Abstract
Enhanced tumor glycolytic activity is a mechanism by which tumors induce an immunosuppressive environment to resist adoptive T cell therapy; therefore, methods of assessing intratumoral glycolytic activity are of considerable clinical interest. In this study, we characterized the relationships among tumor 18F-fluorodeoxyglucose (FDG) retention, tumor metabolic and immune phenotypes, and survival in patients with resected non-small cell lung cancer (NSCLC). We retrospectively analyzed tumor preoperative positron emission tomography (PET) 18F-FDG uptake in 59 resected NSCLCs and investigated correlations between PET parameters (SUVMax, SUVTotal, SUVMean, TLG), tumor expression of glycolysis- and immune-related genes, and tumor-associated immune cell densities that were quantified by immunohistochemistry. Tumor glycolysis-associated immune gene signatures were analyzed for associations with survival outcomes. We found that each 18F-FDG PET parameter was positively correlated with tumor expression of glycolysis-related genes. Elevated 18F-FDG SUVMax was more discriminatory of glycolysis-associated changes in tumor immune phenotypes than other 18F-FDG PET parameters. Increased SUVMax was associated with multiple immune factors characteristic of an immunosuppressive and poorly immune infiltrated tumor microenvironment, including elevated PD-L1 expression, reduced CD57+ cell density, and increased T cell exhaustion gene signature. Elevated SUVMax identified immune-related transcriptomic signatures that were associated with enhanced tumor glycolytic gene expression and poor clinical outcomes. Our results suggest that 18F-FDG SUVMax has potential value as a noninvasive, clinical indicator of tumor immunometabolic phenotypes in patients with resectable NSCLC and warrants investigation as a potential predictor of therapeutic response to immune-based treatment strategies.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02560-5) contains supplementary material, which is available to authorized users.
Keywords: Resected non-small cell lung cancer, Positron emission tomography, Tumor immunometabolic phenotypes, Tumor glycolysis
Introduction
Recent success in the treatment of locally advanced and metastatic non-small cell lung cancer (NSCLC) with immunotherapy has provided a paradigm shift in the management of this disease [1–4]. Unfortunately, however, many patients with NSCLC are refractory to immune-based therapies and the processes by which tumors evade the immune system and become resistant to therapy have not been fully elucidated. Much recent effort has been directed toward the development of clinical methods that may provide tumor- and immune-related information that could inform treatment decisions and maximize the clinical effectiveness of immunotherapy.
Accumulating evidence suggests that tumor metabolism may play a critical role in determining tumor progression and response to immune-based therapies. The activation of oncogenic signaling pathways (e.g., mTOR, BRAF, etc.) has been shown to enhance cancer cell glycolysis and lead to accumulation of lactate in the tumor microenvironment and local immunosuppression [5]. Glucose consumption by tumors may have a detrimental impact on an antitumor immune response by dampening T cell mTOR activity, glycolytic capacity, and IFN-γ production [6]. In addition, acidification of the tumor microenvironment further impairs T cell function [7], drives cancer cell invasion [8], and, moreover, predicts likelihood of metastases, tumor recurrence, and poor patient outcomes [9]. Recently, we reported that tumor glycolysis may impair T cell trafficking and effector functions in human NSCLC and melanoma and that glycolytic end products contribute to resistance of melanoma to T cell-induced killing [10]. However, the approaches used in these studies to characterize the tumor metabolic phenotype have limited clinical applicability.
18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) measures the uptake of radiolabeled 18F-FDG by cells and provides an accurate and noninvasive method to evaluate pulmonary nodules and masses. PET may also be used to guide cancer staging, facilitate the decision-making process, and to quantify responses to therapy [11]. Pretreatment 18F-FDG retention has been shown to have prognostic value for a number of malignancies, including NSCLC [11–14]. By measuring the level of uptake by cancer cells of a radiolabeled glucose analog, 18F-FDG PET has appeal as a readily available clinical method for the functional evaluation of tumor burden, providing important information that can influence the management of several aspects of the disease and guide therapy [15]. However, whether analysis of tumor metabolic phenotypes using 18F-FDG PET provides additional clinical utility remains under investigation.
Here, we investigated whether preoperative 18F-FDG retention is associated with tumor glycolytic gene expression and tumor immune phenotypes in patients with operable early-stage/locally advanced NSCLC. We also determined whether 18F-FDG PET metabolic parameters can be used to identify patients with resectable NSCLC who are at high risk of poor postoperative survival. To test this, we retrospectively examined preoperative 18F-FDG PET parameters, overall tumor expression of glycolysis-related genes in cancer cells and other cell populations within the tumor microenvironment, and immune cell infiltration of tumors in patients who underwent surgical resection of NSCLC.
Materials and methods
Study design, population, and treatment
Patients were considered eligible for analysis if they underwent resection of primary NSCLC (stages I–III) at the University of Texas MD Anderson Cancer Center and were enrolled in the Profiling of Resistance Patterns and Oncogenic Signaling Pathways in Evaluation of Cancers of the Thorax (PROSPECT) study, which has the benefits of full clinical annotation, detailed immune profiling, and long postoperative follow-up [10, 16–18]. To limit potential temporal changes in tumor biology from the time of 18F-FDG PET to that of resection, only patients who underwent PET/computed tomography (CT) within 30 days prior to surgical tumor resection were included (Supplementary Fig. 1) [19]. Postoperatively, all patients underwent periodic surveillance in accordance with guidelines in effect at the time of treatment. All tumors were retrospectively staged using the seventh edition of the International Association for the Study of Lung Cancer staging system [20].
Image acquisition and analysis
All images were obtained using a dedicated PET/CT system (Discovery ST, STe, or RX; GE Medical Systems and GE Healthcare). After fasting for a minimum of 6 h and undergoing confirmation of a blood glucose level less than 200 mg/dL, patients received an intravenous infusion of 259–740 MBq18F-FDG. A CT scan was performed for anatomic correlation and attenuation correction; PET images were subsequently obtained 60–90 min after 18F-FDG infusion. All images were retrospectively reviewed by an experienced board-certified radiologist (B. Amini) who was blinded to the patients’ clinicopathologic characteristics and outcomes. Regions of interest and volumes of interest (VOIs) were identified as pathologic areas on imaging with 18F-FDG uptake exceeding that of the background and segmented using a semiautomated spatial derivative contouring algorithm (MIMVista version 6.6; MIM software) with high accuracy and reproducibility [21, 22]. This contouring method has been validated in a multi-observer study that showed superiority over manual and threshold methods [22]. The maximum standardized uptake value (SUVMax) was defined as the greatest uptake in a single voxel within the semiautomatically defined VOI. The total SUV (SUVTotal) was calculated as the sum of the SUV throughout the VOI, and the mean SUV (SUVMean) was defined as the average SUV throughout the VOI. Total lesion glycolysis (TLG) was calculated as the product of the SUVMean and active tumor volume within the VOI.
mRNA extraction and transcriptomic analysis
To delineate the relationship between 18F-FDG PET metabolic parameters and tumor glycolytic gene expression, expression of 18 genes (ALDOA, ALDOC, BPGM, ENO2, ENO3, FBP1, FBP2, GAPDH, GPI, LDHA, LDHB, PFKM, PFKP, PGAM1, PGAM4, PGK1, PGK2, SLC2A1) involved in the glycolytic pathway was quantified in resected NSCLC tumors [10]. These genes were selected for analysis because our group previously identified their expression to be correlated with tumor immune markers in resected NSCLCs [10]. mRNA was extracted from formalin-fixed, paraffin-embedded (FFPE) NSCLC tumor tissues, and gene expression was quantified using an Illumina Human WG-6 v3 BeadChip according to previously described methods [16–18]. Microarray data have been previously deposited and are publicly available (Gene Expression Omnibus, GSE42127) [10, 16–18, 23]. A signature of overall tumor expression of glycolysis-related genes was defined as the geometric mean expression level of the representative genes GPI and PGAM4, which we have previously shown to be well correlated with increased tumor intrinsic glycolytic activity according to analysis of bioenergetic profiles of tumor cell lines [10]. To further characterize immune phenotypic changes associated with tumor glycolytic metabolism, we analyzed expression of 708 genes well recognized as having roles in antitumor immunity [24]. Pairwise associations between expression of these 708 genes with 18F-FDG PET parameters and overall tumor expression of glycolytic genes were analyzed using Pearson’s correlations, with adjustments for multiple comparisons using the Benjamini–Hochberg method [25]. Gene dysregulation analysis was performed using the R package limma [26] to identify differentially expressed genes (|log-fold change|> 1, P < 0.05) according to each parameter (overall tumor glycolysis gene expression, SUVMax, SUVMean, SUVTotal, TLG). Transcriptomic data were additionally analyzed using Ingenuity Pathway Analysis (QIAGEN Inc) [27], with significantly dysregulated pathways defined as |z score| ≥ 1.5 and –log10(P-value) ≥ 2. To define transcriptomic profiles indicative of exhausted T cell phenotypes, we used two gene signatures: the mean expression levels of a subset of genes in the pan-cancer Tumor Inflammation Signature (TIGIT, LAG3, CD274, PDCD1LG2, CD276) [28], and the mean expression level of CTLA4, HAVCR2, LAG3, PDCD1, and TIGIT [29].
Immunohistochemical analysis of immune cell infiltration of tumors
Immunohistochemical staining was performed using an automated staining system (BOND-MAX; Leica Microsystems) with 4-µm-thick sequential histologic tumor sections of FFPE tumor samples with antibodies against CD3, CD4, CD8, CD57, GZB, CD45RO, FOXP3, CD68, and PD-L1 [10, 30, 31]. Expression of all cell markers was detected using a Novocastra Bond Polymer Refine Detection kit (Leica Microsystems) with a diaminobenzidine reaction to identify antibody labeling and hematoxylin counterstaining. At the same time, tonsil tissue samples were analyzed as positive controls according to the same protocol; negative controls were incubated without primary antibodies.
Slides containing whole tumor sections were digitally scanned at 200 × magnification using a ScanScope Aperio AT Turbo slide scanner (Leica Microsystems) and visualized using the ImageScope software program (Leica Microsystems). Tumor-associated immune cells (TAICs) expressing CD3, CD4, CD8, CD57, GZB, CD45RO, FOXP3, or CD68 were evaluated by counting positive cells in five square areas (1 mm2 each) in the intratumoral compartment, using a nuclear algorithm to identify subpopulations of lymphocytes and a cytoplasmic algorithm to identify macrophages. Each area examined was overlapped with sequential immunohistochemical slides to quantify each marker at the same location of the tumor. The average total number of immune cells positive for each marker in the five square areas was expressed as immune cell density (number of cells/mm2) [30, 31]. A cellular membrane detection algorithm was used to identify expression of PD-L1 by malignant cells using methods that have been described previously; PD-L1 expression was subsequently quantified as an H-score (product of staining intensity [range 0–3 +] and percentage of cells expressing PD-L1 [range 0–100]; possible range 0–300) [30].
Statistics
Pairwise associations among 18F-FDG PET parameters, tumor expression of glycolysis-related genes, and TAIC densities were analyzed using Pearson correlation coefficients after log2 transformation of all values. Differences in continuous variables between groups were analyzed using unpaired t tests. Overall survival (OS) was defined as the time from surgical tumor resection to death due to any cause; patients alive at last follow-up were censored on the date of last contact. Disease-free survival (DFS) was defined as the time from resection to recurrence or death; patients without a DFS event were censored on the date of last contact. Survival was estimated using the Kaplan–Meier method, and differences between groups in time-to-event outcomes were analyzed using the log-rank test. For identification of the transcriptomic Upregulated Immune Signature of Tumors with High Glycolysis (UISTHG), genes were selected if they were significantly upregulated (log-fold change > 1, P < 0.05) according to elevated (> median) overall tumor glycolytic gene expression and were associated with OS (univariable Cox false discovery rate-adjusted P < 0.10). The Downregulated Immune Signature of Tumors with High Glycolysis (DISTHG) included genes that were significantly downregulated (log-fold change < − 1, P < 0.05) according to elevated (> median) overall tumor glycolytic gene expression and were associated with OS (univariable Cox false discovery rate-adjusted P < 0.10). UISTHG and DISTHG were defined as the mean overall expression of their constituent genes, and the cohort was dichotomized according to the median expression level of each signature. Given the number of observed events in the study cohort, multivariable Cox analysis in the PROSPECT cohort was restricted to adjustment for pathologic stage, histology, and receipt of neoadjuvant and/or adjuvant platinum-based chemotherapy in order to avoid model overfitting. For validation of these signatures, we analyzed publicly available transcriptomic data [32] via a web-based tool (https://kmplot.com/analysis). For these analyses, UISTHG and DISTHG gene expression signatures were again defined as the mean expression level of their constituent genes and dichotomized according to the observed median. Multivariable OS analyses were performed with adjustment for stage and histology among the samples with such data available. Statistical significance was defined as a two-tailed P value less than 0.05. All statistical analyses were performed using Python (version 2.7; Python Software Foundation; seaborn [0.8.1] package was used for plotting) and R [33].
Results
Patient characteristics
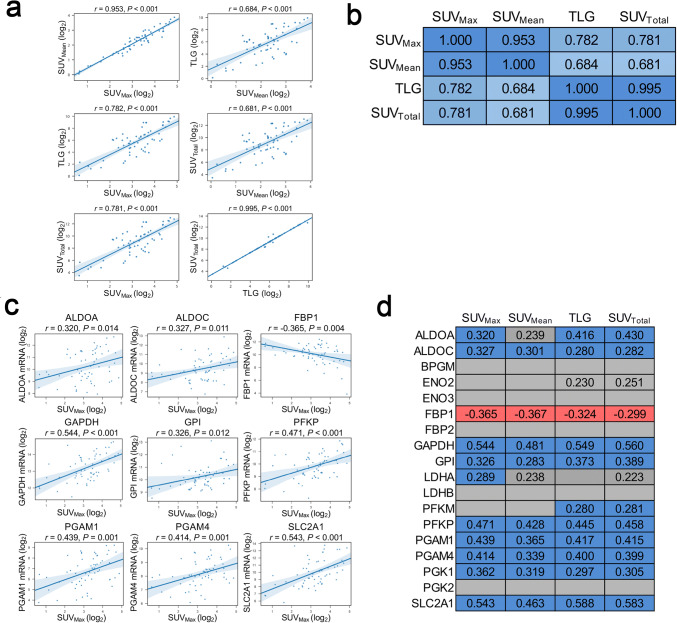
We identified 172 patients with stage I-III NSCLC in the cohort who underwent curative-intent surgery, 123 (72%) of whom had preoperative 18F-FDG PET and available tumor microarray data (Supplementary Fig. 1). Of these patients, we included 59 (48%) who underwent PET within 30 days prior to surgery in the present study (median delay from PET to resection 16.0 days, interquartile range [IQR] 12.0–24.0 days). The clinicopathologic and treatment characteristics of these 59 patients are shown in Supplementary Table 1. Of the patients eligible for analysis, most were men, white, former or current smokers, and treatment-naïve. Adenocarcinomas and early-stage tumors constituted the majority of the tumors examined. Pairwise correlation analysis showed that SUVMax, SUVMean, SUVTotal, and TLG parameters were positively correlated among each other (Fig. 1a, b), with the strongest magnitudes of correlation (as measured by Pearson correlation coefficient) observed between parameters measuring the intensity of tumor 18F-FDG uptake (SUVMax and SUVMean, r = 0.953) and between those reflecting overall tumor burden (SUVTotal and TLG, r = 0.995).
Fig. 1.
Preoperative tumor 18F-FDG uptake is associated with expression of genes encoding enzymes associated with glycolysis (n = 59). a, b Positive correlations between parameters measuring the intensity of tumor 18F-FDG uptake (SUVMax and SUVMean) and those reflecting tumor burden (SUVTotal and TLG). All 18F-FDG PET parameters were log2-transformed. The values provided are Pearson correlation coefficients; a two-tailed P value less than 0.05 was used to determine significance. Blue cells in b reflect statistically significant positive correlations; the intensity of cell color is scaled to the magnitude of the Pearson correlation coefficient. c Correlations of expression of glycolysis-related genes with preoperative SUVMax. d Statistically significant positive (blue) and negative (red) correlations of expression of glycolysis-related genes with four semiquantitative 18F-FDG PET parameters. mRNA expression and all18F-FDG PET parameters were log2-transformed. The values provided in a-d are Pearson correlation coefficients; a two-tailed P value less than 0.05 was used to determine significance. The gray cells in d represent absence of a statistically significant correlation. Trends towards positive correlations (0.05 ≤ P < 0.10) are depicted as gray cells labeled with Pearson correlation coefficients. ALDOA and ALDOC, aldolase A and C; BPGM, bisphosphoglycerate mutase; ENO2 and ENO3, enolase 2 and 3; FBP1 and FBP2, fructose-1,6-bisphosphatase 1 and 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GPI, glucose-6-phosphate isomerase; LDHA and LDHB, lactate dehydrogenase A and B; PFKM, phosphofructokinase, muscle; PFKP, phosphofructokinase, platelet; PGAM1 and PGAM4, phosphoglycerate mutase family members 1 and 4; PGK1 and PGK2, phosphoglycerate kinase 1 and 2; SCL2A1, solute carrier family 2 member 1
18F-FDG PET parameters correlate with transcriptomic quantification of tumor glycolytic gene expression
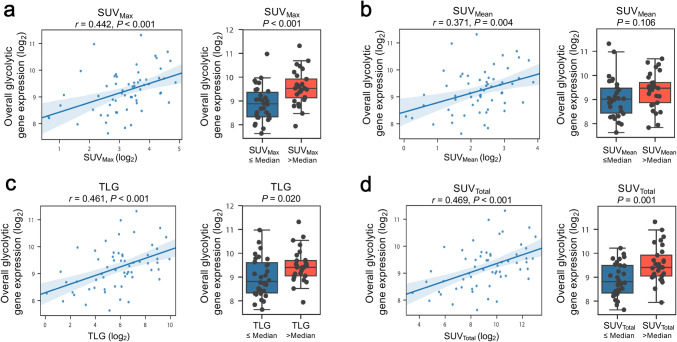
To investigate whether 18F-FDG PET parameters are clinical indicators of tumor glycolytic metabolism, we first examined the association of the 18F-FDG PET parameters with the expression of 18 glycolysis-related genes in tumor tissues from patients with NSCLC. We found that all four parameters were significantly positively correlated with elevated expression levels of glycolysis-related genes, including ALDOA, ALDOC, GAPDH, GPI, LDHA, PFKM, PFKP, PGAM1, PGAM4, PGK1, and SLC2A1 (Fig. 1c, d). We also observed a significant negative correlation between preoperative tumor 18F-FDG retention and the expression of fructose-1,6-bisphosphatase 1 (FBP1), which opposes glycolysis and pyruvate production by catalyzing the conversion of fructose-1,6-bisphosphate to fructose-6-phosphate. We further stratified our cohort based on their histotype and found a similar positive correlation between the expression of glycolysis-related genes and FDG retention parameters in patient with lung adenocarcinomas (n = 43/59, Supplementary Table 1; data not shown). Due to limitations imposed by the small sample size of patients with squamous cell carcinomas (n = 16/59, Supplementary Table 1), the stratified analyses among this subgroup were underpowered to make definitive conclusions (data not shown). By analyzing bioenergetic profiles of cancer cell lines, we previously demonstrated that elevated overall tumor expression of glycolysis-related genes, defined as the mean expression level of the representative genes GPI and PGAM4, is correlated with increased tumor intrinsic glycolytic activity [10]. To examine whether the 18F-FDG PET parameters could be potential clinical indicators of overall tumor glycolysis, we tested correlations between PET parameters and overall glycolytic gene expression (bulk transcripts from both cancer and other components within the tumor microenvironment) (Fig. 2a–d). These analyses revealed positive correlations between all four 18F-FDG PET parameters and the overall glycolytic gene expression (SUVMax: r = 0.442, P < 0.001; SUVMean: r = 0.371, P = 0.004; TLG: r = 0.461, P < 0.001; SUVTotal: r = 0.469, P < 0.001). Collectively, these findings suggest that 18F-FDG PET analysis may also provide valuable information regarding glycolytic metabolic phenotypes of tumors from patients with resectable NSCLC.
Fig. 2.
Correlations between overall tumor expression of glycolysis-related genes and tumor 18F-FDG retention according to a SUVMax, b SUVMean, c TLG, d SUVTotal (n = 59). Overall tumor expression of glycolytic genes was quantified as the geometric mean of GPI and PGAM4 levels (log2-transformed); the 18F-FDG PET parameters were similarly log2-transformed. The values provided in the left panels are Pearson correlation coefficients; a two-tailed P value of less than 0.05 was used to determine significance. In the right panels, the cohort was dichotomized according to the observed median of each 18F-FDG PET parameter (SUVMax, 10.36; SUVTotal, 643.26; TLG, 64.72; SUVMean, 4.90). Boxes depict median and interquartile range (IQR); bars represent minimum and maximum values excluding outliers (median ± 1.5 IQR). P values in the right panels were calculated using unpaired t tests after log2 transformation of glycolytic gene expression levels. GPI, glucose-6-phosphate isomerase; PGAM4, phosphoglycerate mutase family member 4
Preoperative SUVMax discriminates tumor glycolytic phenotypes and is associated with tumor expression of immune-associated genes
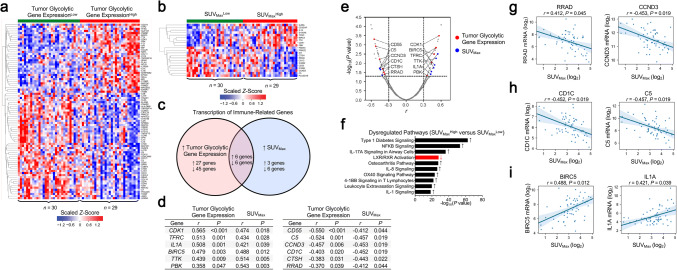
After having established that preoperative 18F-FDG PET parameters are clinical indicators of tumor bulk glycolytic metabolism, we next performed correlative analyses to evaluate whether tumor glycolysis, as determined by evaluation of 18F-FDG PET parameters, is associated with changes in expression of genes involved in an antitumor immune response. Our pairwise correlative analyses between the expression of overall tumor glycolytic genes analyzed from the bulk transcriptome and a panel of 708 immune-related genes [24] identified significant associations with increased mRNA levels of 33 genes (Pearson’s r > 0.0, FDR-adjusted P < 0.05) and reduced expression of 51 genes (r < 0.0, FDR-adjusted P < 0.05) in tumors with high glycolytic gene expression (Fig. 3a, Supplementary Table 2), suggesting that enhanced tumor glycolytic metabolism is associated with immune-related transcriptomic changes in the tumor microenvironment. Next, we sought to identify whether any of the18F-FDG PET parameters SUVMax, SUVMean, SUVTotal, and TLG was a stronger indicator of tumor–glycolysis-associated changes in immune gene expression. We found that preoperative SUVMax had the greatest number of significant pairwise correlations with immune-related genes (9 positively associated [correlation coefficient range r = 0.421–0.543], 12 negatively associated [correlation coefficient range r = – 0.407 to – 0.548] (Fig. 3b; Supplementary Table 3), whereas SUVMean (15 total genes), SUVTotal (6 total genes), and TLG (3 total genes) had fewer individual associations with gene expression (Supplementary Tables 4–6). Moreover, the number of identified overlapping genes significantly associated with glycolytic gene expression was the greatest with SUVMax (12 genes [Fig. 3c–e, SUVMax positive correlation coefficient range r = 0.421–0.543, SUVMax negative correlation coefficient range r = – 0.412 to – 0.457] versus SUVMean [6 genes], SUVTotal [3 genes], and TLG [0 genes]).
Fig. 3.
Preoperative semiquantitative SUVMax identifies changes in transcription of immune-related genes in high and low glycolytic tumors (n = 59). a Hierarchical clustering of immune-related genes that were most strongly associated (|Pearson’s r|≥ 0.3, false discovery rate [FDR]-adjusted P < 0.05) with enhanced overall tumor expression of glycolysis genes. b Hierarchical clustering of immune-related genes associated with preoperative SUVMax according to the same criteria. c Venn diagram depicting the overlap in genes that were significantly correlated (FDR-adjusted P < 0.05) with overall tumor expression of glycolysis-associated genes and with SUVMax. d, e Genes that were significantly correlated (FDR-adjusted P < 0.05) with tumor glycolytic gene expression and SUVMax. f The ten most highly upregulated (black, upward arrow) and downregulated (red, downward arrow) gene signaling pathways among tumors with elevated (> median) SUVMax. g-i Associations between preoperative SUVMax and expression of selected genes in resected NSCLC tumors. For a and b, the study cohort was dichotomized according to the observed median of overall tumor glycolysis gene expression (9.16, a) and SUVMax (10.36, b). The values provided in d, e, and g–i are Pearson coefficients for pairwise correlations between log2-transformed values; a two-tailed FDR-adjusted P value of less than 0.05 was used to determine significance. For f, dysregulated pathways (|z-score|≥ 1.5, − log10[P-value] ≥ 2 according to Ingenuity Pathway Analysis) were ranked in descending order by − log10(P-value). BIRC5, baculoviral IAP repeat containing 5; C5, complement C5; CCND3, cyclin D3; CD1C, CD1c molecule; CD55, CD55 molecule (Cromer blood group); CDK1, cyclin-dependent kinase 1; CTSH, cathepsin H; IL1A, interleukin 1 alpha; PBK, PDZ binding kinase; RRAD, Ras-related glycolysis inhibitor and calcium channel regulator; TFRC, transferrin receptor; TTK, TTK protein kinase
To confirm the findings from the correlative analyses, we next compared the expression of 708 immune-related genes [24] between high and low glycolytic tumors and identified differentially expressed immune-related genes in these two groups. Using overall tumor bulk glycolytic gene expression to dichotomize our study cohort, we identified 25 differentially expressed immune-related genes (|log-fold change|> 1, P < 0.05; 15 genes upregulated, 10 downregulated; Supplementary Table 7). When we dichotomized our study cohort according to SUVMax, we observed 11 total dysregulated genes (Supplementary Table 8; concordance in classification of tumors between overall tumor glycolytic gene expression and SUVMax provided in Supplementary Table 9). Moreover, SUVMax was a more discriminatory indicator of gene dysregulation than SUVMean (10 genes), SUVTotal (5 genes), and TLG (1 gene), and had the greatest overlap of dysregulated genes with tumor bulk glycolytic gene expression (SUVMean, SUVTotal, TLG, Supplementary Table 10). Taken together, our findings suggest that preoperative SUVMax was more discriminatory of glycolysis-associated changes in tumor immune phenotypes than the other 18F-FDG PET parameters.
Examination of the most highly dysregulated pathways in tumors with elevated SUVMax suggested upregulation of several genes known to be involved in proinflammatory signaling (Fig. 3f), including IL-17A [34, 35], IL-8 [36], and NF-κB [37]. Examination of the 12 genes that were significantly correlated with both tumor glycolytic gene expression and SUVMax revealed that highly glycolytic tumors were associated with reduced expression of RRAD, a negative regulator of tumor aerobic glycolysis [38], and CCND3, which shunts glycolytic intermediates toward other metabolic pathways [39] (Fig. 3g). We also observed decreased expression of CD1C and C5, which are involved in antigen presentation and complement activation, respectively, in highly glycolytic tumors (Fig. 3h). Other shared genes suggested that tumors with high glycolytic gene expression and elevated SUVMax were potentially enriched in molecules involved in cell proliferation and proinflammatory signals, including BIRC5 and IL1A (Fig. 3i). Together, these analyses suggest that assessment of tumor 18F-FDG uptake by SUVMax correlates with tumor immunometabolic changes associated with enhanced tumor glycolytic gene expression. Moreover, these results suggest that resected NSCLC tumors with enhanced 18F-FDG retention are potentially characterized by overexpression of factors involved in tumor progression, local inflammation, and cell cycle dysregulation.
Elevated tumor glycolytic activity as assessed by SUVMax is associated with enhanced expression of PD-L1 and an immunosuppressive phenotype
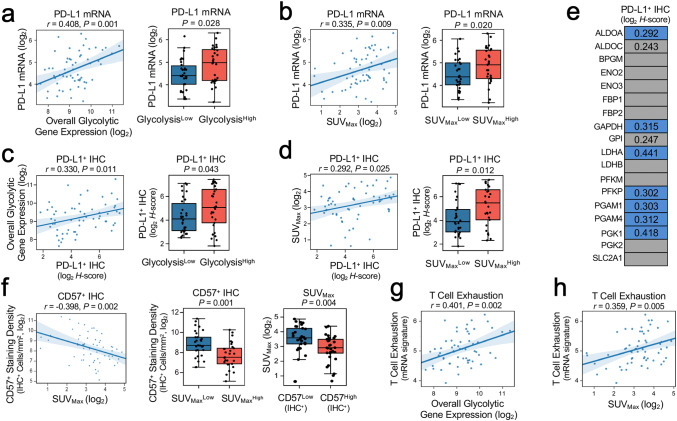
To further confirm the correlations between preoperative SUVMax and tumor immune phenotypes, we determined the associations between SUVMax and the expression of other immune markers using immunohistochemistry (IHC). Previous reports demonstrated that tumor glycolytic metabolism and PD-L1 expression are tightly interlinked [6]. We next asked whether enhanced expression of PD-L1 may be associated with immune evasion within highly glycolytic NSCLC tumors. Transcriptomic analysis of PD-L1 expression (CD274 mRNA) demonstrated statistically significant positive associations with overall tumor bulk glycolytic gene expression (Fig. 4a) and SUVMax (Fig. 4b), indicating that 18F-FDG quantification of tumor bulk glycolysis correlates with enhanced tumor expression of PD-L1.
Fig. 4.
Elevated tumor glycolytic activity as assessed by SUVMax is associated with enhanced PD-L1 expression and an immunosuppressive phenotype (n = 59). a, b Associations between transcription of PD-L1 (CD274 mRNA) with (a, left and right panels) overall tumor glycolysis and (b, left and right panels) preoperative SUVMax. c, d Associations between tumor expression of PD-L1 (IHC H-score, log2-transformed) with (c, left and right panels) overall tumor expression of glycolytic genes and (d, left and right panels) SUVMax. e Pairwise correlations between tumor PD-L1 IHC H-score (log2-transformed) and expression of individual glycolytic genes. f Elevated preoperative SUVMax was associated with reduced intratumoral infiltration by CD57+ immune cells. g The association between overall tumor glycolytic gene expression and an exhausted T cell gene signature (mean expression of TIGIT, LAG3, CD274, PDCD1LG2, and CD276). h The association between SUVMax and exhausted T cell gene signature. Pearson coefficients for pairwise correlations between log2-transformed values are listed; a two-tailed P value of less than 0.05 was used to determine significance. In a-d (right panels) and f (center and right panels), the study cohort is dichotomized according to the observed median of each variable (SUVMax, 10.36; CD57, 330.4 IHC+ cells/mm2; overall tumor expression of glycolytic genes, 9.16). Boxes depict median and interquartile range (IQR); bars represent minimum and maximum values excluding outliers (median ± 1.5 IQR). P values in the right panels were calculated using unpaired t tests. In e, the values provided are Pearson correlation coefficients for statistically significant (two-tailed P < 0.05, blue cells) relationships between expression of individual glycolytic genes and PD-L1 H-score. Gray cells represent absence of a statistically significant correlation. Trends towards positive correlations (0.05 ≤ P < 0.10) are depicted as gray cells labeled with Pearson correlation coefficients. LAG3, lymphocyte activating 3; PDCD1LG2, programmed cell death 1 ligand 2; TIGIT, T cell immunoreceptor with Ig and ITIM domains
To validate our results at the protein level, we quantified PD-L1 expression using IHC. We noted positive correlations between PD-L1 and increased tumor bulk glycolytic gene expression (Fig. 4c) and with 18F-FDG uptake SUVMax (Fig. 4d). Next, we analyzed correlations between tumor PD-L1 protein levels and expression of 18 glycolysis-related genes derived from tumor bulk transcriptome analysis (Fig. 4e) [10]. That analysis revealed statistically significant positive correlations between PD-L1 expression and ALDOA, GAPDH, LDHA, PFKP, PGAM1, PGAM4, and PGK1 levels (correlation coefficient range r = 0.292–0.441). Together, these findings suggest that resected NSCLCs with high glycolytic gene expression and elevated18F-FDG retention are characterized by enhanced expression of PD-L1.
Recently, we demonstrated that increased tumor glycolytic metabolism impairs T cell trafficking and effector function in NSCLC and melanoma tumors [10]. To evaluate whether noninvasive assessment of tumor glycolysis via 18F-FDG PET could provide a readout of reduced intratumoral immune cell infiltration in resected NSCLCs, we analyzed the correlations between 18F-FDG PET parameters and immune cell populations quantified by IHC in resected tumors. We found that increased 18F-FDG retention, as determined by each PET parameter, was associated with reduced tumor infiltration by CD57+ immune cells (SUVMax, r = − 0.398, P = 0.002; Fig. 4f; SUVMean, r = − 0.336, P = 0.009; Supplementary Fig. 2a; SUVTotal, r = − 0.472, P < 0.001; Supplementary Fig. 2b; TLG, r = 0.459, P < 0.001; Supplementary Fig. 2c). We further noted inverse correlations between CD4+ immune cell densities and SUVTotal (r = − 0.289, P = 0.026) and TLG (r = − 0.295, P = 0.023). Although we did not find clear correlations between 18F-FDG retention and intratumoral infiltration of other immune cells (including CD8+ and FOXP3+ cells), NSCLC tumor samples with elevated glycolytic gene expression and SUVMax had increased expression of transcriptomic signatures suggestive of T cell exhaustion (r = 0.401, P = 0.002, Fig. 4g; r = 0.359, P = 0.005, Fig. 4h; r = 0.302, P = 0.020, Supplementary Fig. 3) [28, 29]. Considered in the context of work revealing a relationship between tumor glycolysis and T cell and natural killer cell hyporesponsiveness [6, 10, 40], the findings from this cohort suggest that highly glycolytic resected NSCLCs are characterized by features indicative of a poorly infiltrated and immunosuppressive phenotype and that noninvasive measurement of tumor 18F-FDG retention according to SUVMax may serve as a potential clinical indicator of these characteristics (Supplementary Fig. 4).
Tumor immunometabolic phenotypes are associated with overall survival in patients with resected NSCLC
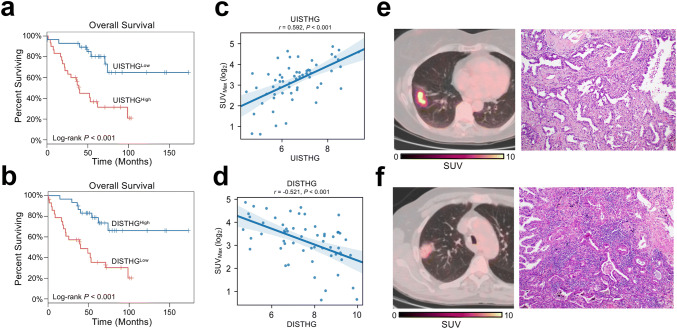
Because pretreatment 18F-FDG retention has been identified as an adverse prognostic factor in NSCLCs and other solid tumors [11], we evaluated whether local tumor glycolysis-related changes are associated with prognosis in NSCLC patients and whether 18F-FDG retention could represent a clinical indicator of outcome in our dataset. By analyzing dysregulated genes that were associated with tumor bulk glycolytic gene expression, we identified a gene signature that was upregulated in highly glycolytic tumors (Upregulated Immune Signature of Tumors with High Glycolysis [UISTHG]: BIRC5, F12, IL1A, PBK, TTK) and a gene signature that was downregulated in NSCLC tumors with high glycolytic gene expression (Downregulated Immune Signature of Tumors with High Glycolysis [DISTHG]: C4BPA, C5, DPP4, PLA2G1B).We tested whether these glycolysis-associated immune gene signatures retained prognostic significance after adjusting for tumor histology, stage, and receipt of neoadjuvant and/or adjuvant platinum-based chemotherapy in the PROSPECT cohort. That analysis demonstrated independent associations between increased (> median) UISTHG (adjusted HR 2.22, 95% CI 1.19–4.15, P = 0.013, Fig. 5a) and DISTHG (adjusted HR 0.36, 95% CI 0.20–0.64, P < 0.001, Fig. 5b) with postoperative OS, as well as with postoperative DFS (UISTHG: adjusted HR 1.61, 95% CI 0.98–2.65, P = 0.063; DISTHG: adjusted HR 0.68, 95% CI 0.47–0.97, P = 0.033). We next examined whether assessment of 18F-FDG uptake according to SUVMax identified tumor expression of these signatures. We identified a strong positive correlation between SUVMax and UISTHG (Fig. 5c) and an inverse association between SUVMax and DISTHG (Fig. 5d). To provide representative clinical examples of the prognostic significance of 18F-FDG retention as quantified by SUVMax in the PROSPECT cohort, we identified two treatment-naïve patients who underwent complete resection of clinical stage I lung adenocarcinoma (Fig. 5e, f; both resected tumors were pathologic stage I). One patient had a highly hypermetabolic tumor and poor survival (SUVMax, 8.41, Fig. 5e; OS duration, 22 months), whereas the other one had a tumor with low 18F-FDG retention and longer survival following tumor resection (SUVMax, 3.26, Fig. 5f; OS duration, 48 months). Histopathologic examination of the resected tumor samples demonstrated that the immune infiltrate of hypermetabolic tumor (Fig. 5e) was much less than that of the tumor with low 18F-FDG retention (Fig. 5f), further illustrating the association between tumor 18F-FDG retention and tumor immune phenotypes.
Fig. 5.
Preoperative SUVMax correlates with tumor glycolysis-associated immune transcriptomic signatures that are prognostic in NSCLC patients. a Reduced overall survival (OS) among NSCLC patients with elevated (> median) overall expression of BIRC5, F12, IL1A, PBK, TTK (Upregulated Immune Signature of Tumors with High Glycolysis, UISTHG). b Improved postoperative OS among NSCLC patients with elevated (> median) overall expression of C4BPA, C5, DPP4, PLA2G1B (Downregulated Immune Signature of Tumors with High Glycolysis, DISTHG). P values were calculated according to the log-rank test. c Pairwise correlation between preoperative SUVMax and the overall expression level of UISTHG. d Inverse association between preoperative SUVMax and the overall expression level of DISTHG. Pearson coefficients for pairwise correlations between log2-transformed values was listed. e Representative axial 18F-FDG PET/CT image (left panel) and hematoxylin and eosin staining image of tumor sample (right panel) of a patient with a hypermetabolic (SUVMax, 8.41) adenocarcinoma of the right lower lobe (clinical and pathologic stage I; postoperative OS duration of 22 months). f Representative axial 18F-FDG PET/CT image (left panel) and hematoxylin and eosin staining image of tumor sample (right panel) of a patient with hypometabolic (SUVMax, 3.26) adenocarcinoma of the right upper lobe (clinical and pathologic stage I; OS duration of 48 months). Color scale bars indicate intensity of 18F-FDG uptake per voxel according to SUV. BIRC5, baculoviral IAP repeat containing 5; C4BPA, complement component 4 binding protein alpha; C5, complement C5; DPP4, dipeptidyl peptidase 4; F12, coagulation factor XII; IL1A, interleukin 1 alpha; PBK, PDZ binding kinase; PLA2G1B, phospholipase A1 group IB; TTK, TTK protein kinase
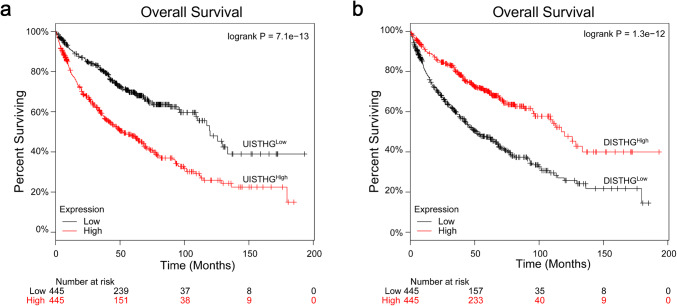
Finally, to validate the prognostic significance of these two gene sets (UISTHG, DISTHG) in an independent cohort, we examined publicly available transcriptomic data (compiled from GEO, TCGA, and caArray datasets) [32], which indicated poor prognosis in patients with elevated (> median) UISTHG (Fig. 6a; adjusted HR 1.66, 95% CI 1.30–2.12, P < 0.001) and improved prognosis in patients with elevated (> median) DISTHG signatures (Fig. 6b; adjusted HR 0.60, 95% CI 0.46–0.76, P < 0.001). Taken together, the results of these analyses support further examinations of the use of preoperative SUVMax as a potential pretreatment means of prognostication and suggest that increased overall tumor glycolytic metabolism, measured according to tumor 18F-FDG uptake by SUVMax, correlates with poor immune cell infiltration and elevated expression of immunosuppressive markers that portended poor prognoses in patients with NSCLC.
Fig. 6.
Prognostic significance of tumor glycolysis-associated immune signatures in NSCLC patients (n = 890) according to analysis of publicly available transcriptomic data. a Reduced overall survival (OS) among NSCLC patients with elevated (> median) overall expression of UISTHG. b Improved postoperative OS among NSCLC patients with elevated (> median) overall expression of DISTHG. P values were calculated according to the log-rank test
Discussion
In this study, we identified a correlation between 18F-FDG PET parameters and tumor metabolic and immune phenotypes in patients with resectable NSCLC by retrospectively analyzing tumor samples and preoperative PET/CT imaging of patients undergoing surgical resection of their tumors. We identified significant associations of 18F-FDG PET parameters with tumor expression of a panel of glycolytic genes previously shown to be associated with a poorly immune-infiltrated tumor phenotype [10]. We also found that increased 18F-FDG PET retention, as quantified by SUVMax, correlated with features associated with tumor progression and immunosuppression. When we stratified patients according to the immunometabolic phenotypes of their tumors, we were able to identify patient subgroups with poorer postoperative survival. Collectively, our results advocate for additional evaluation of PET parameters as potential noninvasive clinical indicators of a highly glycolytic tumor metabolism and an immunosuppressive tumor microenvironment.
Over the past several years, it has become increasingly apparent that metabolic dysregulation is a key driver of oncogenesis and immunoediting [10, 41]. Some tumors may favor glycolysis as a means to meet their metabolic demands despite the presence of adequate oxygen content within the tumor microenvironment [42]. Thus, enhanced glycolytic flux within tumor cells may represent an appealing therapeutic target, and identification of a noninvasive method of assessing the tumor metabolic state remains clinically relevant. Not surprisingly, a variety of imaging modalities using radiolabeled probes to target different metabolic pathways are being explored. However, current technical limitations prohibit ready implementation of these experimental modalities in the clinic, and 18F-FDG PET remains the most widely utilized metabolic imaging modality in clinical practice.
Tumor uptake and intracellular sequestration of 18F-FDG are dependent on transmembrane facilitated diffusion, which is mediated by the SLC2A1 family of transporters and subsequent phosphorylation by hexokinase [43]. Accordingly, much investigative effort has focused on analyzing the relationships between in vivo and in vitro measures of 18F-FDG retention and expression of these proteins in solid tumors [44–47]. However, the relationships between 18F-FDG PET parameters and tumor bulk expression of other glycolysis-related enzymes have not been as fully characterized. Although 18F-FDG PET metabolic parameters identify anatomic areas of enhanced glucose retention and intracellular concentration, whether these parameters can provide information on the actual patterns of intracellular glucose utilization is not well characterized [48, 49]. Here, we identified a positive correlation between increased preoperative 18F-FDG uptake and enhanced tumor bulk glycolysis, as assessed by measuring transcriptome levels of glycolysis-related genes within the tumor tissue, demonstrating that tumors with high 18F-FDG uptake possess elevated expression of genes involved in the glycolytic pathway. Taken in the context of our previous work, which demonstrated that tumor intrinsic glycolysis is associated with the expression of glycolytic genes in melanomas and NSCLCs [10], the results of the present study demonstrate that 18F-FDG PET parameters correlate with tumor glycolysis in resected NSCLC.
By competing for nutrients within the local environment and secreting immunosuppressive metabolites, cancer cells can effectively diminish immune cell trafficking, stimulation, and cytotoxic activity [5–7, 10, 40, 50]. Investigators have demonstrated that tumor infiltration by effector immune cells has prognostic significance in patients with resectable NSCLC [51] and that augmentation of T cell infiltration can be used to overcome resistance to immunotherapies [52]. Consequently, metabolic suppression of the immune response can be expected to have deleterious prognostic effects. Our findings of associations between increased 18F-FDG tumor retention and decreased intratumoral densities of immune cells expressing CD57+ immune cells are consistent with previous reports of inverse correlations between tumor glycolytic metabolism and/or 18F-FDG uptake and tumor infiltration by effector immune cells [14, 40, 53]. Together, our findings indicate that increased 18F-FDG retention may reflect an elevated tumor glycolytic metabolism and an attenuated immune cell-infiltrated tumor microenvironment in patients with resected NSCLC.
Previous mechanistic investigations showed that PD-L1-mediated signaling induces enhanced glycolytic flux in tumors [6]. Therefore, tumor PD-L1 expression contributes to evasion of T cell-mediated adaptive immunity both via direct ligation of PD-1 and promotion of a metabolically unfavorable microenvironment. We found that increased overall tumor bulk glycolysis, expression of individual glycolytic genes, and noninvasive quantification of tumor glycolytic gene expression by SUVMax are all associated with enhanced expression of PD-L1in resected NSCLCs. These findings suggest that 18F-FDG retention may serve as a potential indicator of tumor PD-L1 expression in NSCLC patients; however, whether this association varies across different stages and hystotypes of NSCLC and after different types of therapies remains to be determined. These data are intriguing in their potential application to identify patients for therapies that target the PD-1/PD-L1 axis, as PD-L1 inhibition suppresses tumor glycolytic metabolism [6]. The extent to which response to various immunotherapies, and their mechanisms of action, is a function of glycolysis-driven tumor immunometabolic features requires additional investigation.
Elevated baseline values of PET parameters have been shown to predict a higher risk of recurrence or death in patients with surgical NSCLC [11, 13]. Also, recent studies suggest that 18F-FDG retention early during treatment with immune checkpoint inhibitors (ICIs) or chemoradiation is an independent factor of poor survival and/or response in patients with locoregionally advanced and metastatic NSCLC [54–56]. In this study, we identified SUVMax as the 18F-FDG PET parameter that is most indicative of glycolysis-associated changes in tumor immune phenotypes. Preoperative SUVMax correlated with tumor bulk gene signatures that were independently associated with survival outcomes, supporting the use of SUVMax as a prognostic factor in our cohort. However, the differences in tumor response to cytotoxic therapy and immunotherapy and their effects on the tumor immune microenvironment are incompletely understood and may depend on several factors, such as tumor molecular and histopathologic features, stage, and prior treatment regimens [57]. As immune-based strategies continue to be explored in the perioperative setting for localized NSCLC [4, 58–60] and gain wider clinical use for metastatic disease [3, 61, 62], further investigations will be needed to delineate the ability of 18F-FDG PET parameters to predict outcomes of these diverse disease groups, with respect to tumor stage, histology, genomic aberrations and prior treatments, as well as responses to ICIs in the perioperative setting for NSCLC and other types of cancer [37, 54, 58]. Also, it should be noted that glucose may not be the only or dominant metabolic fuel utilized by NSCLCs. Faubert et al. recently provided direct evidence that tumor cell-autonomous lactate, rather than glucose, uptake is a major contributor to central metabolism in human NSCLCs with high 18F-FDG PET uptake and aggressive oncological behavior [63]. It remains to be determined whether NSCLCs in different stages and with different biological features and PET retention rely on different substrates as major metabolic fuels.
Using the PROSPECT cohort, which has the unique advantages of full clinical annotation, long follow-up, and comprehensive molecular profiling, we confirmed the prognostic relevance of preoperative 18F-FDG retention in surgically resected tumors and identified two prognostic gene expression signatures. However, our analysis has some limitations that include the biases inherent in a retrospective study design and a bulk tumor mass analysis. First, the gene signatures (UISTHG, DISTHG) identified in the present study were derived from bulk transcriptomic analyses and it is unknown whether the expression levels of individual genes are driven by tumor cells or immune cells. It remains to be determined whether cancer cells or immune cells (or both) are the predominant cell type responsible for the metabolic events depicted by FDG retention in resected NSCLC. A more detailed dissection of the contributions of immune cells and cancer cells to the intratumoral metabolic phenotype requires further studies. Second, metabolic reprogramming in NSCLCs is complex and may be heterogeneous across different histologic subtypes, molecular profiles, and within separate regions of individual tumors [64–66]. Therefore, detailed characterization of immunometabolic differences between NSCLC histologic subtypes, disease stages, tumor genomic features, prior therapies, and the impact of these differences on noninvasive assessment of tumor phenotypes according to 18F-FDG uptake requires investigation in larger cohorts [6–8]. Lastly, we previously identified increased tumor glycolytic metabolism to impact the efficacy of adoptive T cell therapy [10]. However, it is challenging to use gene expression levels to assess tumor glycolytic metabolism in the clinical setting. The goal of our study was to identify potential noninvasive approaches to define the immunometabolic phenotype of tumors. This approach can be potentially used to determine the association between tumor glycolytic features and clinical responses to cancer immunotherapy in future studies. Therefore, in the present work, we focused our analyses on indicators of tumor glycolytic phenotypes. An in-depth radiomic assessment of alternative glucose metabolic pathways will be performed as part of future studies.
In summary, our findings establish that elevated 18F-FDG retention is associated with highly glycolytic metabolism, enhanced PD-L1 expression, and an immunosuppressive phenotype in surgically resected NSCLCs. The results of our study suggest that 18F-FDG PET may serve as a noninvasive clinical indicator of tumor metabolic and immune phenotypes in patients with resectable NSCLC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors appreciate the assistance of Mr. Alex Liu with the Division of Information Services, Oncology Care and Research IS at MD Anderson Cancer Center, and Mr. Donald Norwood with the Department of Scientific Publications at MD Anderson Cancer Center for his editorial assistance.
Abbreviations
- 18F-FDG
18F-fluorodeoxyglucose
- CI
Confidence interval
- CT
Computed tomography
- DFS
Disease-free survival
- DISTHG
Downregulated Immune Signature of Tumors with High Glycolysis
- FFPE
Formalin-fixed, paraffin-embedded
- HR
Hazard ratio
- ICI
Immune checkpoint inhibitor
- IHC
Immunohistochemistry
- IQR
Interquartile range
- NSCLC
Non-small cell lung cancer
- OS
Overall survival
- PET
Positron emission tomography
- PROSPECT
Profiling of Resistance Patterns and Oncogenic Signaling Pathways in Evaluation of Cancers of the Thorax
- SUV
Standardized uptake value
- TAIC
Tumor-associated immune cell
- TLG
Total lesion glycolysis
- UISTHG
Upregulated Immune Signature of Tumors with High Glycolysis
- VOI
Volume of interest
Author contributions
WP and TC contributed to conception and design. TC, WP, BA, and ERP contributed to development of methodology. TC, WP, BA, KGM, HK, ERP, PV, CB, AR, YW, JW, AW, CAM, and JF contributed to data acquisition. TC, WP, KGM, BA, ERP, PV, CB, AR, YW, AW, JJL, and DPW contributed to analysis and interpretation of data. KGM, BA, BS, MCBG, ERP, PV, HK, CB, AR, AAV, WLH, SGS, WNW, DLG, IIW, PH, JVH, BWC, YW, JW, JJL, DPW, AW, CAM, JF, GLW, DPW, WP, and TC wrote, reviewed, and revised the manuscript. TC, WP, JVH, and IIW contributed to administrative, technical, or material support. WP and TC contributed to study supervision.
Funding
This work was supported in part by the National Cancer Institute (5 P50 CA070907; P50 CA221703; R01 CA187076; R01 CA184845, P30CA01667), the Cancer Prevention and Research Institute of Texas (RP170401), the American Society of Clinical Oncology 2018 Career Development Award (12895), the Department of Defense (W81XWH-07-1-0306), and the Bob Mayberry Foundation. This work was also supported in part by the Bruton Endowed Chair in Tumor Biology Funds, and the generous philanthropic contributions to the University of Texas MD Anderson Cancer Center Lung Cancer Moon Shot Program, the Khalifa Scholars Program (from Khalifa Bin Zayed Al Nahyan Foundation), the Advanced Scholar Program (from CG Johnson Foundation), and the Physician Scientist Program (from T.J. Martell Foundation).
Compliance with ethical standards
Conflict of interest
M.C.B. Godoy has received research funding from Siemens Healthcare. W.N. William has received honoraria/speaker’s fees and/or participated in advisory boards from Roche/Genentech, Bristol-Myers Squibb, Eli Lilly, Merck, AstraZeneca, and Pfizer. D.L. Gibbons has received research funding from AstraZeneca, Janssen, and Takeda and has participated in advisory boards for AstraZeneca and Sanofi. P. Hwu is a consultant and/or has participated in advisory boards for Immatics, Dragonfly, Sanofi, and GlaxoSmithKline. S.G. Swisher has participated in advisory committees for Ethicon and for the Peter MacCallum Cancer Center. B.S. receives consulting fees from Bristol-Myers Squibb. J.V. Heymach has received research support from AstraZeneca, Bayer, GlaxoSmithKline, and Spectrum; participated in advisory committees for AstraZeneca, Boehringer Ingelheim, Exelixis, Genentech, GlaxoSmithKline, Guardant Health, Hengrui, Lilly, Novartis, Specrtum, EMD Serono, and Synta; and received royalties and/or licensing fees from Spectrum. P. Hwu and W. Peng have received research funding in the form of grants to MD Anderson Cancer Center from GlaxoSmith Kline. T. Cascone has received speaker’s fees from the Society for Immunotherapy of Cancer and Bristol-Myers Squibb; receives consulting/advisory role fees from MedImmune, Bristol-Myers Squibb and EMD Serono, and research funding to MD Anderson Cancer Center from Boehringer Ingelheim, MedImmune and Bristol-Myers Squibb. No potential conflicts of interest are disclosed by the other authors.
Ethical approval
The study was approved by The University of Texas MD Anderson Cancer Center's Institutional Review Board (PROSPECT—LAB07-0233).
Human and animal rights
All human studies were conducted in accordance with the Declaration of Helsinki.
Informed consent
Informed consent was obtained from all study participants.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Weiyi Peng, Email: wpeng2@central.uh.edu.
Tina Cascone, Email: tcascone@mdanderson.org.
References
- 1.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 4.Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378:1976–1986. doi: 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renner K, Singer K, Koehl GE, Geissler EK, Peter K, Siska PJ, Kreutz M. Metabolic hallmarks of tumor and immune cells in the tumor microenvironment. Front Immunol. 2017 doi: 10.3389/fimmu.2017.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang C-H, Qiu J, O’Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer K, Hoffmann P, Voelkl S, et al. Inhibitory effect of tumor cell–derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 8.Estrella V, Chen T, Lloyd M, et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013;73:1524–1535. doi: 10.1158/0008-5472.can-12-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfør K, Rofstad EK, Mueller-Klieser W. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000;60:916–921. [PubMed] [Google Scholar]
- 10.Cascone T, McKenzie JA, Mbofung RM, et al. Increased tumor glycolysis characterizes immune resistance to adoptive T cell therapy. Cell Metab. 2018;27:977–87.e4. doi: 10.1016/j.cmet.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Dong M, Sun X, Li W, Xing L, Yu J. Prognostic value of 18F-FDG PET/CT in surgical non-small cell lung cancer: a meta-analysis. PLoS ONE. 2016;11:e0146195. doi: 10.1371/journal.pone.0146195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pak K, Cheon GJ, Nam HY, Kim SJ, Kang KW, Chung JK, Kim EE, Lee DS. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: A systematic review and meta-analysis. J Nucl Med. 2014;55:884–890. doi: 10.2967/jnumed.113.133801. [DOI] [PubMed] [Google Scholar]
- 13.Im HJ, Pak K, Cheon GJ, Kang KW, Kim SJ, Kim IJ, Chung JK, Kim EE, Lee DS. Prognostic value of volumetric parameters of 18F-FDG PET in non-small-cell lung cancer: a meta-analysis. Eur J Nucl Med Mol Imaging. 2015;42:241–251. doi: 10.1007/s00259-014-2903-7. [DOI] [PubMed] [Google Scholar]
- 14.Kwon HR, Pahk K, Park S, et al. Prognostic value of metabolic information in advanced gastric cancer using preoperative (18)F-FDG PET/CT. Nucl Med Mol Imaging. 2019;53:386–395. doi: 10.1007/s13139-019-00622-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruzzi JF, Munden RF. PET/CT imaging of lung cancer. J Thorac Imaging. 2006;21:123–136. doi: 10.1097/00005382-200605000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Cardnell RJG, Behrens C, Diao L, et al. An integrated molecular analysis of lung adenocarcinomas identifies potential therapeutic targets among TTF1-negative tumors, including DNA repair proteins and Nrf2. Clin Cancer Res. 2015;21:3480–3491. doi: 10.1158/1078-0432.ccr-14-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skoulidis F, Byers LA, Diao L, et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015;5:860–877. doi: 10.1158/2159-8290.cd-14-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang H, Xiao G, Behrens C, et al. A 12-gene set predicts survival benefits from adjuvant chemotherapy in non-small cell lung cancer patients. Clin Cancer Res. 2013;19:1577–1586. doi: 10.1158/1078-0432.ccr-12-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammed N, Kestin LL, Grills IS, Battu M, Fitch DL, C-yO W, Margolis JH, Chmielewski GW, Welsh RJ. Rapid disease progression with delay in treatment of non–small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;79:466–472. doi: 10.1016/j.ijrobp.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 20.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 21.Mhlanga JC, Chirindel A, Lodge MA, Wahl RL, Subramaniam RM. Quantitative PET/CT in clinical practice: assessing the agreement of PET tumor indices using different clinical reading platforms. Nucl Med Commun. 2018;39:154–160. doi: 10.1097/mnm.0000000000000786. [DOI] [PubMed] [Google Scholar]
- 22.Werner-Wasik M, Nelson AD, Choi W, et al. What is the best way to contour lung tumors on PET scans? Multiobserver validation of a gradient-based method using a NSCLC digital PET phantom. Int J Radiat Oncol Biol Phys. 2012;82:1164–1171. doi: 10.1016/j.ijrobp.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilsson MB, Sun H, Diao L, et al. Stress hormones promote EGFR inhibitor resistance in NSCLC: implications for combinations with beta-blockers. Sci Transl Med. 2017 doi: 10.1126/scitranslmed.aao4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cesano A. nCounter® PanCancer immune profiling panel (NanoString Technologies Inc, Seattle, WA) J Immunother Cancer. 2015;3:42. doi: 10.1186/s40425-015-0088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful spproach to multiple testing. J R Stat Soc Ser B (Methodological) 1995;57:289–300. [Google Scholar]
- 26.Law CW, Alhamdoosh M, Su S, Dong X, Tian L, Smyth GK, Ritchie ME. RNA-seq analysis is easy as 1-2-3 with limma, Glimma and edgeR. F1000Res. 2016 doi: 10.12688/f1000research.9005.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krämer A, Green J, Pollard J, Jr, Tugendreich S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics. 2013;30:523–530. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danaher P, Warren S, Lu R, Samayoa J, Sullivan A, Pekker I, Wallden B, Marincola FM, Cesano A. Pan-cancer adaptive immune resistance as defined by the tumor inflammation signature (TIS): results from the cancer genome atlas (TCGA) J Immunother Cancer. 2018;6:63. doi: 10.1186/s40425-018-0367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jerby-Arnon L, Shah P, Cuoco MS, et al. A cancer cell program promotes T cell exclusion and resistance to checkpoint blockade. Cell. 2018;175:984–97.e24. doi: 10.1016/j.cell.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parra ER, Behrens C, Rodriguez-Canales J, et al. Image analysis-based assessment of PD-L1 and tumor-associated immune cells density supports distinct intratumoral microenvironment groups in non-small cell lung carcinoma patients. Clin Cancer Res. 2016;22:6278–6289. doi: 10.1158/1078-0432.ccr-15-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadara H, Choi M, Zhang J, et al. Whole-exome sequencing and immune profiling of early-stage lung adenocarcinoma with fully annotated clinical follow-up. Ann Oncol. 2017;28:75–82. doi: 10.1093/annonc/mdw436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Győrffy B, Surowiak P, Budczies J, Lánczky A. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS ONE. 2013;8:e82241. doi: 10.1371/journal.pone.0082241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. https://www.R-project.org/.
- 34.Chung AS, Wu X, Zhuang G, et al. An interleukin-17–mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med. 2013;19:1114. doi: 10.1038/nm.3291. [DOI] [PubMed] [Google Scholar]
- 35.Charles KA, Kulbe H, Soper R, et al. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. 2009;119:3011–3023. doi: 10.1172/jci39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alfaro C, Teijeira A, Oñate C, et al. Tumor-produced interleukin-8 attracts human myeloid-derived suppressor cells and elicits extrusion of neutrophil extracellular traps (NETs) Clin Cancer Res. 2016;22:3924–3936. doi: 10.1158/1078-0432.Ccr-15-2463. [DOI] [PubMed] [Google Scholar]
- 37.Wellenstein MD, de Visser KE. Cancer-cell-intrinsic mechanisms shaping the tumor immune landscape. Immunity. 2018;48:399–416. doi: 10.1016/j.immuni.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Liu J, Zhang C, Wu R, Lin M, Liang Y, Liu J, Wang X, Yang B, Feng Z. RRAD inhibits the Warburg effect through negative regulation of the NF-KB signaling. Oncotarget. 2015;6:14982–14992. doi: 10.18632/oncotarget.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Nicolay BN, Chick JM, et al. The metabolic function of cyclin D3–CDK6 kinase in cancer cell survival. Nature. 2017;546:426. doi: 10.1038/nature22797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brand A, Singer K, Koehl Gudrun E, et al. LDHA-associated lactic acid productionblunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016;24:657–671. doi: 10.1016/j.cmet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Vander Heiden MG, DeBerardinis RJ. Understanding the intersections between metabolism and cancer biology. Cell. 2017;168:657–669. doi: 10.1016/j.cell.2016.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Timm KN, Kennedy BW, Brindle KM. Imaging tumor metabolism to assess disease progression and treatment response. Clin Cancer Res. 2016;22:5196–5203. doi: 10.1158/1078-0432.ccr-16-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mamede M, Higashi T, Kitaichi M, et al. 18F-FDG Uptake and PCNA, Glut-1, and Hexokinase-II expressions in cancers and inflammatory lesions of the lung. Neoplasia (New York, N. Y.) 2005;7:369–379. doi: 10.1593/neo.04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaira K, Serizawa M, Koh Y, et al. Biological significance of 18F-FDG uptake on PET in patients with non-small-cell lung cancer. Lung Cancer. 2014;83:197–204. doi: 10.1016/j.lungcan.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 46.Zhou X, Chen R, Xie W, Ni Y, Liu J, Huang G. Relationship between 18F-FDG accumulation and lactate dehydrogenase: a expression in lung adenocarcinomas. J Nucl Med. 2014;55:1766–1771. doi: 10.2967/jnumed.114.145490. [DOI] [PubMed] [Google Scholar]
- 47.Goodwin J, Neugent ML, Lee SY, et al. The distinct metabolic phenotype of lung squamous cell carcinoma defines selective vulnerability to glycolytic inhibition. Nat Commun. 2017;8:15503. doi: 10.1038/ncomms15503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 2015;17:351. doi: 10.1038/ncb3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Contractor KB, Aboagye EO. Monitoring predominantly cytostatic treatment response with 18F-FDG PET. J Nucl Med. 2009;50:97S–105S. doi: 10.2967/jnumed.108.057273. [DOI] [PubMed] [Google Scholar]
- 50.Ottensmeier CH, Perry KL, Harden EL, et al. Upregulated glucose metabolism correlates inversely with CD8+ T-cell infiltration and survival in squamous cell carcinoma. Cancer Res. 2016;76:4136–4148. doi: 10.1158/0008-5472.can-15-3121. [DOI] [PubMed] [Google Scholar]
- 51.Soo RA, Chen Z, Yan Teng RS, Tan HL, Iacopetta B, Tai BC, Soong R. Prognostic significance of immune cells in non-small cell lung cancer: meta-analysis. Oncotarget. 2018;9:24801–24820. doi: 10.18632/oncotarget.24835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang H, Wang Y, Chlewicki LK, Zhang Y, Guo J, Liang W, Wang J, Wang X, Fu Y-X. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell. 2016;29:285–296. doi: 10.1016/j.ccell.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lopci E, Toschi L, Grizzi F, et al. Correlation of metabolic information on FDG-PET with tissue expression of immune markers in patients with non-small cell lung cancer (NSCLC) who are candidates for upfront surgery. Eur J Nucl Med Mol Imaging. 2016;43:1954–1961. doi: 10.1007/s00259-016-3425-2. [DOI] [PubMed] [Google Scholar]
- 54.Kaira K, Higuchi T, Naruse I, et al. Metabolic activity by (18)F-FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur J Nucl Med Mol Imaging. 2018;45:56–66. doi: 10.1007/s00259-017-3806-1. [DOI] [PubMed] [Google Scholar]
- 55.Usmanij EA, de Geus-Oei LF, Troost EG, Peters-Bax L, van der Heijden EH, Kaanders JH, Oyen WJ, Schuurbiers OC, Bussink J. 18F-FDG PET early response evaluation of locally advanced non-small cell lung cancer treated with concomitant chemoradiotherapy. J Nucl Med. 2013;54:1528–1534. doi: 10.2967/jnumed.112.116921. [DOI] [PubMed] [Google Scholar]
- 56.Spigel DRCJ, Gettinger S, Chao BH, Dirix L, Schmid P, et al. FIR: Efficacy, safety, and biomarker analysis of a phase II open-label study of atezolizumab in PD-L1-selected patients with NSCLC. J Thorac Oncol. 2018;13:1733–1742. doi: 10.1016/j.jtho.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parra ER, Villalobos P, Behrens C, et al. Effect of neoadjuvant chemotherapy on the immune microenvironment in non-small cell lung carcinomas as determined by multiplex immunofluorescence and image analysis approaches. J Immunother Cancer. 2018;6:48. doi: 10.1186/s40425-018-0368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cascone T, William WN, Weissferdt A et al (2019) Neoadjuvant nivolumab (N) or nivolumab plus ipilimumab (NI) for resectable non-small cell lung cancer (NSCLC): Clinical and correlative results from the NEOSTAR study. J Clin Oncol 37: suppl; abstr 8504. 10.1200/JCO.2019.37.15_suppl.8504
- 59.Kwiatkowski DJ, Rusch VR, Chaft JE et al (2019) Neoadjuvant atezolizumab in resectable non-small cell lung cancer (NSCLC): interim analysis and biomarker data from a multicenter study (LCMC3). J Clin Oncol 37: suppl; abstr 8503. 10.1200/JCO.2019.37.15_suppl.8503
- 60.Provenico M, Nadal E, Insa A et al (2019) Neoadjuvant chemo-immunotherapy for the treatment of stage IIIA resectable non-small cell lung cancer (NSCLC): a phase II multicenter exploratory study-Final data of patients who underwent surgical assessment. J Clin Oncol 37: suppl; abstr 8509. 10.1200/JCO.2019.37.15_suppl.8509
- 61.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 62.Lopes G, Wu Y-L, Kudaba I et al. (2018) Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥ 1%: Open-label, phase 3 KEYNOTE-042 study. J Clin Oncol. 36: LBA4-LBA. 10.1200/JCO.2018.36.18_suppl.LBA4
- 63.Faubert BLK, Cai L, Hensley CT, Kim J, Zacharias LG, et al. Lactate metabolism in human lung tumors. Cell. 2017;171:358–371. doi: 10.1016/j.cell.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Momcilovic M, Jones A, Bailey ST, et al. In vivo imaging of mitochondrial membrane potential in non-small-cell lung cancer. Nature. 2019;575:380–384. doi: 10.1038/s41586-019-1715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hensley Christopher T, Faubert B, Yuan Q, et al. Metabolic heterogeneity in human lung tumors. Cell. 2016;164:681–694. doi: 10.1016/j.cell.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen PH, Cai L, Huffman K, et al. Metabolic diversity in human non-small cell lung cancer cells. Mol Cell. 2019;76:838–51.e5. doi: 10.1016/j.molcel.2019.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.