Abstract
Background:
Severe infections of implant-based breast reconstruction are challenging to treat. Traditional management is removal of the implant with a further attempt at reconstruction months later once the infection has settled. This study evaluates an alternative management protocol using negative pressure wound therapy with instillation (NPWTi).
Methods:
Consecutive patients with severe peri-prosthetic infection following breast reconstruction were managed using the Implant Salvage Protocol: removal of the prosthesis with application of a NPWTi dressing, changed every 3 days until a negative culture was obtained. A new prosthesis was then placed in the pocket. Data were collected on patient demographics, microbiological, hospital/operative information, and overall success of salvage. Descriptive statistics were used for analysis.
Results:
In total, 30 breast prostheses in 28 patients were treated for severe peri-prosthetic infection. Twenty-five (83%) implants were salvaged. Mean time from initial reconstruction surgery to presentation was 49.5 days (median 23, range 7–420). Mean hospital stay was 11.5 days (median 12.0, range 6–22), mean number of returns to the operating theater was 3.7 (median 3.0, range 2–7), and mean number of days to negative culture was 5.2 (median 4.0, range 1–14). The most common organisms were methicillin-sensitive Staphylococcus aureus (n = 9) and Serratia marcescens (n = 4). Most had a tissue expander (n = 24, 80%) or implant (n = 5, 16.7%) placed at the completion of therapy. There was no record of capsular contracture nor recurrent infection during follow-up (mean 39.4 months, range 6–74 months).
Conclusion:
An estimated 83% of prosthetic breast reconstructions with severe infection were successfully salvaged using NPWTi.
INTRODUCTION
Implant loss due to infection is a devastating complication of implant-based breast reconstruction. It is associated with significant psychological and aesthetic morbidity.1–3 Although mild cases of infection readily undergo attempts at salvage, cases of severe peri-prosthetic infection are traditionally considered unsuitable for salvage. In severe infection, the standard approach is to remove the prosthesis, cleanse the pocket, and allow the infection to settle for a number of months before attempting delayed reconstruction. Therefore, implant loss involves not only a protracted process to achieving a breast reconstruction again, but also negative psychological and lifestyle consequences.3
The clinical picture of severe infection includes failure to improve on antibiotics, frank pus drainage, atypical/aggressive organism on culture, and/or systemic infection.4 Procedures described to treat peri-prosthetic infections of this kind include capsule curettage, capsulectomy, pulse lavage, device position change (new pocket), and device exchange.4–14 The limitation of these techniques is that cleaning of the pocket only occurs at the time of surgery, which may not facilitate eradication of biofilm associated with the infection. Also, at the end of the washout process, the surgeon cannot confidently ascertain whether the pocket is clean enough to accept a device without risking recurrent infection.
In 2015, our team described a novel technique called the Implant Salvage Protocol for the treatment of severely infected breast prostheses using negative pressure wound therapy with installation (NPWTi).15 This method facilitates the growth of healthy granulation tissue within the breast pocket due to the negative-pressure component and possible eradication of biofilm due to the continuous irrigation component, as suggested by in vitro studies.16 The dressing is changed at regular intervals in the operating theater, where the surgeon can assess whether the pocket is clean enough for reinsertion of the device.15 NPWTi has been successfully used in other institutions and specialties, particularly for the management of complex wounds such as burns and hardware salvage in orthopedic and spine patients.17–19 Similarly, techniques utilizing NPWTi may play an important role in salvaging the complex clinical problem of severely infected breast prostheses. However, the current evidence is sparse, and more studies are needed to further elucidate its role in breast surgery.15,20
In an initial series of 6 consecutive cases of severe peri-prosthetic infection at our institution, the Implant Salvage Protocol demonstrated a high success rate.15 Based on this initial successful outcome, the protocol continued to be used at Westmead and related private hospitals. Additional experience over 5 years has now been gained. The aim of this study was to examine the outcomes of the Implant Salvage Protocol for the management of severe peri-prosthetic infection following prosthetic breast reconstruction.
METHODS
Consecutive cases of severe peri-prosthetic breast infection following prosthetic breast reconstruction treated at Westmead Hospital and associated private facilities over a 5-year period (2015–2019) were included in a prospectively maintained database. Ethics approval was obtained from the institutional human research ethics committee. (2019/ETH12577).
Severe peri-prosthetic infection was defined as clinical sepsis that persisted despite oral or intravenous antibiotics with or without serial aspirations, frank pus in the implant pocket, and/or gram negative/atypical organisms on culture.9 All cases fulfilling this criteria were managed using the Implant Salvage Protocol. (See appendix, Supplemental Digital Content 1, which displays the implant salvage protocol—outline. http://links.lww.com/PRSGO/B595.)
NPWTi consists of a foam and adhesive dressing system that cycles between the application of sub-atmospheric pressure and the instillation of a topical solution to the wound bed in a pre-programmed manner. The device used in this study was the V.A.C. VeraFlo Therapy (Kinetic Concepts, Inc., San Antonio, Tex.). The foam size is adjustable to maintain the size of the breast pocket. Although normal saline is the usual solution used for instillation, there was flexibility in changing the solution type according to the organism or surgeon/physician preference. The pressure/instillation cycle settings used were: 100–150 mL of topical solution instilled to the breast pocket dependent on breast envelope size; 15 min soaking time; and 3.5 h suction time with −125 mm Hg pressure.15
The antibiotic therapy regimen supporting the Implant Salvage Protocol was determined collaboratively with the Infectious Disease Unit. When microbiological sensitivities were known, antibiotic therapy was rationalized. All patients received intravenous antibiotic therapy during admission and transitioned to at least 3 weeks of oral antibiotics from discharge.
Failure and success of salvage were noted upon completion of the treatment protocol. Failure of salvage was defined by implant loss (and thereby, also loss of the breast envelope) with or without view to delayed reconstruction (autologous or prosthetic). Successful salvage was defined by retention of the breast envelope with restoration of a new breast prosthesis.
Data were collected on patient demographics, initial breast reconstruction surgery, infection information, and NPWTi treatment. Descriptive statistics were used to analyze the data using Microsoft Excel (Microsoft Corp, Redmond, Wa.).
RESULTS
Thirty cases (30 breasts) with severe peri-prosthetic infection following prosthetic breast reconstruction were treated in 28 patients (2 bilateral cases). All patients consented to proceed with the Implant Salvage Protocol. A case example is shown in Figure 1.
Fig. 1.
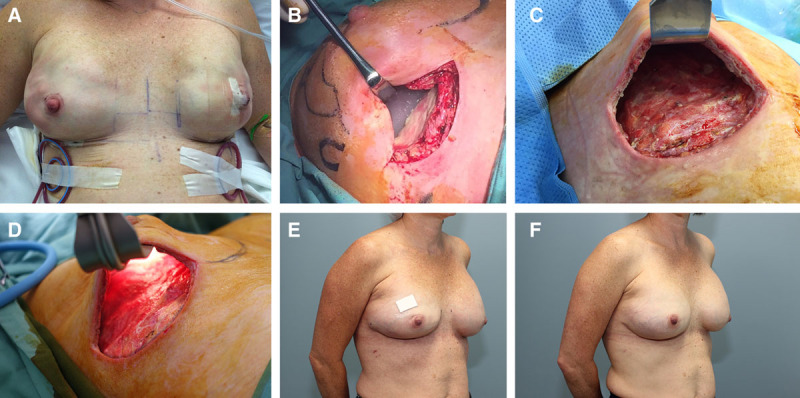
A 43-year-old woman underwent bilateral nipple-sparing mastectomy and direct-to-implant reconstruction with sub-pectoral implants. A, Immediate postoperative result. B, Severe peri-prosthetic infection in the right breast on postoperative day 17 with frank pus in the implant pocket and a culture that grew methicillin-sensitive staphylococcus aureus (MSSA). C, Clean breast pocket after 3 days of negative pressure wound therapy with installation (NPWTi). D, After 7 days of NPWTi, culture was taken, which showed eradication of infection. E, Result following insertion of tissue expander at the completion of NPWTi. F, Final result after exchange of tissue expander for permanent prosthesis.
Patient demographics are listed in Table 1. Notably, the cohort included 1 smoker and 2 cases with previous breast radiotherapy. Most women underwent mastectomy for invasive breast cancer (17/30, 56.7%) followed by risk-reduction (9/30, 30%). Initial breast reconstruction surgery included skin- or nipple-sparing mastectomy. The majority were reconstructed with sub-pectoral prosthesis placement (25/30, 83.3%) and synthetic mesh coverage (17/30, 56.7%). Details with respect to initial breast reconstruction surgery are shown in Table 2.
Table 1.
Patient Demographics (n = 28 Patients; n = 30 Breasts)
| Characteristic | n | % | |
|---|---|---|---|
| Age (y) | Mean | 43.4 | NA |
| Median | 43 | NA | |
| Range | 34–57 | NA | |
| Smoking status | Current smoker | 1 | 3.6 |
| Previous smoker | 4 | 14.3 | |
| Never smoker | 20 | 71.4 | |
| Unknown* | 3 | 10.7 | |
| Diabetes | Yes | 0 | 0 |
| No | 30 | 100 | |
| Connective tissue disease | Yes | 2 | 7.1 |
| No | 26 | 92.9 | |
| Previous radiotherapy | Yes | 2 | 6.6 |
| No | 28 | 93.3 | |
*Not retrieved from review of medical record.
Table 2.
Initial Breast Reconstruction Surgery (n = 30 Breasts)
| Characteristic | n | % | |
|---|---|---|---|
| Indication for mastectomy | Invasive cancer | 17 | 56.7 |
| DCIS | 4 | 13.3 | |
| Risk-reducing | 9 | 30 | |
| Timing of reconstruction | Immediate | 30 | 100 |
| Delayed | 0 | 0 | |
| Initial prosthesis type | Tissue expander | 16 | 53.3 |
| Direct to implant | 14 | 46.7 | |
| Placement of prosthesis | Sub-pectoral | 25 | 83.3 |
| Pre-pectoral | 5 | 16.7 | |
| Coverage of implant | Synthetic* | 17 | 56.7 |
| Biologic† | 6 | 20.0 | |
| Autologous‡ | 5 | 16.7 | |
| None | 2 | 6.7 | |
| Initial prosthesis size (g/mL) | Mean | 350 | NA |
| Median | 335 | NA | |
| Range | 100–650 | NA | |
| Unknown§ | 4 | 13.3 |
*Synthetic mesh included TiLOOP Bra, TiLOOP Bra Pocket, SERI Surgical Scaffold, and TIGR Matrix.
†Biologic mesh included the following Acellular Dermal Matrices—Veritas, Strattice, FlexHD, and Biodesign.
‡Autologous included lipodermal flap.
§Not retrieved from review of medical record.
Most cases had an implant in situ at the time of infection (17/30, 56.7%); the remainder had a tissue expander (13/30, 43.3%). Features at presentation with infection are shown in Table 3. Mean time from initial reconstruction surgery to presentation with severe peri-prosthetic infection was 49.5 days (median 23, range 7–420). An organism was found on culture of peri-prosthetic fluid or swabs of the pocket in 26/30 cases (86.7%); 1 case had no growth, and results were unknown for 3 cases. The most common organism was methicillin-sensitive Staphylococcus aureus (MSSA, n = 9). Other organisms included Serratia marcescens (n = 4), methicillin-resistant Staphylococcus aureus (n = 2) and Pseudomonas aeurginosa (n = 2).
Table 3.
Features at Presentation with Infection (n = 30 Breasts)
| Characteristic | n | % | |
|---|---|---|---|
| Breasts affected | Unilateral | 25 | 83.3 |
| Bilateral | 5 | 16.7 | |
| Days from reconstruction to infection | Mean | 49.5 | NA |
| Median | 23 | NA | |
| Range | 7–420 | NA | |
| White cell count at presentation | Mean | 9.0 | NA |
| Median | 7.9 | NA | |
| Range | 1.8–24.7 | NA | |
| Not requested | 3 | 10 | |
| C-Reactive protein at presentation (mg/L) | Mean | 75.0 | NA |
| Median | 37.0 | NA | |
| Range | 2–380 | NA | |
| Not requested | 2 | 6.7 | |
| Organisms identified on culture | MSSA* | 9 | 30.0 |
| Serratia marcescens | 4 | 13.3 | |
| MRSA† | 2 | 6.7 | |
| Pseudomonas aeurginosa | 2 | 6.7 | |
| Multiple organisms | 3 | 10.0 | |
| Other organisms± | 6 | 20.0 | |
| No growth | 1 | 3.3 | |
| Unknown§ | 3 | 10.0 | |
*methicillin-sensitive Staphylococcus aureus.
†methicillin-resistant Staphylococcus aureus.
±Brevibacterium casei, Corynebacterium imitans, Enterococcus faecalis, Staphylococcus haemolyticus, Klebsiella oxytoca, and Proteus mirabilis.
§Not retrieved from review of medical record.
Treatment information is shown in Table 4. Normal Saline was the most common solution used for instillation of the breast pocket (n = 11). Other solutions included 1% acetic acid (n = 4) and Prontosan (n = 2). The choice of solution used was also determined by microbiology, most notably, for all cases of Pseudomonas, acetic acid solution was chosen. The mean length of hospital stay was 11.5 days (median 12.0, range 6–22). The mean number of returns to theater was 3.7 (median 3.0, range 2–7). The mean number of days to a negative culture was 5.2 days (median 4.0, range 1–14).
Table 4.
Treatment Information, Negative Pressure Wound Therapy with Instillation (n = 30 Breasts)
| Characteristic | n | % | |
|---|---|---|---|
| No. operations | Mean | 3.7 | NA |
| Median | 3 | NA | |
| Range | 2–7 | NA | |
| Days to negative culture | Mean | 5.2 | NA |
| Median | 4 | NA | |
| Range | 1–14 | NA | |
| Unknown* | 4 | 13.3 | |
| Length of hospital stay (d) | Mean | 11.5 | NA |
| Median | 12 | NA | |
| Range | 6–22 | NA | |
| Unknown* | 3 | 10.0 | |
| Irrigation solution | Normal saline | 11 | 36.7 |
| Acetic acid 1% | 4 | 13.3 | |
| Prontosan | 2 | 6.7 | |
| Unknown* | 13 | 43.3 | |
| Prosthesis type in situ at presentation | Implant | 17 | 56.7 |
| Tissue expander | 13 | 43.3 | |
| Prosthesis type inserted at completion of NPWTi treatment | Implant | 5 | 16.7 |
| Tissue expander | 24 | 80.0 | |
| Success of implant salvage | Successful | 25 | 83.0 |
| Not successful | 5 | 17.0 | |
*Not retrieved from review of medical record.
At the completion of treatment with NPWTi, a prosthesis was re-implanted in 29/30 cases (tissue expander in 24 and implant in 5 cases). The tissue expander was exchanged to an implant in 21 cases during follow-up. Of the total 26 cases with restoration of implant, only 1 case failed salvage. The 25 successfully salvaged cases were followed up for an average of 39.4 months (range: 6–74 months). There was no record of capsular contracture nor recurrent infection during this follow-up period.
Overall, the salvage procedure was successful in 25/30 cases (83%). Details of the 5 failed cases were:
Case 1: Protocol abandoned due to concern about delay to adjuvant chemotherapy. The patient had a negative culture, but did not show evidence of healthy granulation tissue of the pocket. There was history of previous breast radiotherapy.
Case 2: Chronic seroma of the prosthesis pocket. A drain was not inserted during reinsertion of prosthesis, which may have contributed to failure of salvage.
Case 3: Device was removed. The reason for removal, however, was not clear because the patient was lost to standard follow-up.
Cases 4 and 5: Both had a skin defect (remote to the incision site) due to flap ischemia or diathermy burn. Despite good granulation tissue within the pocket, the additional area of skin defect did not heal.
DISCUSSION
This study demonstrates an 83% success rate (25/30 cases) over 5 years in salvaging severely infected prosthesis after breast reconstruction using the Implant Salvage Protocol. The high success-rate observed in this study is consistent with the reported experience of NPWTi in other complex wound settings, such as necrotizing fasciitis, chronic ulcers, burns, and infected hardware in orthopedics or spine patients.17–19,21–23 Similarly, NPWTi has been utilized with success in the complex setting of peri-prosthetic infection after breast reconstruction, but has been limited to case reports and small series.15,20 This is the first study to report outcomes over a 5-year period involving 30 cases of severe peri-prosthetic infection.
With most of the existing literature demonstrating variable salvage rates with different techniques for treatment of severe peri-prosthetic infection, the results of this study are encouraging and suggest that the previously considered “unsalvageable” situation of severe peri-prosthetic infection may be rescued. The definition of severe peri-prosthetic infection used in this study has previously been reported as a contra-indication to salvage attempt due to high risk of failure.4–7,12 The conventional wisdom has been that a severely infected breast pocket cannot be adequately sterilized without removal of the implant for an extended period of time. The standard approach recommended for severe infection is removal of the breast prosthesis, washout of the pocket, and review over a number of months awaiting the infection to settle completely before any further attempt at reconstruction.4,24,25
The disadvantage of this approach is the psychological and aesthetic morbidity experienced by patients with implant loss.2–4,26 Furthermore, the majority of patients never return for delayed reconstruction.12,27 If a decision is made for delayed reconstruction, the final aesthetic outcome may be sub-optimal due to the difficulty of lifting the collapsed skin and subcutaneous tissue off a fibrotic chest wall to create a suitable breast pocket.6 These patients usually require a 2-stage reconstruction using a tissue expander to stretch the skin slowly before insertion of a permanent prosthesis or require autologous reconstruction. These options are significantly more protracted compared with direct-to-implant reconstruction. The Implant Salvage Protocol retains the breast envelope during therapy and aims to restore a new prosthesis upon completion of therapy, thereby avoiding protracted implant loss.
Some other techniques described to salvage peri-prosthetic infection include capsule curettage, capsulectomy, pulse lavage, device position change, and/or device exchange.4,5,7–12 These methods also aim to preserve the breast pocket. However, there may be additional advantages to utilizing NPWTi. These include objective assessment regarding eradication of organism/s from the breast pocket, and early evidence suggesting a reduction in biofilm. In vitro studies have suggested that the features of NPWTi, including sub-atmospheric pressure and fluid instillation, assist in the reduction of biofilm and bacterial load.16,28–32 Given that the fluid instillation component of NPWTi allows for flexibility in the type of solution used, future studies could evaluate the effects on bacterial load and biofilm according to different solution types.
The objective assessment of the breast pocket is the determining feature of when to insert a new prosthesis in this treatment protocol. At the time of dressing exchange, a minimum of 2 fluid and tissue samples from different areas of the breast pocket are sent for formal MCS. Intraoperative gram-stain and/or FISH is not utilized in this protocol. At this time, the surgeon also notes the quality of tissue of the breast pocket. If both a negative culture and healthy pink granulation tissue are present, the pocket is ready for insertion of a new prosthesis. Therefore, the duration of the treatment protocol is determined by fulfillment of these 2 criteria. The longest duration to achieving negative culture and healthy granulation in this series was 22 days, involving 1 case. This was one of the first cases salvaged. Pseudomonas was grown on culture in this case, but 1% acetic acid solution was not used until the third dressing change. Therefore, modifications to the solution type for instillation (different solutions may clear organisms at different rates) may reduce the duration of therapy.
Although aesthetic outcome was not formally assessed in this series, there was no record of capsular contracture during follow-up (range: 6–74 months). This has also been observed in other case reports utilizing NPWT/NPWTi.13–15,20 Despite negative pressure applied to the breast pocket, the increased local tissue perfusion known to occur with this therapy may contribute toward a healthier breast envelope possibly preserving cosmetic outcome.28 Nevertheless, long-term results over 10 years utilizing validated tools will be needed to confirm the rate of capsular contracture and aesthetic outcome.
One of the potential criticisms of this treatment protocol is that it requires the patient to have multiple planned returns to the operating theater under general anesthetic and in-patient hospital stay to complete therapy. In all cases, there were no adverse events related to planned return to theater and patients were fully compliant with their treatment. It is possible that the rigorous nature of this protocol is one of the reasons for the high success rate observed. Completing dressing changes under general anesthetic allows for complete debridement of the breast pocket with thorough washout and representative intraoperative sampling for tissue and/or fluid cultures. Hospital stay throughout the duration of therapy also allows for immediate rationalization of intravenous antibiotic therapy and therapeutic solution to the wound bed. In the setting of severe peri-prosthetic infection, these elements of therapy may be important in achieving successful salvage. There is potential to modify the protocol to completion of dressing changes under sedation, and if the patient is not systemically unwell, intravenous antibiotic therapy could be administered at home with return to hospital for dressing changes.
The protocol is not prescriptive with respect to antibiotic therapy. Because patients do not present with the same organisms on culture nor degree of systemic toxicity, the antibiotic therapeutic regimen that supports the protocol is individualized in collaboration with the Infectious Disease Unit. The transition from intravenous therapy to oral therapy was not protocoled. All patients, however, received 3 weeks of oral antibiotics from discharge. Future studies could focus on investigating the decision-making process around antibiotic regimens both during and upon completion of the protocol.
Our experience with this treatment protocol has highlighted some other considerations. Firstly, NPWTi was unable to salvage 1 of the 2 cases of previous radiotherapy. Unfortunately, in breasts affected with previous radiotherapy, development of healthy granulation tissue can be very slow. Secondly, the treatment protocol was also aborted in this case due to the need to proceed with adjuvant therapy for breast cancer treatment. Hence, the decision for salvaging the infected prosthesis must be discussed within the multidisciplinary team. With a move toward neoadjuvant chemotherapy, the concern about delay to adjuvant treatment may become less problematic in the future. Finally, to achieve a good seal of the NPWTi dressing, the breast envelope must be intact and therefore, the NPWTi dressing is not suitable in situations involving skin flap/nipple necrosis that cannot be debrided and re-approximated at the time of initial application of NPWTi.
This study has several limitations. Firstly, as a case series, the strength of evidence may be limited. A recent study of 1293 patients with prosthetic breast reconstruction reported a rate of 8% for any infection.34 Severe peri-prosthetic infection represents a smaller proportion of these patients. Therefore, although a case series by design has inherent limitations, reporting the outcomes of 30 cases (28 patients) of severe infection may possibly be considered a clinically significant representation of experience in treating a challenging and uncommon clinical scenario.
Secondly, a cost–benefit analysis has not been conducted. Potential modifications to the protocol that may reduce actual costs include the administration of intravenous antibiotics at home in an ambulatory setting to avoid inpatient-stay for the systemically well patient and the completion of NPWTi dressing changes under sedation to avoid general anesthesia and further reduce operating theater utilization. The cost-effectiveness of this treatment protocol requires a study into the actual costs of treatment and its impact on value with respect to health outcomes.35 Implant loss has its own costs in terms of both actual costs (associated with delayed reconstruction) and negative long-term psychological/lifestyle impacts.3 Ultimately, the adoption of this technique will be determined not only by cost-effectiveness, but also patient acceptance. All patients in this study demonstrated acceptance of the treatment protocol—there were no cases of aborted treatment due to patient preference, nor did any patient choose implant loss over attempted salvage.
In summary, this study demonstrates an observed high salvage rate and highlights the contributory role of NPWTi within a defined treatment protocol for the salvage of severe peri-prosthetic infection following breast reconstruction.
Supplementary Material
Footnotes
Published online 26 March 2021
Presented at ORBS Meeting 2019, Nottingham, UK; CoBrCa Meeting 2019, San Francisco, Calif.; and World Congress of Surgery 2019, Krakow, Poland.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. No funding was received for this work.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Colakoglu S, Khansa I, Curtis MS, et al. Impact of complications on patient satisfaction in breast reconstruction. Plast Reconstr Surg. 2011; 127:1428–1436 [DOI] [PubMed] [Google Scholar]
- 2.Gopie JP, Timman R, Hilhorst MT, et al. The short-term psychological impact of complications after breast reconstruction. Psychooncology. 2013; 22:290–298 [DOI] [PubMed] [Google Scholar]
- 3.Mahoney B, Walklet E, Bradley E, et al. Experiences of implant loss after immediate implant-based breast reconstruction: qualitative study. BJS Open. 2020; 4:380–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spear SL, Howard MA, Boehmler JH, et al. The infected or exposed breast implant: management and treatment strategies. Plast Reconstr Surg. 2004; 113:1634–1644 [DOI] [PubMed] [Google Scholar]
- 5.Chun JK, Schulman MR. The infected breast prosthesis after mastectomy reconstruction: successful salvage of nine implants in eight consecutive patients. Plast Reconstr Surg. 2007; 120:581–589 [DOI] [PubMed] [Google Scholar]
- 6.Reish RG, Damjanovic B, Austen WG, Jr, et al. Infection following implant-based reconstruction in 1952 consecutive breast reconstructions: salvage rates and predictors of success. Plast Reconstr Surg. 2013; 131:1223–1230 [DOI] [PubMed] [Google Scholar]
- 7.Prince MD, Suber JS, Aya-Ay ML, et al. Prosthesis salvage in breast reconstruction patients with periprosthetic infection and exposure. Plast Reconstr Surg. 2012; 129:42–48 [DOI] [PubMed] [Google Scholar]
- 8.Sforza M, Andjelkov K, Zaccheddu R. A successful salvage protocol for breast implants. Plast Reconstr Surg. 2011; 128:33e–34e [DOI] [PubMed] [Google Scholar]
- 9.Spear SL, Seruya M. Management of the infected or exposed breast prosthesis: a single surgeon’s 15-year experience with 69 patients. Plast Reconstr Surg. 2010; 125:1074–1084 [DOI] [PubMed] [Google Scholar]
- 10.Lapid O. Use of gentamicin collagen sponges for the treatment of periprosthetic breast implant infection. J Plast Reconstr Aesthet Surg. 2011; 64:e313–e316 [DOI] [PubMed] [Google Scholar]
- 11.Yii NW, Khoo CT. Salvage of infected expander prostheses in breast reconstruction. Plast Reconstr Surg. 2003; 111:1087–1092 [DOI] [PubMed] [Google Scholar]
- 12.Bennett SP, Fitoussi AD, Berry MG, et al. Management of exposed, infected implant-based breast reconstruction and strategies for salvage. J Plast Reconstr Aesthet Surg. 2011; 64:1270–1277 [DOI] [PubMed] [Google Scholar]
- 13.Kendrick AS, Chase CW. Salvage of an infected breast tissue expander with an implant sizer and negative pressure wound management. Plast Reconstr Surg. 2008; 121:138e–139e [DOI] [PubMed] [Google Scholar]
- 14.Liao EC, Breuing KH. Breast mound salvage using vacuum-assisted closure device as bridge to reconstruction with inferolateral AlloDerm hammock. Ann Plast Surg. 2007; 59:218–224 [DOI] [PubMed] [Google Scholar]
- 15.Meybodi F, Sedaghat N, French J, et al. Implant salvage in breast reconstruction with severe peri-prosthetic infection. ANZ J Surg. 2017; 87:E293–E299 [DOI] [PubMed] [Google Scholar]
- 16.Tahir S, Malne M, Hu H, et al. The effect of negative pressure wound therapy with and without instillation on mature biofilms in vitro. Materials (Basel). 2018; 11:811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blome-Eberwein S, Lozano D, Amani H. Utility of negative pressure wound therapy with instillation in a burn center. Burns Open. 2018; 2:208–212 [Google Scholar]
- 18.West JM, Jordan SW, Mendel E, et al. Instillation negative pressure wound therapy: an effective tool for complex spine wounds. Adv Wound Care (New Rochelle). 2018; 7:333–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koster G. Management of early periprosthetic infections in the knee using the vacuum instillation therapy. Infection. 2009; 37:18–20 [Google Scholar]
- 20.Cheong JY, Goltsman D, Warrier S. A new method of salvaging breast reconstruction after breast implant using negative pressure wound therapy and instillation. Aesthetic Plast Surg. 2016; 40:745–748 [DOI] [PubMed] [Google Scholar]
- 21.Gabriel A, Shores J, Heinrich C, et al. Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds. Int Wound J. 2008; 5:399–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schintler MV, Prandl EC, Kreuzwirt G, et al. The impact of V.A.C. instill in severe soft tissue infections and necrotizing fasciitis. Infection. 2009; 37:31–42 [Google Scholar]
- 23.Kim PJ, Attinger CE, Steinberg JS, et al. The impact of negative-pressure wound therapy with instillation compared with standard negative-pressure wound therapy: a retrospective, historical, cohort, controlled study. Plast Reconstr Surg. 2014; 133:709–716 [DOI] [PubMed] [Google Scholar]
- 24.Horch RE, Schultz G, Schubert DW, et al. Infectious complications in implant based breast surgery and implications for plastic surgeons. GMS Ger Plast Reconstr Aesthet Surg. 2013; 3:Doc04 [Google Scholar]
- 25.Washer LL, Gutowski K. Breast implant infections. Infect Dis Clin North Am. 2012; 26:111–125 [DOI] [PubMed] [Google Scholar]
- 26.Peled AW, Stover AC, Foster RD, et al. Long-term reconstructive outcomes after expander-implant breast reconstruction with serious infectious or wound-healing complications. Ann Plast Surg. 2012; 68:369–373 [DOI] [PubMed] [Google Scholar]
- 27.Halvorson EG, Disa JJ, Mehrara BJ, et al. Outcome following removal of infected tissue expanders in breast reconstruction: a 10-year experience. Ann Plast Surg. 2007; 59:131–136 [DOI] [PubMed] [Google Scholar]
- 28.Borgquist O, Ingemansson R, Malmsjö M. Wound edge microvascular blood flow during negative-pressure wound therapy: examining the effects of pressures from -10 to -175 mmHg. Plast Reconstr Surg. 2010; 125:502–509 [DOI] [PubMed] [Google Scholar]
- 29.Ngo QD, Vickery K, Deva AK. The effect of topical negative pressure on wound biofilms using an in vitro wound model. Wound Repair Regen. 2012; 20:83–90 [DOI] [PubMed] [Google Scholar]
- 30.Morykwas MJ, Simpson J, Punger K, et al. Vacuum-assisted closure: state of basic research and physiologic foundation. Plast Reconstr Surg. 2006; 1177 suppl121S–126S [DOI] [PubMed] [Google Scholar]
- 31.Bahrs C, Schnabel M, Frank T, et al. Lavage of contaminated surfaces: an in vitro evaluation of the effectiveness of different systems. J Surg Res. 2003; 112:26–30 [DOI] [PubMed] [Google Scholar]
- 32.Brown LL, Shelton HT, Bornside GH, et al. Evaluation of wound irrigation by pulsatile jet and conventional methods. Ann Surg. 1978; 187:170–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Accurso A, Rocco N, Accardo G, et al. Innovative management of implant exposure in ADM/implant-based breast reconstruction with negative pressure wound therapy. Aesthetic Plast Surg. 2017; 41:36–39 [DOI] [PubMed] [Google Scholar]
- 34.Franchelli S, Pesce M, Baldelli I, et al. Analysis of clinical management of infected breast implants and of factors associated to successful breast pocket salvage in infections occurring after breast reconstruction. Int J Infect Dis. 2018; 71:67–72 [DOI] [PubMed] [Google Scholar]
- 35.Negenborn VL, Smit JM, Dikmans REG, et al. Short-term cost-effectiveness of one-stage implant-based breast reconstruction with an acellular dermal matrix versus two-stage expander-implant reconstruction from a multicentre randomized clinical trial. Br J Surg. 2019; 106:586–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


