Abstract
Camels are increasingly becoming the livestock of choice for pastoralists reeling from effects of climate change in semi-arid and arid parts of Kenya. As the population of camels rises, better understanding of their role in the epidemiology of zoonotic diseases in Kenya is a public health priority. Rift Valley fever (RVF), brucellosis and Q fever are three of the top priority diseases in the country but the involvement of camels in the transmission dynamics of these diseases is poorly understood. We analyzed 120 camel serum samples from northern Kenya to establish seropositivity rates of the three pathogens and to characterize the infecting Brucella species using molecular assays. We found seropositivity of 24.2% (95% confidence interval [CI]: 16.5–31.8%) for Brucella, 20.8% (95% CI: 13.6–28.1%) and 14.2% (95% CI: 7.9–20.4%) for Coxiella burnetii and Rift valley fever virus respectively. We found 27.5% (95% CI: 19.5–35.5%) of the animals were seropositive for at least one pathogen and 13.3% (95% CI: 7.2–19.4%) were seropositive for at least two pathogens. B. melitensis was the only Brucella spp. detected. The high sero-positivity rates are indicative of the endemicity of these pathogens among camel populations and the possible role the species has in the epidemiology of zoonotic diseases. Considering the strong association between human infection and contact with livestock for most zoonotic infections in Kenya, there is immediate need to conduct further research to determine the role of camels in transmission of these zoonoses to other livestock species and humans. This information will be useful for designing more effective surveillance systems and intervention measures.
Author summary
Dromedary camels are well adapted to the arid and semi-arid environment that makes up about 80% of Kenya’s landmass. As such, camels play an important role in the socio-economic wellbeing and food security of pastoralists in the country. However, the species remains relatively neglected in scientific research, one of the main reasons being camels are mostly found in remote, low-income, arid regions of Africa and Asia. We carried out a study to determine the levels of exposure of camels in northern Kenya to Brucella spp., Coxiella burnetii and Rift Valley fever virus, three priority zoonotic pathogens in the country. We found high levels of exposure to the three pathogens, indicating the important role camels might play in the epidemiology of the zoonotic diseases in humans and other livestock. Based on the study findings, we argue for the immediate need for investments in disease surveillance and control strategies for priority zoonotic disease in camels in Kenya.
Introduction
The emergence of the Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012 and the subsequent detection of the virus in dromedary camels, brought into focus the role of the species as potential reservoirs of endemic and emerging zoonotic diseases in Kenya [1–4]. As the population of camels and their contribution to food security and economy of pastoralists in the country continues to rise, understanding this role is a public health priority [5–7]. The burden and transmission dynamics of zoonotic diseases in Kenyan camels are poorly understood. This has led to the perception of low burden of camel diseases, the consequence of which is continued neglect of the camel industry in resource allocation for disease control and research [8, 9].
Previous studies have identified camels as reservoirs of three of the top priority zoonotic pathogens in Kenya; Brucella spp, Rift Valley fever virus (RVFv) and Coxiella burnetii, but data on infection patterns of these pathogens, and potential co-infections in camels is limited [2, 8, 10]. Serological studies, however, show that camels have high seroprevalences and may play an important role in the epidemiology of the three pathogens [2, 11]. Camels have been reported to have the highest Brucella and Coxiella burnetii infection rates among Kenyan livestock, with one study reporting more than a two-fold increase in Brucella prevalence in livestock herds with camels versus those with none [8, 12, 13]. Similarly, camels play an important but underappreciated role in the epidemiology of RVFV, one of the pathogens with the highest exposure levels in Kenyan camels [8]. In addition to the high prevalence rates, high camel mortality rates during RVF outbreaks in Kenya and the association of camels with confirmed human RVF cases are indicative of role of camels in the epidemiology of the virus [14–16].
Co-exposure with multiple pathogens has been shown to alter host-pathogen interactions (within and between host pathogen dynamics) which in turn may affect clinical consequences, transmission dynamics and response to prevention and control measures [17–19]. Whereas, advances in diagnostic techniques have increased interest in understanding pathogen interactions, most epidemiological studies in livestock focus on single-pathogen exposures despite the fact that most zoonotic diseases present with similar clinical symptoms and have the same risk factors [20].
Although presence of antibodies for a given pathogen indicates exposure and not necessary active infections, it is an integral component of disease risk assessment. In this study, we investigated the seroprevalences of RVFV, Brucella spp and Coxiella burnetii and characterized Brucella species using molecular techniques in camels from northern Kenya counties of Isiolo and Samburu.
Methods
Ethics statement
This study did not require ethical approvals from an institutional review board because it was conducted as part of an outbreak investigation by the Kenya Ministry of Agriculture, Livestock and Fisheries and Ministry of Health. However, full approval for the study was given by the Director of Veterinary services in Kenya and local county-level Directors of Veterinary Services in the study area. Although individual informed consent was not required for this investigation, all data were handled as a de-identified set to protect farmers’ privacy and confidentiality.
Study area
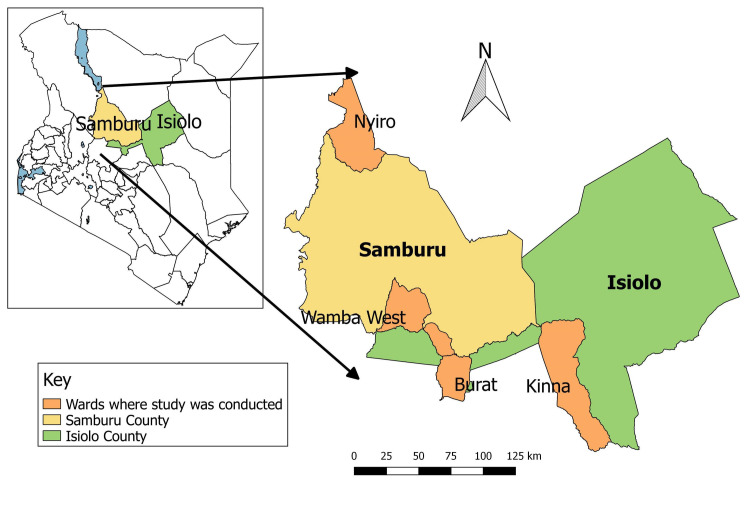
We utilized 120 camel sera samples collected from 16 herds during an outbreak investigation of a respiratory syndrome of unknown aetiology in dromedary camels between 22nd and 28th June 2020. Sera samples were collected from herds with history of at-least one camel presenting with a respiratory syndrome in the 3 months preceding the investigation. The investigation was conducted in Isiolo and Samburu counties, both of which are semi-arid, pastoral regions of northern Kenya (Fig 1). In Isiolo, sampling was conducted in Kinna and Burat wards, while in Samburu camel samples were collected from Wamba West and Nyiro wards. Pastoralism, characterized by transhumance movement is the predominant livestock production system in both study areas. Cattle, goats, sheep and camels are the main species kept in the study area, but goats and sheep form the bulk of livestock in the two counties. Isiolo and Samburu are also home to several wildlife conservancies with significant population of free roaming wildlife and human-animal-wildlife interaction.
Fig 1. Map of the study area showing the sampled wards (in orange).
Shape file used from: https://africaopendata.org/dataset/kenya-counties/shapefile/resource/0b78f25e-494e-4258-96b8-a3ab2b35b121.
Sample collection
Ten millimeters of blood was collected into plain vacutainer tubes from each animal through jugular venipuncture. Serum was then extracted through centrifugation at the laboratories located within county veterinary offices. After serum separation, samples were transferred into 2mls cryovials, labeled and transported in a motorized cool box at 4oc to the International Livestock research Institute, Nairobi for laboratory analysis and testing. Study data was collected electronically in Epi-Info (CDC Atlanta, Georgia, USA). Standardized and pre-tested questionnaires were uploaded to smart phones and administered to key informants; primarily camel owners and herders, to collect data on herd and animal level information such as age and sex.
Laboratory procedure
All serum samples were tested in duplicates for Brucella, RVFV and Coxiella burnetii antibodies using ID screen Brucellosis Serum Indirect ELISA Multispecies kits (IDvet innovative diagnostics, France), ID screen Rift Valley Fever Competition Multispecies ELISA kits (IDvet innovative diagnostics, France) and Q fever (Coxiella burnetii) Antibody test kit (IDEXX Laboratories, Westbrook ME, USA) respectively. Samples that tested positive for Brucella on ELISA test were further subjected to two PCR assays to detect the genus Brucella and a speciation assay to identify Brucella species. All the testing was done according to manufacturer standard operating procedures.
Based on the manufacturer’s instruction, the results were interpreted as negative if the calculated S/P% was <30%, positive if the S/P was ≥40% and doubtful if the S/P was in between 30 and 40%.
Real time PCR detection of Brucella spp.
DNA extraction from serum samples
Total DNA was extracted from all the 32 Brucella sero-positive samples using QIAamp DNA Blood Mini Kit, while following the manufacturer’s instructions. Briefly, 20 μl of proteinase K solution, 200μl of the serum sample and lysis buffer were added into a sterile 1.5ml Eppendorf tube, vortexed and incubated at 56°C for 10 minutes. Absolute ethanol (200μl) was then added into the mixture and vortexed before applying the lysate onto a QIAamp spin column. The columns were centrifuged at 8000 rpm for one minute followed by a two wash steps using 500μl for each buffer AW1 and AW2 respectively. Centrifugation with buffer AW1 was done at 8000 rpm for 1 minute while AW2 was done at maximum speed for 3 minutes. The DNA was eluted from the spin column using buffer 70μl AE and centrifugation at 8000 rpm for one minute. Eluted DNA was spectrophotometrically checked for quality and quantified using NanoDrop 1000 UV-Vis spectrophotometer (Thermo Scientific, Waltham, MA, USA) and stored at -20°C until when required for analysis.
Real-time PCR
Real-time PCR targeting IS711 gene was done to detect the genus Brucella in the extracted DNA samples using oligonucleotide primers and probes (S1 Table). The samples were considered PCR-positive for Brucella if they had a genus-specific amplification with a thresh hold value (<40) in one or all the duplicate samples. The genus PCR-positive samples were subsequently subjected to a multiplex speciation assay with B. abortus, B. melitensis and B. suis species-specific oligonucleotide primers and probes. For both genus and species-specific PCR assays, the test samples were considered positive when they had an amplification with cycle threshold (Ct) values <40 for either or both duplicates (S1 Text).
Statistical analysis
Field and laboratory data were stored in Microsoft Excel files. They were imported to R statistical computing environment [21] for descriptive and statistical analysis. Variables that were available for analysis included (i) three dependent variables that represented exposure states of the three pathogens: RVFV, Brucella spp. and Coxiella burnetii, recorded as either positive or negative, and two independent factors—age and sex of an animal. Herd and animal identification numbers were used for merging the data.
Seroprevalence for each of the three pathogens and their 95% confidence intervals were estimated using binom.confint function. An asymptotic method of estimating these intervals was specified for all the tests. Chi-square or Fisher’s exact test (where values were less than 10) were used to assess the significance of statistical associations between independent variables—age and sex and the dependent variables. Additional dependent variables that represented co-occurrence of two or all the target pathogens were also generated and used in similar analyses [22].
Univariable mixed-effect logistic regression models were fitted to data with age and sex being used as predictors in turns. Variables that were statistically significant (P<0.05) in the univariable analyses were then used in mixed effects logistic regression model that had herd as a random effect. Where age and sex were present and significant, their interaction terms were included and tested for their significance using likelihood ratio tests. The Akaike’s Information Criterion (AIC) was used to analyze the goodness of fit of the model. The data is presented in tables and plots. We used a sjstats package to generate Intracluster Correlation Coefficient (ICC), which measures the degree of correlation of prevalence estimates within herds after running the mixed effects model [23]. Intracluster correlation coefficient is a measure of variability of infection between herds.
Results
We analyzed 120 samples collected from 16 camel herds with no history of vaccination against brucellosis, Coxiella and RVFV. Majority of the camel samples were from females (72%, n = 86) and camels aged above five years (49%, n = 49) as shown in Table 1.
Table 1. Age and sex distribution of sampled camels.
| Sex | Age in years | % (n) |
|---|---|---|
| Female | ||
| <5 | 33% (39) | |
| >5 | 39% (47) | |
| Male | ||
| <5 | 27% (32) | |
| >5 | 2% (2) |
Brucella had the highest seropositivity rate of the three pathogens at 24.2% (95% confidence interval [CI]: 16.5–31.8%), followed by Coxiella burnetii at 20.8% (95% CI: 13.6–28.1%) and RVFV at 14.2% (95% CI: 7.9–20.4%). Co-exposure to two or more pathogens was detected in 15.0% (95% CI: 8.6–21.4%; n = 18) of the samples. Up to 27.5% (95% CI: 19.5–35.5%) of the animals were seropositive for at least one pathogen and 13.3% (95% CI: 7.2–19.4%) were seropositive for at least two pathogens. Of those with co-exposures, 92% (n = 22) had 2 co-exposures detected, the rest of the samples (n = 2) had antibodies to all three pathogens. Co-exposure with Brucella species for both RVFV and Coxiella burnetii were the most common detections. On multivariate analysis, age was found to be significantly associated with co-exposure to Brucella and Coxiella as shown in Table 2.
Table 2. Final multivariable mixed effect model of association between select factors with exposure to one or more pathogens with random effects for herd ID.
| Variable and category | Targeted pathogens and their levels of co-exposure among sampled animals | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brucella spp. | Coxiella burnetii | RVFV | Brucella spp. + RVFV | Brucella spp. + Coxiella burnetii | Coxiella burnetii + RVFV | |||||||
| Odds Ratio (95% CI) | P value | Odds Ratio (95% CI) | P value | Odds Ratio (95% CI) | P value | Odds Ratio (95% CI) | P value | Odds Ratio (95% CI) | P value | Odds Ratio (95% CI) | P value | |
| Fixed Effect | ||||||||||||
| Animal sex | ||||||||||||
| Male | 1 (Ref.) | I (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | ||||||
| Female | 0.7 (2.6–1.9) | 0.499 | 1.8 (0.6–5.5) | 0.278 | 42.6 (1.0–1789.5) | 0.049 | 1.3 (0.1–22.2) | 0.838 | 7.0 (0.5–93.8) | 0.143 | 2.0 (0.1–29.0) | 0.604 |
| Age | ||||||||||||
| <5 years | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | ||||||
| >5 years | 1.6 (0.6–4.3) | 0.388 | 1.9 (0.7–4.7) | 0.200 | 13.1 (1.0–174.3) | 0.052 | 7.1 (0.6–77.4) | 0.108 | 9.4 (1.3–69.8) | 0.028 | 10.2 (0.2–457.2) | 0.220 |
| Variance associated with random effects | 0.77 | 0.019 | 0.03 | <0.001 | 14.68 | 0.005 | 73.03 | 0.032 | 1.67 | 0.002 | 5.96 | 0.034 |
| The intra-herd correlation coefficient (ICC) for herd ID | 0.19 | 0.01 | 0.82 | 0.96 | 0.26 | 0.64 | ||||||
Identification of Brucella species in camels
Thirty-two Brucella (including three borderline positive samples not included in calculation of seropositivity) sero-positive samples were run through PCR for DNA detection. Half of these samples were positive for Brucella on PCR (n = 16). Of these, five samples (32%) were positive for Brucella melitensis. Eleven of 16 samples positive for Brucella genus on PCR could not be confirmed by the Brucella species PCR.
Discussion
The historical neglect of camels and the perception of the species as hardy and resistant to diseases has contributed to limited understanding of their role in the epidemiology of zoonotic pathogens relative to other livestock [8, 24, 25]. Filling this knowledge gap in Kenya; a country with one of the largest camel population globally, is not only key in the economic development and growth of the camel industry, but in reducing the burden of zoonotic diseases in the country [5].
Brucellosis is one of the most important, yet understudied zoonoses in Kenya [26–28]. Our study found more than one in every five camels had been exposed to Brucella spps., which conforms to reported camel brucellosis prevalence range (of up-to 40%) in the East Africa region [26, 29]. Studies conducted in areas of Kenya with similar agro-ecological characteristics and animal husbandry practices in 2012 and 2014, found positivity rates of 11% (ELISA) and 6–18% (serum agglutination test) respectively [30, 31]. The high camel brucellosis prevalence rates in northern Kenya are indicative of the high risk of human infection and interspecies transmission [9, 32]. In literature, camels are reported to be susceptible to B. melitensis and B. abortus [33–35]. In our study however, we only detected B. melitensis after testing for B. abortus, B.suis and B. melitensis. The detection of only B. melitensis is in agreement with similar reports in literature that surmise that B. melitensis is more prevalent in African and Middle East camels than B. abortus [26, 36]. A plausible explanation is that camels are generally herded together with goats as opposed to cattle because of better adaptation of the former to harsher climates; primarily less water requirement, ability to walk longer distances and well adapted dietary preferences [36, 37]. However, our finding should not be deduced to mean that B. melitensis does not affect Kenyan camels, but rather the need for comprehensive studies on the molecular epidemiology of brucellosis in Kenyan camels [26, 38].
Data on the burden of Q fever in northern Kenyan camels is scarce [8, 39]. However, studies in camels in Central Kenya found high Coxiella burnetii positivity rates of between 18.6% - 46%, which is in the range of our 21% prevalence finding [12, 13, 40]. The high Coxiella prevalence in camels is a source of public health concern because of the strong association between Coxiella camel positivity and human infection [41, 42]. Additionally, the high exposure rates indicate camels might play an important role as reservoirs of Coxiella in mixed livestock herds [42–44].
Outbreaks of Rift valley fever have been frequently reported in Kenya since the first detection of the virus in 1931[45, 46]. Traditionally, cattle, sheep and goats are considered to be the species of interest during RVF outbreaks but emerging evidence shows camels play a significant epidemiological role during epizootics [14, 47, 48]. Our study found a positivity rate of 14% which is lower than previously reported rates of 57% and 21% from two studies conducted in 2007 [15, 48]. Other studies in the region have found varying infection rates from 9.6% in Sudan and 45% in Tanzania [49, 50]. The differences in infection rates can be attributed to differences in agro-climatic zones, one of the drivers of RVF infection and different sampling periods; epidemic versus interepidemic period [51, 52]. Our study found a strong association between Brucella and Coxiella co-exposure exposure and camels above five years. This can be explained by the increased likelihood of exposure to the two pathogens over time due to their endemicity [14, 53]. Intra-herd correlation coefficient values are key in calculating sample sizes for multistage sampling [54]. The ICC for RVFv infection was the highest of the three pathogens. This can be inferred to mean RVFv infection within a camel herd is highly correlated. This can be explained by the fact that RVF is a vector borne disease that occurs in explosive outbreaks in very specific ecologies, meaning camels in such areas have much higher exposure rates than those that are not [55]. The persistence of RVFv antibodies in camels compared to the two other pathogens, a hypothesis supported by the fact that age was significantly associated with RVFv exposure in our study, is also a plausible explanation for the high ICC estimate within herds. ICC is an important variable in the calculation of a design effect, a correction factor applied to sample sizes to correct for errors inherent from using cluster sampling, however information on ICCs for most livestock diseases in developing countries are unknown. Given our ICC estimates for exposures and co-exposures with RVFv were higher than the 0.2 value assumed for most infectious diseases, there is need for further investigation and to reconsider the outcome for estimation of ICC in camels [56–58].
Co-exposure with multiple pathogens is a common phenomenon in nature [19]. This is especially true for livestock in pastoral communities in Sub-Saharan Africa with no structured disease control programs, a consequence of which is continuous exposure to endemic pathogens [18, 59]. Extrinsic (environmental) and intrinsic (host-specific) factors influence burden and consequence of co-infections, but these are poorly understood for most endemic livestock diseases in sub-Saharan Africa [59, 60]. Although the outcomes of in vivo pathogen interactions are poorly understood, it is known co-infections can result in change in severity of infection, alteration of transmission dynamics and variation in physiologic response which can change effectiveness of diagnostic techniques, control and prevention efforts [18, 53, 61, 62]. Studies on the outcomes of co-infection in livestock provide evidence of antagonistic effects of co-infection on the host. An example is the Th1 and Th2 immunity response to Toxoplasma gondii and nematode infection or in Trypanomosa cruzi and helminth infections where the former activates Th1 immune response pathways and the latter the Th2 pathways [20, 63]. The three pathogens of interest in the study can affect the immune system; Brucella species and Coxiella burnetii can infect macrophages while RVFv is can affect monocytes [55, 64–67]. Livestock reared under pastoral production system are highly vulnerable to co-exposure to pathogens due to high population densities, widespread movement and frequent contact from sharing pasture and watering points [42, 68]. Our study evaluated co-exposures with the three pathogens in camels as a start to understanding burden of mixed infection and possible outcomes. This is important because zoonotic spillover pathways and clinical presentation of the three infections are very similar and understanding these co-exposures can inform surveillance strategies and integrated disease control and prevention plans in the long term.
Conclusion
This study provides evidence of high exposure rates to the three priority zoonotic pathogens in camels in northern Kenya. Considering the increasing value of camels in food nutrition for pastoralists in Kenya, there is need for more research investment to understand the role of camels in the epidemiology of these diseases. Because the endemicity of these diseases is already established, further research should focus on robust studies to estimate the incidence, transmission between livestock species and from animals to humans, and maintenance hosts for these zoonotic infections to guide future control and elimination programs.
Supporting information
(DOCX)
(DOCX)
(CSV)
Acknowledgments
The authors wish to thank the camel keeping communities of isiolo and Samburu without whom the study would not have been possible, the County governments of Isiolo and Samburu for the logistical support, the Kenya Directorate of Veterinary Services and the Kenya Field Epidemiology and Laboratory Training Program for the technical support.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was part of the project: Co-infection with Rift Valley fever virus, Brucella spp and Coxiella burnetii in humans and animals in Kenya: Disease burden and ecological factors that was funded by the United States Defense Threat Reduction Agency (GRANT 12686246_R, to BB). Additional funding for field work was provided by the Food and Agriculture Organization of the United Nations. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hui DS, Azhar EI, Kim Y-J, Memish ZA, Oh M-D, Zumla A. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis. 2018/04/18. 2018;18: e217–e227. 10.1016/S1473-3099(18)30127-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu S, Zimmerman D, Deem SL. A Review of Zoonotic Pathogens of Dromedary Camels. Ecohealth. 2019/05/28. 2019;16: 356–377. 10.1007/s10393-019-01413-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ommeh S, Zhang W, Zohaib A, Chen J, Zhang H, Hu B, et al. Genetic Evidence of Middle East Respiratory Syndrome Coronavirus (MERS-Cov) and Widespread Seroprevalence among Camels in Kenya. Virol Sin. 2018/12/20. 2018;33: 484–492. 10.1007/s12250-018-0076-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munyua P, Corman VM, Bitek A, Osoro E, Meyer B, Müller MA, et al. No Serologic Evidence of Middle East Respiratory Syndrome Coronavirus Infection Among Camel Farmers Exposed to Highly Seropositive Camel Herds: A Household Linked Study, Kenya, 2013. Am J Trop Med Hyg. 2017;96: 1318–1324. 10.4269/ajtmh.16-0880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamuka PO, Njeruh FM, Gitao GC, Abey KA. Camel health management and pastoralists’ knowledge and information on zoonoses and food safety risks in Isiolo County, Kenya. Pastoralism. 2017;7: 20. 10.1186/s13570-017-0095-z [DOI] [Google Scholar]
- 6.Kagunyu AW, Wanjohi J. Camel rearing replacing cattle production among the Borana community in Isiolo County of Northern Kenya, as climate variability bites. Pastoralism. 2014;4: 13. 10.1186/s13570-014-0013-6 [DOI] [Google Scholar]
- 7.Deem SL. Evaluating Camel Health in Kenya—An Example of Conservation Medicine in Action. Miller RE, Lamberski N, Calle PP, editors. Fowler’s Zoo Wild Anim Med Curr Ther Vol 9. 2018/09/28. 2019; 93–98. [DOI] [Google Scholar]
- 8.Hughes EC, Anderson NE. Zoonotic Pathogens of Dromedary Camels in Kenya: A Systematised Review. Vet Sci. 2020;7: 103. 10.3390/vetsci7030103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson EE, Kochore HH, Dabasso BH. Camels and Climate Resilience: Adaptation in Northern Kenya. Hum Ecol Interdiscip J. 2016/11/12. 2016;44: 701–713. 10.1007/s10745-016-9858-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munyua P, Bitek A, Osoro E, Pieracci EG, Muema J, Mwatondo A, et al. Prioritization of Zoonotic Diseases in Kenya, 2015. PLoS One. 2016;11: e0161576. Available: 10.1371/journal.pone.0161576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devaux CA, Osman IO, Million M, Raoult D. Coxiella burnetii in Dromedary Camels (Camelus dromedarius): A Possible Threat for Humans and Livestock in North Africa and the Near and Middle East?. Frontiers in Veterinary Science. 2020. p. 851. Available: 10.3389/fvets.2020.558481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DePuy W, Benka V, Massey A, Deem SL, Kinnaird M, O’Brien T, et al. Q Fever Risk Across a Dynamic, Heterogeneous Landscape in Laikipia County, Kenya. Ecohealth. 2014;11: 429–433. 10.1007/s10393-014-0924-0 [DOI] [PubMed] [Google Scholar]
- 13.Larson PS, Espira L, Grabow C, Wang CA, Muloi D, Browne AS, et al. The sero-epidemiology of Coxiella burnetii (Q fever) across livestock species and herding contexts in Laikipia County, Kenya. Zoonoses Public Health. 2019/02/20. 2019;66: 316–324. 10.1111/zph.12567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassan A, Muturi † Mathew, Mwatondo A, Omolo J, Bett B, Gikundi S, et al. Epidemiological Investigation of a Rift Valley Fever Outbreak in Humans and Livestock in Kenya, 2018. Am J Trop Med Hyg. 2020; 1–7. 10.4269/ajtmh.20-0387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Britch SC, Binepal YS, Ruder MG, Kariithi HM, Linthicum KJ, Anyamba A, et al. Rift Valley Fever Risk Map Model and Seroprevalence in Selected Wild Ungulates and Camels from Kenya. PLoS One. 2013;8: e66626. Available: 10.1371/journal.pone.0066626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macharia J, Murithi RM, Wainwright S, Breiman RF, Munyua P, Bloland P, et al. Rift Valley Fever Outbreak in Livestock in Kenya, 2006–2007. Am J Trop Med Hyg. 2010;83: 58–64. 10.4269/ajtmh.2010.09-0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Susi H, Barrès B, Vale PF, Laine A-L. Co-infection alters population dynamics of infectious disease. Nat Commun. 2015;6: 5975. 10.1038/ncomms6975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McArdle AJ, Turkova A, Cunnington AJ. When do co-infections matter? Curr Opin Infect Dis. 2018;31. Available: https://journals.lww.com/co-infectiousdiseases/Fulltext/2018/06000/When_do_co_infections_matter_.2.aspx 10.1097/QCO.0000000000000447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thumbi SM, Bronsvoort BM de C, Poole EJ, Kiara H, Toye PG, Mbole-Kariuki MN, et al. Parasite co-infections and their impact on survival of indigenous cattle. PLoS One. 2014;9: e76324–e76324. 10.1371/journal.pone.0076324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaumourin E, Vourc’h G, Gasqui P, Vayssier-Taussat M. The importance of multiparasitism: examining the consequences of co-infections for human and animal health. Parasit Vectors. 2015;8: 545. 10.1186/s13071-015-1167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R: The R Project for Statistical Computing. [cited 21 Sep 2020]. Available: https://www.r-project.org/
- 22.Sprent P. Fisher Exact Test. In: Lovric M, editor. International Encyclopedia of Statistical Science. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. pp. 524–525. 10.1007/978-3-642-04898-2_253 [DOI] [Google Scholar]
- 23.Lüdecke D. sjstats: Statistical functions for regression models. R Packag version 014 0. 2018.
- 24.Abbas B, Omer O. Review of infectious disease of the camel. Vet Bull. 2005;75: 1N–16N. [Google Scholar]
- 25.Miguel Eve, Ahmed El Idrissi Véronique Chevalier, Caron Alexandre, Faye Bernard, Peiris Malik, et al. Ecological and epidemiological roles of camels: lessons from existing and emerging viral infections contents. Empres Heal. 2016; 4–8. Available: http://www.fao.org/3/a-i6184e.pdf [Google Scholar]
- 26.Wernery U. Camelid brucellosis: A review. OIE Rev Sci Tech. 2014;33: 839–857. 10.20506/rst.33.3.2322 [DOI] [PubMed] [Google Scholar]
- 27.Franc KA, Krecek RC, Häsler BN, Arenas-Gamboa AM. Brucellosis remains a neglected disease in the developing world: a call for interdisciplinary action. BMC Public Health. 2018;18: 125. 10.1186/s12889-017-5016-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ducrotoy M, Bertu WJ, Matope G, Cadmus S, Conde-Álvarez R, Gusi AM, et al. Brucellosis in Sub-Saharan Africa: Current challenges for management, diagnosis and control. Acta Trop. 2017;165: 179–193. 10.1016/j.actatropica.2015.10.023 [DOI] [PubMed] [Google Scholar]
- 29.Zewdie Wakene W. Review on Epidemiology of Camel and Human Brucellosis in East Africa, Igad Member Countries. Sci J Clin Med. 2017;6: 109. 10.11648/j.sjcm.20170606.13 [DOI] [Google Scholar]
- 30.Osoro EM, Munyua P, Omulo S, Ogola E, Ade F, Mbatha P, et al. Strong Association Between Human and Animal Brucella Seropositivity in a Linked Study in Kenya, 2012–2013. Am J Trop Med Hyg. 2015/06/22. 2015;93: 224–231. 10.4269/ajtmh.15-0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wanjohi M, Gitao CG, Bebora L. The prevalence of Brucella spp. in camel milk marketed from north Eastern Province, Kenya. 2012.
- 32.Njeru J, Wareth G, Melzer F, Henning K, Pletz MW, Heller R, et al. Systematic review of brucellosis in Kenya: disease frequency in humans and animals and risk factors for human infection. BMC Public Health. 2016;16: 853. 10.1186/s12889-016-3532-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sprague LD, Al-Dahouk S, Neubauer H. A review on camel brucellosis: a zoonosis sustained by ignorance and indifference. Pathog Glob Health. 2012;106: 144–149. 10.1179/2047773212Y.0000000020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abbas B, Agab H. A review of camel brucellosis. Prev Vet Med. 2002;55: 47–56. 10.1016/s0167-5877(02)00055-7 [DOI] [PubMed] [Google Scholar]
- 35.Kaltungo BY, Saidu SNA, Musa IW, Baba AY. Brucellosis: a neglected zoonosis. Microbiol Res J Int. 2014; 1551–1574. [Google Scholar]
- 36.Gwida M, El-Gohary A, Melzer F, Khan I, Rösler U, Neubauer H. Brucellosis in camels. Res Vet Sci. 2012;92: 351–355. 10.1016/j.rvsc.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 37.Rutagwenda T, Lechner-Doll M, Kaske M, Engelhardt W, Schultka W, Schwartz H. Adaptation strategies of camels on a thornbush savannah pasture: Comparison with other domestic animals. Options Mèditerranéennes- Ser Semin. 1989;2: 69–73. [Google Scholar]
- 38.Dadar M, Alamian S. Isolation of Brucella melitensis from seronegative camel: potential implications in brucellosis control. Prev Vet Med. 2020;185: 105194. 10.1016/j.prevetmed.2020.105194 [DOI] [PubMed] [Google Scholar]
- 39.Njeru J, Henning K, Pletz MW, Heller R, Neubauer H. Q fever is an old and neglected zoonotic disease in Kenya: a systematic review. BMC Public Health. 2016;16: 297. 10.1186/s12889-016-2929-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Browne AS, Fèvre EM, Kinnaird M, Muloi DM, Wang CA, Larsen PS, et al. Serosurvey of Coxiella burnetii (Q fever) in Dromedary Camels (Camelus dromedarius) in Laikipia County, Kenya. Zoonoses Public Health. 2017/02/08. 2017;64: 543–549. 10.1111/zph.12337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanderburg S, Rubach MP, Halliday JEB, Cleaveland S, Reddy EA, Crump JA. Epidemiology of Coxiella burnetii Infection in Africa: A OneHealth Systematic Review. PLoS Negl Trop Dis. 2014;8: e2787. Available: 10.1371/journal.pntd.0002787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schelling E, Diguimbaye C, Daoud S, Nicolet J, Boerlin P, Tanner M, et al. Brucellosis and Q-fever seroprevalences of nomadic pastoralists and their livestock in Chad. Prev Vet Med. 2003;61: 279–293. 10.1016/j.prevetmed.2003.08.004 [DOI] [PubMed] [Google Scholar]
- 43.Mohammed OB, Jarelnabi AA, Aljumaah RS, Alshaikh MA, Bakhiet AO, Omer SA, et al. Coxiella burnetii, the causative agent of Q fever in Saudi Arabia: molecular detection from camel and other domestic livestock. Asian Pac J Trop Med. 2014;7: 715–719. doi: 10.1016/S1995-7645(14)60122-X [DOI] [Google Scholar]
- 44.Klemmer J, Njeru J, Emam A, El-Sayed A, Moawad AA, Henning K, et al. Q fever in Egypt: Epidemiological survey of Coxiella burnetii specific antibodies in cattle, buffaloes, sheep, goats and camels. PLoS One. 2018;13: e0192188. Available: 10.1371/journal.pone.0192188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daubney R, Hudson JR. Enzootic Hepatitis or Rift Valley Fever. An Un-described Virus Disease of Sheep, Cattle and Man from East Africa. J Pathol Bacteriol. 1931;34: 545–579. [Google Scholar]
- 46.MURITHI RM, MUNYUA P, ITHONDEKA PM, MACHARIA JM, HIGHTOWER A, LUMAN ET, et al. Rift Valley fever in Kenya: history of epizootics and identification of vulnerable districts. Epidemiol Infect. 2010/05/18. 2011;139: 372–380. 10.1017/S0950268810001020 [DOI] [PubMed] [Google Scholar]
- 47.Linthicum KJ, Britch SC, Anyamba A. Rift Valley Fever: An Emerging Mosquito-Borne Disease. Annu Rev Entomol. 2016;61: 395–415. 10.1146/annurev-ento-010715-023819 [DOI] [PubMed] [Google Scholar]
- 48.Bird BH, Githinji JWK, Macharia JM, Kasiiti JL, Muriithi RM, Gacheru SG, et al. Multiple Virus Lineages Sharing Recent Common Ancestry Were Associated with a Large Rift Valley Fever Outbreak among Livestock in Kenya during 2006–2007. J Virol. 2008;82: 11152 LP– 11166. 10.1128/JVI.01519-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdallah MMM, Adam IA, Abdalla TM, Abdelaziz SA, Ahmed ME, Aradaib IE. A survey of rift valley fever and associated risk factors among the one-humped camel (Camelus dromedaries) in Sudan. Ir Vet J. 2016;69: 6. 10.1186/s13620-016-0065-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swai ES, Sindato C. Seroprevalence of Rift Valley fever virus infection in camels (dromedaries) in northern Tanzania. Trop Anim Health Prod. 2015;47: 347–352. 10.1007/s11250-014-0726-y [DOI] [PubMed] [Google Scholar]
- 51.Nanyingi MO, Munyua P, Kiama SG, Muchemi GM, Thumbi SM, Bitek AO, et al. A systematic review of Rift Valley Fever epidemiology 1931–2014. Infect Ecol Epidemiol. 2015;5: 28024. 10.3402/iee.v5.28024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin V, Chevalier V, Ceccato P, Anyamba A, De Simone L, Lubroth J, et al. The impact of climate change on the epidemiology and control of Rift Valley fever. Rev Sci Tech Int des épizooties. 2008;27: 413–426. [PubMed] [Google Scholar]
- 53.Kairu-Wanyoike S, Nyamwaya D, Wainaina M, Lindahl J, Ontiri E, Bukachi S, et al. Positive association between Brucella spp. seroprevalences in livestock and humans from a cross-sectional study in Garissa and Tana River Counties, Kenya. PLoS Negl Trop Dis. 2019;13: e0007506. Available: 10.1371/journal.pntd.0007506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Segura J, Domínguez-Díaz D, Avalos-Ramírez R, Argáez-Sosa J. Intraherd correlation coefficients and design effects for bovine viral diarrhoea, infectious bovine rhinotracheitis, leptospirosis and neosporosis in cow-calf system herds in North-eastern Mexico. Prev Vet Med. 2010;96: 272–275. 10.1016/j.prevetmed.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 55.Ikegami T, Makino S. The pathogenesis of Rift Valley fever. Viruses. 2011;3: 493–519. 10.3390/v3050493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bett B, Lindahl J, Sang R, Wainaina M, Kairu-Wanyoike S, Bukachi S, et al. Association between Rift Valley fever virus seroprevalences in livestock and humans and their respective intra-cluster correlation coefficients, Tana River County, Kenya. Epidemiol Infect. 2018;147: 1–9. 10.1017/S0950268818003242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alimohamadi Y, Sepandi M. Considering the design effect in cluster sampling. J Cardiovasc Thorac Res. 2019/02/17. 2019;11: 78. 10.15171/jcvtr.2019.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Otte MJ, Gumm ID. Intra-cluster correlation coefficients of 20 infections calculated from the results of cluster-sample surveys. Prev Vet Med. 1997;31: 147–150. 10.1016/s0167-5877(96)01108-7 [DOI] [PubMed] [Google Scholar]
- 59.Petney TN, Andrews RH. Multiparasite communities in animals and humans: frequency, structure and pathogenic significance. Int J Parasitol. 1998;28: 377–393. 10.1016/s0020-7519(97)00189-6 [DOI] [PubMed] [Google Scholar]
- 60.Abbate JL, Galan M, Razzauti M, Sironen T, Voutilainen L, Henttonen H, et al. Pathogen community composition and co-infection patterns in a wild community of rodents. bioRxiv. 2020; 2020.02.09.940494. 10.1101/2020.02.09.940494 [DOI] [Google Scholar]
- 61.Cox FEG, Chappell LH. Concomitant infections. 2001.
- 62.Drake LJ, Bundy DAP. Multiple helminth infections in children: impact and control. PARASITOLOGY-CAMBRIDGE-. 2001;122: S73–S82. 10.1017/s0031182000017662 [DOI] [PubMed] [Google Scholar]
- 63.Ahmed N, French T, Rausch S, Kühl A, Hemminger K, Dunay IR, et al. Toxoplasma Co-infection Prevents Th2 Differentiation and Leads to a Helminth-Specific Th1 Response. Front Cell Infect Microbiol. 2017;7: 341. 10.3389/fcimb.2017.00341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nfon C, Marszal P, Zhang S, Weingartl H. Innate Immune Response to Rift Valley Fever Virus in Goats. PLoS Negl Trop Dis. 2012;6: e1623. 10.1371/journal.pntd.0001623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Schaik EJ, Chen C, Mertens K, Weber MM, Samuel JE. Molecular pathogenesis of the obligate intracellular bacterium Coxiella burnetii. Nat Rev Microbiol. 2013/06/24. 2013;11: 561–573. 10.1038/nrmicro3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Christopher S, Umapathy BL, Ravikumar KL. Brucellosis: review on the recent trends in pathogenicity and laboratory diagnosis. J Lab Physicians. 2010;2: 55. 10.4103/0974-2727.72149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melenotte C, Million M, Audoly G, Gorse A, Dutronc H, Roland G, et al. B-cell non-Hodgkin lymphoma linked to Coxiella burnetii. Blood. 2015;127: 113–122. 10.1182/blood-2015-04-639617 [DOI] [PubMed] [Google Scholar]
- 68.McDermott JJ, Arimi SM. Brucellosis in sub-Saharan Africa: epidemiology, control and impact. Vet Microbiol. 2002;90: 111–134. 10.1016/s0378-1135(02)00249-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(CSV)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.