Introdcution
The first case series of skin manifestations of COVID-19 was reported by Recalcati et al. from Italy in March 2020. A wide variety of cutaneous manifestations have been reported in patients diagnosed with COVID-19, including maculopapular or perifollicular rash, urticaria, vesicles, petechiae, purpura, livedo racemosa, and pseudo-chilblains, which are often referred to as the “COVID toes.”1,2 We hereby present a case that we initially diagnosed as a possible COVID-19 with skin manifestations; however, later we found an increase in the patient's liver enzymes and the presence of atypical lymphocytes along with positive serology tests for Epstein-Barr virus (EBV), cytomegalovirus (CMV), and parvovirus B19. This case highlights the importance of a thorough evaluation to distinguish between COVID-19-associated complications and manifestations of reactivated viral co-infections.
Case report
A 28-year-old woman with no past medical history presented to our clinic complaining of a skin rash on her extremities. Two weeks prior to presentation, she first noticed fatigue and decreased tolerance to alcohol, which caused nausea and easy inebriation. She then noticed the rash initially developing on her feet. She had no fever or respiratory symptoms but felt lethargic. One month earlier, her older sister had developed flu-like symptoms, which were suspected to reflect COVID-19.
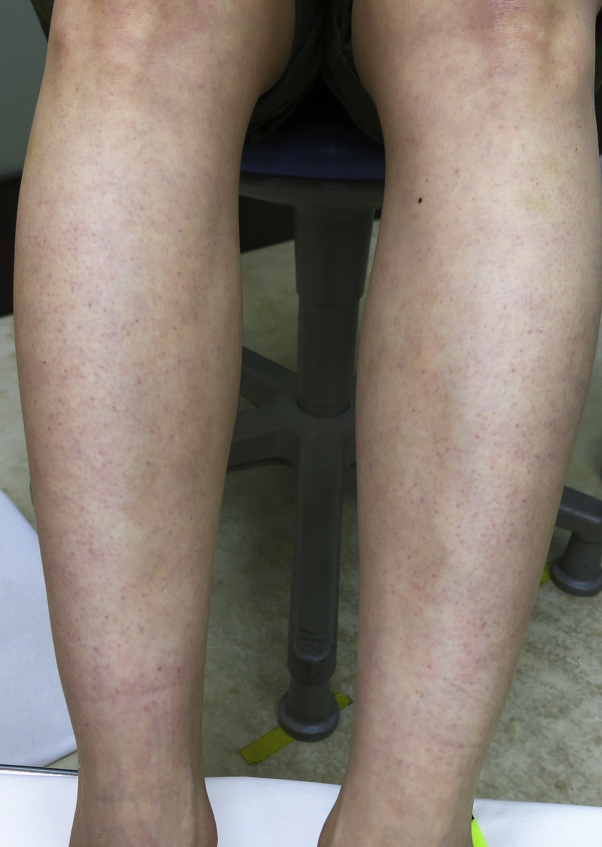
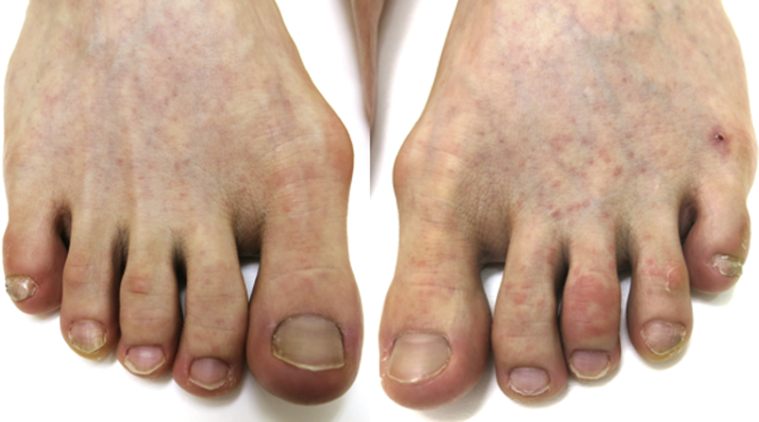
On physical examination, she was afebrile with stable vital signs. Heart, lung, and abdominal examinations were normal. On skin examination, she had faint, erythematous, nonpalpable, perifollicular eruption on the lower extremities (Fig 1). Acral cyanosis was observed on her toes as well as a small ulceration on the dorsal aspect of her left foot (Fig 2). All lesions were neither pruritic nor painful. Her complete blood count showed normal a white blood cell count (9900/μL) with an increase in lymphocytes (63%), of which 12% were atypical lymphocytes. She also had elevated liver enzymes (aspartate transaminase, 122 U/L; alanine aminotransferase, 199 U/L), lactate dehydrogenase (486 U/L), ferritin (152 ng/mL), C-reactive protein (0.36 mg/dL), and D-dimer (3.0 μg/mL).
Fig 1.
Faint, erythematous, nonpalpable, perifollicular eruption on the lower extremities.
Fig 2.
Acral cyanosis on the toes and a small ulceration on the dorsum of the left foot.
On the following day, her temperature had slightly increased to 37.5 °C, and her lethargy had worsened. We suspected COVID-19, and made a request to the regional hospital for confirmatory diagnostic testing. A reverse-transcriptase polymerase chain reaction (RT-PCR) for SARS-CoV-2 on DNA from a nasopharyngeal swab was negative; however, an antibody test (IgG) for SARS-CoV-2 was positive. Antigen and IgM antibody testing were not performed. Moreover, serology for EBV was positive for antiviral capsid antigen (VCA)-IgM, anti-VCA-IgG, and anti-Epstein-Barr nuclear antigen (EBNA)-IgG antibodies (1.2, 9.7, and 2.3 respectively). Serology tests for CMV and parvovirus B19 were also positive (CMV-IgG [EIA], 12.3 S/CO; CMV-IgM [EIA], 5.14 S/CO; parvovirus B19-IgM [EIA], 2.32). These results were consistent with current infection or previous infection with reactivation. The results for IgA, IgG, and IgM for Chlamydia pneumoniae and Mycoplasma antibodies were negative.
Discussion
Given the positive anti-SARS-CoV-2 IgG antibody test, perifollicular exanthema of the lower legs, and chilblain-like lesions of the toes, the skin manifestations in this patient were initially attributed to COVID-19. Positive serology tests for EBV, CMV, and parvovirus, however, presented the possibility of current or reactivated infection by these viruses, possibly co-occurring with COVID-19. On laboratory evaluation, the elevation of D-dimer was compatible with COVID-19,3 whereas the elevation of atypical lymphocytes was more suggestive of infection by EBV or CMV. Alcohol intolerance has been reported in association with EBV-associated hepatitis, further supporting EBV infection in our patient.4
Regarding the skin findings, aside from chilblain-like lesions, the exanthema noted was relatively nonspecific. Parvovirus B19 however was felt to be a less likely cause in view of the absence of typical petechial rash in a glove-and-stocking distribution, reticular erythema, and “slapped cheek” facial findings. Thus, our patient most plausibly reflected a case of co-infection by SARS-CoV-2 with reactivated EBV, and, possibly, reactivated CMV.
Recently, co-infection with SARS-CoV-2 and other viruses have been reported, including the herpes viruses EBV (HHV-4), CMV (HHV-5), HHV-6, and HHV-7.5 This is an interesting immunological phenomenon caused by the SARS-CoV-2 virus, which could perhaps be explained by the simultaneous occurrence of cytokine storm and immunosuppression facilitating the reactivation of other viruses. Lymphopenia is frequently observed in COVID-19 with significantly reduced CD8 levels and elevated CD4/CD8 ratio, which are predictive of disease severity.6,7 This leads to an immunosuppressed state, and it may induce the reactivation of another latent viral infection. Thus, there is a possibility of SARS-CoV-2 infection leading to immunocompromised status and co-reactivation of latent viruses, which could well aggravate the COVID-19 disease course.
In our case, the diagnosis was challenging, as the RT-PCR for SARS-CoV-2 was negative at the time of presentation. However, it was reported that the clinical sensitivity of PCR decreases with days post-symptom onset (70% on days 9-11 and 30% on Day 21).8 IgG antibody positivity was demonstrated using the Abbott SARS-CoV-2 CLIA IgG assay, which is reported to have a sensitivity of 94%-100% and a specificity of 99%-100% more than 2 weeks after symptom onset when the viral load becomes undetectable by RT-PCR.9 This makes it very likely that the patient's symptoms of the toes and elevated D-dimer were related to COVID-19. Furthermore, a low rate of PCR positivity (less than 50%) has been reported in COVID-19 cases with skin manifestations, especially for patients with chilblain-like lesions.10
In summary, we hypothesize that in our case, 3 conditions occurred concurrently. Firstly, our patient had mild systemic COVID-19 with “COVID toes.” By the time she visited our clinic 2 weeks after onset of her general symptoms, she tested SARS-CoV-2 PCR-negative but IgG-positive, results compatible with the later stage of the disease. Second, reactivation of other pathogens, which are usually latent, was found due to the immunosuppressed state. Third, the exanthem on the lower limbs could have been due to the reactivation of other viruses, that is, EBV and/or CMV. Most skin manifestations from COVID-19 are nonspecific, and caution is recommended as to how they are interpreted in the setting of diagnosed or suspected COVID-19. As the cases of COVID-19 increase, there could be more atypical cases with comorbidities and co-infections.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Galván Casas C., Català A., Carretero Hernández G. Classification of the cutaneous manifestations of COVID-19: A rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71–77. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hubiche T., Duff F.L., Chiaverini C., Giordanengo V., Passeron T. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions. Lancet Infect Dis. 2021;21(3):315–316. doi: 10.1016/S1473-3099(20)30518-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Velavan T.P., Meyer C.G. Mild versus severe COVID-19: Laboratory markers. Int J Infect Dis. 2020;95:304–307. doi: 10.1016/j.ijid.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambore S., McSherry J., Kraus A.S. Acute and chronic symptoms of mononucleosis. J Fam Pract. 1991;33(1):33–37. [PubMed] [Google Scholar]
- 5.Drago F., Ciccarese G., Rebora A., Parodi A. Human herpesvirus 6, 7 and Epstein Barr virus reactivation in pityriasis rosea during COVID-19. J Med Virol. 2021;93(4):1850–1851. doi: 10.1002/jmv.26549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang M., Guo Y., Wan L. T-Cell subset counts in peripheral blood can be used as discriminatory biomarkers for diagnosis and severity prediction of coronavirus disease 2019. J Infect Dis. 2020;222(2):198–202. doi: 10.1093/infdis/jiaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang F., Nie J., Wang H. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020;221(11):1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller T.E., Garcia Beltran W.F.G., Bard A.Z. Clinical sensitivity and interpretation of PCR and serological COVID-19 diagnostics for patients presenting to the hospital. FASEB J. 2020;34(10):13877–13884. doi: 10.1096/fj.202001700RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicol T., Lefeuvre C., Serri O. Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: Two automated immunoassays (Euroimmun and Abbott) and one rapid lateral flow immunoassay (NG Biotech) J Clin Virol. 2020;129:104511. doi: 10.1016/j.jcv.2020.104511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrascosa J.M., Morillas V., Bielsa I., Munera-Campos M. Cutaneous manifestations in the context of SARS-CoV-2 infection (COVID-19) Actas Dermosifiliogr. 2020;111(9):734–742. doi: 10.1016/j.ad.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]