Abstract
The rapid light response of electron transport rate (ETRR), obtained from chlorophyll fluorescence parameters by short illumination periods (10–30 s) at each light level, can provide a rapid and easy measurement of photosynthetic light response in plants. However, the relationship between ETRR and the steady-state light response of CO2 exchange rate (AS) of terrestrial plants has not been studied in detail. In this study, we compared the ETRR and AS for five woody and four fern species with different light and/or water adaptations. Under well-watered conditions, a constant temperature (25 °C) and with stomatal conductance (gs) not being a main limiting factor for photosynthesis, ETRR and AS were closely related, even when merging data for regression analysis for a species grown under different light conditions and measured under different light intensity and air humidity. However, when Alnus formosana was treated with low soil water and air humidity, because of the decrease in AS mainly due to stomatal closure, the ETRR–AS relation was not so close. In addition, at both 100 and 2000 μmol m−2 s−1 photosynthetic photon flux density (PPFD), ETRR and AS were significantly correlated within a plant group (i.e., woody plants and ferns) regardless of the broad difference in AS due to different species or environmental factors. The results indicate that the relationship between the ETRR and AS is varied by species. We concluded that 1) ETRR could reflect the variation in AS at each irradiance level within a species under well-watered conditions and 2) ETRR at 100 μmol m−2 s−1 PPFD (as the efficiency of light capture) or 2000 μmol m−2 s−1 PPFD (as a maximum photosynthetic parameter) could be used to compare the photosynthetic capacity within a plant group, such as woody plants and ferns.
Keywords: electron transport rate, fern, photosynthetic rate, rapid light curve, stomatal conductance, tree
1. Introduction
Photosynthesis is a major determinant of biomass production and terrestrial carbon budgets [1]. Sunlight is the energy source of plant photosynthesis; however, the response of photosynthesis to light intensity varies by species and environmental conditions. Plants adapted or acclimated to high light often have a high light compensation point, light saturation point, and maximal photosynthetic rate [1,2,3]. Light-response curves (LC) reveal the photosynthetic properties of plants. They can be used to characterize CO2 assimilation, photochemistry, photoacclimation, photoinhibition, and photoprotective mechanisms in different light conditions. LC are widely used to describe the physiological plasticity of plants. Thus, the LC of photosynthesis is fundamental for plant ecophysiological research [1,2,3,4].
Traditionally, the LC of photosynthesis has been measured by the rate of steady-state photosynthesis under a range of relevant light intensity. Thus, the measurement is limited by the long measurement time and cumbersome leaf gas exchange techniques, especially in the field [5]. Recently, chlorophyll fluorescence quenching analysis has been found to be a fast, simple, non-invasive, and reliable method to assess changes in photosystem II (PSII) function under different environmental and physiological conditions [6,7,8]. Among chlorophyll fluorescence parameters, electron transport rate (ETR), calculated from the product of PSII efficiency and absorbed light, expresses the relative rate of electron transport through PSII [9,10]. Two ways to obtain light-response data for ETR are steady-state light curve (SLC) and rapid light curve (RLC) methods.
ETR obtained by SLC methods (ETRS) is under steady-state conditions at a given strength of illumination. Because CO2 fixation (As) is a major sink for electrons from PSII, when A is inhibited by environmental and/or physiological factors, leaves may downregulate their PSII efficiency, mainly by xanthophyll-dependent non-photochemical quenching to avoid damage caused by excessively absorbed energy [11,12,13,14]. Even if electrons from PSII have several energy sinks (e.g., photorespiration and the water–water cycle) [15,16,17], the allocation of electron flow between A and other alternative sinks remains unchanged under many conditions. Examples are C4 plants (with photorespiration mostly restricted) and C3 plants under conditions with approximate temperature as well as CO2 and O2 concentrations but varied light intensity [3,18,19,20]. Because both CO2 fixation and photorespiration are major sinks for electrons from PSII in C3 plants, the ratio of ETR to As (or PSII efficiency/photosynthetic rate per absorbed quantum) greatly increases with decreasing CO2 partial pressure [21], increasing temperature [3,22], and O2 partial pressure [20] because of increased photorespiration.
In contrast to ETRS, ETR obtained by RLC methods (ETRR) involves short illumination periods (10–30 s) at each light level, so the RLC can be measured within 1.5–2 min, but leaves do not achieve steady-state conditions during each light step [23,24]. Nevertheless, ETRR can provide reliable information about cardinal points of photosynthesis [5,25]. It can use to investigate short-term responses to rapid changes in the light environment [4]. Aquatic photosynthetic organisms often show a parallel change in light responses of ETRR and steady-state photosynthetic rate (AS); thus, ETRR is widely used to assess the photosynthetic activity and biomass productivity [26,27,28,29] and to investigate light acclimation [30,31,32,33].
For terrestrial plants, ETRR is used to study environmental acclimation [23,34,35,36,37], stress responses [35,38,39,40,41], and estimate photosynthetic efficiency [25,42]. However, in addition to irradiance, stomatal conductance (gs) is another important limiting factor in the photosynthesis of terrestrial plants. To prevent water loss and facilitate CO2 diffusion to mesophyll cells, guard cells may monitor the plant water status and the CO2 demand from the mesophyll [1,43]. Stomatal behavior is influenced strongly by water and light conditions. In general, A and gs may decrease with decreasing light intensity [44,45], as well as soil water content [20,46] and air moisture [2,20]. In addition, the response of stomata to environmental and physiological conditions varies among species. For example, stomata of xerophytic species are more sensitive, and those of hygrophytic species are more insensitive to water deficits than are mesophytic species [47,48]. Moreover, ferns have a lower ability to respond to increases in CO2 concentration and decreases to water, for lower As/gs ratio, than angiosperms [49,50]. In higher plants grown under low light and/or in dry seasons, the maximum values of AS and ETRR may decrease together [35]. However, the induction of As and gs requires several minutes (e.g., [51,52]), and the time required for these inductions were varied among species with different light-adaptation capabilities [49]. However, during ETRR measurement, leaves are exposed to only 10–30 s of actinic light at each step. Thus, the effect of gs on ETRR may not be as large as on AS, and the ETRR–AS relation may vary among species.
Studies elucidating the relation of ETRR to AS or productivity of terrestrial plants are rare [35,36], as are those investigating the effect of gs on the ETRR–AS relation among species across a wide taxonomic range and environmental adaptation and acclimation capability. Due to the difference of light adaptation and acclimation, plants could be broadly divided into sun- and shade-tolerant plants as well as xerophytic and hygrophytic species. Plant species adapted to different light and water regimes show differential photosynthetic characteristics. To obtain a simple, fast, non-invasive, and reliable method to assess photosynthesis under different environmental and physiological conditions [49], we compared the ETRR and AS for five woody and four fern species with different light and/or water adaptations. In this study, we examined four fern species, three broad-leaved tree species, and two broad-leaved understory shrubs with different light and/or water adaptation capabilities to investigate these aspects.
2. Results
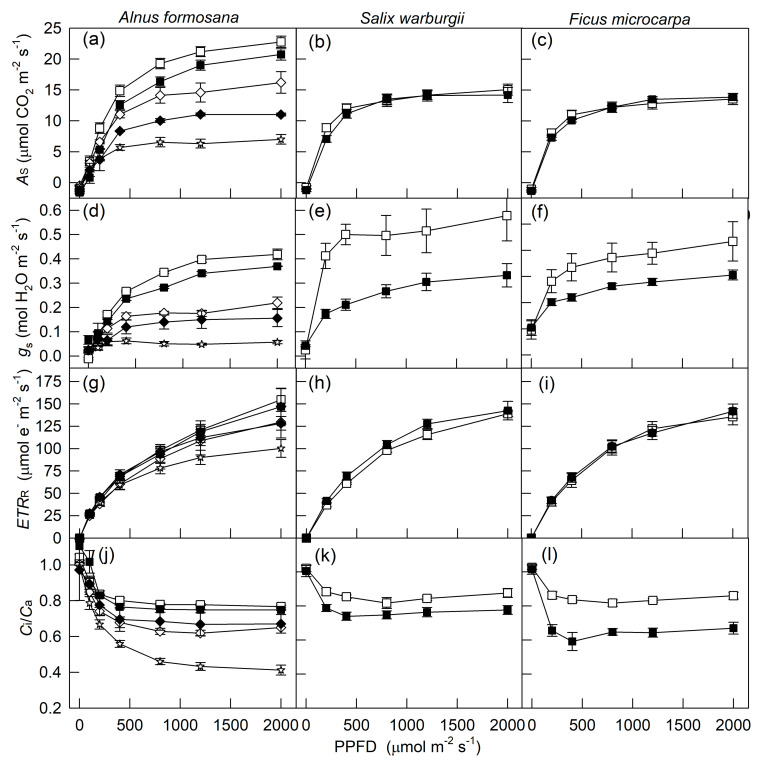
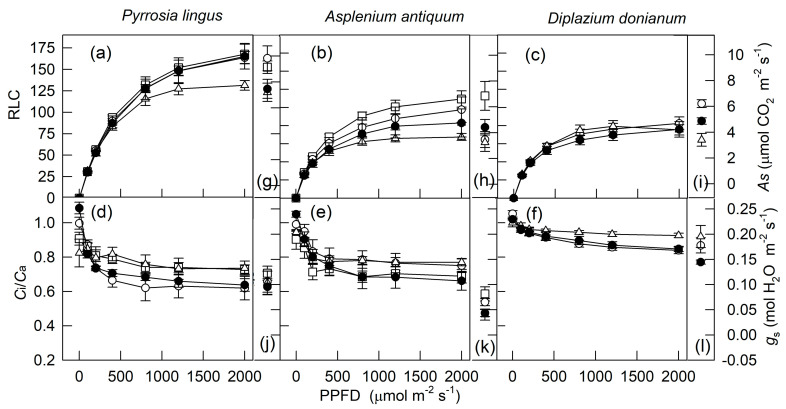
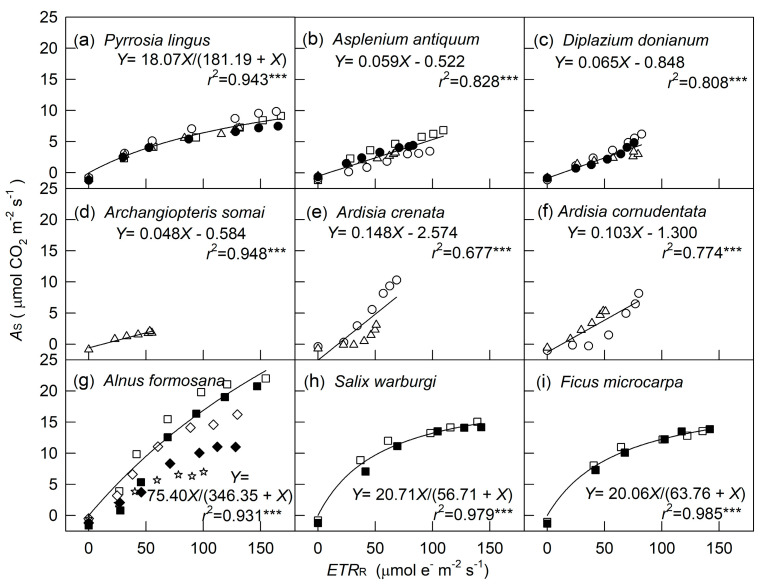
Figure 1 shows the LCs of AS, gs, ETRR, and intercellular and atmospheric CO2 concentration (Ci/Ca) for three tree species measured at 80% and 40% relative humidity (RH). AS and gs for four ferns and two understory shrubs, measured under well-watered conditions and 75% RH, were described previously [3]. Thus, only the LCs of ETRR and Ci/Ca for Pyrrosia lingus, Asplenium antiquum, and Diplazium donianum, measured at 80% and 40% RH, were selected, as shown in Figure 2a–f. To compare the AS and gs, these two variables for three ferns measured at 2000 μmol m−2 s−1 photosynthetic photon flux density (PPFD) are also shown in Figure 2g–l. In addition, the relation between AS and ETRR for all tested species under different PPFD, RH, and soil water conditions is shown in Figure 3. Generally, AS, gs, and ETRR for all tested species showed a hyperbolic increase with increasing PPFD. However, these LCs varied by species and environmental conditions during cultivation and measurement. Under well-watered conditions, a pioneer tree, Alnus formosana, had the highest light saturation point and maximal value of photosynthesis, followed by a hemiepiphytic tree, Ficus microcarpa, and a hygrophytic tree, Salix warburgii, then by two understory shrubs, Ardisia crenata and Ardisia cornudentata (Figure 1, Figure 2 and Figure 3 and [3]). In addition, for two understory shrubs, 50% sunlight-grown plants showed a higher maximal value of photosynthesis than 10% sunlight-grown plants (Figure 3e–f). Ferns adapted or acclimated to high light always had a higher light saturation point and maximal photosynthetic rate [3]. Only three trees grown under 100% sunlight and three ferns grown under 50% sunlight were measured under both high and low RH. Under well-watered conditions, the AS for A. formosana was inhibited only slightly by 40% RH but not for S. warburgii and F. microcarpa; even the gs for these two species was largely inhibited. Both AS and gs were not affected or were decreased slightly under 50% RH for three ferns. Both AS and gs were inhibited for A. formosana treated with both low soil water content and air moisture. Thus, findings for AS and gs were similar (Figure 1a,d).
Figure 1.
Light-response curves of As (a–c), gs (d–f), ETRR (g–i), and Ci/Ca (j–l) for three tree species measured at 25 °C and 80% (open symbols) and 40% (closed symbols) relative humidity. AS and gs indicate the net photosynthetic rate and stomatal conductance, respectively, obtained from steady-state light response; ETRR indicates electron transport rate obtained from rapid light response; Ci and Ca indicate intercellular and atmospheric CO2 concentration, respectively, obtained from steady-state light response. Squares, diamonds, and stars gs indicate measured under well-watered conditions, mild and severe drought, respectively. Data are mean ± SE.
Figure 2.
Light-response curves for ETRR and Ci/Ca (a–f) as well as AS and gs measured at 2000 μmol m−2 s−1 photosynthetic photon flux (PPFD; g–l) for three fern species at 25 °C and 75% (open symbols, data from [3]) and 50% (closed symbols) relative humidity. AS and gs indicate the net photosynthetic rate and stomatal conductance, respectively, from steady-state light response; ETRR indicates electron transport rate from rapid light response; Ci and Ca indicate intercellular and atmospheric CO2 concentration, respectively. Squares, circles, and triangles indicate cultivated under 100%, 50%, and 10% sunlight, respectively. Data are mean ± SE.
Figure 3.
Relationship between net photosynthetic rate from a steady-state light response (AS) and electron transport rates from a rapid light response (ETRR) for four ferns (a–d), two understory shrubs (e–f), and three tree (g–i) species. Squares, circles, and triangles indicate cultivated under 100%, 50%, and 10% sunlight, respectively, and measured under well-watered conditions; diamonds and stars (g) indicate cultivated under 100% sunlight and measured under mild and severe drought conditions, respectively. Open and closed symbols indicate measured under 75% and 50% relative humidity, respectively, for ferns and understory shrubs, and 80% and 40%, respectively, for trees. AS of ferns and understory shrubs measured at 75% relative humidity were from [3]. The regression line in g was fitted for well-watered conditions only. *** is significant at p < 0.001.
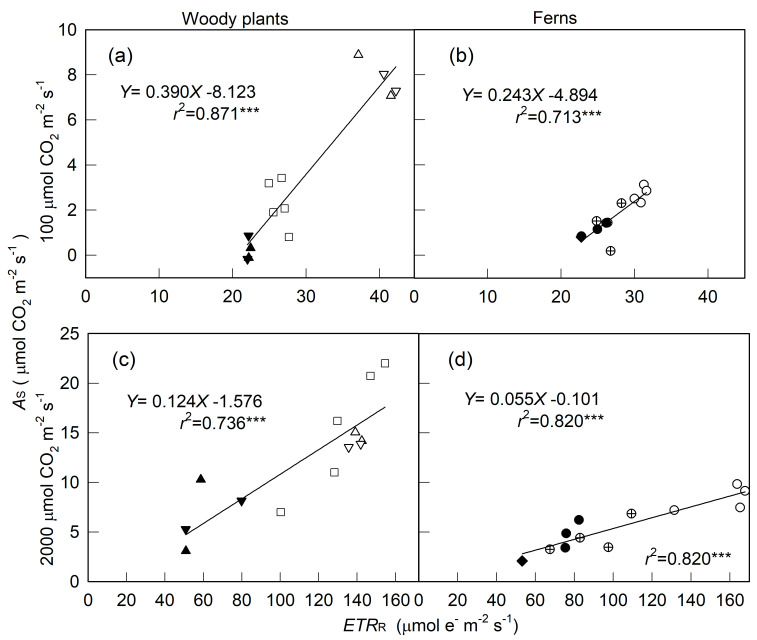
In contrast to AS, which for most plants was saturated at 800–1200 μmol m−2 s−1 PPFD, ETRR for high-light- and slight-shade-adapted species did not reach saturation until 2000 μmol m−2 s−1 PPFD (Figure 1 and Figure 2). Nevertheless, when merging data from the same species measured under different light and moisture conditions, the AS for three trees (high-light-adapted) and P. lingus, a slight-shade-adapted fern, showed a hyperbolic relation with ETRR: the AS–ETRR relation could be best fitted by the equation Y = aX/(b + X) (Y = AS, X = PPFD, r2 = 0.943–0.985, p < 0.001, Figure 3a,g–i). This relation for the other medium- to heavy-shade-adapted ferns and two understory shrubs was linear (r2 = 0.677–0.948, p < 0.001). The AS of A. formosana was inhibited largely by low soil water content and low RH, but its ETRR was not as inhibited as AS; thus, the AS–ETRR relation was not as close as for the other tested species. At both 100 and 2000 μmol m−2 s−1 PPFD, the leaves with high AS always had high ETRR, regardless of species or environmental factors. However, the slope of the AS–ETRR regression line was higher for woody plants than ferns (Figure 4).
Figure 4.
Relationship between net photosynthetic rate from steady-state light response (AS) and electron transport rates from a rapid light response (ETRR) for all tested materials at 100 (a,b) and 2000 (c,d) μmol m−2 s−1 PPFD. □ is Alnus formosana; △ is Salix Warburgii; ▽ is Ficus microcarpa; ▲ is Ardisia crenata; ▼ is Ardisia cornudentata; ○ is Pyrrosia lingus; ⊕ is Asplenium antiquum; ● is Diplazium donianum; ◆ is Archangiopteris somai. AS for ferns and understory shrubs measured at 75% relative humidity were from [3]. *** is significant at p < 0.001.
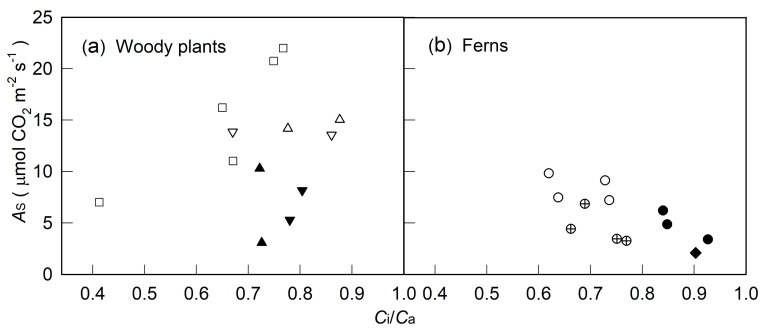
The Ci/Ca for all measurements decreased with increasing PPFD and stabilized somewhat at about 800 μmol m−2 s−1 PPFD with most treatments (Figure 1j–l and Figure 2d–f). At 2000 μmol m−2 s−1 PPFD, the AS–Ci/Ca relation could be divided into four groups: (1) four ferns, (2) two understory shrubs, (3) Ficus microcarpa and Salix Warburgii, and (4) Alnus formosana (Figure 5). The AS for ferns was decreased, and that for A. formosana was increased with increasing Ci/Ca. However, the AS for F. microcarpa and S. warburgii was not affected by Ci/Ca. As well, although the AS for two understory shrubs was inhibited by 10% sunlight during growth, their Ci/Ca was not greatly affected.
Figure 5.
The relationship between net photosynthetic rate (AS) and the ratio of intercellular CO2 concentration (Ci) to atmospheric CO2 concentration (Ca) for woody plants (a), and ferns (b). All data were obtained from the steady-state light response at 2000 μmol m−2 s−1 PPFD. □ is Alnus formosana; △ is Salix Warburgii; ▽ is Ficus microcarpa; ▲ is Ardisia crenata; ▼ is Ardisia cornudentata; ○ is Pyrrosia lingus; ⊕ is Asplenium antiquum; ● is Diplazium donianum; ◆ is Archangiopteris somai. AS for ferns and understory shrubs measured at 75% relative humidity were from [3].
3. Discussion
The ETRR–AS relation of terrestrial plants has not been studied in detail, especially among species across a wide taxonomic range and environmental adaptation capability. In this study, we compared the AS and ETRR for five woody and four fern species with different light and/or water adaptations. The obtained data showed a broad range of AS because of the specific differences of species and the environmental conditions under which materials were cultivated and measured. Under the steady-state, plants adapted or acclimated to high light always had high values of both light saturation point and maximal photosynthetic rate (Figure 1 and Figure 2 and [3]), which agreed with previous results (e.g., [1,2]).
ETR is calculated as the product of PSII efficiency and absorbed light. Many studies used the empirical mean of α (0.84) to calculate ETR and compare differences in ETR among species [5] and under different growth irradiances [35]. However, the α value may vary by leaf pigment content and anatomical structures. Previously, we examined leaves with a broad range of chlorophyll content (0.18–0.55 g m−2) and found a similar association of AS and ETR regardless of the use of α = 0.84 or 0.80–0.89 (from an empirical regression equation between α and chlorophyll content) to calculate ETR (Weng et al. unpublished data). In addition, our plants featured no specific anatomical structures. So we chose the empirical mean α of 0.84 [5].
Measurement of SLC requires light steps long enough to allow for stabilization of the photosynthetic processes under each irradiance level. RLC only requires 10 to 30 s at each light level; nevertheless, the difference in AS between high- and low-light-grown materials can be defined by ETRR [24,31,35]. However, in addition to a long-term photoacclimation status, ETRR also depends on the short-term (min) light history of photosynthetic organisms immediately before measurement as well as illumination time for each light level during measurement. Maximum ETRR value is very low after long-term dark adaptation, but when organisms are exposed to light immediately before RLC measurement, maximum ETRR increases with increasing illumination time and to a stable level within 8 to 15 min of illumination [23]. In addition, maximum ETRR increases with increasing light intensity immediately before measurement [30] but may decrease under high light (2000 μmol m−2 s−1 PPFD) pre-irradiance [23]. During measurement, maximum ETRR increases with increasing illumination time at each light level [24,53].
Our materials were acclimated to 1 to 3 levels of light intensity for at least five months. To minimize the effects of light history immediately before measurement on the ETRR and AS, we used overnight dark-adapted leaves for measurement of ETRR and then measured leaves were kept in the dark until measurement of AS. Even for leaves not exposed to light until the measurement and with short (10-s) steps of increasing irradiance, ETRR was still high (50–168 μmol e- m−2 s−1 for all materials at 2000 μmol m−2 s−1 PPFD) and closely related to AS (Figure 3 and Figure 4). Thus, ETRR could reflect the broad range in AS among different species and environmental conditions.
Photosynthesis is limited by both stomatal and non-stomatal factors. The former is associated with decreased leaf Ci caused by stomata closure, and the latter with a decrease in photochemical efficiency and CO2 fixation [1,46]. The gs value often decreases with decreasing light intensity [44,45] as well as soil water content [20,46,54] and air humidity [2,20]. However, photosynthetic electron transport and CO2 fixation are also inhibited by low light [3,5,23] and low soil water content [20,54]. In the present study, all AS and gs values decreased with decreasing light intensity (Figure 1 and Figure 2). However, the effect of low RH on gs varied by species. Under well-watered conditions, the gs for F. microcarpa and S. warburgii was largely inhibited by 40% RH (Figure 1e,f). Nevertheless, AS did not differ greatly at 80% and 40% RH for two trees (Figure 1b,c). In contrast, only A. formosana was treated with low soil water content, and its AS, gs, and ETRR values were markedly inhibited (Figure 1a,d,g). Thus, we revealed a combination of stomatal and nonstomatal effects on photosynthesis.
At 2000 μmol m−2 s−1, the AS for four ferns decreased with increasing Ci/Ca when AS was affected by specific differences of species and environmental conditions during cultivation and measurement (Figure 5b). Based on the relation between Ci/Ca and AS [46,51,55], indicating non-stomatal factors were the main cause of the difference in AS for the four ferns. In contrast, the AS for A. formosana increased with increasing Ci/Ca under low RH and low soil water content (Figure 5a). The decrease in AS was explained mainly by stomatal closure (Figure 1d). The AS for F. microcarpa and S. warburgii was not affected by Ci/Ca (Figure 5a), so neither stomatal nor non-stomatal factors were a main limiting factor for the AS for these two species, even if their gs value was markedly limited by low RH (Figure 1e,f). As well, the Ci/Ca for two 10%-sunlight-grown understory shrubs was not affected by decreasing AS (Figure 5a), so AS decreased with decreasing gs.
ETRR is related to environmental acclimation [23,34,35,36] and stress responses [35,38,39] of terrestrial plants but is rarely used to elucidate the relation with the photosynthetic rate [35]. When data in Figure 1, Figure 2 and Figure 3 and Figure 5 were combined, stomatal limitation played an important role in affecting the ETRR–AS relation within a species. When stomatal closure was not a main limiting factor for photosynthesis, ETRR and AS were closely related across a wide range of PPFD, even when merging data for a species grown under different light conditions and measured under different light intensity and RH (Figure 3). This finding may explain why almost all research involving ETRR to assess photosynthetic activity or biomass productivity is limited to species without stomatal limitations to CO2 uptake, such as algae [26,27,29] and coral [28]. However, the induction of A and ETR requires several minutes to reach stability (e.g., [51,52]), whereas leaves are exposed to only 10–30 s of actinic light at each step during ETRR measurement. Thus, ETRR represents only the potential response of steady-state photosynthesis under a range of light conditions [30].
Our results indicate that the ETRR–AS relationship varies by species. The increase in LC in the light-limiting region, as well as light-saturation and maximum photosynthetic variables, have been used for research into plant ecophysiology [24,30,31,35]. Because the ETRR for some species did not reach saturation until 2000 μmol m−2 s−1 PPFD, we could not compare the light-saturation variable among species. Here, we used only data obtained at 100 μmol m−2 s−1 PPFD for the efficiency of light capture and data obtained at 2000 μmol m−2 s−1 PPFD as a maximum photosynthetic variable to elucidate the interspecific relationship between ETRR and AS. A significant ETRR–AS relation could be found within a plant group (i.e., woody plants and ferns) (Figure 4). Therefore, the ETRR value obtained at both 100 and 2000 μmol m−2 s−1 PPFD could be used to compare the photosynthetic capacity within the same plant group, regardless of a broad difference in AS due to different species or environmental factors, with the empirical mean of α (0.84) used to calculate ETR for all tested materials. Moreover, slopes were higher for woody plants than ferns for both AS–ETRR (Figure 4) and AS–ETRS [3] on regression analysis, which might somewhat be caused by the difference of light absorptivity of leaf among species. However, even with α value changed from 0.80 to 0.89 (chlorophyll content from 0.18 to 0.55 g m−2, Weng et al. unpublished data), there was only a 1.1-fold difference between ETR with α = 0.81 and 0.89 used for calculation. However, the difference in slopes for the AS–ETRR regression between woody plants and fern was much higher than 1.1-fold (1.6- and 2.3-fold at 100 and 2000 μmol m−2 s−1 PPFD, respectively, Figure 4). Thus, we prefer to explain that tested woody species could share more electrons for CO2 fixation at a given ETR level than ferns. This finding might be due to differences in allocation portion between CO2 fixation and alternative electronic pathways [19,22], such as photorespiration [15], water–water cycle [16], and cyclic electron flow within PSII [17] as well as nitrogen [56] and sulfur [57] assimilation.
In the present study, we found that 1) ETRR could reflect the variation in AS at each irradiance level within a species under well-watered conditions and 2) ETRR obtained at 100 μmol m−2 s−1 PPFD (as the efficiency of light capture) or 2000 μmol m−2 s−1 PPFD (as a maximum photosynthetic parameter) could be used to compare the photosynthetic capacity within a plant group, such as woody plants and ferns. Because ETRR can be measured within 1.5–2 min, it might be a useful tool for ecophysiological research. However, we investigated only five woody plants and four fern species. The number of species may not be enough to argue the taxonomic distinctions, and more comparisons might be needed. In addition, photorespiration is another major sink for electrons from PSII in C3 plants. The AS/ETRR ratio may vary on changing the CO2 and O2 concentration as well as temperature because the allocation of electrons between CO2 fixation and photorespiration may vary.
4. Materials and Methods
4.1. Plant Materials
We examined 4 fern species with different light adaptation (ranked from high to low light adaptation: Pyrrosia lingus (Thunb.) Farw., Asplenium antiquum Makino, Diplazium donianum (Mett.) Tard. -Blot., and Archangiopteris somai Hayata), 3 broad-leaved tree species with different water adaptation (Alnus formosana (Burkill) Makino, a pioneer tree; Salix warburgii O. Seem., a hygrophyte, and Ficus microcarpa L., a hemiepiphyte) and 2 broad-leaved understory shrubs (Ardisia crenata Sims. and Ardisia cornudentata Mez.) in this study [48,49,58]. In addition, A. formosana and S. warburgii are usually distributed near the rivers or in gullies; P. lingus and F. microcarpa can survive in the dry environment; whereas D. donianum, Arc. Somai, and S. warburgii are sensitive to drought [3]. Four ferns (adult plants, about 30 cm tall), 2 understory shrubs (adult plants, about 60 cm tall), and A. formosana (1- to 2-year-old seeding, about 30–50 cm tall) were the same as we used previously, and collected from central Taiwan [3]. The other 2 trees were only used in the present study and were propagated from cuttings (about 30–50 cm tall). All plants were collected in March and then transplanted to pots (16-cm diameter, 12-cm depth, 1 plant per pot for the five woody species and As. antiquum, and 1 rhizome with 3–4 leaves per pot for the other 3 ferns) filled with organic soil and maintained outdoors in the nursery of the Endemic Species Research Institute, Chichi Township, Nantou County, Taiwan (23°49′ N, 120°48′ E, 250 m a.s.l.). Materials were regularly watered and fertilized (half-strength Hoagland’s nutrient solution per month) and received up to 3 levels of light intensity (i.e., 100%, 50%, and 10% (beneath shade cloth)), according to the light condition of their habitat, i.e., 3 trees received 100% sunlight; 2 slight- to medium-shade ferns, P. lingus and As. antiquum, received 100%, 50%, and 10% sunlight; 1 medium-to-heavy shade fern, D. donianum, and 2 understory shrubs received 10% and 50% sunlight; and 1 heavy-shade fern, Arc. somai received 10% sunlight. The average elevation and temperature were about 250 m and 20 °C. The average annual rainfall and air humidity were about 2200 mm and 80%. During the growth period of the materials (March–November), the average hourly values of daily maximum photosynthetic photon flux density (PPFD) ranged from 1296−1456 μmol m−2 s−1 (Mar.−Aug.) and 1150−770 μmol m−2 s−1 (Sept.−Nov.) (data from the Endemic Species Research Institute). Only A. formosana was treated with mild and severe drought immediately before photosynthetic measurement by withholding water, until AS values were reduced to about 70% and 30%, respectively, of the maximum (AS under well-watered conditions: 100%) [54].
4.2. Measurements
Measurements were carried out from September to November in a laboratory at the Endemic Species Research Institute. At nightfall of 1 day before the measurement, potted materials were dark-adapted overnight (room temperature about 25 °C). On the next day, fully expanded younger leaves were selected for measurements. First, the measurement of ETRR was at dawn at room temperature and involved the software of the PAM-2000 fluorometer (Walz, Effeltrich, Germany). Nine steps of active light from about 60–2300 μmol m−2 s−1 PPFD were applied at each irradiation step for 10 s [23,34,35]. The actual (F) and maximal (Fm’) levels of fluorescence were measured at the end of each irradiance level. The F was determined under each PPFD level, and the Fm’ was determined by applying a 0.8-s pulse of saturating flashes of approximately 6000 μmol quanta m−2 s−1. Actual PSII efficiency (ΦPSII) was calculated as (Fm’−F)/Fm’, and ETRR was calculated as ΦPSII × PPFDD × 0.5 × α [8]. We used the mean value of leaf absorption (α) of 0.84 for green leaves [59] (see Discussion section). ETRR at 200, 400, 800, 1200, and 2000 μmol m−2 s−1 PPFD was calculated from linear interpolation between the 2 nearest values. After the measurement of ETRR, the measured leaves were kept in the dark until the measurement of the steady-state light response of CO2 exchange. From 09:30 h to 15:00 h, photosynthesis and stomatal conductance were measured by use of a portable, open-flow gas exchange system (LI-6400, LI-COR, Lincoln, NE, USA), and an integrated fluorescence LI-6400-40 chamber head stepwise from low to high levels of PPFD (i.e., 0, 100, 200, 400, 800, 1200, and 2000 μmol m−2 s−1). The values of AS (net photosynthetic rate), gs, and intercellular CO2 concentration/ambient CO2 concentration (Ci/Ca) were recorded when the gas exchange was stable (about 4 min in the dark and 10–20 min under each level of illumination). Throughout the measurements, leaf temperature and CO2 concentration were kept at 25 °C and 350–400 μmol mol−1 (no control), respectively, for all materials. Relative humidity (RH) in the chamber was taken at 75% and 50% (air entering chamber controlled by passing temperature-controlled water) for ferns and understory shrubs, and 80% and 40% for trees.
4.3. Statistical Analysis
Four to 6 fully expanded younger leaves from 4 plants of each species grown in each light condition were measured. Each leaf was taken as 1 replicate for statistical analyses. The results are expressed as the mean ± standard error (SE). The light-response curve of photosynthetic rate was fitted by sigmoidal or hyperbolic equations. Data were analyzed by linear or curve–linear regression. All statistical analyses involved the use of Sigma Plot v10.0.
Author Contributions
S.-L.W. performed the experiments and collected all data sets; J.-H.W. designed the full experiment; S.-L.W. and J.-H.W. provided laboratory facilities for all analysis and interpreted the data. M.-Y.H. and J.-H.W. wrote the manuscript and reviewed the final manuscript for journal submission. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully acknowledge the grant: “108-2621-B-034-002-MY2” by the Ministry of Science and Technology (MOST) in Taiwan.
Conflicts of Interest
All the authors declare that they have no conflict of interest regarding this manuscript. Everyone contributed with positive manners and agreed to publish the work.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Berry J.A., Downton W.J.S. Environmental regulation of photosynthesis. In: Govindjee, editor. Photosynthesis. Volume 2. Academic Press; London, UK: 1982. pp. 263–343. [Google Scholar]
- 2.Hölscher D., Leuschner C., Bohman K., Hagemeier M., Juhrbandt J., Tjitrosemito S. Leaf gas exchange of trees in old-growth and young secondary forest stands in Sulawesi, Indonesia. Trees. 2006;20:278–285. doi: 10.1007/s00468-005-0040-4. [DOI] [Google Scholar]
- 3.Wong S.L., Chen C.W., Huang H.W., Weng J.H. Using combined measurements of gas exchange and chlorophyll fluorescence to investigate the photosynthetic light responses of plant species adapted to different light regimes. Photosynthetica. 2012;50:206–214. doi: 10.1007/s11099-012-0027-5. [DOI] [Google Scholar]
- 4.Coe R.A., Lin H.C. Light-Response Curves in Land Plants. In: Covshoff S., editor. Photosynthesis: Methods and Protocols. Springer Science + Business Media; New York, NY, USA: 2018. pp. 83–93. [Google Scholar]
- 5.Rascher U., Liebig M., Lüttge U. Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant Cell Environ. 2000;23:1397–1405. doi: 10.1046/j.1365-3040.2000.00650.x. [DOI] [Google Scholar]
- 6.Schreiber U., Schliwa U., Bilger W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986;10:51–62. doi: 10.1007/BF00024185. [DOI] [PubMed] [Google Scholar]
- 7.Roháček K., Barták M. Technique of the modulated chlorophyll fluorescence: Basic concepts, useful parameters, and some applications. Photosynthetica. 1999;37:339–363. doi: 10.1023/A:1007172424619. [DOI] [Google Scholar]
- 8.Maxwell K., Johnson G.N. Chlorophyll fluorescence-a practical guide. J. Exp. Bot. 2000;51:659–668. doi: 10.1093/jexbot/51.345.659. [DOI] [PubMed] [Google Scholar]
- 9.Krall J.P., Edwards G.E. Relationship between photosystem II activity and CO2 fixation in leaves. Physiol. Plant. 1992;86:180–187. doi: 10.1111/j.1399-3054.1992.tb01328.x. [DOI] [Google Scholar]
- 10.Baker N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008;59:89–113. doi: 10.1146/annurev.arplant.59.032607.092759. [DOI] [PubMed] [Google Scholar]
- 11.Demmig-Adams B., Adams W.W., III, Barker D.H., Logan B.A., Bowlong D.R., Verhoeven A.S. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol. Plant. 1996;98:253–264. doi: 10.1034/j.1399-3054.1996.980206.x. [DOI] [Google Scholar]
- 12.Kato M.C., Hikosaka K., Hirotsu N., Makino A., Hirose T. The excess light energy that is neither utilized in photosynthesis nor dissipated by photoprotective mechanisms determines the rate of photoinactivation in photosystem II. Plant Cell Physiol. 2003;44:318–325. doi: 10.1093/pcp/pcg045. [DOI] [PubMed] [Google Scholar]
- 13.Adams W.W., III, Zarter C.R., Ebbert V., Demmig-Adams B. Photoprotective strategies of overwintering evergreens. BioScience. 2004;54:41–49. doi: 10.1641/0006-3568(2004)054[0041:PSOOE]2.0.CO;2. [DOI] [Google Scholar]
- 14.Demmig-Adams B., Stewart J.J., López-Pozo M., Polutchko S.K., Adams W.W., III Zeaxanthin, a molecule for photoprotection in many different environments. Molecules. 2020;25:5825. doi: 10.3390/molecules25245825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson R.B. Regulation of electron transport in photosystems I and II in C3, C3-C4, and C4 species of Panicum in response to changing irradiance and O2 levels. Plant Physiol. 1994;105:349–356. doi: 10.1104/pp.105.1.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asada K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Phys. Plant Mol. Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- 17.Miyake C., Okamura M. Cyclic electron flow within PSII protects PSII from its photoinhibition in thylakoid membranes from Spinach chloroplasts. Plant Cell Physiol. 2003;44:457–462. doi: 10.1093/pcp/pcg053. [DOI] [PubMed] [Google Scholar]
- 18.Cheng L., Fuchigami L.H., Breen P.J. The relationship between photosystem II efficiency and quantum yield for CO2 assimilation is not affected by nitrogen content in apple leaves. J. Exp. Bot. 2001;52:1865–1872. doi: 10.1093/jexbot/52.362.1865. [DOI] [PubMed] [Google Scholar]
- 19.Pérez-Torres E., Bravo L.A., Corcuera L.J., Johnson G.N. Is electron transport to oxygen an important mechanism in photoprotection? Contrasting responses from Antarctic vascular plants. Physiol. Plant. 2007;130:185–194. doi: 10.1111/j.1399-3054.2007.00899.x. [DOI] [Google Scholar]
- 20.Ripley B.S., Gilbert M.E., Ibrahim D.G., Osborne C.P. Drought constraints on C4 photosynthesis: Stomatal and metabolic limitations in C3 and C4 subspecies of Alloteropsis semialata. J. Exp. Bot. 2007;58:1351–1363. doi: 10.1093/jxb/erl302. [DOI] [PubMed] [Google Scholar]
- 21.Cornic G., Briantais J.M. Partitioning of photosynthetic electron flow between CO2 and O2 reduction in a C3 leaf (Phaseolus vulgaris L.) at different CO2 concentrations and during drought stress. Planta. 1991;183:178–184. doi: 10.1007/BF00197786. [DOI] [PubMed] [Google Scholar]
- 22.Oberhuber W., Edwards G.E. Temperature dependence of the linkage of quantum yield of photosystem II to CO2 fixation in C4 and C3 plants. Plant Physiol. 1993;101:507–512. doi: 10.1104/pp.101.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White A.J., Critchley C. Rapid light curves: A new fluorescence method to assess the state of the photosynthetic apparatus. Photosynth. Res. 1999;59:63–72. doi: 10.1023/A:1006188004189. [DOI] [Google Scholar]
- 24.Ralph P.J., Gademann R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005;82:222–237. doi: 10.1016/j.aquabot.2005.02.006. [DOI] [Google Scholar]
- 25.Pleban J.R., Guadagno C.R., Mackay D.S., Weinig C., Ewers B.E. Rapid chlorophyll a fluorescence light response curves mechanistically inform photosynthesis modeling. Plant Physiol. 2020;183:602–619. doi: 10.1104/pp.19.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longstaff B.J., Kildea T., Runcie J.W., Cheshire A., Dennison W.C., Hurd C., Kana T., Raven J.A., Larkum A.W.D. An in situ study of photosynthetic oxygen exchange and electron transport rate in the marine macroalga Ulva lactuca (Chlorophyta) Photosynth. Res. 2002;74:281–293. doi: 10.1023/A:1021279627409. [DOI] [PubMed] [Google Scholar]
- 27.Carr H., Björk M. A methodological comparison of photosynthetic oxygen evolution and estimated electron transport rate in tropical Ulva (Chlorophyceae) species under different light and inorganic carbon conditions. J. Phycol. 2003;39:1125–1131. doi: 10.1111/j.0022-3646.2003.02-077.x. [DOI] [Google Scholar]
- 28.Lesser M.P., Slattery M., Stat M., Ojimi M., Gates R.D., Grottoli A. Photoacclimatization by the coral Montastraea cavernosa in the mesophotic zone: Light, food, and genetics. Ecology. 2010;91:990–1003. doi: 10.1890/09-0313.1. [DOI] [PubMed] [Google Scholar]
- 29.Suggett D.J., Moore C.M., Geider R.J. Estimating aquatic productivity from active fluorescence measurements. In: Suggett D.J., Borowitzka M.A., Prášil O., editors. Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications. Springer Science + Business Media B.V.; Dordrecht, The Netherland: 2011. pp. 103–127. [Google Scholar]
- 30.Serôdio J., Vieira S., Cruz S., Coelho H. Rapid light-response curves of chlorophyll fluorescencein microalgae: Relationship to steady-state light curves and non-photochemical quenching in benthic diatom-dominated assemblages. Photosynth. Res. 2006;90:29–43. doi: 10.1007/s11120-006-9105-5. [DOI] [PubMed] [Google Scholar]
- 31.Cruz S., Serôdio J. Relationship of rapid light curves of variable fluorescence to photoacclimation and non-photochemical quenching in a benthic diatom. Aquat. Bot. 2008;88:256–264. doi: 10.1016/j.aquabot.2007.11.001. [DOI] [Google Scholar]
- 32.Yarnold J., Ross I.L., Hankamer B. Photoacclimation and productivity of Chlamydomonas reinhardtii grown in fluctuating light regimes which simulate outdoor algal culture conditions. Algal Res. 2016;13:182–194. doi: 10.1016/j.algal.2015.11.001. [DOI] [Google Scholar]
- 33.Houliez E., Lefebvre S., Lizon F., Schmitt F.G. Rapid light curves (RLC) or non-sequential steady-state light curves (N-SSLC): Which fluorescence-based light response curve methodology robustly characterizes phytoplankton photosynthetic activity and acclimation status? Mar. Biol. 2017;164:175. doi: 10.1007/s00227-017-3208-8. [DOI] [Google Scholar]
- 34.Liu N., Lin Z.F., Guan L.L., Lin G.Z., Peng C.L. Light acclimation and HSO3− damage on photosynthetic apparatus of three subtropical forest species. Ecotoxicology. 2009;18:929–938. doi: 10.1007/s10646-009-0356-8. [DOI] [PubMed] [Google Scholar]
- 35.Liang K.M., Lin Z.F., Ren H., Liu N., Zhang Q.M., Wang J., Wang Z.F., Guan L.L. Characteristics of sun- and shade-adapted populations of an endangered plant Primulina tabacum Hance. Photosynthetica. 2010;48:494–506. doi: 10.1007/s11099-010-0066-8. [DOI] [Google Scholar]
- 36.Fu W., Li P., Wu Y. Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci. Hort. 2012;135:45–51. doi: 10.1016/j.scienta.2011.12.004. [DOI] [Google Scholar]
- 37.Sma-Air S., Ritchie R.J. Photosynthesis in a Vanda sp orchid with photosynthetic roots. J. Plant Physiol. 2020;251:153187. doi: 10.1016/j.jplph.2020.153187. [DOI] [PubMed] [Google Scholar]
- 38.Waldhoff D., Furch B., Junk W.J. Fluorescence parameters, chlorophyll concentration, and anatomical features as indicators for flood adaptation of an abundant tree species in Central Amazonia: Symmeria paniculata. Environ. Exp. Bot. 2002;48:225–235. doi: 10.1016/S0098-8472(02)00037-0. [DOI] [Google Scholar]
- 39.Li Q.M., Liu B., Wu Y., Zou Z.R. Interactive effects of drought stresses and elevated CO2 concentration on photochemistry efficiency of cucumber seedlings. J. Integr. Plant Biol. 2008;50:1307–1317. doi: 10.1111/j.1744-7909.2008.00686.x. [DOI] [PubMed] [Google Scholar]
- 40.Azhar A., Makihara D., Naito H., Asano K., Takagi M., Unoki S., Tomita R., Abbas B., Ehara H. Sago palm (Metroxylon sagu Rottb.) response to drought condition in terms of leaf gas exchange and chlorophyll a fluorescence. Plant Prod. Sci. 2020;24:1794914. doi: 10.1080/1343943X.2020.1794914. [DOI] [Google Scholar]
- 41.Zheng L., Steppe K., Labeke M.V. Spectral quality of monochromatic LED affects photosynthetic acclimation to high-intensity sunlight of Chrysanthemum and Spathiphyllum. Physiol. Plant. 2020;169:10–26. doi: 10.1111/ppl.13067. [DOI] [PubMed] [Google Scholar]
- 42.Quinnell R., Howell D., Ritchie R.J. Photosynthesis of an epiphytic resurrection fern Davallia angustata (Wall. ex Hook. & Grev.) Aust. J Bot. 2017;65:348–356. [Google Scholar]
- 43.Vavasseur A., Raghavendra A.S. Guard cell metabolism and CO2 sensing. New Phytol. 2005;165:665–682. doi: 10.1111/j.1469-8137.2004.01276.x. [DOI] [PubMed] [Google Scholar]
- 44.Yu Q., Zhang Y.Q., Liu Y.F., Shi P.L. Simulation of the stomatal conductance of winter wheat in response to light temperature and CO2 changes. Ann. Bot. 2004;93:435–441. doi: 10.1093/aob/mch023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang J., Boerner R.E.J., Rebbeck J. Ecophysiological responses of two herbaceous species to prescribed burning, alone or in combination with overstory thinning. Am. J. Bot. 2007;94:755–763. doi: 10.3732/ajb.94.5.755. [DOI] [PubMed] [Google Scholar]
- 46.Brodribb T. Dynamics of changing intercellular CO2 concentration (ci) during drought and determination of minimum functional ci. Plant Physiol. 1996;111:179–185. doi: 10.1104/pp.111.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larcher W. Physiological Plant Ecology. 3rd ed. Springer; Berlin, Germany: 1995. [Google Scholar]
- 48.Weng J.H., Chien C.T., Chen C.W., Lai X.M. Effects of osmotic and high light stresses on PSII efficiency of attached and detached leaves of three tree species adapted to different water regimes. Photosynthetica. 2011;49:555–563. doi: 10.1007/s11099-011-0072-5. [DOI] [Google Scholar]
- 49.Brodribb T.J., McAdam S.A.M. Evolution of the Stomatal Regulation of Plant Water Content. Plant Physiol. 2017;174:639–649. doi: 10.1104/pp.17.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haworth M., Elliott-Kingston C., McElwain J.C. Stomatal control as a driver of plant evolution. J. Exp. Bot. 2011;62:2419–2423. doi: 10.1093/jxb/err086. [DOI] [PubMed] [Google Scholar]
- 51.Wong S.H., Chen C.W., Huang H.W., Weng J.H. Using combined measurements for comparison of light induction of stomatal conductance, electron transport rate and CO2 fixation in woody and fern species adapted to different light regimes. Tree Physiol. 2012;32:535–544. doi: 10.1093/treephys/tps037. [DOI] [PubMed] [Google Scholar]
- 52.Allen M.T., Pearcy R.W. Stomatal behavior and photosynthetic performance under dynamic light regimes in a seasonally dry tropical rain forest. Oecologia. 2000;122:470–478. doi: 10.1007/s004420050968. [DOI] [PubMed] [Google Scholar]
- 53.Herlory O., Richard P., Blanchard G. Methodology of light response curves: Application of chlorophyll fluorescence to microphytobenthic biofilms. Mar. Biol. 2007;153:91–101. doi: 10.1007/s00227-007-0787-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gulías J., Flexas J., Abadía A., Medrano H. Photosynthetic responses to water deficit in six Mediterranean sclerophyll species: Possible factors explaining the declining distribution of Rhamnus ludovici-salvatoris, an endemic Balearic species. Tree Physiol. 2002;22:687–697. doi: 10.1093/treephys/22.10.687. [DOI] [PubMed] [Google Scholar]
- 55.Ishida A., Toma T., Marjenah M. Limitation of leaf carbon gain by stomatal and photochemical processes in the top canopy of Macaranga conifera, a tropical pioneer tree. Tree Physiol. 1999;19:467–473. doi: 10.1093/treephys/19.7.467. [DOI] [PubMed] [Google Scholar]
- 56.Robinson J.M. Nitrite photoreduction in vivo is inhibited by oxygen. Plant Physiol. 1990;92:862–865. doi: 10.1104/pp.92.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saito K. Sulfur assimilatory metabolism. The long and smelling road. Plant Physiol. 2004;136:2443–2450. doi: 10.1104/pp.104.046755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liao T.S., Weng J.H. Ecophysiological characteristics of Taiwan alder (Alnus formosana Makino) adapted to the subtropical region. Tree Physiol. 2002;22:355–362. doi: 10.1093/treephys/22.5.355. [DOI] [PubMed] [Google Scholar]
- 59.Björkman O., Demmig B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta. 1987;170:489–504. doi: 10.1007/BF00402983. [DOI] [PubMed] [Google Scholar]