as the acidic form, Δ9-tetrahydrocannabinolic acid (THCA) [6]. To be considered hemp, Cannabis must possess a low percentage of THC relative to the total dry weight of flowers, with this low THC percentage varying from country to country. In order to be legally cultivated as hemp, the cultivated plants must possess less than 0.3% THC (w/w) in Canada [4,7] and China [8], whereas since 2001, the European Union determined that the THC content (w/w) of hemp must be below 0.2% [6].
Hemp has traditionally been bred as a source for textile products due to the strong, elongated bast fibres present in the phloem of the stem. More recently, the elevated cellulosic content of hemp cell walls has garnered interest in the plant as a source for the development of sustainable biofuel production [6]. Hempseed, and hempseed oil, have historically been utilised as a food source, with more contemporary research revealing their unique dietary value. In particular, the essential polyunsaturated fatty acids (PUFAs), linoleic acid (LA) and linolenic acid (LNA), comprise 50–70% and 15–25% of the total fatty acid content of hempseed, respectively; a 3:1 ratio promoted as nutritionally optimal [9,10,11,12,13]. PUFAs found in hempseed oil are incorporated into phospholipid bilayers and are integral to membrane fluidity and the maintenance of its permeability [14]. Moreover, the two proteins, edestin and albumin found in hempseed, contain rich amino acid profiles comparable to that of high-quality soybean and egg white [15]. Given the functions and importance of both fatty and amino acids, hempseed and hempseed oil may have some potential, albeit minor, for reducing the incidence of certain diseases, while in parallel conferring a range of health benefits [15,16,17]. Alternatively, marijuana has traditionally been bred for its recreational intoxication properties derived from the THCA-containing resin produced on the protruding secretory hair-like structures known as trichomes which are predominantly located on female reproductive parts of the Cannabis plant [18,19]. The sticky resin produced from these specialised epidermal glands is a rich mix of cannabinoid and non-cannabinoid constituents, numbering at least 104 and 441, respectively [20,21]. Most recently, two novel cannabinoids, namely Δ9-tetrahydrocannabiphorol (Δ9-THCP) and cannabidiphorol (CBDP), near identical in structure to THC and cannabidiol (CBD), respectively, were identified [22]. Notably, Δ9-THCP was demonstrated to possess higher cannabimimetic activity than THC, and its recent discovery is therefore postulated as a potential candidate cannabinoid responsible for variation in pharmacological properties observed in uncharacterised Cannabis varieties. This also identifies the likelihood of secondary metabolites present in Cannabis resin that remain to be discovered.
In addition to possessing a range of phenotypic and chemotypic traits of interest to the textile, medicinal, food and energy industries as an agricultural crop, Cannabis is extremely versatile and hardy, hence the application of the colloquial term for this species, ‘weed’. The phenotypic flexibility of Cannabis provides it with the capacity to adapt and survive a range of abiotic and biotic insults, such as drought [23], heavy metal stress [24], high temperature [25], poor soil nutrient content [3], high plant density [26], and stem damage from the larva of Ostrinia nubilalis, the European corn borer [27]. Tolerance to a range of abiotic stress conditions is exemplified by the tap root of Cannabis which is able to adapt to highly variable edaphic conditions, either penetrating deep (greater than 2 metres) into dry soil, or developing an extensive lateral root network in response to its growth in soil that has a high moisture content [26]. Further, the widespread legalisation of medicinal application and recreational use of Cannabis is driving the growth of diverse research programs encompassing the broad scope, from plant breeding to clinical trials. In the United States of America (USA), for example, to date, 33 states have approved the medicinal use of Cannabis, while 14 states and territories have legalised the recreational use of marijuana by adults. At the federal level in the USA, however, Cannabis remains a ‘Schedule I Substance’. In direct contrast to the heavy legislation of Cannabis in the USA, its direct neighbour, Canada, legalised the use of Cannabis across the country in 2018 under the ‘Cannabis Act’ [28]. As the legislative approval of Cannabis use increases worldwide, there will be an increasing need for interdisciplinary research to characterise secondary metabolites of interest and to increase the production of Cannabis to meet the demand for medicinal and recreational products.
Currently, there exists an extant literature on the medical potential for the best characterised cannabinoids, THC and CBD [29,30,31,32,33,34]. Significantly less attention in medical research has been paid to the potential for the minor phytocannabinoids to treat illnesses, and there is still the need for methods to produce these cannabinoids cost-effectively for commercial production. In particular, the medical Cannabis industry faces significant challenges in multiple aspects of product development. For instance, THC is associated with multiple side effects, and furthermore, pharmaceutical-standard THC and CBD are expensive to produce. Due to these hurdles, many companies around the world which have attempted to capitalise on the increasing legality of Cannabis have been unsuccessful [35]. Therefore, here we review the current literature describing emerging research concerning the medical potential of the minor cannabinoids, as well as to outline the agricultural and production considerations that will be necessary to meet the needs of the growing medical market. Readers interested primarily in the effects of CBD and THC should consult any of the substantial reviews on these topics that are published elsewhere and referred to here in Section 2.2. It should also be noted that there are some recent review articles on the molecular targets of the minor cannabinoids [36,37], but to the best of our knowledge, no published review of the current literature has combined this research with the potential for improving Cannabis yield and extraction efficacy to make these possibilities economically and logistically pragmatic. This review therefore presents a novel, interdisciplinary perspective on the practical possibilities for improving the Cannabis species for its utilisation in the cannabinoid industry in the near future.
2. The Endocannabinoid System and Its Associated Molecular Targets
2.1. An Overview of the Endocannabinoid System
The discovery of the endogenous cannabinoid system followed the initial isolation [38] and synthesis [39] of the primary psychoactive compound in Cannabis, THC. Following on from this in the late 1980s, and into the early 1990s, two cannabinoid receptors, CB1 and CB2, were identified [40,41]. Surprisingly, it was discovered that CB1 was highly abundant in the central nervous system (CNS), and in the CNS, CB1 is one of the most profuse G protein-coupled receptors [42]. The identification of these two CB receptors subsequently led to the discovery of an endogenous receptor ligand termed arachidonylethanolamide (anandamide), a receptor ligand accurately predicted to exist based on the presence of the CB receptors themselves [43]. A second receptor ligand, 2-arachidonoylglycerol (2-AG) was later identified [44,45]. Anandamide and 2-AG are both synthesised from arachidonic acid. Synthesis of anandamide is complex, and therefore remains to be elucidated, though it is thought to occur largely via the cleavage of arachidonic acid by a phospholipase D from its membrane precursor, N-arachidonoyl phosphatidylethanolamine [46]. The synthesis of 2-AG occurs following the conversion of diacylglycerol by the metabolic enzyme, diacylglycerol lipase (DAGL). Hydrolysis of anandamide occurs via the enzyme activity of fatty acid amide hydrolase (FAAH), whereas 2-AG is hydrolysed by both FAAH and monoacylglycerol lipase (MAGL) [47]. Inhibition of these enzymes increases anandamide and 2-AG concentrations and has therapeutic potential [48,49,50]. Similarly, it is possible that modulation of precursory compounds of anandamide and 2-AG may have therapeutic potential [51].
Previous investigations into CB receptor distribution within the fetal, neonatal and adult human brain revealed that the CB receptors were primarily localised to areas responsible for; (1) higher cognitive function; (2) movement, and; (3) control of sensory and motor functions of the autonomic nervous system [52]. Protein crystallisation has revealed the structure of CB1 [53] and CB2 [54] to assist in the characterisation of the molecular binding of ligands, such as THC, and potentially other key cannabinoids, both naturally or synthetically produced. Using radiolabelled synthetic cannabinoids, it was shown that the highest density of cannabinoid binding, and thus CB receptor localisation, appeared in the basal ganglia, hippocampus and cerebellum [42]. Cannabinoids were shown to function on hippocampal presynaptic receptors, via regulating the release of γ-aminobutyric acid (GABA) to modulate higher cognitive functions, while also increasing the activity of p38 mitogen-activated protein kinases [55,56]. Similarly, GABA modulation in the basal ganglia, specifically the presynaptic striatal projection neuron axons and their termini, was found to be stimulated to differing degrees by either endocannabinoids or synthetic cannabinoids [57,58]. The binding of the CB1 receptor by both endogenous and exogenous cannabinoids also modulates excitatory synaptic transmission in Purkinje cells located in the cerebellum [59,60,61,62]. Crucially, endocannabinoid signalling was recognised as the mediatory secondary messenger responsible for long-term potentiation, and depression [49,63], which are both fundamental to the control of synaptic transmission. CB1 receptors and endocannabinoid signalling also interacts with other systems in the brain, such as the dopaminergic [64], and glucocorticoid [65] pathways, to modulate stress response and associative learning processes.
While early understanding of receptor distribution suggested exclusive ‘central’ aggregation in specific regions of the brain, it is now understood that there is a more extensive presence of CB1 type receptors in peripheral tissues. Two CB1 receptor isoforms have since been identified, both of which display distinct expression patterns in pancreatic β-cells and liver hepatocytes [66]. Antagonism of peripheral CB receptors located in skeletal muscles was shown to trigger glucose uptake, while simultaneously initiating lipid mobilisation in white adipose tissue [67]. Though the protein expression pattern of CB1 does show some overlap with CB2 in peripheral tissues, and conversely some CB2 receptors are cerebrally positioned [68,69,70,71,72], peripheral receptors are predominantly CB2 type receptors. Analysis of CB2 transcript levels has previously revealed its expression in the tonsils, spleen, and peripheral blood mononuclear cells, where further cell isolation showed detectable CB2 transcript levels in polymorphonuclear neutrophils (PMN), T4 cells, T8 cells, natural killer (NK) cells, macrophages, and B cells. However, at the protein level, the CB2 receptor appears to be restricted to B cells [73]. Similarly, CB2 receptor binding has been observed in other immune system regions, namely the lymph node cortex, as well as in the Peyer’s patches, which are areas of B lymphocyte aggregation [74]. The expression and/or localisation of functional CB2 protein has also been reported for mast cells, modulating their initial activation, or downregulating their activity post their initial activation, an activity change which can in turn provoke an anti-inflammatory response [75]. Anandamide and 2-AG, as well as their metabolic enzymes, are detectable in blood [76,77], hair [78,79,80], saliva [81,82,83], breast milk [84,85], and reproductive fluids [84,86]. Compounded with the peripheral anti-inflammatory response, CB2 receptor agonists can mediate peripheral antinociception without the psychotropic CNS effects associated with phytocannabinoid CB1 receptor binding [87,88]. This characteristic of exerting medically beneficial effects, while simultaneously avoiding any psychotropic responses, is likely to form a key focus of future cannabinoid research.
2.2. The Expanded Cannabinoid System and Its Less Characterised Receptors
It has been clearly demonstrated that the collective effects of cannabinoid administration cannot be explained solely by the presence of CB receptors. Conversely, it has been increasingly recognised that cannabinoids have the potential to affect other molecular targets and receptor types, particularly given their role as presynaptic secondary messengers on various neuron species [89,90] (Table 1). One such receptor is the G protein-coupled receptor (GPCR), GPR55, with the GPR55 transcript identified in the adrenals, jejunum, and ileum in mammalian systems [91]. Studies on canine, rat and mouse gastrointestinal systems collectively suggest that GPR55 may be involved in smooth muscle contractions and colonic motility, especially when activated by CBD, pointing to a potential target for treatment of some gastrointestinal disorders [92,93,94,95]. Human embryonic kidney 293 (HEK293) cells expressing the GPR55 protein have been assessed for their response when treated with the lysolipid, L-α-lysophosphatidylinositol (LPI), as well as following their treatment with endogenous, synthetic or phytocannabinoids. LPI was found to induce phosphorylation of the protein, extracellular signal-related kinase (ERK) in GPR55-expressing cells, while also initiating a transient Ca2+ signal involved in downstream messaging and intracellular processing [96]. The degree of elevation in the concentration of Ca2+ increases in HEK293 cells when mediated by GPR55-phospholipase C coupling varied depending on whether THC, anandamide, methanandamide or the CB2 agonist, JWH015 was administered [97]. However, there was no Ca2+ response initiated by CBD, the CBD regioisomer abnormal CBD, the endogenous cannabinoids, 2-arachidonoylglycerol and O-arachidonoyl ethanolamine, or the synthetic cannabinoids, WIN55,212-2 and CP55,940 [97]. Beyond Ca2+ transients, cannabinoid ligand interaction with the GPR55 receptor promotes ERK phosphorylation, as well as the varied activation of cyclic adenosine monophosphate (cAMP) response element binding protein (CREB), nuclear factor-κB (NF-κB) and nuclear factor of activated T-cell (NFAT) transcription factors, the latter two of which are involved in inflammation of endothelial cells and irritable bowel syndrome (IBS) [98,99,100,101]. The GPR55 transcript can also be found in the basal ganglia, hippocampus, forebrain, cerebellum, cortex and large dorsal root ganglion (DRG) [97,102,103,104]. The expression of GPR55 in these tissues significantly broadens the potential for its therapeutic application. For instance, activation of the GPR55 receptor by THC enhances neuronal excitability and reduces the M-type potassium current, which when combined with the expression pattern of GPR55 in the large DRG, indicates a nociceptive role [97]. Inflammatory pain was modulated by abnormal CBD through GPR55 antagonism in acute arthritis models in rats [105]. Evidence of pro-nociception was observed in rats when the abundance of GPR55-dependent Ca2+ increased in periaqueductal grey neurons and which preceded a pain threshold reduction [106]. However, another study [107] reported that GPR55 knockout mice show no difference to wild-type mice in neuropathic pain models.
Table 1.
Receptor modulation by cannabinoids and studies outlining their potential involvement in disease treatment.
| Receptor | Cannabinoid | Disease/Interaction | Study Type | Reference |
|---|---|---|---|---|
| CB1 | Anandamide | Appetite | Murine models | [173,174] |
| Met-F-AEA | Thyroid cancer | in vitro human | [175] | |
| THCB (PA) | Pain | Murine models | [176] | |
| THC (PA) | Epilepsy | Murine models | [177] | |
| Sleep | Various studies | [178] | ||
| THCP (Ag) | Pain, anxiety, hypothermia, catalepsy | Murine models | [22] | |
| THCV (^) | Pain, anxiety, hypothermia, catalepsy | Murine models | [179,180] | |
| Parkinson’s disease | Murine models | [181] | ||
| Obesity | Murine models | [182] | ||
| Epilepsy | in vitro murine | [183] | ||
| THC, WIN55,212-2, CP55, 940 |
Emesis | Animal models | [184,185,186,187,188] | |
| WIN55,212-2 | Parkinson’s disease | Murine model | [189] | |
| Prostate cancer | in vitro human | [190] | ||
| WIN55,212-2, JWH-133 | Breast, lung cancer | in vitro human | [191,192] | |
| CB2 | CBC (Ag) | Inflammation | in vitro models | [193] |
| CBG (PA) | Inflammatory bowel disease | Murine models | [194] | |
| HU-308, AM630 | Parkinson’s disease | Murine models | [195,196] | |
| THCP (Ag) | Pain, anxiety, hypothermia, catalepsy | Murine models | [22] | |
| THCV (^) | Inflammation | Murine models | [180] | |
| CB2 | THCV (^) | Parkinson’s disease | Murine models | [181] |
| Pain, anxiety, hypothermia, catalepsy | Murine models | [179] | ||
| WIN55,212-2 | Prostate cancer | in vitro human | [190] | |
| WIN55,212-2, JWH-133 | Breast, lung cancer | in vitro human | [191,192] | |
| GPR55 | Abnormal CBD | Parkinson’s disease | Murine models | [103] |
| GPR55 | Abnormal CBD | Pain/arthritis | Murine models | [105] |
| CBD (An) | Gastrointestinal disorders | Canine, murine models | [93,94,95,96] | |
| CBDV (An) | Rett syndrome | Murine models | [197] | |
| LPI inhibitor | in vitro | [198] | ||
| THC, anandamide, JWH015 | Pain | in vitro HEK239 | [97] | |
| TRPV1 | CBDV (Ag) | Anti-seizure | in vitro HEK239 | [199] |
| CBG (Ag), CBGV, CBD (Ag), CBDV (Ag), THCV (Ag) | Receptor desensitisation | in vitro HEK239 | [200] | |
| TRPV2 | CBD (Ag), CBGV, CBG (Ag), THCV (Ag), CBDV (Ag), CBN (Ag) | Receptor desensitisation | in vitro HEK239 | [200] |
| TRPV3 | CBGV, CBGA (Ag) | Receptor desensitisation | in vitro HEK239 | [201] |
| TRPV4 | CBGV, CBGA, CBN, CBG | Receptor desensitisation | in vitro HEK239 | [201] |
| TRPM8 | CBG (An), CBC (An), CBD (An), CBDV (An), THC (An), THCA (An) | Colorectal cancer | in vitro model | [200,202,203] |
| TRPA1 | CBC (Ag), CBN (Ag), THC (Ag), THCV (Ag), THCA (Ag), CBDA, CBG (Ag) | Receptor desensitisation | in vitro HEK239 | [200,202] |
| CBDV (Ag) | Ulcerative colitis | in vitro human | [204] | |
| Muscular dystrophy | in vitro studies | [205] |
PA = Partial Agonist, Ag = Agonist, ^ = Dose Dependent, An = Antagonist.
Another seven-transmembrane G protein-coupled receptor, termed GPR18, was first identified in canine gastric mucosa and a human colonic cancer cell line, with a high abundance of the GPR18 transcript detected in human testis and spleen tissue [108]. The candidate ligand was later suggested to be N-arachidonoyl glycine (NAGly), an anandamide metabolite, which was first detected when GPR18-expressing cell lines, including the L929, K562 and Chinese hamster ovary (CHO) cell lines produced, high levels of intracellular Ca2+ and inhibited the production of cAMP following NAGly exposure [109]. In addition, quantitative real-time PCR analysis revealed high levels of GPR18 expression in peripheral lymphocytes, further supporting the suggestion of a role in immune system function [109].
The transient receptor potential vanilloid (TRPV) channels are a subfamily of transmembrane ligand-gated ion channels that mediate signal transduction processes initiated by a broad range of noxious stimuli in animals, with the TRPVs, TRPV1 through to TRPV4, activated to varying degrees via cannabinoid application. TRPV expression in several human tissues and the documented role of TRPVs in human disease is a current avenue of interest. The capsaicin and temperature (~42 °C) responsive TRPV1, displays an ambiguous expression profile. However, the weight of evidence suggests that its expression domain is rather broad in animal systems. Specifically, the TRPV1 protein was observed to be localised to the dorsal root and trigeminal ganglions [110], thermoregulatory tissue smooth muscle cells [111], urothelial cells [112], corneal fibroblasts [113], and a broad distribution profile in the brain, including the hippocampus, cortex and olfactory bulb [114]. Sharing 50% sequence identity to TRPV1, TRPV2 has been demonstrated to respond to high-intensity thermal stimuli (~52 °C). However, unlike TRPV1, TRPV2 is insensitive to capsaicin [115]. Given its sensory involvement, TRPV2 localisation in the ganglia is unsurprising. However, TRPV2 is also localised to the brain, lung, spleen, intestine, mast cells and lymphocytes [115,116,117,118], which, when considered together, infers additional TRPV2 function beyond heat sensing, and by extension, activation by non-thermal receptor modulators. The initiation of signal cascades via TRPV2 are potentially involved in diseases and physiological responses including cancer [119], the innate and adaptive immune responses [116,117,120,121], cardiomyopathy [122,123], muscular dystrophy [124,125], and insulin secretion response [126,127,128].
The cannabinoid-responsive TRPVs, TRPV3 and TRPV4, are also temperature sensitive proteins. The responsive temperature range (27–40 °C) for these two receptors is below that of TRPV1 and TRPV2, but they do closely overlap with one another [129,130,131,132]. Their thermosensory involvement localises these two TRPVs to keratinocytes, where they sense warmth on the skin and transmit a signal to nearby neurons [133,134,135,136,137,138]. In the tongue and nasal epithelium, TRPV3 is activated by the ‘pungent’ carvacrol as well as by thymol and camphor [133,139], whereas the mevalonate (MVA) pathway product and cannabinoid/terpenoid precursor, isopentenyl diphosphate (IPP), has been shown to inhibit TRPV3 activity [140]. TRPV4, in association with aquaporin 5 (AQP5), is additionally involved in osmosensing and regulatory volume decrease in cells following swelling in hypotonic environments [141,142,143,144]. Located in the brain [145,146], kidneys [147], CNS [148], and endocardium [149], TRPV4 activity is also modulated by phorbol esters and arachidonic acid expanding its activation beyond physical stimuli [150,151].
In addition to the vanilloid subtype of the transient receptor potential channels are the melastatin and ankyrin subtypes. Of the melastatin type, transient receptor potential melastatin 8 (TRPM8) is a cold/menthol-responsive channel located in the DRG and trigeminal ganglia [152,153]. Of the ankyrin subtype, transient receptor potential ankyrin 1 (TRPA1) acts similarly to TRPM8 in response to cold stimuli covering a similar temperature range (~8–28 °C). However, it is suggested that TRPA1 contributes to sensation of lower temperatures, and is also similarly localised in sensory neurons [154,155,156,157]. TRPA1 is additionally activated by formalin and allyl isothiocyanates such as mustard oil [158,159], and has further been implicated in eliciting inflammatory pain [160,161,162,163].
Multiple other targets show notable interactions with the endocannabinoid system; however, a comprehensive description of all interactions is beyond the scope of this review. Briefly, other notable molecular interactions include glycine receptors with anandamide, and in addition, CBD and THC have also been shown to activate glycine receptors [164,165]. Further, THC appears to exhibit dose-dependent effects on glycine receptor activation [166]. The activation of peroxisome proliferator-activated receptors (PPAR), in particular the α and γ subtypes, is responsible for many of the metabolic, analgesic, neuroprotective, and other health-related benefits of cannabinoids [167]. Cannabinoids have also been shown to interact with serotonergic sites, particularly with the 5-HT1A [168] and 5-HT2A [169,170] receptors, and these interactions are strongly associated with disorders such as anxiety and post-traumatic stress [171,172]. Consequently, the spectrum of potential therapeutic applications is very broad for cannabinoids and would require a specifically dedicated and lengthy review in its own right. Currently lacking are robust, double-blind in vivo and clinical studies of the constituents of the broader cannabinoid profile that target specific diseases, and/or can be used to treat the symptoms of these diseases, possibly via targeting the interactions between cannabinoids and these other putative or lesser-known receptors.
2.3. Examples of the Potential Medicinal Use of Cannabinoids
While research into the cannabinoids and their role in human disease is still in its infancy, the field abounds in promising preliminary studies. Cannabinoids, both of the endo- and phytocannabinoid categories, have been demonstrated to provide protection against further neurodegeneration in lesioned neurons post-treatment with toxic doses of 6-hydroxydopamine, as well as the neuron degeneration linked to Parkinson’s disease [189,206]. Moreover, symptoms of dyskinesia associated with Parkinson’s disease and other movement disorders, originating from deficiencies in the cannabinoid receptor-rich basal ganglia in marmosets, and reserpine-treated rats, have been reduced by CB1 receptor stimulation-mediated suppression of involuntary motor behaviour [189,207,208,209,210]. Central nervous system activation of the CB2 receptor has exhibited promising results in combating the inflammation and oxidative stress of Parkinson’s disease which is associated with dopaminergic neuron loss in the substantia nigra pars compacta in nonhuman models [195,196].
Studies into the treatment of a variety of cancers through cannabinoid use have also proved valuable. For example, CB1 and CB2 activation by either endogenous or synthetic receptor ligands has inhibited prostate [190] and pancreatic [211] adenocarcinoma growth, as well as breast [191] and thyroid [175] tumour growth. Modulation of non-CB receptors by the minor cannabinoids is also under investigation for their role in the initiation of oncogenic signalling cascades that may induce the arrest of the cell cycle, or inhibit the growth of tumours [212]. Endocannabinoid-mediated breast cancer cell proliferation has been inhibited by a reduction in prolactin action at the receptor level [213], and CB1 and CB2 receptor activation has induced apoptosis of cancerous cells in the breast [191] and colon [214]. In non-small-cell lung cancer cell lines, treatment with agonists targeting CB1 and CB2, or specifically CB2, were demonstrated to induce apoptosis, and to attenuate chemotaxis, metastatic growth and development, metastatic proliferation, and angiogenesis [192]. Similarly, cannabinoid activity against vascularization was also observed in human grade glioma cells in mice, with CB2 activation reducing tumour angiogenesis by inhibiting vascular endothelial cell migration and the suppression of pro-angiogenic factors in tumour cells [215].
First alluded to over 40 years ago, the use of Cannabis as a treatment for epilepsy has garnered traction in recent years and several comprehensive reviews have recently described the efficacy of cannabinoids in the treatment and/or management of epilepsy [216,217,218]. Further evidence of the involvement of the endocannabinoid systems in seizure mitigation is suggested with inactivation of the endocannabinoid degrading, FAAH, with FAAH shown to reduce both kainic acid associated seizure activity, and synaptic decline and damage to cytoskeletal elements in the hippocampus of rat models [219,220]. A double-blind, placebo-controlled study of 218 patients in which CBD was administered at a dose of 10 and 20 mg per kg reduced the frequency of drop seizures in both children and adults with Lennox-Gastaut syndrome, when compared to conventional epilepsy treatment [221]. A similar double-blind, placebo-controlled study of 120 children with the epilepsy disorder, Dravet syndrome, saw a significant reduction in the frequency of convulsive seizures when treated with CBD, as compared with those administered the placebo [222]. In a retrospective, open-labelled study, Press et al. [223] reported improvements in seizure control and frequency reduction in paediatric patients using oral Cannabis extracts, as well as additional improvements in some off-target metrics, including alertness and motor skill usage also observed. Use of a THC extract has attenuated seizure duration and termination via the activation of CB1. However, inhibition of CB1 receptor activity has also been demonstrated to increase the frequency and duration of seizures in non-human models, findings which firmly identify a role for CB1 in seizure responses [177]. Indeed, transgenic CB1 overexpressing mice were reported to have reduced kainic acid-induced seizure severity and mortality with reduced hippocampal neuron damage [224]. While these examples suggest promise in the efficacy of cannabinoids, or the modulation of cannabinoid receptor activity against epilepsy, there currently remains deficiencies in access to data emerging from large, controlled clinical studies.
The treatment of Parkinson’s disease, cancer and epilepsy are persistently pursued and remain ‘high-value’ targets for researchers. However, the importance of treating other less deleterious ailments, or the treatment of the negative side effects that originate from the aggressive treatment strategies of major diseases such as cancer, chemotherapy for example, is not without utility. A suite of clinical trials have supported the ability of Cannabis-derived metabolite constituents to (1) act as effective antiemetics [184,185,186,187,188], (2) ease the spasticity symptoms associated with Motor Neuron Disease and Multiple Sclerosis [225], (3) stimulate appetite [173,174,226,227,228,229], (4) help regulate sleep patterns [178,230,231,232], (5) initiate analgesia [233,234,235,236], (6) act as an anxiolytic to alleviate the psychotic symptoms of schizophrenia [237,238,239,240,241], (7) treat anxiety and post-traumatic stress disorders [31,171,242], (8) be utilised as palliative care agents [243,244], (9) aid in the acute inflammatory response and its protracted recovery [245], and (10) mitigate the effects of opioid addiction [246,247].
A full review of the current understanding of cannabis in the medical sphere is beyond the scope of this review and has been published elsewhere [90,248]. Despite much of the current research remaining in the preliminary stages, requiring a greater amount of more stringent, double-blind studies, the medicinal promise of Cannabis is readily evident. Meta-analyses relating to the legitimacy of medical Cannabis, specifically the use of CBD and THC in control randomised trials, have been conducted. Studies surrounding the use of CBD indicate that the drug is well tolerated with minimal serious adverse side effects and drug–drug interactions [249]. CBD is described as effective in the treatment of refractory seizures, but scientifically stringent data are lacking to claim effectiveness for other indications, with concerns remaining about the quality control in drug preparation and long-term safety [250]. It has been noted that inconsistencies across current studies relating to dosage and administration methods limit the conclusions that can be drawn to direct medical intervention using CBD [251]. Currently, cannabinoid therapies for sleep quality and mental health-related disorders also suggest that while preliminary evidence may indicate positive outcomes, the collation of eligible studies provides insufficient evidence to suggest efficacy or promote usage until additional, and more stringent studies have been conducted [252,253]. Although more stringent studies on the effectiveness of cannabinoids to control pain and spasticity exist, additional comprehensive studies demonstrating improvements in the treatment of chemotherapy associated nausea, sleep disorders, weight gain, and Tourette’s syndrome, and which also note the risk of short-term adverse events of cannabinoid treatment, are still required [32].
3. The Cannabinoid and Terpene Pathways of Cannabis
It is clear that modulation of the endocannabinoid system can be achieved outside of THC, CBD, and their CB receptors. Despite this, the majority of research conducted to date has sought to understand how these two cannabinoids interact with the various constituents of the expanded endocannabinoid system. However, significant knowledge exists concerning what further compounds can be extracted from Cannabis as well as an emerging understanding of how such compounds can be efficiently extracted from the Cannabis plant. To date, the most studied phytochemicals in Cannabis are the cannabinoids and terpenes. Together, these two classes of phytochemical comprise approximately 41% of the total number of known secondary metabolites identified in Cannabis [21,22]. Cannabinoid and terpenoid biosynthesis occurs in hair-like capitate stalked glandular trichomes [254,255], which cover the female floral organs, and exhibit a particularly high density on the bracts (a specialised leaf of the floral organs; Figure 1).
Figure 1.
A close up of the female floral architecture of mature Cannabis sativa plants. The cannabinoid-containing glandular trichomes are visible in the magnified image, and are characterised by a globular head which is connected to the plant via a stalk. Colouration of the heads ranges from translucent, to a creamy white, to brown.
In trichome development, a protodermal cell is enlarged vertically out from the epidermis and subsequently undergoes anticlinal division, prior to a series of periclinal division events to create a secretory and auxiliary tier of cells atop the epidermal basal cells [256,257,258,259]. Additional division events develop the secretory tier of disc cells that form a cavity on the external surface of the trichome from a portion of the outer wall. This cavity then enlarges as the secretory vesicles that harbour a diverse payload of secondary metabolites are extruded into the expanding waxy cavity. Post their cellular release, the secreted vesicles disintegrate upon contact with the thickened outer cuticle wall to release their contents [256,257,258,259].
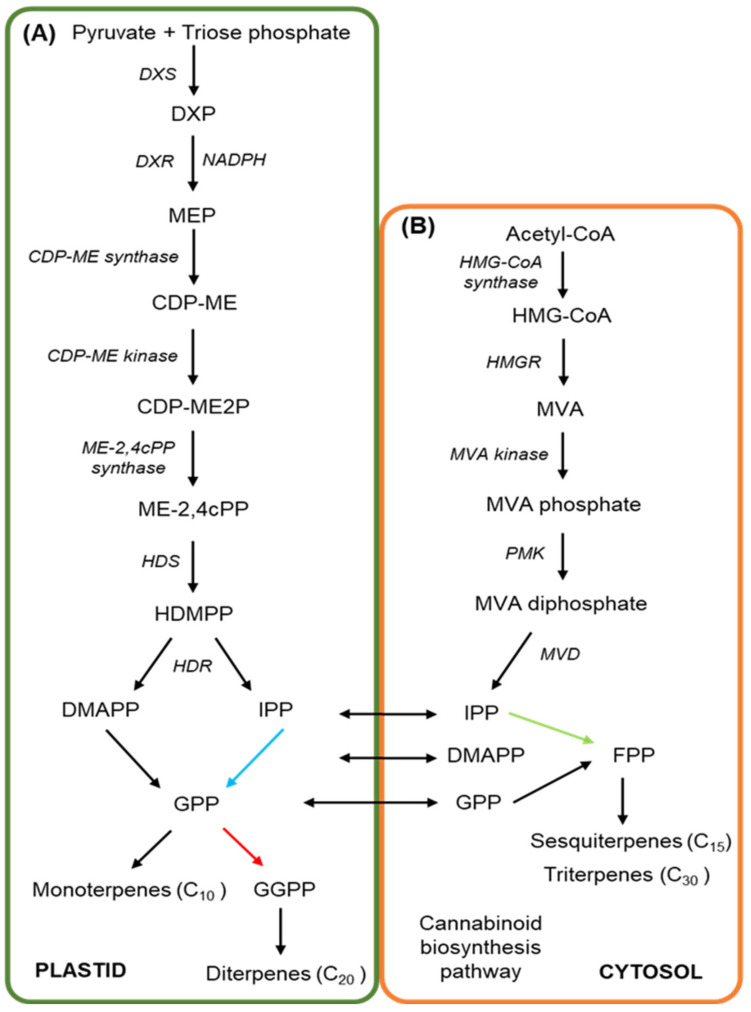
The complete biosynthetic pathway of how the prenylated polyketides, particularly minor cannabinoids, are derived from precursor molecules still requires further elucidation, particularly in view of the recent discovery of the two novel cannabinoids, THCP and CBDP [22]. Cannabigerolic acid (CBGA), the key intermediate substrate required for the synthesis of the three primary cannabinoids—cannabichromenic acid (CBCA), THCA and CBDA—arises from molecular products of the polyketide and methylerythritol 4-phosphate (MEP) pathways. A schematic representation of the MEP pathway is provided in Figure 2A. More specifically, the MEP pathway begins in the plastid via the condensation of the substrates, pyruvate and triose phosphate, a reaction that is catalysed by 1-deoxy-D-xylulose-5-synthase (DXS), and which produces 1-deoxy-D-xylulose-5-phosphate (DXP) [260,261,262]. Via the action of 1-deoxy-D-xylulose-5-reductase (DXR) in the presence of the co-factor NADPH, DXP is next reduced to MEP [263] and subsequently, MEP is converted to CDP-ME by the action of the enzyme, 4-diphosphocytidyl-2-C-methyl-D-erythritol (CDP-ME) synthase. The kinase, DCP-ME kinase then phosphorylates CDP-ME to produce 4-diphospho-cytidyl-2-C-methyl-D-erythritol-2-phosphate (CDP-ME2P) [264,265]. CDP-ME2P is subsequently converted to 2-C-methyl-D-erythritol 2,4-cyclodiphosphate (ME-2,4cPP) via the activity of the enzyme, ME-2,4cPP synthase, prior to another synthase, 4-hydroxy-3-methylbut-2-enyl diphosphate synthase (HDS), converting ME-2,4cPP to 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate (HDMPP). In the final step of the MEP pathway, HDMPP is used as a substrate by 4-hydroxy-3-methylbut-2-enyl diphosphate reductase (HDR) to produce IPP and dimethylallyl diphosphate (DMAPP) [264,265,266].
Figure 2.
An overview of the mevalonate and methylerythritol 4-phosphate pathways in Cannabis sativa. The MEP (A) and MVA (B) pathways both produce terpenoid precursors, as well as the substrate for cannabinoid production, GPP. (A) The MEP pathway begins in the plastid with the condensation of pyruvate and glyceraldehyde 3-phosphate by DXS to produce DXP, prior to a series of enzymatic reactions to produce HDMPP. HDR then converts HDMPP to IPP and DMAPP, serving as the precursor to GPP, GGPP, and subsequently monoterpene and diterpene production. (B) The cytosolic MVA pathway is initiated by the conversion of acetyl-CoA to HMG-CoA and then to MVA, catalysed by the regulated, and rate-limiting enzyme, HMGR. MVA undergoes phosphorylation and then is decarboxylated to produce IPP, which is then converted to FPP as the basis for sesquiterpene and triterpene synthesis, or for GPP production for use in the cannabinoid biosynthesis pathway.
The HDR enzyme is essential for the in planta production of IPP and DMAPP, with over 98% of these two molecules produced by the MEP pathway. IPP and DMAPP both form essential precursor substrates for the biosynthesis of cannabinoids and terpenoids [261]. In the cytosol, IPP is also produced by the MVA pathway (Figure 2B). At the start of the MVA pathway, acetyl-CoA is converted to 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) by the enzyme, HMG-CoA synthase. Next, HMG-CoA is converted to MVA in the highly rate-limiting step of the MVA pathway, a step that is regulated via the activity of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) [267,268,269]. MVA is then converted to MVA phosphate by MVA kinase (MVK), and subsequently, MVA phosphate is converted to its diphosphate form via the activity of phospho-MVA kinase (PMK). MVA diphosphate is subsequently converted to IPP via its decarboxylation by mevalonate 5-diphosphate decarboxylase (MVD) [270,271,272]. Via the use of yellow fluorescent protein (YFP) fusion constructs, the activity of PMK and MVD has been observed in the peroxisome in Catharanthus roseus (Madagascar periwinkle) and Arabidopsis thaliana (Arabidopsis) to strongly indicate peroxisomal localisation of these two enzymes in planta, and not in the cytosol [270,271,273]. IPP isomerase catalyses the conversion between IPP and DMAPP, a conversion reaction that provides the building blocks for terpene biosynthesis [274,275,276]. Geranyl diphosphate synthase (GPPS) catalyses the production of the ten-carbon (C10) molecule, geranyl diphosphate (GPP), via the condensation of one molecule each of DMAPP and IPP [277,278]. Similarly, formation of the C15 molecule, farnesyl diphosphate (FPP), and the C20 molecule, geranylgeranyl-diphosphate (GGPP), is catalysed by their specific synthases, farnesyl diphosphate synthase (FPPS) and geranylgeranyl diphosphate synthase (GGPPS), respectively, which condense either 2 or 3 molecules of IPP together with a single molecule of DMAPP [279,280,281]. Together, GPP, FPP and GGPP form the precursors necessary for monoterpene or CBGA biosynthesis (GPP precursor), or the numerous sesqui-, di-, tri-, or tetra-terpene products (FPP or GGPP precursors) found in Cannabis [282,283].
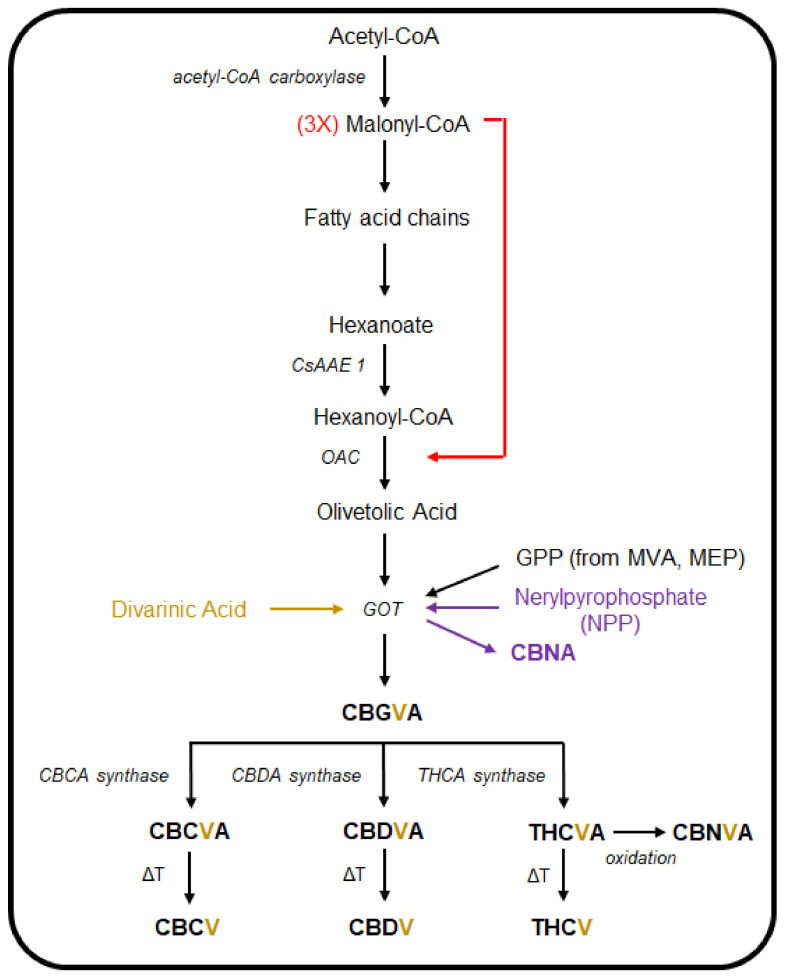
The polyketide pathway is initiated when acetyl-CoA is carboxylated to malonyl-CoA, which in turn serves as the precursor for the fatty acid chains used to produce hexanoate (Figure 3) [254,255,261]. The acyl-activating enzyme (AAE), which in Cannabis is encoded by two putative genes, termed CsAAE1 and CsAAE3, with the encoded proteins localised to the cytoplasm and peroxisome, respectively, where they function to catalyse the synthesis of hexanoyl-CoA from hexanoate [255]. Condensation of hexanoyl-CoA, together with three malonyl-CoA molecules, is subsequently catalysed by the polyketide synthases, tetraketide synthase (TKS), or olivetol synthase [284,285]. The product of these two synthases, and post a final round of aldol cyclisation by the olivetolic acid cyclase (OAC) enzyme, is olivetolic acid (OA) [284]. Via the utilisation of GPP from the MVA pathway, OA is then prenylated by geranylpyrophosphate:olivetolate geranyltransferase (GOT), to produce CBGA [286,287,288]. The cis isomer of GPP, neryl diphosphate (NPP), can be used as a substrate by GOT in place of GPP, to produce cannabinolic acid (CBNA) [289]. CBGA then serves as the primary cannabinoid precursor for the synthesis of cannabichromenic acid (CBCA), THCA and CBDA, with the production of each of these three acids catalysed by a specific oxidocyclisation enzyme, namely the CBCA, THCA and CBDA synthases [289,290,291,292,293]. The use of divarinic acid as a substitute for OA by GOT, putatively produces the propyl cannabinoid homolog, cannabigerovarinic acid (CBGVA) [286,294]. The aforementioned cannabinoid-specific synthases that yield CBCA, CBDA, and THCA can all recruit CBGVA to produce cannabidivarinic acid (CBDVA), cannabichromevarinic acid (CBCVA) and Δ9-tetrahydrocannabivarinic acid (THCVA), respectively [294,295,296]. The resulting cannabinoids are maintained in their acidic forms until they are thermally decarboxylated to convert them into their neutral forms [297,298,299,300].
Figure 3.
An overview of the cannabinoid biosynthesis pathway in Cannabis sativa. Malonyl-CoA, formed from acetyl-CoA, is used downstream with hexanoyl-CoA to produce olivetolic acid (OA). Next, OA is used as substrate along with other biomolecules by the GOT enzyme to produce the major cannabinoid precursor, CBGA. When GOT uses substrates additional to OA, such as divarinic acid or nerylpyrophosphate, a range of other minor cannabinoids are produced.
Research to date has primarily focused on the biosynthetic pathways and putative medical benefits of the two major cannabinoids, THC and CBD. Therefore, the medical and biological potential of the minor cannabinoids that also contribute to the total cannabinoid profile of the Cannabis plant have been largely overlooked. The small proportion that these minor cannabinoids contribute to the total cannabinoid profile of the Cannabis plant presents a significant obstacle for in-depth analysis of their effects when consumed. A comprehensive, and ever-increasing list of naturally occurring minor phytocannabinoids has been compiled based upon their derivation from THC, CBD, CBG (cannabigerol) and CBC, which represent the diversity that stems from variations to the three fundamental components of cannabinoids, including the (1) resorcinyl core; (2) isoprenyl residue, and; (3) resorcinyl side chain [20,301]. Eighty-two individual cannabinoids from 10 cannabinoid types, specifically the (1) CBG; (2) CBC; (3) CBD; (4) Δ9-THC; (5) Δ8-THC; (6) cannabicyclol (CBL); (7) cannabielsoin (CBE); (8) cannabinol (CBN); (9) cannabinodol (CBND), and; (10) cannabitriol (CBT) types, in addition to the miscellaneous types, and their transformation products, as well as terpenoids, hydrocarbons, sugars and fatty acids are among the constituents that comprise the chemical cornucopia of glandular trichomes. Further, several minor oxygenated cannabinoids, cannabinoid metabolites, and cannabinoid esters present in Cannabis have yet to be isolated and/or experimentally validated but have been identified using a variety of spectroscopic techniques [302,303,304]. In addition, a number of interesting structural formations have been observed in some of the minor cannabinoids. For example, cannabioxepane (CBX) has a tetracyclic skeleton with a seven-membered ring, a structure not previously reported for a characterised cannabinoid, while cannabisol is a Δ9-THC dimer with a methylene bridge. However, it must be noted that the binding affinity for specific CB receptors for these minor cannabinoids remains unknown, with some potentially not recognised, and therefore not bound by any known CB receptor [305,306]. The CBD derivative, cannabimovone, and the farnesyl prenylogue of CBG, sesquicannabigerol, were also spectroscopically characterised, with CB receptor binding assays predicting receptor–cannabinoid affinity, highlighting the structural and potential psychoactive diversity among the minor phytocannabinoids [307,308]. In addition to the identification of their parent cannabinoid precursors, plausible biochemistry behind the synthesis of these compounds is offered. However, the actual enzymatic production of many of these minor cannabinoids remains to be determined. Furthermore, the non-enzymatic formation of some of the minor cannabinoids is certainly likely, but it remains of interest to understand whether there is a greater portion of enzyme-catalysed reactions in the production of the minor cannabinoids, or indeed whether there are alternative pathways, or even additional pathway entry points in the biosynthesis of cannabinoids, both minor and primary.
4. Minor Cannabinoids and Their Biological Interactions
There is mounting evidence that the minor cannabinoids described above share combinations of many of the same molecular targets as THC and CBD, and therefore may potentially have unique medical applications that cannot be achieved by THC or CBD alone. The THC propyl homologue, THCV, is a CB1 and CB2 competitive antagonist against CP55,940 and WIN55,21–2, acting with similar potency to that of THC [309,310]. THCV also antagonised anandamide and methanandamide in mice vas deferens, attenuating stimulated contractile responses [309]. More recently, THCV was shown to similarly displace CP55,940 from CB1 and CB2 in CHO cells, and contrary to previous assumptions, was shown to be a weak partial CB1 agonist at high doses [179]. Moreover, Zagzoog et al. [310] showed THCV to produce anxiolytic, hypothermic, anti-nociceptive, hypolocomotive, and cataleptic effects in vivo in mice. CB2 agonism by THCV was demonstrated to reduce inflammation and attenuate hyperalgesia in mice following injection of carrageenan and formalin, respectively [180]. Neuroprotective properties were observed in 6-hydroxydopamine lesioned rats, where THCV administration preceded maintenance of tyrosine hydroxylase-positive neurons in this Parkinsonian model [311]. Similarly, THCV delayed onset of abnormal involuntary movements associated with Parkinson’s disease in mice, and reduced their severity after administration following symptom onset [181]. The in vitro demonstrated inhibition of GABA release by WIN55,21-2 at Purkinje cell synapses was reversed by THCV, which also prevented the action of WIN55,212–2 when used in pre-incubation [312,313]. In vitro studies of insulin-resistant human hepatocytes showed THCV restoration of insulin signaling mediated by CB1, while also improving glucose tolerance and increased sensitivity to insulin in mice obesity models [182]. Antiepileptic properties were also established in vitro, specifically when THCV reduced both the frequency and amplitude of epileptiform activity in rat piriform cortex slices [183]. The majority of published studies have focused on the CB1 and CB2 receptors, but the in vitro activity of THCV has been observed for the TRPV1 to TRPV4 group of receptors, as well as for the TRPA1 receptor [200,201,202]. THCV can enhance 5-HT1A receptor activation to produce antipsychotic-like effects in rats [314], but does not affect other endocannabinoid system constituents such as PPARγ [315], FAAH [200], or MAGL [200]. One clinical trial in humans where THCV was administered once daily for five days followed by intravenous administration of THC suggested that THCV inhibited an increase in heart rate, protected against verbal recall impairment, and reduced the subjective psychoactive intensity induced by THC [316]. Further, THCV affects brain regions associated with reward and aversive stimuli, as well as areas associated with cognitive control [317,318]
Recently, a four-carbon side chain variant, Δ9-tetrahydrocannabutol (THCB), was isolated and which showed CB1 and CB2 binding affinities similar to those of THC, with in vivo mice studies suggesting potential analgesic and anti-inflammatory properties [176]. Similarly, the recently identified seven-carbon side chain variant, THCP, was shown to be able to bind to both CB1 and CB2 with 33 and 5 times greater affinity than THC, respectively, as well as to initiate catalepsy, hypothermia, analgesia, and reduce locomotion; all indications of potent full CB1 agonism [22]. THCA has been shown in rodent culture supernatants to reduce the abundance of inflammatory and oxidant markers [319,320], though no other research to our knowledge of this nature has been published. In addition, Δ8-THC has been shown to possess higher antiemetic effects than THC [188], and has been successfully trialed for repressing emesis in children [184]. Furthermore, in humans, Δ8-THC appears required to be administered at higher doses than THC to display a similar degree of psychoactive properties [321]. THCA is a 5-HT1A agonist [322], a PPARγ agonist [323], and displays the same properties against TRP channels as does THC [200]. However, little pharmacological, pharmacokinetic, or recent safety data are available for any of these compounds.
Improvements in seizure frequency has been reported in an epileptic patient coinciding with increased CBDV serum levels, after which in vitro studies confirmed that CBDV, at least, possesses the ability to influence GABA receptors; a finding that indicates a potential avenue for anticonvulsant properties [324]. Further, in vitro analyses revealed CBDV to have anticonvulsant effects in four seizure models, namely the (1) maximal electroshock-, (2) audiogenic-, (3) penytylenetetrazole-(PTZ), and (4) pilocarpine-induced seizure models [325,326]. Using rat brain tissue samples, PTZ-induced seizures coincided with an increase in Early growth response 1 (Egr1), Activity-regulated cytoskeleton-associated protein (Arc), Chemokine (C-C motif) ligand 4 (Ccl4), Brain-derived neurotrophic factor (Bdnf), and FBJ osteosarcoma oncogene (Fos) gene expression [327]. Interestingly, the administration of CBDV was shown to reduce the expression of all of these genes [327]. Additional seizure studies identified TRPV1 as the potential receptor modulating anti-seizure effects via the use of trpv1 knockout mice which showed a reduced response to CBDV [328]. Desensitisation of TRPV1, in addition to TRPV2, by both CBDV and CBD has been observed [199], while Ca2+ transients were induced in TRVP2-expressing HEK293 cells more potently by CBD than by CBDV. However, THC was a more potent inducer of Ca2+ transients than either CBDV or CBD [200]. Another study did alternately suggest that CBD was the more potent agonist of TRPV2 than THC, but this study did not include the assessment of CBDV [329]. Cannabinoid administration improved symptoms in mice models of Rett syndrome, including motor control and sociability [197], and through TRPA1, CBDV mediates anti-inflammatory effects in intestinal tissue of humans with ulcerative colitis [204]. Similar to CBD, CBDV inhibits FAAH and anandamide reuptake [200]. However, unlike CBD, CBDV does not show affinity for the CB1 or CB2 receptors [180]. CBDV may confer some benefit in patients with Autism Spectrum Disorder [330] and Duchenne muscular dystrophy [205]. CBDV did, however, fail to alleviate the neuropathic pain associated with human immunodeficiency virus (HIV) [331], and in another study, the administration of CBDV induced DNA damage in human cell lines at concentrations similar to those observed in Cannabis consumers [332], indicating carcinogenicity potential for CBDV. However, CBDV has been safely trialed in humans at a single 600 mg oral dose [330], and it remains to be determined whether CBDV will be efficacious for other illnesses in clinical trial.
CBG has shown partial agonism of CB1 and CB2, α2-adrenceptor agonism and 5-HT1A antagonism, while exerting some minor anti-nociceptive and anxiolytic properties in vivo [179,333]. Mice models of inflammatory bowel disease (IBD) showed positive outcomes with CBG treatment including reductions in the level of reactive oxygen species in intestinal cells, as well as reduced nitric oxide concentration in macrophages through CB2 modulation [194]. Further in vivo animal studies provided evidence for neuroprotectivity against symptoms of Huntington’s disease in 3-nitropropionate treated mice, with improvement in motor function, reduction in proinflammatory marker upregulation and increased antioxidant defenses, with R6/2 mice showing a reduction in the expression profiles of several genes linked to the disease following CBG treatment [334]. Similarly, in vitro analysis of NSC-34 neuronal cells showed that CBG pre-treatment reduced both inflammation and the expression of pro-inflammatory cytokines, and inhibited cell death resulting from the cell culture medium of lipopolysaccharide (LPS) stimulated RAW 264.7 macrophages [335]. CBG shows a similar profile at TRP channels compared to CBD, with agonist properties at TRPV1 through to TRPV4, and at TRPA1, but antagonism at TRPM8 [200]. It is also an anandamide reuptake inhibitor [336], and an LPI inhibitor at GRP55 [97]. As for the propyl analogue of CBG, CBGV, very little information surrounding its clinical application exists, except to show that CBGV has activity at GPR55, TRPV3 and TRPV4 [198,201].
CBC use in a clinical setting, or in human trials, appears to be untested currently, and additionally, cannabichromevarin (CBCV) currently has even fewer studies dedicated to it. However, CBC has seen some use in animal models and in vitro studies. CBC has been shown to inhibit FAAH, MAGL, and anandamide reuptake [200,337], but has been demonstrated to have no effect at TRPV1 or TRPV2. Further, CBC is a very weak CB1 agonist [338,339,340], and only exhibits modest agonist properties at CB2. An early study suggested that CBC, CBCV, and a CBC variant which lacks a carbon side chain, possessed anti-inflammatory properties in rat edema models and varying anti-bacterial and anti-fungal properties [341]. More recently, CBC was seen to produce anti-inflammatory effects in LPS paw edema models in mice in CB1- and CB2-independent pathways and also produce hypothermia, catalepsy, and locomotor suppression [342]. The authors went on to suggest that the effects of CBC were altered in the presence of THC, with an additive effect against inflammation [342] and similarly, tail-flick tests revealed that subtle analgesic properties of CBC were potentiated by its combination with THC [343]. Selective CB2, but not CB1 agonism, was exhibited by CBC on mouse pituitary tumour cells, and the persistent administration of CBC caused desensitisation of CB2 receptors [344]. Intestinal studies suggest that CBC confers some benefit against inflammation. However, this was potentially independent of CB1, CB2, or TRPA1, the expression of which were all downregulated in the presence of CBC in one study, but shown to be unchanged in another study [193,345]. Colorectal cancer cell viability was attenuated through TRPM8 antagonism by CBG, as well as by the administration of CBD, CBDV, and CBC, albeit to lesser degrees [203]. Other studies have indicated that CBC is not a potent antagonist of TRPM8, and instead suggest that CBD, CBG, THC, and THCA are more effective antagonists of TRPM8 [200,202]. Additionally, CBC, CBN, THC, THCV, THCA, CBDA, and CBG all induced intracellular Ca2+ increases in HEK293 and rat DRG neurons through TRPA1 [200,202]. CBC has also shown promise in increasing neural stem cell viability in animal models (in vitro), mediated through ERK phosphorylation [346]. However, it is concerning that large amounts of CBC are required to produce pharmacological effects [90], which implies that CBC may be difficult to implement in a human health context.
The binding affinity of CBN, and of its primary derivatives, to the two main cannabinoid receptors was established in 2000, and showed rather unsurprisingly that alterations at carbon atom positions 1, 3, and 9, resulted in significantly different affinities at both receptors [347]. An earlier study indicated CBN to have cataleptic, hypothermic, and locomotive effects, as did 11-hydroxy-CBN; a hepatic microsome CYP2C- and CYP3A4-catalysed metabolite [348,349]. Additionally, CBN directly inhibited the activity of the human cytochrome P450 family 1 (CYP1) enzymes, CYP1A2 and CYP1B1 [350]. Assays of cultured neuronal cells expressing an inducible disease conferring huntingtin (Htt) protein, suggest that CBN has protective effects against cell death in vivo, with low toxicity even at the high concentrations required for protectivity [351]. Interestingly, cannabinoid receptor loss has been indicated as a pathophysiology of Huntington’s disease [352,353], which may suggest that the purported protective action of cannabinoids is independent of cannabinoid receptor binding. Subcutaneously delivered CBN delayed the onset of amyotrophic lateral sclerosis (ALS) symptoms in murine models but failed to affect survival, so was postulated to mask the early spasticity associations without affecting disease progression [354]. A synergistic effect of CBN with CBD at reducing mechanical sensitisation in rat masseter muscles was observed in one study, however high concentrations of CBD ameliorated the efficacy of CBN [355]. CBN has been reported to have no effect at FAAH, MAGL, or TRPV1, but acts as an agonist at TRPA1 and TRPV2 [200].
Via the use of in silico analyses, the even lesser-known cannabinoids, cannabiripsol (CBR) and CBT, are predicted to have cytochrome P450 inhibitor activity [356]. In another in silico study, CBL, CBT, and CBE were assessed, and ranked in this order, to have acetylcholinesterase-inhibiting function. However, their inhibitory effects were less than those of THC, CBN, and CBDV [357]. Exactly how well in silico studies translate to clinical relevance, or even to in vitro and/or in vivo studies, restricts what conclusions can be accurately drawn. Minor phytocannabinoids do represent an understudied portion of the Cannabis plant. Very few studies exist that have utilised an in vivo approach to ascertain the viability of minor cannabinoids to potentially produce any significant medical benefits, and fewer still cover any human clinical trials. There has been indication that some cannabinoids exhibit synergistic action, and as a result there may be value in investigating the interactions among cannabinoids or constituents of the Cannabis plant.
5. Directions in Cannabis Development for Secondary Metabolite Production
The establishment of superior varieties of Cannabis has been the target for plant breeders since the domestication of this species. To produce new medically relevant Cannabis varieties with elevated concentrations of specific minor cannabinoids, or to develop techniques to manipulate the cannabinoid biosynthetic pathway in other organisms, a deeper understanding of the genetics of the Cannabis plant is first required. Here we outline the progress in relation to (1) the sequencing of the Cannabis genome, and (2) the potential to molecularly manipulate the Cannabis plant itself for the altered production of specific cannabinoids. In this regard, we highlight the established success in Papaver somniferum (opium poppy), as a parallel example for maximising yield and the concentration of key secondary metabolites of medical and commercial relevance.
5.1. Next-Generation Sequencing of the Cannabis Plant and Its Potential for Genetic Manipulation
Over the last 25 years, various experimental approaches have been employed to unveil the wealth of information contained in the Cannabis genome. Using early DNA sequencing and karyotyping techniques, the X and Y sex chromosome characteristics of Cannabis were uncovered, as were the diploid (2n = 20) genome sizes for male and female plants [358,359]. The female Cannabis plant was revealed to have a genome size of 818 megabase (Mb), while the male Cannabis plant was determined to have a larger genome size of 843 Mb; specifically due to the larger size of the Y chromosome, compared to the X chromosome of female plants [358]. Microsatellite markers have been employed as a tool for DNA typing Cannabis, and these polymorphic short tandem repeat (STR) markers have been utilised as a measurement of genetic relationships among cultivars [360,361,362]. More recently, the rapid change in technologies surrounding Next-Generation Sequencing (NGS) platforms has meant that studies can unravel whole genomes in a fraction of the time required via the use of older methods. As a result, the first draft Cannabis reference genome, and transcriptome, were constructed in 2011 using the high THCA, low CBDA cultivar, ‘Purple Kush’, and the high CBDA, low THCA hemp strains, ‘Finola’ and ‘USO-31’ [363]. Using a PacBio long-read sequencing platform, the Purple Kush and Finola genomes were again sequenced in 2019 to generate a physical and genetic map for Cannabis, and further distinguish the genes, and importantly the gene products (specifically, the encoded enzymes), underpinning the secondary metabolite profiles responsible for the divergent chemotype between hemp and marijuana cultivars [364,365].
Earlier work surrounding the chemotypic variance of cannabinoids observed in Cannabis unveiled the relationship between THCA and CBDA synthase expression, describing a single locus (B), with two codominant alleles, BD and BT [295]. A 1:1:2 segregation ratio results in the production of three chemotypes of the B locus, including the (1) pure CBD (BD/BD homozygote), (2) pure THC (BT/BT homozygote), and (3) mixed CBD/THC (BD/BT heterozygote) chemotypes [295]. However, later studies based around NGS platforms indicated an alternate genetic model of synthase gene duplication and rearrangement at multiple linked loci, and that CBDA synthase is more ancient, has a greater affinity for the CBGA substrate, and that the CBDA synthase locus is solely responsible for the cannabinoid chemotypes observed in Cannabis [363,365,366,367,368,369]. In an attempt to classify variability in chemotypes, and to associate genotype to chemotype in a diverse germplasm collection, DNA sequence characterised amplified region (SCAR) markers associated with THCA/CBDA synthases were assessed in 22 Cannabis varieties representing 2 fibre and 1 drug type plants from East (n = 8), Central (n = 1), and South (n = 2) Asia, as well as from Europe (n = 7) and of mixed (n = 4) domestication status [370]. This approach revealed a variability in cannabinoid profiles (CBD:THC) across ‘chemotype II’, or BD/BT equivalent plants, more than three-fold greater than previously observed, supporting the allelic variant and multiple loci prediction, when assuming that a heterozygote plant in a single locus model would have a 1:1 CBD:THC ratio [370].
Other large-scale genetic diversity studies using NGS, and which compared the evolutionary relationships between 340 Cannabis varieties from existing datasets, and from other novel multiplexed libraries, highlighted the murky ancestry of the Cannabis plant resulting from generations of repeated rounds of selective breeding, and also provides an extensive data platform for future genotyping efforts [371]. Moreover, Lynch et al. [371] classed their assessed Cannabis varieties into three genetic groups, including (1) hemp, (2) narrow leaflet, and (3) broad leaflet drug types, in order to determine the genomic and genetic variation of their population for the potential use of varieties from each group in either agricultural or medicinal applications. The authors indicated unique cannabinoid and terpenoid profiles for each group, structured loosely around geographic origin of each species, and noted the requirement for the inclusion of the putative Cannabis species, C. ruderalis, in future studies to fully elucidate their genetic distinction and ancestral lineage [371]. The development of expressed sequence tag simple sequence repeat (EST-SSR) markers to assess genetic diversity of 115 Cannabis genotypes also revealed geographical-based clustering into 4 groupings, including the Northern China, Southern China, Central China and Europe groupings [372]. Interestingly, a genetic similarity coefficient derived from 45 of 117 randomly selected EST-SSRs markers revealed that despite physical proximity to the other Chinese varieties, Northern Chinese varieties had a greater similarity coefficient to the European grouping, predicted to be related to latitude and day length [372]. The analysis of inter simple sequence repeats (ISSR) of 27 native Chinese hemp varieties identified a similar geographic distribution to genetic distance relationship, while also revealing the hemp varieties were genetically diverse, yet primitive, a finding which adds further weight to the suggestion that the Cannabis plant originated in southern China and then spread north [373].
The recent assembly and annotation of the mitochondrial genome of Cannabis using NGS methods will also allow for similar studies to be performed to determine the extent of the genetic diversity among Cannabis varieties [374]. In addition, the assembly of two chloroplast genomes from different Cannabis varieties will aid in validating the phylogenetic relationship of Cannabis among the Rosales order of the Plantae kingdom [375]. However, as with all sequencing, repeated efforts across diverse genotypic populations compared against reference genomes will increase the accuracy and reliability of publicly available repositories. RNA sequencing as a tool for differentiating strains has been used with some success, where the transcriptome isolated from cannabinoid-containing glandular trichomes from different varieties allows for comparative analysis based on the cannabinoid and terpenoid chemical profiles [376,377]. As the regulatory landscape surrounding the use of Cannabis evolves, and the value of the unique chemical profile of specific Cannabis varieties is realised, breeders are likely to use these sequencing techniques to rapidly characterise and protect their ‘strains’. The development of such highly targeted databases provides the platform for precise manipulation of phenotypic or chemotypic traits in Cannabis to deliver improved medical efficacy or novel therapeutics.
A forward and/or reverse genetics approach with the application of chemical mutagenesis agents, such as ethyl methanesulfonate (EMS), a mutagen that introduces point mutations into the plant genome, is an effective approach for functional genomic assessments and effective plant breeding regimes, and has been successfully demonstrated in a variety of plant species, including hemp [9,378,379,380,381,382,383]. The application of alkylating agents such as EMS in a time-dependent manner causes a larger number of point mutations across the genome, compared to an irradiating method such as X-ray, or fast neutron bombardment, both of which produce much larger genome deletions and/or chromosome rearrangements [384,385,386]. Deletions ranging from 0.8 to 12 kilobases (kb) were produced in Arabidopsis using fast neutron bombardment, a widely used model plant species with an average gene density of one gene per 4.8 kb. The size of the genome alterations produced by this approach can, however, potentially cause the loss of function, or significantly altered expression of more than one gene. Therefore, a considerable drawback of using such an approach is the time and effort required post-mutagenesis to identify a ‘causative mutation’. While the Arabidopsis genome is comparatively smaller than that of Cannabis, a similar post-mutagenesis investigative strategy would likely be required in other plant species with nuclear genomes either of a similar or significantly larger-size [385,387]. Regardless, these types of methods require rather large numbers of plants to be effective as deletions and point mutations are not site directed, which is a considerable limitation as even rapid standard screening techniques demand intensive laboratory work [388,389,390].
Since the advent of the CRISPR/Cas9 gene-editing system in late 2012 [391], the ability to manipulate plant genomes has become more cost efficient and less experimentally tedious when compared to the traditional genetic engineering approaches used by plant breeders in other crop species [392]. The CRISPR/Cas9 system effectively directs site-specific genome editing using RNA-guided, microbial-derived nucleases that initiate double-stranded DNA breaks in eukaryotic and bacterial systems [391,393]. The specificity of this system greatly reduces the amount of off-target genome alterations compared to more traditional transformation techniques. However, off-targeting has also been observed with CRISPR/Cas9 use, an inherent challenge when manipulating any biological system [394,395,396,397]. Earlier work was directed towards human applications, but increasingly this system has been utilised in plant systems, with examples in Arabidopsis, tobacco (Nicotiana tabacum), rice (Oryza sativa), lettuce (Lactuca sativa), maize (Zea mays), soybean (Glycine max) and wheat (Triticum aestivum) now documented [398,399,400,401,402,403,404,405,406,407,408,409,410]. By no means an exhaustive list of CRISPR/Cas9-facilitated manipulation in plants, the above does, however, highlight the potential applicability of this targeted mutagenesis approach to modulate specific biosynthetic pathways in Cannabis to produce superior varieties that display phenotypic and chemotypic traits of interest, and as a tool to discover key genes involved the production of minor cannabinoids. Transformation technologies has thus far been conducted in hemp varieties only, and therefore require further development and considerable refinement for application in other Cannabis varieties. The first report of successful hemp transformation emerged in 2001 [411], and two years later, a protocol for successful Agrobacterium tumefaciens-mediated transformation of tissue cultured hemp callus was implemented [412]. More recently, Wahby et al. [413,414] successfully transformed hemp using both A. tumefaciens and A. rhizogenes, establishing the initial protocol for hairy root culture in Cannabis, a system used for the production of key phytochemicals. Despite these successes, Cannabis has proven to be a difficult plant species to transform with such variables as variety, plant age and the explant used for callus production, all demonstrated to be crucial factors underpinning transformant regeneration efficiency [415]. As with any novel plant transformation system, in order to overcome poor transformation efficiency, optimised protocols with respect to culture media, experimental approach, and selected explant material, will be required for routine and robust transformation of Cannabis.
5.2. Synthetic Production of Cannabinoids
Recently, the synthetic biology approach utilising microorganisms to produce high-quality cannabinoid products has removed the requirement for plant material [287,416]. Luo and colleagues [287] were successful in producing CBG, CBD, THC and Δ9-THCV from galactose, via manipulation of the native MVA pathway of the yeast Saccharomyces cerevisiae post the introduction of Cannabis genes encoding cannabinoid synthases, olivetolic acid synthase and geranylpyrophosphate: olivetolate geranyltransferase. Production of THCA from CBGA through functional THCA synthase expression in the two yeast species, S. cerevisiae and Pichia pastoris, has been demonstrated. However, attempts to introduce the same functionality in Escherichia coli, a bacterium, have proved unsuccessful [293,417]. Over-expression of genes encoding enzymes in the MVA and prenyl diphosphate pathways, also in S. cerevisiae, produced prenyl alcohol precursors required for terpenoid and cannabinoid synthesis [418], while expression of a functional aromatic prenyltransferase from Streptomyces resulted in THCA production from OA and DPP in the yeast, Komagataella phaffi [419]. These approaches present an attractive alternative with the ability to conceivably produce large quantities of minor cannabinoids that are only found in trace amounts in planta, while also reducing and/or removing the costs, carbon emissions (associated with indoor growth; [420]) and environmental variables associated with the agricultural crop production. However, it should be noted that due to the criminalisation of Cannabis since the early 1930s, there are very few studies analysing water and energy use associated with the cultivation of Cannabis, although undoubtedly, as research in this area becomes more prevalent, efficient horticultural practices will reduce the consumption of water and energy for the large-scale cultivation of Cannabis.
5.3. Phenotypic Parameters Affecting Cannabis Yield and Potency
In Cannabis plants exhibiting an illicit drug chemotype (high THC), a primary concern, in conjunction with desired cannabinoid content, is overall biomass yield of female floral tissue. Consistent with other agriculturally significant species, Cannabis is sensitive to environmental variations which alter physiological characteristics affecting plant growth and yield potential. Early work on Cannabis flowering, uncovered the response to photoperiodism [421,422], which has subsequently been exploited, particularly by illicit indoor growers, who can cultivate Cannabis year-round by manipulating the response to reduced photoperiod length [423]. Photoperiodism is a well-known biological response critical for development of branching and floral architecture in Cannabis, and as a result, has implications for yield potential [423,424]. A reduction in day length from 18 to 12 h induces flowering, and maintenance of this regime for 8 weeks produces an acceptable floral yield [423]. Elevated light intensity from 400 watts per square metre (W m−2), to 600 W m−2, produced a higher yield of floral tissue per plant in several chemotypes when grown indoors [424]. In addition, an increase in plant density from 16 to 20 plants m−2 reduced biomass yield of floral tissue in all 600 W m−2 treated plants [425]; a finding that indicates that light interception is compromised at the lower canopy level in crowded growth conditions. The use of different artificial lighting systems in controlled environment greenhouse applications also affects yield, but there are ‘trade offs’ when using light emitting diode (LED), versus high-intensity discharge (HID) light sources. HID lighting is generally of lower cost and generates greater photon flux density between 400 and 700 nm, while LED lighting has greater configurability for specified needs and emits substantially less heat than HID lighting; with both lighting options having similar electricity to photosynthetic photon conversion efficiencies, expressed as, µmol J−1 [426,427,428]. The importance of light quality has been demonstrated in cucumber (Cucumis sativus) where a significant increase in dry weight was measured in plants grown under an ‘artificial solar spectrum’, produced by sulfur plasma and quartz-halogen lamps irradiating a light spectrum that emulated standard sunlight, when compared with those plants provided with either fluorescent or HID lighting [429]. Photosynthetic photon flux density significantly affects harvestable floral biomass yield, while elevated UV-B radiation and electrical lighting power density (W m−2) increased the ‘potency’ of Cannabis through an elevation in THC concentration; all of which highlight the importance of light quantity and quality capture by the photosynthetic apparatus of this species to improve the harvestable output of cultivated Cannabis [423,430,431,432,433].
Manipulating temperature conditions in indoor growth facilities has revealed a relationship with factors affecting plant growth and development. Rate of photosynthesis, water use efficiency, rate of transpiration, and leaf stomatal conductance, all increased in Cannabis plants with a temperature increase from 20 to 30 °C, suggesting an optimal temperature range for cultivation [431]. Temperature and photosynthetic rate are tightly linked with the photosynthetic apparatus sensitive to fluctuations in temperature, responding particularly with reduced Ribulose 1,5-bisphosphate (RuBP) regeneration and lowered stomatal aperture, which together decreased CO2 uptake; both rate reducing outcomes [434,435,436]. It is worthwhile to note that Cannabis varieties are similarly sensitive to temperature where photosynthetic rate, water use efficiency, leaf number, and stem elongation, are modulated in response to temperature change [431,437,438]. Mineral supplementation via fertilizer application has produced mixed results in terms of biomass and secondary metabolite concentration and/or profile composition in Cannabis. Cannabis was shown to be sensitive to nitrogen (N), phosphorus (P) and potassium (K) (NPK) supplementation, as well as the plant biostimulant, humic acid. The application of NPK reduced THC, CBN and CBD content, but increased CBG content in the Cannabis inflorescence, while the application of humic acid was found to significantly lower the THC, CBD, CBG, THCV, CBC, CBL and CBT content of the Cannabis inflorescence [439]. However, N supplementation alone increased hemp seed yield, plant height, chlorophyll content, while decreasing fibre yield [440]. The application of exogenous hormones during distinct developmental phases of Cannabis growth has also produced mixed results in relation to secondary metabolite content and biomass. Gibberellic acid (GA) application to whole flowering plants with developed, resinous trichomes reduced chlorophyll levels, DXS activity, mono- and sesquiterpene levels, and THC content, while increasing HMGR activity, to suggest a degree of interference (either directly or indirectly) by GA to both the MVA and MEP pathways [441,442]. Abscisic acid (ABA) application at the vegetative stage of Cannabis development, increased chlorophyll a content, but reduced HMGR, THC and CBD content. In contrast, ABA application at the flowering stage of development decreased total chlorophyll and HMGR content, and increased DXS activity and the content of THC in the flowers of female Cannabis plants, findings which again indicated either direct or indirect phytohormone-mediated interference of both the MVA and MEP pathways [441,442].
Alterations of the architecture of the Cannabis flower via the application of molecular-assisted breeding, or genetic engineering, are potential strategies to increase the floral yield of Cannabis. Alternatively, directed manipulation of the biosynthetic pathways by application of similar approaches leading to increased cannabinoid or terpenoid content would provide greater value via the targeted elevation of the exact concentration of specific secondary metabolites. Currently, research describing the implementation of such strategies in Cannabis are scarce. However, investigations of trichome development in Arabidopsis and other plant species are not. The extremely well-annotated genome of Arabidopsis, combined with the ease that Arabidopsis can be genetically manipulated, identifies Arabidopsis for use in baseline studies that are potentially applicable to more valuable agricultural species. Indeed, Arabidopsis-based studies of trichome development have revealed a cohort of genes of interest. As with the development of any specialised cell type, it is underpinned by a complex gene network, and in Arabidopsis, the protein products encoded by the GLABROUS1 (GL1), GL2, GL3 and TRANSPARENT TESTA GLABROUS loci are responsible for various aspects of trichome morphogenesis, maturation, branching and spatial variation [443,444,445,446]. Additional gene products have been identified as essential for correct branching patterns and trichome responses to hormones, with EMS-induced mutation to the MYB encoding gene, TRIPTYCHON, resulting in the ‘nesting’, or grouping of trichomes with higher local densities [447,448]. A gene encoding a zinc-finger transcription factor from Arabidopsis, GLABROUS INFLORESCENCE STEMS, increased glandular trichome density on the leaves, sepals, inflorescence and its branches, while also increasing the content of nicotine secretion into the glandular heads when over-expressed in tobacco plants [449]. Similarly, overexpression of a serine proteinase inhibitor, SaPIN2a, from American nightshade (Solanum americanum) in transformed tobacco, significantly increased the branching and density of glandular trichomes [450]. Regulation of the expression of the gene encoding the DXS synthase 2 (DXS2) enzyme, which is active in the MEP pathway in Cannabis, and also in tomato (Solanum lycopersicum) via a RNA silencing approach, resulted in an increase in trichome density on tomato leaves and reduced the accumulation of the monoterpene, β-phellandrene [451]. In cotton (Gossypium spp.), a mutation in the PIGMENT GLAND FORMATION locus, resulted in the expression of the glandless phenotype: a strategy adopted to remove toxic gossypol from cotton seeds for human consumption [452]. While the opposite phenotypic outcome of increased trichome density would be the desired result in Cannabis experimentation, when taken together, these findings highlight the importance of targeting specific genetic networks for molecular manipulation to initiate the expression of desired and/or designer plant phenotypes.
Increasing the biomass of agriculturally valuable species is not a novel undertaking, and anthropogenic selection has perhaps inadvertently, been conducted by humans since the dawn of agriculture. Plant height is identified as a target for manipulation in relation to overall biomass yield in maize and sorghum (Sorghum bicolor) [453], and in Cannabis grown for fibre, stem length is an important parameter for fibre yield which is affected by plant density and soil N content [454,455]. The inverse is true for Cannabis varieties grown for their cannabinoid content, where reduced stem lengths produce a shorter overall plant stature and correlates with a greater photoassimilate input into reproductive tissues leading to the development of floral architecture with increased accumulation of cannabinoids and terpenoids [456]. Small [3] suggests that the value of drug chemotype varieties is linked to the development of ‘semi-dwarf Cannabis germplasm’, characterised by compact, congested flowers on short branches. Such plants ultimately produce more cannabinoids due to greater resource partitioning into floral and trichome development and are of short enough stature that they can be grown at high indoor densities where the artificial environment is readily manipulated to produce greater amounts of secondary metabolites. The combination of key phenotypic traits associated with increased secondary metabolite accumulation, including dense compact floral arrangements, and semi-dwarf stature, and with novel chemotypic traits that confer targeted medical efficacy epitomises the new varieties (chemovars) to be pursued as part of a highly focused research strategy. Similar strategies that use marker assisted breeding and EMS to provide the molecular basis to generate plants that produce elevated levels of desired compounds have been undertaken in other medically significant plant species. Quantitative trait loci mapping of Artemisia annua L. (sweet wormwood), a plant species which produces the anti-malarial compound, artemisinin, provided the platform for marker assisted breeding programs to increase artemisinin yield [457], and by extension, revealed both the pathway for similar research that would later be undertaken in opium poppy and the avenues for the future development of similar strategies in Cannabis.
5.4. Papaver somniferum: Potential Parallels for Future Cannabis Research
With significant change surrounding the societal views and scientific inquiry into Cannabis on the horizon, it is important to look at past endeavours to envisage future directions. While Cannabis is a unique plant for its utility, Papaver somniferum (Papaver; opium poppy) rivals the versatility seen across Cannabis varieties, and given its long history of human use, it is an excellent comparison to investigate. Papaver, otherwise known as opium poppy, is responsible for the production of the most medically significant alkaloids, including morphine, codeine, thebaine, oripavine and noscapine. These opioids accumulate in the phloem, particularly the mesocarp capsule of Papaver aerial tissues in specialised cells called lactifers, which join to form a latex-containing network of anastomosing vessels [458,459,460]. The therapeutic efficacy of Papaver-derived opioids is better understood than the secondary metabolites of Cannabis, and the scope of their effects is far reaching. Morphine has been utilised for decades as one of the most widely used analgesics, effective in the post-operative clinical setting [461,462,463]. Codeine has been shown to be a less effective analgesic than morphine [464,465] but has historically been accepted as the prevailing antitussive [466]. More recent evidence suggests however, that there are more effective treatments, especially for chronic coughing disorders [467,468,469]. Additionally, noscapine, another Papaver alkaloid, displays antitussive properties, and is also suggested to potentially mitigate stroke mortality and induce apoptosis in a broad set of cancers [470,471,472,473]. Thebaine and oripavine are not themselves used therapeutically. However, they are precursors for a wide range of semi-synthetic opioids including, but not limited to, hydrocodone, oxycodone and hydromorphone, as well as naloxone, which is interestingly employed to treat the acute effects of opioid overdose [474,475,476,477,478,479].
Given the multitude of efficacious compounds produced by Papaver, and the commercial value emanating from such, the desire to generate plant varieties that produce specific chemical profiles is one that is mirrored in Cannabis. While the latter is currently reliant on years of predominantly illicit breeding programs to produce plants with increased psychoactive properties, the development of novel Papaver varieties has already been established. EMS treatment of poppy seeds preceded the identification of a variety termed top1 (thebaine oripavine poppy 1) which harboured a mutation leading to premature arrest of the morphine and codeine biosynthesis pathway. The resulting top1 plants displayed a pigmented latex, and the enhanced accumulation of thebaine and oripavine, but failed to produce either codeine or morphine [480]. Similarly, a reduction in codeine 3-O-demethylase (CODM) activity, via either a viral-induced gene silencing (VIGS) strategy, or a fast neutron bombardment mutagenesis approach, yielded Papaver plants with enhanced codeine accumulation, but which were unable to synthesise morphine from a codeine substrate [481,482,483]. These high codeine Papaver varieties that harbour CODM polymorphisms, provided a basis for a marker-assisted breeding platform, and to produce Papaver chemotypes accumulating novel alkaloid profiles [483]. Similar actions utilising Cannabis may also mediate alterations to the cannabinoid biosynthesis pathways to produce varieties with elevated minor cannabinoid content. Sequencing of a high noscapine variety of Papaver, termed HN1, led to the discovery of a 10 gene cluster responsible for noscapine biosynthesis that was absent in either a high morphine (HM1)- or high thebaine (HT1)-producing variety of Papaver [484]. Generation of an F2 mapping population from HN1 and HM1 parents showed tight linkage of this gene cluster, revealing high noscapine-producing progeny that were homozygous for the HN1 gene cluster, while heterozygosity, or absence of the HN1 gene cluster, was associated with plant lines that produced low or undetectable levels of noscapine, respectively [484]. The identification of the STORR ([S]- to [R]-reticuline) locus led to the development of high noscapine Papaver varieties with a non-functioning cytochrome P450-oxidoreductase fusion protein, inhibiting the [S]-reticuline conversion to [R]-reticuline necessary for completion of the morphinan pathway [485,486,487,488]. A VIGS approach has been successfully utilised to individually regulate the expression of six genes encoding enzymes involved in the final six conversion steps of [R]-reticuline to morphine, each of which were shown to alter the major alkaloid profile [489]. An RNA silencing approach which employed a chimeric hairpin RNA to target all members of the multigene codeinone reductase family produced a non-narcotic, [S]-reticuline-accumulating variety of Papaver [490]. In the exploitation of the versatility of Papaver beyond narcotics, varieties with high food value have been established through EMS and gamma ray mutagenesis breeding programs to produce increased seed yield (5.66 g/capsule versus the 3.39 g/capsule of control plants) with elevated levels of unsaturated seed oil and no narcotic production [491]. While this is not an exhaustive list of selectively bred, or engineered Papaver varieties, the long-standing and successful development of Papaver varieties with superior phenotypic and/or chemotypic traits of interest certainly provides a reference for guiding Cannabis research strategies, which at present are comparatively in their infancy. Development of varieties producing high levels of alkaloid biosynthetic pathway intermediates is a promising indicator for the potential production of Cannabis varieties that reliably produce high levels of minor cannabinoids or intermediates in the cannabinoid biosynthesis pathway.
6. Conclusions
In summary, we have reviewed the current literature of several important aspects of cannabinoid research outside of THC and CBD, which dominate discussion in the Cannabis research field. Emerging research has begun to reveal the pharmacology and molecular targets of the minor cannabinoids. Due to the wide spectrum of molecular effects involved with cannabinoid consumption, it is clear that there are a range of medical ailments that could be addressed through endocannabinoid augmentation using secondary metabolites of Cannabis. Here, we have illustrated that via the utilisation of specific minor cannabinoids, which share some, but not all targets of THC and CBD, the medical reach of cannabinoid-containing pharmaceuticals could potentially be broadened. However, there are many challenges that currently impede this possibility, even outside of the international legal environment. Firstly, there is further room for significant characterisation of minor cannabinoid pharmacology, and currently, disease-orientated preclinical and clinical trials are lacking. Critically, techniques for producing cannabinoid isolates—even CBD and THC—are still in their infancy, and this remains a clear barrier to large-scale commercialisation of pharmaceutical cannabinoids. Here, we have reviewed the currently available literature which covers the processes involved in the biosynthesis of cannabinoids, as well as the techniques involved in the production of novel Cannabis chemotypes, including methods of improving yield that might be adopted from historically similar cases, such as the opioid industry. Based on this historical example, and the existing literature, it is likely that a molecular genetic modification approach will be applied to Cannabis to generate new opportunities for the improved yield of specific minor and major cannabinoids in the near future. In conclusion, there are multiple enticing and potentially profitable opportunities for commercial and academic growth in the Cannabis market outside of THC and CBD, and here, we highlight some of the most important current perspectives of this growing industry.
Author Contributions
Conceptualization and design, J.M.J.O., J.L.P., T.A.B., A.L.E. and C.P.L.G.; writing, J.M.J.O., J.L.P., T.A.B., A.L.E., L.J.N. and C.P.L.G.; review and editing, J.M.J.O., J.L.P., T.A.B., A.L.E., L.J.N. and C.P.L.G. All authors have read and agreed to the published version of the manuscript.
Funding
J.M.J.O.’s research is supported by CannaPacific Pty Ltd., Australia.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Clarke R.C., Merlin M.D. Letter to the Editor: Small, Ernest. 2015. Evolution and Classification of Cannabis sativa (Marijuana, Hemp) in Relation to Human Utilization. Bot. Rev. 2015;81:295–305. doi: 10.1007/s12229-015-9158-2. [DOI] [Google Scholar]
- 2.Schultes R.E., Klein W.M., Plowman T., Lockwood T.E. Cannabis: An Example of Taxonomic Neglect. Bot. Museum Leafl. Harvard Univ. 1974;23:337–367. [Google Scholar]
- 3.Small E. Evolution and Classification of Cannabis sativa (Marijuana, Hemp) in Relation to Human Utilization. Bot. Rev. 2015;81:189–294. doi: 10.1007/s12229-015-9157-3. [DOI] [Google Scholar]
- 4.Small E., Cronquist A. A Practical and Natural Taxonomy for Cannabis. Taxon. 1976;25:405–435. doi: 10.2307/1220524. [DOI] [Google Scholar]
- 5.Small E., Naraine S.G.U. Size Matters: Evolution of Large Drug-Secreting Resin Glands in Elite Pharmaceutical Strains of Cannabis sativa (Marijuana) Genet. Resour. Crop Evol. 2016;254:349–359. doi: 10.1007/s10722-015-0254-2. [DOI] [Google Scholar]
- 6.Salentijn E.M.J., Zhang Q., Amaducci S., Yang M., Trindade L.M. New Developments in Fiber Hemp (Cannabis sativa L.) Breeding. Ind. Crops Prod. 2015;68:32–41. doi: 10.1016/j.indcrop.2014.08.011. [DOI] [Google Scholar]
- 7.Mead A. The Legal Status of Cannabis (Marijuana) and Cannabidiol (CBD) under U.S. Law. Epilepsy Behav. 2017;70:288–291. doi: 10.1016/j.yebeh.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 8.Amaducci S., Scordia D., Liu F.H., Zhang Q., Guo H., Testa G., Cosentino S.L. Key Cultivation Techniques for Hemp in Europe and China. Ind. Crop. Prod. 2015;68:2–16. doi: 10.1016/j.indcrop.2014.06.041. [DOI] [Google Scholar]
- 9.Bielecka M., Kaminski F., Adams I., Poulson H., Sloan R., Li Y., Larson T.R., Winzer T., Graham I.A. Targeted Mutation of Δ12 and Δ15 Desaturase Genes in Hemp Produce Major Alterations in Seed Fatty Acid Composition Including a High Oleic Hemp Oil. Plant Biotechnol. J. 2014;12:613–623. doi: 10.1111/pbi.12167. [DOI] [PubMed] [Google Scholar]
- 10.Callaway J.C., Tennilä T., Pate D.W. Occurence of “Omega 3” Stearidonic Acid (Cis-6,9,12,15-Ocadecatetraenoic Acid) in Hemp (Cannabis sativa L.) Seed. J. Int. Hemp Assoc. 1996;3:61–63. [Google Scholar]
- 11.Dimić E., Romanić R., Vujasinović V. Essential Fatty Acids, Nutritive Value and Oxidative Stability of Cold Pressed Hempseed (Cannabis sativa L.) Oil from Different Varieties. Acta Aliment. 2009;38:229–236. doi: 10.1556/AAlim.2008.0035. [DOI] [Google Scholar]
- 12.Deferne J., Pate D.W. Hemp Seed Oil: A Source of Valuable Essential Fatty Acids. J. Int. Hemp Assoc. 1996;3:4–7. [Google Scholar]
- 13.Erasmus U. Fats That Heal, Fats That Kill: The Complete Guide to Fats, Oils, Cholesterol, and Human Health. 3rd ed. Alive Books; Burnaby, BC, Canada: 1993. [Google Scholar]
- 14.Stubbs C.D., Smith A.D. The Modification of Mammalian Membrane Polyunsaturated Fatty Acid Composition in Relation to Membrane Fluidity and Function. Biochim. Biophys. Acta. 1984;779:89–137. doi: 10.1016/0304-4157(84)90005-4. [DOI] [PubMed] [Google Scholar]
- 15.Callaway J.C. Hempseed as a Nutritional Resource: An Overview. Euphytica. 2004;140:65–72. doi: 10.1007/s10681-004-4811-6. [DOI] [Google Scholar]
- 16.Harbige L.S., Layward L., Morris-Downes M.M., Dumonde D.C., Amor S. The Protective Effects of Omega-6 Fatty Acids in Experimental Autoimmune Encephalomyelitis (EAE) in Relation to Transforming Growth Factor-Beta 1 (TGF-Β1) up-Regulation and Increased Prostaglandin E2 (PGE2) Production. Clin. Exp. Immunol. 2000;122:445–452. doi: 10.1046/j.1365-2249.2000.01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prociuk M.A., Edel A.L., Richard M.N., Gavel N.T., Ander B.P., Dupasquier C.M.C., Pierce G.N. Cholesterol-Induced Stimulation of Platelet Aggregation Is Prevented by a Hempseed-Enriched Diet. Can. J. Physiol. Pharmacol. 2008;86:153–159. doi: 10.1139/Y08-011. [DOI] [PubMed] [Google Scholar]
- 18.Clarke R.C., Merlin M.D. Cannabis Domestication, Breeding History, Present-Day Genetic Diversity, and Future Prospects. CRC Crit. Rev. Plant Sci. 2016;35:293–327. doi: 10.1080/07352689.2016.1267498. [DOI] [Google Scholar]
- 19.Dayanandan P., Kaufman P.B. Trichomes of Cannabis sativa L. (Cannabaceae) Am. J. Bot. 1976;63:578–591. doi: 10.1002/j.1537-2197.1976.tb11846.x. [DOI] [Google Scholar]
- 20.ElSohly M.A., Slade D. Chemical Constituents of Marijuana: The Complex Mixture of Natural Cannabinoids. Life Sci. 2005;78:539–548. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Pertwee R.G., editor. Handbook of Cannabis. Oxford University Press; Oxford, UK: 2015. [DOI] [Google Scholar]
- 22.Citti C., Linciano P., Russo F., Luongo L., Iannotta M., Maione S., Laganà A., Capriotti A.L., Forni F., Vandelli M.A., et al. A Novel Phytocannabinoid Isolated from Cannabis sativa L. with an in Vivo Cannabimimetic Activity Higher than Δ9-Tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol. Sci. Rep. 2019;9:20335. doi: 10.1038/s41598-019-56785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babaei M., Ajdanian L. Screening of Different Iranian Ecotypes of Cannabis under Water Deficit Stress. Sci. Hortic. 2020;260 doi: 10.1016/j.scienta.2019.108904. [DOI] [Google Scholar]
- 24.Linger P., Ostwald A., Haensler J. Cannabis sativa L. Growing on Heavy Metal Contaminated Soil: Growth, Cadmium Uptake and Photosynthesis. Biol. Plant. 2005;49:567–576. doi: 10.1007/s10535-005-0051-4. [DOI] [Google Scholar]
- 25.Bouquet R.J. Cannabis. Bull. Narc. 1950;2:14–30. [Google Scholar]
- 26.Amaducci S., Zatta A., Raffanini M., Venturi G. Characterisation of Hemp (Cannabis sativa L.) Roots under Different Growing Conditions. Plant Soil. 2008;313 doi: 10.1007/s11104-008-9695-0. [DOI] [Google Scholar]
- 27.Small E., Marcus D., Butler G., McElroy A.R. Apparent Increase in Biomass and Seed Productivity in Hemp (Cannabis sativa) Resulting from Branch Proliferation Caused by the European Corn Borer (Ostrinia nubilalis) J. Ind. Hemp. 2007;12:15–26. doi: 10.1300/J237v12n01_03. [DOI] [Google Scholar]
- 28.Government of Canada Department of Justice. [(accessed on 15 January 2020)]; Available online: https://www.justice.gc.ca/eng/cj-jp/cannabis/
- 29.Ney L.J., Matthews A., Bruno R., Felmingham K.L. Cannabinoid Interventions for PTSD: Where to Next? Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2019;93:124–140. doi: 10.1016/j.pnpbp.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 30.Lattanzi S., Brigo F., Trinka E., Zaccara G., Cagnetti C., Del Giovane C., Silvestrini M. Efficacy and Safety of Cannabidiol in Epilepsy: A Systematic Review and Meta-Analysis. Drugs. 2018;78:1791–1804. doi: 10.1007/s40265-018-0992-5. [DOI] [PubMed] [Google Scholar]
- 31.Patel S., Hill M.N., Cheer J.F., Wotjak C.T., Holmes A. The Endocannabinoid System as a Target for Novel Anxiolytic Drugs. Neurosci. Biobehav. Rev. 2017;76:56–66. doi: 10.1016/j.neubiorev.2016.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whiting P.F., Wolff R.F., Deshpande S., Di Nisio M., Duffy S., Hernandez A.V., Keurentjes J.C., Lang S., Misso K., Ryder S., et al. Cannabinoids for Medical Use: A Systematic Review and Meta-Analysis. JAMA J. Am. Med. Assoc. 2015;313:2456–2473. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- 33.Wade D.T., Collin C., Stott C., Duncombe P. Meta-Analysis of the Efficacy and Safety of Sativex (Nabiximols), on Spasticity in People with Multiple Sclerosis. Mult. Scler. 2010;16:707–714. doi: 10.1177/1352458510367462. [DOI] [PubMed] [Google Scholar]
- 34.Stevens A.J., Higgins M.D. A Systematic Review of the Analgesic Efficacy of Cannabinoid Medications in the Management of Acute Pain. Acta Anaesthesiol. Scand. 2017;61:268–280. doi: 10.1111/aas.12851. [DOI] [PubMed] [Google Scholar]
- 35.Morales P., Jagerovic N. Novel Approaches and Current Challenges with Targeting the Endocannabinoid System. Expert Opin. Drug Discov. 2020;15:917–930. doi: 10.1080/17460441.2020.1752178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morales P., Hurst D.P., Reggio P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog. Chem. Org. Nat. Prod. 2017;103:103–131. doi: 10.1007/978-3-319-45541-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sampson P.B. Phytocannabinoid Pharmacology: Medicinal Properties of Cannabis sativa Constituents Aside from the “Big Two”. J. Nat. Prod. 2020;84:142–160. doi: 10.1021/acs.jnatprod.0c00965. [DOI] [PubMed] [Google Scholar]
- 38.Gaoni Y., Mechoulam R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964;86:1646–1647. doi: 10.1021/ja01062a046. [DOI] [Google Scholar]
- 39.Mechoulam R., Gaoni Y. A Total Synthesis of Dl-Δ1-Tetrahydrocannabinol, the Active Constituent of Hashish. J. Am. Chem. Soc. 1965;87:3273–3275. doi: 10.1021/ja01092a065. [DOI] [PubMed] [Google Scholar]
- 40.Devane W.A., Dysarz F.A., Johnson M.R., Melvin L.S., Howlett A.C. Determination and Characterization of a Cannabinoid Receptor in Rat Brain. Mol. Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- 41.Munro S., Thomas K.L., Abu-Shaar M. Molecular Characterization of a Peripheral Receptor for Cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 42.Herkenham M., Lynn A.B., Little M.D., Johnson M.R., Melvin L.S., De Costa B.R., Rice K.C. Cannabinoid Receptor Localization in Brain. Proc. Natl. Acad. Sci. USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Devane W.A., Hanuš L., Breuer A., Pertwee R.G., Stevenson L.A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 44.Mechoulam R., Ben-Shabat S., Hanus L., Ligumsky M., Kaminski N.E., Schatz A.R., Gopher A., Almog S., Martin B.R., Compton D.R., et al. Identification of an Endogenous 2-Monoglyceride, Present in Canine Gut, That Binds to Cannabinoid Receptors. Biochem. Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-D. [DOI] [PubMed] [Google Scholar]
- 45.Sugiura T., Kondo S., Sukagawa A., Nakane S., Shinoda A., Itoh K., Yamashita A., Waku K. 2-Arachidonoylglycerol: A Possible Endogenous Cannabinoid Receptor Ligand in Brain. Biochem. Biophys. Res. Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 46.Liu J., Wang L., Harvey-White J., Osei-Hyiaman D., Razdan R., Gong Q., Chan A.C., Zhou Z., Huang B.X., Kim H.Y., et al. A Biosynthetic Pathway for Anandamide. Proc. Natl. Acad. Sci. USA. 2006;103:13345–13350. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mechoulam R., Parker L.A. The Endocannabinoid System and the Brain. Annu. Rev. Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 48.Di Marzo V. The Endocannabinoid System: Its General Strategy of Action, Tools for Its Pharmacological Manipulation and Potential Therapeutic Exploitation. Pharmacol. Res. 2009;60:77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 49.Kano M., Ohno-Shosaku T., Hashimotodani Y., Uchigashima M., Watanabe M. Endocannabinoid-Mediated Control of Synaptic Transmission. Physiol. Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 50.Tripathi R.K.P. A Perspective Review on Fatty Acid Amide Hydrolase (FAAH) Inhibitors as Potential Therapeutic Agents. Eur. J. Med. Chem. 2020;188:111953. doi: 10.1016/j.ejmech.2019.111953. [DOI] [PubMed] [Google Scholar]
- 51.Ligresti A., Cascio M.G., Pryce G., Kulasegram S., Beletskaya I., De Petrocellis L., Saha B., Mahadevan A., Visintin C., Wiley J.L., et al. New Potent and Selective Inhibitors of Anandamide Reuptake with Antispastic Activity in a Mouse Model of Multiple Sclerosis. Br. J. Pharmacol. 2006;147:83–91. doi: 10.1038/sj.bjp.0706418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glass M., Dragunow M., Faull R.L.M. Cannabinoid Receptors in the Human Brain: A Detailed Anatomical and Quantitative Autoradiographic Study in the Fetal, Neonatal and Adult Human Brain. Neuroscience. 1997;77:299–318. doi: 10.1016/S0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- 53.Hua T., Vemuri K., Pu M., Qu L., Han G.W., Wu Y., Zhao S., Shui W., Li S., Korde A., et al. Crystal Structure of the Human Cannabinoid Receptor CB1. Cell. 2016;167:750–762. doi: 10.1016/j.cell.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li X., Hua T., Vemuri K., Ho J.H., Wu Y., Wu L., Popov P., Benchama O., Zvonok N., Locke K., et al. Crystal Structure of the Human Cannabinoid Receptor CB2. Cell. 2019;176:459–467. doi: 10.1016/j.cell.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katona I., Sperlágh B., Sík A., Käfalvi A., Vizi E.S., Mackie K., Freund T.F. Presynaptically Located CB1 Cannabinoid Receptors Regulate GABA Release from Axon Terminals of Specific Hippocampal Interneurons. J. Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Derkinderen P., Ledent C., Parmentier M., Girault J.A. Cannabinoids Activate P38 Mitogen-Activated Protein Kinases through CB1 Receptors in Hippocampus. J. Neurochem. 2001;77:957–960. doi: 10.1046/j.1471-4159.2001.00333.x. [DOI] [PubMed] [Google Scholar]
- 57.Herkenham M., Lynn A.B., de Costa B.R., Richfield E.K. Neuronal Localization of Cannabinoid Receptors in the Basal Ganglia of the Rat. Brain Res. 1991;547:267–274. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- 58.Mátyás F., Yanovsky Y., Mackie K., Kelsch W., Misgeld U., Freund T.F. Subcellular Localization of Type 1 Cannabinoid Receptors in the Rat Basal Ganglia. Neuroscience. 2006;137:337–361. doi: 10.1016/j.neuroscience.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 59.Bidaut-Russell M., Devane W.A., Howlett A.C. Cannabinoid Receptors and Modulation of Cyclic AMP Accumulation in the Rat Brain. J. Neurochem. 1990;55:21–26. doi: 10.1111/j.1471-4159.1990.tb08815.x. [DOI] [PubMed] [Google Scholar]
- 60.Kawamura Y., Fukaya M., Maejima T., Yoshida T., Miura E., Watanabe M., Ohno-Shosaku T., Kano M. The CB1 Cannabinoid Receptor Is the Major Cannabinoid Receptor at Excitatory Presynaptic Sites in the Hippocampus and Cerebellum. J. Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kreitzer A.C., Regehr W.G. Cerebellar Depolarization-Induced Suppression of Inhibition Is Mediated by Endogenous Cannabinoids. J. Neurosci. 2001;21:RC174. doi: 10.1523/JNEUROSCI.21-20-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lévénès C., Daniel H., Soubrié P., Crépel F. Cannabinoids Decrease Excitatory Synaptic Transmission and Impair Long-Term Depression in Rat Cerebellar Purkinje Cells. J. Physiol. 1998;510:867–879. doi: 10.1111/j.1469-7793.1998.867bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohno-Shosaku T., Maejima T., Kano M. Endogenous Cannabinoids Mediate Retrograde Signals from Depolarized Postsynaptic Neurons to Presynaptic Terminals. Neuron. 2001;29:729–738. doi: 10.1016/S0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 64.Ney L.J., Akhurst J., Bruno R., Laing P.A.F., Matthews A., Felmingham K.L. Dopamine, Endocannabinoids and Their Interaction in Fear Extinction and Negative Affect in PTSD. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2021;105:110118. doi: 10.1016/j.pnpbp.2020.110118. [DOI] [PubMed] [Google Scholar]
- 65.Balsevich G., Petrie G.N., Hill M.N. Endocannabinoids: Effectors of Glucocorticoid Signaling. Front. Neuroendocrinol. 2017;47:86–108. doi: 10.1016/j.yfrne.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 66.González-Mariscal I., Krzysik-Walker S.M., Doyle M.E., Liu Q.R., Cimbro R., Santa-Cruz Calvo S., Ghosh S., Cieala A., Moaddel R., Carlson O.D., et al. Human CB1 Receptor Isoforms, Present in Hepatocytes and β-Cells, Are Involved in Regulating Metabolism. Sci. Rep. 2016;6:33302. doi: 10.1038/srep33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nogueiras R., Veyrat-Durebex C., Suchanek P.M., Klein M., Tschöp J., Caldwell C., Woods S.C., Wittmann G., Watanabe M., Liposits Z., et al. Peripheral, but Not Central, CB1 Antagonism Provides Food Intake-Independent Metabolic Benefits in Diet-Induced Obese Rats. Diabetes. 2008;57:2977–2991. doi: 10.2337/db08-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ashton J.C., Friberg D., Darlington C.L., Smith P.F. Expression of the Cannabinoid CB2 Receptor in the Rat Cerebellum: An Immunohistochemical Study. Neurosci. Lett. 2006;396:113–116. doi: 10.1016/j.neulet.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 69.Núñez E., Benito C., Pazos M.R., Barbachano A., Fajardo O., González S., Tolón R.M., Romero J. Cannabinoid CB2 Receptors Are Expressed by Perivascular Microglial Cells in the Human Brain: An Immunohistochemical Study. Synapse. 2004;53:208–213. doi: 10.1002/syn.20050. [DOI] [PubMed] [Google Scholar]
- 70.Ross R.A., Coutts A.A., McFarlane S.M., Anavi-Goffer S., Irving A.J., Pertwee R.G., MacEwan D.J., Scott R.H. Actions of Cannabinoid Receptor Ligands on Rat Cultured Sensory Neurones: Implications for Antinociception. Neuropharmacology. 2001;40:221–232. doi: 10.1016/S0028-3908(00)00135-0. [DOI] [PubMed] [Google Scholar]
- 71.Van Sickle M.D., Duncan M., Kingsley P.J., Mouihate A., Urbani P., Mackie K., Stella N., Makriyannis A., Piomelli D., Davison J.S., et al. Identification and Functional Characterization of Brainstem Cannabinoid CB2 Receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 72.Wotherspoon G., Fox A., McIntyre P., Colley S., Bevan S., Winter J. Peripheral Nerve Injury Induces Cannabinoid Receptor 2 Protein Expression in Rat Sensory Neurons. Neuroscience. 2005;135:235–245. doi: 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 73.Galiègue S., Mary S., Marchand J., Dussossoy D., Carrière D., Carayon P., Bouaboula M., Shire D., LE Fur G., Casellas P. Expression of Central and Peripheral Cannabinoid Receptors in Human Immune Tissues and Leukocyte Subpopulations. Eur. J. Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- 74.Lynn A.B., Herkenham M. Localization of Cannabinoid Receptors and Nonsaturable High-Density Cannabinoid Binding Sites in Peripheral Tissues of the Rat: Implications for Receptor-Mediated Immune Modulation by Cannabinoids. J. Pharmacol. Exp. Ther. 1994;268:1612–1623. [PubMed] [Google Scholar]
- 75.Facci L., Dal Toso R., Romanello S., Buriani A., Skaper S.D., Leon A. Mast Cells Express a Peripheral Cannabinoid Receptor with Differential Sensitivity to Anandamide and Palmitoylethanolamide. Proc. Natl. Acad. Sci. USA. 1995;92:3376–3380. doi: 10.1073/pnas.92.8.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lam P.M.W., Marczylo T.H., El-Talatini M., Finney M., Nallendran V., Taylor A.H., Konje J.C. Ultra Performance Liquid Chromatography Tandem Mass Spectrometry Method for the Measurement of Anandamide in Human Plasma. Anal. Biochem. 2008;380:195–201. doi: 10.1016/j.ab.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 77.Fanelli F., Di Lallo V.D., Belluomo I., De Iasio R., Baccini M., Casadio E., Gasparini D.I., Colavita M., Gambineri A., Grossi G., et al. Estimation of Reference Intervals of Five Endocannabinoids and Endocannabinoid Related Compounds in Human Plasma by Two Dimensional-LC/MS/MS. J. Lipid Res. 2012;53:481–493. doi: 10.1194/jlr.M021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krumbholz A., Anielski P., Reisch N., Schelling G., Thieme D. Diagnostic Value of Concentration Profiles of Glucocorticosteroids and Endocannabinoids in Hair. Ther. Drug Monit. 2013;35:600–607. doi: 10.1097/FTD.0b013e3182953e43. [DOI] [PubMed] [Google Scholar]
- 79.Mwanza C., Chen Z., Zhang Q., Chen S., Wang W., Deng H. Simultaneous HPLC-APCI-MS/MS Quantification of Endogenous Cannabinoids and Glucocorticoids in Hair. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016;1028:1–10. doi: 10.1016/j.jchromb.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 80.Voegel C.D., Baumgartner M.R., Kraemer T., Wüst S., Binz T.M. Simultaneous Quantification of Steroid Hormones and Endocannabinoids (ECs) in Human Hair Using an Automated Supported Liquid Extraction (SLE) and LC-MS/MS—Insights into EC Baseline Values and Correlation to Steroid Concentrations. Talanta. 2021;222:121499. doi: 10.1016/j.talanta.2020.121499. [DOI] [PubMed] [Google Scholar]
- 81.Ney L.J., Felmingham K.L., Bruno R., Matthews A., Nichols D.S. Simultaneous Quantification of Endocannabinoids, Oleoylethanolamide and Steroid Hormones in Human Plasma and Saliva. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020;1152:122252. doi: 10.1016/j.jchromb.2020.122252. [DOI] [PubMed] [Google Scholar]
- 82.Ney L.J., Stone C., Nichols D., Felmingham K.L., Bruno R., Matthews A. Endocannabinoid Reactivity to Acute Stress: Investigation of the Relationship between Salivary and Plasma Levels. Biol. Psychol. 2021;159:108022. doi: 10.1016/j.biopsycho.2021.108022. [DOI] [PubMed] [Google Scholar]
- 83.Matias I., Gatta-Cherifi B., Tabarin A., Clark S., Leste-Lasserre T., Marsicano G., Piazza P.V., Cota D. Endocannabinoids Measurement in Human Saliva as Potential Biomarker of Obesity. PLoS ONE. 2012;7:e42399. doi: 10.1371/journal.pone.0042399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schuel H., Burkman L.J., Lippes J., Crickard K., Forester E., Piomelli D., Giuffrida A. N-Acylethanolamines in Human Reproductive Fluids. Chem. Phys. Lipids. 2002;121:211–227. doi: 10.1016/S0009-3084(02)00158-5. [DOI] [PubMed] [Google Scholar]
- 85.Lam M.P.Y., Siu S.O., Lau E., Mao X., Sun H.Z., Chiu P.C.N., Yeung W.S.B., Cox D.M., Chu I.K. Online Coupling of Reverse-Phase and Hydrophilic Interaction Liquid Chromatography for Protein and Glycoprotein Characterization. Anal. Bioanal. Chem. 2010;398:791–804. doi: 10.1007/s00216-010-3991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lewis S.E.M., Rapino C., Di Tommaso M., Pucci M., Battista N., Paro R., Simon L., Lutton D., Maccarrone M. Differences in the Endocannabinoid System of Sperm from Fertile and Infertile Men. PLoS ONE. 2012;7:e47704. doi: 10.1371/journal.pone.0047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malan T.P., Ibrahim M.M., Deng H., Liu Q., Mata H.P., Vanderah T., Porreca F., Makriyannis A. CB2 Cannabinoid Receptor-Mediated Peripheral Antinociception. Pain. 2001;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- 88.Jaggar S.I., Hasnie F.S., Sellaturay S., Rice A.S.C. The Anti-Hyperalgesic Actions of the Cannabinoid Anandamide and the Putative CB2 Receptor Agonist Palmitoylethanolamide in Visceral and Somatic Inflammatory Pain. Pain. 1998;76:189–199. doi: 10.1016/S0304-3959(98)00041-4. [DOI] [PubMed] [Google Scholar]
- 89.Katona I., Freund T.F. Multiple Functions of Endocannabinoid Signaling in the Brain. Annu. Rev. Neurosci. 2012;35:529–558. doi: 10.1146/annurev-neuro-062111-150420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ligresti A., De Petrocellis L., Di Marzo V. From Phytocannabinoids to Cannabinoid Receptors and Endocannabinoids: Pleiotropic Physiological and Pathological Roles through Complex Pharmacology. Physiol. Rev. 2016;96:1593–1659. doi: 10.1152/physrev.00002.2016. [DOI] [PubMed] [Google Scholar]
- 91.Ryberg E., Larsson N., Sjögren S., Hjorth S., Hermansson N.O., Leonova J., Elebring T., Nilsson K., Drmota T., Greasley P.J. The Orphan Receptor GPR55 Is a Novel Cannabinoid Receptor. Br. J. Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li K., Fichna J., Schicho R., Saur D., Bashashati M., MacKie K., Li Y., Zimmer A., Göke B., Sharkey K.A., et al. A Role for O-1602 and G Protein-Coupled Receptor GPR55 in the Control of Colonic Motility in Mice. Neuropharmacology. 2013;71:255–263. doi: 10.1016/j.neuropharm.2013.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ross G.R., Lichtman A., Dewey W.L., Akbarali H.I. Evidence for the Putative Cannabinoid Receptor (GPR55)-Mediated Inhibitory Effects on Intestinal Contractility in Mice. Pharmacology. 2012;90:55–65. doi: 10.1159/000339076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin X.H., Yuece B., Li Y.Y., Feng Y.J., Feng J.Y., Yu L.Y., Li K., Li Y.N., Storr M. A Novel CB Receptor GPR55 and Its Ligands Are Involved in Regulation of Gut Movement in Rodents. Neurogastroenterol. Motil. 2011;23:862-e342. doi: 10.1111/j.1365-2982.2011.01742.x. [DOI] [PubMed] [Google Scholar]
- 95.Galiazzo G., Giancola F., Stanzani A., Fracassi F., Bernardini C., Forni M., Pietra M., Chiocchetti R. Localization of Cannabinoid Receptors CB1, CB2, GPR55, and PPARα in the Canine Gastrointestinal Tract. Histochem. Cell Biol. 2018;150:187–205. doi: 10.1007/s00418-018-1684-7. [DOI] [PubMed] [Google Scholar]
- 96.Oka S., Nakajima K., Yamashita A., Kishimoto S., Sugiura T. Identification of GPR55 as a Lysophosphatidylinositol Receptor. Biochem. Biophys. Res. Commun. 2007;362:928–934. doi: 10.1016/j.bbrc.2007.08.078. [DOI] [PubMed] [Google Scholar]
- 97.Lauckner J.E., Jensen J.B., Chen H.Y., Lu H.C., Hille B., Mackie K. GPR55 Is a Cannabinoid Receptor That Increases Intracellular Calcium and Inhibits M Current. Proc. Natl. Acad. Sci. USA. 2008;105:2699–2704. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Waldeck-Welermair M., Zoratti C., Osibow K., Balenga N., Goessnitzer E., Waldhoer M., Malli R., Graier W.F. Integrin Clustering Enables Anandamide-Induced Ca2+ Signaling in Endothelial Cells via GPR55 by Protection against CB1-Receptor-Triggered Repression. J. Cell Sci. 2008;121:1704–1717. doi: 10.1242/jcs.020958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu Z., Lee J., Krummey S., Lu W., Cai H., Lenardo M.J. The Kinase LRRK2 Is a Regulator of the Transcription Factor NFAT That Modulates the Severity of Inflammatory Bowel Disease. Nat. Immunol. 2011;12:1063–1070. doi: 10.1038/ni.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Henstridge C.M., Balenga N.A., Schröder R., Kargl J.K., Platzer W., Martini L., Arthur S., Penman J., Whistler J.L., Kostenis E., et al. GPR55 Ligands Promote Receptor Coupling to Multiple Signalling Pathways. Br. J. Pharmacol. 2010;160:604–614. doi: 10.1111/j.1476-5381.2009.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Badrichani A.Z., Stroka D.M., Bilbao G., Curiel D.T., Bach F.H., Ferran C. Bcl-2 and Bcl-X(L) Serve an Anti-Inflammatory Function in Endothelial Cells through Inhibition of NF-ΚB. J. Clin. Investig. 1999;103:543–553. doi: 10.1172/JCI2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu C.S., Chen H., Sun H., Zhu J., Jew C.P., Wager-Miller J., Straiker A., Spencer C., Bradshaw H., Mackie K., et al. GPR55, a G-Protein Coupled Receptor for Lysophosphatidylinositol, Plays a Role in Motor Coordination. PLoS ONE. 2013;8:e60314. doi: 10.1371/journal.pone.0060314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Celorrio M., Rojo-Bustamante E., Fernández-Suárez D., Sáez E., Estella-Hermoso de Mendoza A., Müller C.E., Ramírez M.J., Oyarzábal J., Franco R., Aymerich M.S. GPR55: A Therapeutic Target for Parkinson’s Disease? Neuropharmacology. 2017;125:319–332. doi: 10.1016/j.neuropharm.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 104.Sawzdargo M., Nguyen T., Lee D.K., Lynch K.R., Cheng R., Heng H.H.Q., George S.R., O’Dowd B.F. Identification and Cloning of Three Novel Human G Protein-Coupled Receptor Genes GPR52, ΨGPR53 and GPR55: GPR55 Is Extensively Expressed in Human Brain. Mol. Brain Res. 1999;64:193–198. doi: 10.1016/S0169-328X(98)00277-0. [DOI] [PubMed] [Google Scholar]
- 105.Schuelert N., McDougall J.J. The Abnormal Cannabidiol Analogue O-1602 Reduces Nociception in a Rat Model of Acute Arthritis via the Putative Cannabinoid Receptor GPR55. Neurosci. Lett. 2011;500:72–76. doi: 10.1016/j.neulet.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 106.Deliu E., Sperow M., Console-Bram L., Carter R.L., Tilley D.G., Kalamarides D.J., Kirby L.G., Brailoiu G.C., Brailoiu E., Benamar K., et al. The Lysophosphatidylinositol Receptor GPR55 Modulates Pain Perception in the Periaqueductal Gray. Mol. Pharmacol. 2015;88:265–272. doi: 10.1124/mol.115.099333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carey L.M., Gutierrez T., Deng L., Lee W.H., Mackie K., Hohmann A.G. Inflammatory and Neuropathic Nociception Is Preserved in GPR55 Knockout Mice. Sci. Rep. 2017;7:944. doi: 10.1038/s41598-017-01062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gantz I., Muraoka A., Yang Y.K., Samuelson L.C., Zimmerman E.M., Cook H., Yamada T. Cloning and Chromosomal Localization of a Gene (GPR18) Encoding a Novel Seven Transmembrane Receptor Highly Expressed in Spleen and Testis. Genomics. 1997;42:462–466. doi: 10.1006/geno.1997.4752. [DOI] [PubMed] [Google Scholar]
- 109.Kohno M., Hasegawa H., Inoue A., Muraoka M., Miyazaki T., Oka K., Yasukawa M. Identification of N-Arachidonylglycine as the Endogenous Ligand for Orphan G-Protein-Coupled Receptor GPR18. Biochem. Biophys. Res. Commun. 2006;347:827–832. doi: 10.1016/j.bbrc.2006.06.175. [DOI] [PubMed] [Google Scholar]
- 110.Kobayashi K., Fukuoka T., Obata K., Yamanaka H., Dai Y., Tokunaga A., Noguchi K. Distinct Expression of TRPM8, TRPA1, and TRPV1 MRNAs in Rat Primary Afferent Neurons with Aδ/C-Fibers and Colocalization with Trk Receptors. J. Comp. Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 111.Cavanaugh D.J., Chesler A.T., Jackson A.C., Sigal Y.M., Yamanaka H., Grant R., O’Donnell D., Nicoll R.A., Shah N.M., Julius D., et al. Trpv1 Reporter Mice Reveal Highly Restricted Brain Distribution and Functional Expression in Arteriolar Smooth Muscle Cells. J. Neurosci. 2011;31:5067–5077. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Avelino A., Cruz F. TRPV1 (Vanilloid Receptor) in the Urinary Tract: Expression, Function and Clinical Applications. Naunyn. Schmiedebergs. Arch. Pharmacol. 2006;373:287–299. doi: 10.1007/s00210-006-0073-2. [DOI] [PubMed] [Google Scholar]
- 113.Yang Y., Yang H., Wang Z., Mergler S., Wolosin J.M., Reinach P.S. Functional TRPV1 Expression in Human Corneal Fibroblasts. Exp. Eye Res. 2013;107:121–129. doi: 10.1016/j.exer.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tóth A., Boczán J., Kedei N., Lizanecz E., Bagi Z., Papp Z., Édes I., Csiba L., Blumberg P.M. Expression and Distribution of Vanilloid Receptor 1 (TRPV1) in the Adult Rat Brain. Mol. Brain Res. 2005;135:162–168. doi: 10.1016/j.molbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 115.Caterina M.J., Rosen T.A., Tominaga M., Brake A.J., Julius D. A Capsaicin-Receptor Homologue with a High Threshold for Noxious Heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 116.Frederick J., Buck M.E., Matson D.J., Cortright D.N. Increased TRPA1, TRPM8, and TRPV2 Expression in Dorsal Root Ganglia by Nerve Injury. Biochem. Biophys. Res. Commun. 2007;358:1058–1064. doi: 10.1016/j.bbrc.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 117.Shimosato G., Amaya F., Ueda M., Tanaka Y., Decosterd I., Tanaka M. Peripheral Inflammation Induces Up-Regulation of TRPV2 Expression in Rat DRG. Pain. 2005;119:225–232. doi: 10.1016/j.pain.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 118.Saunders C.I., Kunde D.A., Crawford A., Geraghty D.P. Expression of Transient Receptor Potential Vanilloid 1 (TRPV1) and 2 (TRPV2) in Human Peripheral Blood. Mol. Immunol. 2007;44:1429–1435. doi: 10.1016/j.molimm.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 119.Santoni G., Amantini C., Maggi F., Marinelli O., Santoni M., Nabissi M., Morelli M.B. The TRPV2 Cation Channels: From Urothelial Cancer Invasiveness to Glioblastoma Multiforme Interactome Signature. Lab. Investig. 2020;100:186–198. doi: 10.1038/s41374-019-0333-7. [DOI] [PubMed] [Google Scholar]
- 120.Link T.M., Park U., Vonakis B.M., Raben D.M., Soloski M.J., Caterina M.J. TRPV2 Has a Pivotal Role in Macrophage Particle Binding and Phagocytosis. Nat. Immunol. 2010;11:232–241. doi: 10.1038/ni.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang D., Spielmann A., Wang L., Ding G., Huang F., Gu Q., Schwarz W. Mast-Cell Degranulation Induced by Physical Stimuli Involves the Activation of Transient-Receptor-Potential Channel TRPV2. Physiol. Res. 2012;61:113–124. doi: 10.33549/physiolres.932053. [DOI] [PubMed] [Google Scholar]
- 122.Iwata Y., Ohtake H., Suzuki O., Matsuda J., Komamura K., Wakabayashi S. Blockade of Sarcolemmal TRPV2 Accumulation Inhibits Progression of Dilated Cardiomyopathy. Cardiovasc. Res. 2013;99:760–768. doi: 10.1093/cvr/cvt163. [DOI] [PubMed] [Google Scholar]
- 123.Lorin C., Vögeli I., Niggli E. Dystrophic Cardiomyopathy: Role of TRPV2 Channels in Stretch-Induced Cell Damage. Cardiovasc. Res. 2015;106:153–162. doi: 10.1093/cvr/cvv021. [DOI] [PubMed] [Google Scholar]
- 124.Iwata Y., Katanosaka Y., Arai Y., Shigekawa M., Wakabayashi S. Dominant-Negative Inhibition of Ca2+ Influx via TRPV2 Ameliorates Muscular Dystrophy in Animal Models. Hum. Mol. Genet. 2009;18:84–834. doi: 10.1093/hmg/ddn408. [DOI] [PubMed] [Google Scholar]
- 125.Iwata Y., Wakabayashi S., Ito S., Kitakaze M. Production of TRPV2-Targeting Functional Antibody Ameliorating Dilated Cardiomyopathy and Muscular Dystrophy in Animal Models. Lab. Investig. 2020;100:324–337. doi: 10.1038/s41374-019-0363-1. [DOI] [PubMed] [Google Scholar]
- 126.Hisanaga E., Nagasawa M., Ueki K., Kulkarni R.N., Mori M., Kojima I. Regulation of Calcium-Permeable TRPV2 Channel by Insulin in Pancreatic β-Cells. Diabetes. 2009;58:174–184. doi: 10.2337/db08-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kanzaki M., Zhang Y.Q., Mashima H., Li L., Shibata H., Kojima I. Translocation of a Calcium-Permeable Cation Channel Induced by Insulin-like Growth Factor-I. Nat. Cell Biol. 1999;1:165–170. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]
- 128.Aoyagi K., Ohara-Imaizumi M., Nishiwaki C., Nakamichi Y., Nagamatsu S. Insulin/Phosphoinositide 3-Kinase Pathway Accelerates the Glucose-Induced First-Phase Insulin Secretion through TrpV2 Recruitment in Pancreatic β-Cells. Biochem. J. 2010;432:375–386. doi: 10.1042/BJ20100864. [DOI] [PubMed] [Google Scholar]
- 129.Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The Capsaicin Receptor: A Heat-Activated Ion Channel in the Pain Pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 130.Smith G.D., Gunthorpe M.J., Kelsell R.E., Hayes P.D., Reilly P., Facer P., Wright J.E., Jerman J.C., Walhin J.P., Ooi L., et al. TRPV3 Is a Temperature-Sensitive Vanilloid Receptor-like Protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- 131.Güler A.D., Lee H., Iida T., Shimizu I., Tominaga M., Caterina M. Heat-Evoked Activation of the Ion Channel, TRPV4. J. Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu H., Ramsey I.S., Kotecha S.A., Moran M.M., Chong J.A., Lawson D., Ge P., Lilly J., Silos-Santiago I., Xie Y., et al. TRPV3 Is a Calcium-Permeable Temperature-Sensitive Cation Channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- 133.Moqrich A., Hwang S.W., Earley T.J., Petrus M.J., Murray A.N., Spencer K.S.R., Andahazy M., Story G.M., Patapoutian A. Impaired Thermosensation in Mice Lacking TRPV3, a Heat and Camphor Sensor in the Skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- 134.Mandadi S., Sokabe T., Shibasaki K., Katanosaka K., Mizuno A., Moqrich A., Patapoutian A., Fukumi-Tominaga T., Mizumura K., Tominaga M. TRPV3 in Keratinocytes Transmits Temperature Information to Sensory Neurons via ATP. Pflugers Arch. Eur. J. Physiol. 2009;458:1093–1102. doi: 10.1007/s00424-009-0703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chung M.K., Lee H., Mizuno A., Suzuki M., Caterina M.J. TRPV3 and TRPV4 Mediate Warmth-Evoked Currents in Primary Mouse Keratinocytes. J. Biol. Chem. 2004;279:21569–21575. doi: 10.1074/jbc.M401872200. [DOI] [PubMed] [Google Scholar]
- 136.Todaka H., Taniguchi J., Satoh J.I., Mizuno A., Suzuki M. Warm Temperature-Sensitive Transient Receptor Potential Vanilloid 4 (TRPV4) Plays an Essential Role in Thermal Hyperalgesia. J. Biol. Chem. 2004;279:35133–35138. doi: 10.1074/jbc.M406260200. [DOI] [PubMed] [Google Scholar]
- 137.Watanabe H., Vriens J., Suh S.H., Benham C.D., Droogmans G., Nilius B. Heat-Evoked Activation of TRPV4 Channels in a HEK293 Cell Expression System and in Native Mouse Aorta Endothelial Cells. J. Biol. Chem. 2002;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- 138.Peier A.M., Reeve A.J., Andersson D.A., Moqrich A., Earley T.J., Hergarden A.C., Story G.M., Colley S., Hogenesch J.B., McIntyre P., et al. A Heat-Sensitive TRP Channel Expressed in Keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- 139.Xu H., Delling M., Jun J.C., Clapham D.E. Oregano, Thyme and Clove-Derived Flavors and Skin Sensitizers Activate Specific TRP Channels. Nat. Neurosci. 2006;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- 140.Bang S., Yoo S., Yang T.J., Cho H., Hwang S.W. Isopentenyl Pyrophosphate Is a Novel Antinociceptive Substance That Inhibits TRPV3 and TRPA1 Ion Channels. Pain. 2011;152:1156–1164. doi: 10.1016/j.pain.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 141.Liu X., Bandyopadhyay B., Nakamoto T., Singh B., Liedtke W., Melvin J.E., Ambudkar I. A Role for AQP5 in Activation of TRPV4 by Hypotonicity: Concerted Involvement of AQP5 and TRPV4 in Regulation of Cell Volume Recovery. J. Biol. Chem. 2006;281:15485–15495. doi: 10.1074/jbc.M600549200. [DOI] [PubMed] [Google Scholar]
- 142.Becker D., Blase C., Bereiter-Hahn J., Jendrach M. TRPV4 Exhibits a Functional Role in Cell-Volume Regulation. J. Cell Sci. 2005 doi: 10.1242/jcs.02372. [DOI] [PubMed] [Google Scholar]
- 143.Vriens J., Watanabe H., Janssens A., Droogmans G., Voets T., Nilius B. Cell Swelling, Heat, and Chemical Agonists Use Distinct Pathways for the Activation of the Cation Channel TRPV4. Proc. Natl. Acad. Sci. USA. 2004;101:396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liedtke W., Choe Y., Martí-Renom M.A., Bell A.M., Denis C.S., Šali A., Hudspeth A.J., Friedman J.M., Heller S. Vanilloid Receptor-Related Osmotically Activated Channel (VR-OAC), a Candidate Vertebrate Osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/S0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shibasaki K., Tominaga M., Ishizaki Y. Hippocampal Neuronal Maturation Triggers Post-Synaptic Clustering of Brain Temperature-Sensor TRPV4. Biochem. Biophys. Res. Commun. 2015;458:168–173. doi: 10.1016/j.bbrc.2015.01.087. [DOI] [PubMed] [Google Scholar]
- 146.Zhang L., Papadopoulos P., Hamel E. Endothelial TRPV4 Channels Mediate Dilation of Cerebral Arteries: Impairment and Recovery in Cerebrovascular Pathologies Related to Alzheimer’s Disease. Br. J. Pharmacol. 2013;170:661–670. doi: 10.1111/bph.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wissenbach U., Bödding M., Freichel M., Flockerzi V. Trp12, a Novel Trp Related Protein from Kidney. FEBS Lett. 2000;485:127–134. doi: 10.1016/S0014-5793(00)02212-2. [DOI] [PubMed] [Google Scholar]
- 148.Liedtke W., Friedman J.M. Abnormal Osmotic Regulation in Trpv4-/- Mice. Proc. Natl. Acad. Sci. USA. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Heckel E., Boselli F., Roth S., Krudewig A., Belting H.G., Charvin G., Vermot J. Oscillatory Flow Modulates Mechanosensitive Klf2a Expression through Trpv4 and Trpp2 during Heart Valve Development. Curr. Biol. 2015;25:1354–1361. doi: 10.1016/j.cub.2015.03.038. [DOI] [PubMed] [Google Scholar]
- 150.Watanabe H., Davis J.B., Smart D., Jerman J.C., Smith G.D., Hayes P., Vriens J., Cairns W., Wissenbach U., Prenen J., et al. Activation of TRPV4 Channels (HVRL-2/MTRP12) by Phorbol Derivatives. J. Biol. Chem. 2002;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- 151.Watanabe H., Vriens J., Prenen J., Droogmans G., Voets T., Nillus B. Anandamide and Arachidonic Acid Use Epoxyeicosatrienoic Acids to Activate TRPV4 Channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 152.McKemy D.D., Neuhausser W.M., Julius D. Identification of a Cold Receptor Reveals a General Role for TRP Channels in Thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 153.Peier A.M., Moqrich A., Hergarden A.C., Reeve A.J., Andersson D.A., Story G.M., Earley T.J., Dragoni I., McIntyre P., Bevan S., et al. A TRP Channel That Senses Cold Stimuli and Menthol. Cell. 2002;108:705–715. doi: 10.1016/S0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 154.Del Camino D., Murphy S., Heiry M., Barrett L.B., Earley T.J., Cook C.A., Petrus M.J., Zhao M., D’Amours M., Deering N., et al. TRPA1 Contributes to Cold Hypersensitivity. J. Neurosci. 2010;30:15165–15174. doi: 10.1523/JNEUROSCI.2580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Story G.M., Peier A.M., Reeve A.J., Eid S.R., Mosbacher J., Hricik T.R., Earley T.J., Hergarden A.C., Andersson D.A., Hwang S.W., et al. ANKTM1, a TRP-like Channel Expressed in Nociceptive Neurons, Is Activated by Cold Temperatures. Cell. 2003;112:819–829. doi: 10.1016/S0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 156.Bandell M., Story G.M., Hwang S.W., Viswanath V., Eid S.R., Petrus M.J., Earley T.J., Patapoutian A. Noxious Cold Ion Channel TRPA1 Is Activated by Pungent Compounds and Bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/S0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 157.Karashima Y., Talavera K., Everaerts W., Janssens A., Kwan K.Y., Vennekens R., Nilius B., Voets T. TRPA1 Acts as a Cold Sensor in Vitro and in Vivo. Proc. Natl. Acad. Sci. USA. 2009;106:1273–1278. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.McNamara C.R., Mandel-Brehm J., Bautista D.M., Siemens J., Deranian K.L., Zhao M., Hayward N.J., Chong J.A., Julius D., Moran M.M., et al. TRPA1 Mediates Formalin-Induced Pain. Proc. Natl. Acad. Sci. USA. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jordt S.E., Bautista D.M., Chuang H.H., McKemy D.D., Zygmunt P.M., Högestätt E.D., Meng I.D., Julius D. Mustard Oils and Cannabinoids Excite Sensory Nerve Fibres through the TRP Channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 160.Dai Y., Wang S., Tominaga M., Yamamoto S., Fukuoka T., Higashi T., Kobayashi K., Obata K., Yamanaka H., Noguchi K. Sensitization of TRPA1 by PAR2 Contributes to the Sensation of Inflammatory Pain. J. Clin. Investig. 2007;117:1979–1987. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nagata K., Duggan A., Kumar G., García-Añoveros J. Nociceptor and Hair Cell Transducer Properties of TRPA1, a Channel for Pain and Hearing. J. Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bautista D.M., Jordt S.E., Nikai T., Tsuruda P.R., Read A.J., Poblete J., Yamoah E.N., Basbaum A.I., Julius D. TRPA1 Mediates the Inflammatory Actions of Environmental Irritants and Proalgesic Agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 163.Kremeyer B., Lopera F., Cox J.J., Momin A., Rugiero F., Marsh S., Woods C.G., Jones N.G., Paterson K.J., Fricker F.R., et al. A Gain-of-Function Mutation in TRPA1 Causes Familial Episodic Pain Syndrome. Neuron. 2010;66:671–680. doi: 10.1016/j.neuron.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xiong W., Cui T., Cheng K., Yang F., Chen S.R., Willenbring D., Guan Y., Pan H.L., Ren K., Xu Y., et al. Cannabinoids Suppress Inflammatory and Neuropathic Pain by Targeting Α3 Glycine Receptors. J. Exp. Med. 2012;209:1121–1134. doi: 10.1084/jem.20120242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hejazi N., Zhou C., Oz M., Sun H., Jiang H.Y., Zhang L. Δ9-Tetrahydrocannabinol and Endogenous Cannabinoid Anandamide Directly Potentiate the Function of Glycine Receptors. Mol. Pharmacol. 2006;69:991–997. doi: 10.1124/mol.105.019174. [DOI] [PubMed] [Google Scholar]
- 166.Xiong W., Cheng K., Cui T., Godlewski G., Rice K.C., Xu Y., Zhang L. Cannabinoid Potentiation of Glycine Receptors Contributes to Cannabis-Induced Analgesia. Nat. Chem. Biol. 2011;7:296–303. doi: 10.1038/nchembio.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.O’Sullivan S.E. An Update on PPAR Activation by Cannabinoids. Br. J. Pharmacol. 2016;173:1899–1910. doi: 10.1111/bph.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Russo E.B., Burnett A., Hall B., Parker K.K. Agonistic Properties of Cannabidiol at 5-HT1a Receptors. Neurochem. Res. 2005;30:1037–1043. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- 169.Franklin J.M., Carrasco G.A. Cannabinoid-Induced Enhanced Interaction and Protein Levels of Serotonin 5-HT2A and Dopamine D2 Receptors in Rat Prefrontal Cortex. J. Psychopharmacol. 2012;26:1333–1347. doi: 10.1177/0269881112450786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Franklin J.M., Carrasco G.A. Cannabinoid Receptor Agonists Upregulate and Enhance Serotonin 2A (5-HT2A) Receptor Activity via ERK1/2 Signaling. Synapse. 2013;67:145–159. doi: 10.1002/syn.21626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hill M.N., Campolongo P., Yehuda R., Patel S. Integrating Endocannabinoid Signaling and Cannabinoids into the Biology and Treatment of Posttraumatic Stress Disorder. Neuropsychopharmacology. 2018;43:80–102. doi: 10.1038/npp.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Blessing E.M., Steenkamp M.M., Manzanares J., Marmar C.R. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics. 2015;12:825–836. doi: 10.1007/s13311-015-0387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Harrold J.A., Elliott J.C., King P.J., Widdowson P.S., Williams G. Down-Regulation of Cannabinoid-1 (CB-1) Receptors in Specific Extrahypothalamic Regions of Rats with Dietary Obesity: A Role for Endogenous Cannabinoids in Driving Appetite for Palatable Food? Brain Res. 2002;952:232–238. doi: 10.1016/S0006-8993(02)03245-6. [DOI] [PubMed] [Google Scholar]
- 174.Jamshidi N., Taylor D.A. Anandamide Administration into the Ventromedial Hypothalamus Stimulates Appetite in Rats. Br. J. Pharmacol. 2001;134:1151–1154. doi: 10.1038/sj.bjp.0704379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Portella G., Laezza C., Laccetti P., De Petrocellis L., Di Marzo V., Bifulco M. Inhibitory Effects of Cannabinoid CB1 Receptor Stimulation on Tumor Growth and Metastatic Spreading: Actions on Signals Involved in Angiogenesis and Metastasis. FASEB J. 2003;17:1771–1773. doi: 10.1096/fj.02-1129fje. [DOI] [PubMed] [Google Scholar]
- 176.Linciano P., Citti C., Luongo L., Belardo C., Maione S., Vandelli M.A., Forni F., Gigli G., Laganà A., Montone C.M., et al. Isolation of a High-Affinity Cannabinoid for the Human CB1 Receptor from a Medicinal Cannabis sativa Variety: Δ9-Tetrahydrocannabutol, the Butyl Homologue of Δ9-Tetrahydrocannabinol. J. Nat. Prod. 2020;83:88–98. doi: 10.1021/acs.jnatprod.9b00876. [DOI] [PubMed] [Google Scholar]
- 177.Wallace M.J., Blair R.E., Falenski K.W., Martin B.R., DeLorenzo R.J. The Endogenous Cannabinoid System Regulates Seizure Frequency and Duration in a Model of Temporal Lobe Epilepsy. J. Pharmacol. Exp. Ther. 2003;307:129–137. doi: 10.1124/jpet.103.051920. [DOI] [PubMed] [Google Scholar]
- 178.Murillo-Rodríguez E. The Role of the CB1 Receptor in the Regulation of Sleep. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2008;32:1420–1427. doi: 10.1016/j.pnpbp.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 179.Zagzoog A., Mohamed K.A., Kim H.J.J., Kim E.D., Frank C.S., Black T., Jadhav P.D., Holbrook L.A., Laprairie R.B. In Vitro and in Vivo Pharmacological Activity of Minor Cannabinoids Isolated from Cannabis sativa. Sci. Rep. 2020;10:20405. doi: 10.1038/s41598-020-77175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bolognini D., Costa B., Maione S., Comelli F., Marini P., Di Marzo V., Parolaro D., Ross R.A., Gauson L.A., Cascio M.G., et al. The Plant Cannabinoid Δ 9-Tetrahydrocannabivarin Can Decrease Signs of Inflammation and Inflammatory Pain in Mice. Br. J. Pharmacol. 2010;160:677–687. doi: 10.1111/j.1476-5381.2010.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Espadas I., Keifman E., Palomo-Garo C., Burgaz S., García C., Fernández-Ruiz J., Moratalla R. Beneficial Effects of the Phytocannabinoid Δ9-THCV in L-DOPA-Induced Dyskinesia in Parkinson’s Disease. Neurobiol. Dis. 2020;141:104892. doi: 10.1016/j.nbd.2020.104892. [DOI] [PubMed] [Google Scholar]
- 182.Wargent E.T., Zaibi M.S., Silvestri C., Hislop D.C., Stocker C.J., Stott C.G., Guy G.W., Duncan M., Di Marzo V., Cawthorne M.A. The Cannabinoid Δ9-Tetrahydrocannabivarin (THCV) Ameliorates Insulin Sensitivity in Two Mouse Models of Obesity. Nutr. Diabetes. 2013;3:e68. doi: 10.1038/nutd.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hill A.J., Weston S.E., Jones N.A., Smith I., Bevan S.A., Williamson E.M., Stephens G.J., Williams C.M., Whalley B.J. 9-Tetrahydrocannabivarin Suppresses in Vitro Epileptiform and in Vivo Seizure Activity in Adult Rats. Epilepsia. 2010;51:1522–1532. doi: 10.1111/j.1528-1167.2010.02523.x. [DOI] [PubMed] [Google Scholar]
- 184.Abrahamov A., Abrahamov A., Mechoulam R. An Efficient New Cannabinoid Antiemetic in Pediatric Oncology. Life Sci. 1995;56:2097–2102. doi: 10.1016/0024-3205(95)00194-B. [DOI] [PubMed] [Google Scholar]
- 185.Darmani N.A. Δ9-Tetrahydrocannabinol and Synthetic Cannabinoids Prevent Emesis Produced by the Cannabinoid CB1 Receptor Antagonist/Inverse Agonist SR 141716A. Neuropsychopharmacology. 2001;24:198–203. doi: 10.1016/S0893-133X(00)00197-4. [DOI] [PubMed] [Google Scholar]
- 186.Darmani N.A. The Cannabinoid CB1 Receptor Antagonist SR 141716A Reverses the Antiemetic and Motor Depressant Actions of WIN 55, 212–2. Eur. J. Pharmacol. 2001;430:49–58. doi: 10.1016/S0014-2999(01)01355-3. [DOI] [PubMed] [Google Scholar]
- 187.Darmani N.A., Sim-Selley L.J., Martin B.R., Janoyan J.J., Crim J.L., Parekh B., Breivogel C.S. Antiemetic and Motor-Depressive Actions of CP55,940: Cannabinoid CB1 Receptor Characterization, Distribution, and G-Protein Activation. Eur. J. Pharmacol. 2003;459:83–95. doi: 10.1016/S0014-2999(02)02815-7. [DOI] [PubMed] [Google Scholar]
- 188.Darmani N.A., Janoyan J.J., Crim J., Ramirez J. Receptor Mechanism and Antiemetic Activity of Structurally-Diverse Cannabinoids against Radiation-Induced Emesis in the Least Shrew. Eur. J. Pharmacol. 2007;563:187–196. doi: 10.1016/j.ejphar.2007.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lastres-Becker I., Cebeira M., De Ceballos M.L., Zeng B.Y., Jenner P., Ramos J.A., Fernández-Ruiz J.J. Increased Cannabinoid CB1 Receptor Binding and Activation of GTP-Binding Proteins in the Basal Ganglia of Patients with Parkinson’s Syndrome and of MPTP-Treated Marmosets. Eur. J. Neurosci. 2001;14:1827–1832. doi: 10.1046/j.0953-816x.2001.01812.x. [DOI] [PubMed] [Google Scholar]
- 190.Sarfaraz S., Afaq F., Adhami V.M., Mukhtar H. Cannabinoid Receptor as a Novel Target for the Treatment of Prostate Cancer. Cancer Res. 2005;65:1635–1641. doi: 10.1158/0008-5472.CAN-04-3410. [DOI] [PubMed] [Google Scholar]
- 191.Qamri Z., Preet A., Nasser M.W., Bass C.E., Leone G., Barsky S.H., Ganju R.K. Synthetic Cannabinoid Receptor Agonists Inhibit Tumor Growth and Metastasis of Breast Cancer. Mol. Cancer Ther. 2009;8:3117–3129. doi: 10.1158/1535-7163.MCT-09-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Preet A., Qamri Z., Nasser M.W., Prasad A., Shilo K., Zou X., Groopman J.E., Ganju R.K. Cannabinoid Receptors, CB1 and CB2, as Novel Targets for Inhibition of Non-Small Cell Lung Cancer Growth and Metastasis. Cancer Prev. Res. 2011;4:65–75. doi: 10.1158/1940-6207.CAPR-10-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Izzo A.A., Capasso R., Aviello G., Borrelli F., Romano B., Piscitelli F., Gallo L., Capasso F., Orlando P., Di Marzo V. Inhibitory Effect of Cannabichromene, a Major Non-Psychotropic Cannabinoid Extracted from Cannabis sativa, on Inflammation-Induced Hypermotility in Mice. Br. J. Pharmacol. 2012;166:1444–1460. doi: 10.1111/j.1476-5381.2012.01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Borrelli F., Fasolino I., Romano B., Capasso R., Maiello F., Coppola D., Orlando P., Battista G., Pagano E., Di Marzo V., et al. Beneficial Effect of the Non-Psychotropic Plant Cannabinoid Cannabigerol on Experimental Inflammatory Bowel Disease. Biochem. Pharmacol. 2013;85:1306–1316. doi: 10.1016/j.bcp.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 195.Gómez-Gálvez Y., Palomo-Garo C., Fernández-Ruiz J., García C. Potential of the Cannabinoid CB2 Receptor as a Pharmacological Target against Inflammation in Parkinson’s Disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;64:200–208. doi: 10.1016/j.pnpbp.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 196.Javed H., Azimullah S., Haque M.E., Ojha S.K. Cannabinoid Type 2 (CB2) Receptors Activation Protects against Oxidative Stress and Neuroinflammation Associated Dopaminergic Neurodegeneration in Rotenone Model of Parkinson’s Disease. Front. Neurosci. 2016;10:321. doi: 10.3389/fnins.2016.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Vigli D., Cosentino L., Raggi C., Laviola G., Woolley-Roberts M., De Filippis B. Chronic Treatment with the Phytocannabinoid Cannabidivarin (CBDV) Rescues Behavioural Alterations and Brain Atrophy in a Mouse Model of Rett Syndrome. Neuropharmacology. 2018;140:121–129. doi: 10.1016/j.neuropharm.2018.07.029. [DOI] [PubMed] [Google Scholar]
- 198.Anavi-Goffer S., Baillie G., Irving A.J., Gertsch J., Greig I.R., Pertwee R.G., Ross R.A. Modulation of L-α-Lysophosphatidylinositol/GPR55 Mitogen-Activated Protein Kinase (MAPK) Signaling by Cannabinoids. J. Biol. Chem. 2012;287:91–104. doi: 10.1074/jbc.M111.296020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Iannotti F.A., Hill C.L., Leo A., Alhusaini A., Soubrane C., Mazzarella E., Russo E., Whalley B.J., Di Marzo V., Stephens G.J. Nonpsychotropic Plant Cannabinoids, Cannabidivarin (CBDV) and Cannabidiol (CBD), Activate and Desensitize Transient Receptor Potential Vanilloid 1 (TRPV1) Channels in Vitro: Potential for the Treatment of Neuronal Hyperexcitability. ACS Chem. Neurosci. 2014;5:1131–1141. doi: 10.1021/cn5000524. [DOI] [PubMed] [Google Scholar]
- 200.De Petrocellis L., Ligresti A., Moriello A.S., Allarà M., Bisogno T., Petrosino S., Stott C.G., Di Marzo V. Effects of Cannabinoids and Cannabinoid-Enriched Cannabis Extracts on TRP Channels and Endocannabinoid Metabolic Enzymes. Br. J. Pharmacol. 2011;163:1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.De Petrocellis L., Orlando P., Moriello A.S., Aviello G., Stott C., Izzo A.A., di Marzo V. Cannabinoid Actions at TRPV Channels: Effects on TRPV3 and TRPV4 and Their Potential Relevance to Gastrointestinal Inflammation. Acta Physiol. 2012;204:255–266. doi: 10.1111/j.1748-1716.2011.02338.x. [DOI] [PubMed] [Google Scholar]
- 202.De Petrocellis L., Vellani V., Schiano-Moriello A., Marini P., Magherini P.C., Orlando P., Di Marzo V. Plant-Derived Cannabinoids Modulate the Activity of Transient Receptor Potential Channels of Ankyrin Type-1 and Melastatin Type-8. J. Pharmacol. Exp. Ther. 2008;325:1007–1015. doi: 10.1124/jpet.107.134809. [DOI] [PubMed] [Google Scholar]
- 203.Borrelli F., Pagano E., Romano B., Panzera S., Maiello F., Coppola D., De Petrocellis L., Buono L., Orlando P., Izzo A.A. Colon Carcinogenesis Is Inhibited by the TRPM8 Antagonist Cannabigerol, a Cannabis-Derived Non-Psychotropic Cannabinoid. Carcinogenesis. 2014;35:2787–2797. doi: 10.1093/carcin/bgu205. [DOI] [PubMed] [Google Scholar]
- 204.Pagano E., Romano B., Iannotti F.A., Parisi O.A., D’Armiento M., Pignatiello S., Coretti L., Lucafò M., Venneri T., Stocco G., et al. The Non-Euphoric Phytocannabinoid Cannabidivarin Counteracts Intestinal Inflammation in Mice and Cytokine Expression in Biopsies from UC Pediatric Patients. Pharmacol. Res. 2019;149:104464. doi: 10.1016/j.phrs.2019.104464. [DOI] [PubMed] [Google Scholar]
- 205.Iannotti F.A., Pagano E., Moriello A.S., Alvino F.G., Sorrentino N.C., D’Orsi L., Gazzerro E., Capasso R., De Leonibus E., De Petrocellis L., et al. Effects of Non-Euphoric Plant Cannabinoids on Muscle Quality and Performance of Dystrophic Mdx Mice. Br. J. Pharmacol. 2019;176:1568–1584. doi: 10.1111/bph.14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.García-Arencibia M., González S., de Lago E., Ramos J.A., Mechoulam R., Fernández-Ruiz J. Evaluation of the Neuroprotective Effect of Cannabinoids in a Rat Model of Parkinson’s Disease: Importance of Antioxidant and Cannabinoid Receptor-Independent Properties. Brain Res. 2007;1134:162–170. doi: 10.1016/j.brainres.2006.11.063. [DOI] [PubMed] [Google Scholar]
- 207.Di Marzo V. Enhanced Levels of Endogenous Cannabinoids in the Globus Pallidus Are Associated with a Reduction in Movement in an Animal Model of Parkinson’s Disease. FASEB J. 2000;14:1432–1438. doi: 10.1096/fasebj.14.10.1432. [DOI] [PubMed] [Google Scholar]
- 208.Fox S.H., Henry B., Hill M., Crossman A., Brotchie J. Stimulation of Cannabinoid Receptors Reduces Levodopa-Induced Dyskinesia in the MPTP-Lesioned Nonhuman Primate Model of Parkinson’s Disease. Mov. Disord. 2002;17:1180–1187. doi: 10.1002/mds.10289. [DOI] [PubMed] [Google Scholar]
- 209.Morgese M.G., Cassano T., Cuomo V., Giuffrida A. Anti-Dyskinetic Effects of Cannabinoids in a Rat Model of Parkinson’s Disease: Role of CB1 and TRPV1 Receptors. Exp. Neurol. 2007;208:110–119. doi: 10.1016/j.expneurol.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sañudo-Peña M.C., Patrick S.L., Khen S., Patrick R.L., Tsou K., Walker J.M. Cannabinoid Effects in Basal Ganglia in a Rat Model of Parkinson’s Disease. Neurosci. Lett. 1998;248:171–174. doi: 10.1016/S0304-3940(98)00368-1. [DOI] [PubMed] [Google Scholar]
- 211.Donadelli M., Dando I., Zaniboni T., Costanzo C., Dalla Pozza E., Scupoli M.T., Scarpa A., Zappavigna S., Marra M., Abbruzzese A., et al. Gemcitabine/Cannabinoid Combination Triggers Autophagy in Pancreatic Cancer Cells through a ROS-Mediated Mechanism. Cell Death Dis. 2011;2:E152. doi: 10.1038/cddis.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Afrin F., Chi M., Eamens A.L., Duchatel R.J., Douglas A.M., Schneider J., Gedye C., Woldu A.S., Dun M.D. Can Hemp Help? Low-THC Cannabis and Non-THC Cannabinoids for the Treatment of Cancer. Cancers. 2020;12 doi: 10.3390/cancers12041033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.De Petrocellis L., Melck D., Palmisano A., Bisogno T., Laezza C., Bifulco M., Di Marzo V. The Endogenous Cannabinoid Anandamide Inhibits Human Breast Cancer Cell Proliferation. Proc. Natl. Acad. Sci. USA. 1998;95:8375–8380. doi: 10.1073/pnas.95.14.8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cianchi F., Papucci L., Schiavone N., Lulli M., Magnelli L., Vinci M.C., Messerini L., Manera C., Ronconi E., Romagnani P., et al. Cannabinoid Receptor Activation Induces Apoptosis through Tumor Necrosis Factor α-Mediated Ceramide de Novo Synthesis in Colon Cancer Cells. Clin. Cancer Res. 2008;14:7691–7700. doi: 10.1158/1078-0432.CCR-08-0799. [DOI] [PubMed] [Google Scholar]
- 215.Blázquez C., Casanova M.L., Planas A., Del Pulgar T.G., Villanueva C., Fernández-Aceñero M.J., Aragonés J., Huffman J.W., Jorcano J.L., Guzmán M. Inhibition of Tumor Angiogenesis by Cannabinoids. FASEB J. 2003;17:529–531. doi: 10.1096/fj.02-0795fje. [DOI] [PubMed] [Google Scholar]
- 216.Cunha J.M., Carlini E.A., Pereira A.E., Ramos O.L., Pimentel C., Gagliardi R., Sanvito W.L., Lander N., Mechoulam R. Chronic Administration of Cannabidiol to Healthy Volunteers and Epileptic Patients. Pharmacology. 1980;21:175–185. doi: 10.1159/000137430. [DOI] [PubMed] [Google Scholar]
- 217.Rosenberg E.C., Tsien R.W., Whalley B.J., Devinsky O. Cannabinoids and Epilepsy. Neurotherapeutics. 2015;12:747–768. doi: 10.1007/s13311-015-0375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Stockings E., Zagic D., Campbell G., Weier M., Hall W.D., Nielsen S., Herkes G.K., Farrell M., Degenhardt L. Evidence for Cannabis and Cannabinoids for Epilepsy: A Systematic Review of Controlled and Observational Evidence. J. Neurol. Neurosurg. Psychiatry. 2018;89:741–753. doi: 10.1136/jnnp-2017-317168. [DOI] [PubMed] [Google Scholar]
- 219.Karanian D.A., Karim S.L., Wood J.A.T., Williams J.S., Lin S., Makriyannis A., Bahr B.A. Endocannabinoid Enhancement Protects against Kainic Acid-Induced Seizures and Associated Brain Damage. J. Pharmacol. Exp. Ther. 2007;322:1059–1066. doi: 10.1124/jpet.107.120147. [DOI] [PubMed] [Google Scholar]
- 220.Naidoo V., Karanian D.A., Vadivel S.K., Locklear J.R., Wood J.A.T., Nasr M., Quizon P.M.P., Graves E.E., Shukla V., Makriyannis A., et al. Equipotent Inhibition of Fatty Acid Amide Hydrolase and Monoacylglycerol Lipase—Dual Targets of the Endocannabinoid System to Protect against Seizure Pathology. Neurotherapeutics. 2012;9:801–813. doi: 10.1007/s13311-011-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Devinsky O., Patel A.D., Cross J.H., Villanueva V., Wirrell E.C., Privitera M., Greenwood S.M., Roberts C., Checketts D., VanLandingham K.E., et al. Effect of Cannabidiol on Drop Seizures in the Lennox–Gastaut Syndrome. N. Engl. J. Med. 2018;378:1888–1897. doi: 10.1056/NEJMoa1714631. [DOI] [PubMed] [Google Scholar]
- 222.Devinsky O., Cross J.H., Laux L., Marsh E., Miller I., Nabbout R., Scheffer I.E., Thiele E.A., Wright S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017;376:2011–2020. doi: 10.1056/NEJMoa1611618. [DOI] [PubMed] [Google Scholar]
- 223.Press C.A., Knupp K.G., Chapman K.E. Parental Reporting of Response to Oral Cannabis Extracts for Treatment of Refractory Epilepsy. Epilepsy Behav. 2015;45:49–52. doi: 10.1016/j.yebeh.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 224.Guggenhuber S., Monory K., Lutz B., Klugmann M. AAV Vector-Mediated Overexpression of CB1 Cannabinoid Receptor in Pyramidal Neurons of the Hippocampus Protects against Seizure-Induced Excitoxicity. PLoS ONE. 2010;5:e15707. doi: 10.1371/journal.pone.0015707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Riva N., Mora G., Sorarù G., Lunetta C., Ferraro O.E., Falzone Y., Leocani L., Fazio R., Comola M., Comi G., et al. Safety and Efficacy of Nabiximols on Spasticity Symptoms in Patients with Motor Neuron Disease (CANALS): A Multicentre, Double-Blind, Randomised, Placebo-Controlled, Phase 2 Trial. Lancet Neurol. 2019;18:155–164. doi: 10.1016/S1474-4422(18)30406-X. [DOI] [PubMed] [Google Scholar]
- 226.Beal J.E., Olson R., Laubenstein L., Morales J.O., Bellman P., Yangco B., Lefkowitz L., Plasse T.F., Shepard K.V. Dronabinol as a Treatment for Anorexia Associated with Weight Loss in Patients with AIDS. J. Pain Symptom Manag. 1995;10:89–97. doi: 10.1016/0885-3924(94)00117-4. [DOI] [PubMed] [Google Scholar]
- 227.Foltin R.W., Fischman M.W., Byrne M.F. Effects of Smoked Marijuana on Food Intake and Body Weight of Humans Living in a Residential Laboratory. Appetite. 1988;11:1–14. doi: 10.1016/S0195-6663(88)80017-5. [DOI] [PubMed] [Google Scholar]
- 228.Mattes R.D., Engelman K., Shaw L.M., Elsohly M.A. Cannabinoids and Appetite Stimulation. Pharmacol. Biochem. Behav. 1994;49:187–195. doi: 10.1016/0091-3057(94)90475-8. [DOI] [PubMed] [Google Scholar]
- 229.Williams C.M., Rogers P.J., Kirkham T.C. Hyperphagia in Pre-Fed Rats Following Oral Δ9-THC. Physiol. Behav. 1998;65:343–346. doi: 10.1016/S0031-9384(98)00170-X. [DOI] [PubMed] [Google Scholar]
- 230.Feinberg I., Jones R., Walker J.M., Cavness C., March J. Effects of High Dosage Delta-9-Tetrahydrocannabinol on Sleep Patterns in Man. Clin. Pharmacol. Ther. 1975;14:458–466. doi: 10.1002/cpt1975174458. [DOI] [PubMed] [Google Scholar]
- 231.Freemon F.R. The Effect of Chronically Administered Delta-9-Tetrahydrocannabinol upon the Polygraphically Monitored Sleep of Normal Volunteers. Drug Alcohol Depend. 1982;10:345–353. doi: 10.1016/0376-8716(82)90036-9. [DOI] [PubMed] [Google Scholar]
- 232.Pivik R.T., Zarcone V., Dement W.C., Hollister L.E. Delta-9-Tetrahydrocannabinol and Synhexl: Effects on Human Sleep Patterns. Clin. Pharmacol. Ther. 1972;13:426–435. doi: 10.1002/cpt1972133426. [DOI] [PubMed] [Google Scholar]
- 233.Agarwal N., Pacher P., Tegeder I., Amaya F., Constantin C.E., Brenner G.J., Rubino T., Michalski C.W., Marsicano G., Monory K., et al. Cannabinoids Mediate Analgesia Largely via Peripheral Type 1 Cannabinoid Receptors in Nociceptors. Nat. Neurosci. 2007;10:870–879. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Meng I.D., Manning B.H., Martin W.J., Fields H.L. An Analgesia Circuit Activated by Cannabinoids. Nature. 1998;395:381–383. doi: 10.1038/26481. [DOI] [PubMed] [Google Scholar]
- 235.Ottani A., Leone S., Sandrini M., Ferrari A., Bertolini A. The Analgesic Activity of Paracetamol Is Prevented by the Blockade of Cannabinoid CB1 Receptors. Eur. J. Pharmacol. 2006;531:280–281. doi: 10.1016/j.ejphar.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 236.Walker J.M., Hohmann A.G., Martin W.J., Strangman N.M., Huang S.M., Tsou K. The Neurobiology of Cannabinoid Analgesia. Life Sci. 1999;65:665–673. doi: 10.1016/S0024-3205(99)00289-1. [DOI] [PubMed] [Google Scholar]
- 237.Leweke F.M., Piomelli D., Pahlisch F., Muhl D., Gerth C.W., Hoyer C., Klosterkötter J., Hellmich M., Koethe D. Cannabidiol Enhances Anandamide Signaling and Alleviates Psychotic Symptoms of Schizophrenia. Transl. Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lutz B., Marsicano G., Maldonado R., Hillard C.J. The Endocannabinoid System in Guarding against Fear, Anxiety and Stress. Nat. Rev. Neurosci. 2015;16:705–718. doi: 10.1038/nrn4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Navarro M., Hernández E., Muñoz R.M., Del Arco I., Villanúa M.A., Carrera M.R.A., Rodríguez De Fonseca F. Acute Administration of the CB1 Cannabinoid Receptor Antagonist SR 141716A Induces Anxiety-like Responses in the Rat. Neuroreport. 1997;8:491–496. doi: 10.1097/00001756-199701200-00023. [DOI] [PubMed] [Google Scholar]
- 240.Moreira F.A., Aguiar D.C., Guimarães F.S. Anxiolytic-like Effect of Cannabinoids Injected into the Rat Dorsolateral Periaqueductal Gray. Neuropharmacology. 2007;52:958–965. doi: 10.1016/j.neuropharm.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 241.Rey A.A., Purrio M., Viveros M.P., Lutz B. Biphasic Effects of Cannabinoids in Anxiety Responses: CB1 and GABA B Receptors in the Balance of Gabaergic and Glutamatergic Neurotransmission. Neuropsychopharmacology. 2012;37:2624–2634. doi: 10.1038/npp.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ney L.J., Matthews A., Bruno R., Felmingham K.L. Modulation of the Endocannabinoid System by Sex Hormones: Implications for Posttraumatic Stress Disorder. Neurosci. Biobehav. Rev. 2018;94:302–320. doi: 10.1016/j.neubiorev.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 243.Carter G.T., Flanagan A.M., Earleywine M., Abrams D.I., Aggarwal S.K., Grinspoon L. Cannabis in Palliative Medicine: Improving Care and Reducing Opioid-Related Morbidity. Am. J. Hosp. Palliat. Med. 2011;28:297–303. doi: 10.1177/1049909111402318. [DOI] [PubMed] [Google Scholar]
- 244.Bar-Sela G., Vorobeichik M., Drawsheh S., Omer A., Goldberg V., Muller E. The Medical Necessity for Medicinal Cannabis: Prospective, Observational Study Evaluating the Treatment in Cancer Patients on Supportive or Palliative Care. Evid.-Based Complement. Altern. Med. 2013;2013:510392. doi: 10.1155/2013/510392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Motwani M.P., Bennett F., Norris P.C., Maini A.A., George M.J., Newson J., Henderson A., Hobbs A.J., Tepper M., White B., et al. Potent Anti-Inflammatory and Pro-Resolving Effects of Anabasum in a Human Model of Self-Resolving Acute Inflammation. Clin. Pharmacol. Ther. 2018;104:675–686. doi: 10.1002/cpt.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lucas P. Rationale for Cannabis-Based Interventions in the Opioid Overdose Crisis. Harm Reduct. J. 2017;14:58. doi: 10.1186/s12954-017-0183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Boehnke K.F., Litinas E., Clauw D.J. Medical Cannabis Use Is Associated with Decreased Opiate Medication Use in a Retrospective Cross-Sectional Survey of Patients with Chronic Pain. J. Pain. 2016;17:739–744. doi: 10.1016/j.jpain.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 248.Groce E. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. National Academies Press; Washington, DC, USA: 2017. [DOI] [PubMed] [Google Scholar]
- 249.Dos Santos R.G., Guimarães F.S., Crippa J.A.S., Hallak J.E.C., Rossi G.N., Rocha J.M., Zuardi A.W. Serious Adverse Effects of Cannabidiol (CBD): A Review of Randomized Controlled Trials. Expert Opin. Drug Metab. Toxicol. 2020;16:517–526. doi: 10.1080/17425255.2020.1754793. [DOI] [PubMed] [Google Scholar]
- 250.White C.M. A Review of Human Studies Assessing Cannabidiol’s (CBD) Therapeutic Actions and Potential. J. Clin. Pharmacol. 2019;59:923–934. doi: 10.1002/jcph.1387. [DOI] [PubMed] [Google Scholar]
- 251.Pauli C.S., Conroy M., Vanden Heuvel B.D., Park S.H. Cannabidiol Drugs Clinical Trial Outcomes and Adverse Effects. Front. Pharmacol. 2020;11:63. doi: 10.3389/fphar.2020.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kuhathasan N., Dufort A., MacKillop J., Gottschalk R., Minuzzi L., Frey B.N. The Use of Cannabinoids for Sleep: A Critical Review on Clinical Trials. Exp. Clin. Psychopharmacol. 2019;27:383–401. doi: 10.1037/pha0000285. [DOI] [PubMed] [Google Scholar]
- 253.Black N., Stockings E., Campbell G., Tran L.T., Zagic D., Hall W.D., Farrell M., Degenhardt L. Cannabinoids for the Treatment of Mental Disorders and Symptoms of Mental Disorders: A Systematic Review and Meta-Analysis. Lancet Psychiatry. 2019;6:995–1010. doi: 10.1016/S2215-0366(19)30401-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Marks M.D., Tian L., Wenger J.P., Omburo S.N., Soto-Fuentes W., He J., Gang D.R., Weiblen G.D., Dixon R.A. Identification of Candidate Genes Affecting Δ9-Tetrahydrocannabinol Biosynthesis in Cannabis sativa. J. Exp. Bot. 2009;60:3715–3726. doi: 10.1093/jxb/erp210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Stout J.M., Boubakir Z., Ambrose S.J., Purves R.W., Page J.E. The Hexanoyl-CoA Precursor for Cannabinoid Biosynthesis Is Formed by an Acyl-Activating Enzyme in Cannabis sativa Trichomes. Plant J. 2012;71:353–365. doi: 10.1111/j.1365-313X.2012.04949.x. [DOI] [PubMed] [Google Scholar]
- 256.Kim E.-S., Mahlberg P.G. Secretory Cavity Development in Glandular Trichomes of Cannabis sativa L. (Cannabaceae) Am. J. Bot. 1991;78:220–229. doi: 10.1002/j.1537-2197.1991.tb15749.x. [DOI] [Google Scholar]
- 257.Kim E.S., Mahlberg P.G. Secretory Vesicle Formation in the Secretory Cavity of Glandular Trichomes of Cannabis sativa L. (Cannabaceae) Mol. Cells. 2003;15:387–395. doi: 10.1142/S0578563403000816. [DOI] [PubMed] [Google Scholar]
- 258.Mahlberg P.G., Eun S.K. Accumulation of Cannabinoids in Glandular Trichomes of Cannabis (Cannabaceae) J. Ind. Hemp. 2004;9:15–36. doi: 10.1300/J237v09n01_04. [DOI] [Google Scholar]
- 259.Mahlberg P.G., Kim E.-S. Cuticle Development on Glandular Trichomes of Cannabis sativa (Cannabaceae) Am. J. Bot. 1991;78:1113–1122. doi: 10.1002/j.1537-2197.1991.tb14518.x. [DOI] [Google Scholar]
- 260.Arigoni D., Sagner S., Latzel C., Eisenreich W., Bacher A., Zenk M.H. Terpenoid Biosynthesis from 1-Deoxy-D-Xylulose in Higher Plants by Intramolecular Skeletal Rearrangement. Proc. Natl. Acad. Sci. USA. 1997;94:10600–10605. doi: 10.1073/pnas.94.20.10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Fellermeier M., Eisenreich W., Bacher A., Zenk M.H. Biosynthesis of Cannabinoids: Incorporation Experiments with 13C-Labeled Glucoses. Eur. J. Biochem. 2001;268:1596–1604. doi: 10.1046/j.1432-1327.2001.02030.x. [DOI] [PubMed] [Google Scholar]
- 262.Schwender J., Zeidler J., Gröner R., Müller C., Focke M., Braun S., Lichtenthaler F.W., Lichtenthaler H.K. Incorporation of 1-Deoxy-D-Xylulose into Isoprene and Phytol by Higher Plants and Algae. FEBS Lett. 1997 doi: 10.1016/S0014-5793(97)01002-8. [DOI] [PubMed] [Google Scholar]
- 263.Botella-Pavía P., Besumbes Ó., Phillips M.A., Carretero-Paulet L., Boronat A., Rodríguez-Concepción M. Regulation of Carotenoid Biosynthesis in Plants: Evidence for a Key Role of Hydroxymethylbutenyl Diphosphate Reductase in Controlling the Supply of Plastidial Isoprenoid Precursors. Plant J. 2004;40:188–199. doi: 10.1111/j.1365-313X.2004.02198.x. [DOI] [PubMed] [Google Scholar]
- 264.Phillips M.A., León P., Boronat A., Rodríguez-Concepción M. The Plastidial MEP Pathway: Unified Nomenclature and Resources. Trends Plant Sci. 2008;13:619–623. doi: 10.1016/j.tplants.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 265.Hsieh M.H., Chang C.Y., Hsu S.J., Chen J.J. Chloroplast Localization of Methylerythritol 4-Phosphate Pathway Enzymes and Regulation of Mitochondrial Genes in IspD and IspE Albino Mutants in Arabidopsis. Plant Mol. Biol. 2008;66:663–673. doi: 10.1007/s11103-008-9297-5. [DOI] [PubMed] [Google Scholar]
- 266.Bick J.A., Lange B.M. Metabolic Cross Talk between Cytosolic and Plastidial Pathways of Isoprenoid Biosynthesis: Unidirectional Transport of Intermediates across the Chloroplast Envelope Membrane. Arch. Biochem. Biophys. 2003;415:146–154. doi: 10.1016/S0003-9861(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 267.Buhaescu I., Izzedine H. Mevalonate Pathway: A Review of Clinical and Therapeutical Implications. Clin. Biochem. 2007;40:575–584. doi: 10.1016/j.clinbiochem.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 268.Goldstein J.L., Brown M.S. Regulation of the Mevalonate Pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 269.Miziorko H.M. Enzymes of the Mevalonate Pathway of Isoprenoid Biosynthesis. Arch. Biochem. Biophys. 2011;505:131–143. doi: 10.1016/j.abb.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Guirimand G., Simkin A.J., Papon N., Besseau S., Burlat V., St-Pierre B., Giglioli-Guivarc’h N., Clastre M., Courdavault V. Cycloheximide as a Tool to Investigate Protein Import in Peroxisomes: A Case Study of the Subcellular Localization of Isoprenoid Biosynthetic Enzymes. J. Plant Physiol. 2012;169:825–829. doi: 10.1016/j.jplph.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 271.Simkin A.J., Guirimand G., Papon N., Courdavault V., Thabet I., Ginis O., Bouzid S., Giglioli-Guivarc’h N., Clastre M. Peroxisomal Localisation of the Final Steps of the Mevalonic Acid Pathway in Planta. Planta. 2011;234:903–914. doi: 10.1007/s00425-011-1444-6. [DOI] [PubMed] [Google Scholar]
- 272.Vranová E., Coman D., Gruissem W. Network Analysis of the MVA and MEP Pathways for Isoprenoid Synthesis. Annu. Rev. Plant Biol. 2013;64:665–700. doi: 10.1146/annurev-arplant-050312-120116. [DOI] [PubMed] [Google Scholar]
- 273.Thabet I., Guirimand G., Courdavault V., Papon N., Godet S., Dutilleul C., Bouzid S., Giglioli-Guivarc’h N., Clastre M., Simkin A.J. The Subcellular Localization of Periwinkle Farnesyl Diphosphate Synthase Provides Insight into the Role of Peroxisome in Isoprenoid Biosynthesis. J. Plant Physiol. 2011;168:2110–2116. doi: 10.1016/j.jplph.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 274.Campbell M., Hahn F.M., Poulter C.D., Leustek T. Analysis of the Isopentenyl Disphosphate Isomerase Gene from Arabidopsis thaliana. Plant Mol. Biol. 1998;36:323–328. doi: 10.1023/A:1005935516274. [DOI] [PubMed] [Google Scholar]
- 275.Okada K., Kasahara H., Yamaguchi S., Kawaide H., Kamiya Y., Nojiri H., Yamane H. Genetic Evidence for the Role of Isopentenyl Diphosphate Isomerases in the Mevalonate Pathway and Plant Development in Arabidopsis. Plant Cell Physiol. 2008;49:604–616. doi: 10.1093/pcp/pcn032. [DOI] [PubMed] [Google Scholar]
- 276.Sapir-Mir M., Mett A., Belausov E., Tal-Meshulam S., Frydman A., Gidoni D., Eya Y. Peroxisomal Localization of Arabidopsis Isopentenyl Diphosphate Isomerases Suggests That Part of the Plant Isoprenoid Mevalonic Acid Pathway Is Compartmentalized to Peroxisomes. Plant Physiol. 2008;148:1219–1228. doi: 10.1104/pp.108.127951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Burke C.C., Wildung M.R., Croteau R. Geranyl Diphosphate Synthase: Cloning, Expression, and Characterization of This Prenyltransferase as a Heterodimer. Proc. Natl. Acad. Sci. USA. 1999;96:13062–13067. doi: 10.1073/pnas.96.23.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Bouvier F., Suire C., D’Harlingue A., Backhaus R.A., Camara B. Molecular Cloning of Geranyl Diphosphate Synthase and Compartmentation of Monoterpene Synthesis in Plant Cells. Plant J. 2000;24:241–252. doi: 10.1046/j.1365-313x.2000.00875.x. [DOI] [PubMed] [Google Scholar]
- 279.Ogura K., Koyama T. Enzymatic Aspects of Isoprenoid Chain Elongation. Chem. Rev. 1998;98:1263–1276. doi: 10.1021/cr9600464. [DOI] [PubMed] [Google Scholar]
- 280.Ohnuma S.I., Hirooka K., Tsuruoka N., Yano M., Ohto C., Nakane H., Nishino T. A Pathway Where Polyprenyl Diphosphate Elongates in Prenyltransferase: Insight into a Common Mechanism of Chain Length Determination of Prenyltransferases. J. Biol. Chem. 1998;273:26705–26713. doi: 10.1074/jbc.273.41.26705. [DOI] [PubMed] [Google Scholar]
- 281.Wang K., Ohnuma S.I. Chain-Length Determination Mechanism of Isoprenyl Diphosphate Synthases and Implications for Molecular Evolution. Trends Biochem. Sci. 1999;24:445–451. doi: 10.1016/S0968-0004(99)01464-4. [DOI] [PubMed] [Google Scholar]
- 282.Booth J.K., Bohlmann J. Terpenes in Cannabis sativa—From Plant Genome to Humans. Plant Sci. 2019;284:67–72. doi: 10.1016/j.plantsci.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 283.Oldfield E., Lin F.Y. Terpene Biosynthesis: Modularity Rules. Angew. Chem. Int. Ed. Engl. 2012;51:1124–1137. doi: 10.1002/anie.201103110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Gagne S.J., Stout J.M., Liu E., Boubakir Z., Clark S.M., Page J.E. Identification of Olivetolic Acid Cyclase from Cannabis sativa Reveals a Unique Catalytic Route to Plant Polyketides. Proc. Natl. Acad. Sci. USA. 2012;109:12811–12816. doi: 10.1073/pnas.1200330109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Taura F., Tanaka S., Taguchi C., Fukamizu T., Tanaka H., Shoyama Y., Morimoto S. Characterization of Olivetol Synthase, a Polyketide Synthase Putatively Involved in Cannabinoid Biosynthetic Pathway. FEBS Lett. 2009;583:2061–2066. doi: 10.1016/j.febslet.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 286.Fellermeier M., Zenk M.H. Prenylation of Olivetolate by a Hemp Transferase Yields Cannabigerolic Acid, the Precursor of Tetrahydrocannabinol. FEBS Lett. 1998;427:283–285. doi: 10.1016/S0014-5793(98)00450-5. [DOI] [PubMed] [Google Scholar]
- 287.Luo X., Reiter M.A., D’Espaux L., Wong J., Denby C.M., Lechner A., Zhang Y., Grzybowski A.T., Harth S., Lin W., et al. Complete Biosynthesis of Cannabinoids and Their Unnatural Analogues in Yeast. Nature. 2019;567:123–126. doi: 10.1038/s41586-019-0978-9. [DOI] [PubMed] [Google Scholar]
- 288.Valliere M.A., Korman T.P., Woodall N.B., Khitrov G.A., Taylor R.E., Baker D., Bowie J.U. A Cell-Free Platform for the Prenylation of Natural Products and Application to Cannabinoid Production. Nat. Commun. 2019;10:565. doi: 10.1038/s41467-019-08448-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Taura F., Morimoto S., Shoyama Y. Purification and Characterization of Cannabidiolic-Acid Synthase from Cannabis sativa L. Biochemical Analysis of a Novel Enzyme That Catalyzes the Oxidocyclization of Cannabigerolic Acid to Cannabidiolic Acid. J. Biol. Chem. 1996;271:17411–17416. doi: 10.1074/jbc.271.29.17411. [DOI] [PubMed] [Google Scholar]
- 290.Morimoto S., Komatsu K., Taura F., Shoyama Y. Purification and Characterization of Cannabichromenic Acid Synthase from Cannabis sativa. Phytochemistry. 1998;49:1525–1529. doi: 10.1016/S0031-9422(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 291.Shoyama Y., Tamada T., Kurihara K., Takeuchi A., Taura F., Arai S., Blaber M., Shoyama Y., Morimoto S., Kuroki R. Structure and Function of Δ1-Tetrahydrocannabinolic Acid (THCA) Synthase, the Enzyme Controlling the Psychoactivity of Cannabis sativa. J. Mol. Biol. 2012;423:96–105. doi: 10.1016/j.jmb.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 292.Taura F., Morimoto S., Shoyama Y., Mechoulam R. First Direct Evidence for the Mechanism of Δ1-Tetrahydrocannabinolie Acid Biosynthesis. J. Am. Chem. Soc. 1995;117:9766–9767. doi: 10.1021/ja00143a024. [DOI] [Google Scholar]
- 293.Taura F., Dono E., Sirikantaramas S., Yoshimura K., Shoyama Y., Morimoto S. Production of Δ1-Tetrahydrocannabinolic Acid by the Biosynthetic Enzyme Secreted from Transgenic Pichia Pastoris. Biochem. Biophys. Res. Commun. 2007;361:675–680. doi: 10.1016/j.bbrc.2007.07.079. [DOI] [PubMed] [Google Scholar]
- 294.De Zeeuw R.A., Wijsbeek J., Brejmer D.D., Vree T.B., Van Ginneken C.A.M., Van Rossum J.M. Cannabinoids with a Propyl Side Chain in Cannabis: Occurrence and Chromatographic Behavior. Science. 1972;175:778–779. doi: 10.1126/science.175.4023.778. [DOI] [PubMed] [Google Scholar]
- 295.De Meijer E.P.M., Bagatta M., Carboni A., Crucitti P., Moliterni V.M.C., Ranalli P., Mandolino G. The Inheritance of Chemical Phenotype in Cannabis sativa L. Genetics. 2003;163:335–346. doi: 10.1093/genetics/163.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Shoyama Y., Hirano H., Nishioka I. Biosynthesis of Propyl Cannabinoid Acid and Its Biosynthetic Relationship with Pentyl and Methyl Cannabinoid Acids. Phytochemistry. 1984;23:1909–1984. doi: 10.1016/S0031-9422(00)84939-0. [DOI] [Google Scholar]
- 297.Kanter S.L., Musumeci M.R., Hollister L.E. Quantitative Determination of Δ9-Tetrahydrocannabinol and Δ9-Tetrahydrocannabinolic Acid in Marihuana by High-Pressure Liquid Chromatography. J. Chromatogr. A. 1979;171:504–508. doi: 10.1016/S0021-9673(01)95346-4. [DOI] [PubMed] [Google Scholar]
- 298.Perrotin-Brunel H., Buijs W., Van Spronsen J., Roosmalen M.J.E.V., Peters C.J., Verpoorte R., Witkamp G.J. Decarboxylation of Δ9-Tetrahydrocannabinol: Kinetics and Molecular Modeling. J. Mol. Struct. 2011;987:67–73. doi: 10.1016/j.molstruc.2010.11.061. [DOI] [Google Scholar]
- 299.Shoyama Y., Yagi M., Nishioka I., Yamauchi T. Biosynthesis of Cannabinoid Acids. Phytochemistry. 1975;14:2189–2192. doi: 10.1016/S0031-9422(00)91096-3. [DOI] [Google Scholar]
- 300.Veress T., Szanto J.I., Leisztner L. Determination of Cannabinoid Acids by High-Performance Liquid Chromatography of Their Neutral Derivatives Formed by Thermal Decarboxylation. I. Study of the Decarboxylation Process in Open Reactors. J. Chromatogr. A. 1990 doi: 10.1016/0021-9673(90)85118-F. [DOI] [Google Scholar]
- 301.Hanuš L.O., Meyer S.M., Muñoz E., Taglialatela-Scafati O., Appendino G. Phytocannabinoids: A Unified Critical Inventory. Nat. Prod. Rep. 2016;33:1357–1392. doi: 10.1039/C6NP00074F. [DOI] [PubMed] [Google Scholar]
- 302.Ahmed S.A., Ross S.A., Slade D., Radwan M.M., Khan I.A., ElSohly M.A. Structure Determination and Absolute Configuration of Cannabichromanone Derivatives from High Potency Cannabis sativa. Tetrahedron Lett. 2008;49:6050–6053. doi: 10.1016/j.tetlet.2008.07.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Ahmed S.A., Ross S.A., Slade D., Radwan M.M., Zulfiqar F., ElSohly M.A. Cannabinoid Ester Constituents from High-Potency Cannabis sativa. J. Nat. Prod. 2008;71:536–542. doi: 10.1021/np070454a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Radwan M.M., Ross S.A., Slade D., Ahmed S.A., Zulfiqar F., Elsohly M.A. Isolation and Characterization of New Cannabis Constituents from a High Potency Variety. Planta Med. 2008;74:267–272. doi: 10.1055/s-2008-1034311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Pagani A., Scala F., Chianese G., Grassi G., Appendino G., Taglialatela-Scafati O. Cannabioxepane, a Novel Tetracyclic Cannabinoid from Hemp, Cannabis sativa L. Tetrahedron. 2011;67:3369–3373. doi: 10.1016/j.tet.2011.03.062. [DOI] [Google Scholar]
- 306.Zulfiqar F., Ross S.A., Slade D., Ahmed S.A., Radwan M.M., Ali Z., Khan I.A., Elsohly M.A. Cannabisol, a Novel Δ 9-THC Dimer Possessing a Unique Methylene Bridge, Isolated from Cannabis sativa. Tetrahedron Lett. 2012;53:3560–3562. doi: 10.1016/j.tetlet.2012.04.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Pollastro F., Taglialatela-Scafati O., Allarà M., Muñoz E., Di Marzo V., De Petrocellis L., Appendino G. Bioactive Prenylogous Cannabinoid from Fiber Hemp (Cannabis sativa) J. Nat. Prod. 2011;74:2019–2022. doi: 10.1021/np200500p. [DOI] [PubMed] [Google Scholar]
- 308.Taglialatela-Scafati O., Pagani A., Scala F., De Petrocellis L., Di Marzo V., Grassi G., Appendino G. Cannabimovone, a Cannabinoid with a Rearranged Terpenoid Skeleton from Hemp (Eur. J. Org. Chem. 11/2010) Eur. J. Org. Chem. 2010;2010:2023. doi: 10.1002/ejoc.201090025. [DOI] [Google Scholar]
- 309.Thomas A., Stevenson L.A., Wease K.N., Price M.R., Baillie G., Ross R.A., Pertwee R.G. Evidence That the Plant Cannabinoid Δ 9-Tetrahydrocannabivarin Is a Cannabinoid CB 1 and CB 2 Receptor Antagonist. Br. J. Pharmacol. 2005;146:917–926. doi: 10.1038/sj.bjp.0706414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Pertwee R.G., Thomas A., Stevenson L.A., Ross R.A., Varvel S.A., Lichtman A.H., Martin B.R., Razdan R.K. The Psychoactive Plant Cannabinoid, Δ 9-Tetrahydrocannabinol, Is Antagonized by Δ 8- and Δ 9-Tetrahydrocannabivarin in Mice in Vivo. Br. J. Pharmacol. 2007;150:586–594. doi: 10.1038/sj.bjp.0707124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.García C., Palomo-Garo C., García-Arencibia M., Ramos J.A., Pertwee R.G., Fernández-Ruiz J. Symptom-Relieving and Neuroprotective Effects of the Phytocannabinoid Δ 9-THCV in Animal Models of Parkinson’s Disease. Br. J. Pharmacol. 2011;163:1495–1506. doi: 10.1111/j.1476-5381.2011.01278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Ma Y.L., Weston S.E., Whalley B.J., Stephens G.J. The Phytocannabinoid Δ 9-Tetrahydrocannabivarin Modulates Inhibitory Neurotransmission in the Cerebellum. Br. J. Pharmacol. 2008;154:204–215. doi: 10.1038/bjp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Dennis I., Whalley B.J., Stephens G.J. Effects of Δ 9-Tetrahydrocannabivarin on [35S]GTPγS Binding in Mouse Brain Cerebellum and Piriform Cortex Membranes. Br. J. Pharmacol. 2008 doi: 10.1038/bjp.2008.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Cascio M.G., Zamberletti E., Marini P., Parolaro D., Pertwee R.G. The Phytocannabinoid, Δ9-Tetrahydrocannabivarin, Can Act through 5-HT1A Receptors to Produce Antipsychotic Effects. Br. J. Pharmacol. 2015;172:1305–1318. doi: 10.1111/bph.13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.O’Sullivan S.E., Bennett A.J., Kendall D.A., Randall M.D. Cannabinoids and Peroxisome Proliferator-Activated Receptor γ (PPARg); Proceedings of the 16th Annual Symposium on the Cannabinoids; Tihany, Hungary. 24–28 June 2006; [Google Scholar]
- 316.Englund A., Atakan Z., Kralj A., Tunstall N., Murray R., Morrison P. The Effect of Five Day Dosing with THCV on THC-Induced Cognitive, Psychological and Physiological Effects in Healthy Male Human Volunteers: A Placebo-Controlled, Double-Blind, Crossover Pilot Trial. J. Psychopharmacol. 2016;30:140–151. doi: 10.1177/0269881115615104. [DOI] [PubMed] [Google Scholar]
- 317.Tudge L., Williams C., Cowen P.J., McCabe C. Neural Effects of Cannabinoid CB1 Neutral Antagonist Tetrahydrocannabivarin on Food Reward and Aversion in Healthy Volunteers. Int. J. Neuropsychopharmacol. 2015;18:Pyu094. doi: 10.1093/ijnp/pyu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Rzepa E., Tudge L., McCabe C. The CB1 Neutral Antagonist Tetrahydrocannabivarin Reduces Default Mode Network and Increases Executive Control Network Resting State Functional Connectivity in Healthy Volunteers. Int. J. Neuropsychopharmacol. 2016;19:Pyv092. doi: 10.1093/ijnp/pyv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Moldzio R., Pacher T., Krewenka C., Kranner B., Novak J., Duvigneau J.C., Rausch W.D. Effects of Cannabinoids Δ(9)-Tetrahydrocannabinol, Δ(9)-Tetrahydrocannabinolic Acid and Cannabidiol in MPP+ Affected Murine Mesencephalic Cultures. Phytomedicine. 2012;19:819–824. doi: 10.1016/j.phymed.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 320.Verhoeckx K.C.M., Korthout H.A.A.J., Van Meeteren-Kreikamp A.P., Ehlert K.A., Wang M., Van Der Greef J., Rodenburg R.J.T., Witkamp R.F. Unheated Cannabis sativa Extracts and Its Major Compound THC-Acid Have Potential Immuno-Modulating Properties Not Mediated by CB1 and CB2 Receptor Coupled Pathways. Int. Immunopharmacol. 2006;6:656–665. doi: 10.1016/j.intimp.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 321.Hollister L.E., Gillespie H.K. Delta-8- and Delta-9-Tetrahydrocannabinol Comparison in Man by Oral and Intravenous Administration. Clin. Pharmacol. Ther. 1973;14:353–357. doi: 10.1002/cpt1973143353. [DOI] [PubMed] [Google Scholar]
- 322.Rock E.M., Limebeer C.L., Navaratnam R., Sticht M.A., Bonner N., Engeland K., Downey R., Morris H., Jackson M., Parker L.A. A Comparison of Cannabidiolic Acid with Other Treatments for Anticipatory Nausea Using a Rat Model of Contextually Elicited Conditioned Gaping. Psychopharmacology. 2014;231:3207–3215. doi: 10.1007/s00213-014-3498-1. [DOI] [PubMed] [Google Scholar]
- 323.Nadal X., del Río C., Casano S., Palomares B., Ferreiro-Vera C., Navarrete C., Sánchez-Carnerero C., Cantarero I., Bellido M.L., Meyer S., et al. Tetrahydrocannabinolic Acid Is a Potent PPARγ Agonist with Neuroprotective Activity. Br. J. Pharmacol. 2017;174:4263–4276. doi: 10.1111/bph.14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Morano A., Cifelli P., Nencini P., Antonilli L., Fattouch J., Ruffolo G., Roseti C., Aronica E., Limatola C., Di Bonaventura C., et al. Cannabis in Epilepsy: From Clinical Practice to Basic Research Focusing on the Possible Role of Cannabidivarin. Epilepsia Open. 2016;1:145–151. doi: 10.1002/epi4.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Hill T.D.M., Cascio M.G., Romano B., Duncan M., Pertwee R.G., Williams C.M., Whalley B.J., Hill A.J. Cannabidivarin-Rich Cannabis Extracts Are Anticonvulsant in Mouse and Rat via a CB1 Receptor-Independent Mechanism. Br. J. Pharmacol. 2013;170:679–692. doi: 10.1111/bph.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Hill A.J., Mercier M.S., Hill T.D.M., Glyn S.E., Jones N.A., Yamasaki Y., Futamura T., Duncan M., Stott C.G., Stephens G.J., et al. Cannabidivarin Is Anticonvulsant in Mouse and Rat. Br. J. Pharmacol. 2012;167:1629–1642. doi: 10.1111/j.1476-5381.2012.02207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Amada N., Yamasaki Y., Williams C.M., Whalley B.J. Cannabidivarin (CBDV) Suppresses Pentylenetetrazole (PTZ)-Induced Increases in Epilepsy-Related Gene Expression. PeerJ. 2013;1:E214. doi: 10.7717/peerj.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Huizenga M.N., Sepulveda-Rodriguez A., Forcelli P.A. Preclinical Safety and Efficacy of Cannabidivarin for Early Life Seizures. Neuropharmacology. 2019;148:189–198. doi: 10.1016/j.neuropharm.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Qin N., Neeper M.P., Liu Y., Hutchinson T.L., Lubin M.L., Flores C.M. TRPV2 Is Activated by Cannabidiol and Mediates CGRP Release in Cultured Rat Dorsal Root Ganglion Neurons. J. Neurosci. 2008;28:6231–6238. doi: 10.1523/JNEUROSCI.0504-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Pretzsch C.M., Voinescu B., Lythgoe D., Horder J., Mendez M.A., Wichers R., Ajram L., Ivin G., Heasman M., Edden R.A.E., et al. Effects of Cannabidivarin (CBDV) on Brain Excitation and Inhibition Systems in Adults with and without Autism Spectrum Disorder (ASD): A Single Dose Trial during Magnetic Resonance Spectroscopy. Transl. Psychiatry. 2019;9:313. doi: 10.1038/s41398-019-0654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Eibach L., Scheffel S., Cardebring M., Lettau M., Özgür Celik M., Morguet A., Roehle R., Stein C. Cannabidivarin for HIV-Associated Neuropathic Pain: A Randomized, Blinded, Controlled Clinical Trial. Clin. Pharmacol. Ther. 2020:1–8. doi: 10.1002/cpt.2016. [DOI] [PubMed] [Google Scholar]
- 332.Russo C., Ferk F., Mišík M., Ropek N., Nersesyan A., Mejri D., Holzmann K., Lavorgna M., Isidori M., Knasmüller S. Low Doses of Widely Consumed Cannabinoids (Cannabidiol and Cannabidivarin) Cause DNA Damage and Chromosomal Aberrations in Human-Derived Cells. Arch. Toxicol. 2019;93:179–188. doi: 10.1007/s00204-018-2322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Cascio M.G., Gauson L.A., Stevenson L.A., Ross R.A., Pertwee R.G. Evidence That the Plant Cannabinoid Cannabigerol Is a Highly Potent α 2-Adrenoceptor Agonist and Moderately Potent 5HT 1A Receptor Antagonist. Br. J. Pharmacol. 2010;159:129–141. doi: 10.1111/j.1476-5381.2009.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Valdeolivas S., Navarrete C., Cantarero I., Bellido M.L., Muñoz E., Sagredo O. Neuroprotective Properties of Cannabigerol in Huntington’s Disease: Studies in R6/2 Mice and 3-Nitropropionate-Lesioned Mice. Neurotherapeutics. 2015;12:185–199. doi: 10.1007/s13311-014-0304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Gugliandolo A., Pollastro F., Grassi G., Bramanti P., Mazzon E. In Vitro Model of Neuroinflammation: Efficacy of Cannabigerol, a Non-Psychoactive Cannabinoid. Int. J. Mol. Sci. 2018;19:1992. doi: 10.3390/ijms19071992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Kathmann M., Flau K., Redmer A., Tränkle C., Schlicker E. Cannabidiol Is an Allosteric Modulator at Mu- and Delta-Opioid Receptors. Naunyn. Schmiedebergs. Arch. Pharmacol. 2006;372:354–361. doi: 10.1007/s00210-006-0033-x. [DOI] [PubMed] [Google Scholar]
- 337.Ligresti A., Moriello A.S., Starowicz K., Matias I., Pisanti S., De Petrocellis L., Laezza C., Portella G., Bifulco M., Di Marzo V. Antitumor Activity of Plant Cannabinoids with Emphasis on the Effect of Cannabidiol on Human Breast Carcinoma. J. Pharmacol. Exp. Ther. 2006;318:1375–1387. doi: 10.1124/jpet.106.105247. [DOI] [PubMed] [Google Scholar]
- 338.Booker L., Naidu P.S., Razdan R.K., Mahadevan A., Lichtman A.H. Evaluation of Prevalent Phytocannabinoids in the Acetic Acid Model of Visceral Nociception. Drug Alcohol Depend. 2009;105:42–47. doi: 10.1016/j.drugalcdep.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Esposito G., Scuderi C., Valenza M., Togna G.I., Latina V., de Filippis D., Cipriano M., Carratù M.R., Iuvone T., Steardo L. Cannabidiol Reduces Aβ-Induced Neuroinflammation and Promotes Hippocampal Neurogenesis through PPARγ Involvement. PLoS ONE. 2011;6:e28668. doi: 10.1371/journal.pone.0028668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Rosenthaler S., Pöhn B., Kolmanz C., Nguyen Huu C., Krewenka C., Huber A., Kranner B., Rausch W.D., Moldzio R. Differences in Receptor Binding Affinity of Several Phytocannabinoids Do Not Explain Their Effects on Neural Cell Cultures. Neurotoxicol. Teratol. 2014;46:49–56. doi: 10.1016/j.ntt.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 341.Turner C.E., Elsohly M.A. Biological Activity of Cannabichromene, Its Homologs and Isomers. J. Clin. Pharmacol. 1981;21:283S–291S. doi: 10.1002/j.1552-4604.1981.tb02606.x. [DOI] [PubMed] [Google Scholar]
- 342.DeLong G.T., Wolf C.E., Poklis A., Lichtman A.H. Pharmacological Evaluation of the Natural Constituent of Cannabis sativa, Cannabichromene and Its Modulation by Δ9-Tetrahydrocannabinol. Drug Alcohol Depend. 2010;112:126–133. doi: 10.1016/j.drugalcdep.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Davis W.M., Hatoum N.S. Neurobehavioral Actions of Cannabichromene and Interactions with Δ9-Tetrahydrocannabinol. Gen. Pharmacol. 1983;14:247–252. doi: 10.1016/0306-3623(83)90004-6. [DOI] [PubMed] [Google Scholar]
- 344.Udoh M., Santiago M., Devenish S., McGregor I.S., Connor M. Cannabichromene Is a Cannabinoid CB2 Receptor Agonist. Br. J. Pharmacol. 2019;176:4537–4547. doi: 10.1111/bph.14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Romano B., Borrelli F., Fasolino I., Capasso R., Piscitelli F., Cascio M.G., Pertwee R.G., Coppola D., Vassallo L., Orlando P., et al. The Cannabinoid TRPA1 Agonist Cannabichromene Inhibits Nitric Oxide Production in Macrophages and Ameliorates Murine Colitis. Br. J. Pharmacol. 2013;169:213–229. doi: 10.1111/bph.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Shinjyo N., Di Marzo V. The Effect of Cannabichromene on Adult Neural Stem/Progenitor Cells. Neurochem. Int. 2013;63:432–437. doi: 10.1016/j.neuint.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 347.Mahadevan A., Siegel C., Martin B.R., Abood M.E., Beletskaya I., Razdan R.K. Novel Cannabinol Probes for CB1 and CB2 Cannabinoid Receptors. J. Med. Chem. 2000;43:3778–3785. doi: 10.1021/jm0001572. [DOI] [PubMed] [Google Scholar]
- 348.Yamamoto I., Watanabe K., Kuzuoka K., Narimatsu S., Yoshimura H. The Pharmacological Activity of Cannabinol and Its Major Metabolite, 11-Hydroxycannabinol. Chem. Pharm. Bull. 1987;35:2144–2147. doi: 10.1248/cpb.35.2144. [DOI] [PubMed] [Google Scholar]
- 349.Watanabe K., Yamaori S., Funahashi T., Kimura T., Yamamoto I. Cytochrome P450 Enzymes Involved in the Metabolism of Tetrahydrocannabinols and Cannabinol by Human Hepatic Microsomes. Life Sci. 2007;80:1415–1419. doi: 10.1016/j.lfs.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 350.Yamaori S., Kushihara M., Yamamoto I., Watanabe K. Characterization of Major Phytocannabinoids, Cannabidiol and Cannabinol, as Isoform-Selective and Potent Inhibitors of Human CYP1 Enzymes. Biochem. Pharmacol. 2010;79:1691–1698. doi: 10.1016/j.bcp.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 351.Aiken C.T., Tobin A.J., Schweitzer E.S. A Cell-Based Screen for Drugs to Treat Huntington’s Disease. Neurobiol. Dis. 2004;16:546–555. doi: 10.1016/j.nbd.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 352.Glass M., Faull R.L.M., Dragunow M. Loss of Cannabinoid Receptors in the Substantia Nigra in Huntington’s Disease. Neuroscience. 1993;56:523–527. doi: 10.1016/0306-4522(93)90352-G. [DOI] [PubMed] [Google Scholar]
- 353.Blázquez C., Chiarlone A., Sagredo O., Aguado T., Pazos M.R., Resel E., Palazuelos J., Julien B., Salazar M., Börner C., et al. Loss of Striatal Type 1 Cannabinoid Receptors Is a Key Pathogenic Factor in Huntington’s Disease. Brain. 2011;134:119–136. doi: 10.1093/brain/awq278. [DOI] [PubMed] [Google Scholar]
- 354.Weydt P., Hong S., Witting A., Möller T., Stella N., Kliot M. Cannabinol Delays Symptom Onset in SOD1 (G93A) Transgenic Mice without Affecting Survival. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2005;6:182–184. doi: 10.1080/14660820510030149. [DOI] [PubMed] [Google Scholar]
- 355.Wong H., Cairns B.E. Cannabidiol, Cannabinol and Their Combinations Act as Peripheral Analgesics in a Rat Model of Myofascial Pain. Arch. Oral Biol. 2019;104:33–39. doi: 10.1016/j.archoralbio.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 356.Baroi S., Saha A., Bachar R., Bachar S.C. Cannabinoid as Potential Aromatase Inhibitor through Molecular Modeling and Screening for Anti-Cancer Activity. Dhaka Univ. J. Pharm. Sci. 2020;19:47–58. doi: 10.3329/dujps.v19i1.47818. [DOI] [Google Scholar]
- 357.Furqan T., Batool S., Habib R., Shah M., Kalasz H., Darvas F., Kuca K., Nepovimova E., Batool S., Nurulain S.M. Cannabis Constituents and Acetylcholinesterase Interaction: Molecular Docking, in Vitro Studies and Association with CNR1 RS806368 and ACHE RS17228602. Biomolecules. 2020;10:758. doi: 10.3390/biom10050758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Sakamoto K., Akiyama Y., Fukui K., Kamada H., Satoh S. Characterization; Genome Sizes and Morphology of Sex Chromosomes in Hemp (Cannabis sativa L.) Cytologia. 1998;635:459–464. doi: 10.1508/cytologia.63.459. [DOI] [Google Scholar]
- 359.Sakamoto K., Shimomura K., Komeda Y., Kamada H., Satoh S. A Male-Associated DNA Sequence in a Dioecious Plant, Cannabis sativa L. Plant Cell Physiol. 1995;36:1549–1554. doi: 10.1093/oxfordjournals.pcp.a078920. [DOI] [PubMed] [Google Scholar]
- 360.Alghanim H.J., Almirall J.R. Development of Microsatellite Markers in Cannabis sativa for DNA Typing and Genetic Relatedness Analyses. Anal. Bioanal. Chem. 2003;376:1225–1233. doi: 10.1007/s00216-003-1984-0. [DOI] [PubMed] [Google Scholar]
- 361.Gilmore S., Peakall R., Robertson J. Short Tandem Repeat (STR) DNA Markers Are Hypervariable and Informative in Cannabis sativa: Implications for Forensic Investigations. Forensic Sci. Int. 2003;131:65–74. doi: 10.1016/S0379-0738(02)00397-3. [DOI] [PubMed] [Google Scholar]
- 362.Hsieh H.M., Hou R.J., Tsai L.C., Wei C.S., Liu S.W., Huang L.H., Kuo Y.C., Linacre A., Lee J.C.I. A Highly Polymorphic STR Locus in Cannabis sativa. Forensic Sci. Int. 2003;131:53–58. doi: 10.1016/S0379-0738(02)00395-X. [DOI] [PubMed] [Google Scholar]
- 363.Van Bakel H., Stout J.M., Cote A.G., Tallon C.M., Sharpe A.G., Hughes T.R., Page J.E. The Draft Genome and Transcriptome of Cannabis sativa. Genome Biol. 2011;12:R102. doi: 10.1186/gb-2011-12-10-r102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Gao S., Wang B., Xie S., Xu X., Zhang J., Pei L., Yu Y., Yang W., Zhang Y. A High-Quality Reference Genome of Wild Cannabis sativa. Hortic. Res. 2020;7:73. doi: 10.1038/s41438-020-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Laverty K.U., Stout J.M., Sullivan M.J., Shah H., Gill N., Holbrook L., Deikus G., Sebra R., Hughes T.R., Page J.E., et al. A Physical and Genetic Map of Cannabis sativa Identifies Extensive Rearrangements at the THC/CBD Acid Synthase Loci. Genome Res. 2019;29:146–156. doi: 10.1101/gr.242594.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Kojoma M., Seki H., Yoshida S., Muranaka T. DNA Polymorphisms in the Tetrahydrocannabinolic Acid (THCA) Synthase Gene in “Drug-Type” and “Fiber-Type” Cannabis sativa L. Forensic Sci. Int. 2006;159:132–140. doi: 10.1016/j.forsciint.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 367.McKernan K., Helbert Y., Tadigotla V., McLaughlin S., Spangler J., Zhang L., Smith D. Single Molecule Sequencing of THCA Synthase Reveals Copy Number Variation in Modern Drug-Type Cannabis sativa L. bioRxiv. 2015:28654. doi: 10.1101/028654. [DOI] [Google Scholar]
- 368.Onofri C., De Meijer E.P.M., Mandolino G. Sequence Heterogeneity of Cannabidiolic- and Tetrahydrocannabinolic Acid-Synthase in Cannabis sativa L. and Its Relationship with Chemical Phenotype. Phytochemistry. 2015;116:57–68. doi: 10.1016/j.phytochem.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 369.Weiblen G.D., Wenger J.P., Craft K.J., ElSohly M.A., Mehmedic Z., Treiber E.L., Marks M.D. Gene Duplication and Divergence Affecting Drug Content in Cannabis sativa. New Phytol. 2015;208:1241–1250. doi: 10.1111/nph.13562. [DOI] [PubMed] [Google Scholar]
- 370.Welling M.T., Liu L., Shapter T., Raymond C.A., King G.J. Characterisation of Cannabinoid Composition in a Diverse Cannabis sativa L. Germplasm Collection. Euphytica. 2016;208:463–475. doi: 10.1007/s10681-015-1585-y. [DOI] [Google Scholar]
- 371.Lynch R.C., Vergara D., Tittes S., White K., Schwartz C.J., Gibbs M.J., Ruthenburg T.C., DeCesare K., Land D.P., Kane N.C. Genomic and Chemical Diversity in Cannabis. CRC. Crit. Rev. Plant Sci. 2016;35:349–363. doi: 10.1080/07352689.2016.1265363. [DOI] [Google Scholar]
- 372.Gao C., Xin P., Cheng C., Tang Q., Chen P., Wang C., Zang G., Zhao L. Diversity Analysis in Cannabis sativa based on Large-Scale Development of Expressed Sequence Tag-Derived Simple Sequence Repeat Markers. PLoS ONE. 2014;9:e110638. doi: 10.1371/journal.pone.0110638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Zhang L.G., Chang Y., Zhang X.F., Guan F.Z., Yuan H.M., Yu Y., Zhao L.J. Analysis of the Genetic Diversity of Chinese Native Cannabis sativa Cultivars by Using ISSR and Chromosome Markers. Genet. Mol. Res. 2014;13:10490–10500. doi: 10.4238/2014.December.12.10. [DOI] [PubMed] [Google Scholar]
- 374.White K.H., Vergara D., Keepers K.G., Kane N.C. The Complete Mitochondrial Genome for Cannabis sativa. Mitochondrial DNA Part B Resour. 2016;1:715–716. doi: 10.1080/23802359.2016.1155083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Oh H., Seo B., Lee S., Ahn D.H., Jo E., Park J.K., Min G.S. Two Complete Chloroplast Genome Sequences of Cannabis sativa Varieties. Mitochondrial DNA. 2015;27:2835–2837. doi: 10.3109/19401736.2015.1053117. [DOI] [PubMed] [Google Scholar]
- 376.Booth J.K., Page J.E., Bohlmann J. Terpene Synthases from Cannabis sativa. PLoS ONE. 2017;12:e0173911. doi: 10.1371/journal.pone.0173911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Zager J.J., Lange I., Srividya N., Smith A., Markus Lange B. Gene Networks Underlying Cannabinoid and Terpenoid Accumulation in Cannabis. Plant Physiol. 2019;180:1877–1897. doi: 10.1104/pp.18.01506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Amano E., Smith H.H. Mutations Induced by Ethyl Methanesulfonate in Maize. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1965;2:344–351. doi: 10.1016/0027-5107(65)90070-9. [DOI] [PubMed] [Google Scholar]
- 379.Froese-Gertzen E.E., Konzak C.F., Nilan R.A., Heiner R.E. The Effect of Ethyl Methanesulfonate on the Growth Response, Chromosome Structure and Mutation Rate in Barley. Radiat. Bot. 1964;4:61–69. doi: 10.1016/S0033-7560(64)80050-8. [DOI] [Google Scholar]
- 380.Jander G., Baerson S.R., Hudak J.A., Gonzalez K.A., Gruys K.J., Last R.L. Ethylmethanesulfonate Saturation Mutagenesis in Arabidopsis to Determine Frequency of Herbicide Resistance. Plant Physiol. 2003;131:139–146. doi: 10.1104/pp.102.010397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Luan Y.S., Zhang J., Gao X.R., An L.J. Mutation Induced by Ethylmethanesulphonate (EMS), in Vitro Screening for Salt Tolerance and Plant Regeneration of Sweet Potato (Ipomoea batatas L.) Plant Cell. Tissue Organ Cult. 2007;88:77–81. doi: 10.1007/s11240-006-9183-2. [DOI] [Google Scholar]
- 382.Stavreva D.A., Ptáček O., Plewa M.J., Gichner T. Single Cell Gel Electrophoresis Analysis of Genomic Damage Induced by Ethyl Methanesulfonate in Cultured Tobacco Cells. Mutat Res. 1998;422:323–330. doi: 10.1016/S0027-5107(98)00213-9. [DOI] [PubMed] [Google Scholar]
- 383.Watanabe S., Mizoguchi T., Aoki K., Kubo Y., Mori H., Imanishi S., Yamazaki Y., Shibata D., Ezura H. Ethylmethanesulfonate (EMS) Mutagenesis of Solanum Lycopersicum Cv. Micro-Tom for Large-Scale Mutant Screens. Plant Biotechnol. 2007;24:33–38. doi: 10.5511/plantbiotechnology.24.33. [DOI] [Google Scholar]
- 384.Koornneeff M., Dellaert L.W.M., van der Veen J.H. EMS- and Relation-Induced Mutation Frequencies at Individual Loci in Arabidopsis thaliana (L.) Heynh. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1982;93:109–123. doi: 10.1016/0027-5107(82)90129-4. [DOI] [PubMed] [Google Scholar]
- 385.Li X., Song Y., Century K., Straight S., Ronald P., Dong X., Lassner M., Zhang Y. A Fast Neutron Deletion Mutagenesis-Based Reverse Genetics System for Plants. Plant J. 2001;27:235–242. doi: 10.1046/j.1365-313x.2001.01084.x. [DOI] [PubMed] [Google Scholar]
- 386.Shirley B.W., Hanley S., Goodman H.M. Effects of Ionizing Radiation on a Plant Genome: Analysis of Two Arabidopsis Transparent Testa Mutations. Plant Cell. 1992;4:333–347. doi: 10.1105/tpc.4.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Bevan M., Bancroft I., Bent E., Love K., Goodman H., Dean C., Bergkamp R., Dirkse W., Van Staveren M., Stiekema W., et al. Analysis of 1.9 Mb of Contiguous Sequence from Chromosome 4 of Arabidopsis thaliana. Nature. 1998;391:485–488. doi: 10.1038/35140. [DOI] [PubMed] [Google Scholar]
- 388.Bolon Y.T., Haun W.J., Xu W.W., Grant D., Stacey M.G., Nelson R.T., Gerhardt D.J., Jeddeloh J.A., Stacey G., Muehlbauer G.J., et al. Phenotypic and Genomic Analyses of a Fast Neutron Mutant Population Resource in Soybean. Plant Physiol. 2011;156:240–253. doi: 10.1104/pp.110.170811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Colbert T., Till B.J., Tompa R., Reynolds S., Steine M.N., Yeung A.T., McCallum C.M., Comai L., Henikoff S. High-Throughput Screening for Induced Point Mutations. Plant Physiol. 2001;126:480–484. doi: 10.1104/pp.126.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Jander G., Norris S.R., Joshi V., Fraga M., Rugg A., Yu S., Li L., Last R.L. Application of a High-Throughput HPLC-MS/MS Assay to Arabidopsis Mutant Screening; Evidence That Threonine Aldolase Plays a Role in Seed Nutritional Quality. Plant J. 2004;39:465–475. doi: 10.1111/j.1365-313X.2004.02140.x. [DOI] [PubMed] [Google Scholar]
- 391.Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J.A., Charpentier E. A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Jaganathan D., Ramasamy K., Sellamuthu G., Jayabalan S., Venkataraman G. CRISPR for Crop Improvement: An Update Review. Front. Plant Sci. 2018;9:985. doi: 10.3389/fpls.2018.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Mali P., Yang L., Esvelt K.M., Aach J., Guell M., DiCarlo J.E., Norville J.E., Church G.M. RNA-Guided Human Genome Engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Fu Y., Foden J.A., Khayter C., Maeder M.L., Reyon D., Joung J.K., Sander J.D. High-Frequency off-Target Mutagenesis Induced by CRISPR-Cas Nucleases in Human Cells. Nat. Biotechnol. 2013;31:822–826. doi: 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Hsu P.D., Scott D.A., Weinstein J.A., Ran F.A., Konermann S., Agarwala V., Li Y., Fine E.J., Wu X., Shalem O., et al. DNA Targeting Specificity of RNA-Guided Cas9 Nucleases. Nat. Biotechnol. 2013;31:827–832. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Ran F.A., Hsu P.D., Wright J., Agarwala V., Scott D.A., Zhang F. Genome Engineering Using the CRISPR-Cas9 System. Nat. Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Tsai S.Q., Nguyen N.T., Malagon-Lopez J., Topkar V.V., Aryee M.J., Joung J.K. CIRCLE-Seq: A Highly Sensitive in Vitro Screen for Genome-Wide CRISPR-Cas9 Nuclease off-Targets. Nat. Methods. 2017;14:607–614. doi: 10.1038/nmeth.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Woo J.W., Kim J., Kwon S.I., Corvalán C., Cho S.W., Kim H., Kim S.G., Kim S.T., Choe S., Kim J.S. DNA-Free Genome Editing in Plants with Preassembled CRISPR-Cas9 Ribonucleoproteins. Nat. Biotechnol. 2015;33:1162–1164. doi: 10.1038/nbt.3389. [DOI] [PubMed] [Google Scholar]
- 399.Lowder L.G., Zhang D., Baltes N.J., Paul J.W., Tang X., Zheng X., Voytas D.F., Hsieh T.F., Zhang Y., Qi Y. A CRISPR/Cas9 Toolbox for Multiplexed Plant Genome Editing and Transcriptional Regulation. Plant Physiol. 2015;169:971–985. doi: 10.1104/pp.15.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Jiang W., Zhou H., Bi H., Fromm M., Yang B., Weeks D.P. Demonstration of CRISPR/Cas9/SgRNA-Mediated Targeted Gene Modification in Arabidopsis, Tobacco, Sorghum and Rice. Nucleic Acids Res. 2013;41:e188. doi: 10.1093/nar/gkt780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Feng Z., Zhang B., Ding W., Liu X., Yang D.L., Wei P., Cao F., Zhu S., Zhang F., Mao Y., et al. Efficient Genome Editing in Plants Using a CRISPR/Cas System. Cell Res. 2013;23:1229–1232. doi: 10.1038/cr.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Bortesi L., Fischer R. The CRISPR/Cas9 System for Plant Genome Editing and Beyond. Biotechnol. Adv. 2015;33:41–52. doi: 10.1016/j.biotechadv.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 403.Podevin N., Davies H.V., Hartung F., Nogué F., Casacuberta J.M. Site-Directed Nucleases: A Paradigm Shift in Predictable, Knowledge-Based Plant Breeding. Trends Biotechnol. 2013;31:375–383. doi: 10.1016/j.tibtech.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 404.Ma X., Zhang Q., Zhu Q., Liu W., Chen Y., Qiu R., Wang B., Yang Z., Li H., Lin Y., et al. A Robust CRISPR/Cas9 System for Convenient, High-Efficiency Multiplex Genome Editing in Monocot and Dicot Plants. Mol. Plant. 2015;8:1274–1284. doi: 10.1016/j.molp.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 405.Ali Z., Abulfaraj A., Idris A., Ali S., Tashkandi M., Mahfouz M.M. CRISPR/Cas9-Mediated Viral Interference in Plants. Genome Biol. 2015;16 doi: 10.1186/s13059-015-0799-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Xing H.L., Dong L., Wang Z.P., Zhang H.Y., Han C.Y., Liu B., Wang X.C., Chen Q.J. A CRISPR/Cas9 Toolkit for Multiplex Genome Editing in Plants. BMC Plant Biol. 2014;14:327. doi: 10.1186/s12870-014-0327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Wang Y., Cheng X., Shan Q., Zhang Y., Liu J., Gao C., Qiu J.L. Simultaneous Editing of Three Homoeoalleles in Hexaploid Bread Wheat Confers Heritable Resistance to Powdery Mildew. Nat. Biotechnol. 2014;32:947–951. doi: 10.1038/nbt.2969. [DOI] [PubMed] [Google Scholar]
- 408.Li J.F., Norville J.E., Aach J., McCormack M., Zhang D., Bush J., Church G.M., Sheen J. Multiplex and Homologous Recombination-Mediated Genome Editing in Arabidopsis and Nicotiana Benthamiana Using Guide RNA and Cas9. Nat. Biotechnol. 2013;31:688–691. doi: 10.1038/nbt.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Belhaj K., Chaparro-Garcia A., Kamoun S., Patron N.J., Nekrasov V. Editing Plant Genomes with CRISPR/Cas9. Curr. Opin. Biotechnol. 2015;32:76–84. doi: 10.1016/j.copbio.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 410.Li Z., Liu Z.B., Xing A., Moon B.P., Koellhoffer J.P., Huang L., Ward R.T., Clifton E., Falco S.C., Cigan A.M. Cas9-Guide RNA Directed Genome Editing in Soybean. Plant Physiol. 2015;169:960–970. doi: 10.1104/pp.15.00783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Mackinnon L., McDougall G., Aziz N., Millam S. Progress Towards Transformation of Fibre Hemp. Scottish Crop Research Institute; Dundee, UK: 2000. pp. 84–86. Scottish Crop Research Institute Annual Report 2000/2001. [Google Scholar]
- 412.Feeney M., Punja Z.K. Tissue Culture and Agrobacterium-Mediated Transformation of Hemp (Cannabis sativa L.) Vitr. Cell. Dev. Biol. Plant. 2003;39:578–585. doi: 10.1079/IVP2003454. [DOI] [Google Scholar]
- 413.Wahby I., Caba J.M., Ligero F. Agrobacterium Infection of Hemp (Cannabis sativa L.): Establishment of Hairy Root Cultures. J. Plant Interact. 2013;8:312–320. doi: 10.1080/17429145.2012.746399. [DOI] [Google Scholar]
- 414.Srivastava S., Srivastava A.K. Hairy Root Culture for Mass-Production of High-Value Secondary Metabolites. Crit. Rev. Biotechnol. 2007;27:29–43. doi: 10.1080/07388550601173918. [DOI] [PubMed] [Google Scholar]
- 415.Ślusarkiewicz-Jarzina A., Ponitka A., Kaczmarek Z. Influence of Cultivar, Explant Source and Plant Growth Regulator on Callus Induction and Plant Regeneration of Cannabis sativa L. Acta Biol. Cracoviensia Ser. Bot. 2005;47:145–151. [Google Scholar]
- 416.Carvalho Â., Hansen E.H., Kayser O., Carlsen S., Stehle F. Designing Microorganisms for Heterologous Biosynthesis of Cannabinoids. FEMS Yeast Res. 2017;17:Fox037. doi: 10.1093/femsyr/fox037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Zirpel B., Stehle F., Kayser O. Production of Δ9-Tetrahydrocannabinolic Acid from Cannabigerolic Acid by Whole Cells of Pichia (Komagataella) Pastoris Expressing Δ9-Tetrahydrocannabinolic Acid Synthase from Cannabis sativa L. Biotechnol. Lett. 2015;37:1869–1875. doi: 10.1007/s10529-015-1853-x. [DOI] [PubMed] [Google Scholar]
- 418.Ohto C., Muramatsu M., Obata S., Sakuradani E., Shimizu S. Overexpression of the Gene Encoding HMG-CoA Reductase in Saccharomyces Cerevisiae for Production of Prenyl Alcohols. Appl. Microbiol. Biotechnol. 2009;82:837–845. doi: 10.1007/s00253-008-1807-5. [DOI] [PubMed] [Google Scholar]
- 419.Zirpel B., Degenhardt F., Martin C., Kayser O., Stehle F. Engineering Yeasts as Platform Organisms for Cannabinoid Biosynthesis. J. Biotechnol. 2017;259:204–212. doi: 10.1016/j.jbiotec.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 420.Mills E. The Carbon Footprint of Indoor Cannabis Production. Energy Policy. 2012;46:58–67. doi: 10.1016/j.enpol.2012.03.023. [DOI] [Google Scholar]
- 421.Borthwick H.A., Scully N.J. Photoperiodic Responses of Hemp. Bot. Gaz. 1954;116:14–29. doi: 10.1086/335843. [DOI] [Google Scholar]
- 422.Schaffner J.H. The Influence of Relative Length of Daylight on the Reversal of Sex in Hemp. Ecology. 1923;4:323–334. doi: 10.2307/1929179. [DOI] [Google Scholar]
- 423.Potter D.J., Duncombe P. The Effect of Electrical Lighting Power and Irradiance on Indoor-Grown Cannabis Potency and Yield. J. Forensic Sci. 2012;57:618–622. doi: 10.1111/j.1556-4029.2011.02024.x. [DOI] [PubMed] [Google Scholar]
- 424.Spitzer-Rimon B., Duchin S., Bernstein N., Kamenetsky R. Architecture and Florogenesis in Female Cannabis sativa Plants. Front. Plant Sci. 2019;10:350. doi: 10.3389/fpls.2019.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Vanhove W., Van Damme P., Meert N. Factors Determining Yield and Quality of Illicit Indoor Cannabis (Cannabis Spp.) Production. Forensic Sci. Int. 2011;212:1. doi: 10.1016/j.forsciint.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 426.Viršile A., Olle M., Duchovskis P. Light Emitting Diodes for Agriculture: Smart Lighting. Springer; Singapore: 2017. LED Lighting in Horticulture; pp. 113–147. [DOI] [Google Scholar]
- 427.Nelson J.A., Bugbee B. Economic Analysis of Greenhouse Lighting: Light Emitting Diodes vs. High Intensity Discharge Fixtures. PLoS ONE. 2014;9:e99010. doi: 10.1371/journal.pone.0099010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Tamulaitis G., Duchovskis P., Bliznikas Z., Breive K., Ulinskaite R., Brazaityte A., Novičkovas A., Žukauskas A. High-Power Light-Emitting Diode Based Facility for Plant Cultivation. J. Phys. D. Appl. Phys. 2005;38:3182–3187. doi: 10.1088/0022-3727/38/17/S20. [DOI] [Google Scholar]
- 429.Hogewoning S.W., Douwstra P., Trouwborst G., Van Ieperen W., Harbinson J. An Artificial Solar Spectrum Substantially Alters Plant Development Compared with Usual Climate Room Irradiance Spectra. J. Exp. Bot. 2010;61:1267–1276. doi: 10.1093/jxb/erq005. [DOI] [PubMed] [Google Scholar]
- 430.Backer R., Schwinghamer T., Rosenbaum P., McCarty V., Eichhorn Bilodeau S., Lyu D., Ahmed M.B., Robinson G., Lefsrud M., Wilkins O., et al. Closing the Yield Gap for Cannabis: A Meta-Analysis of Factors Determining Cannabis Yield. Front. Plant Sci. 2019;10 doi: 10.3389/fpls.2019.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Chandra S., Lata H., Khan I.A., Elsohly M.A. Photosynthetic Response of Cannabis sativa L. to Variations in Photosynthetic Photon Flux Densities, Temperature and CO2 Conditions. Physiol. Mol. Biol. Plants. 2008;14:299–306. doi: 10.1007/s12298-008-0027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Chandra S., Lata H., Mehmedic Z., Khan I.A., ElSohly M.A. Light Dependence of Photosynthesis and Water Vapor Exchange Characteristics in Different High Δ9-THC Yielding Varieties of Cannabis sativa L. J. Appl. Res. Med. Aromat. Plants. 2015;2:39–47. doi: 10.1016/j.jarmap.2015.03.002. [DOI] [Google Scholar]
- 433.Lydon J., Teramura A.H., Coffman C.B. UV-B Radiation Effects on Photosynthesis, Growth and Cannabinoid Production of Two Cannabis sativa Chemotypes. Photochem. Photobiol. 1987;46:201–206. doi: 10.1111/j.1751-1097.1987.tb04757.x. [DOI] [PubMed] [Google Scholar]
- 434.Berry J., Bjorkman O. Photosynthetic Response and Adaptation to Temperature in Higher Plants. Annu. Rev. Plant Physiol. 1980;31:491–543. doi: 10.1146/annurev.pp.31.060180.002423. [DOI] [Google Scholar]
- 435.Larcher W. Photosynthesis as a Tool for Indicating Temperature Stress Events. In: Schulze E., Caldwell M.M., editors. Ecophysiology of Photosynthesis. Springer; Berlin/Heidelberg, Germany: 1995. pp. 261–277. [DOI] [Google Scholar]
- 436.Hikosaka K., Ishikawa K., Borjigidai A., Muller O., Onoda Y. Temperature Acclimation of Photosynthesis: Mechanisms Involved in the Changes in Temperature Dependence of Photosynthetic Rate. J. Exp. Bot. 2006;57:291–302. doi: 10.1093/jxb/erj049. [DOI] [PubMed] [Google Scholar]
- 437.Chandra S., Lata H., Khan I.A., ElSohly M.A. Temperature Response of Photosynthesis in Different Drug and Fiber Varieties of Cannabis sativa L. Physiol. Mol. Biol. Plants. 2011;17:297–303. doi: 10.1007/s12298-011-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Van der Werf H.M.G., Brouwer K., Wijlhuizen M., Withagen J.C.M. The Effect of Temperature on Leaf Appearance and Canopy Establishment in Fibre Hemp (Cannabis sativa L.) Ann. Appl. Biol. 1995;126:551–561. doi: 10.1111/j.1744-7348.1995.tb05389.x. [DOI] [Google Scholar]
- 439.Bernstein N., Gorelick J., Zerahia R., Koch S. Impact of N, P, K, and Humic Acid Supplementation on the Chemical Profile of Medical Cannabis (Cannabis sativa L) Front. Plant Sci. 2019;10 doi: 10.3389/fpls.2019.00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Maļceva M., Vikmane M., Stramkale V. Changes of Photosynthesis-Related Parameters and Productivity of Cannabis sativa under Different Nitrogen Supply. Environ. Exp. Biol. 2011;9:61–69. [Google Scholar]
- 441.Mansouri H., Asrar Z. Effects of Abscisic Acid on Content and Biosynthesis of Terpenoids in Cannabis sativa at Vegetative Stage. Biol. Plant. 2012;56:153–156. doi: 10.1007/s10535-012-0033-2. [DOI] [Google Scholar]
- 442.Mansouri H., Asrar Z., Mehrabani M. Effects of Gibberellic Acid on Primary Terpenoids and Δ9-Tetrahydrocannabinol in Cannabis sativa at Flowering Stage. J. Integr. Plant Biol. 2009;51:553–561. doi: 10.1111/j.1744-7909.2009.00833.x. [DOI] [PubMed] [Google Scholar]
- 443.Larkin J.C., Oppenheimer D.G., Lloyd A.M., Paparozzi E.T., Marks M.D. Roles of the GLABROUS1 and TRANSPARENT TESTA GLABRA Genes in Arabidopsis Trichome Development. Plant Cell. 1994;6:1065–1076. doi: 10.2307/3869885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Payne C.T., Zhang F., Lloyd A.M. GL3 Encodes a BHLH Protein That Regulates Trichome Development in Arabidopsis through Interaction with GL1 and TTG1. Genetics. 2000;156:1349–1362. doi: 10.1093/genetics/156.3.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Rerie W.G., Feldmann K.A., Marks M.D. The GLABRA2 Gene Encodes a Homeo Domain Protein Required for Normal Trichome Development in Arabidopsis. Genes Dev. 1994;8:1388–1399. doi: 10.1101/gad.8.12.1388. [DOI] [PubMed] [Google Scholar]
- 446.Szymanski D.B., Jilk R.A., Pollock S.M., Marks M.D. Control of GL2 Expression in Arabidopsis Leaves and Trichomes. Development. 1998;125:1161–1171. doi: 10.1242/dev.125.7.1161. [DOI] [PubMed] [Google Scholar]
- 447.Hülskamp M., Miséra S., Jürgens G. Genetic Dissection of Trichome Cell Development in Arabidopsis. Cell. 1994;76:555–566. doi: 10.1016/0092-8674(94)90118-X. [DOI] [PubMed] [Google Scholar]
- 448.Perazza D., Herzog M., Hülskamp M., Brown S., Dorne A.M., Bonneville J.M. Trichome Cell Growth in Arabidopsis thaliana Can Be Derepressed by Mutations in at Least Five Genes. Genetics. 1999;152:461–476. doi: 10.1093/genetics/152.1.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Liu Y., Liu D., Hu R., Hua C., Ali I., Zhang A., Liu B., Wu M., Huang L., Gan Y. AtGIS, a C2H2 Zinc-Finger Transcription Factor from Arabidopsis Regulates Glandular Trichome Development through GA Signaling in Tobacco. Biochem. Biophys. Res. Commun. 2017;483:209–215. doi: 10.1016/j.bbrc.2016.12.164. [DOI] [PubMed] [Google Scholar]
- 450.Luo M., Wang Z., Li H., Xia K.F., Cai Y., Xu Z.F. Overexpression of a Weed (Solanum Americanum) Proteinase Inhibitor in Transgenic Tobacco Results in Increased Glandular Trichome Density and Enhanced Resistance to Helicoverpa Armigera and Spodoptera Litura. Int. J. Mol. Sci. 2009;10:1896–1910. doi: 10.3390/ijms10041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Paetzold H., Garms S., Bartram S., Wieczorek J., Urós-Gracia E.M., Rodríguez-Concepción M., Boland W., Strack D., Hause B., Walter M.H. The Isogene 1-Deoxy-D-Xylulose 5-Phosphate Synthase 2 Controls Isoprenoid Profiles, Precursor Pathway Allocation, and Density of Tomato Trichomes. Mol. Plant. 2010;3:904–916. doi: 10.1093/mp/ssq032. [DOI] [PubMed] [Google Scholar]
- 452.Ma D., Hu Y., Yang C., Liu B., Fang L., Wan Q., Liang W., Mei G., Wang L., Wang H., et al. Genetic Basis for Glandular Trichome Formation in Cotton. Nat. Commun. 2016;7:10456. doi: 10.1038/ncomms10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Salas Fernandez M.G., Becraft P.W., Yin Y., Lübberstedt T. From Dwarves to Giants? Plant Height Manipulation for Biomass Yield. Trends Plant Sci. 2009;14:454–461. doi: 10.1016/j.tplants.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 454.Campiglia E., Radicetti E., Mancinelli R. Plant Density and Nitrogen Fertilization Affect Agronomic Performance of Industrial Hemp (Cannabis sativa L.) in Mediterranean Environment. Ind. Crop. Prod. 2017;100:246–254. doi: 10.1016/j.indcrop.2017.02.022. [DOI] [Google Scholar]
- 455.Van der Werf H.M.G., Wijlhuizen M., de Schutter J.A.A. Plant Density and Self-Thinning Affect Yield and Quality of Fibre Hemp (Cannabis sativa L.) Field Crop. Res. 1995;40:153–164. doi: 10.1016/0378-4290(94)00103-J. [DOI] [Google Scholar]
- 456.Small E. Dwarf Germplasm: The Key to Giant Cannabis Hempseed and Cannabinoid Crops. Genet. Resour. Crop Evol. 2018;65:1071–1107. doi: 10.1007/s10722-017-0597-y. [DOI] [Google Scholar]
- 457.Graham L.A., Besser K., Blumer S., Branigan C.A., Czechowski T., Elias L., Guterman I., Harvey D., Isaac P.G., Khan A.M., et al. The Genetic Map of Artemisia Annua L Identifies Loci Affecting Yield of the Antimalarial Drug Artemisinin. Science. 2010;327:328–331. doi: 10.1126/science.1182612. [DOI] [PubMed] [Google Scholar]
- 458.Fairbairn J., Kapoor L. The Lactiferous Vessels of Papaver Somniferum L. Planta Med. 1960;8:49–61. doi: 10.1055/s-0028-1101536. [DOI] [Google Scholar]
- 459.Nessler C.L., Mahlberg P.G. Laticifers in Stamens of Papaver Somniferum L. Planta. 1976;129:83–85. doi: 10.1007/BF00390918. [DOI] [PubMed] [Google Scholar]
- 460.Weid M., Ziegler J., Kutchan T.M. The Roles of Latex and the Vascular Bundle in Morphine Biosynthesis in the Opium Poppy, Papaver Somniferum. Proc. Natl. Acad. Sci. USA. 2004;101:13957–13962. doi: 10.1073/pnas.0405704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Fuller J.G., McMorland G.H., Douglas M.J., Palmer L. Epidural Morphine for Analgesia after Caesarean Section: A Report of 4880 Patients. Can. J. Anaesth. 1990;37:636–640. doi: 10.1007/BF03006481. [DOI] [PubMed] [Google Scholar]
- 462.Stenseth K., Sellevold O., Breivik H. Epidural Morphine for Postoperative Pain: Experience with 1085 Patients. Acta Anaesthesiol. Scand. 1985;29:148–156. doi: 10.1111/j.1399-6576.1985.tb02176.x. [DOI] [PubMed] [Google Scholar]
- 463.Walder B., Schafer M., Henzi I., Tramèr M.R. Efficacy and Safety of Patient-Controlled Opioid Analgesia for Acute Postoperative Pain. Acta Anaesthesiol. Scand. 2001;45:795–804. doi: 10.1034/j.1399-6576.2001.045007795.x. [DOI] [PubMed] [Google Scholar]
- 464.Goldsack C., Scuplak S.M., Smith M. A Double-Blind Comparison of Codeine and Morphine for Postoperative Analgesia Following Intracranial Surgery. Anaesthesia. 1996;51:1029–1032. doi: 10.1111/j.1365-2044.1996.tb14997.x. [DOI] [PubMed] [Google Scholar]
- 465.Walker D.J., Zacny J.P. Subjective, Psychomotor, and Analgesic Effects of Oral Codeine and Morphine in Healthy Volunteers. Psychopharmacology. 1998;140:191–201. doi: 10.1007/s002130050757. [DOI] [PubMed] [Google Scholar]
- 466.Sevelius H., McCoy J.F., Colmore J.P. Dose Response to Codeine in Patients with Chronic Cough. Clin. Pharmacol. Ther. 1971;12:449–455. doi: 10.1002/cpt1971123449. [DOI] [PubMed] [Google Scholar]
- 467.Bolser D.C., Davenport P.W. Codeine and Cough: An Ineffective Gold Standard. Curr. Opin. Allergy Clin. Immunol. 2007;7:32–36. doi: 10.1097/ACI.0b013e3280115145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Freestone C., Eccles R. Assessment of the Antitussive Efficacy of Codeine in Cough Associated with Common Cold. J. Pharm. Pharmacol. 1997;49:1045–1049. doi: 10.1111/j.2042-7158.1997.tb06039.x. [DOI] [PubMed] [Google Scholar]
- 469.Takahama K., Shirasaki T. Central and Peripheral Mechanisms of Narcotic Antitussives: Codeine-Sensitive and -Resistant Coughs. Cough. 2007;152:349–355. doi: 10.1186/1745-9974-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Jackson T., Chougule M.B., Ichite N., Patlolla R.R., Singh M. Antitumor Activity of Noscapine in Human Non-Small Cell Lung Cancer Xenograft Model. Cancer Chemother. Pharmacol. 2008;63:117–126. doi: 10.1007/s00280-008-0720-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Joshi H.C., Zhou J. Noscapine and Analogues as Potential Chemotherapeutic Agents. Drug News Perspect. 2000;13:543–546. doi: 10.1358/dnp.2000.13.9.858482. [DOI] [PubMed] [Google Scholar]
- 472.Rida P.C.G., Livecche D., Ogden A., Zhou J., Aneja R. The Noscapine Chronicle: A Pharmaco-Historic Biography of the Opiate Alkaloid Family and Its Clinical Applications. Med. Res. Rev. 2015;35:1072–1096. doi: 10.1002/med.21357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Ye K., Ke Y., Keshava N., Shanks J., Kapp J.A., Tekmal R.R., Petros J., Joshi H.C. Opium Alkaloid Noscapine Is an Antitumor Agent That Arrests Metaphase and Induces Apoptosis in Dividing Cells. Proc. Natl. Acad. Sci. USA. 1998;95:1601–1606. doi: 10.1073/pnas.95.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Carroll R.J., Leisch H., Rochon L., Hudlicky T., Cox D.P. One-Pot Conversion of Thebaine to Hydrocodone and Synthesis of Neopinone Ketal. J. Org. Chem. 2009;74:747–752. doi: 10.1021/jo802454v. [DOI] [PubMed] [Google Scholar]
- 475.Endoma-Arias M.A.A., Cox D.P., Hudlicky T. General Method of Synthesis for Naloxone, Naltrexone, Nalbuphone, and Nalbuphine by the Reaction of Grignard Reagents with an Oxazolidine Derived from Oxymorphone. Adv. Synth. Catal. 2013;355:1869–1873. doi: 10.1002/adsc.201300284. [DOI] [Google Scholar]
- 476.MacHara A., Werner L., Endoma-Arias M.A., Cox D.P., Hudlicky T. Improved Synthesis of Buprenorphine from Thebaine and/or Oripavine via Palladium-Catalyzed N-Demethylation/Acylation and/or Concomitant O-Demethylation. Adv. Synth. Catal. 2012;354:613–626. doi: 10.1002/adsc.201100807. [DOI] [Google Scholar]
- 477.Murphy B., Šnajdr I., Machara A., Endoma-Arias M.A.A., Stamatatos T.C., Cox D.P., Hudlický T. Conversion of Thebaine to Oripavine and Other Useful Intermediates for the Semisynthesis of Opiate-Derived Agents: Synthesis of Hydromorphone. Adv. Synth. Catal. 2014;356:2679–2687. doi: 10.1002/adsc.201400445. [DOI] [Google Scholar]
- 478.Orman J.S., Keating G.M. Buprenorphine/Naloxone: A Review of Its Use in the Treatment of Opioid Dependence. Drugs. 2009;69:577–607. doi: 10.2165/00003495-200969050-00006. [DOI] [PubMed] [Google Scholar]
- 479.Werner L., Wernerova M., MacHara A., Endoma-Arias M.A., Duchek J., Adams D.R., Cox D.P., Hudlicky T. Unexpected N-Demethylation of Oxymorphone and Oxycodone N-Oxides Mediated by the Burgess Reagent: Direct Synthesis of Naltrexone, Naloxone, and Other Antagonists from Oxymorphone. Adv. Synth. Catal. 2012;354:2706–2712. doi: 10.1002/adsc.201200676. [DOI] [Google Scholar]
- 480.Millgate A.G., Pogson B.J., Wilson I.W., Kutchan T.M., Zenk M.H., Gerlach W.L., Fist A.J., Larkin P.J. Morphine-Pathway Block in Top1 Poppies. Nature. 2004;431:413–414. doi: 10.1038/431413a. [DOI] [PubMed] [Google Scholar]
- 481.Hagel J.M., Facchini P.J. Dioxygenases Catalyze the O-Demethylation Steps of Morphine Biosynthesis in Opium Poppy. Nat. Chem. Biol. 2010;6:273–275. doi: 10.1038/nchembio.317. [DOI] [PubMed] [Google Scholar]
- 482.Fist A.J., Miller J.A.C., Gregory D. Papaver Somniferum with High Concentration of Codeine. WO2009143574. 2009 Dec 3;
- 483.Winzer T., Walker T.C., Meade F., Larson T.R., Graham I.A. Modified Plant. WO2017122011. 2017 Jul 20;
- 484.Winzer T., Gazda V., He Z., Kaminski F., Kern M., Larson T.R., Li Y., Meade F., Teodor R., Vaistij F.E., et al. A Papaver Somniferum 10-Gene Cluster for Synthesis of the Anticancer Alkaloid Noscapine. Science. 2012;336:1704–1708. doi: 10.1126/science.1220757. [DOI] [PubMed] [Google Scholar]
- 485.Guo L., Winzer T., Yang X., Li Y., Ning Z., He Z., Teodor R., Lu Y., Bowser T.A., Graham I.A., et al. The Opium Poppy Genome and Morphinan Production. Science. 2018;362:343–347. doi: 10.1126/science.aat4096. [DOI] [PubMed] [Google Scholar]
- 486.Winzer T., Graham I.A., Walker T.C. Genes Involved in Noscapine Production. WO2013136057. 2013 Sep 19;
- 487.Winzer T., Kern M., King A.J., Larson T.R., Teodor R.I., Donninger S.L., Li Y., Dowle A.A., Cartwright J., Bates R., et al. Morphinan Biosynthesis in Opium Poppy Requires a P450-Oxidoreductase Fusion Protein. Science. 2015;349:309–3012. doi: 10.1126/science.aab1852. [DOI] [PubMed] [Google Scholar]
- 488.Winzer T., Graham I.A., Walker T.C. Production of Noscapine. WO2016207643. 2016 Dec 29;
- 489.Wijekoon C.P., Facchini P.J. Systematic Knockdown of Morphine Pathway Enzymes in Opium Poppy Using Virus-Induced Gene Silencing. Plant J. 2012;69:1052–1063. doi: 10.1111/j.1365-313X.2011.04855.x. [DOI] [PubMed] [Google Scholar]
- 490.Allen R.S., Millgate A.G., Chitty J.A., Thisleton J., Miller J.A.C., Fist A.J., Gerlach W.L., Larkin P.J. RNAi-Mediated Replacement of Morphine with the Nonnarcotic Alkaloid Reticuline in Opium Poppy. Nat. Biotechnol. 2004;22:1559–1566. doi: 10.1038/nbt1033. [DOI] [PubMed] [Google Scholar]
- 491.Sharma J.R., Lal R.K., Gupta A.P., Misra H.O., Pant V., Singh N.K., Pandey V. Development of Non-Narcotic (Opiumless and Alkaloid-Free) Opium Poppy, Papaver Somniferum. Plant Breed. 1999;118:449–452. doi: 10.1046/j.1439-0523.1999.00419.x. [DOI] [Google Scholar]