Abstract
In people recovering from COVID-19, there is concern regarding potential long-term pulmonary sequelae and associated impairment of functional capacity. Data published thus far indicate that spirometric indices appear to be generally well preserved, but that a defect in diffusing capacity (DLco) is a prevalent abnormality identified on follow-up lung function; present in 20–30% of those with mild to moderate disease and 60% in those with severe disease. Reductions in total lung capacity were commonly reported. Functional capacity is also often impaired, with data now starting to emerge detailing walk test and cardiopulmonary exercise test outcome at follow-up. In this review, we evaluate the published evidence in this area, to summarise the impact of COVID-19 infection on pulmonary function and relate this to the clinico-radiological findings and disease severity.
Current Opinion in Physiology 2021, 21:29–35
This review comes from a themed issue on Physiology of the diseased lung
Edited by Alexander Larcombe and Peter Noble
For a complete overview see the Issue
Available online 26th March 2021
https://doi.org/10.1016/j.cophys.2021.03.005
2468-8673/© 2021 Elsevier Ltd. All rights reserved.
Introduction
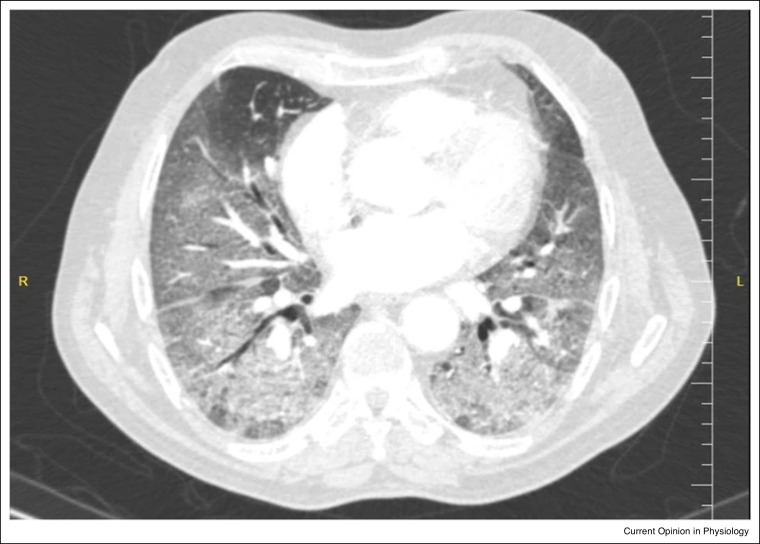
The COVID-19 pandemic, arising from the novel coronavirus, SARS-CoV-2, is associated with significant morbidity and mortality and continues to place unprecedented challenges on global healthcare systems. It is now apparent that whilst most individuals with COVID-19 appear to develop a mild to moderate self-limiting viral illness, over one in ten develop severe respiratory manifestations and most commonly a viral pneumonic process, often leading to hypoxaemic respiratory failure [1]. In this context, computer tomography (CT) imaging often shows diffuse interstitial changes with ground glass type opacities in 75% of patients hospitalised with COVID-19 [2] (Figure 1 ). Moreover, patients with COVID-19 pneumonitis frequently display features of vascular pathology on CT [3] and a high prevalence of coexisting pulmonary venous thrombosis is now recognised [4].
Figure 1.
An axial chest CT from a 60-year-old man with COVID-19 pneumonia with extensive ground-glass opacities observed in both lungs [33].
In people recovering from COVID-19, there is concern regarding potential long-term pulmonary sequelae and associated impairment of functional capacity. There is also increasing recognition that between 30–60% of individuals report protracted symptoms, such as fatigue and dyspnoea, following resolution of their acute illness [5••,6••]; variably termed the ‘long-COVID’ or the post-COVID syndrome. Exertional dyspnoea and exercise intolerance are prominent clinical features reported in this syndrome [7,8] and there is increasing interest in how best to objectively characterise and followed-up this issue with objective tests. Similar concerns were apparent following the first SARS pandemic with SARS-CoV-1 infection, in 2003. Pulmonary function testing (PFT) in patients following SARS-CoV-1 pneumonitis often revealed impaired in gas transfer indices [9], and a disconnect between this impairment and exercise limitation on cardio-pulmonary exercise testing (CPET) [10].
Pulmonary function follow-up data are now emerging after SARS-CoV-2 infection and over the last few months several centres, across the world and the aim of this brief review is twofold; firstly, to provide a summary of recent key findings regarding PFT and COVID-19 and to relate this to pathophysiology and radiological findings, and secondly, to describe future research priorities and identify unmet needs.
Studies evaluating the impact of COVID-19 on pulmonary function
Despite the fact that approximately 120 million people have now been infected with SARS-CoV-2, pulmonary function data in those affected still remains relatively scarce, which may in part be explained by the temporary closure of PFT laboratories in the early phases of the pandemic [11]. Although several observational studies are now available, most still only provide limited insight, on the basis that the majority only describe a relatively low sample size (n = 30–150) in heterogeneous populations [5••,6••,12, 13, 14,15•]. At the time of writing, only one randomised controlled trial including pulmonary function data had been conducted [16••].
In published studies, most characterise the severity of COVID-19 pulmonary disease as per World Health Organisation (WHO) interim guidance [17] and based on requirement for hospital ± critical care support.
Pulmonary function following COVID-19 infection
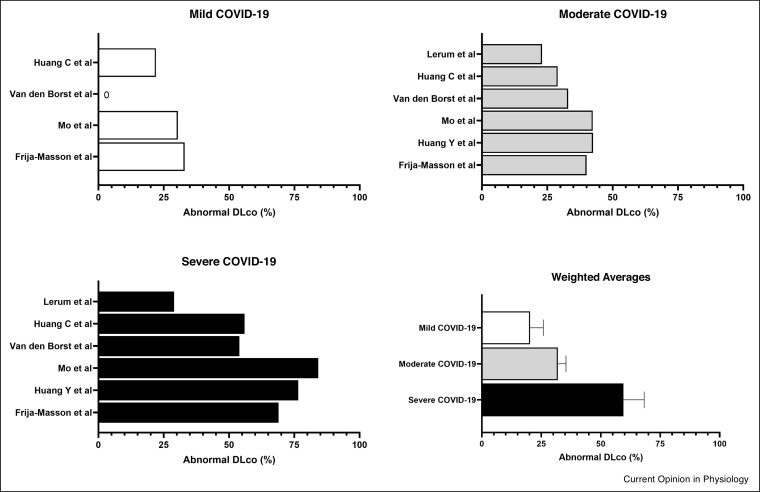
To the best of our knowledge, the first study to describe PFT in patients following COVID-19 was published by Mo et al. [15•] and reported findings from 110 patients (age: 49 ± 14 years; 50% female), who underwent testing, 27 ± 9 days after symptom onset, in April 2020 in Guangzhou, China. The clinical cohort was largely previously healthy individuals with mild (n = 24), moderate (n = 67) and severe disease (n = 19), only three patients had pre-existing lung disease. The key finding from this study was of a reduced pulmonary diffusing capacity (DLco; data presented in Figure 2 ), but also impaired total lung capacity and residual volumes, primarily in patients with severe disease. Importantly, less than 15% of the cohort had spirometric defects and no significant differences were observed in airflow spirometric measurements based on disease severity. Taken together, these findings align broadly with historical data from the SARS-CoV-1 pandemic [9] and are consistent with the typical PFT defect that might be expected in an individual with an interstitial-based process.
Figure 2.
Percentage of COVID-19 patients with abnormal diffusing capacity stratified according to disease severity (weighted average is presented as mean ± SD).
Similar observations were reported from 45 patients (age: 54 ± 8 years; 44% female), with mild (n = 12), moderate (n = 17) and severe disease (n = 16), by Frija-Masson and colleagues [13] who also, as expected, reported that the rate of PFT abnormality was higher in patients with severe disease. In this cohort of patients, 81% had abnormal PFTs with the majority displaying a reduced TLC and reduced DLco. Similarly, Huang et al. [14] compared the pulmonary function of 57 patients (age: 46 ± 13 years; 55% female) with severe disease (n = 17) to those with mild or moderate disease (n = 40), and found a higher prevalence of DLco impairment in those with severe disease. In contrast, Lerum et al. [18] stratified patients according to requirement for intensive care unit admission and DLco was below the lower limit of normal in almost one quarter (24%) of all patients, yet no significant difference was reported between groups; this may be explained by the inclusion criteria.
In one of the largest European studies to date, Van den Borst et al. [6••] obtained PFT measurements from 124 patients with COVID-19 infection approximately three months following hospital discharge. In this cohort, no differences in spirometry or static lung volumes were observed when comparing disease severity. DLco was within normal limits in all patients with mild disease, yet was significantly impaired in over one third in those with moderate disease (33%) and over half in those with critical (55%) or severe (54%) disease.
The overall evidence to date therefore indicates that DLco is the primary PFT defect seen post COVID-19 and relates to disease severity; all of the available data that is compared to the LLN is aggregated in Figure 2. The studies combined a total of 768 PFTs post-COVID-19; 20.3% of 152 patients with mild disease had abnormally low DLco measurements at follow-up; DLco was low in 32% of 435 patients with moderate disease, and 59.5% of 181 patients with severe disease. In contrast, spirometric indices appear to be generally well preserved and thus simple clinic-based pulmonary function evaluation (e.g., with a hand-held spirometer) is not likely to detect or characterise the sequalae of COVID-19 infection.
Longer-term surveillance following COVID-19
COVID-19 was first described in November 2019 and therefore long-term data are not yet available. Despite this, one prospective, multi-centre, observational trial evaluating PFT data in 145 patients at 60 (visit 1) and 100 (visit 2) days post COVID-19 onset has been published [5••]. At visit one, 42% of patients demonstrated PFT impairment, 27% of which had a restrictive pattern on spirometry, 11% had a reduced TLC via body plethysmography, and 31% had a reduced DLco. At the second visit, 36% of patients still demonstrated lung function impairment; 22% had restrictive spirometry, 11% had reduced TLC, and 21% had a reduced DLco, meaning that 30% of those patients with reduced DLco at baseline had improved.
Liu et al. [16••] investigated the impact of pulmonary rehabilitation in 36 male and female patients recovering from COVID-19 (age: 69 ± 8 years). DLco and 6MWT distance improved by approximately 30% versus controls with six weeks of respiratory muscle training. None of the measurements in the control group showed significant improvement six-weeks post-discharge. Acute and follow-up studies evaluating the impact of COVID on pulmonary function are summarised in Table 1 .
Table 1.
Studies evaluating pulmonary function following SARS-CoV-2 infection (n = 30+ participants)
| Author (Date; Country) | Title | Methodology | Measurements | Main findings |
|---|---|---|---|---|
| Mo et al. (April 2020; China) [15•] | Abnormal pulmonary function in COVID-19 patients at time of hospital discharge | Prospective observational study measuring pulmonary function at discharge across the severity spectrum (n = 110) | Spirometry, diffusing capacity, static lung volumes | Spirometry was normal, reduced lung volume was more frequent in those with severe disease (47% having a TLC lower than 80% predicted). Reduced DLco was the most common pulmonary function finding (47% of all patients) and was increasingly more common in more severe symptoms (84% in severe disease) |
| Liu Kai et al. (April 2020; China) [16••] | Respiratory rehabilitation in elderly patients with COVID-19: A randomised controlled study | RCT with six weeks of pulmonary rehabilitation compared with controls (n = 72) | Spirometry, diffusing capacity, 6MWT, CT, SF-36 | DLco was reduced in both groups before intervention. Pulmonary rehabilitation led to an improvement in DLco, spirometry and 6MWT compared to controls |
| Lv et al. (April 2020; China) [34] | Pulmonary function of patients with 2019 novel coronavirus induced pneumonia: A Retrospective Cohort Study | Retrospective analysis of pulmonary function data measured 14 days after discharge (n = 137) | Spirometry | Patients with severe disease had lower FVC and FEV1 with preserved FEV1/FVC ratio suggesting a restrictive pattern |
| Daher et al. (October 2020; Germany) [12] | Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae | Prospective observational study following patients with severe COVID-19 disease (n = 33) | Spirometry, diffusing capacity, body plethysmography, 6MWT, TTE, ABG, SGRQ | At follow-up, spirometry and static lung volumes were normal. Median DLco was 65% predicted (IQR 53-73). 45% of patients were below the LLN for 6MWT distance and 45% complained about persistent fatigue |
| Van den Borst et al. (November 2020; The Netherlands) [6••] | Comprehensive health assessment three months after recovery from acute COVID-19 | Prospective observational study looking at pulmonary function across COVID-19 disease severity spectrum (n = 124) | Spirometry, diffusing capacity, body plethysmography, CT, 6MWT, clinical frailty score, SF-36 | DLco was below LLN in 42% of patients; DLco was lower in those with severe and critical disease than those with mild or moderate disease. 86% of patient demonstrated ground glass opacities on CT |
| Sonnweber et al. (December 2020; Austria) [5••] | Cardiopulmonary recovery after COVID-19 — an observational prospective multi-centre trial | Prospective multi-centre observational study with measurements at 60 and 100 days after COVID-19 onset (n = 145) | Spirometry, diffusing capacity, body plethysmography, CBG, TTE, mMRC dyspnoea score, CT | 60 days after COVID-19 onset, 42% of patients demonstrated abnormal lung function — most common was a reduced DLco. After 100 days of COVID-19 onset, 41% of patients exhibited persistent symptoms and 36% had abnormal lung function findings; DLco had improved in 30% of patients that were abnormal at 60 days |
6MWT = 6-min walk test; ABG = arterial blood gas; CBG = capillary blood gas; DLco = diffusing capacity for carbon monoxide; FEV1 = forced expiratory volume in 1 s; FVC = forced vital capacity; LLN = lower limit of normal; mMRC = Modified British Medical Research Council; QoL = Quality of life; RCT = randomised controlled trial; SF-36 = Short Form Health Survey; SGRQ = St. George's Respiratory Questionnaire; TLC = total lung capacity; TTE = transthoracic echocardiography.
Functional and exercise capacity following COVID-19
Early studies evaluating functional capacity report detail on propensity to desaturate on field-based exercise testing. Fuglebjerg et al. [19] collected data from 26 patients (age: 63 ± 30 years; 84% female) at the time of discharge and reported that 50% of patients demonstrated significant desaturation (SpO2 < 90%) on exertion during 6MWT. A further study by Daher et al. [12] reported that 60-days following discharge, no patients significantly desaturated during a 6MWT, but 79% of patients were unable to reach their predicted walk distance and 45% of patients were below the LLN. Three months post-discharge, 16% of patients desaturated with 22% below the LLN for walk distance [6••].
More recently, studies have characterised functional capacity following COVID-19 infection utilising cardiopulmonary exercise testing (CPET). This approach was used in the SARS-CoV-1 pandemic and revealed that whilst exercise tolerance was impaired in 57% of patients, a disconnect was often evident between individuals with impaired diffusion capacity, such that those with impaired peak oxygen uptake on CPET were not always the same group of individuals with impaired gas exchange [10]. In the SARS-CoV-2 pandemic, Clavario et al. [7] utilised CPET in 110 patients (age: 61.7 ± 8 years) and reported impaired aerobic capacity (defined as a peak oxygen consumption of <85% predicted) in 34% of non-severe (defined as those that did not require ITU) patients three months after discharge which was attributed predominantly to muscular impairment. Similarly, one month following discharge, patients demonstrated impaired aerobic capacity which was not explained by pulmonary function changes, but likely attributable to physical deconditioning [20] and 70% of patients were limited by decreases in peripheral muscle mass [21]. Raman et al. [8] observed, in 58 patients (age: 55.4 ± 13.2 years), that aerobic capacity was lower in COVID-19 patients 2–3 months after symptom onset than controls (54% versus 7.4%). Another study found that quadricep force production was reduced in 86% of COVID-19 patients at the point of discharge [22].
Discussion
The respiratory community has made significant efforts to undertake physiological assessment in patients following COVID-19 infection, in order to describe and understand the impact of this disease on functional outcome and capacity. To date, however, the available data remains heterogeneous, with relatively small sample sizes from single centres and predominantly observational methodology. Irrespective, it is apparent that the overarching abnormality or defect in PFT encountered following SARS-CoV-2 respiratory infection is contained within the interstitial compartment rather than the airways (i.e., it is uncommon to encounter defects in spirometry or airflow indices). This finding aligns with the clinico-patho-radiological findings of a disease that in most cases causes a diffuse airspace/interstitial-based process, with inflammatory substrate impacting the gas exchange surface and DLco.
Whilst a reduction in DLco was the most common finding, some published work also reports a reduction in TLC in patients following COVID-19 infection. Unfortunately, most did not present Kco and VA alongside DLco, and this precludes interpretation of the type of pulmonary restriction (intra-pulmonary versus extra-pulmonary thoracic restriction). Moreover, data concerning functional impairment appears to be related to physical deconditioning ± muscular impairment secondary to severe illness or inflammation and the data from Liu et al. [16••] suggest this may be responsive to physical rehabilitation. There is a dearth of information related to respiratory muscle function post-COVID-19. We in the respiratory community could do more to measure respiratory muscle function particularly after mechanical ventilation and/or severe illness [23].
The long-term impact that SAR-CoV-2 infection has on PFT will continue to emerge over the coming years. Data published thus far have managed to capture the pulmonary function of patients recovering from COVID-19 at discharge [15•], several weeks after discharge [6••,12,13], after six weeks of pulmonary rehabilitation [16••], and at two time points 60 and 100 days after symptom onset [5••]. There is however no data yet on longer-term follow-up or with respect to the impact of targeted interventions, for example, use of systemic steroid treatment.
A further key limitation in this area is the lack of knowledge regarding baseline (i.e., pre-morbid) PFT and exercise capacity. Studies have shown that baseline cardiorespiratory fitness is predictive of need for hospital-based care for COVID infection [24]. Thus, it is likely that there will be selection bias when following-up post-COVID cardiorespiratory functional parameters in the respect that those developing more severe COVID-19 infection are likely to have poor baseline functional status. The impact of pre-existing pulmonary disease ± pulmonary function impairment on post COVID-19 PFT outcome is also in need of study. Certainly, pulmonary function impairment before hospitalisation predicts COVID-19 disease outcomes in sarcoidosis [25] and other chronic comorbid pulmonary conditions have been associated with poor outcome in COVID-19 such as COPD and ILD [26, 27, 28, 29]. Ippolito et al. [30], using quantitative CT analysis, found that disease severity and need for invasive ventilation was correlated with measures of lung volume. If lung volume measurements and pulmonary function can predict severity, it may be that static lung volumes and/or diffusing capacity abnormalities are not the consequence of COVID-19 disease severity, but that pre-existing abnormality contributes to COVID-19 disease severity.
As data continues to emerge, there needs to be a systematic review and attempts to aggregate all the available pulmonary function data and synthesise high-quality evidence. The data currently available regarding rehabilitation and physical activity post-infection are limited, but offer promise in terms of interrogating the factors limiting physical exercise performance and PFT; likewise, studies following the pulmonary function of groups receiving different interventions (i.e., post RCT for steroid-based therapy) will be informative. Symptoms of exercise intolerance and dyspnoea are common and focus in the future of COVID-19 research needs to be directed at the impact of rehabilitation. There also needs to be attention directed to the underlying cause of the reduction in diffusing capacity observed, and the impact the disease has on the pulmonary vasculature requires investigation. The measurement of the transfer factor for nitric oxide (DLno) concurrently with the transfer factor for carbon monoxide can provide insights into the diffusing membrane capacity and capillary blood volume [31], and these measurements may hold the key for understanding how diffusing capacity is impaired in COVID-19. Barisione and Brusasco [32] have very recently performed such measurements and observed that DLno impairment exceeded DLco impairment, but both values were lower with increasing disease severity. In all severities, the DLno/DLco ratio was similar to controls. The findings indicate that this is most probably an issue with the alveolar membrane (related to loss of alveolar units and damage to the membrane) rather than altered capillary volume.
In conclusion, COVID-19 related pulmonary disease is associated with impaired diffusing capacity that appears related to disease severity and in keeping with radiological interstitial change. This defect appears to improve in most during a 3–6-month convalescent period and this improvement may be accelerated with rehabilitative exercise. More work is now needed to better understand the mechanisms underpinning and the impact of premorbid functional capacity on recovery from COVID-19.
Author contribution
All authors contributed equally to conception. MT drafted the manuscript and figures and OP and JH reviewed and contributed to final drafting.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
References
- 1.World Health Organisation . WHO; 2020. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) pp. 1–9. Available from: https://www.who.int/publications/m/item/who-convened-global-study-of-the-origins-of-sars-cov-2. [Google Scholar]
- 2.Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N., et al. Chest CT findings in Coronavirus Disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295 doi: 10.1148/radiol.2020200463. Available from: http://pubs.rsna.org/doi/10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai H.X., Hsieh B., Xiong Z., Halsey K., Choi J.W., Tran T.M.L., et al. Performance of radiologists in differentiating COVID-19 from non-COVID-19 viral pneumonia at chest CT. Radiology. 2020;296:E46–E54. doi: 10.1148/radiol.2020200823. Available from: http://pubs.rsna.org/doi/10.1148/radiol.2020200823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Provencher S., Potus F., Bonnet S. COVID-19 and the pulmonary vasculature. Pulm Circ. 2020;10 doi: 10.1177/2045894020933088. Available from: http://journals.sagepub.com/doi/10.1177/2045894020933088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5••.Sonnweber T., Sahanic S., Pizzini A., Luger A., Schwabl C., Sonnweber B., et al. Cardiopulmonary recovery after COVID-19 – an observational prospective multi-center trial. Eur Respir J. 2020;57 doi: 10.1183/13993003.03481-2020. Available from: http://erj.ersjournals.com/lookup/doi/10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; Prospective multi-centre observational study with PFT measurements at 60 and 100 days after COVID-19 onset. Total abnormality at visit 1 was 42% and at visit 2 was 36%. DLco was <80% predicted in 31% at visit 1 and 21% at visit 2 suggesting an improvement with convalescence.
- 6••.Van den Borst B., Peters J., Brink M., Schoon Y., Bleeker-Rovers C., Schers H., et al. Comprehensive health assessment three months after recovery from acute COVID-19. Ann Med. 2020;0:1–14. doi: 10.1080/07853890.2020.1840620. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]; Prospective multi-centre observational study that presented PFT data for 124 patients across COVID-19 severity spectrum. DLco was below LLN in 42% of patients; DLco was lower in those with severe and critical disease than those with mild or moderate disease. 86% of patients demonstrated ground glass opacities on CT.
- 7.Clavario P., de Marzo V., Lotti R., Barbara C., Porcile A., Russo C., et al. Assessment of functional capacity with cardiopulmonary exercise testing in non-severe COVID-19 patients at three months follow-up. medRxiv. 2020 doi: 10.1101/2020.11.15.20231985. (Dimi). Available from: [DOI] [Google Scholar]
- 8.Raman B., Cassar M.P., Tunnicliffe E.M., Filippini N., Griffanti L., Alfaro-Almagro F., et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31 doi: 10.1016/j.eclinm.2020.100683. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2589537020304272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed H., Patel K., Greenwood D., Halpin S., Lewthwaite P., Salawu A., et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med. 2020;52 doi: 10.2340/16501977-2694. Available from: https://www.medicaljournals.se/jrm/content/abstract/10.2340/16501977-2694. [DOI] [PubMed] [Google Scholar]
- 10.Su M.-C., Hsieh Y.-T., Wang Y.-H., Lin A.-S., Chung Y.-H., Lin M.-C. Exercise capacity and pulmonary function in hospital workers recovered from severe acute respiratory syndrome. Respiration. 2007;74:511–516. doi: 10.1159/000095673. Available from: https://www.karger.com/Article/FullText/95673. [DOI] [PubMed] [Google Scholar]
- 11.Hull J.H., Lloyd J.K., Cooper B.G. Lung function testing in the COVID-19 endemic. Lancet Respir Med. 2020;8:666–667. doi: 10.1016/S2213-2600(20)30246-0. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2213260020302460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daher A., Balfanz P., Cornelissen C., Müller A., Bergs I., Marx N., et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): pulmonary and extrapulmonary disease sequelae. Respir Med. 2020;174 doi: 10.1016/j.rmed.2020.106197. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0954611120303371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frija-Masson J., Debray M.-P., Gilbert M., Lescure F.-X., Travert F., Borie R., et al. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur Respir J. 2020;56 doi: 10.1183/13993003.01754-2020. Available from: http://erj.ersjournals.com/lookup/doi/10.1183/13993003.01754-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y., Tan C., Wu J., Chen M., Wang Z., Luo L., et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21:163. doi: 10.1186/s12931-020-01429-6. Available from: https://respiratory-research.biomedcentral.com/articles/10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Mo X., Jian W., Su Z., Chen M., Peng H., Peng P., et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55 doi: 10.1183/13993003.01217-2020. Available from: http://erj.ersjournals.com/lookup/doi/10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; First paper to publish pulmonary function data in patients who have suffered with COVID-19.
- 16••.Liu K., Zhang W., Yang Y., Zhang J., Li Y., Chen Y. Respiratory rehabilitation in elderly patients with COVID-19: a randomized controlled study. Complement Ther Clin Pract. 2020;39 doi: 10.1016/j.ctcp.2020.101166. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1744388120304278. [DOI] [PMC free article] [PubMed] [Google Scholar]; The only RCT with pulmonary function data to date. PFT, QoL, and functional capacity were measured before and after six weeks of rehabilitative exercises post-discharge and compared with controls with no intervention. Significant improvements demonstrated in essentially all domains, most notably a 30% improvement in DLco and 6MWT distance. No changes observed in the control arm.
- 17.World Health Organisation . WHO; 2020. Clinical Management of COVID-19: Interrim Guidance. Available from: https://www.who.int/publications/i/item/clinical-management-of-covid-19. [Google Scholar]
- 18.Lerum T.V., Aaløkken T.M., Brønstad E., Aarli B., Ikdahl E., Lund K.M.A., et al. Dyspnoea, lung function and CT findings three months after hospital admission for COVID-19. Eur Respir J. 2020;57 doi: 10.1183/13993003.03448-2020. Available from: http://erj.ersjournals.com/lookup/doi/10.1183/13993003.03448-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuglebjerg N.J.U., Jensen T.O., Hoyer N., Ryrsø C.K., Lindegaard B., Harboe Z.B. Silent hypoxia in patients with SARS CoV-2 infection before hospital discharge. Int J Infect Dis. 2020;99:100–101. doi: 10.1016/j.ijid.2020.07.014. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao Y., Chen R., Geng Q., Mo X., Zhan C., Jian W., et al. Cardiopulmonary exercise testing might be helpful for interpretation of impaired pulmonary function in recovered COVID-19 patients. Eur Respir J. 2021;57 doi: 10.1183/13993003.04265-2020. Available from: http://erj.ersjournals.com/lookup/doi/10.1183/13993003.04265-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blokland I.J., Ilbrink S., Houdijk H., Dijkstra J.-W., van Bennekom C.A.M., Fickert R., et al. Exercise capacity after mechanical ventilation because of COVID-19: cardiopulmonary exercise tests in clinical rehabilitation. Ned Tijdschr Geneeskd. 2020;164 Available from: http://www.ncbi.nlm.nih.gov/pubmed/33331718. [PubMed] [Google Scholar]
- 22.Paneroni M., Simonelli C., Saleri M., Bertacchini L., Venturelli M., Troosters T., et al. Muscle strength and physical performance in patients without previous disabilities recovering from COVID-19 pneumonia. Am J Phys Med Rehabil. 2021;100:105–109. doi: 10.1097/PHM.0000000000001641. Available from: https://journals.lww.com/10.1097/PHM.0000000000001641. [DOI] [PubMed] [Google Scholar]
- 23.Severin R., Arena R., Lavie C.J., Bond S., Phillips S.A. Respiratory muscle performance screening for infectious disease management following COVID-19: a highly pressurized situation. Am J Med. 2020;133:1025–1032. doi: 10.1016/j.amjmed.2020.04.003. Available from: https://www.embase.com/search/results?subaction=viewrecord&id=L2006130357&from=export. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brawner C.A., Ehrman J.K., Bole S., Kerrigan D.J., Parikh S.S., Lewis B.K., et al. Inverse relationship of maximal exercise capacity to hospitalization secondary to coronavirus disease 2019. Mayo Clin Proc. 2021;96:32–39. doi: 10.1016/j.mayocp.2020.10.003. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgenthau A.S., Levin M.A., Freeman R., Reich D.L., Klang E. Moderate or severe impairment in pulmonary function is associated with mortality in sarcoidosis patients infected with SARS-CoV-2. Lung. 2020;198:771–775. doi: 10.1007/s00408-020-00392-9. Available from: http://link.springer.com/10.1007/s00408-020-00392-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He Y., Xie M., Zhao J., Liu X. Clinical characteristics and outcomes of patients with severe COVID-19 and chronic obstructive pulmonary disease (COPD) Med Sci Monit. 2020;26 doi: 10.12659/MSM.927212. Available from: https://www.embase.com/search/results?subaction=viewrecord&id=L2007962199&from=export. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu W., Dong M., Xiong M., Zhao D., Zhao Y., Wang M., et al. Clinical courses and outcomes of patients with chronic obstructive pulmonary disease during the COVID-19 epidemic in Hubei, China. Int J Chron Obstruct Pulmon Dis. 2020;15:2237–2248. doi: 10.2147/COPD.S265004. Available from: https://www.dovepress.com/clinical-courses-and-outcomes-of-patients-with-chronic-obstructive-pul-peer-reviewed-article-COPD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drake T.M., Docherty A.B., Harrison E.M., Quint J.K., Adamali H., Agnew S., et al. Outcome of hospitalization for COVID-19 in patients with interstitial lung disease. An international multicenter study. Am J Respir Crit Care Med. 2020;202:1656–1665. doi: 10.1164/rccm.202007-2794OC. Available from: https://www.atsjournals.org/doi/10.1164/rccm.202007-2794OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esposito A.J., Menon A.A., Ghosh A.J., Putman R.K., Fredenburgh L.E., El-Chemaly S.Y., et al. Increased odds of death for patients with interstitial lung disease and COVID-19: a case–control study. Am J Respir Crit Care Med. 2020;202:1710–1713. doi: 10.1164/rccm.202006-2441LE. Available from: https://www.atsjournals.org/doi/10.1164/rccm.202006-2441LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ippolito D., Ragusi M., Gandola D., Maino C., Pecorelli A., Terrani S., et al. Computed tomography semi-automated lung volume quantification in SARS-CoV-2-related pneumonia. Eur Radiol. 2020 doi: 10.1007/s00330-020-07271-0. Available from: http://link.springer.com/10.1007/s00330-020-07271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guenard H., Varene N., Vaida P. Determination of lung capillary blood volume and membrane diffusing capacity in man by the measurements of NO and CO transfer. Respir Physiol. 1987;70:113–120. doi: 10.1016/s0034-5687(87)80036-1. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0034568787800361. [DOI] [PubMed] [Google Scholar]
- 32.Barisione G., Brusasco V. Lung diffusing capacity for nitric oxide and carbon monoxide following mild-to-severe COVID-19. Physiol Rep. 2021;9:1–10. doi: 10.14814/phy2.14748. Available from: https://onlinelibrary.wiley.com/doi/10.14814/phy2.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohamed Y.G., Mohamud M.F.Y., Medişoğlu M.S., Atamaca I.Y., Ali I.H. Clinical and chest CT presentations from 27 patients with COVID-19 pneumonia in Mogadishu, Somalia: a descriptive study. Egypt J Radiol Nucl Med. 2020;51:184. Available from: https://ejrnm.springeropen.com/articles/10.1186/s43055-020-00302-2. [Google Scholar]
- 34.Lv D., Chen X., Wang X., Mao L., Sun J., Wu G., et al. Pulmonary function of patients with 2019 novel coronavirus induced-pneumonia: a retrospective cohort study. Ann Palliat Med. 2020;9:3447–3452. doi: 10.21037/apm-20-1688. Available from: http://apm.amegroups.com/article/view/52209/html. [DOI] [PubMed] [Google Scholar]