Abstract
Background
COVID-19 is characterized by a rapid change in the patient’s condition, with major changes occurring over a few days. We aimed to develop and evaluate an emergency system for monitoring patients with COVID-19, which may be useful in hospitals where more severe patients stay in their homes.
Methodology/Principal findings
The system consists of the home-based patient unit, which is set up around the patient and the hospital unit, which enables the medical staff to telemonitor the patient’s condition and help to send medical recommendations. The home unit allows the data transmission from the patient to the hospital, which is performed using a cell phone application. The hospital unit includes a virtual instrument developed in LabVIEW® environment that can provide a real-time monitoring of the oxygen saturation (SpO2), beats per minute (BPM), body temperature (BT), and peak expiratory flow (PEF). Abnormal events may be fast and automatically identified. After the design details are described, the system is validated by a 30-day home monitoring study in 12 controls and 12 patients with COVID-19 presenting asymptomatic to mild disease. Patients presented reduced SpO2 (p<0.0001) and increased BPM values (p<0.0001). Three patients (25%) presented PEF values between 50 and 80% of the predicted. Three of the 12 monitored patients presented events of desaturation (SpO2<92%). The experimental results were in close agreement with the involved pathophysiology, providing clear evidence that the proposed system can be a useful tool for the remote monitoring of patients with COVID-19.
Conclusions
An emergency system for home monitoring of patients with COVID-19 was developed in the current study. The proposed system allowed us to quickly respond to early abnormalities in these patients. This system may contribute to conserving hospital resources for those most in need while simultaneously enabling early recognition of patients under acute deterioration, requiring urgent assessment.
1. Introduction
We are experiencing a global pandemic due to COVID-19 of devastating consequences. The highly infectious pathogen that causes COVID-19, SARS-CoV-2, has infected most of the countries in the world, with over 62.7 million confirmed cases and near 1.460.000 documented deaths as of December 1, 2020 [1].
As the hospital environment becomes more crowded, the criteria for hospital admission become progressively stricter and, as a consequence, more severe patients stay in their homes awaiting improvement or worsening. It was pointed out previously that a rapid clinical deterioration may occur in the initial phase of COVID-19 due to the development of arterial hypoxemia without a concomitant increase in work of breathing [2]. This can prevent an adequate perception by the patient of the real magnitude of the problem. In this context, patients have emerged who silently and rapidly decompensate respiratory function at home, progressing to death even before receiving specialized care. Thus, it is essential to obtain severity markers, especially to predict and prevent the evolution to hospitalization in ICU and death. In this emergency scenario, a consensus has emerged in the literature on the need to institute home monitoring of these patients [3–5], enabling early identification of those who deteriorate acutely and require urgent assessment.
In this context, we aimed to develop an emergency system for monitoring patients with COVID-19, which may be useful in hospitals where more severe patients stay in their homes. We hypothesized that an emergency system based on a smartphone application and specific instruments that allow oxygen saturation, body temperature, and peak expiratory flow could be useful as a COVID-19 home-monitoring tool in clinical practice.
2. Proposed emergency home monitoring system
Based on previous studies monitoring respiratory diseases [6] and the specific characteristics of COVID-19 [7, 8], the following variables were selected for monitoring:
2.1. Pulse oximetry
In the particular case of COVID-19, this monitoring is essential because in about 10% of cases, especially in the elderly and people with comorbidities, hypoxemia can develop quickly, implying intensive therapy with mechanical ventilation. It is also recognized that hypoxemia is at the very heart of the most severe cases of COVID-19 [9]. In addition, there is now fairly strong evidence that the mortality risk increased with the oxygen saturation reduction [10], which highlights the importance of continuous monitoring of this parameter.
2.2. Heart rate
Increased heart rate (HR) is a common manifestation in many respiratory infections [11] and is typically elevated by COVID-19 [11, 12]. The exact pathophysiological mechanism underlying myocardial injury caused by COVID-19 is not yet fully understood. However, there is evidence that this injury may be closely associated with inflammatory pathogenesis during the disease progress. This may reduce coronary blood flow, decreases in oxygen supply, destabilization of coronary plaque, and microthrombogenesis [13]. HR monitoring’s relevance is highlighted in the study conducted by Mishra and collaborators, which showed that HR is a sensitive parameter that may help in the pre-symptomatic detection of COVID-19 [11].
2.3. Body temperature
It is known that increased body temperature (BT) is a marker of infection, so temperature provides an essential orientation of health professionals during the COVID 19 disease course [14]. A recent report by the World Health Organization showed that of the approximately 56,000 laboratory confirmed cases studied in China, among the typical signs and symptoms of COVID-19, increased BT is the most common [15]. Thus, BT is widely recognized in the literature as an essential measurement in the COVID-19 pandemic [14–16] that can be used as an easily obtained prognostic indicator [14, 17].
2.4. Peak expiratory flow
As this is the first study to investigate the use of PEF in COVID-19, this section shortly describes how this method was successfully used in other diseases to solve problems that are crucial in COVID-19. This includes home monitoring the progression of the disease and its treatment and identifying patients predisposed to worse clinical outcomes.
The monitoring of peak expiratory flow (PEF) is widely recommended in international guidelines for asthma management. In these patients, the predicted percentage of PEF correlates reasonably well with the predicted percentage for forced expiratory volume in the first second (FEV1) and provides an objective measure of airflow limitation when spirometry is not available [18]. The value of PFE can be measured using portable meters, which are affordable and relatively simple to handle. This method is widely recognized as suitable for monitoring the disease’s progression and its treatment, using the initial values obtained from the patient as a control [19–21]. The monitoring of PEF also helps to monitor the patient’s improvement after a particular mode of treatment [21]. In general, PEF is reduced in all types of respiratory diseases. Ignacio-Garcia et al. observed a significant improvement in morbidity parameters in the 35 patients with asthma monitored through peak flow at home [22].
In asthma, the drop in PEF values indicates a decrease in the condition of patients. From the initial measurements of PEF, the fall in its value by up to 20% indicates caution, but there is no danger, as this variation is not unexpected in 24 hours. A drop of 20 to 50% indicates that the patient is at risk of suffering an exacerbation. If the drop exceeds 50%, patients are at imminent risk of exacerbation. Reduced PEF values in patients with asthma precede the presence of shortness of breath or even the signs of wheezing and snoring detected by the stethoscope. Thus, correct knowledge of PEF predicts the patient’s condition and offers valuable time and opportunity to take all necessary measures to prevent adverse effects of the disease. The integration of PEF monitoring would allow identifying fall limits of the PEF suitable for monitoring the COVID-19, reducing hospitalizations and death. In this sense, previous studies show that the daily monitoring of PEF was a useful tool in the identification of patients with COPD predisposed to worse clinical outcomes [23].
Previous studies describing the physiological changes in COVID-19 have shown rapid reductions in lung volumes in the presence of edema [8, 24], which highlights the importance of monitoring the PEF of these patients.
2.5. System architecture
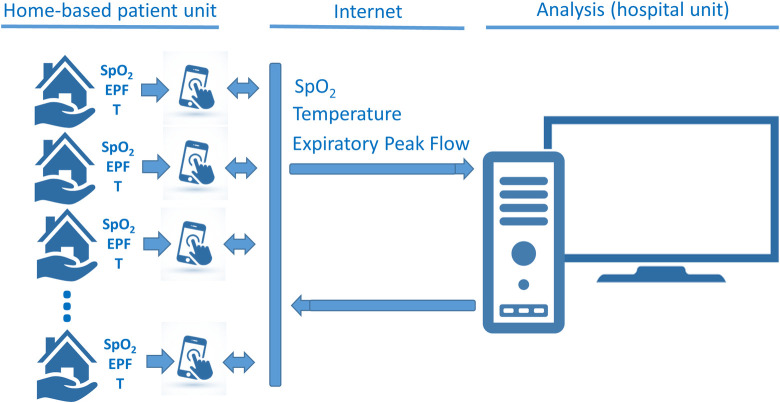
The general architecture of the system is reported in Fig 1. The system consists mainly of two parts: 1) the home-based patient unit, which is set up around the patient to acquire data and to receive medical recommendations, and 2) the hospital unit, which enables the medical staff to telemonitor the patient’s condition and helps to send medical recommendations.
Fig 1. General architecture of the proposed system describing the home-based patient unit, which is set up around the patient to acquire data and the hospital unit, which enables the medical staff to telemonitor the patient’s condition.
Considering the urgency of patient care, the home-based patient unit was developed using readily available commercial instruments. In order to simplify the use of the system by patients, easy-to-use instruments were selected. Thus, patients were assessed using a portable pulse oximeter (finger type, BIC model YK-80A) together with a disposable peak flow meter (Medicate, model 72000M). The used thermometer was the one owned by the patients.
The home unit allows the data transmission from the patient to the hospital, which is performed using a cell phone application. The application was developed in Java using the integrated development environment Android Studio (version 3.6.3). It is based on a form that is filled out and sent by the patient. To make clinical use easier for non-technical personnel, a dedicated user-friendly front panel was developed in the smartphone environment. This interface is shown in Fig 2.
Fig 2. User interface in the cell phone application observed in the home unit.
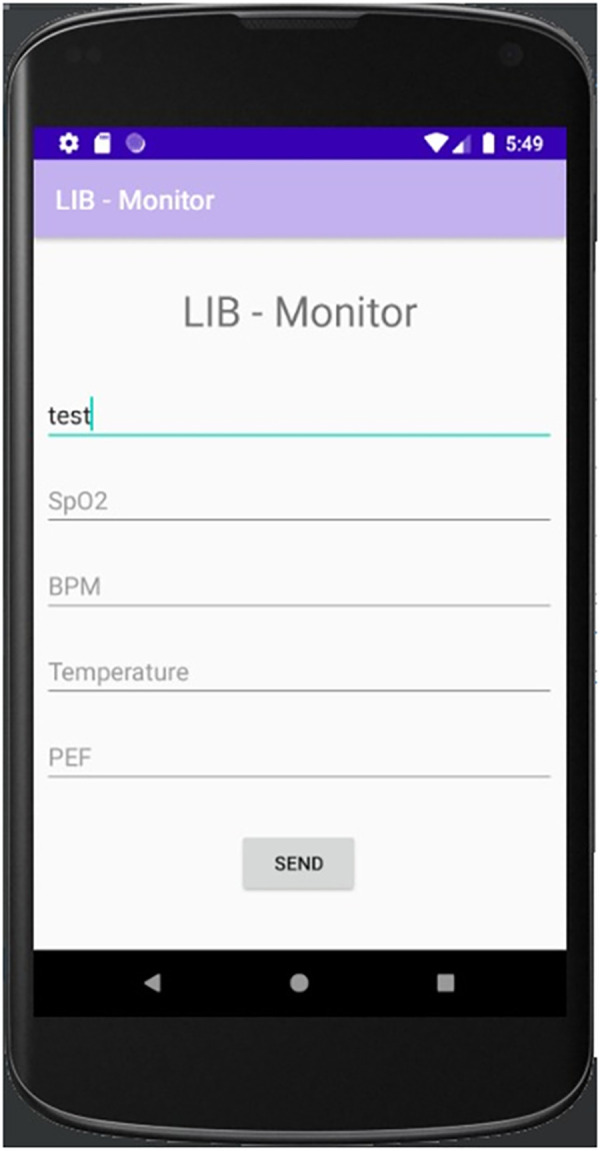
This application allows data transmission from the patient to the hospital. To make clinical use easier for non-technical personnel, a dedicated user-friendly front panel was developed.
After filled out, this form is sent from the application to an online script from Google, which saves the data in a worksheet according to the patient ID. To this end, the application uses Google Sheets (spreadsheet where data is saved) and Google Scrips (that integrates the application and Google Spreadsheet). As a result, the application creates an Excel file with one spreadsheet for each patient, which maximum size is 5 million cells.
Additionally, the values obtained in these exams are also recorded by the patients in a follow-up paper personal diary, which is available in S1 Personal diary. These diaries are provided to patients at the beginning of the monitoring. This redundancy is vital to maintain the system’s perfect functioning even in case of failures in the Internet or other system components.
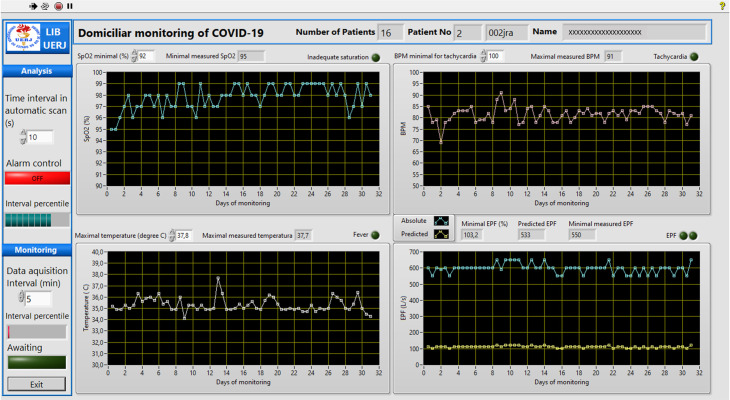
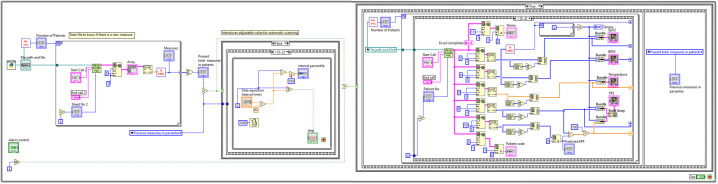
On the other hand, in the hospital environment, the hardware platform was constituted by an Intel Core i7-8750H, 2.2 GHz computer with 16 GB of RAM, a hard disk of 1 TB, and a Microsoft Windows 10 operating system. The software was developed in the LABVIEW 2020 environment (National Instruments, Austin, TX). A user-friendly front panel was also developed to be used in the hospital environment. This interface is shown in Fig 3. Its use is described in the flow diagram presented in Fig 4, and the basic LabVIEW program is described in Fig 5.
Fig 3. User-friendly front panel that was developed to be used in the hospital environment.
The program automatically downloads the patient’s measurements and searches for abnormal values. The limits of abnormality may be adjusted by the user. In the presence of abnormal values, a red indicator light starts flashing, simultaneously with an alarm beep. More detail of this program may be obtained in the flow diagram presented in Fig 4, and the basic LabVIEW program described in Fig 5.
Fig 4. Flow diagram describing the basic operation of the program used in the hospital environment.

Fig 5. Basic LabVIEW program used in the hospital environment.
The application installed on patients’ cell phones sends the data obtained over the monitoring periods to the hospital’s analysis center, where they are stored. Initially, the program (Fig 5) reads the first patient’s data and elaborates the graphs of SpO2, BPM, temperature, and PEF (Fig 4). This provides a graphical description of these readings against time (Fig 3), allowing the user to detect trends toward normality or abnormal changes in the mentioned parameters by visual analysis.
The program allows the user to select the minimum SpO2 value considered appropriate. Previous studies used a minimum normal SpO2 of 92% [25, 26], while others report that these values are between 93% and 94% [10]. The program automatically searches for the minimum SpO2 values presented by this patient and compared them with the selected value. In the presence of saturation values smaller than the user’s value, the red indicator light starts flashing, simultaneously with an alarm beep.
Similar analyses are performed in terms of BPM and temperature. The user can select the maximum BPM value considered appropriate and the maximum temperature considered normal. In this sense, values above 100 BPM indicate tachycardia [27], and body temperatures above 37°C are considered abnormal [14, 27]. The system automatically identifies abnormal values presented by this patient. In the presence of these events, specific red light indicators start flashing, indicating the nature of the abnormal event, simultaneously with an alarm sound, similar to the indication of abnormal values in SpO2.
EPF analyses are performed, taking into account the measured absolute values (blue trace in the EPF chart in Fig 9) and the percent of the predicted values [28] for each monitored patient (a yellow trace in the EPF chart in Fig 9). We perform an initial analysis adapting a traditionally used methodology in asthmatic patients [29]. A zoning scheme similar to a traffic light system (green-yellow-red zones) was used to evaluate the predicted EPF values (EPFp) obtained by the patient. The green zone is characterized by PEF readings between 100 to 80% of the EPFp, and signals "all clear." The yellow zone includes reading from 80 to 50% of the EPFp, and signals "caution," while the red zone (below 50% of the EPFp) signals "medical alert." The limits used in the cited zones are probably not adequate for COVID-19 patients, and we hope that they can be rapidly adjusted as the experience of using the system accumulates.
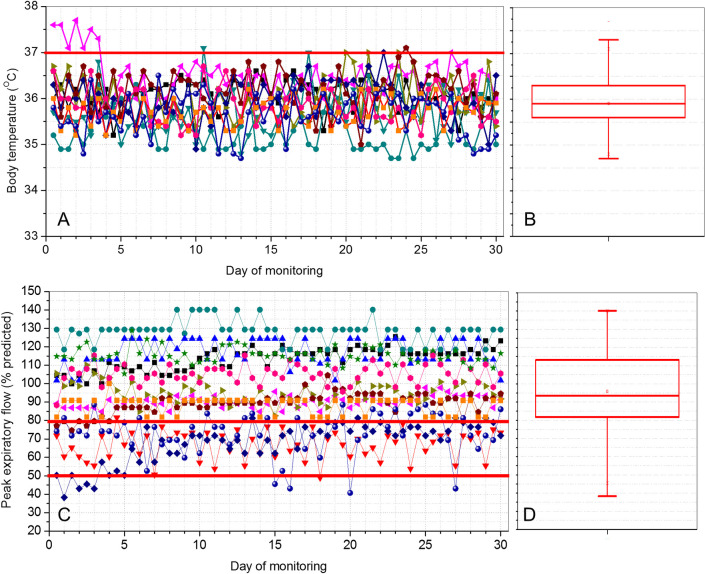
Fig 9.
Results obtained in the daily telemonitoring of temperature (A) and peak expiratory flow (C) in COVID-19–positive patients during 30 days. Box-plot descriptions of these results are also presented for the temperature (B) and peak expiratory flow (D). The top and the bottom of the box plot represent the 25th- to 75th-percentile values while the circle represents the mean value, and the bar across the box represents the 50th-percentile value.
When the system is started and used for the first time on the day, the cited analyses are performed for all patients under home monitoring (Fig 4). To simplify the visual analysis of the results from non-technical personnel, the software allows the user to perform this first evaluation with an automatic increment among patients and a constant time window for each patient (2 minutes, for example, Fig 3). The automatic increment process can be interrupted if more intriguing results that require a more extended analysis time are observed in a given patient. The patient details, monitoring values, and the cited analysis may be saved for further analysis (Fig 4).
After the first scan of patient results, the system automatically updates the results whenever a patient reports new measurements (Fig 4). This is an important feature as it allows the real-time identification of adverse events, and, as a consequence, the clinician may quickly implement the treatment plan. This can be very useful in COVID-19, since this disease is characterized by a rapid deterioration in the patient conditions.
The system also allows the analysis of the measurements’ results in the measurements performed in patients in an asynchronous manner while monitoring is performed. The medical recommendations, obtained from the analysis of the results sent previously, may be sent using e-mail messages. In case of severely abnormal values, emergency cell phone contact may be used. To help this contact, the system also automatically makes available the patient’s phone number. To receive recommendations in this manner could be a significant benefit for the patient, allowing the fast and easy adjustment of medical treatments. The front panel of this program is similar to that used in the online monitoring system. The interested reader may find a detailed description of this front panel in the S1 Fig.
All the programs used in the current study are available from the link: XXXX.
3. Methods
The Research Ethics Committee of the Pedro Ernesto University Hospital (HUPE) approved the study that obeys the Declaration of Helsinki. The written post-informed consent of all volunteers was obtained before inclusion in the study.
3.1. Monitoring protocol
Based on previous studies on asthma, patients are asked to measure and record their SpO2, BPM, body temperature, and PEF two times daily, in the morning and the afternoon, for thirty days. The SpO2 measurements were performed in the sitting position, after five minutes of rest, and without moving the hand where the instrument was adapted. All of the instruments were provided at no cost to the patients. Patients completed a simple satisfaction/usability questionnaire scored on a 0–10 numerical rating scale including the following questions:
1—How safe did you feel with the monitoring?
(0—not safe; 5 –moderately safe; 10—very safe);
2—How difficult was it to use the Peak-Flow device?
(0—very easy; 5 –moderately easy; 10—very difficult);
3—How difficult was it to use the oximeter?
(0—very easy; 5 –moderately easy; 10—very difficult);
4—How difficult was it to maintain the monitoring protocol?
(0—very easy; 5 –moderately easy; 10—very difficult).
3.2. Preliminary study
Twenty-four volunteers were selected for the study, including 12 controls and 12 diagnosed with COVID-19. The control group was composed of healthy subjects with no history of COVID-19, tobacco use, and cardiac or pulmonary disease. Among COVID-19 patients, all presented asymptomatic to mild disease. All patients were older than 18 years of age and were enrolled if they had positive COVID-19 testing, which was performed using reverse transcriptase–polymerase chain reaction (RT-PCR) of an oropharyngeal or nasopharyngeal swab. Hospital evaluations after RT-PCR tests showed that all patients had resting SpO2 ≥ 94% before the home-monitoring period. Physiotherapists supervised by pulmonologists managed the dashboard. In the presence of values indicating adverse events during this period, it was quickly informed, and the contact with the patient and the resulting clinical actions were performed under the discretion of the physician evaluating the patient, independent of this study. None of the studied patients required noninvasive or invasive ventilation during or after the monitoring period.
3.3. Statistics
Initially, the sample distribution characteristics were assessed using Shapiro-Wilk’s test. Since data were non-normally distributed, non-parametric analyses (Mann-Whitney test) were performed. Differences with p≤0.05 were considered statistically significant. These analyses were performed using Origin® 8.0 (Microcal Software Inc., Northampton, Massachusetts, United States). Graphs were elaborated using MedCalc 13.1, and the results are present as the median and interquartile range.
4. Preliminary results
The studied subjects’ biometric characteristics are described in Table 1, while the past medical history and medication use of the patients with COVID-19 are described in Table 2.
Table 1. Mean ± SD of the biometric characteristics of control individuals and patients with COVID-19.
| Age (years) | Body mass (kg) | Height (cm) | BMI (kg/m2) | Gender (M/F) | |
|---|---|---|---|---|---|
| Control (n = 12) | 38.2±15.0 | 68.2±10.7 | 167.8±7.2 | 24.2±3.1 | 4/8 |
| COVID-19 (n = 12) | 37.2±13.3 | 77.6±16.4 | 163.8±8.2 | 28.9±5.4 | 3/9 |
| p | ns | ns | ns | 0.02 | - |
Table 2. Characteristics of COVID-19 patients.
| N | (%) | |
|---|---|---|
| Smoking | 1 | 8.3 |
| Overweight | 7 | 58.3 |
| Obesity | 3 | 25.0 |
| Controlled diabetes | 1 | 8.3 |
| Pre-diabetes | 1 | 8.3 |
| Hypertension | 2 | 16.7 |
| Epilepsy | 1 | 8.3 |
| Cardiac arrhythmias | 1 | 8.3 |
| Essential thrombocytosis | 1 | 8.3 |
| Received hydroxychloroquine and azithromycin | 5 | 41.2 |
| Ivermectin | 3 | 25.0 |
Overweight was defined as body mass index (BMI) between 25 and 30 kg/m2; Obesity was defined as BMI ≥ 30 kg/m2; N, number of patients; %: percentage of the total number of patients.
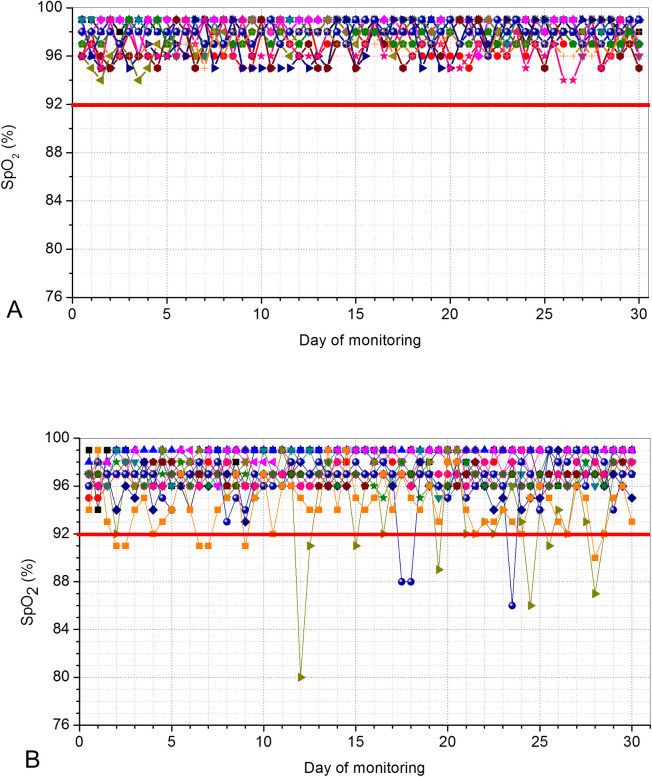
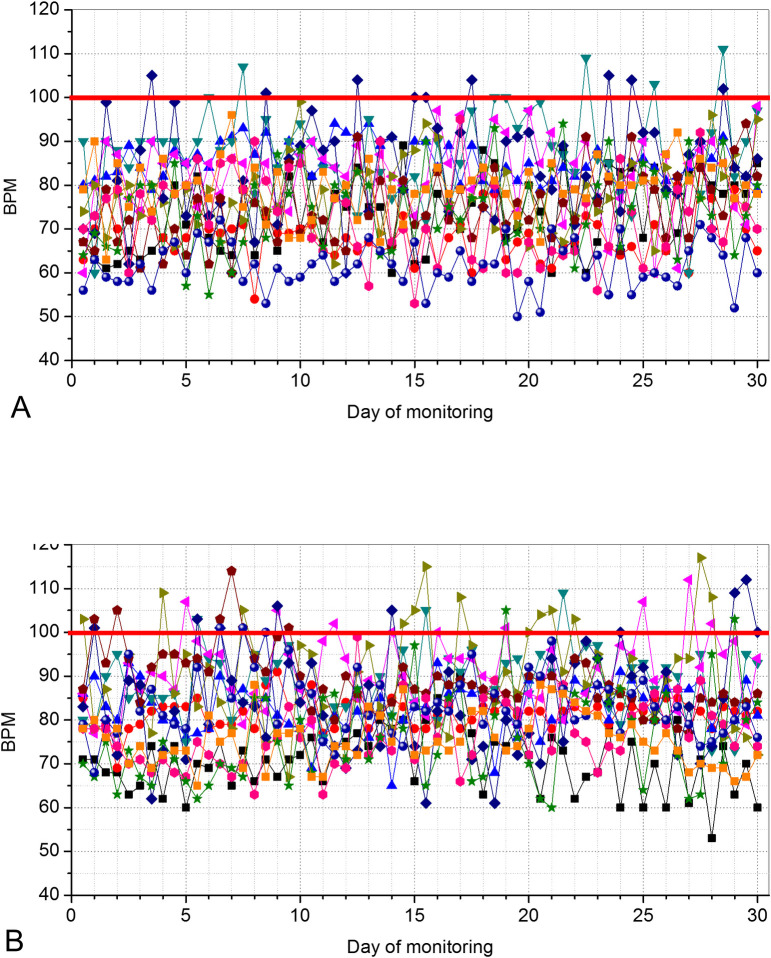
Figs 6 and 7 describes the results of the SpO2 and BPM in controls and patients, respectively.
Fig 6.
Home pulse oximetry values telemonitored during a 30-days period in controls (A) and in COVID-19–positive patients. (B). The red line describes the limit considered as a minimum normal value of saturation (92%). Three patients presented values below this limit.
Fig 7.
Beats per minute (BPM) values telemonitored during a 30-days period in controls (A) and in COVID-19–positive patients (B). The red line describes the limit considered as a maximum normal value of BPM (100 BPM). Seven patients presented values above this limit along with this home readings.
COVID-19 resulted in a significant reduction in SpO2 (Fig 8A, p<0.0001) and increased values of BPM (Fig 8B, p<0.0001).
Fig 8.
Median and 95% confidence intervals of the home monitored oxygen saturation (A) and BPM (B) in controls and COVID-19–positive patients.
Fig 9 shows the results obtained in the daily monitoring of temperature and PEF in patients with COVID-19 during 30 days. Most of the studied patients presented PEF values above 80% during the monitoring period (8 patients, 66.7%), while 3 patients (25%) had values between 50 and 80% of predicted (Fig 9). Two of the 12 patients (17%) showed transient values below 50%, which subsequently evolved to values between 50 and 80% of the predicted values.
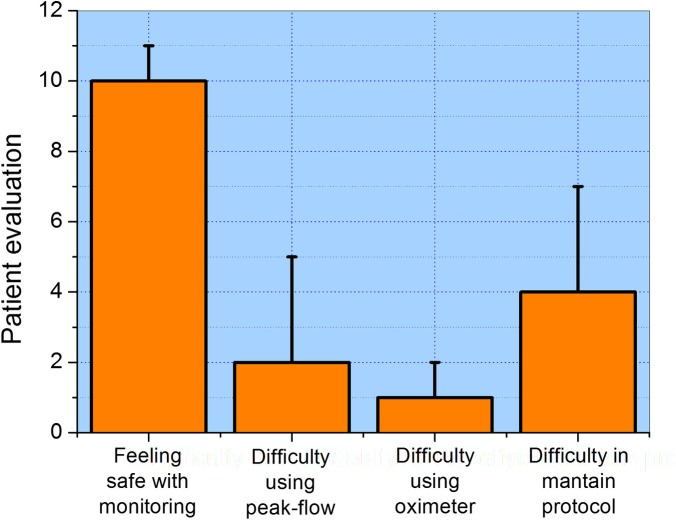
The satisfaction questionnaire was completed in 12 (100%) patients. A hundred percent of patients felt very safe when using the telemonitoring system (Fig 10). The use of peak flow and oximeter was considered easy to very easy in 92% of the patients, while 67% considered it easy to maintain the protocol during the 30 days of monitoring.
Fig 10. Results obtained in the evaluation of satisfaction/usability by the patients (median and 95% confidence intervals).
5. Discussion
To the best of our knowledge, this is the first study to provide a detailed description of an emergency remote monitoring system for individuals with COVID-19. The system is able to provide real-time monitoring of oximetry, BPM, body temperature, and peak expiratory flow. The preliminary results obtained in the 30-day validation study showed that patients with COVID-19 presented reduced SpO2 and PEF values, as well as increased BPM values. This was also the first study to investigate the use of PEF in COVID-19. The proposed system allowed us to quickly respond to early abnormalities in patients with COVID-19.
During the 30-days period of the initial tests of the proposed system, 720 data-points regarding SpO2 were remotely obtained, resulting in a total of 16 alerts among the 12 monitored patients (Fig 6). It can be seen in Fig 6B that 3 patients presented multiple events of desaturation. It was observed that these alerts resulted from an abrupt drop in SpO2 rather than a gradual decline. This is consistent with previous results [26] and is probably associated with the rapid deterioration caused by a surge in proinflammatory molecules in the “cytokine storm” phase of COVID-19 [30].
The values of SpO2 were reduced in patients with COVID-19 in comparison with controls (Fig 8A). This finding is consistent with the observation that in the initial phase of COVID-19, there is an increase in V/Q mismatch and thus persistence of pulmonary arterial blood flow to non-ventilated alveoli. The current understanding is that this results from the infection, which leads to a modest local interstitial edema and loss of surfactant. These factors are associated with alveolar collapse and intrapulmonary shunting [2]. The results observed in Fig 6 are in line with that obtained by O’Carroll and collaborators [31] investigating the remote monitoring of SpO2 in individuals with COVID-19.
It was pointed out previously that data lacks for young adults who often present with mild or asymptomatic disease, a part of the population considered highly contagious [32]. Figs 7 and 8B contribute to elucidate this question, providing evidence that COVID-19 introduces increased BPM values compared with control subjects (Fig 8B). These results are consistent with a previous review showing that COVID-19 is related to several cardiovascular complications [33] and the use of increased heart rate as clinical criteria for hospital admission in COVID-19 pneumonia [34].
It is known that the degree of temperature elevation might reflect the severity of inflammation [14]. Previous studies suggest that poor BT control during the COVID 19 disease course is a marker of poor prognosis, and BT can be used as an easily obtained prognostic indicator [14]. However, at this point in the COVID-19 outbreak, a specific fever pattern associated with this disease has not yet been identified. There is a paucity of data on temperature management for COVID-19 [17]. The present study (Fig 9A and 9B) indicates that the proposed system can help fill this literature’s critical gap. Low fever events were observed in 5 of the 12 studied patients (41.7%), consistent with the characteristics of asymptomatic to a mild disease of the studied COVID-19 group.
Fig 9C and 9D show that most of the studied patients presented normal PEF values during the monitoring period. Patients who have been infected by the Coronavirus may develop pulmonary edema and atelectasis, which may result in a reduction in lung volume, and a restrictive pattern. Thus, it may be speculated that this reduction may introduce a limitation of the expiratory flow influencing the values obtained in peak-flow measurements. This process would be similar to that observed in other restrictive diseases where the volumes exhaled are reduced [35]. These effects may explain, at least in part, the decrease in PEF presented in three of the studied patients, as well as the transient values below 50% of the predicted values observed in two patients, as described in Fig 9A. It is noteworthy that one of the patients with EPF <50% also had desaturation <92%.
There is general agreement in the literature that, given the severity of the ongoing global pandemic, the ability to remotely monitor patients who do not require hospitalization is essential for optimal utilization of health care resources [26]. To contribute in this direction, this study presents a low-cost open-architecture emergency system for remote monitoring of patients with COVID-19. There are currently no data to guide the use of home pulse oximetry in COVID-19 patients or its validity in identifying disease progression [26]. There is also a paucity of data on temperature management for COVID-19. No previous study has investigated the use of PEF in COVID-19. The system presented in this study may help to quickly accumulate data on SpO2, body temperature, and EPF, contributing to the development of the guidelines for these clinical practices.
Remote monitoring systems have been increasingly used in respiratory diseases [6, 36–39]. In the particular case of COVID-19, an essential factor is that the disease is characterized by a rapid change in the patient’s condition, with major changes occurring over a few days. The proposed system may provide early detection of patient’s conditions deteriorating at home. This technology allowed us to facilitate discharge in patients with mild-to-moderate disease safely and appropriately. This procedure holds the potential to increase bed availability without compromising safe patient care.
In the present study, home telemonitoring was considered a patient-friendly tool in mild-to-moderate COVID-19 patients (Fig 10). The proposed system positively contributed to patient safety feeling and enabled them to recover in their home environment. These results are in close agreement with those obtained by Grutters et al. [40] and Annis et al. [41], indicating that home telemonitoring may reduce hospitalization’s mean duration.
The used protocol was based on previous studies in asthma. This temporal resolution (about every 12 hours) was adequate in this sample of patients, which present asymptomatic to mild disease. This is less burdensome to these patients. However, in more severe COVID-19 patients, in which there is a risk of rapid desaturation, the frequency of measurements will need to be increased. The proposed system allows this change to be easily implemented, and it is only necessary to ask the patient to perform the measurements in the desired time interval.
We acknowledge the limitations of our study, including unknown methods of temperature measurement. Nevertheless, a clear trend in increased mortality among the patients with poor temperature control highlights the usefulness of this noninvasively and easily obtained parameter for evaluating patients’ prognoses.
Secondly, one could argue that the study presents a small sample size, and additional studies, including a more significant number of subjects are necessary. These studies would allow us to perform a detailed investigation concerning the utilization of home pulse oximetry, body temperature, and peak expiratory flow monitoring to identify robust predictors of hospitalization.
Cost-effectiveness analysis may help decision-makers to choose the interventions and programs which maximize health for the available resources. Previous studies showed that home telemonitoring in COVID-19 introduced a reduction of approximately 4-fold comparing with the estimated costs of the saved admission days [40]. A similar analysis in the present system would help decision-makers potentially improve their health systems’ performance. We are planning to do these analyses in the next steps of this research.
An important open question is; how fast are changes that require hospitalization? Unfortunately, the present study cannot answer this question because the investigated sample of patients presents asymptomatic to mild disease. To elucidate this question, it is necessary to monitor a significant number of patients who require hospitalization. This is a work in progress, and we believe that soon we will be able to contribute to elucidating this important question.
Finally, the system validation was performed in subjects from a Brazilian population at a single practice site, which affects the study’s generalizability. Therefore, multicenter studies are necessary for the future to expand the generalizability of these findings. The study used broad inclusion criteria and was performed in a typical setting under usual clinical procedures, which enhanced its generalizability.
6. Conclusion
An emergency system for home monitoring of SpO2, body temperature, and PEF in patients with COVID-19 was developed in the current study. This was the first study to propose such a system and evaluate the use of PEF in COVID-19. Using this system, the cited signals’ acquisition and analysis can be performed remotely through the Internet. The system’s ability to detect abnormal events was initially validated by a 30-day monitoring study in normal subjects and patients with COVID-19. In close agreement with previous results and physiological fundamentals, the presence of COVID-19 resulted in reduced values of SpO2, increased BPM, fever events in 41.7% of the patients, and decreased PEF in 33% of the studied patients.
The proposed system may contribute to conserving hospital resources for those most in need while simultaneously enabling early recognition of patients under acute deterioration, requiring urgent assessment.
Based on these promising results, future work includes a clinical trial in which we will perform a follow up in well-defined groups of patients with COVID-19. This will provide a detailed evaluation of the home monitoring approach’s clinical contribution in improving the patient’s care and outcomes.
Supporting information
(TIF)
(PDF)
Acknowledgments
We thank all study participants who made this work possible. We also thank Mônica Luz Villar Martins and Eduarda Martins de Faria for support with the patents.
Data Availability
Data was deposited in Open Science Framework, and is available in the following link: https://osf.io/zwbns/.
Funding Statement
LPM, PPFS, BMB, JLMA, LGM, MNB, MRF, RM, RAN, PLM received funding from Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ under grant E-26/010.000166/2020, http://www.faperj.br/. PLM received funding from Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ under grant 210.099/2020, http://www.faperj.br/. PLM received funding from Conselho Nacional de Desenvolvimento Científico e Tecnológico under grant 409380/2018-0, http://www.cnpq.br/. PLM also received funding from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, https://www.gov.br. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO Coronavirus Disease (COVID-19) Dashboard 2020 [cited 2020 11/04/2020]. Available from: https://covid19.who.int/.
- 2.Dhont S, Derom E, Van Braeckel E, Depuydt P, Lambrecht BN. The pathophysiology of ’happy’ hypoxemia in COVID-19. Respiratory research. 2020;21(1):198. 10.1186/s12931-020-01462-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Portnoy J, Waller M, Elliott T. Telemedicine in the Era of COVID-19. The journal of allergy and clinical immunology In practice. 2020. 10.1016/j.jaip.2020.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bashshur R, Doarn CR, Frenk JM, Kvedar JC, Woolliscroft JO. Telemedicine and the COVID-19 Pandemic, Lessons for the Future. Telemedicine journal and e-health: the official journal of the American Telemedicine Association. 2020. 10.1089/tmj.2020.29040.rb . [DOI] [PubMed] [Google Scholar]
- 5.Nakshbandi G, Moor CC, Wijsenbeek MS. Home monitoring for patients with ILD and the COVID-19 pandemic. The Lancet Respiratory medicine. 2020. 10.1016/S2213-2600(20)30452-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pare G, Jaana M, Sicotte C. Systematic review of home telemonitoring for chronic diseases: the evidence base. Journal of the American Medical Informatics Association: JAMIA. 2007;14(3):269–77. 10.1197/jamia.M2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. 10.1016/S0140-6736(20)30183-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marini JJ, Gattinoni L. Management of COVID-19 Respiratory Distress. Jama. 2020. 10.1001/jama.2020.6825 . [DOI] [PubMed] [Google Scholar]
- 9.Tobin MJ, Laghi F, Jubran A. Why COVID-19 Silent Hypoxemia Is Baffling to Physicians. American journal of respiratory and critical care medicine. 2020;202(3):356–60. 10.1164/rccm.202006-2157CP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torjesen I. Covid-19: Patients to use pulse oximetry at home to spot deterioration. Bmj. 2020. Epub 27 October 2020. 10.1136/bmj.m4151 [DOI] [Google Scholar]
- 11.Mishra T, Wang M, Metwally AA, Bogu GK, Brooks AW, Bahmani A, et al. Pre-symptomatic detection of COVID-19 from smartwatch data. Nature biomedical engineering. 2020;4(12):1208–20. 10.1038/s41551-020-00640-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Natarajan A, Su HW, Heneghan C. Assessment of physiological signs associated with COVID-19 measured using wearable devices. NPJ digital medicine. 2020;3(1):156. 10.1038/s41746-020-00363-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA cardiology. 2020;5(7):811–8. 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tharakan S, Nomoto K, Miyashita S, Ishikawa K. Body temperature correlates with mortality in COVID-19 patients. Critical care. 2020;24(1):298. 10.1186/s13054-020-03045-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.W.H.O. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19)2020.
- 16.Hsiao SH, Chen TC, Chien HC, Yang CJ, Chen YH. Measurement of body temperature to prevent pandemic COVID-19 in hospitals in Taiwan: repeated measurement is necessary. The Journal of hospital infection. 2020;105(2):360–1. 10.1016/j.jhin.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drewry AM, Hotchkiss R, Kulstad E. Response to "Body temperature correlates with mortality in COVID-19 patients". Critical care. 2020;24(1):460. 10.1186/s13054-020-03186-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization WHO. GINA–Global Initiative for Asthma2020 04-07-2015. [Google Scholar]
- 19.Shingo S, Zhang J, Reiss TF. Correlation of airway obstruction and patient-reported endpoints in clinical studies. The European respiratory journal. 2001;17(2):220–4. 10.1183/09031936.01.17202200 . [DOI] [PubMed] [Google Scholar]
- 20.Goldberg S., Springer C, Avital A, Godfrey S, Bar-Yishay E. Can peak expiratory flow measurements estimate small airway function in asthmatic children? CHEST. 2001;120(2):482–8. 10.1378/chest.120.2.482 [DOI] [PubMed] [Google Scholar]
- 21.Lenfant C., AL. S. Objective measures of lung function. J ALLERGY CLIN IMMUNOL. 1991;88(3(2)):439–46. [Google Scholar]
- 22.Ignacio-Garcia JM, Gonzalez-Santos P. Asthma self-management education program by home monitoring of peak expiratory flow. American journal of respiratory and critical care medicine. 1995;151(2 Pt 1):353–9. 10.1164/ajrccm.151.2.7842191 . [DOI] [PubMed] [Google Scholar]
- 23.o Jennifer Y. S, Lastra Alejandra C., Zhao Huaqing, Marchetti N, Criner GJ. Daily peak flow monitoring can be a useful tool in identifying COPD patients predisposed to worse outcomes. Chronic Obstr Pulm Dis 2016;3(1):398–405. [Google Scholar]
- 24.Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID‑19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020. 10.1007/s00134-020-06033-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pilcher J, Ploen L, McKinstry S, Bardsley G, Chien J, Howard L, et al. A multicentre prospective observational study comparing arterial blood gas values to those obtained by pulse oximeters used in adult patients attending Australian and New Zealand hospitals. BMC pulmonary medicine. 2020;20(1):7. 10.1186/s12890-019-1007-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah S, Majmudar K, Stein A, Gupta N, Suppes S, Karamanis M, et al. Novel Use of Home Pulse Oximetry Monitoring in COVID-19 Patients Discharged From the Emergency Department Identifies Need for Hospitalization. ACADEMIC EMERGENCY MEDICINE. 2020;27:681–92. 10.1111/acem.14053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall JE. Guyton and hall textbook of medical physiology. 14. ed. pages cm p.
- 28.Leiner GC, Abramowitz S, Small MJ, Stenby VB, Lewis WA. Expiratory Peak Flow Rate. Standard Values for Normal Subjects. Use as a Clinical Test of Ventilatory Function. The American review of respiratory disease. 1963;88:644–51. 10.1164/arrd.1963.88.5.644 . [DOI] [PubMed] [Google Scholar]
- 29.Gerald LB, Tara FC. Peak expiratory flow monitoring in asthma2019 Accessed on November, 5, 2020. Available from: https://www.uptodate.com/contents/peak-expiratory-flow-monitoring-in-asthma.
- 30.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. The Journal of infection. 2020;80(6):607–13. 10.1016/j.jinf.2020.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Carroll O, MacCann R, O’Reilly A, Dunican EM, Feeney ER, Ryan S, et al. Remote monitoring of oxygen saturation in individuals with COVID-19 pneumonia. The European respiratory journal. 2020;56(2). 10.1183/13993003.01492-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bielecki M, Crameri GAG, Schlagenhauf P, Buehrer TW, Deuel JW. Body temperature screening to identify SARS-CoV-2 infected young adult travellers is ineffective. Travel medicine and infectious disease. 2020;37:101832. 10.1016/j.tmaid.2020.101832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. The American journal of emergency medicine. 2020;38(7):1504–7. 10.1016/j.ajem.2020.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenhalgh T, Koh GCH, Car J. Covid-19: a remote assessment in primary care. Bmj. 2020;368:m1182. 10.1136/bmj.m1182 . [DOI] [PubMed] [Google Scholar]
- 35.Altalag A, Road J, Wilcox P, Aboulhosn K. Pulmonary Function Tests in Clinical Practice. Switzerland: Springer Nature Switzerland AG; 2019. [Google Scholar]
- 36.Maiolo C, Mohamed EI, Fiorani CM, De Lorenzo A. Home telemonitoring for patients with severe respiratory illness: the Italian experience. J Telemed Telecare. 2003;9(2):67–71. 10.1258/135763303321327902 . [DOI] [PubMed] [Google Scholar]
- 37.Graham LE, Zimmerman M, Vassallo DJ, Patterson V, Swinfen P, Swinfen R, et al. Telemedicine—the way ahead for medicine in the developing world. Tropical doctor. 2003;33(1):36–8. 10.1177/004947550303300118 . [DOI] [PubMed] [Google Scholar]
- 38.Morlion B, Verbandt Y, Paiva M, Estenne M, Michils A, Sandron P, et al. A telemanagement system for home follow-up of respiratory patients. IEEE Eng Med Biol Mag. 1999;18(4):71–9. 10.1109/51.775491 . [DOI] [PubMed] [Google Scholar]
- 39.Pedone C, Chiurco D, Scarlata S, Incalzi RA. Efficacy of multiparametric telemonitoring on respiratory outcomes in elderly people with COPD: a randomized controlled trial. BMC Health Serv Res. 2013;13:82. 10.1186/1472-6963-13-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grutters LA, Majoor KI, Mattern ESK, Hardeman JA, van Swol CFP, Vorselaars ADM. Home telemonitoring makes early hospital discharge of COVID-19 patients possible. Journal of the American Medical Informatics Association: JAMIA. 2020;27(11):1825–7. 10.1093/jamia/ocaa168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Annis T, Pleasants S, Hultman G, Lindemann E, Thompson JA, Billecke S, et al. Rapid implementation of a COVID-19 remote patient monitoring program. Journal of the American Medical Informatics Association: JAMIA. 2020;27(8):1326–30. 10.1093/jamia/ocaa097 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(PDF)
Data Availability Statement
Data was deposited in Open Science Framework, and is available in the following link: https://osf.io/zwbns/.