Abstract
Background
Pregnant and lactating women were excluded from initial coronavirus disease 2019 vaccine trials; thus, data to guide vaccine decision making are lacking.
Objective
This study aimed to evaluate the immunogenicity and reactogenicity of coronavirus disease 2019 messenger RNA vaccination in pregnant and lactating women compared with: (1) nonpregnant controls and (2) natural coronavirus disease 2019 infection in pregnancy.
Study Design
A total of 131 reproductive-age vaccine recipients (84 pregnant, 31 lactating, and 16 nonpregnant women) were enrolled in a prospective cohort study at 2 academic medical centers. Titers of severe acute respiratory syndrome coronavirus 2 spike and receptor-binding domain immunoglobulin G, immunoglobulin A, and immunoglobulin M were quantified in participant sera (n=131) and breastmilk (n=31) at baseline, at the second vaccine dose, at 2 to 6 weeks after the second vaccine, and at delivery by Luminex. Umbilical cord sera (n=10) titers were assessed at delivery. Titers were compared with those of pregnant women 4 to 12 weeks from the natural infection (n=37) by enzyme-linked immunosorbent assay. A pseudovirus neutralization assay was used to quantify neutralizing antibody titers for the subset of women who delivered during the study period. Postvaccination symptoms were assessed via questionnaire. Kruskal-Wallis tests and a mixed-effects model, with correction for multiple comparisons, were used to assess differences among groups.
Results
Vaccine-induced antibody titers were equivalent in pregnant and lactating compared with nonpregnant women (pregnant, median, 5.59; interquartile range, 4.68–5.89; lactating, median, 5.74; interquartile range, 5.06–6.22; nonpregnant, median, 5.62; interquartile range, 4.77–5.98, P=.24). All titers were significantly higher than those induced by severe acute respiratory syndrome coronavirus 2 infection during pregnancy (P<.0001). Vaccine-generated antibodies were present in all umbilical cord blood and breastmilk samples. Neutralizing antibody titers were lower in umbilical cord than maternal sera, although this finding did not achieve statistical significance (maternal sera, median, 104.7; interquartile range, 61.2–188.2; cord sera, median, 52.3; interquartile range, 11.7–69.6; P=.05). The second vaccine dose (boost dose) increased severe acute respiratory syndrome coronavirus 2–specific immunoglobulin G, but not immunoglobulin A, in maternal blood and breastmilk. No differences were noted in reactogenicity across the groups.
Conclusion
Coronavirus disease 2019 messenger RNA vaccines generated robust humoral immunity in pregnant and lactating women, with immunogenicity and reactogenicity similar to that observed in nonpregnant women. Vaccine-induced immune responses were statistically significantly greater than the response to natural infection. Immune transfer to neonates occurred via placenta and breastmilk.
Key words: antibodies, breastfeeding, breastmilk, cord blood, COVID-19 vaccine, maternal immunity, mRNA, neonatal immunity, pregnancy
Introduction
More than 73,600 infections and 80 maternal deaths have occurred in pregnant women in the United States alone as of March 1, 2021.1 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is more severe in pregnant women compared with their nonpregnant counterparts, with an increased risk of hospital admission, intensive care unit stay, and death.2 Despite their higher risk, pregnant and lactating women were not included in any initial coronavirus disease 2019 (COVID-19) vaccine trials, although the first vaccine trial began in pregnant women in February 2021 (Pfizer/BioNTech, ClinicalTrials.gov identifier: NCT04754594).
AJOG at a Glance.
Why was this study conducted?
Because pregnant and lactating women were excluded from initial coronavirus disease 2019 (COVID-19) vaccine trials, data are lacking regarding vaccine efficacy and infant humoral protection in this population.
Key findings
Pregnant and lactating women elicited comparable vaccine-induced humoral immune responses with nonpregnant controls and generated higher antibody titers than those observed after severe acute respiratory syndrome coronavirus 2 infection in pregnancy. Vaccine-generated antibodies were present in umbilical cord blood and breastmilk after maternal vaccination.
What does this add to what is known?
This study provides data from a large cohort on maternal antibody generation in response to COVID-19 vaccination, compares vaccine-generated immunity with that from natural infection in pregnancy, and suggests that vaccination of pregnant and lactating women can confer robust maternal and neonatal immunity.
The COVID-19 pandemic has given rise to hundreds of vaccine platforms in development to fight SARS-CoV-2.3 , 4 However, few of these platforms have been tested or are specifically designed to elicit immunity in vulnerable populations, including pregnant women. Pregnant women have long been left out of therapeutic and vaccine research, reportedly owing to heightened safety concerns in this population.5, 6, 7, 8 Although the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine encouraged the Food and Drug Administration to include pregnant women in the COVID-19 vaccine emergency use authorization (EUA) owing to the risk of increased disease severity in this population, evidence about vaccine immunogenicity to guide patient decision making and provider counseling is lacking.10, 11, 9 In particular, given the novelty of the first emergency approved COVID-19 vaccines, both of which use messenger RNA (mRNA) to deliver SARS-CoV-2 spike to educate the immune system,12 , 13 it remains unclear whether this novel vaccine approach will drive immunity in the context of pregnancy and whether antibodies will be transferred efficiently to neonates via the cord and breastmilk. Here, vaccine-induced immunity was profiled in vaccinated pregnant, lactating, and nonpregnant controls compared with women infected with SARS-CoV-2 during pregnancy.
Materials and Methods
Study design
Women at 2 tertiary care centers were approached for enrollment in an institutional review board–approved COVID-19 pregnancy and lactation biorepository study between December 17, 2020, and February 23, 2021. Eligible women were: (1) pregnant, (2) lactating, or (3) nonpregnant and of reproductive age (18–45 years), 18 years old, able to provide an informed consent, and receiving the COVID-19 vaccine.
Participants and procedures
Eligible study participants were identified by practitioners at the participating hospitals or were self-referred. A study questionnaire was administered to assess pregnancy and lactation status, previous SARS-CoV-2 infection, timing of COVID-19 vaccine doses, type of COVID-19 vaccine received (BNT162b2 Pfizer/BioNTech or mRNA-1273 Moderna/National Institutes of Health [NIH]), and side effects after each vaccine dose (injection site soreness, injection site skin reaction or rash, headache, myalgias, fatigue, fever or chills, allergic reaction, or others [reaction detailed]). A cumulative symptom and reactogenicity score was generated by assigning 1 point to each side effect.
Sample collection and processing
Blood and breastmilk from lactating women were collected at V0 (at the time of the first vaccine dose/baseline), at V1 (at the time of the second vaccine dose/“prime” profile), at V2 (2–6 weeks after the second vaccine dose/“boost” profile), and at delivery (for pregnant participants who delivered during the study time frame). Umbilical cord blood was also collected at delivery for pregnant participants. The V2 time point reflects full antibody complement, achieved 1 week after Pfizer/BioNTech and 2 weeks after Moderna/NIH.12 , 13 Blood was collected by venipuncture (or from the umbilical vein following delivery for cord blood) into serum separator tubes. Blood was centrifuged at 1000 g for 10 min at room temperature. Sera were aliquoted into cryogenic vials and stored at −80°C. Breastmilk was collected by the lactating participant into study-provided breastmilk bottles or breastmilk bags depending on volume. Breastmilk was centrifuged at 2000 rpm at 4°C for 25 minutes, supernatant was aliquoted into cryogenic vials and stored at −80°C.
Antibody quantification
Antibody quantification was performed as described previously.14 Briefly, a multiplexed Luminex assay was used to determine relative titer of antigen-specific isotypes and subclasses using the following antigens: SARS-CoV-2 receptor-binding domain (RBD), S1, and S2 (all Sino Biologic, Beijing, China), and SARS-CoV-2 spike (LakePharma, Inc, San Carlos, CA). Antigen-specific antibody titers were log10 transformed for time course analyses. Phosphate-buffer saline (PBS) background intensity was reported for each antigen as a threshold for positivity. Titers resulting from natural infection and vaccination-induced antibodies against SARS-CoV-2 RBD and spike were quantified from the same plate using enzyme-linked immunosorbent assay as previously described.15 Additional detail regarding antibody quantification may be found in Supplemental Methods.
Antibody neutralization assay
On the morning of the experiment, 17,000 angiotensin-converting enzyme 2 (ACE2) cells were plated in each well of a flat-bottom 96-well plate in 100 μL of D10 (Dulbecco’s modified Eagle medium+10% fetal bovine serum). Notably, 6 hours later, the serum samples were heat inactivated by incubation at 56°C for 1 hour. A solution containing virus at 1.9 ng equivalent of p24 per μL was prepared in D10. The heat-inactivated serum was diluted in this virus-containing media 1:5-fold, and then 3-fold serial dilutions were done in the same virus-containing media. The virus and serum samples were incubated at 37°C for 2 hours; 50 μL of the virus-serum mix was then added to the ACE2 cells. Therefore, the lowest final dilution of each serum sample is 15-fold. The cells were incubated at 37°C for 48 hours, and the red fluorescent protein was quantified using the flow cytometer (BD Accuri C6, BD Biosciences, San Jose, CA). Additional details about this assay may be found in the Supplemental Methods.
Statistical analyses
Participant characteristics were summarized with frequency statistics. Continuous outcome measures were reported as either mean (standard deviation) or median (interquartile range [IQR]). Correlation analyses were performed using Spearman coefficients. Within- and between-group analyses of log10 transformed antibody levels in serum or breastmilk across multiple time points were evaluated by a repeated measures mixed-effects model, followed by post hoc Tukey’s multiple comparisons test. Differences between paired maternal and cord sera immunoglobulin (Ig) G and neutralization titers were evaluated by Wilcoxon matched-pairs signed rank test. Statistical significance was defined as P<.05. Statistical analyses were performed using GraphPad Prism 9 (San Diego, CA) and Stata/IC version 16.1 (College Station, TX).
Results
From December 17, 2020, to March 2, 2021, samples were obtained from 131 enrolled participants: 84 pregnant, 31 lactating, and 16 nonpregnant reproductive-age women. Of the pregnant vaccine recipients, 13 delivered during the study time frame, and cord blood was collected at delivery from 10. Banked sera from 37 pregnant women infected with SARS-CoV-2 in pregnancy and enrolled between March 24, 2020, and December 11, 2020, were included as a second comparison group.
Participant characteristics
Participant demographic and clinical characteristics, sampling time points, and side effect profiles are presented in Table 1 . The study population consisted primarily of white, non-Hispanic women, reflecting the healthcare worker population at the 2 hospitals. A total of 5 participants reported previous SARS-CoV-2 infection: 2 pregnant, 2 lactating, and 1 nonpregnant. The characteristics of the comparison group with natural SARS-CoV-2 infection in pregnancy are detailed in Supplemental Table 1. These participants all had symptomatic SARS-CoV-2 with known timing of infection.
Table 1.
Cohort demographic characteristics
| Characteristic | Nonpregnant (n=16), n (%) | Pregnant (n=84), n (%) | Lactating (n=31), n (%) |
|---|---|---|---|
| Participant age, mean (SD), y | 38.4 (8.3) | 34.1 (3.3) | 34.6 (2.6) |
| Race | |||
| White | 12 (75) | 75 (89) | 27 (87) |
| Black | 2 (12) | 2 (2) | 0 (0) |
| Asian | 0 (0) | 6 (7) | 2 (6) |
| Multiracial | 0 (0) | 1 (1) | 1 (3) |
| Other | 1 (6) | 0 (0) | 1 (3) |
| Unknown | 1 (6) | 0 (0) | 0 (0) |
| Ethnicity | |||
| Hispanic or Latino | 0 (0) | 5 (6) | 2 (6) |
| Not Hispanic or Latino | 14 (88) | 79 (94) | 28 (90) |
| Unknown or not reported | 2 (12) | 0 (0) | 1 (3) |
| Maternal comorbidities | |||
| Chronic hypertension | 1 (6) | 3 (4) | 3 (10) |
| Diabetes mellitus or gestational diabetes | 0 (0) | 3 (4) | 3 (10) |
| BMI of >30 kg/m2 | 2 (12) | 10 (12) | 3 (10) |
| Asthma | 2 (12) | 16 (19) | 7 (23) |
| Immunosuppression/cancer | 0 (0) | 3 (4) | 0 (0) |
| Previous SARS-CoV-2 infection | 1 (6) | 2 (2) | 2 (6) |
| Vaccine type | |||
| Pfizer-BioNTech | 8 (50) | 41 (49) | 16 (52) |
| Moderna | 8 (50) | 43 (51) | 15 (48) |
| Gestational age at first vaccine dose | n/a | 23.2 (16.3–32.1) | n/a |
| Trimester of first vaccine dose | n/a | n/a | |
| - First | 11 (13) | ||
| - Second | 39 (46) | ||
| - Third | 34 (40) | ||
| Time points for blood collection | |||
| - Baseline/at first dose (V0) | 1 (6) | 31 (37) | 14 (45) |
| - At second dose (V1) | 15 (94) | 78 (93) | 26 (84) |
| - 2–5.5 wk after second dose (V2) | 16 (100) | 17 (20) | 13 (42) |
| Time points for milk collection | |||
| - Baseline or at first dose (V0) | — | 3 (4) | 16 (52) |
| - At second dose (V1) | — | 26 (31) | 28 (90) |
| - 2–5.5 wk after second dose (V2) | — | 0 (0) | 13 (42) |
| Side effects at first vaccine dosea | |||
| - Injection site soreness | 12 (75) | 73 (88) | 20 (67) |
| - Injection site reaction or rash | 0 (0) | 1 (1) | 0 (0) |
| - Headache | 5 (31) | 7 (8) | 9 (30) |
| - Muscle aches | 2 (12) | 2 (2) | 4 (13) |
| - Fatigue | 6 (38) | 12 (14) | 4 (13) |
| - Fever or chills | 1 (6) | 1 (1) | 1 (3) |
| - Allergic reaction | 0 (0) | 0 (0) | 0 (0) |
| - Otherb | 2 (6) | 3 (4) | 0 (0) |
| Side effects at second vaccine dosec | |||
| - Injection site soreness | 12 (75) | 44 (57) | 17 (61) |
| - Injection site reaction or rash | 0 (0) | 1 (1) | 0 (0) |
| - Headache | 6 (38) | 25 (32) | 11 (39) |
| - Muscle aches | 7 (44) | 37 (48) | 16 (57) |
| - Fatigue | 9 (56) | 41 (53) | 14 (50) |
| - Fever or chills | 8 (50) | 25 (32) | 12 (43) |
| - Allergic reaction | 0 (0) | 1 (1) | 0 (0) |
| - Otherd | 2 (12) | 7 (9) | 7 (25) |
BMI, body mass index; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
Gray et al. Coronavirus disease 2019 vaccination in pregnancy and lactation. Am J Obstet Gynecol 2021.
Not all participants provided side effect data after the first dose: 2 patients (1 pregnant, 1 lactating) did not provide information. Thus, percentages are based off of 16 nonpregnant, 79 pregnant, and 30 lactating participants
“Other” side effects reported after vaccine dose 1: elevated heart rate, joint pain, nausea, swollen lymph node, or sore throat
Not all participants received the second dose at the time of analysis: 16 nonpregnant, 80 pregnant, and 29 lactating patients received the second dose. Of those who received the second dose, 4 did not provide side effect data (3 pregnant, 1 lactating). Thus, percentages are based off of 16 nonpregnant, 77 pregnant, and 28 lactating participants
“Other” side effects reported after the vaccine dose 2: joint pain, nausea, sore throat, dizziness/light headedness, stomach ache, night sweats, clogged ears, or swollen eyes.
Vaccination characteristics
At the time of the study, 2 COVID-19 vaccines had received EUA: Pfizer/BioNTech (Mainz, Germany) and Moderna (Cambridge, MA). Both vaccines use mRNA to deliver the SARS-CoV-2 spike antigen to the immune system,12 , 13 representing a novel vaccine platform never before tested in pregnancy. Although mRNA vaccines have shown highly effective immune induction in nonpregnant adults, the immunogenicity and reactogenicity of this platform in pregnancy remain unclear. An equivalent number of pregnant women receiving the Pfizer/BioNTech and Moderna vaccines were included in our study. Of pregnant participants, the mean gestational age at the first vaccine dose was 23.2 weeks, with 11 women (13%) receiving their first vaccine dose in the first trimester, 39 (46%) in the second trimester, and 34 (40%) in the third trimester. Side effect profiles between participant groups following vaccination were similar and are detailed in Table 1. The cumulative symptom score after the first dose in all 3 groups was low. After the second dose, there was no significant difference between groups with respect to cumulative symptom score (median, 2 (IQR, 1–3); 3 (IQR, 2–4); and 2.5 (IQR, 1–4.5) in pregnant, lactating, and nonpregnant groups respectively; P=.40). Vaccine-related fevers or chills were reported by 32% of pregnant women (25 of 77) after the boost dose and 50% of nonpregnant women (8 of 16) (P=.25).
Delivery outcomes and characteristics of lactating women
Delivery information for the 13 pregnant participants who delivered during the study period is detailed in Table 2 . All 13 were vaccinated in the third trimester. Notably, 3 women delivered at hospitals other than the study sites, and cord blood samples were not available. Of the 10 umbilical cord blood samples available for analysis, 9 of 10 mothers had received both vaccine doses (median, 36.5 days (IQR, 30–42) from the first vaccine and 14 days (IQR, 11–16) from the second vaccine). One participant delivered 17 days after vaccine 1, with spontaneous preterm labor at 35 weeks’ gestation. Lactating participant characteristics are detailed in Table 2.
Table 2.
Characteristics of pregnant, delivered vaccine recipients and lactating vaccine recipients
| Pregnant, delivered vaccine recipients (n=13) | |
|---|---|
| Characteristic | |
| Gestational age at delivery, median (IQR), wk | 39.3 (39–40.3) |
| Days from the first vaccine to delivery, median (IQR) | 36.5 (30–42) |
| Days from the second vaccine to delivery, median (IQR)a | 14 (11–16) |
| Labor | 11 (85) |
| Mode of delivery | |
| Vaginal | 10 (77) |
| Cesarean | 3 (23) |
| Birthweight, g | 3452 (563) |
| Adverse pregnancy outcome | |
| Fetal growth restriction | 0 (0) |
| Preeclampsia/gestational hypertension | 0 (0) |
| Preterm delivery | 1 (8) |
| - Spontaneous | 1 |
| - Medically indicated | 0 |
| Composite infant morbidityb | |
| Supplemental oxygen/CPAP | 1 (8) |
| TTN | 1 (8) |
| Special care nursery admission | 0 |
| NICU admission | 2 (15) |
| Respiratory distress syndrome | 0 |
| Necrotizing enterocolitis | 0 |
| Sepsis | 0 |
| Assisted ventilation | 0 |
| Seizure | 0 |
| Grade 3/4 intraventricular hemorrhage | 0 |
| Death | 0 |
| Lactating vaccine recipients (n=31) | |
|---|---|
| Characteristic | |
| Months after delivery, median (IQR) | 7.3 (3.8–10.8) |
| Months after delivery | |
| 0–3 | 5 (16) |
| 3–6 | 6 (19) |
| 6–>9 | 18 (58) |
| Unknown | 2 (6) |
Values are expressed as number (percentage) unless indicated otherwise.
CPAP, continuous positive airway pressure; IQR, interquartile range; NICU, neonatal intensive care unit; TTN, transient tachypnea of the newborn.
Gray et al. Coronavirus disease 2019 vaccination in pregnancy and lactation. Am J Obstet Gynecol 2021.
Two patients delivered before receiving the second dose (17 days after V1 and 14 days after V1, cord blood only available for the patient delivering 17 days after V1)
The 1 preterm delivery accounted for the documented cases of supplemental oxygen, TTN, and 1 of the 2 NICU admissions. The other NICU admission was a term infant with growth restriction admitted for persistent hypoglycemia.
The maternal vaccine response
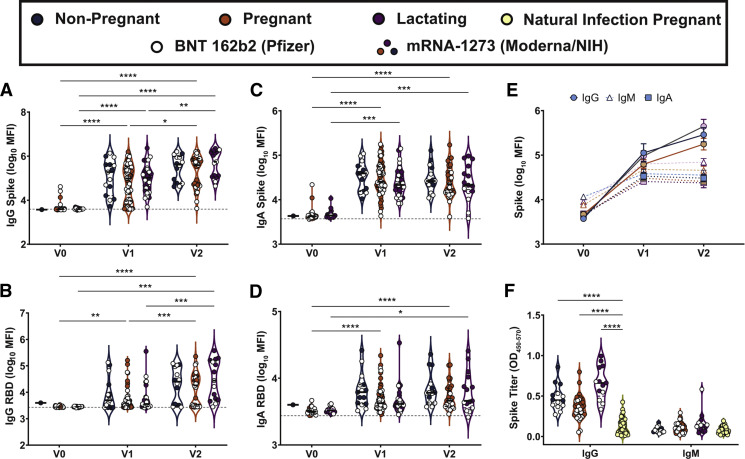
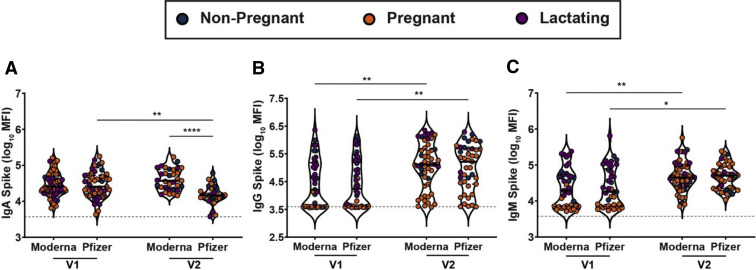
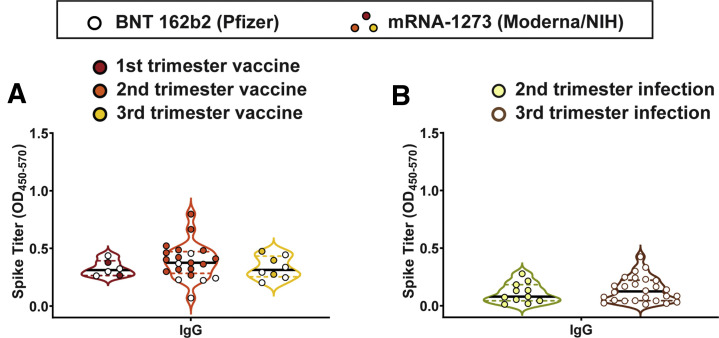
IgM, IgG, and IgA responses to the spike (S), RBD, S1 segment of S, and S2 segment of S were measured. A significant rise in all isotypes across all antigens was observed from V0 to V1, with a further rise in IgG levels from V1 to V2 in both the pregnant and lactating groups (Figure 1 , A–D; Supplemental Figure 1). Spike titers rose more rapidly than RBD titers after the first (V1/prime time point) and second (V2/boost time point) vaccine dose, but the magnitude of the response did not differ across pregnant or lactating women. In contrast to IgG responses, IgM and IgA responses were induced robustly after the prime and were poorly induced after boosting, across all groups (Figure 1, C and D). Higher S- and RBD-specific IgA responses were noted in Moderna vaccinees than Pfizer/BioNTech vaccinees (Supplemental Figure 2, A–C), potentially related to the extended boosting window used for the Moderna vaccine. By 2 weeks after the second vaccine, the dominant serum antibody response was IgG for pregnant, lactating, and nonpregnant women (Figure 1, E; Supplemental Figure 1, C). Vaccine-induced maternal antibody titers in sera did not differ by trimester of vaccination (Supplemental Figure 3). Strikingly higher levels of SARS-CoV-2 antibodies were observed in all vaccinated women compared with pregnant women with natural infection 4 to 12 weeks before (Figure 1, F) (Kruskal-Wallis P<.001), highlighting the robust humoral immune responses induced by mRNA vaccination.
Figure 1.
Maternal vaccination induces a robust SARS-CoV-2–specific antibody response
A–D, Violin plots show the log10 transformed mean fluorescence intensity (MFI) for (A) IgG spike-, (B) IgG RBD-, (C) IgA spike-, and (D) IgA RBD–specific titers across V0, V1, and V2 time points collected from nonpregnant of reproductive age (blue), pregnant (orange), or lactating (purple) participants. Participants who received BNT 162b2 from Pfizer/BioNTech are depicted as open circles, and participants who received mRNA-1273 from Moderna/NIH are depicted as closed circles. Differences across time points and groups were assessed by repeated measures mixed-effects model followed by post hoc Tukey’s multiple comparisons test. The asterisk indicates P<.05, the double asterisk indicates P<.01, the triple asterisk indicates P<.001, and the quadruple asterisk indicates P<.0001. E, Line graph showing the log10 transformed relative spike-specific titers across V0, V1, and V2 time points collected from nonpregnant (blue), pregnant (orange), or lactating (purple) participants for IgG (circles and solid lines), IgM (open triangles and dashed lines), and IgA (squares and dotted lines). F, Violin plots show the IgG and IgM spike–specific titer in nonpregnant (blue), pregnant (orange), lactating (purple), and naturally-infected pregnant (yellow) participants. Participants who received BNT 162b2 from Pfizer/BioNTech are depicted as open circles, and participants who received mRNA-1273 from Moderna/NIH are depicted as closed circles. Differences across groups were assessed by Kruskal-Wallis test followed by post hoc Dunn’s multiple comparisons test. The quadruple asterisk indicates P<.0001 compared with natural infection in pregnant women.
Ig, immunoglobulin; mRNA, messenger RNA; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Gray et al. Coronavirus disease 2019 vaccination in pregnancy and lactation. Am J Obstet Gynecol 2021.
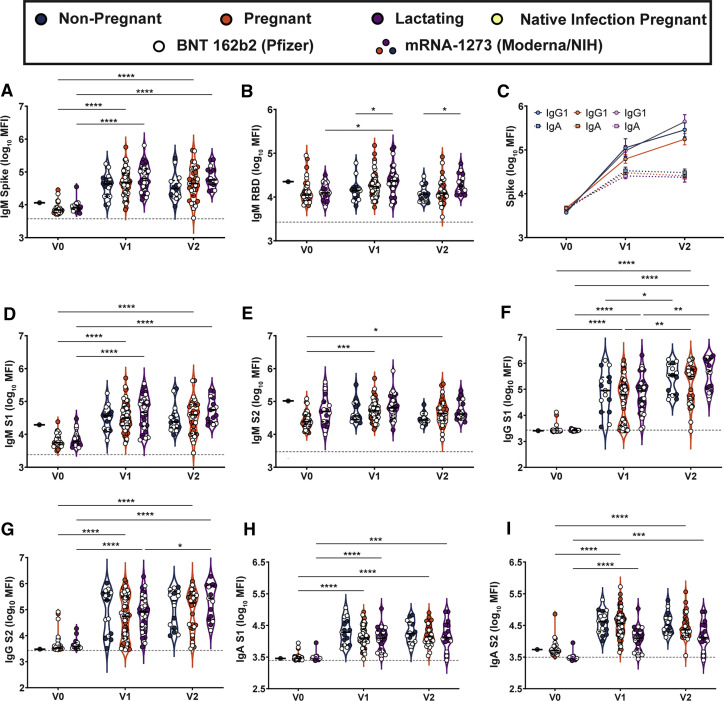
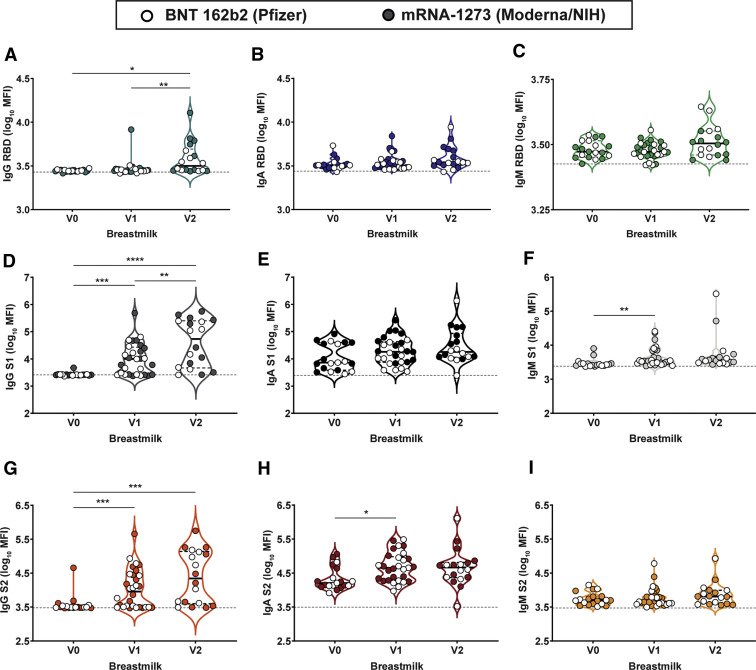
Supplemental Figure 1.
Maternal vaccination induces robust SARS-CoV-2–specific antibodies in maternal serum
A–B, Violin plots show the log10 transformed mean fluorescence intensity (MFI) for (A) IgM spike– and (B) IgM RBD–specific titers across V0, V1, and V2 time points collected from nonpregnant controls (blue), pregnant (orange), or lactating (purple) patients. Participants injected with BNT 162b2 from Pfizer/BioNTech are depicted as open circles, and participants injected with mRNA-1273 from Moderna/NIH are depicted as closed circles. Differences across time points and groups were assessed by repeated measures mixed-effects model followed by post hoc Tukey’s multiple comparisons test. The asterisk indicates P<.05, the double asterisk indicates P<.01, the triple asterisk indicates P<.001, and the quadruple asterisk indicates P<.0001. The dotted line depicts PBS background level. C, Line graph showing the log10 transformed relative spike-specific titers across V0, V1, and V2 time points collected from nonpregnant controls (blue), pregnant (orange), or lactating (purple) patients for IgG (circles and solid lines) and IgA (squares and dotted lines). The dotted line depicts PBS background level. D–I, Violin plots show the log10 transformed (D) IgM S1–, (E) IgM S2–, (F) IgG S1–, (G), IgG S2–, (H) IgA S1–, and (I) IgA S2–specific titers across V0, V1, and V2 time points collected from nonpregnant controls (blue), pregnant (orange), or lactating (purple) patients. Participants injected with BNT 162b2 from Pfizer/BioNTech are depicted as open circles, and participants injected with mRNA-1273 from Moderna/NIH are depicted as closed circles. Differences across time points and groups were assessed by repeated measures mixed-effects model followed by post hoc Tukey’s multiple comparisons test. The asterisk indicates P<.05, the double asterisk indicates P<.01, the triple asterisk indicates P<.001, the quadruple asterisk indicates P<.0001.
Ig, immunoglobulin; NIH, National Institutes of Health; PBS, phosphate-buffer saline; SARS-CoV-2, severe acute respiratory syndrome coronavirus.
Gray et al. Coronavirus disease 2019 vaccination in pregnancy and lactation. Am J Obstet Gynecol 2021.
Supplemental Figure 2.
mRNA-1273 (Moderna/NIH) induces a greater IgA response than does BNT 162b2 (Pfizer/BioNTech)
A–C, Violin plots show the log10 transformed mean fluorescence intensity (MFI) for (A) IgA spike–, (B) IgG spike–, and (C) IgM spike–specific titers across V1 and V2 time points collected from nonpregnant (blue), pregnant (orange), or lactating (purple) participants receiving either mRNA-1273 (Moderna) or BNT 162b2 (Pfizer). Differences across time points and groups were assessed by repeated measures mixed-effects model followed by post hoc Tukey’s multiple comparisons test. The dotted line depicts PBS background level. The asterisk indicates P<.05, the double asterisk indicates P<.01, the triple asterisk indicates P<.001, the quadruple asterisk indicates P<.0001.
Ig, immunoglobulin; mRNA, messenger RNA; NIH, National Institutes of Health; PBS, phosphate-buffer saline.
Gray et al. Coronavirus disease 2019 vaccination in pregnancy and lactation. Am J Obstet Gynecol 2021.
Supplemental Figure 3.
Neither trimester of infection nor vaccination affect SARS-CoV-2 antibody production
A, Violin plots show the IgG spike–specific titer induced by vaccination during the first trimester (red), second trimester (orange), or third trimester (yellow). Participants who received BNT 162b2 from Pfizer/BioNTech are depicted as open circles, and participants who received mRNA-1273 from Moderna/NIH are depicted as closed circles. Differences across groups were assessed by Kruskal-Wallis test. Kruskal-Wallis P=.48. B, Violin plots show the IgG spike–specific of naturally-infected pregnant women infected during the second trimester (yellow) or third trimester (brown). Differences across groups were assessed by Mann-Whitney test. Mann-Whitney P=.48.
Ig, immunoglobulin; mRNA, messenger RNA; OD, optical density; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Gray et al. Coronavirus disease 2019 vaccination in pregnancy and lactation. Am J Obstet Gynecol 2021.
Impact of maternal vaccination on breastmilk antibody transfer
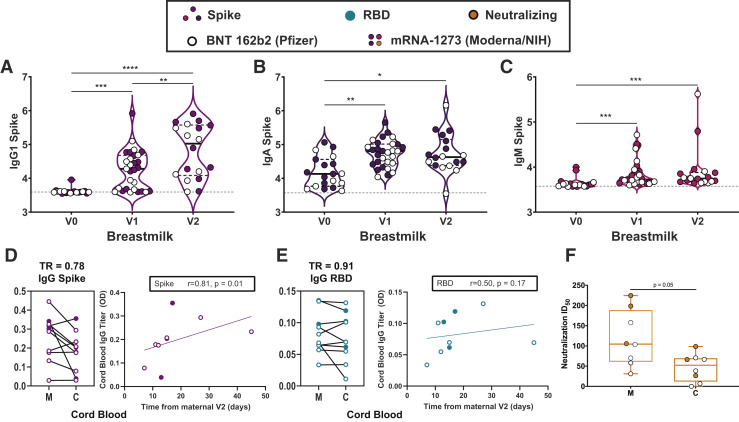
mRNA vaccination resulted in the induction of antibodies in the circulation of vaccinated women (Figure 1). However, whether these antibodies were transferred efficiently to infants remained unclear. Thus, we next examined the levels of antibodies in breastmilk of lactating mothers (Figure 2 , A–C). Robust induction of IgG, IgA, and IgM was observed after the prime and boost. Interestingly, IgA and IgM levels did not increase with boosting, in synchrony with a minimal boost in these isotypes in serum (Figure 1, C and D; Supplemental Figure 1, A–E). However, a boost in breastmilk IgG levels was observed (Figure 2, A), concomitant with the boost observed systemically/in maternal serum (Figure 1, A). IgG1 RBD rose significantly from V0 to V2 (3.44–3.50; P=.002) but not V0 to V1 (3.44–3.45; P=.7) in breastmilk, and there was no significant rise in anti-RBD IgA or IgM in breastmilk after either dose (Supplemental Figure 4). Overall, these data suggest that the boost may drive enhanced breastmilk transfer of IgG, in the setting of consistent unboosted IgA transfer.
Figure 2.
Placental and breastmilk transfer of vaccine-induced SARS-CoV-2 antibodies
A–C, Violin plots show the log10 transformed mean fluorescence intensity (MFI) for (A) IgG1-, (B) IgA-, and (C) IgM spike–specific breastmilk titers across V0, V1, and V2 time points. Differences across time points were assessed with repeated measures mixed-effects model followed by post hoc Tukey’s multiple comparisons test. Participants who received BNT 162b2 from Pfizer/BioNTech are depicted as open circles, and participants who received mRNA-1273 from Moderna/NIH are depicted as closed circles. The asterisk indicates P<.05, the double asterisk indicates P<.01, the triple asterisk indicates P<.001, and the quadruple asterisk indicates P<.0001. D–E, Dot plots showing relative (D) spike- and (E) RBD-specific maternal blood (M) and cord blood (C) titers of IgG1. Wilcoxon matched-pairs signed rank test was performed to determine significance. At the right of each panel, the x-axis shows the time from the second vaccine to delivery and the y-axis shows cord blood log10 transformed titer for (D) IgG spike (purple) and (E) IgG RBD (turquoise). Correlation was determined by Spearman correlation test. PBS background subtraction was used to determine corrected optical density (OD) of 0.0. F, Neutralizing antibody titers (50% inhibitory dose) of maternal blood (M) and cord blood (C) are presented. Wilcoxon matched-pairs signed rank test was performed to determine significance.
Ig, immunoglobulin; PBS, phosphate-buffer saline; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TR, transfer ratio.
Gray et al. Coronavirus disease 2019 vaccination in pregnancy and lactation. Am J Obstet Gynecol 2021.
Supplemental Figure 4.
Maternal vaccination induces SARS-CoV-2–specific antibodies in breastmilk
A–I, Violin plots show the log10 transformed mean fluorescence intensity (MFI) for (A) IgG1 RBD–, (B) IgA RBD–, (C) IgM RBD, (D) IgG1 S1–, (E) IgA S1–, and (F) IgM S1–, (G) IgG1 S2–, (H) IgA S2–, and (I) IgM S2–specific breastmilk titers across V0, V1, and V2 time points. Differences across time points were assessed with repeated measures mixed-effects model followed by post hoc Tukey’s multiple comparisons test. Participants injected with BNT 162b2 from Pfizer/BioNTech are depicted as open circles, and participants injected with mRNA-1273 from Moderna/NIH are depicted as closed circles. The dotted line depicts PBS background level. The asterisk indicates P<.05, the double asterisk indicates P<.01, the triple asterisk indicates P<.001, and the quadruple asterisk indicates P<.0001.
Ig, immunoglobulin; PBS, phosphate-buffer saline; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Gray et al. Coronavirus disease 2019 vaccination in pregnancy and lactation. Am J Obstet Gynecol 2021.
Impact of maternal vaccination on placental antibody transfer
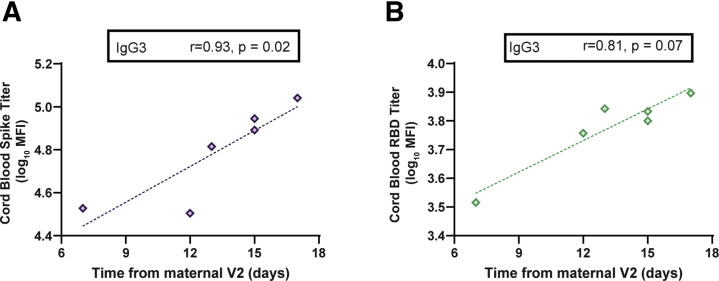
Maternal IgG is also capable of crossing the placenta to confer immunity to the neonate. Spike- and RBD-specific IgG were detectable in 10 of 10 umbilical cords after maternal vaccination (Figure 2, D and E). The cord with the lowest spike- and RBD-specific IgG belonged to a mother who delivered between the first and second vaccine doses and had received her first vaccine dose 17 days before delivery, suggesting that 2 doses may be essential to optimize humoral immune transfer to the neonate. Neutralizing antibody (NAb) titers were lower in umbilical cord than maternal serum, although this finding did not achieve statistical significance (Figure 2, F) (maternal sera, median, 104.7; IQR, 61.2–188.2; cord sera, median, 52.3; IQR, 11.7–69.6; P=.05). Notably, 2 umbilical cords had undetectable NAbs: in 1 case, the mother had not yet received vaccine 2 (17 days from V1) and in the other, the mother was 7 days from the boost dose. Interestingly, there was a significant improvement of transfer of S-, but not RBD-, specific IgG1 into the cord with time from boost (Figure 2, D and E), suggesting that time from vaccination may be an important determinant of transfer rates of specific IgG subpopulations after immunization in pregnancy (Supplemental Figure 5, A and B).
Supplemental Figure 5.
Transfer of SARS-CoV-2–specific antibodies from maternal to umbilical cord blood following maternal vaccination
A–B, The x-axis shows the time from V2 until delivery and the y-axis shows cord blood log10 transformed titer for (A) IgG3 spike (purple) and (B) IgG3 RBD (turquoise). Significance and rho were determined by Spearman’s correlation test.
Ig, immunoglobulin; MFI, mean fluorescence intensity; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Gray et al. Coronavirus disease 2019 vaccination in pregnancy and lactation. Am J Obstet Gynecol 2021.
Vaccine reactogenicity in pregnancy and lactation
Composite reactogenicity score after boost dose of vaccine was significantly positively correlated with both maternal serum and breastmilk antibody titers. Composite symptom score after vaccination was significantly positively correlated with maternal serum spike- and RBD-specific IgG1 and IgG3; breastmilk anti-spike IgG1, IgG3, and IgA; and breastmilk anti-RBD IgG1 (Supplemental Table 2). Within the pregnant women, medical comorbidities were not significantly associated with maternal serum antibody titers, although there were relatively few medical comorbidities in this group.
Discussion
Principal findings
Here, robust and comparable IgG titers were observed across pregnant, lactating, and nonpregnant controls, all of which were significantly higher than those observed in pregnant women with previous SARS-CoV-2 infection. Boosting resulted in augmented IgG levels in the blood, translating to transfer of IgG to the neonate through the placenta and breastmilk.
Results
The lack of boosting of IgM was likely related to an expected class switching to IgG, observed with increasing IgG titers observed after the boost. Conversely, the lack of boosting of IgA observed across all women in this study was unexpected. This lack of IgA augmentation may be related to the intramuscular administration of the vaccine, which triggers a robust induction of systemic, but not mucosal, antibodies. However, higher levels of IgA were noted after the boost in pregnant Moderna recipients, potentially attributable to enhanced class switching after a longer boosting interval. Robust IgG levels were noted in all vaccinees, and vaccine-induced IgG was transferred across the placenta to the fetus, as has been noted in the setting of influenza, pertussis, and other vaccination in pregnancy.16, 17, 18 The presence of NAb transfer in nearly all cords and improved transfer with increased time from vaccination point to the promise of mRNA vaccine-induced delivery of immunity to neonates. Transfer would perhaps be optimized if vaccination is administered earlier during gestation, although this needs to be directly examined in future studies. Although the transferred levels of IgA through breastmilk did not increase with boosting, IgG transfer increased significantly with boost, resulting in the delivery of high levels of IgG to the neonate through breastmilk. Importantly, emerging data point to a critical role for breastmilk IgG in neonatal immunity against several other vaccinatable viral pathogens including HIV, respiratory syncytial virus, and influenza.19, 20, 21 In contrast, IgA dominates breastmilk profiles in natural SARS-CoV-2 infection.22 The different isotype transfer profile for breastmilk (IgG in vaccine, IgA in natural infection) likely reflects differences in antibody profile programming across mucosally acquired natural SARS-CoV-2 infection vs intramuscular vaccination. Whether breastmilk IgG or IgA will be more critical for neonatal protection remains unclear.
Based on what is known about other vaccines, the amount of maternal IgG transferred across the placenta to the cord is likely to differ by trimester of vaccination.16 , 17 Based on data from natural infection,14 qualitative changes in vaccine-elicited antibodies are likely to profoundly alter antibody transfer, and immunization with a de novo antigen earlier in pregnancy is likely to increase placental transfer. Understanding vaccine-induced antibody transfer kinetics across all pregnancy trimesters will be an important direction for future research. Although timing maternal COVID-19 vaccination may not be possible during this phase of the pandemic, understanding optimal timing of vaccination to augment neonatal humoral immunity remains important. Unlike vaccines that aim to boost preexisting antibodies (eg, influenza and pertussis vaccines), optimal timing for de novo vaccine administration remains unclear. Thus, as the prevalence of SARS-CoV-2 community spread decreases, different factors such as optimizing neonatal immunity via placental or breastmilk transfer may be weighted more heavily to inform future vaccine deployment.
After EUA for the COVID-19 mRNA vaccines, safety information has been tracked by the Centers for Disease Control and Prevention using the V-safe smartphone application. Consistent with our observations, the V-safe data indicate no significant differences in postvaccination reactions in pregnant vs nonpregnant women at the age of 16 to 54 years.23 Although the side effect profile of pregnant women receiving the COVID-19 vaccines was not significantly different from nonpregnant women, the relatively high incidence of fever (up to 32% after the second dose) raises a theoretical concern for pregnant recipients,24 , 25 although the level of risk remains controversial.26
Clinical implications
When considering vaccination in pregnancy, evidence regarding maternal and fetal benefit and potential maternal and fetal harm and effects on pregnancy outcomes should be weighed carefully. Although the absolute risk of severe COVID-19 is low in pregnant women, pregnancy is a risk factor for severe disease.27 , 28 There are well-documented maternal, neonatal, and obstetrical risks of SARS-CoV-2 infection during pregnancy.29, 30, 31, 32, 33 These data provide a compelling argument that COVID-19 mRNA vaccines induce similar humoral immunity in pregnant and lactating women as in the nonpregnant population. These data do not elucidate potential risks to the fetus.
Research implications
Future studies, in larger populations spanning vaccine administration across all 3 trimesters and evaluating associated fetal/neonatal transfer of IgG via cord and breastmilk, may enhance our ability to develop evidence-based recommendations for the administration of vaccines and particularly different platforms during pregnancy. Although limited evidence of antibody-dependent enhancement has been observed in the context of preexisting natural or vaccine immunity in adults, future studies should carefully examine the impact of transferred immunity on infant immune response and should define the optimal window for immunization to empower infants with robust immunity.
Strengths and limitations
This study was limited by the select population of primarily healthcare workers from 1 city in the United States, the focused time frame with limited number of delivered participants, inability to assess persistent immunity, and the exclusive focus on antibody titers rather than T cell–driven or other functional immunity. Future work examining T cells and other immune functions may provide additional insights on mRNA vaccine–induced immunity in pregnancy and lactation. The strengths of this work include the provision of longitudinal data profiling vaccine-induced immune response across contemporaneously-recruited pregnant, lactating, and nonpregnant women; the ability to compare vaccine-induced IgG titers to those from previous SARS-CoV-2 infection; and the inclusion of 10 maternal/neonatal dyads, demonstrating transfer of vaccine-induced IgG (including NAbs) to the neonate, with improved cord titers achieved as interval from vaccination increased.
Conclusions
COVID-19 vaccination in pregnancy and lactation generated robust humoral immunity similar to that observed in nonpregnant women with similar side effect profiles. Although humoral immune response and side effects are only 2 of many considerations for pregnant women and their care providers in weighing whether or not to be vaccinated against COVID-19 in pregnancy, these data confirm that the COVID-19 mRNA vaccines result in comparable humoral immune responses in pregnant and lactating women with those observed in nonpregnant populations.
Glossary.
SARS-CoV-2: a single-stranded RNA virus that causes COVID-19
SARS-CoV-2 spike protein: a virus surface protein that mediates viral entry into cells and is composed of S1 and S2 subunits
SARS-CoV-2 receptor-binding domain (RBD): a region of the spike protein that binds to the angiotensin-converting enzyme 2 receptor on human cells for viral entry into cells.
SARS-CoV-2 nucleocapsid (N) antigen: an antigen important for eliciting antibodies against SARS-CoV-2 during infection. A critical protein in many parts of the viral life cycle.
COVID-19 mRNA vaccine: a vaccine designed by packaging messenger RNA (mRNA) that encodes for the SARS-CoV-2 spike protein into an injection. The mRNA elicits an immune response against the spike protein which allows a vaccinated individual’s immune system to become trained to recognize the spike protein and prevent infection with SARS-CoV-2.
Antibody titers: a measurement of the antibody levels generated in response to exposure to an antigen.
Immunoglobulins (IgG, IgM, IgA): antibodies are referred to by immunoglobulin type, including IgG, IgM and IgA. IgG is the most abundant type of immunoglobulin-- it is found in all body fluids and can cross the placenta. IgM is primarily found in blood and lymph and is the first type of antibody to be generated in response to a new infection. IgA is found in mucous membranes including the respiratory and gastrointestinal tracts, saliva, and tears. IgA is the main type of antibody found in breastmilk.
Prime vaccine dose: the first dose of a vaccine that “primes” the body to respond to a subsequent exposure.
Boost vaccine dose: an additional dose of vaccine given to “boost” the immune system. A boost dose is currently given for both approved COVID-19 mRNA vaccines 3 to 4 weeks after the prime vaccine dose.
Immunogenicity: the ability of a foreign substance (eg, antigen or vaccine) to elicit an immune response in an individual.
Reactogenicity: the degree of physical effects following vaccination owing to the body’s immune response. These include the adverse reaction of fever and injection site soreness.
Acknowledgments
We thank Dr Anjali Kaimal and Dr Jeff Ecker for their assistance starting the COVID-19 Pregnancy Biorepository at Massachusetts General Hospital.
Footnotes
K.J.G., E.A.B., and C.A. contributed equally to this work.
G.A. and A.G.E. contributed equally to this work.
K.J.G. has consulted for Illumina, BillionToOne, and Aetion outside the submitted work. A.F. reported serving as a cofounder of and owning stock in Alba Therapeutics and serving on scientific advisory boards for NextCure and Viome outside the submitted work. G.A. reported serving as a founder of Systems SeromYx. M.A.E. reported serving as medical advisor for Mirvie. The remaining authors report no conflict of interest.
This work was supported by the National Institutes of Health, including the Eunice Kennedy ShriverNational Institute of Child Health and Human Development (grants R01HD100022 and 3R01HD100022-02S20 to A.G.E.) and the National Heart, Lung, and Blood Institute (grants K08HL1469630-02 and 3K08HL146963-02S1 to K.J.G.). Additional support was provided by the National Institute of Allergy and Infectious Diseases (3R37AI080289-11S1, R01AI146785, U19AI42790-01, U19AI135995-02, U19AI42790-01, and 1U01CA260476-01 to G.A.; R01A1145840 supplement to M.A.E.); the Gates Foundation; the Massachusetts Consortium on Pathogen Readiness; the Musk Foundation; the Ragon Institute of Massachusetts General Hospital, Massachusetts Institute of Technology, and the Massachusetts General Hospital; and Brigham and Women’s Hospital Departments of Obstetrics and Gynecology.
Cite this article as: Gray KJ, Bordt EA, Atyeo C, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol 2021;225:303.e1-17.
Supplementary Data
Supplemental Methods
Luminex-based antibody quantification
Antibody quantification was performed as described previously.14 Briefly, a multiplexed Luminex assay was used to determine relative titer of antigen-specific isotypes and subclasses using the following antigens: severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) receptor-binding domain (RBD), S1, S2 (all Sino Biologic), and SARS-CoV-2 spike (LakePharma). Antigens were covalently linked to carboxyl-modified Magplex Luminex beads using Sulfo-NHS (N-hydroxysulfosuccinimide, Pierce) and ethyl dimethylaminopropyl carbodiimide hydrochloride. Antigen-coupled microspheres were blocked, washed, resuspended in phosphate-buffer saline (PBS), and stored at 4°C.
To form immune complexes, appropriately diluted plasma (1:100 for immunoglobulin (Ig) G2/3, IgA1, IgM; 1:500 for IgG1) or breastmilk (1:5 for IgG1, IgA1, and IgM) was added to the antigen-coupled microspheres, and plates were incubated overnight at 4˚C, shaking at 700 rpm. The following day, plates were washed with 0.1% BSA 0.02% Tween-20. PE-coupled mouse antihuman detection antibodies (Southern Biotech, Birmingham, AL) were used to detect antigen-specific antibody binding. Fluorescence was acquired using an Intellicyt iQue, and relative antigen-specific antibody titer was log10 transformed for time course blood and breastmilk analyses. PBS background intensity was reported for each antigen as a threshold for positivity.
Antibody quantification using enzyme-linked immunosorbent assay
Antibodies against SARS-CoV-2 RBD and spike were quantified using enzyme-linked immunosorbent assay (ELISA) as previously described.15 Briefly, plates were coated with 500 ng/mL per well of SARS-CoV-2 RBD, SARS-CoV-2 spike, or SARS-CoV-2 N. Plates were incubated for 30 minutes at room temperature and washed in wash buffer (0.05% Tween-20, 400 mM NaCl, 50 mM Tris, pH 8.0). Plates were blocked with a 1% BSA solution then washed again with wash buffer. Serum samples were diluted at 1:100 and added to the plates. Plates were incubated at 37°C for 30 minutes. After incubation, plates were washed, and antihuman IgG or antihuman IgM coupled to horseradish peroxidase (Bethyl Laboratories, Montgomery, TX) was added for detection. Plates were incubated for 30 minutes at room temperature and washed. The ELISA was developed with 3,3′,5,5′-tetramethylbenzidine and stopped with sulfuric acid. The signal was read at 450 nm and background corrected from a reference wavelength of 570 nm. Units had an optical density of 450 to 570.
Neutralization assay
Cell lines
HEK-ACE2 are clonal cells expressing ACE2 receptor and are generously provided by Michael Farzan.
Plasmids and viral constructs
The lentiviruses pseudotyped with the spike protein of SARS-CoV-2 are made by cotransfecting HEK293T cells with 3 plasmids: The psPAX2 was generously provided by Didlier Trono (Addgene Plasmid #12260, Addgene, Watertown, MA) and is a second-generation lentivirus packaging vector. The pSin-DsRed-IRES-Puro are a modification of the pSin-EF-Sox2-Puro, which was generously provided by James Thomson (Addgene plasmid # 16577). The sox2 ORF was replaced with DsRed using standard cloning techniques. The vectors expressing the spike are made from PiggyBac (PB) vector generously provided by Sahand Hormoz. The codon-optimized spike gene was amplified from a plasmid obtained from Sino Biologic (VG40589-UT) and cloned into the PB vector. The C-terminal 19 amino acids of the spike protein were deleted and replaced with the HA tag (YPYDVPDYA). The D614G mutation is then made in this vector using the Quickchange XL Site-directed Mutagenesis (Agilent Technologies, Inc, Santa Clara, CA).
Pseudotyped virus production and quantification
HEK293T cells were plated in T150 flasks 1 night before transfection at a confluency of approximately 50%. The next day, the cells were cotransfected with the abovementioned 3 plasmids at 1:1:1 molar ratio for a total DNA concentration of 40 μg using the TransIT-LT1 Transfection Reagent (Mirus Bio, Madison, WI). Three days later, the supernatant was collected and the virus was pelleted by ultracentrifugation (100,000×g over a 20% sucrose cushion, for 2 hours). The virus was then quantified using the Lenti-X p24 Rapid Titer Kit (Takara Bio Mountain View, CA) and aliquots were frozen at −80°C for future use.
Neutralization assay
On the morning of the experiment, 17,000 ACE2 cells were plated in each well of a flat-bottom 96-well plate in 100 μL of D10 (Dulbecco's Modified Eagle Medium+10% fetal bovine serum). A total of 6 hours later, the serum samples were heat inactivated by incubation at 56°C for 1 hour. A solution containing virus at 1.9 ng equivalent of p24 per μL was prepared in D10. The heat-inactivated serum was diluted in this virus-containing media 1:5-fold, and then 3-fold serial dilutions were done in the same virus-containing media. The virus and serum samples were incubated at 37°C for 2 hours. Notably, 50 μL of the virus-serum mix was then added to the ACE2 cells. Therefore, the lowest final dilution of each serum sample is 15-fold. The cells were incubated at 37°C for 48 hours, and the red fluorescent protein was quantified using the flow cytometer (BD Accuri C6, BD Biosciences, San Jose, CA).
Supplemental Table 1.
Characteristics of pregnant women with natural COVID-19 infection
| Characteristic | Natural COVID-19 infection in pregnancya (N=37) |
|---|---|
| Participant age, mean (SD) | 32.5 (5.3) |
| Race | |
| White | 14 (39) |
| Black | 5 (14) |
| Asian | 1 (3) |
| Multiracial | 1 (3) |
| Other | 14 (39) |
| Unknown | 1 (3) |
| Ethnicity | |
| Hispanic or Latino | 16 (44) |
| Not Hispanic or Latino | 19 (53) |
| Unknown or not reported | 1 (3) |
| Gravidity (including current pregnancy), median (IQR) | 2 (2–3) |
| Parity (excluding current delivery), median (IQR) | 1 (0–1) |
| COVID severity | |
| Mild | 18 (50) |
| Moderate | 11 (31) |
| Severe | 7 (19) |
| Gestational age at COVID diagnosis in wk, median (IQR) | 30.1 (26.9–33.8) |
| Days from symptom onset to blood draw in d, median (IQR) | 62.5 (39.5–84) |
COVID-19, coronavirus disease 2019; IQR, interquartile range; RT-PCR, reverse transcription-polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
Gray et al. Coronavirus disease 2019 vaccination in pregnancy and lactation. Am J Obstet Gynecol 2021.
Only symptomatic women or those with clinical COVID-19 included for the timing of infection from symptom onset. All women had a positive RT-PCR test result for SARS-CoV-2 by nasopharyngeal swab.
Supplemental Table 2.
SARS-CoV-2–specific antibody titer correlation with composite participant symptom score after vaccine dose 2
| V2 time point antibodies (2–6 wk after vaccination) | ||
|---|---|---|
| Spearman rho | P value | |
| Maternal serum | ||
| Spike IgG1 | 0.25 | .05 |
| Spike IgG3 | 0.45 | .0003 |
| RBD IgG1 | 0.29 | .02 |
| RBD IgG3 | 0.36 | .005 |
| Breastmilk | ||
| Spike IgG1 | 0.49 | .04 |
| Spike IgG3 | 0.42 | .04 |
| Spike IgA | 0.42 | .04 |
| RBD IgG1 | 0.42 | .04 |
Ig, immunoglobulin; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus.
Gray et al. Coronavirus disease 2019 vaccination in pregnancy and lactation. Am J Obstet Gynecol 2021.
References
- 1.CDC COVID-19 Response Team; Food and Drug Administration Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine—United States, December 21, 2020-January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:125–129. doi: 10.15585/mmwr.mm7004e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zambrano L.D., Ellington S., Strid P., et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 4.Creech C.B., Walker S.C., Samuels R.J. SARS-CoV-2 vaccines. JAMA. 2021 doi: 10.1001/jama.2021.3199. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Bianchi D.W., Kaeser L., Cernich A.N. Involving pregnant individuals in clinical research on COVID-19 vaccines. JAMA. 2021;325:1041–1042. doi: 10.1001/jama.2021.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riley L.E., Jamieson D.J. Inclusion of pregnant and lactating persons in COVID-19 vaccination efforts. Ann Intern Med. 2021 doi: 10.7326/M21-0173. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beigi R.H., Krubiner C., Jamieson D.J., et al. The need for inclusion of pregnant women in COVID-19 vaccine trials. Vaccine. 2021;39:868–870. doi: 10.1016/j.vaccine.2020.12.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein S.L., Creisher P.S., Burd I. COVID-19 vaccine testing in pregnant females is necessary. J Clin Invest. 2021;131 doi: 10.1172/JCI147553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minkoff H., Ecker J. Balancing risks: making decisions for maternal treatment without data on fetal safety. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2021.01.025. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stafford I.A., Parchem J.G., Sibai B.M. The coronavirus disease 2019 vaccine in pregnancy: risks, benefits, and recommendations. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2021.01.022. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zipursky J.S., Greenberg R.A., Maxwell C., Bogler T. Pregnancy, breastfeeding and the SARS-CoV-2 vaccine: an ethics-based framework for shared decision-making. CMAJ. 2021;193:E312–E314. doi: 10.1503/cmaj.202833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahin U., Muik A., Derhovanessian E., et al. COVID-19 vaccine BNT162b1 elicits human antibody and T H 1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 13.Jackson L.A., Anderson E.J., Rouphael N.G., et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atyeo C., Pullen K.M., Bordt E.A., et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell. 2021;184:628–642.e10. doi: 10.1016/j.cell.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edlow A.G., Li J.Z., Collier A.-R.Y., et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies During the COVID-19 pandemic. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.30455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmeira P., Quinello C., Silveira-Lessa A.L., Zago C.A., Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:985646. doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilcox C.R., Holder B., Jones C.E. Factors affecting the FcRn-mediated transplacental transfer of antibodies and implications for vaccination in pregnancy. Front Immunol. 2017;8:1294. doi: 10.3389/fimmu.2017.01294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fouda G.G., Martinez D.R., Swamy G.K., Permar S.R. The Impact of IgG transplacental transfer on early life immunity. Immunohorizons. 2018;2:14–25. doi: 10.4049/immunohorizons.1700057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fouda G.G., Yates N.L., Pollara J., et al. HIV-specific functional antibody responses in breast milk mirror those in plasma and are primarily mediated by IgG antibodies. J Virol. 2011;85:9555–9567. doi: 10.1128/JVI.05174-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazur N.I., Horsley N.M., Englund J.A., et al. Breast milk Prefusion F immunoglobulin G as a correlate of protection against respiratory syncytial virus acute respiratory illness. J Infect Dis. 2019;219:59–67. doi: 10.1093/infdis/jiy477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demers-Mathieu V., Huston R.K., Markell A.M., McCulley E.A., Martin R.L., Dallas D.C. Impact of pertussis-specific IgA, IgM, and IgG antibodies in mother’s own breast milk and donor breast milk during preterm infant digestion. Pediatr Res. 2020 doi: 10.1038/s41390-020-1031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pace R.M., Williams J.E., Järvinen K.M., et al. Characterization of SARS-CoV-2 RNA, antibodies, and neutralizing capacity in milk produced by women with COVID-19. mBio. 2021;12 doi: 10.1128/mBio.03192-20. e03192–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention Vaccine safety. V-safe after vaccination health checker. 2021. https://www.cdc.gov/vaccinesafety Available at:
- 24.Graham J.M., Jr., Edwards M.J., Edwards M.J. Teratogen update: gestational effects of maternal hyperthermia due to febrile illnesses and resultant patterns of defects in humans. Teratology. 1998;58:209–221. doi: 10.1002/(SICI)1096-9926(199811)58:5<209::AID-TERA8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 25.Dreier J.W., Andersen A.M., Berg-Beckhoff G. Systematic review and meta-analyses: fever in pregnancy and health impacts in the offspring. Pediatrics. 2014;133:e674–e688. doi: 10.1542/peds.2013-3205. [DOI] [PubMed] [Google Scholar]
- 26.Sass L., Urhoj S.K., Kjærgaard J., Dreier J.W., Strandberg-Larsen K., Nybo Andersen A.M. Fever in pregnancy and the risk of congenital malformations: a cohort study. BMC Pregnancy Childbirth. 2017;17:413. doi: 10.1186/s12884-017-1585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellington S., Strid P., Tong V.T., et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:769–775. doi: 10.15585/mmwr.mm6925a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douedi S., Miskoff J. Novel coronavirus 2019 (COVID-19): a case report and review of treatments. Medicine (Baltimore) 2020;99:e20207. doi: 10.1097/MD.0000000000020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeBolt C.A., Bianco A., Limaye M.A., et al. Pregnant women with severe or critical coronavirus disease 2019 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.11.022. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hantoushzadeh S., Shamshirsaz A.A., Aleyasin A., et al. Maternal death due to COVID-19. Am J Obstet Gynecol. 2020;223:109.e1–109.e16. doi: 10.1016/j.ajog.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierce-Williams R.A.M., Burd J., Felder L., et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: a United States cohort study. Am J Obstet Gynecol MFM. 2020;2:100134. doi: 10.1016/j.ajogmf.2020.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juan J., Gil M.M., Rong Z., Zhang Y., Yang H., Poon L.C. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecol. 2020;56:15–27. doi: 10.1002/uog.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dashraath P., Wong J.L.J., Lim M.X.K., et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol. 2020;222:521–531. doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.