Abstract
Simple Summary
Defective insulin secretion by pancreatic beta cells is key for the development of type 2 diabetes but the precise mechanisms involved are poorly understood. Metallothioneins are metal binding proteins whose precise biological roles have not been fully characterized. Available evidence indicated that Metallothioneins are protective cellular effectors involved in heavy metal detoxification, metal ion homeostasis and antioxidant defense. This concept has however been challenged by emerging evidence in different medical research fields revealing novel negative roles of Metallothioneins, including in the context of diabetes. In this review, we gather and analyze the available knowledge regarding the complex roles of Metallothioneins in pancreatic beta cell biology and insulin secretion. We comprehensively analyze the evidence showing positive effects of Metallothioneins on beta cell function and survival as well as the emerging evidence revealing negative effects and discuss the possible underlying mechanisms. We expose in parallel findings from other medical research fields and underscore unsettled questions. Then, we propose some future research directions to improve knowledge in the field.
Abstract
Metallothioneins (MTs) are low molecular weight, cysteine-rich, metal-binding proteins whose precise biological roles have not been fully characterized. Existing evidence implicated MTs in heavy metal detoxification, metal ion homeostasis and antioxidant defense. MTs were thus categorized as protective effectors that contribute to cellular homeostasis and survival. This view has, however, been challenged by emerging evidence in different medical fields revealing novel pathophysiological roles of MTs, including inflammatory bowel disease, neurodegenerative disorders, carcinogenesis and diabetes. In the present focused review, we discuss the evidence for the role of MTs in pancreatic beta-cell biology and insulin secretion. We highlight the pattern of specific isoforms of MT gene expression in rodents and human beta-cells. We then discuss the mechanisms involved in the regulation of MTs in islets under physiological and pathological conditions, particularly type 2 diabetes, and analyze the evidence revealing adaptive and negative roles of MTs in beta-cells and the potential mechanisms involved. Finally, we underscore the unsettled questions in the field and propose some future research directions.
Keywords: metallothionein, pancreatic beta-cell, insulin secretion, beta-cell compensation, beta-cell decompensation, stress response, diabetes
1. Introduction
Type 2 diabetes (T2D) is a complex metabolic disorder involving the interaction of predisposing genetic factors and environmental risk elements, and pancreatic beta-cell failure is central to the development of the disease. Indeed, while peripheral insulin resistance is a key clinical predictor of T2D, progression to frank diabetes mellitus requires a further impairment of insulin secretion. In support of this view, non-diabetic obese-insulin resistant subjects can cope with the important metabolic demand by enhanced insulin synthesis and secretion and increased beta-cell mass, thereby preserving normal blood glucose levels at the cost of hyperinsulinemia. This stage is termed beta-cell adaptation or compensation. However, in a subpopulation of obese subjects, this phase is overtaken by a second phase where beta-cells fail to sustain an optimal secretory function, thereby leading to hyperglycemia. The ensuing vicious cycle of glucotoxicity contributes to further alterations of the beta-cell differentiated phenotype and the progressive worsening of the disease overtime. This second phase is termed beta-cell decompensation (these concepts are extensively reviewed in [1,2,3,4,5]). While the magnitude of the compensatory response is thought to be predetermined genetically, the precise molecular mechanisms involved in the progression from successful beta-cell compensation to beta-cell decompensation are poorly understood. A key challenge in diabetes research is the identification and characterization of these mechanisms towards a better understanding of beta-cell pathophysiology, and the development of novel targeted therapeutic strategies to preserve and/or restore the functional beta-cell mass in (pre)T2D subjects.
We have unveiled a novel role of metallothionein 1 (MT1) in beta-cell biology as a negative regulator of glucose-stimulated insulin secretion (GSIS) [6]. Using complementary models and in vivo and ex vivo/in vitro evidence, we demonstrated that Mt1 inhibition potentiated GSIS and improved glucose tolerance, while Mt1 overexpression attenuated the secretory response. These novel findings, together with previous findings in our research field and from other medical research fields, converge to underscore emerging pathophysiological roles of MTs that are beyond and above their classical beneficial involvement in metal homeostasis and antioxidant defense.
In this focused review, we introduce the MT gene family and their characterized biological roles. We then focus on MT gene expression, regulation, and roles in beta-cells. We describe the evidence showing positive effects of MTs on the beta-cell phenotype as well as the emerging evidence revealing negative effects. We also discuss the potential mechanisms underlying the negative effects of MTs on the beta-cell phenotype and expose in parallel findings from other medical research fields. Finally, we underscore unsettled questions and propose some future research directions needed to understand the complex role of MTs in beta-cell pathophysiology.
2. Metallothioneins: The Guardians of Metal and Redox Homeostasis
MTs are highly conserved proteins from prokaryotes to higher vertebrates. In mice, there are four MT isoforms encoded by Mt1, Mt2, Mt3 and Mt4 genes located on chromosome 8. In humans, there are eight different active MT1 genes (MT1A, MT1B, MT1E, MT1F, MT1G, MT1M, MT1X) in addition to MT2 (usually known as MT2A), MT3 and MT4 for a total of 11 functional MT isoforms (Table 1). The human MT genes are located on chromosome 16. While the expression of Mt1 and Mt2 is ubiquitous, Mt3 is mainly expressed in neuronal cells and Mt4 in squamous epithelium cells. Despite important sequence homology, several lines of evidence suggest specific regulatory mechanisms and roles of distinct MT isoforms within various contexts and cell types.
Table 1.
Human metallothionein (MT) protein sequences.
| Protein | ID | AA | Protein Sequences |
|---|---|---|---|
| MT1A | P04731 | 61 | MDPNCSCAT-GGSCTCTGSCKCKECKCTSCKKSCCSCCPMSCAKCAQGCICKGAS------EKCSCCA |
| MT1B | P07438 | 61 | MDPNCSCTT-GGSCACAGSCKCKECKCTSCKKCCCSCCPVGCAKCAQGCVCKGSS------EKCRCCA |
| MT1E | P04732 | 61 | MDPNCSCA-TGGSCTCAGSCKCKECKCTSCKKSCCSCCPVGCAKCAQGCVCKGAS------EKCSCCA |
| MT1F | P04733 | 61 | MDPNCSCA-AGVSCTCAGSCKCKECKCTSCKKSCCSCCPVGCSKCAQGCVCKGAS------EKCSCCD |
| MT1G | P13640 | 62 | MDPNCSCAAAGVSCTCASSCKCKECKCTSCKKSCCSCCPVGCAKCAQGCICKGAS------EKCSCCA |
| MT1H | P80294 | 61 | MDPNCSCEA-GGSCACAGSCKCKKCKCTSCKKSCCSCCPLGCAKCAQGCICKGAS------EKCSCCA |
| MT1L | Q93083 | 61 | MDPNCSCAT-GGSCSCASSCKCKECKCTSCKKSCCSCCPMGCAKCAQGCVCKGAS------EKCSCCA |
| MT1M | Q8N339 | 61 | MDPNCSCTT-GVSCACTGSCTCKECKCTSCKKSCCSCCPVGCAKCAHGCVCKGTL------ENCSCCA |
| MT1X | P80297 | 61 | MDPNCSCSPV-GSCACAGSCKCKECKCTSCKKSCCSCCPVGCAKCAQGCICKGTS------DKCSCCA |
| MT2A | P02795 | 61 | MDPNCSCA-AGDSCTCAGSCKCKECKCTSCKKSCCSCCPVGCAKCAQGCICKGAS------DKCSCCA |
| MT3 | P25713 | 68 | MDPETCPCPSGGSCTCADSCKCEGCKCTSCKKSCCSCCPAECEKCAKDCVCKGGEAAEAEAEKCSCCQ |
| MT4 | P47944 | 62 | MDPRECVCMSGGICMCGDNCKCTTCNCKTYWKSCCPCCPPGCAKCARGCICKGGS------DKCSCCP |
AA: amino acids. Conserved residues between all human MT proteins are highlighted in green. Sequence alignment was performed using the Align tool of the Uniprot database.
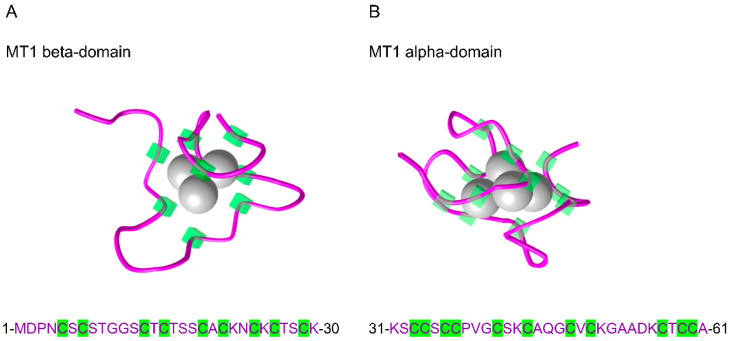
The MT proteins present unique molecular features. First, they have a low molecular mass of about 7 kDa. Second, they are rich in cysteine residues. For example, mouse MT1 is composed of 61 amino acids, of which 19 are cysteines (about 31%) (Figure 1A,B). Third, because of this important thiol content, MTs are able to bind different heavy metal elements, both essential, such as zinc (Zn) and copper (Cu), and toxic, such as cadmium (Cd). Evidence has demonstrated that vertebrate MTs exhibit a specific 3D structure with two distinct functional domains that bind divalent metals. For example, mouse MT1 has an alpha domain and a beta domain that can bind four and three divalent metal atoms, respectively (Figure 1A,B).
Figure 1.
Three-dimensional solution structure of mouse [Cd7]-metallothionein 1 (MT1) obtained by NMR spectroscopy. (A) Mouse MT1 beta-domain with 3 Cd atoms and (B) mouse MT1 alpha-domain with 4 Cd atoms. The amino acid sequence is indicated below each domain structure. The protein sequence is represented in purple, and the cysteine residues are highlighted in green. Data were obtained from the NCBI’s Molecular Modeling Database (MMDB ID 12319 and 12320).
Owing to these characteristics, MTs play a fundamental role in heavy metal detoxification through the chelation of these elements and the reduction of their intracellular levels. Indeed, MT has been identified for the first time in the equine renal cortex as a Cd-rich protein [7,8], and mice lacking Mt1 and Mt2 genes displayed increased sensitivity to Cd and other heavy metal toxicity [9,10,11,12,13,14]. In contrast, mice overexpressing Mt1 were protected against Cd lethality and hepatotoxicity [15]. MT gene promoters enclose metal response element (MRE) motifs [16,17], and their expression is markedly induced by heavy metals. Consequently, MTs have been proposed as useful biomarkers of metal exposure [18,19,20,21,22]. On the other hand, MTs also play key roles in physiological metal homeostasis, in particular Zn homeostasis. Zn is a trace element involved in key cellular processes, including insulin crystallization and storage within the secretory granules [23,24,25], and an important cofactor necessary for the proper function of about 10% of human proteins, including various enzymes and transcription factors [26]. Together with different Zn transporters, MTs are involved in the tight and dynamic regulation of intracellular Zn availability via the processes of chelation/storage, transfer, and release [27,28].
MTs are also involved in antioxidant defense and the regulation of cellular redox homeostasis. Through their sulfhydryl groups, they can scavenge reactive oxygen and nitrogen species (ROS, RNS) [29,30,31]. A subsequent release of Zn from the oxidized proteins leads to the activation of metal responsive transcription factor 1 (MTF1), binding to MREs, and ensuing upregulation of MT gene expression. Moreover, evidence suggested the involvement of oxidized glutathione in Zn release from MT [32], and a role of Zn in glutathione biosynthesis has also been proposed [33]. Zn is also a cofactor of the antioxidant enzymes superoxide dismutase 1 (SOD1) and SOD3, and evidence has shown the possible transfer of Zn from MT to SOD1 [34]. Besides MTF1, MT gene promoters also encompass antioxidant response element (ARE) motifs and can thus be regulated under oxidative stress conditions by other transcription factors [17,35,36,37]. Noteworthy, in comparison to reduced glutathione on a molar basis, the antioxidant activity of MT has been shown to be about 50 times higher against oxidative DNA damage and about 10 times higher against lipid peroxidation [38], and corroborative evidence supported the protective role of MTs against oxidative injury in various experimental models [30,39,40,41,42,43,44,45,46,47,48,49,50,51].
Moreover, MTs have been shown to play complex immunomodulatory roles and have been connected to inflammation and defense against infection [52]. While the precise pathways involved are only partially identified, it has been proposed that MT regulation of immune cell development and function may depend, at least in part, on their metal handling properties and antioxidant function [53].
In pancreatic beta-cells, oxidative stress is a central mechanism involved in the alteration of the beta-cell differentiated phenotype under chronic hyperglycemia [3,54,55,56,57]. Moreover, metal ions play essential physiological roles in beta-cells, and metal dyshomeostasis has been linked with the beta-cell demise in experimental models and was associated with diabetes and its complications in human subjects [58,59,60,61,62,63]. Interestingly, several clinical and epidemiological reports have shown that both type 1 diabetes (T1D) and T2D states are characterized by hypozincemia [64,65,66,67,68,69]. Furthermore, polymorphisms in MT1A and MT2A genes have been associated with increased risk for T2D and diabetic complications in humans [70,71,72]. However, while several studies have shown protective effects of MTs under different stress conditions both in vitro/ex vivo and in vivo, especially in the models of chemically induced diabetes and in the context of islet transplantation, emerging evidence, in contrast, revealed negative effects of MTs on beta-cell function and diabetes development via noncanonical mechanisms.
Before discussing and analyzing this emerging evidence, it is important to first describe the pattern of MT gene expression in rodents and human beta-cells.
3. Metallothioneins in Pancreatic Beta Cells
3.1. Expression of Metallothionein Genes in Mouse and Human Beta Cells
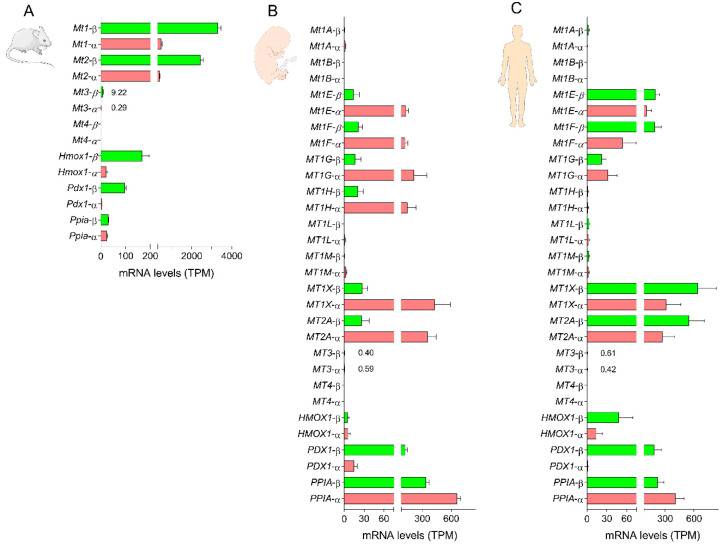
The presence of constitutive and/or inducible MT expression in mouse and human islets has been known for decades [73,74,75,76]. Analysis of more recent RNA sequencing data of purified mouse islet cell preparations revealed that Mt1 and Mt2 were strongly expressed in beta-cells in comparison to the stress response gene Hmox1, the beta-cell enriched gene Pdx1 or the house-keeping gene Ppia, for example, with Mt1 exhibiting relatively higher mRNA levels than Mt2. On the other hand, Mt3 was expressed to a much lower extent, while Mt4 mRNAs were not detected (Figure 2A). We observed a similar expression pattern in whole mouse islets [6]. In alpha cells, Mt1 and Mt2 were also markedly expressed, yet without a difference between Mt1 and Mt2 and to a lower level in comparison to beta-cells, while Mt3 was barely detectable and Mt4 was absent (Figure 2A). The elevated basal expression of Mt1 and Mt2 in mouse beta-cells suggests the potential essential biological roles of these genes in insulin-secreting cells.
Figure 2.
Pattern of metallothionein gene expression in pancreatic beta and alpha cells. (A) Changes in the mRNA levels of Mt1-4, Hmox1, Pdx1 and Ppia in purified mouse beta and alpha cell preparations. Data are the means of the average number of transcripts per million (TPM) ± SEM for 5 (beta) and 3 (alpha) preparations. RNA sequencing data were obtained from the Gene Expression Omnibus database under the accession number GSE80673 [77]. (B) Changes in the mRNA levels of MT1A, MT1B, MT1E, MT1F, MT1G, MT1M, MT1X, MT2A, MT3, MT4, HMOX1, PDX1 and PPIA in purified human fetal beta and alpha cell preparations. (C) Changes in the mRNA levels of MT1A, MT1B, MT1E, MT1F, MT1G, MT1M, MT1X, MT2A, MT3, MT4, HMOX1, PDX1 and PPIA in purified human adult beta and alpha cell preparations. Data are the means of the average number of TPM ± SEM for 5 (fetal alpha), 6 (adult alpha and fetal beta) and 7 (adult beta) preparations. RNA sequencing data were obtained from the Gene Expression Omnibus database under the accession number GSE67543 [78].
In humans, analysis of RNA sequencing data of purified islet cell preparations from fetal and adult donors revealed interesting findings. Thus, in immature fetal cells, MT1A, MT1B, MT1L, MT1M, MT3 and MT4 were either barely expressed or absent in both alpha and beta-cells. In contrast, MT1E, MT1F, MT1G, MT1H, MT1X and MT2A were expressed in both cell types, but about 6–16 times higher in fetal alpha vs. beta-cells (Figure 2B). Strikingly, in adult mature beta-cells, MT1E, MT1F, MT1X and MT2A mRNA levels were strongly upregulated by about 9–23-fold in comparison to fetal beta-cells and were also slightly higher in comparison to adult alpha cells (Figure 2C). Such maturity-dependent regulation appears to be unique to beta-cells since there was only a minor decrease in the expression of these genes in adult vs. fetal alpha cells. On the other hand, MT1G mRNA levels were slightly increased in adult beta-cells and markedly downregulated in adult alpha cells in comparison to fetal cells, while MT1H expression was strongly repressed in both cell types (Figure 2C). These observations underscore the specific pattern of MT gene expression in human alpha and beta-cells and suggest that MT1E, MT1F, MT1X and MT2A are important traits of differentiated mature human beta-cells and that they may play significant biological roles.
Ours and others’ evidence has demonstrated that MT gene expression in beta-cells is regulated by various physiological and stress stimuli, including metals and glucose.
3.2. Physiological and Pathological Regulation of MT Gene Expression in Beta Cells
3.2.1. Regulation by Metals
As explained in Section 2, it is well established that MTs are highly responsive to physiological and toxic metal exposure in different organ systems and cell types. Unsurprisingly, this is also the case in pancreatic islets and beta-cells. Thus, earlier evidence has shown that Cd administration upregulated MT protein levels in the mouse pancreas [73]. In agreement, exposure of the mouse beta-cell line MIN6 to Cd augmented MT1 and MT2 protein levels [79]. Moreover, Zn exposure markedly increased Mt1 mRNA levels in mouse, rat and chicken pancreatic endocrine and exocrine cells [80]. Furthermore, intraperitoneal injection of Zn salts in mice upregulated MT protein levels in pancreatic islets [74,75]. Similarly, exposure of mouse and rat islets to Zn salts ex vivo strongly increased Mt1 and Mt2 mRNA and protein levels [81,82,83]. In the rat beta-cell line INS1E, the mRNA levels of Mt1a, but not Mt3, were upregulated by ZnCl2 treatment and reduced by Zn chelation [84].
Thus, congruent evidence has demonstrated the regulation of Mt1 and Mt2 gene expression in pancreatic beta-cells in vitro/ex vivo and in vivo by metal exposure. As will be discussed in Section 4, this regulatory mechanism has been exploited to protect beta-cells from injury in some experimental models. Besides metals, MT gene expression in pancreatic beta-cells is also regulated by glucose.
3.2.2. Regulation by Glucose
Glucose is the key driver of beta-cell function and homeostasis. Indeed, regular physiological stimulation by glucose and other nutrients is necessary to maintain the beta-cell differentiated phenotype and preserve beta-cell responsiveness to the next glucose challenge [85]. Conversely, prolonged exposure to under- or supra-physiological glucose levels negatively affects the beta-cell differentiated phenotype in parallel with the activation of various stress response pathways, impaired GSIS, and increased cell death [3,55,86].
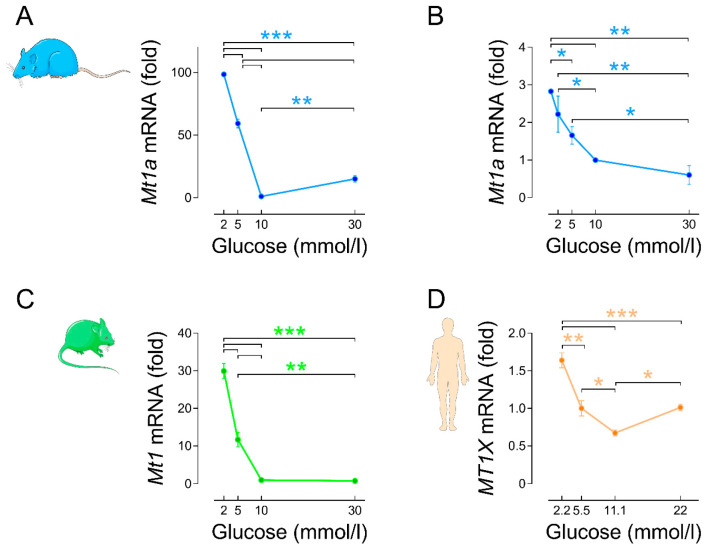
We have previously found that Mt1a and Mt2a (the equivalent of mouse Mt1 and Mt2) were among the 10 most-affected glucose-regulated genes in the transcriptome of rat islets [87]. Interestingly, Mt1a and Mt2a mRNA levels were strongly downregulated by 18 h culture in the presence of 10 mmol/L glucose (G10; the optimal concentration for rodent islet function and survival ex vivo [87,88,89,90]) instead of G2-5 (low non-stimulatory of insulin secretion). On the other hand, they were upregulated by culture in G30 vs. G10, thereby displaying an asymmetric V-shape expression profile with stronger downregulation (G10 vs. G2-5) than upregulation (G30 vs. G10) [87] (Figure 3A).
Figure 3.
Mt1a, Mt1 and MT1X mRNA levels are downregulated by glucose stimulation. (A) Changes in the mRNA levels of Mt1a in rat islets cultured for 18 h in the presence of increasing glucose concentrations (G2, G5, G10, G30). Islet isolation and culture, RNA extraction and RT-real time PCR were performed as previously described [87]. The primers used for Mt1a and the housekeeping gene Tbp are the same as in [87]. Data are means ± SEM of n = 4 experiments. (B) Changes in the mRNA levels of Mt1a in the rat beta-cell line INS 832/13 cultured for 24 h in the presence of increasing glucose concentrations (G0.5, G2, G5, G10, G30). INS 832/13 cells were kindly provided by Dr. Christopher Newgard, Duke University. Cell culture, RNA extraction and real-time PCR were performed as previously described [91]. Data are means ± SEM of n = 3 experiments. (C) Changes in the mRNA levels of Mt1 in mouse islets cultured for 24 h in the presence of increasing glucose concentrations (G2, G5, G10, G30). Data were taken from [6] with permission of the publisher. Data are means ± SEM of n = 4 experiments. (D) Changes in the mRNA levels of MT1X in human islets from non-diabetic donors cultured for 24 h in the presence of increasing glucose concentrations (G2.2, G5.5, G11.1, G22). Data were taken from [6] with permission of the publisher. Data are means ± SEM of n = 3 experiments. * p < 0.05, ** p < 0.01, *** p < 0.001 for the indicated comparisons; one-way ANOVA with Newman–Keuls post hoc test.
This upregulation is transient since no significant difference was observed after prolonged culture under G30 in comparison to G10 [83]. We also confirmed the glucose-dependent inhibition of Mt1a gene expression in the rat beta-cell line INS1 832/13 (Figure 3B). In mouse islets, Mt1 and Mt2 mRNA and protein levels were also markedly downregulated by culture in G10-30 vs. G2-5 [6,82] (Figure 3D). In contrast, Mt3 mRNA levels were increased by glucose stimulation in rat and mouse islets [6,87]. In human islets, we found that glucose also downregulated MT1E, MT1X and MT2A mRNA levels in a concentration-dependent manner after culture in the presence of G2.2, G5.5 (the optimal concentration for human islets ex vivo [92,93]) and G11.1. Culture in the presence of G22 vs. G11.1 slightly upregulated MT1E and MT1X mRNA levels to a similar extent as with G5.5 [6] (Figure 3E). Collectively, these observations indicate that glucose stimulation overall inhibits MT gene expression in rodent and human islets. Noteworthy, this effect is in an inverse relationship with the stimulation of insulin secretion.
In contrast to negative glucose regulation, evidence has demonstrated that MT gene expression in beta-cells is markedly induced by various stress stimuli, including oxidative stress, proinflammatory cytokines, endoplasmic reticulum (ER) stress, and hypoxia.
3.2.3. Regulation by Stress Stimuli
Earlier studies have shown that MT expression in mouse islets is upregulated ex vivo and in vivo by streptozotocin (STZ) treatment [74,81]. STZ is a glucosamine-nitrosourea alkylating agent that is particularly toxic to beta-cells [94], most likely via increased ROS generation and subsequent oxidative damage [95,96,97,98]. In agreement, we observed that exposure of rat islets to H2O2 markedly increased Mt1a mRNA levels [86]. Oxidative stress is a master mechanism involved in beta-cell demise under diabetes, not only because of the important macromolecular damage induced but also because ROS and RNS can activate other deleterious stress pathways. Alternatively, the activation of different stress pathways can lead to oxidative stress, and inflammation is one of these pathways.
Thus, we found that rat islet Mt1a mRNA levels were strongly upregulated by treatment with the proinflammatory cytokine IL1β [86]. MT protein levels were also upregulated by IL1β treatment in rat islets and beta-cell lines and by the combination of IL1β + TNFα in mouse islets [99]. Similarly, analysis of the transcriptome of purified rat beta-cells exposed to the proinflammatory cytokines IL1β, IL1β + IFNγ or TNFα + IFNγ also revealed marked and time-dependent upregulation of Mt1a mRNA levels [100,101]. Upregulation of Mt1a by proinflammatory cytokines may involve oxidative stress [102,103]. Interestingly, in the latter transcriptomics studies, upregulation of Mt1a mRNA levels was paralleled by upregulation of other oxidative stress-responsive genes, including catalase, several glutathione s-transferase genes and Sod2 [100,101]. In contrast, MT induction by IL1β in the mouse beta-cell line βHC9 was not altered by inducible nitric oxide synthase inhibitors, while the NO generator sodium nitroprusside failed to significantly affect MT protein levels thereby suggesting that NO may not play a significant role in MT induction [99]. On the other hand, MT gene promoters enclose signal transducer and activator of transcription (STAT) binding sites and may thus be induced via this pathway upon cytokine stimulation in beta-cells [17,104].
Besides cytokines, evidence indicated that MT gene expression is also induced by glucocorticoid hormones. Thus, mouse Mt1 and Mt2 and several human MT gene promoters have been shown to enclose glucocorticoid response element (GRE) motifs [17,105,106]. In rodent islets and insulinoma cell lines, dexamethasone treatment time-dependently upregulated MT protein levels and displayed a synergistic effect when combined with cytokines [99].
Evidence also has suggested a role for lipotoxicity in the regulation of MT gene expression. Thus, exposure of human islets to 500 µmol/L palmitate acutely and transiently upregulated the mRNA levels of several MT genes [107]. Moreover, chronic exposure of human islets to 400 µmol/L oleates increased MT1F mRNA levels in association with increased ROS generation and upregulation of catalase [108]. Elevated levels of free fatty acids (FFAs) may induce beta-cell oxidative stress, likely via a mechanism involving peroxisomal H2O2 generation [109]. Alternatively, it is well established that FFAs trigger beta-cell ER stress [110]. In support of the role of ER stress in the regulation of MT gene expression, we found that the pharmacological ER stress inducer thapsigargin strongly increased the mRNA levels of Mt1a in rat islets [86].
Rodent and human islet MT gene expression is also regulated by hypoxic stress. Thus, rat islet Mt1a mRNA levels were significantly upregulated by culture in the presence of 5% instead of 20% O2 [86]. A stronger effect was observed for Mt1 when mouse islets were cultured in the presence of 1% vs. 20% O2 [6]. Likewise, mouse islet Mt2 mRNA levels were upregulated by hypoxia, while, in contrast, Mt3 mRNA levels were downregulated [111]. In human islets, hypoxia (1% O2) also markedly upregulated MT1A and, to a stronger extent, MT2A mRNA levels [111].
These types of stress characterize the (pre)diabetic milieu, and some of them are already present at the stage of beta-cell compensation in db/db mice, including oxidative stress and inflammation [112]. However, unexpectedly, we found that Mt1 and Mt2 are differentially regulated in conditions of beta-cell compensation and failure in vivo.
3.2.4. Regulation during Beta Cell Compensation and Failure
To examine the temporal changes of islet Mt1 and Mt2 gene expression under the states of beta-cell compensation and failure, we used two mouse models of obesity with an opposite propensity for the development of diabetes: (1) the diabetes resistant ob/ob mice on the C57BL/6J genetic background and (2) the diabetes-prone db/db mice on the C57BL/KsJ genetic background, which exhibit a progressive age-dependent beta-cell decompensation and development of hyperglycemia [112,113,114]. Thus, we found that Mt1 and Mt2 mRNA levels were markedly decreased in the islets of compensating ob/ob mice at 6 and 16 weeks of age vs. age-matched lean control mice. Interestingly, in db/db mice, islet Mt1 and Mt2 mRNA levels were similarly decreased in 6-week-old prediabetic mice (compensation) but were increased in 16-week-old diabetic mice (beta-cell failure) in comparison to age-matched lean control mice [6]. Moreover, we found that increased plasma insulin levels (beta-cell compensation) correlated with a strong downregulation of Mt1 and Mt2 mRNA levels in the islets of diet-induced obese (DIO) mice (6 weeks of high-fat diet) [6].
In humans, a previous study examining the transcriptome of islet tissue obtained by laser capture microdissection from non-diabetic subjects and subjects with T2D revealed a significant upregulation of MT1E, MT1M, MT1X and MT2A mRNA levels in the diabetic group. In this study, MT1E, MT1X and MT2A displayed the strongest array signals among the MT gene isoforms [115]. In agreement, we subsequently found a significant increase in MT1X mRNA levels in the islets of donors with T2D vs. non-diabetic donors [6].
While upregulation of islet MT genes under diabetes may be explained by an effect of the unfavorable glucolipotoxic and proinflammatory diabetic milieu, the downregulation of islet Mt1 and Mt2 mRNA levels in compensating 6-week-old db/db mice and DIO mice, despite the presence of an evident stress signature in these islets [112,116,117], is intriguing and suggests a different regulatory mechanism and a biological role other than antioxidant defense in this context.
Collectively, these in vivo findings highlight a negative correlation between beta-cell compensation and Mt1-Mt2 gene expression. Taken together with the inhibitory effect of glucose on islet MT gene expression ex vivo, these observations raise the hypothesis that changes in Mt1 and/or Mt2 gene expression may play a role in the modulation of beta-cell function. However, if upregulation of MT gene expression has a negative impact on beta-cell function, then upregulation of MT gene expression in diabetes may play both adaptive and negative roles.
4. Adaptive Roles of MTs in Beta Cells
Past evidence has shown that exposure of isolated mouse islets to Cd reduced GSIS. However, when mice were pretreated with Cd prior to islet isolation which upregulated MT protein levels in the pancreas, subsequent exposure of isolated islet to Cd did not reduce GSIS. This was the first study suggesting a protective role of MT in beta-cell against acute Cd toxicity [73]. Similarly, pretreatment of mice with Zn-enriched drinking water increased islet MT protein levels and partially protected against diabetes induced by multiple low doses of STZ [75]. In isolated rat islets, supplementation of culture medium with ZnCl2 markedly augmented Mt1a and Mt2a gene expression and significantly reduced beta-cell apoptosis induced by prolonged culture in the presence of G5 or G30 instead of G10 [83]. Several Zn supplementation strategies have been tested in experimental models of diabetes as well as in humans [118]. Some of these studies revealed beneficial effects and improvement of glycemic control, while other studies showed no significant effect or even a negative effect [119,120,121,122]. Importantly, the observed beneficial effects in some of these studies cannot be ascribed with certainty to the induction of MTs given the pleiotropic effects of Zn, and hence transgenic mouse models have provided more solid insights about the protective actions of MTs in beta-cell.
Thus, in comparison to control FVB mice, transgenic mice overexpressing the human MT2A gene under the control of the human insulin promoter (MT2A-Tg) exhibited reduced hyperglycemia after STZ injection. Moreover, in comparison to control islets, transgenic islets showed reduced DNA fragmentation, NAD+ depletion, degranulation, and necrosis upon STZ treatment ex vivo [123]. When transferred on the C57BL/6 J background, these transgenic mice also displayed a marked reduction of hyperglycemia after STZ treatment [124]. Moreover, in response to acute exposure to various prooxidant agents, MT2A-Tg dispersed islet cells exhibited reduced intracellular ROS levels vs. control FVB islet cells. A similar result was obtained upon exposure of whole islets to hypoxia (1% O2) in association with higher cell viability in MT2A-Tg islets. In agreement, when transplanted into diabetic mice, MT2A-Tg islets extended the duration of euglycemia, and transgenic islet grafts exhibited reduced nitrotyrosine staining and higher insulin staining. Furthermore, NO-induced damage was also attenuated in MT2A-Tg islets vs. control FVB islets ex vivo [125]. In contrast, Mt1-Mt2-KO islets showed a higher cell death rate under hypoxia (1% O2) in comparison to control islets [111].
In parallel with transgenic mouse evidence, another strategy uses recombinant MT proteins fused with protein transduction domains (also known as cell-penetrating peptides). Owing to their positive charge, these peptides can bind negatively charged plasma membrane, thereby enhancing the intracellular delivery of the fusion protein. Thus, using a basic domain of HIV-1 Tat protein, Tat-MT1A fusion protein was developed and has been shown to slightly protect INS1 cells against glucolipotoxicity and hypoxia [126]. The same group showed in a subsequent study a protective effect of Tat-MT1A pretreatment against glucolipotoxicity in INS1 cells and rat islets. This effect was associated with reduced ROS production and nuclear factor κB activation [127]. In vivo, intraperitoneal injection of Tat-MT1A in mice showed possible delivery into islets, and mice receiving 8 injections of Tat-MT1A over 11 days were partially protected against diabetes induced by multiple low doses of STZ. Moreover, Tat-MT1A treatment every 3 days for 18 weeks slightly improved glucose tolerance in OLETF rats and delayed diabetes onset [127]. The combination Tat-MT1A + Tat-SOD1 also showed a protective effect against islet injury and delayed diabetes development in vivo [128]. In the context of islet transplantation, this strategy has been shown to enhance islet graft survival and glycemic control [128,129,130].
All in all, these cumulative findings reveal an important protective antioxidant role of MT in beta-cells and suggest that MT gene induction in the islets of diabetic animal models and human subjects may play a key adaptive role. Nevertheless, this evidence has been challenged by previous and recent emerging findings revealing negative roles of MTs in beta-cells.
5. Negative Roles of MTs in Beta Cells
Proinflammatory cytokines are involved in beta-cell demise in T1D and T2D, and this effect is mediated, at least in part, via increased ROS and RNS production and subsequent oxidative damage. In addition, infiltrating immune cells can also produce ROS and RNS, thereby exacerbating islet oxidative stress. Therefore, overexpression of MT has been tested as a strategy to protect beta-cells in the T1D model of non-obese diabetic (NOD) mice. These MT2A-Tg NOD mice displayed normal glucose tolerance before diabetes induction, and their islets had normal expression of ER stress genes (Hspa5, Ddit3) [131]. Surprisingly, in comparison to control NOD mice, MT2A-Tg NOD mice exhibited a marked acceleration of diabetes onset after cyclophosphamide injection (used to accelerate and synchronize diabetes development in this model). This was associated with reduced pancreatic insulin content and increased cleaved-caspase 3 immunostaining. Moreover, spontaneous diabetes onset was also accelerated in male, but not female, MT2A-Tg NOD mice. Furthermore, isolated MT2A-Tg NOD islets were more sensitive to the proinflammatory cytokines IL1β + IFNγ + TNFα. Thus, despite a clear attenuation of cytokine-induced ROS generation, these islets displayed increased cleaved-caspase 3 protein levels, reduced AKT and FOXO1 phosphorylation, reduced PDX1 protein levels, and reduced metabolic activity. The addition of vanadate to the culture medium preserved MT2A-Tg NOD islets shape and metabolic activity under cytokine treatment, thereby suggesting a potential role of protein tyrosine phosphatase activity in the sensitivity of MT2A-Tg NOD islets to proinflammatory cytokines [131]. Accelerated diabetes onset in NOD mice also has been triggered, though to a lesser extent, by catalase, but not SOD2, transgenic overexpression (no difference in the latter model) [131]. Nevertheless, in the same model, other studies have shown a protective effect of beta-cell transgenic overexpression of Hmox1 [132] and islet overexpression of Txn [133] and Sod2 [134]. This indicates that the negative impact of MT2A overexpression is unlikely to be related to ROS scavenging properties.
On the C57BL/6 J background, MT2A-Tg mice exhibited an age-dependent worsening of glucose tolerance in association with reduced plasma insulin levels during intraperitoneal glucose tolerance test (ipGTT), reduced pancreatic insulin content and reduced islet size. This phenotype was more pronounced in males than in females. Moreover, GSIS was abolished in the transgenic islets in comparison to control islets. Abrogation of GSIS in this model seems to result from an important alteration of the beta-cell differentiated phenotype. Indeed, the MT2A-Tg male islets displayed a marked reduction in the mRNA levels of several beta-cell enriched genes (Ins1, Pdx1, Neruod1, Slc2a2, Gck) in parallel with significant upregulation of stress response genes (Hmox1, Trib3, Tnfrsf1b) [124]. Beta-cell demise in this model may be a consequence of ER and/or oxidative stress. The latter may stem from the high expression level driven by the human insulin promoter and subsequent high biosynthesis rate of MT2A that exceeds the ER folding capacity. A role of (islet resident) macrophages or other immune cells is also possible given that MTs have chemokine-like actions and can activate immune cells, which may, in turn, affect beta-cells by producing proinflammatory cytokines and ROS/RNS (discussed in Section 6).
Although expressed to a much lesser degree in comparison to Mt1 and Mt2, Mt3 may also play a negative role in beta-cells. One study has shown that Mt3-KO mice were protected against STZ-induced diabetes and Mt3-KO islets were resistant to STZ and NO toxicity ex vivo. This effect has been proposed to involve a partial reduction of phosphodiesterase 3A expression [135]. However, other mechanisms may be involved. Moreover, it is unclear if these islets presented a compensatory upregulation of other MT gene isoforms.
On the other hand, given the differential expression of Mt1 and Mt2 in the contexts of beta-cell compensation and failure in vivo, and the inverse correlation between Mt1-Mt2 gene expression and insulin secretion in isolated islets, we verified whether changes in Mt1 and/or Mt2 gene expression might have an impact on beta-cell function using a comprehensive approach and complementary models [6].
Thus, in Mt1-Mt2-KO mice on the Sv129 genetic background [10], we observed a significantly improved glucose tolerance during ipGTT in association with increased plasma insulin levels during the test. In agreement, an overnight fasting-1 h refeeding test revealed that Mt1-Mt2-KO mice displayed lower blood glucose levels after refeeding. Of note, Mt1-Mt2-KO mice showed a higher daily food intake in comparison to control mice. In parallel, insulin tolerance tests did not reveal a difference between control and Mt1-Mt2-KO mice, thereby suggesting that improved glucose tolerance in these mice stems from an effect of Mt1-Mt2 deletion on insulin secretion and not insulin action. In support of this mechanism, isolated Mt1-Mt2-KO islets showed enhanced GSIS in comparison to control islets without a difference in islet insulin content. Furthermore, in MIN6 cells, Mt1 knockdown, but not Mt2, also augmented GSIS, thereby underscoring the role of MT1 in the negative regulation of insulin secretion. Changes in cytosolic free calcium (Ca2+) and Zn2+ concentrations in response to acute stepwise increases in glucose levels were similar between control and Mt1-Mt2-KO islets. Similarly, changes in intracellular NAD(P)H levels in response to acute glucose stimulation were not different between the two types of islets, thereby suggesting that the enhanced secretory phenotype involves a mechanism downstream of glucose metabolism and Ca2+ influx. Importantly, besides glucose, the potentiation of insulin secretion in Mt1-Mt2-KO vs. control islets was also observed in response to 30 mmol/L potassium salt (KCl). High K+ levels depolarize the beta-cell plasma membrane and trigger insulin secretion independently from glucose metabolism [6]. Together, these findings point to a potential effect of Mt1-Mt2 deletion on the secretory machinery.
Moreover, to assess whether Mt1 overexpression results in the opposite phenotype to Mt1 deletion, we used transgenic mice overexpressing mouse Mt1 under the control of its natural promoter in a position-independent and copy-dependent manner on the C57BL/6 J background (Mt1-Tg) [136,137]. In comparison to MT2A-Tg mice [124], this model presents the advantage of overexpressing the mouse Mt1 gene rather than the human MT2A gene. It is important here to emphasize that MT1 and MT2 are not always redundant and present differences at the level of amino acid sequence, protein–protein interactions as well as metal affinities, and hence exhibit functional differences as we saw in our study [138,139]. The use of the strong human insulin promoter and subsequently enhanced MT2A protein synthesis may explain, at least in part, the stress signature observed in MT2A-Tg islets [124]. Indeed, our Mt1-Tg islets displayed a normal expression of beta-cell enriched genes and stress response genes [6]. Moreover, Mt1-Tg mice had normal fed and fasted blood glucose levels and normal glucose tolerance. However, they displayed lower blood glucose levels during the insulin tolerance test, an effect that may result from an impact of MT1 overexpression on peripheral tissues. Importantly, isolated Mt1-Tg islet exhibited a significant attenuation of GSIS vs. control islets. This effect was independent of changes in islet insulin content and intracellular free Ca2+, Zn2+ and NAD(P)H levels, thereby further supporting the assumption that MT1 impacts GSIS at a level downstream of glucose metabolism and Ca2+ influx, in harmony with the results from Mt1-Mt2-KO islets [6].
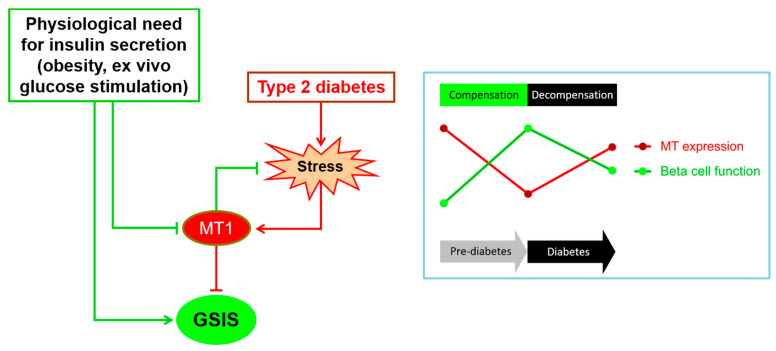
Taken together, these corroborative findings strongly support the involvement of MT1 in the negative regulation of insulin secretion in mouse beta-cells. Therefore, Mt1 downregulation in obesity may be an important component of the beta-cell compensatory response. In contrast, its upregulation in T2D may contribute to impaired secretory function, although it may have a parallel antioxidant role (Figure 4). However, several questions remain unsettled.
Figure 4.
The proposed model of the role of MT1 in the pathogenesis of type 2 diabetes. Under physiological conditions requiring the stimulation of insulin secretion, Mt1 gene expression is repressed, thereby allowing enhanced secretory response to meet the metabolic needs. In contrast, under diabetes conditions, the unfavorable diabetic environment and subsequent induction of oxidative stress and other stress pathways lead to the upregulation of Mt1 gene expression. The latter is a double-edged sword that will, on one hand, counter beta-cell stress, at least in part through scavenging of ROS, and on the other hand, inhibit GSIS. These events occur sequentially during the natural history of diabetes, as depicted in the insert. In the prediabetic phase (beta-cell compensation), the beta-cell function is markedly enhanced in parallel with significant downregulation of MT gene expression. Conversely, in the diabetic phase (beta-cell decompensation), beta-cell function declines in parallel with significant upregulation of MT gene expression. GSIS: glucose-stimulated insulin secretion.
6. Unsettled Questions and Future Research Directions
The precise molecular pathway(s) underlying the inhibitory action of MT1 on insulin secretion is(are) elusive at this stage. Our functional data support an impact on exocytosis. One plausible mechanism may involve an interaction of MT1 with component(s) of the secretory machinery to modulate insulin granule dynamics. In this regard, MT3 has previously been shown to interact with RAB3A and has been proposed to play a role in presynaptic vesicle trafficking [140]. MT3 has also been shown to interact with SEC84 (also known as Exo84p), a subunit of the exocyst that is involved in secretory vesicle targeting and docking at the plasma membrane [141,142]. An interaction between MT and α-synuclein has also been proposed [143]. The latter has been shown to play a role in the regulation of insulin secretion in beta-cells [144,145,146]. Most of the investigation in this area has focused on MT3-protein interactions in the central nervous system [138]. Therefore, further investigation is needed to decipher the precise interacting partners of MT1 in beta-cells. It would also be important to determine the precise subcellular localization of MT1 under non-stimulatory and glucose stimulatory conditions. These experiments will be important to better understand the role of MT1 in beta-cell biology. Unfortunately, because of important sequence homology between MT1 and MT2 proteins, reliable specific antibodies against MT1 are at present unavailable. The development of a specific MT1 antibody would be important not only for immunolocalization studies but also from a future therapeutic perspective.
In humans, it is currently unknown whether MT1X and/or other MT gene isoforms are affected in the islets of obese compensating subjects and how. However, noteworthy, evidence has shown downregulation of MT1X and MT1G expression in lymphoblastoid cells of male subjects with non-syndromic obesity in comparison to lean subjects [147]. Moreover, it is unclear whether MT1X (or another human MT isoform) plays a role in human beta-cells similar to that of MT1 in mouse beta-cells. Thus, further gene inhibition/overexpression studies in human beta-cell lines and primary islets are required to shed light on the potential involvement of MT1X in the inhibition of insulin secretion in humans.
Upregulation of islet MT gene expression in T2D is associated with alteration of the beta-cell dedifferentiated phenotype. The phenomenon of dedifferentiation has been proposed as an adaptive mechanism to mitigate cell death at the expense of insulin secretory dysfunction under specific diabetic stress conditions [55]. It is possible that MT contributes to this phenomenon via parallel attenuation of insulin secretion and protection against oxidative stress, but it is unclear whether beta-cell dedifferentiation plays a role upstream of MT gene induction.
Another big question concerns the mechanisms underlying the downregulation of Mt1 and Mt2 during beta-cell compensation. The potential involvement of metal dyshomeostasis in this regulation is not supported by a previous study that showed no elemental difference in the beta cells of prediabetic Chinese hamsters [148]. Additionally, it has been proposed that diabetes development in db/db mice was not associated with reliable elemental changes in beta-cells [149]. However, the use of the novel synchrotron X-ray fluorescence imaging system to compare the elemental profiles of whole cryofrozen islets from compensating and decompensating mice may reveal novel insights [150]. Otherwise, in other cell systems, several mechanisms have been proposed to repress MT gene expression, including transcriptional repressors and promoter methylation [17,151]. These mechanisms may play a role in the regulation of Mt1 and Mt2 under the states of beta-cell compensation and decompensation. Understanding these molecular events and potential upstream regulators (hormones, metabolism/metabolites, metals?) is important from a fundamental and translational point of view.
Similarly, the mechanism(s) involved in the downregulation of islet Mt1 and Mt2 gene expression by glucose stimulation ex vivo is (are) also unclear. Interestingly, pharmacological glucokinase activation under low glucose conditions also downregulated Mt1a mRNA levels in rat islets [152]. This indicates that it is not glucose per se that inhibits Mt1a gene expression but rather signals engendered by the acceleration of mitochondrial metabolism and/or the stimulation of insulin secretion. Reduced mitochondrial superoxide anion production upon acute glucose stimulation may also play a role [153,154,155]. Whether changes in metals are also involved is uncertain. However, we observed that the Zn transporter gene Slc30a1 (also known as Znt1), which is involved in Zn efflux from the cell, is also markedly downregulated by glucose stimulation in parallel to Mt1a and Mt2a, which may reflect changes in Zn intracellular levels [87]. In addition, Mt1a and Mt2a mRNA upregulation by culture in G5 vs. G10 was abrogated by Zn chelation [83]. Prolonged culture in the presence of low glucose concentrations may trigger lysosomal insulin granule degradation [156]. Therefore, it is possible that insulin granule degradation under low nutrient conditions releases Zn ions that lead to upregulation of MT gene expression. In contrast, nutrient stimulation and subsequent increase in proinsulin biosynthesis may channel cellular Zn to the secretory granules for insulin crystallization and packaging, which may contribute to reduced MT gene expression. This tempting hypothesis is, however, in disagreement with the reported increase in cytosolic free Zn in mouse beta-cells cultured for 24h in G16.7 vs. G3 [82]. But since cellular Zn dynamics are complex and differences may exist between the different subcellular compartments, further investigation using complementary models and novel technological tools is needed to uncover the precise mechanisms involved in the downregulation of MT gene expression by glucose stimulation and determine if Zn plays a role or not. Besides Zn, several reports revealed Cu dyshomeostasis in T1D and T2D subjects [63,64,67,68,157,158]. Cu is an important cofactor for different enzymes, including SOD1 and mitochondrial cytochrome c oxidase. However, the presence of high concentrations can play a role in oxidative stress (Fenton reaction). Cu also induces MT gene expression, and MTs are involved in the regulation of Cu homeostasis [139]. In cultured rat islets, we previously observed that changes in the mRNA levels of the Cu high-affinity transporter Slc31a1 (also known as Ctr1) in response to increasing glucose concentrations paralleled those of Mt1a and Mt2a, which may reflect parallel changes in Cu intracellular levels [87]. Therefore, the role of Cu in the regulation of MT gene expression under these conditions cannot be excluded.
Interestingly, MTs may also have a chemokine-like role. They have been shown to be present in different extracellular body fluids and potentially secreted by cells, although the mechanism involved is unknown (they do not have an N-terminal signal peptide sequence). They may also bind a receptor(s) on the surface of neuronal, renal and immune cells [159,160,161,162,163]. In the context of inflammatory bowel disease, MT1 and/or MT2 have been proposed to play the role of danger signals released by dead intestinal epithelial cells that attract and activate leukocytes. Indeed, Mt1-Mt2-KO mice displayed reduced severity of colitis in association with reduced leukocyte infiltration. Moreover, blockade of extracellular MT1 and MT2 with a monoclonal antibody reduced macrophage infiltration in control mice. In agreement, the Boyden chamber migration assay revealed that the attraction of leukocytes to dead intestinal epithelial cell supernatant was also abolished with the anti-MT1/MT2 antibody [164]. In our context, a previous clinical trial revealed that Zn supplementation in T2D patients reduced glucose disposal, increased fasting glucose and fructosamine levels, and increased the reactivity of their T-lymphocytes to phytohemagglutinin [119]. However, it is unclear if MT1 and/or MT2 play such chemokine-like roles in the islets of diabetic mice and human subjects. This is an attractive hypothesis that is compatible with the observed increase in islet macrophages in diabetic db/db mice, but not ob/ob compensating mice. Interestingly, macrophages from diabetic db/db islets also displayed a distinct proinflammatory gene expression pattern in comparison to those from ob/ob islets [165].
7. Conclusions
Contrary to the general perception that MTs are protective cellular effectors under stress conditions, several lines of evidence from in vitro/ex vivo models and animal studies have revealed complex negative roles of MTs in pancreatic beta-cells that are far from being completely understood. In light of this emerging evidence, the safety and usefulness of therapeutic strategies that augment MT levels in beta-cells should be considered with caution. More studies in human beta-cells and the development of novel animal models and technical tools are needed to fill the gaps of knowledge in this field and answer the unsettled questions. A better understanding of the role of MTs in beta-cell pathophysiology may lead to a novel targeted therapeutic strategy to preserve/enhance insulin secretion in obese (pre)diabetic subjects.
Author Contributions
M.B. conceived the paper, analyzed data and wrote the first draft of the manuscript. D.R.L. and J.-C.J. analyzed data and critically reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kahn S.E. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 2.Weir G.C., Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53(Suppl. 3):S16–S21. doi: 10.2337/diabetes.53.suppl_3.S16. [DOI] [PubMed] [Google Scholar]
- 3.Bensellam M., Laybutt D.R., Jonas J.-C. The molecular mechanisms of pancreatic β-cell glucotoxicity: Recent findings and future research directions. Mol. Cell. Endocrinol. 2012;364:1–27. doi: 10.1016/j.mce.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Esser N., Utzschneider K.M., Kahn S.E. Early beta cell dysfunction vs. insulin hypersecretion as the primary event in the pathogenesis of dysglycaemia. Diabetologia. 2020;63:2007–2021. doi: 10.1007/s00125-020-05245-x. [DOI] [PubMed] [Google Scholar]
- 5.Prentki M., Peyot M.-L., Masiello P., Madiraju S.M. Nutrient-Induced Metabolic Stress, Adaptation, Detoxification, and Toxicity in the Pancreatic β-Cell. Diabetes. 2020;69:279–290. doi: 10.2337/dbi19-0014. [DOI] [PubMed] [Google Scholar]
- 6.Bensellam M., Shi Y.-C., Chan J.Y., Laybutt D.R., Chae H., Abou-Samra M., Pappas E.G., Thomas H.E., Gilon P., Jonas J.-C. Metallothionein 1 negatively regulates glucose-stimulated insulin secretion and is differentially expressed in conditions of beta cell compensation and failure in mice and humans. Diabetologia. 2019;62:2273–2286. doi: 10.1007/s00125-019-05008-3. [DOI] [PubMed] [Google Scholar]
- 7.Margoshes M., Vallee B.L. A Cadmium Protein from Equine Kidney Cortex. J. Am. Chem. Soc. 1957;79:4813–4814. doi: 10.1021/ja01574a064. [DOI] [Google Scholar]
- 8.Kagi J.H., Valee B.L. Metallothionein: A cadmium- and zinc-containing protein from equine renal cortex. J. Biol. Chem. 1960;235:3460–3465. doi: 10.1016/S0021-9258(18)64490-4. [DOI] [PubMed] [Google Scholar]
- 9.Michalska A.E., Choo K.H. Targeting and germ-line transmission of a null mutation at the metallothionein I and II loci in mouse. Proc. Natl. Acad. Sci. USA. 1993;90:8088–8092. doi: 10.1073/pnas.90.17.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masters B.A., Kelly E.J., Quaife C.J., Brinster R.L., Palmiter R.D. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc. Natl. Acad. Sci. USA. 1994;91:584–588. doi: 10.1073/pnas.91.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly E.J., Palmiter R.D. A murine model of Menkes disease reveals a physiological function of metallothionein. Nat. Genet. 1996;13:219–222. doi: 10.1038/ng0696-219. [DOI] [PubMed] [Google Scholar]
- 12.Liu J., Liu Y., Habeebu S.S., Klaassen C.D. Metallothionein-Null Mice Are Highly Susceptible to the Hematotoxic and Immunotoxic Effects of Chronic CdCl2 Exposure. Toxicol. Appl. Pharmacol. 1999;159:98–108. doi: 10.1006/taap.1999.8718. [DOI] [PubMed] [Google Scholar]
- 13.Liu J., Liu Y., Goyer R.A., Achanzar W., Waalkes M.P. Metallothionein-I/II Null Mice Are More Sensitive than Wild-Type Mice to the Hepatotoxic and Nephrotoxic Effects of Chronic Oral or Injected Inorganic Arsenicals. Toxicol. Sci. 2000;55:460–467. doi: 10.1093/toxsci/55.2.460. [DOI] [PubMed] [Google Scholar]
- 14.Waalkes M.P., Liu J., Goyer R.A., Diwan B.A. Metallothionein-I/II Double Knockout Mice Are Hypersensitive to Lead-Induced Kidney Carcinogenesis: Role of inclusion body formation. Cancer Res. 2004;64:7766–7772. doi: 10.1158/0008-5472.CAN-04-2220. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y., Liu J., Iszard M., Andrews G., Palmiter R., Klaassen C. Transgenic Mice That Overexpress Metallothionein-I Are Protected from Cadmium Lethality and Hepatotoxicity. Toxicol. Appl. Pharmacol. 1995;135:222–228. doi: 10.1006/taap.1995.1227. [DOI] [PubMed] [Google Scholar]
- 16.Richards R.I., Heguy A., Karin M. Structural and functional analysis of the human metallothionein-IA gene: Differential induction by metal ions and glucocorticoids. Cell. 1984;37:263–272. doi: 10.1016/0092-8674(84)90322-2. [DOI] [PubMed] [Google Scholar]
- 17.Laukens D., Waeytens A., De Bleser P., Cuvelier C., De Vos M. Human metallothionein expression under normal and pathological conditions: Mechanisms of gene regulation based on in silico promoter analysis. Crit. Rev. Eukaryot. Gene Expr. 2009;19:301–317. doi: 10.1615/CritRevEukarGeneExpr.v19.i4.40. [DOI] [PubMed] [Google Scholar]
- 18.Tohyama C., Shaikh Z.A., Nogawa K., Kobayashi E., Honda R. Elevated urinary excretion of metallothionein due to environmental cadmiun exposure. Toxicology. 1981;20:289–297. doi: 10.1016/0300-483X(81)90036-6. [DOI] [PubMed] [Google Scholar]
- 19.Amiard J.-C., Amiard-Triquet C., Barka S., Pellerin J., Rainbow P.S. Metallothioneins in aquatic invertebrates: Their role in metal detoxification and their use as biomarkers. Aquat. Toxicol. 2006;76:160–202. doi: 10.1016/j.aquatox.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Prozialeck W.C., Edwards J.R. Early biomarkers of cadmium exposure and nephrotoxicity. BioMetals. 2010;23:793–809. doi: 10.1007/s10534-010-9288-2. [DOI] [PubMed] [Google Scholar]
- 21.Shariati F., Sari A.E., Mashinchian A., Pourkazemi M. Metallothionein as Potential Biomarker of Cadmium Exposure in Persian Sturgeon (Acipenser persicus) Biol. Trace Element Res. 2011;143:281–291. doi: 10.1007/s12011-010-8877-9. [DOI] [PubMed] [Google Scholar]
- 22.Pizzino G., Bitto A., Interdonato M., Galfo F., Irrera N., Mecchio A., Pallio G., Ramistella V., De Luca F., Minutoli L., et al. Oxidative stress and DNA repair and detoxification gene expression in adolescents exposed to heavy metals living in the Milazzo-Valle del Mela area (Sicily, Italy) Redox Biol. 2014;2:686–693. doi: 10.1016/j.redox.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emdin S.O., Dodson G.G., Cutfield J.M., Cutfield S.M. Role of zinc in insulin biosynthesis. Some possible zinc-insulin interactions in the pancreatic B-cell. Diabetologia. 1980;19:174–182. doi: 10.1007/BF00275265. [DOI] [PubMed] [Google Scholar]
- 24.Hill C.P., Dauter Z., Dodson E.J., Dodson G.G., Dunn M.F. X-ray structure of an unusual Ca2+ site and the roles of Zn2+ and Ca2+ in the assembly, stability, and storage of the insulin hexamer. Biochemistry. 1991;30:917–924. doi: 10.1021/bi00218a006. [DOI] [PubMed] [Google Scholar]
- 25.Dunn M.F. Zinc–Ligand Interactions Modulate Assembly and Stability of the Insulin Hexamer—A Review. BioMetals. 2005;18:295–303. doi: 10.1007/s10534-005-3685-y. [DOI] [PubMed] [Google Scholar]
- 26.Andreini C., Banci L., Bertini I., Rosato A. Counting the Zinc-Proteins Encoded in the Human Genome. J. Proteome Res. 2006;5:196–201. doi: 10.1021/pr050361j. [DOI] [PubMed] [Google Scholar]
- 27.Maret W. Zinc Biochemistry: From a Single Zinc Enzyme to a Key Element of Life. Adv. Nutr. 2013;4:82–91. doi: 10.3945/an.112.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimura T., Kambe T. The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective. Int. J. Mol. Sci. 2016;17:336. doi: 10.3390/ijms17030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thornalley P.J., Vašák M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzym. 1985;827:36–44. doi: 10.1016/0167-4838(85)90098-6. [DOI] [PubMed] [Google Scholar]
- 30.Schwarz M.A., Lazo J.S., Yalowich J.C., Allen W.P., Whitmore M., Bergonia H.A., Tzeng E., Billiar T.R., Robbins P.D., Lancaster J.R., Jr., et al. Metallothionein protects against the cytotoxic and DNA-damaging effects of nitric oxide. Proc. Natl. Acad. Sci. USA. 1995;92:4452–4456. doi: 10.1073/pnas.92.10.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumari M.R., Hiramatsu M., Ebadi M. Free radical scavenging actions of metallothionein isoforms I and II. Free. Radic. Res. 1998;29:93–101. doi: 10.1080/10715769800300111. [DOI] [PubMed] [Google Scholar]
- 32.Maret W., Vallee B.L. Thiolate ligands in metallothionein confer redox activity on zinc clusters. Proc. Natl. Acad. Sci. USA. 1998;95:3478–3482. doi: 10.1073/pnas.95.7.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cortese M.M., Suschek C.V., Wetzel W., Kröncke K.-D., Kolb-Bachofen V. Zinc protects endothelial cells from hydrogen peroxide via Nrf2-dependent stimulation of glutathione biosynthesis. Free. Radic. Biol. Med. 2008;44:2002–2012. doi: 10.1016/j.freeradbiomed.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki K.T., Kuroda T. Transfer of copper and zinc from ionic and metallothionein-bound forms to Cu, Zn--superoxide dismutase. Res. Commun. Mol. Pathol. Pharmacol. 1995;87:287–296. [PubMed] [Google Scholar]
- 35.Dalton T., Palmiter R.D., Andrews G.K. Transcriptional induction of the mouse metallothionein-I gene in hydrogen peroxide-treated Hepa cells involves a composite major late transcription factor/antioxidant response element and metal response promoter elements. Nucleic Acids Res. 1994;22:5016–5023. doi: 10.1093/nar/22.23.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campagne M.V.L., Thibodeaux H., Van Bruggen N., Cairns B., Lowe D.G. Increased Binding Activity at an Antioxidant-Responsive Element in the Metallothionein-1 Promoter and Rapid Induction of Metallothionein-1 and -2 in Response to Cerebral Ischemia and Reperfusion. J. Neurosci. 2000;20:5200–5207. doi: 10.1523/JNEUROSCI.20-14-05200.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohtsuji M., Katsuoka F., Kobayashi A., Aburatani H., Hayes J.D., Yamamoto M. Nrf1 and Nrf2 Play Distinct Roles in Activation of Antioxidant Response Element-dependent Genes. J. Biol. Chem. 2008;283:33554–33562. doi: 10.1074/jbc.M804597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miura T., Muraoka S., Ogiso T. Antioxidant activity of metallothionein compared with reduced glutathione. Life Sci. 1997;60:301–309. doi: 10.1016/S0024-3205(97)00156-2. [DOI] [PubMed] [Google Scholar]
- 39.Chubatsu L.S., Meneghini R. Metallothionein protects DNA from oxidative damage. Pt 1Biochem. J. 1993;291:193–198. doi: 10.1042/bj2910193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lazo J.S., Kondo Y., Dellapiazza D., Michalska A.E., Choo K.H.A., Pitt B.R. Enhanced Sensitivity to Oxidative Stress in Cultured Embryonic Cells from Transgenic Mice Deficient in Metallothionein I and II Genes. J. Biol. Chem. 1995;270:5506–5510. doi: 10.1074/jbc.270.10.5506. [DOI] [PubMed] [Google Scholar]
- 41.Pitt B.R., Schwarz M., Woo E.S., Yee E., Wasserloos K., Tran S., Weng W., Mannix R.J., Watkins S.A., Tyurina Y.Y., et al. Overexpression of metallothionein decreases sensitivity of pulmonary endothelial cells to oxidant injury. Am. J. Physiol. Content. 1997;273:L856–L865. doi: 10.1152/ajplung.1997.273.4.L856. [DOI] [PubMed] [Google Scholar]
- 42.Viarengo A., Burlando B., Cavaletto M., Marchi B., Ponzano E., Blasco J. Role of metallothionein against oxidative stress in the mussel Mytilus galloprovincialis. Am. J. Physiol. Content. 1999;277:R1612–R1619. doi: 10.1152/ajpregu.1999.277.6.r1612. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki Y., Apostolova M.D., Cherian M.G. Astrocyte cultures from transgenic mice to study the role of metallothionein in cytotoxicity of tert-butyl hydroperoxide. Toxicology. 2000;145:51–62. doi: 10.1016/S0300-483X(99)00220-6. [DOI] [PubMed] [Google Scholar]
- 44.Chimienti F., Jourdan E., Favier A., Sève M. Zinc resistance impairs sensitivity to oxidative stress in hela cells: Protection through metallothioneins expression. Free. Radic. Biol. Med. 2001;31:1179–1190. doi: 10.1016/S0891-5849(01)00701-8. [DOI] [PubMed] [Google Scholar]
- 45.Wang G.W., Klein J.B., Kang Y.J. Metallothionein inhibits doxorubicin-induced mitochondrial cytochrome c release and caspase-3 activation in cardiomyocytes. J. Pharmacol. Exp. Ther. 2001;298:461–468. [PubMed] [Google Scholar]
- 46.Liang Q., Carlson E.C., Donthi R.V., Kralik P.M., Shen X., Epstein P.N. Overexpression of metallothionein reduces diabetic cardiomyopathy. Diabetes. 2002;51:174–181. doi: 10.2337/diabetes.51.1.174. [DOI] [PubMed] [Google Scholar]
- 47.Molinero A., Penkowa M., Hernández J., Camats J., Giralt M., Lago N., Carrasco J., Campbell I.L., Hidalgo J. Metallothionein-I overexpression decreases brain pathology in transgenic mice with astrocyte-targeted expression of interleukin-6. J. Neuropathol. Exp. Neurol. 2003;62:315–328. doi: 10.1093/jnen/62.3.315. [DOI] [PubMed] [Google Scholar]
- 48.Sharma S.K., Ebadi M. Metallothionein Attenuates 3-Morpholinosydnonimine (SIN-1)-Induced Oxidative Stress in Dopaminergic Neurons. Antioxidants Redox Signal. 2003;5:251–264. doi: 10.1089/152308603322110832. [DOI] [PubMed] [Google Scholar]
- 49.Reinecke F., Levanets O., Olivier Y., Louw R., Semete B., Grobler A., Hidalgo J., Smeitink J., Olckers A., Van Der Westhuizen F.H. Metallothionein isoform 2A expression is inducible and protects against ROS-mediated cell death in rotenone-treated HeLa cells. Biochem. J. 2006;395:405–415. doi: 10.1042/BJ20051253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cavalca E., Cesani M., Gifford J.C., Esteves M.S., Terreni M.R., Leoncini G., Peviani M., Biffi A., Gifford J.C. Metallothioneins are neuroprotective agents in lysosomal storage disorders. Ann. Neurol. 2018;83:418–432. doi: 10.1002/ana.25161. [DOI] [PubMed] [Google Scholar]
- 51.Wang K., Dai X., He J., Yan X., Yang C., Fan X., Sun S., Chen J., Xu J., Deng Z., et al. Endothelial Overexpression of Metallothionein Prevents Diabetes-Induced Impairment in Ischemia Angiogenesis Through Preservation of HIF-1α/SDF-1/VEGF Signaling in Endothelial Progenitor Cells. Diabetes. 2020;69:1779–1792. doi: 10.2337/db19-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lynes M.A., Hidalgo J., Manso Y., Devisscher L., Laukens D., Lawrence D.A. Metallothionein and stress combine to affect multiple organ systems. Cell Stress Chaperon. 2014;19:605–611. doi: 10.1007/s12192-014-0501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Subramanian Vignesh K., Deepe G.S., Jr. Metallothioneins: Emerging Modulators in Immunity and Infection. Int. J. Mol. Sci. 2017;18:2197. doi: 10.3390/ijms18102197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robertson R.P. Chronic Oxidative Stress as a Central Mechanism for Glucose Toxicity in Pancreatic Islet Beta Cells in Diabetes. J. Biol. Chem. 2004;279:42351–42354. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- 55.Bensellam M., Jonas J.-C., Laybutt D.R. Mechanisms of β-cell dedifferentiation in diabetes: Recent findings and future research directions. J. Endocrinol. 2018;236:R109–R143. doi: 10.1530/JOE-17-0516. [DOI] [PubMed] [Google Scholar]
- 56.Ježek P., Jabůrek M., Plecitá-Hlavatá L. Contribution of Oxidative Stress and Impaired Biogenesis of Pancreatic β-Cells to Type 2 Diabetes. Antioxidants Redox Signal. 2019;31:722–751. doi: 10.1089/ars.2018.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roma L.P., Jonas J.-C. Nutrient Metabolism, Subcellular Redox State, and Oxidative Stress in Pancreatic Islets and β-Cells. J. Mol. Biol. 2020;432:1461–1493. doi: 10.1016/j.jmb.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 58.Hansen J.B.J., Tonnesen M.F.M., Madsen A.N.A., Hagedorn P.H., Friberg J., Grunnet L.G.L., Heller R.S.R., Nielsen A.O.A., Størling J., Baeyens L., et al. Divalent Metal Transporter 1 Regulates Iron-Mediated ROS and Pancreatic β Cell Fate in Response to Cytokines. Cell Metab. 2012;16:449–461. doi: 10.1016/j.cmet.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 59.Chimienti F. Zinc, pancreatic islet cell function and diabetes: New insights into an old story. Nutr. Res. Rev. 2013;26:1–11. doi: 10.1017/S0954422412000212. [DOI] [PubMed] [Google Scholar]
- 60.Gilon P., Chae H.-Y., Rutter G.A., Ravier M.A. Calcium signaling in pancreatic β-cells in health and in Type 2 diabetes. Cell Calcium. 2014;56:340–361. doi: 10.1016/j.ceca.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Rutter G.A., Chabosseau P., Bellomo E.A., Maret W., Mitchell R.K., Hodson D.J., Solomou A., Hu M. Intracellular zinc in insulin secretion and action: A determinant of diabetes risk? Proc. Nutr. Soc. 2015;75:61–72. doi: 10.1017/S0029665115003237. [DOI] [PubMed] [Google Scholar]
- 62.Farooq M. Zinc Deficiency is Associated with Poor Glycemic Control. J. Coll. Physicians Surg. Pak. 2019;29:253–257. doi: 10.29271/jcpsp.2019.03.253. [DOI] [PubMed] [Google Scholar]
- 63.Yin J., Wang X., Li S., Zhu Y., Chen S., Li P., Luo C., Huang Y., Li X., Hu X., et al. Interactions between plasma copper concentrations and SOD1 gene polymorphism for impaired glucose regulation and type 2 diabetes. Redox Biol. 2019;24:101172. doi: 10.1016/j.redox.2019.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walter R.M., Jr., Uriu-Hare J.Y., Olin K.L., Oster M.H., Anawalt B.D., Critchfield J.W., Keen C.L. Copper, Zinc, Manganese, and Magnesium Status and Complications of Diabetes Mellitus. Diabetes Care. 1991;14:1050–1056. doi: 10.2337/diacare.14.11.1050. [DOI] [PubMed] [Google Scholar]
- 65.Garg V.K., Gupta R., Goyal R.K. Hypozincemia in diabetes mellitus. J. Assoc. Physicians India. 1994;42:720–721. [PubMed] [Google Scholar]
- 66.Taylor C.G. Zinc, the Pancreas, and Diabetes: Insights from Rodent Studies and Future Directions. BioMetals. 2005;18:305–312. doi: 10.1007/s10534-005-3686-x. [DOI] [PubMed] [Google Scholar]
- 67.Viktorínová A., Tošerová E., Križko M., Ďuračková Z. Altered metabolism of copper, zinc, and magnesium is associated with increased levels of glycated hemoglobin in patients with diabetes mellitus. Metabolism. 2009;58:1477–1482. doi: 10.1016/j.metabol.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 68.Basaki M., Saeb M., Nazifi S., Shamsaei H.A. Zinc, Copper, Iron, and Chromium Concentrations in Young Patients with Type 2 Diabetes Mellitus. Biol. Trace Element Res. 2012;148:161–164. doi: 10.1007/s12011-012-9360-6. [DOI] [PubMed] [Google Scholar]
- 69.Jansen J., Rosenkranz E., Overbeck S., Warmuth S., Mocchegiani E., Giacconi R., Weiskirchen R., Karges W., Rink L. Disturbed zinc homeostasis in diabetic patients by in vitro and in vivo analysis of insulinomimetic activity of zinc. J. Nutr. Biochem. 2012;23:1458–1466. doi: 10.1016/j.jnutbio.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 70.Giacconi R., Bonfigli A.R., Testa R., Sirolla C., Cipriano C., Marra M., Muti E., Malavolta M., Costarelli L., Piacenza F., et al. +647 A/C and +1245 MT1A polymorphisms in the susceptibility of diabetes mellitus and cardiovascular complications. Mol. Genet. Metab. 2008;94:98–104. doi: 10.1016/j.ymgme.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 71.Yang L., Li H., Yu T., Zhao H., Cherian M.G., Cai L., Liu Y. Polymorphisms in metallothionein-1 and -2 genes associated with the risk of type 2 diabetes mellitus and its complications. Am. J. Physiol. Metab. 2008;294:E987–E992. doi: 10.1152/ajpendo.90234.2008. [DOI] [PubMed] [Google Scholar]
- 72.Raudenska M., Gumulec J., Podlaha O., Sztalmachova M., Babula P., Eckschlager T., Adam V., Kizek R., Masarik M. Metallothionein polymorphisms in pathological processes. Metallomics. 2014;6:55–68. doi: 10.1039/C3MT00132F. [DOI] [PubMed] [Google Scholar]
- 73.Yau E.T., Mennear J.H. Pancreatic metallothionein: Protection against cadmium-induced inhibition of insulin secretory activity. Toxicol. Appl. Pharmacol. 1977;39:515–520. doi: 10.1016/0041-008X(77)90142-9. [DOI] [PubMed] [Google Scholar]
- 74.Zimny S., Gogolin F., Abel J., Gleichmann H. Metallothionein in isolated pancreatic islets of mice: Induction by zinc and streptozotocin, a naturally occurring diabetogen. Arch. Toxicol. 1993;67:61–65. doi: 10.1007/BF02072037. [DOI] [PubMed] [Google Scholar]
- 75.Ohly P., Dohle C., Abel J., Seissler J., Gleichmann H. Zinc sulphate induces metallothionein in pancreatic islets of mice and protects against diabetes induced by multiple low doses of streptozotocin. Diabetologia. 2000;43:1020–1030. doi: 10.1007/s001250050009. [DOI] [PubMed] [Google Scholar]
- 76.Tomita T., Matsubara O. Immunocytochemical Localization of Metallothionein in Human Pancreatic Islets. Pancreas. 2000;20:21–24. doi: 10.1097/00006676-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 77.DiGruccio M.R., Mawla A.M., Donaldson C.J., Noguchi G.M., Vaughan J., Cowing-Zitron C., van der Meulen T., Huising M.O., van der Meulen T. Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. Mol. Metab. 2016;5:449–458. doi: 10.1016/j.molmet.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blodgett D.M., Nowosielska A., Afik S., Pechhold S., Cura A.J., Kennedy N.J., Kim S., Kucukural A., Davis R.J., Kent S.C., et al. Novel Observations From Next-Generation RNA Sequencing of Highly Purified Human Adult and Fetal Islet Cell Subsets. Diabetes. 2015;64:3172–3181. doi: 10.2337/db15-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.El Muayed M., Raja M.R., Zhang X., MacRenaris K.W., Bhatt S., Chen X., Urbanek M., O’Halloran T.V., Lowe J.W.L., Jr. Accumulation of cadmium in insulin-producing β cells. Islets. 2012;4:405–416. doi: 10.4161/isl.23101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andrews G.K., Kage K., Palmiter-Thomas P., Sarras M.P. Metal Ions Induce Expression Pancreatic Exocrine and of Met allot hionein in Endocrine Cells. Pancreas. 1990;5:548–554. doi: 10.1097/00006676-199009000-00009. [DOI] [PubMed] [Google Scholar]
- 81.Ohly P., Gleichmann H. Metallothionein: In Vitro Induction With Zinc and Streptozotocin in Pancreatic Islets of Mice. Exp. Clin. Endocrinol. Diabetes. 1995;103(Suppl. 2):79–82. doi: 10.1055/s-0029-1211399. [DOI] [PubMed] [Google Scholar]
- 82.Bellomo E.A., Meur G., Rutter G.A. Glucose Regulates Free Cytosolic Zn2+ Concentration, Slc39 (ZiP), and Metallothionein Gene Expression in Primary Pancreatic Islet β-Cells. J. Biol. Chem. 2011;286:25778–25789. doi: 10.1074/jbc.M111.246082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Duprez J., Roma L.P., Close A.-F., Jonas J.-C. Protective Antioxidant and Antiapoptotic Effects of ZnCl2 in Rat Pancreatic Islets Cultured in Low and High Glucose Concentrations. PLoS ONE. 2012;7:e46831. doi: 10.1371/journal.pone.0046831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nygaard S.B., Larsen A., Knuhtsen A., Rungby J., Smidt K. Effects of zinc supplementation and zinc chelation on in vitro β-cell function in INS-1E cells. BMC Res. Notes. 2014;7:84. doi: 10.1186/1756-0500-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hinke S.A., Hellemans K., Schuit F.C. Plasticity of the β cell insulin secretory competence: Preparing the pancreatic β cell for the next meal. J. Physiol. 2004;558:369–380. doi: 10.1113/jphysiol.2004.064881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jonas J.-C., Bensellam M., Duprez J., Elouil H., Guiot Y., Pascal S.M.A. Glucose regulation of islet stress responses and β-cell failure in type 2 diabetes. Diabetes Obes. Metab. 2009;11(Suppl. 4):65–81. doi: 10.1111/j.1463-1326.2009.01112.x. [DOI] [PubMed] [Google Scholar]
- 87.Bensellam M., Van Lommel L., Overbergh L., Schuit F.C., Jonas J.-C. Cluster analysis of rat pancreatic islet gene mRNA levels after culture in low-, intermediate- and high-glucose concentrations. Diabetologia. 2009;52:463–476. doi: 10.1007/s00125-008-1245-z. [DOI] [PubMed] [Google Scholar]
- 88.Andersson A. Isolated mouse pancreatic islets in culture: Effects of serum and different culture media on the insulin production of the islets. Diabetologia. 1978;14:397–404. doi: 10.1007/BF01228134. [DOI] [PubMed] [Google Scholar]
- 89.Ling Z., Pipeleers D.G. Preservation of glucose-responsive islet beta-cells during serum-free culture. Endocrinol. 1994;134:2614–2621. doi: 10.1210/endo.134.6.7515006. [DOI] [PubMed] [Google Scholar]
- 90.Efanova I.B., Zaitsev S.V., Zhivotovsky B., Köhler M., Efendić S., Orrenius S., Berggren P.-O. Glucose and Tolbutamide Induce Apoptosis in Pancreatic β-Cells. A process dependent on intracellular Ca2+ concentration. J. Biol. Chem. 1998;273:33501–33507. doi: 10.1074/jbc.273.50.33501. [DOI] [PubMed] [Google Scholar]
- 91.Bensellam M., Duvillié B., Rybachuk G., Laybutt D.R., Magnan C., Guiot Y., Pouysségur J., Jonas J.-C. Glucose-Induced O2 Consumption Activates Hypoxia Inducible Factors 1 and 2 in Rat Insulin-Secreting Pancreatic Beta-Cells. PLoS ONE. 2012;7:e29807. doi: 10.1371/journal.pone.0029807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eizirik D.L., Korbutt G.S., Hellerström C. Prolonged exposure of human pancreatic islets to high glucose concentrations in vitro impairs the beta-cell function. J. Clin. Investig. 1992;90:1263–1268. doi: 10.1172/JCI115989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ling Z., Pipeleers D.G. Prolonged exposure of human beta cells to elevated glucose levels results in sustained cellular activation leading to a loss of glucose regulation. J. Clin. Investig. 1996;98:2805–2812. doi: 10.1172/JCI119108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schnedl W.J., Ferber S., Johnson J.H., Newgard C.B. STZ Transport and Cytotoxicity: Specific Enhancement in GLUT2-Expressing Cells. Diabetes. 1994;43:1326–1333. doi: 10.2337/diab.43.11.1326. [DOI] [PubMed] [Google Scholar]
- 95.Takasu N., Komiya I., Asawa T., Nagasawa Y., Yamada T. Streptozocin- and Alloxan-Induced H2O2 Generation and DNA Fragmentation in Pancreatic Islets: H2O2 as Mediator for DNA Fragmentation. Diabetes. 1991;40:1141–1145. doi: 10.2337/diab.40.9.1141. [DOI] [PubMed] [Google Scholar]
- 96.Gille L., Schott-Ohly P., Friesen N., Walde S.S.I., Udilova N., Nohl H., Gleichmann H. Generation of Hydroxyl Radicals Mediated by Streptozotocin in Pancreatic Islets of Mice in vitro. Pharmacol. Toxicol. 2002;90:317–326. doi: 10.1034/j.1600-0773.2002.900605.x. [DOI] [PubMed] [Google Scholar]
- 97.Buchau A.S., Schott-Ohly P., Lgssiar A., Gleichmann H., Friesen N.T.E. Generation of hydrogen peroxide and failure of antioxidative responses in pancreatic islets of male C57BL/6 mice are associated with diabetes induced by multiple low doses of streptozotocin. Diabetologia. 2004;47:676–685. doi: 10.1007/s00125-004-1367-x. [DOI] [PubMed] [Google Scholar]
- 98.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2007;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 99.Laychock S.G., Duzen J., Simpkins C.O. Metallothionein induction in islets of Langerhans and insulinoma cells. Mol. Cell. Endocrinol. 2000;165:179–187. doi: 10.1016/S0303-7207(00)00247-1. [DOI] [PubMed] [Google Scholar]
- 100.Cardozo A.K., Kruhøffer M., Leeman R., Ørntoft T., Eizirik D.L. Identification of Novel Cytokine-Induced Genes in Pancreatic -Cells by High-Density Oligonucleotide Arrays. Diabetes. 2001;50:909–920. doi: 10.2337/diabetes.50.5.909. [DOI] [PubMed] [Google Scholar]
- 101.Ortis F., Naamane N., Flamez D., Ladrière L., Moore F., Cunha D.A., Colli M.L., Thykjaer T., Thorsen K., Ørntoft T.F., et al. Cytokines Interleukin-1 and Tumor Necrosis Factor- Regulate Different Transcriptional and Alternative Splicing Networks in Primary -Cells. Diabetes. 2009;59:358–374. doi: 10.2337/db09-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mandrup-Poulsen T., Zumsteg U., Reimers J., Pociot F., Mørch L., Helqvist S., Dinarello C.A., Nerup J. Involvement of interleukin 1 and interleukin 1 antagonist in pancreatic β-cell destruction in insulin-dependent diabetes mellitus. Cytokine. 1993;5:185–191. doi: 10.1016/1043-4666(93)90003-N. [DOI] [PubMed] [Google Scholar]
- 103.Tabatabaie T., Vasquez-Weldon A., Moore D.R., Kotake Y. Free Radicals and the Pathogenesis of Type 1 Diabetes: -Cell Cytokine-Mediated Free Radical Generation Via Cyclooxygenase-2. Diabetes. 2003;52:1994–1999. doi: 10.2337/diabetes.52.8.1994. [DOI] [PubMed] [Google Scholar]
- 104.Lee D.K., Carrasco J., Hidalgo J., Andrews G.K. Identification of a signal transducer and activator of transcription (STAT) binding site in the mouse metallothionein-I promoter involved in interleukin-6-induced gene expression. Pt 1Biochem. J. 1999;337:59–65. doi: 10.1042/bj3370059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Karin M., Haslinger A., Holtgreve H., Richards R.I., Krauter P., Westphal H.M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nat. Cell Biol. 1984;308:513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- 106.Kelly E.J., Sandgren E.P., Brinster R.L., Palmiter R.D. A pair of adjacent glucocorticoid response elements regulate expression of two mouse metallothionein genes. Proc. Natl. Acad. Sci. USA. 1997;94:10045–10050. doi: 10.1073/pnas.94.19.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sargsyan E., Cen J., Roomp K., Schneider R., Bergsten P. Identification of early biological changes in palmitate-treated isolated human islets. BMC Genom. 2018;19:1–11. doi: 10.1186/s12864-018-5008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bikopoulos G., da Silva Pimenta A., Lee S.C., Lakey J.R., Der S.D., Chan C.B., Ceddia R.B., Wheeler M.B., Rozakis-Adcock M. Ex vivo transcriptional profiling of human pancreatic islets following chronic exposure to monounsaturated fatty acids. J. Endocrinol. 2007;196:455–464. doi: 10.1677/JOE-07-0174. [DOI] [PubMed] [Google Scholar]
- 109.Elsner M., Gehrmann W., Lenzen S. Peroxisome-Generated Hydrogen Peroxide as Important Mediator of Lipotoxicity in Insulin-Producing Cells. Diabetes. 2010;60:200–208. doi: 10.2337/db09-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Biden T.J., Boslem E., Chu K.Y., Sue N. Lipotoxic endoplasmic reticulum stress, β cell failure, and type 2 diabetes mellitus. Trends Endocrinol. Metab. 2014;25:389–398. doi: 10.1016/j.tem.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 111.Gerber P.A., Bellomo E.A., Hodson D.J., Meur G., Solomou A., Mitchell R.K., Hollinshead M., Chimienti F., Bosco M., Hughes S.J., et al. Hypoxia lowers SLC30A8/ZnT8 expression and free cytosolic Zn2+ in pancreatic beta cells. Diabetologia. 2014;57:1635–1644. doi: 10.1007/s00125-014-3266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chan J.Y., Luzuriaga J., Bensellam M., Biden T.J., Laybutt D.R. Failure of the Adaptive Unfolded Protein Response in Islets of Obese Mice Is Linked with Abnormalities in β-Cell Gene Expression and Progression to Diabetes. Diabetes. 2013;62:1557–1568. doi: 10.2337/db12-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shafrir E., Ziv E., Mosthaf L. Nutritionally induced insulin resistance and receptor defect leading to beta-cell failure in animal models. Ann. New York Acad. Sci. 1999;892:223–246. doi: 10.1111/j.1749-6632.1999.tb07798.x. [DOI] [PubMed] [Google Scholar]
- 114.Kjørholt C., Åkerfeldt M.C., Biden T.J., Laybutt D.R. Chronic Hyperglycemia, Independent of Plasma Lipid Levels, Is Sufficient for the Loss of -Cell Differentiation and Secretory Function in the db/db Mouse Model of Diabetes. Diabetes. 2005;54:2755–2763. doi: 10.2337/diabetes.54.9.2755. [DOI] [PubMed] [Google Scholar]
- 115.Marselli L., Thorne J., Dahiya S., Sgroi D.C., Sharma A., Bonner-Weir S., Marchetti P., Weir G.C. Gene Expression Profiles of Beta-Cell Enriched Tissue Obtained by Laser Capture Microdissection from Subjects with Type 2 Diabetes. PLoS ONE. 2010;5:e11499. doi: 10.1371/journal.pone.0011499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roat R., Rao V., Doliba N.M., Matschinsky F.M., Tobias J.W., Garcia E., Ahima R.S., Imai Y. Alterations of Pancreatic Islet Structure, Metabolism and Gene Expression in Diet-Induced Obese C57BL/6J Mice. PLoS ONE. 2014;9:e86815. doi: 10.1371/journal.pone.0086815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hatanaka M., Anderson-Baucum E., Lakhter A., Kono T., Maier B., Tersey S.A., Tanizawa Y., Evans-Molina C., Mirmira R.G., Sims E.K. Chronic high fat feeding restricts islet mRNA translation initiation independently of ER stress via DNA damage and p53 activation. Sci. Rep. 2017;7:3758. doi: 10.1038/s41598-017-03869-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maret W. Zinc in Pancreatic Islet Biology, Insulin Sensitivity, and Diabetes. Prev. Nutr. Food Sci. 2017;22:1–8. doi: 10.3746/pnf.2017.22.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Raz I., Karsai D., Katz M. The influence of zinc supplementation on glucose homeostasis in NIDDM. Diabetes Res. (Edinburgh, Scotland) 1989;11:73–79. [PubMed] [Google Scholar]
- 120.Minami T., Shimizu M., Tanaka H., Okazaki Y., Cherian M. Metallothionein does not protect mouse endocrine cells from damage induced by alloxan injection. Toxicology. 1999;132:33–41. doi: 10.1016/S0300-483X(98)00138-3. [DOI] [PubMed] [Google Scholar]
- 121.De Sena K.C.M., Arrais R.F., das Gracas Almeida M., De Araújo A.D.M., Dos Santos M.M., De Lima V.T., Pedrosa L.D.F.C. Effects of Zinc Supplementation in Patients with Type 1 Diabetes. Biol. Trace Element Res. 2005;105:1–9. doi: 10.1385/BTER:105:1-3:001. [DOI] [PubMed] [Google Scholar]
- 122.El Dib R., Gameiro O.L.F., Ogata M.S.P., Módolo N.S.P., Braz L.G., Jorge E.C., Junior P.D.N., Beletate V., Nascimento P.D. Zinc supplementation for the prevention of type 2 diabetes mellitus in adults with insulin resistance. Cochrane Database Syst. Rev. 2015 doi: 10.1002/14651858.CD005525.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen H., Carlson E.C., Pellet L., Moritz J.T., Epstein P.N. Overexpression of metallothionein in pancreatic beta-cells reduces streptozotocin-induced DNA damage and diabetes. Diabetes. 2001;50:2040–2046. doi: 10.2337/diabetes.50.9.2040. [DOI] [PubMed] [Google Scholar]
- 124.Chen S., Han J., Liu Y. Dual Opposing Roles of Metallothionein Overexpression in C57BL/6J Mouse Pancreatic β-Cells. PLoS ONE. 2015;10:e0137583. doi: 10.1371/journal.pone.0137583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li X., Chen H., Epstein P.N. Metallothionein Protects Islets from Hypoxia and Extends Islet Graft Survival by Scavenging Most Kinds of Reactive Oxygen Species. J. Biol. Chem. 2004;279:765–771. doi: 10.1074/jbc.M307907200. [DOI] [PubMed] [Google Scholar]
- 126.Lim K.S., Won Y.-W., Park Y.S., Kim Y.-H. Preparation and functional analysis of recombinant protein transduction domain-metallothionein fusion proteins. Biochimie. 2010;92:964–970. doi: 10.1016/j.biochi.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 127.Park L., Min D., Kim H., Chung H.-Y., Lee C.-H., Park I.-S., Kim Y., Park Y. Tat-enhanced delivery of metallothionein can partially prevent the development of diabetes. Free. Radic. Biol. Med. 2011;51:1666–1674. doi: 10.1016/j.freeradbiomed.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 128.Park L., Min D., Kim H., Park J., Choi S., Park Y. The combination of metallothionein and superoxide dismutase protects pancreatic β cells from oxidative damage. Diabetes/Metabolism Res. Rev. 2011;27:802–808. doi: 10.1002/dmrr.1254. [DOI] [PubMed] [Google Scholar]
- 129.Jung H.S., Lim K.S., Kim M.J., Hwang Y.H., Yoo C., Lee Y.-K., Kim Y.-H., Lee D.Y. Hypoxic resistance of hypodermically transplanted pancreatic islets by using cell-absorbable antioxidant Tat-metallothionein. J. Control. Release. 2013;172:1092–1101. doi: 10.1016/j.jconrel.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 130.Kim M.J., Hwang Y.H., Kim Y.H., Lee D.Y. Immunomodulation of cell-penetrating tat-metallothionein for successful outcome of xenotransplanted pancreatic islet. J. Drug Target. 2016;25:350–359. doi: 10.1080/1061186X.2016.1258704. [DOI] [PubMed] [Google Scholar]
- 131.Li X., Chen H., Epstein P.N. Metallothionein and Catalase Sensitize to Diabetes in Nonobese Diabetic Mice: Reactive Oxygen Species May Have a Protective Role in Pancreatic -Cells. Diabetes. 2006;55:1592–1604. doi: 10.2337/db05-1357. [DOI] [PubMed] [Google Scholar]
- 132.Huang S.H., Chu C.H., Yu J.C., Chuang W.C., Lin G.J., Chen P.L., Chou F.-C., Chau L.Y., Sytwu H.K. Transgenic expression of haem oxygenase-1 in pancreatic beta cells protects non-obese mice used as a model of diabetes from autoimmune destruction and prolongs graft survival following islet transplantation. Diabetologia. 2010;53:2389–2400. doi: 10.1007/s00125-010-1858-x. [DOI] [PubMed] [Google Scholar]
- 133.Chou F.-C., Sytwu H.-K. Overexpression of thioredoxin in islets transduced by a lentiviral vector prolongs graft survival in autoimmune diabetic NOD mice. J. Biomed. Sci. 2009;16:71. doi: 10.1186/1423-0127-16-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bertera S., Crawford M.L., Alexander A.M., Papworth G.D., Watkins S.C., Robbins P.D., Trucco M. Gene transfer of manganese superoxide dismutase extends islet graft function in a mouse model of autoimmune diabetes. Diabetes. 2003;52:387–393. doi: 10.2337/diabetes.52.2.387. [DOI] [PubMed] [Google Scholar]
- 135.Byun H.-R., Choi J.A., Koh J.-Y. The role of metallothionein-3 in streptozotocin-induced beta-islet cell death and diabetes in mice. Metallomics. 2014;6:1748–1757. doi: 10.1039/C4MT00143E. [DOI] [PubMed] [Google Scholar]
- 136.Palmiter R.D., Sandgren E.P., Koeller D.M., Brinster R.L. Distal regulatory elements from the mouse metallothionein locus stimulate gene expression in transgenic mice. Mol. Cell. Biol. 1993;13:5266–5275. doi: 10.1128/MCB.13.9.5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Iszard M., Liu J., Liu Y., Dalton T., Andrews G., Palmiter R., Klaassen C. Characterization of Metallothionein-I-Transgenic Mice. Toxicol. Appl. Pharmacol. 1995;133:305–312. doi: 10.1006/taap.1995.1155. [DOI] [PubMed] [Google Scholar]
- 138.Artells E., Palacios Ò., Capdevila M., Atrian S. Mammalian MT1 and MT2 metallothioneins differ in their metal binding abilities. Metallomics. 2013;5:1397–1410. doi: 10.1039/c3mt00123g. [DOI] [PubMed] [Google Scholar]
- 139.Atrian S., Capdevila M. Metallothionein-protein interactions. Biomol. Concepts. 2013;4:143–160. doi: 10.1515/bmc-2012-0049. [DOI] [PubMed] [Google Scholar]
- 140.Knipp M., Meloni G., Roschitzki A.B., Vasák M. Zn7Metallothionein-3 and the Synaptic Vesicle Cycle: Interaction of Metallothionein-3 with the Small GTPase Rab3A†. Biochemistry. 2005;44:3159–3165. doi: 10.1021/bi047636d. [DOI] [PubMed] [Google Scholar]
- 141.Guo W., Grant A., Novick P. Exo84p Is an Exocyst Protein Essential for Secretion. J. Biol. Chem. 1999;274:23558–23564. doi: 10.1074/jbc.274.33.23558. [DOI] [PubMed] [Google Scholar]
- 142.El Ghazi I., Martin B.L., Armitage I.M. New Proteins Found Interacting with Brain Metallothionein-3 Are Linked to Secretion. Int. J. Alzheimer Dis. 2011;2011:208634. doi: 10.4061/2011/208634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zuo P., Qu W., Cooper R.N., Goyer R.A., Diwan B.A., Waalkes M.P. Potential Role of α-Synuclein and Metallothionein in Lead-Induced Inclusion Body Formation. Toxicol. Sci. 2009;111:100–108. doi: 10.1093/toxsci/kfp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Geng X., Lou H., Wang J., Li L., Swanson A.L., Sun M., Beers-Stolz D., Watkins S., Perez R.G., Drain P. α-Synuclein binds the KATP channel at insulin-secretory granules and inhibits insulin secretion. Am. J. Physiol. Metab. 2011;300:E276–E286. doi: 10.1152/ajpendo.00262.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Steneberg P., Bernardo L., Edfalk S., Lundberg L., Backlund F., Östenson C.-G., Edlund H. The Type 2 Diabetes-Associated Gene Ide Is Required for Insulin Secretion and Suppression of -Synuclein Levels in -Cells. Diabetes. 2013;62:2004–2014. doi: 10.2337/db12-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rodriguez-Araujo G., Nakagami H., Takami Y., Katsuya T., Akasaka H., Saitoh S., Shimamoto K., Morishita R., Rakugi H., Kaneda Y. Low alpha-synuclein levels in the blood are associated with insulin resistance. Sci. Rep. 2015;5:12081. doi: 10.1038/srep12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Butler M.G., Wang K., Naggert J.K., Rethmeyer J.A., Gunewardena S.S., Manzardo A.M., Marshall J.D. Coding and noncoding expression patterns associated with rare obesity-related disorders: Prader–Willi and Alström syndromes. Adv. Genom. Genet. 2015;5:53–75. doi: 10.2147/AGG.S74598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Juntti-Berggren L., Lindh U., Berggren P.-O., Frankel B.J. Elemental Composition in the Pancreatic B Cell Is Normal in the Prediabetic Chinese Hamster. Pancreas. 1992;7:11–14. doi: 10.1097/00006676-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 149.Juntti-Berggren L., Lindh U., Berggren P.-O., Berglund O., Frankel B.J. Diabetes is not associated with a change in the elemental composition of the pancreatic B cell in diabetic C57BL KsJ-db/db mice. Biosci. Rep. 1990;10:217–223. doi: 10.1007/BF01116581. [DOI] [PubMed] [Google Scholar]
- 150.De Samber B., Bensellam M., Van Malderen S.J.M., Seiboth F., Brückner D., Garrevoet J., Falkenberg G., Jonas J.-C., Vincze L. Proof-of-concept for 2D/CT element analysis of entire cryofrozen islets of Langerhans using a cryoloop synchrotron X-ray fluorescence setup. J. Anal. At. Spectrom. 2020;35:1368–1379. doi: 10.1039/D0JA00067A. [DOI] [Google Scholar]
- 151.Takahashi S. Positive and negative regulators of the metallothionein gene (Review) Mol. Med. Rep. 2012;12:795–799. doi: 10.3892/mmr.2015.3459. [DOI] [PubMed] [Google Scholar]
- 152.Roma L.P., Duprez J., Jonas J.-C. Glucokinase activation is beneficial or toxic to cultured rat pancreatic islets depending on the prevailing glucose concentration. Am. J. Physiol. Metab. 2015;309:E632–E639. doi: 10.1152/ajpendo.00154.2015. [DOI] [PubMed] [Google Scholar]
- 153.Martens G.A., Cai Y., Hinke S.A., Stangé G., Van De Casteele M., Pipeleers D. Glucose Suppresses Superoxide Generation in Metabolically Responsive Pancreatic β Cells. J. Biol. Chem. 2005;280:20389–20396. doi: 10.1074/jbc.M411869200. [DOI] [PubMed] [Google Scholar]
- 154.Roma L.P., Pascal S.M., Duprez J., Jonas J.-C. Mitochondrial oxidative stress contributes differently to rat pancreatic islet cell apoptosis and insulin secretory defects after prolonged culture in a low non-stimulating glucose concentration. Diabetologia. 2012;55:2226–2237. doi: 10.1007/s00125-012-2581-6. [DOI] [PubMed] [Google Scholar]
- 155.Deglasse J.-P., Roma L.P., Pastor-Flores D., Gilon P., Dick T.P., Jonas J.-C. Glucose Acutely Reduces Cytosolic and Mitochondrial H2O2 in Rat Pancreatic Beta Cells. Antioxidants Redox Signal. 2019;30:297–313. doi: 10.1089/ars.2017.7287. [DOI] [PubMed] [Google Scholar]
- 156.Goginashvili A., Zhang Z., Erbs E., Spiegelhalter C., Kessler P., Mihlan M., Pasquier A., Krupina K., Schieber N., Cinque L., et al. Insulin secretory granules control autophagy in pancreatic cells. Science. 2015;347:878–882. doi: 10.1126/science.aaa2628. [DOI] [PubMed] [Google Scholar]
- 157.Car N., Car A., Granic M., Skrabalo Z., Momčilović B. Zinc and copper in the serum of diabetic patients. Biol. Trace Element Res. 1992;32:325–329. doi: 10.1007/BF02784618. [DOI] [PubMed] [Google Scholar]
- 158.Squitti R., Negrouk V., Perera M., Llabre M.M., Ricordi C., Rongioletti M.C.A., Mendez A.J. Serum copper profile in patients with type 1 diabetes in comparison to other metals. J. Trace Elements Med. Biol. 2019;56:156–161. doi: 10.1016/j.jtemb.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 159.El Refaey H., Ebadi M., Kuszynski C.A., Sweeney J., Hamada F.M., Hamed A. Identification of metallothionein receptors in human astrocytes. Neurosci. Lett. 1997;231:131–134. doi: 10.1016/S0304-3940(97)00548-X. [DOI] [PubMed] [Google Scholar]
- 160.Klassen R.B., Crenshaw K., Kozyraki R., Verroust P.J., Tío L., Atrian S., Allen P.L., Hammond T.G. Megalin mediates renal uptake of heavy metal metallothionein complexes. Am. J. Physiol. Physiol. 2004;287:F393–F403. doi: 10.1152/ajprenal.00233.2003. [DOI] [PubMed] [Google Scholar]
- 161.Yin X., Knecht D.A., Lynes M.A. Metallothionein mediates leukocyte chemotaxis. BMC Immunol. 2005;6:21. doi: 10.1186/1471-2172-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wolff N.A., Abouhamed M., Verroust P.J., Thévenod F. Megalin-Dependent Internalization of Cadmium-Metallothionein and Cytotoxicity in Cultured Renal Proximal Tubule Cells. J. Pharmacol. Exp. Ther. 2006;318:782–791. doi: 10.1124/jpet.106.102574. [DOI] [PubMed] [Google Scholar]
- 163.Chung R.S., Penkowa M., Dittmann J., King C.E., Bartlett C., Asmussen J.W., Hidalgo J., Carrasco J., Leung Y.K.J., Walker A.K., et al. Redefining the Role of Metallothionein within the Injured Brain: Extracellular metallothioneins play an important role in the astrocyte-neuron response to injury. J. Biol. Chem. 2008;283:15349–15358. doi: 10.1074/jbc.M708446200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Devisscher L., Hindryckx P., Lynes M.A., Waeytens A., Cuvelier C., De Vos F., Vanhove C., De Vos M., Laukens D. Role of metallothioneins as danger signals in the pathogenesis of colitis. J. Pathol. 2014;233:89–100. doi: 10.1002/path.4330. [DOI] [PubMed] [Google Scholar]
- 165.Chan J.Y., Lee K., Maxwell E.L., Liang C., Laybutt D.R. Macrophage alterations in islets of obese mice linked to beta cell disruption in diabetes. Diabetologia. 2019;62:993–999. doi: 10.1007/s00125-019-4844-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.