Abstract
Autophagy is a catabolic process that ensures homeostasis in the cells of our organism. It plays a crucial role in protecting eye cells against oxidative damage and external stress factors. Ocular pathologies of high incidence, such as age-related macular degeneration, cataracts, glaucoma, and diabetic retinopathy are of multifactorial origin and are associated with genetic, environmental factors, age, and oxidative stress, among others; the latter factor is one of the most influential in ocular diseases, directly affecting the processes of autophagy activity. Alteration of the normal functioning of autophagy processes can interrupt organelle turnover, leading to the accumulation of cellular debris and causing physiological dysfunction of the eye. The aim of this study is to review research on the role of autophagy processes in the main ocular pathologies, which have a high incidence and result in high costs for the health system. Considering the role of autophagy processes in cell homeostasis and cell viability, the control and modulation of autophagy processes in ocular pathologies could constitute a new therapeutic approach.
Keywords: autophagy, ocular pathology, glaucoma, cataract, diabetic retinopathy, AMD
1. Introduction
Autophagy, a catabolic process that is conserved in the evolutionary process, involves the degradation and recycling of cellular components to maintain the cells’ interior under appropriate conditions for their survival [1]. The process of autophagy is a double-edged sword, as it is involved in both cell death and cell survival [1,2]. Through digestion, autophagy can eliminate debris that may be toxic, causing cell death. It can also digest cellular organelles, which are altered for subsequent recycling [3]. Autophagy also removes defective or aggregated proteins and lipid droplets from the cytoplasm via double membrane vesicles that are transported to and fused with lysosomes for enzymatic degradation [4]. After the digestion process, metabolites that can serve as the basis for the synthesis of new organelles or essential molecules for the cell are generated.
Various stress conditions, such as infections, ionizing radiation, hypoxia, and even chemotherapeutics, can cause the induction of autophagy [5]. Yoshinori Oshumi won the Nobel Prize in 2016 for his research work on autophagy processes. In the early 1990s, he identified genes related to autophagy processes in yeast (Saccharomyces cerevisiae) [6], subsequently characterizing the proteins encoded by these genes. He later conducted similar research in mammals [7]. Since the beginning of the 21st century, this catabolic process has been one of the main targets in cellular research in order to study some types of cancer, metabolic, inflammatory, and neurodegenerative diseases as well as cellular aging [1]. In addition, it is known that alterations in autophagy processes are involved in the development of certain ocular pathologies; therefore, treatments focused on the regulation of autophagy processes could offer a therapeutic alternative for the treatment of ophthalmologic pathologies [8].
2. Overview of Autophagy
2.1. Autophagy Types
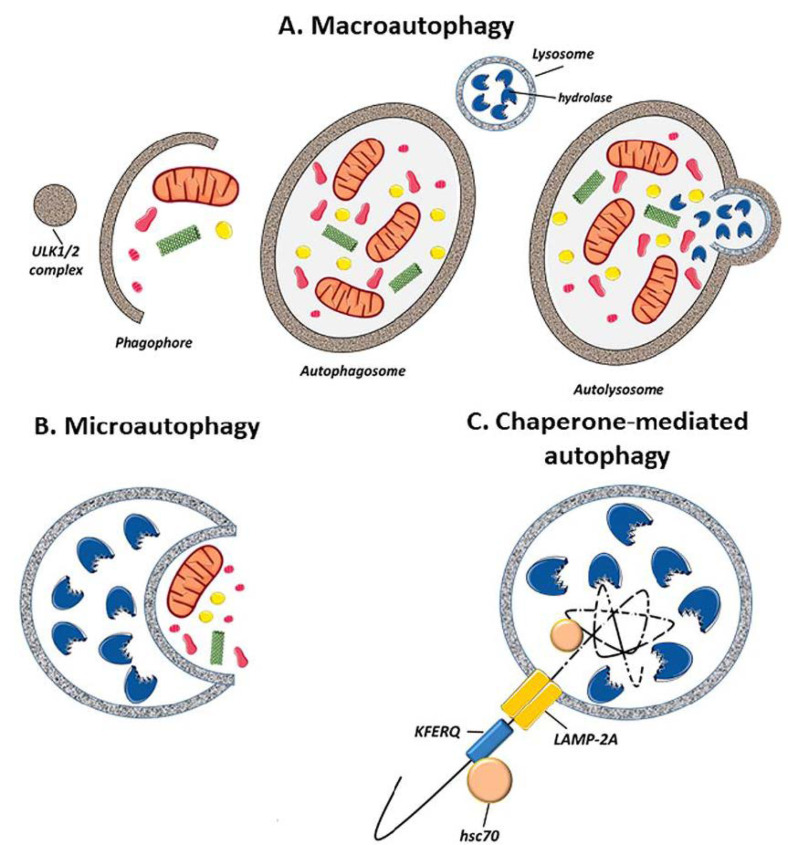
Autophagy can be classified into three different types: macro-autophagy, micro-autophagy, or chaperone-mediated autophagy (CMA) (Figure 1). The difference between the three depends on how the components to be degraded are transferred to the lysosomes:
Figure 1.
Schematic representation of the different types of autophagy. (A) Macro-autophagy: the material to be degraded is enclosed in a double-membraned cytosolic vesicle called the autophagosome. (B) Micro-autophagy: cytosol components are enclosed by lysosomes through membrane invaginations. (C) Chaperone-mediated autophagy: proteins are detected by the lysosome membrane through a chaperone called hsc70. This complex binds with lysosome-associated membrane protein 2 (LAMP-2A) and is introduced into the lysosome. (Created in part with smart.servier.com, accessed date 20 February 2021).
(i) Macro-autophagy: The process of macro-autophagy is important in physiological processes such as development, aging, and apoptosis, as well as in pathologies including cancer, infections, and degenerative, metabolic, and inflammatory diseases, among others. The cytoplasmic material is captured by double membrane vesicles (autophagosomes) and transported to the lysosomes for degradation. When autophagosomes fuse with lysosomes, the resulting vesicles (single membrane vesicles) are called autolysosomes [9] (Figure 1A).
(ii) Micro-autophagy: The lysosomal membrane invaginates to envelop small portions of the material to be degraded, introducing them into the lysosome. [10] (Figure 1B).
(iii) Chaperone-mediated autophagy: this type of autophagy is only observed in mammals. Soluble proteins are incorporated into the lysosome by a transporter located in the lysosome membrane. The transporter is a chaperone complex of the 70 KDa heat shock protein family (hsp70) that recognizes the specific amino acid sequence signal in proteins: KFERQ (pentapeptide: Lysine, Phenylalanine, Glutamic Acid, Arginine and Glutamine). The protein is then translocated into the lysosome. This type of autophagy does not affect organelles or other macromolecules [11] (Figure 1C).
Autophagy can be selective or non-selective [12].
(i) Selective autophagy: this can achieve the specific removal of harmful substances, cellular organelles, and specific proteins [13]. These components can be recognized by specific receptors. The three types of autophagy mentioned above can act in the following way [14]. There are different nomenclatures for the elimination of different types of organelles, such as "mitophagy" for the elimination of mitochondria. This is very important because its alteration can be related to some neurodegenerative diseases, some of them with ocular involvement [9].
(ii) Non-selective autophagy: this is a survival mechanism that is activated by the stress caused by the absence of nutrients. It serves to randomly recycle cellular components and compensate for this deficit. It can be used through both macrophagy and microphagy [15].
2.2. Regulation of Autophagy: Signaling Pathways
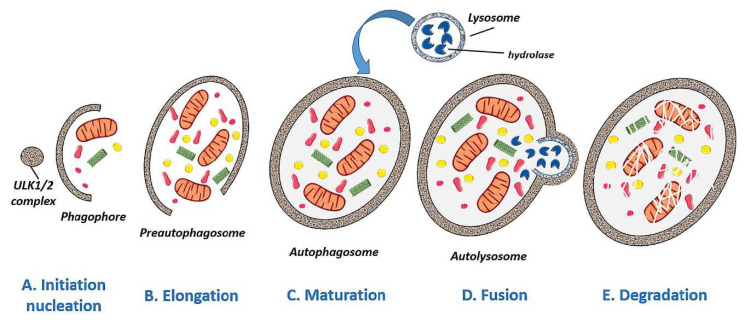
Autophagy can be divided into several phases: initiation/nucleation, elongation, maturation, fusion, and degradation. A nutrient deficiency induces the initiation or nucleation of autophagy and with it the formation of an insulating membrane that envelops damaged proteins and organelles (Figure 2A). During elongation, the double membrane of the phagophore extends and eventually closes around the contents to be degraded (Figure 2B), becoming a mature autophagosome (Figure 2C). Finally, the mature autophagosome will fuse with a lysosome to form an autolysosome (Figure 2D) and degrade its contents by proteases and lipases [9] (Figure 2E).
Figure 2.
Schematic representation of the different phases of autolysosome formation. (A) Initiation/nucleation: a deficiency of nutrients induces autophagy and with it the formation of an insulating membrane to envelop damaged proteins and organelles. (B,C) Elongation and maturation: the phagophore elongates its double membrane to become a fully-closed mature autophagosome. (D,E) Fusion and degradation: the autophagosome fuses with a lysosome to form an autolysosome, digesting the material by enzymes (lipases and proteases). (Created in part with smart.servier.com, accessed date 20 February 2021).
The mTOR (mammalian Target of Rapamycin) complex is the most important regulator and prevents the initiation of the autophagy pathway by inhibiting the serine/threonine kinase ULK1 when growth factors and nutrients are abundant [16,17]. When nutrients are scarce, the ATP level is greatly reduced, causing the ratio of ATP to AMP to decompensate. This decompensation leads to the activation of AMPK (AMP-activated protein kinase) and, subsequently, to the phosphorylation of ULK1, initiating autophagy [18]. Next, the ULK1 complex binds to membranes containing autophagy-related protein 9 (ATG9), followed by ULK1-dependent phosphorylation of ATG9, thus forming the phagophore [19]. Vesicle nucleation is then initiated by phospholipid incorporation, and a multiprotein complex with phosphatidylinositol 3-kinase (PI3K) activity is recruited. For elongation, the vacuolar protein VPS34 synthesizes phosphatidylinositol 3-phosphate (PI3P) [20]. Subsequently, the autophagosome membranes bind to PI3P-binding ATG proteins and beta-transducin (WD) repeat protein elements that interact with the phosphoinositide (WIPI) family, resulting in the phagosome membranes being completely closed [21]. This mature autophagosome can move and fuse with lysosomes, creating an autolysosome. The contents are then degraded by lysosomal hydrolases to produce energy or synthesize new structures [22]. mTOR signaling (the most important autophagic regulator) is inhibited by p53 and ATG (autophagy-related gene) proteins [23]. Another signaling pathway that regulates autophagy is eukaryotic initiation factor 2α (eIF2α). This factor can be activated in response to endoplasmic reticulum stress, nutrient deficiency, and the presence of double-stranded RNA [24].
2.3. Physiological Functions of Autophagy
Many factors are capable of inducing the autophagy process, including oxygen deprivation, nutrient deficiency, or infectious processes. Therefore, it is necessary for this process to be well regulated to provide correct cellular and tissue homeostasis [9]. Autophagy is involved in processes that are essential for cell survival, as it is able to remove altered cellular organelles, eliminate viruses and bacteria from cells, and prevent the accumulation of altered proteins [25,26]. In fact, it has been shown that if two regulators of autophagy are removed, damaged organelles and proteins accumulate, thus increasing the level of reactive oxygen species (ROS) and damaging intracellular components [27,28,29]. In addition, autophagy plays an important role in the maintenance of metabolism and energy levels in both adult and newborn tissues, as has been observed during delivery when the maternal nutrient supply through the placenta is interrupted [30]. In the immune system, autophagy stimulates cytosolic antigen presentation mediated by the major histocompatibility complex class II (MHC II) and affects T-cell and B-cell homeostasis [31]. Other physiological functions of autophagy include protection against oxidative damage to aged membranes and nervous system proteins [32,33], ischemia, hypertension, and response to tumor suppressor genes [34].
Regarding the physiological role of autophagy in the human eye, autophagy also has an important function in cellular homeostasis. Many cells in this organ have a high cellular metabolism rate, a low rate of cell division, or are highly differentiated non-dividing cells [35]. These cells exist in a highly oxidative environment provided by exposure to visible light and ultraviolet radiation which can induce cellular damage [36]. To cope with this oxidative damage, most cell types in the eye make use of autophagy as a cytoprotective method [37]. It has been shown that macro-autophagy is a widespread process that occurs to a greater or lesser degree in different regions of the eye, and selective processes such as mitophagy play fundamental roles in mitochondrial homeostasis [38].
Autophagy-related proteins are highly expressed in the cells of ocular tissues, and in the retina there is greater expression of these proteins during the day [32]. The diurnal variation of autophagosome formation is highly dependent on light; in fact, it has been shown that the concentration of autophagosomes decreases greatly in animals kept in constant darkness [39]. In addition, autophagy processes in retinal pigment epithelium (RPE) cells have been shown to be essential for the functioning of these cells, as they are involved in the phagocytosis of photoreceptor outer segments [40].
2.4. Pathological Implications of Autophagy
Autophagy is a process that needs to be under controlled balance. If there is any failure in autophagy pathways, serious pathologies could occur in the organism. An imbalance in autophagy can stimulate the initiation of neurodegenerative diseases such as Huntington’s, Parkinson’s and Alzheimer’s diseases [32,33,41]. Autophagy is essential for cell survival in many solid tumors, as it may be involved in deactivation of apoptosis processes, inducing tumor progression [42,43]. In addition, oncogenes that activate mTOR, and thereby inhibit autophagy, are known to exist [44]. Furthermore, in ocular pathologies, a reduction in autophagy efficiency is associated with diseases such as cataracts, glaucoma, diabetic retinopathy (DR), and age-related macular degeneration (AMD). Therefore, an understanding of the functional roles of autophagy processes in ocular tissues is necessary to allow them to be incorporated into therapeutic strategies for certain ocular pathologies. It is very important to understand the mechanisms and functional roles associated with autophagy in specific cells of the eye before using it as a therapeutic strategy [45].
3. Autophagy in Ocular Diseases
Under normal conditions, autophagy plays an important role in the maintenance of cellular homeostasis through the recycling of cytoplasmic proteins and cellular organelles [2]. However, disruption of normal autophagy processes is implicated in the pathophysiology of major ocular diseases [46,47,48,49].
3.1. Glaucoma
Oxidative stress plays a crucial role in the development of glaucoma by damaging the cells of the trabecular meshwork (TM), which are responsible for regulating the outflow of aqueous humor and maintaining intraocular pressure (IOP) [50]. Therefore, as an individual ages, oxidative damage accumulates, contributing to cell death by apoptosis and the development of glaucoma [51]. To protect themselves from constant oxidative stress and maintain intracellular homeostasis, TM cells activate autophagy to eliminate damaged proteins and organelles. Autophagic activity begins to saturate during the lifetime of an individual, since non-degradable materials accumulate in the lysosome compartment, which consequently causes a decrease in lysosomal activity. This decrease in autophagy leads to progressive dysfunction of TM cells and may contribute to the development of a glaucomatous pathology [52,53,54,55]. Elevated IOP can induce autophagy independently of mTOR to maintain cellular homeostasis in TM cells and cope with the mechanical forces by which they are affected. Porter et al. (2014) [53] conducted an in vitro experiment with pig eyes in which they applied a normal physiological pressure (8 mmHg) to some eyes (control eyes) and a high pressure to others (30 mmHg). Compared with controls, eyes subjected to high pressure showed increased levels of LC3-II (Microtubule-associated protein-1 light chain 3-Type II), a membrane component that is converted from LC3-I (Type I) to initiate autophagosome formation and elongation. In addition, ultrastructural analysis showed the presence of autophagosomes and autolysosomes in TM cells in those eyes subjected to high pressure, a result that was not observed in control eyes.
During the process of optic nerve cell damage in glaucoma, elevated IOP induces the activation of genes related to autophagosome formation (including Atg5, Atg7, Atg12) and autophagy markers such as Beclin-1 and LC3, to increase dendritic autophagic activity [47]. Optineurin has been identified as an autophagy receptor that is involved in cytosolic bacterial removal and the regulation of selective and nonselective autophagy [56,57,58,59,60]. Mutations in the optineurin gene have been associated with normotensive glaucoma [61]. In a study conducted on transgenic mice, overexpression of the most common mutation of the optineurin gene (OPTN E50K) was shown to cause a loss of retinal ganglion cells (RGCs) and other cell types [62,63]. In cultured TM cells from donors without an ophthalmic pathology, it was observed that the amount of p62 protein was higher in cells from young individuals than in those from old individuals (over 60 years of age), indicating that autophagy in TM decreases with the aging process [54]. The increased levels of oxidative stress present in glaucoma as a consequence of mitochondrial alteration would be counteracted by mitophagy, which could exert a neuroprotective effect on RGCs [64,65,66].
The optic nerve axotomy induces the death of RGCs, a process that occurs in glaucoma, and therefore serves as a model to study the development of this disease. A previous study showed that, five days after mouse optic nerve axotomy, an increase in autophagy occurred and this was associated with autophagosome formation and overexpression of the autophagy regulator Atg5. It was demonstrated that autophagy stimulated RGC survival after axotomy [65]. In this study, mitochondria within autophagosomes were also observed in RGCs. In a similar model of optic nerve axotomy developed in aged autophagy-deficient transgenic mice, it was shown that old individuals were more susceptible to damage compared with young individuals. These aged mice showed alterations in the oxidative stress response and mitochondrial alterations that contributed to increased axonal damage in RGCs [67]. In another study, in a rat model of glaucoma, autophagy was shown to first occur in the dendrites of RGCs rather than in the cell cytoplasm. In addition, autophagosomes located in the dendrites were found to contain organelles, suggesting that mitophagic activity might be active in dendrites [68]. This provides evidence that autophagy could first be activated by cytoprotective stress, but if such stress reaches a limit, apoptosis may be activated. However, in another study, it was shown that, one hour after optic nerve axotomy in rats, autophagosomes appeared in RGC axons. Furthermore, when the autophagy process was inhibited, injury-induced axonal degeneration was enhanced [69].
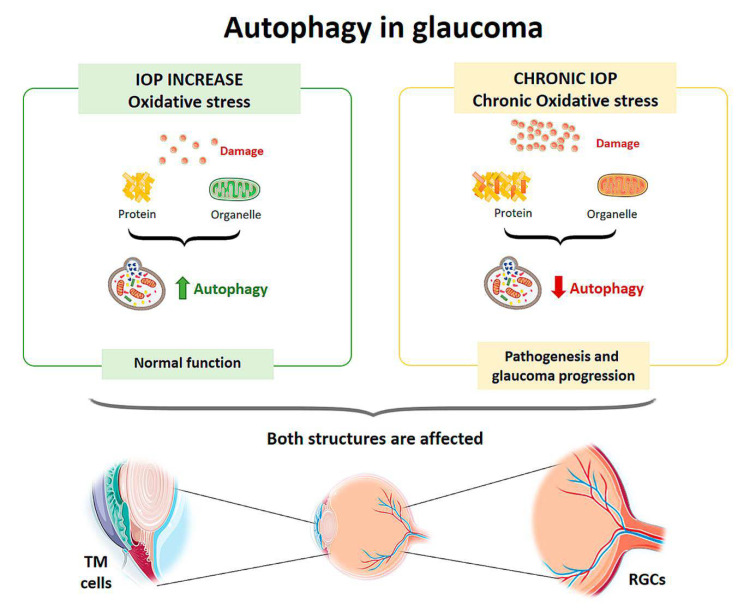
In cultures of human TM cells derived from glaucomatous eyes in which IOP had been elevated for a long time, when cells were exposed to high oxygen concentrations (40% O2), autophagy processes were not activated [55]. Control TM cells from the healthy eye showed an autophagic response to hyperoxia, as there was an upregulation of LC3-II levels, but cells from glaucomatous eyes did not show a variation in this structural protein. Increased LC3 immunoreactivity in the RGC layer as well as LC3-II accumulation was also observed between 6 and 24 h after the transient increase in IOP in an experimental model developed in rats [70,71]. Impairment of the autophagy process by calpain-mediated cleavage of beclin-1 was also demonstrated in this same experimental model [72]. In a transgenic mouse model with altered basal autophagic flux by ablation of the Beclin-1-regulated autophagy-activated molecule (AMBRA-1), increased RGC loss in response to ischemia was observed after a transient increase in IOP [73]. Furthermore, in a transgenic mouse model with impaired autophagic flux and chronic hypertension DBA/2J::GFP-LC3, which express the autophagosome LC3, higher IOP values, further RGC loss, and aggravated axonal impairment were observed compared with the spontaneous ocular hypertensive DBA/2J mouse model [74]. All of these studies demonstrate that autophagy is rapidly activated at the onset of the disease in response to elevated IOP, but if these conditions were chronic autophagic activity would become saturated and cease to function properly. Therefore, the disruption of autophagic flux could be involved in the pathogenesis and progression of glaucoma [75] (Figure 3).
Figure 3.
Scheme of the main autophagy changes related to glaucoma. IOP: intraocular pressure; TM cells: trabecular meshwork cells; RGCs: retinal ganglion cells. (Created in part with smart.servier.com, accessed date 20 February 2021).
3.2. Cataract
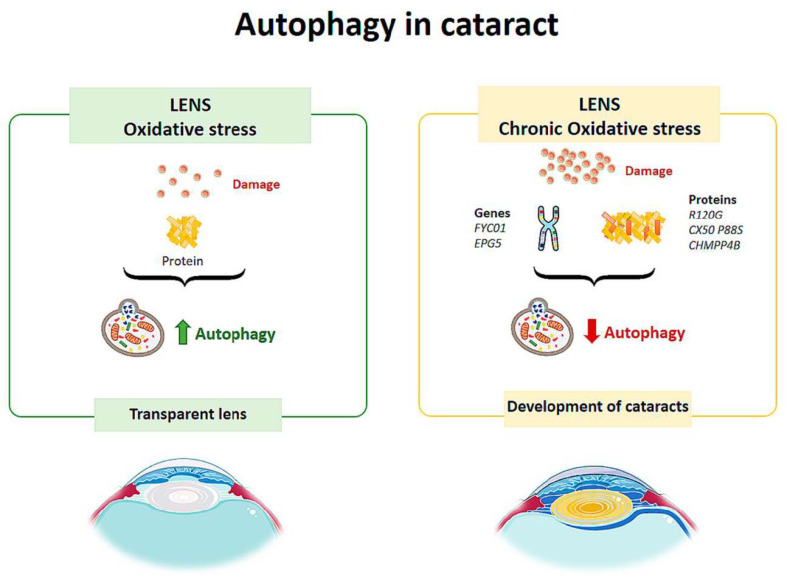
Autophagic vesicles containing mitochondria or fragments of mitochondria have been observed in human lens fibers. Costello et al. [76] performed a study to confirm the presence of autophagic activity in the lens and demonstrated its presence. Therefore, autophagy helps to maintain the integrity of the cells by contributing to the maintenance of the physical properties of the lens [76] (Figure 4). Disturbances in the elimination of organelles in the cytoplasm of lens fibers increase ROS, altering the homeostasis of the lens, decreasing its transparency and leading to the development of cataracts [77] (Figure 4).
Figure 4.
Scheme of the main autophagy changes related to cataract. (Created in part with smart.servier.com, accessed date 20 February 2021).
TBC1D20 (TBC1 domain family member 20) is a key regulator of autophagosome maturation. There is evidence that regulation by TBC1D20 maintains lens transparency. A study conducted on mice tested what would happen if TBC1D20-mediated autophagic flux was altered [78] and found that the animals presented cataracts in the lens nucleus, but the cortex remained transparent. Over time, the pathology progressed, and cataracts completely occupied the lens. In addition, it was observed that the lens fibers were highly degenerated and disorganized, and there was an accumulation of ubiquitin aggregates. Therefore, alteration of the autophagic flux mediated by TBC1D20 resulted in the accumulation of autophagic material in the lens fibers and the formation of cataracts [78]. In another study, Atg5 (autophagy-related protein) knock-out mice showed opacity in the cortical region of the lens after 6–9 months, and by 21 months they had cataracts [79]. The lenses of these mice showed age-dependent cellular disorganization and accumulation of abnormal materials. Accumulation of insoluble p62, ubiquitin aggregates and oxidized proteins was also observed in the lens fibers [79]. These results suggest that autophagy is critical for maintaining intracellular homeostasis in the lens.
Congenital cataracts are directly related to mutations in several genes. These include recessive mutations in the FYCO1 gene (FYVE domain and autophagy adaptor coiled-coil domain 1), which codes for a binding protein that regulates adaptor protein (AP) transport [80], a process that is essential for autolysosome formation. There are other mutations in this pathology that may be related to autophagy (Figure 4). Vsp34 is a kinase that plays a role in autophagy, so its loss results in the accumulation of ubiquitin and p62 aggregates in lens cells, affecting tissue transparency [79]. In an inherited cataract model, the R120G mutation in αβ-crystallin (a chaperone that binds to misfolded proteins to prevent their aggregation) results in increases in the LC3-II level and autophagosome size and up-regulates the level of p62 in lens fibers and lens epithelial cells [81]. The CX50 P88S mutation results in a failure of autophagic activity, causing a type of congenital cataract [82]. The EPG5 gene encodes for a specific autophagic protein. Its deletion in experimental animals mimics the symptoms of Vici syndrome, which is an inherited disease that causes cataracts, among other pathologies. In a 2010 study by Tian et al., it was found that the deletion of EPG5 in C. elegans resulted in the accumulation of autolysosomes that did not degrade their contents [83]; therefore, it was demonstrated that EPG5 is important for the maturation of the autolysosome and that autophagy is involved in this hereditary pathology. Components of the endosomal sorting complex required for transport (ESCRT) are necessary for autophagy and the completion of cytokinesis. The ESCRT subunit called CHMPP4B localizes micronuclei (structures derived from an altered cell division), which are subsequently encircled by lysosomes, and autophagosomes, playing a fundamental role in the process of autophagy [84]. The CHMPP4B mutation is associated with congenital cataracts. This mutation prevents the localization of micronuclei, preventing correct autophagic degradation. Thus, it is suggested as a possible pathway for the development of this pathology.
3.3. Diabetic Retinopathy
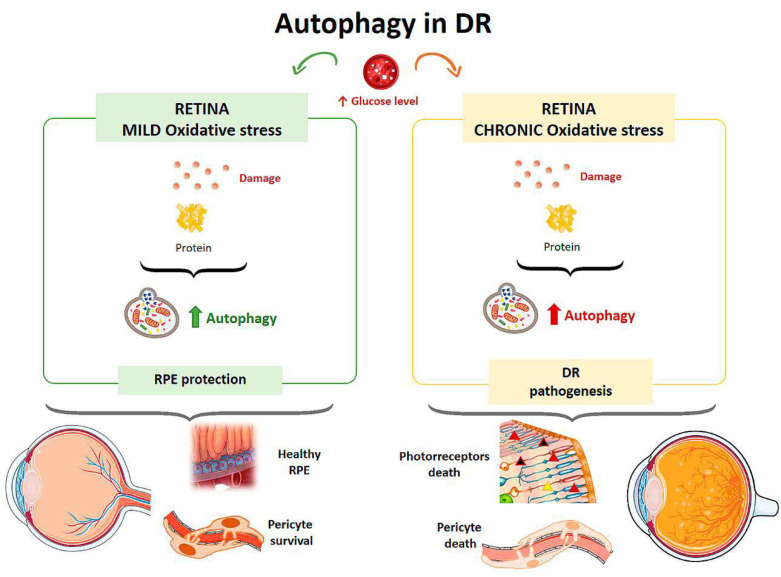
In diabetic patients, at the cellular level, hyperglycemia causes the activation of metabolic pathways, resulting in an increase in reactive oxygen species, the appearance of advanced glycation end products, and the release of proinflammatory cytokines, all of which lead to cell death mediated by apoptosis, necroptosis, and autophagy [85]. Therefore, the retinal damage suffered by diabetic patients is related to autophagic events [66].
Autophagy is responsible for removing damaged proteins, providing a defense mechanism against RPE damage, but under conditions of excess metabolic stress autophagic activity is impaired [85] (Figure 5). High glucose levels also affect Müller cells, which are the major source of VEGF (Vascular Endothelial Growth Factor) in DR. In a study on the effect of hyperglycemia on the retina, rat retinal Müller cells were treated with normal or high glucose levels with/without inhibitors and activators for p62 for 24 hours [86]. Cells in contact with high glucose levels responded with increased autophagic activity and endoplasmic reticulum stress associated with increased apoptosis. By inhibiting autophagy in cells treated with high glucose levels, there was an increased number of Müller cells in apoptosis [86]. In a diabetic mouse model, the physiological autophagic flux was observed to be altered, with increased LC3 at the level of the outer plexiform layer and up-regulation of the autophagic proteins Beclin-1 and Atg5 [87].
Figure 5.
Scheme of the main autophagy changes related to diabetic retinopathy (DR). RPE: retinal pigment epithelium. (Created in part with smart.servier.com, accessed date 20 February 2021).
The effect of hyperglycemia on autophagic activity has been demonstrated in cultured human RPE cells [88]. Cultures were incubated in media with a normal glucose concentration (5 mM) and media with a high glucose concentration (30 mM). Cells treated with high glucose levels showed an increased amount of double membrane vacuoles, typical of autophagosomes, at 48 hours after incubation compared with the culture with a normal glucose concentration. In addition, after exposure to hyperglycemic conditions, cells showed higher levels of LC3 in the cytoplasm, indicating an elevated autophagic response. Autophagy induced by high glucose levels was found to be mTOR-dependent but mainly regulated by ROS-mediated endoplasmic reticulum stress signals. Therefore, under a scenario of oxidative stress induced by high glucose levels, autophagy is required to eliminate damaged proteins and provide a mechanism to prevent RPE induced damage [88] (Figure 5). Compared to a control culture, cells that were treated with a high concentration of glucose and were deficient in autophagic processes exhibited a strong reduction in cell viability, suggesting that autophagy plays a protective role for RPE cells in the presence of a DR-common environment [88].
The loss of pericytes characteristic of DR leads to increased capillary permeability. Studies on the effect of autophagy performed in a mouse model of diabetes and hypercholesterolemia [89] showed that when low-density lipoproteins (LDL) leaving the vessels accumulate, several cycles of cellular damage are triggered. LDLs were found to cause endoplasmic reticulum stress, oxidative stress, and mitochondrial dysfunction, and these effects induced apoptosis in RPE cells. Autophagy plays a dual role on human retinal capillary pericytes. Thus, autophagy stimulates pericyte survival under mild stress, but if the stress is chronic, excessive autophagic activity results in pericyte necrosis [89] (Figure 5).
In mice in which RD was induced, photoreceptor death occurred before retinal vascular alterations appeared, possibly due to the increase in autophagic activity observed in these cells [87] (Figure 5). The circadian rhythm and diurnal variations in the expression of autophagic proteins are characteristic properties of the retina. In DR, it has been demonstrated that there is an alteration of this biological clock that affects cellular processes such as the regulation of inflammation or lipid metabolism [90,91,92].
3.4. Age-Related Macular Degeneration
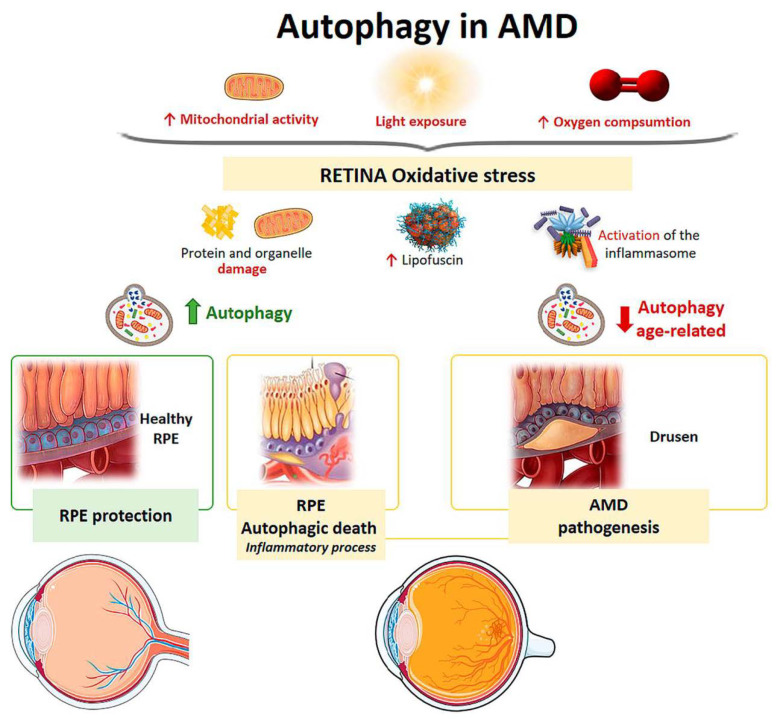
Recent studies have shown that RPE cells undergo important pathological changes in AMD, and the alteration of autophagy processes in these cells may play a key role in the development of this pathology [93]. In RPE cells from AMD donors, autophagy levels were found to be decreased with respect to RPE cells from healthy controls [94]. This shows that autophagy is closely related to the pathogenesis of AMD [95].
Mitochondria from human RPE cells exposed to blue light release ROS, resulting in increased damage to the mitochondrial DNA of these cells [96]. Endogenous ROS generation induces autophagy, and it has been observed that, under conditions of oxidative stress, RPE cells in AMD patients generate elevated levels of ROS compared with healthy RPE cells [94]. When oxidative stress occurs continuously over time, mitophagy is activated as a mechanism to protect the cell against this stress. RPE cell dysfunction in AMD donor samples has been linked to mitochondrial DNA injury, and as the disease severity increases, there is greater damage and reduced ability to repair [97]. Therefore, selective removal of damaged mitochondria by autophagy (mitophagy) could be essential for cell survival (Figure 6).
Figure 6.
Scheme of the main autophagy changes related to age related macular degeneration (AMD). RPE: retinal pigment epithelium. (Created in part with smart.servier.com, accessed date 20 February 2021).
The retina is under intense oxidative stress due to high oxygen consumption, light exposure and high mitochondrial activity leading to the release of ROS in the RPE. To defend themselves, these cells increase the production of anti-oxidants, which decrease with aging, causing severe oxidative damage and progression of AMD. Oxidative stress can be induced by H2O2. In vitro models have shown that autophagy-associated cell death occurs in ARPE-19 cells and primary human RPE cells by serum deprivation, and H2O2 (2 h, 1 mM) [98,99]. The phenomenon means, firstly, an induction of a high level of autophagy, which is the cause of cell death, thus autophagic death occurs. Furthermore, these RPE cells undergoing autophagy-associated cell death participate in elimination mechanisms guided by professional and nonprofessional phagocytes and are accompanied by the induction of inflammation. Therefore, this process may be involved in the pathogenesis of AMD [98,99] (Figure 6).
Retinal neovascularization may be related to oxidative stress, pyroptosis (a highly inflammatory form of programmed cell death), and impaired autophagy [100]. Another pathological feature of AMD is the accumulation of lipofuscin in the lysosomes of RPE cells [101]. Lipofuscin is a heterogeneous mixture of lipoproteins that sensitize RPE cells to light exposure and oxidative stress, leading to apoptosis [32,86,102]. A2E (N-retinylidene-N-retinyl-ethanolamine) is a lipofuscin fluorophore that is toxically deposited in the RPE cells of AMD patients. Zhang et al. found that A2E triggers autophagy in RPE cells during the early stages of AMD. Inhibition of autophagy in RPE cells with A2E results in elevated levels of proinflammatory and proangiogenic factors [103]. Therefore, an increase in autophagic activity protects against the harmful effects of A2E.
It has also been observed that reduced autophagic activity is involved in the susceptibility of RPE cells to photo-oxidative damage and, consequently, in the dysfunction of these cells [104,105,106]. Protein aggregates induce the activation of inflammasomes (multiprotein complexes involved in inflammatory processes and pyroptosis) in RPE cells. Increased autophagic activity reduces intracellular debris and causes a decrease in inflammasome generation that could prevent the inflammatory response in AMD [107]. Therefore, if high protein aggregation contributes to inflammasome activation and tissue damage, a decrease in autophagy together with elevated inflammation could be involved in drusen generation [108,109] (Figure 6).
It has been shown that in RPE cells and photoreceptors of wild-type mice, autophagic levels remain elevated and that exposure to light triggers an autophagic response in these cell types [106]. During the early phase of AMD, autophagic activity acts as protection against damage to organelles and oxidative stress (Figure 6). In human and old mouse AMD samples, LC3, Atg5 were found to be elevated in RPE cells and in drusen [106]. RPE cells from AMD patients have been shown to have impaired mitophagy, resulting in tissue aging [110]. The role of autophagy in the homeostasis of aged RPE cells was demonstrated in mice through a deletion in the gene encoding for the RB1CC1 protein, which is essential for autophagic induction [111]. These mice developed AMD with altered RPE characterized by atrophic patches, subretinal migration of microglia, oxidatively damaged protein deposition, and choroidal neovascularization [111]. RPE degeneration was associated with the loss of neighboring photoreceptors, demonstrating the importance of autophagy in the aging process. βA3/A1-crystallin is a protein that regulates lysosome-mediated degradation in the EPR through the activity of V-ATPase, a proton pump that acidifies the lysosomal lumen through the AKT/mTOR signaling pathway [112,113]. This has been observed in animals deficient in βA3/A1-crystallin, encoded by the Cryba-1 gene, who present a decrease in the lysosomal activity of the EPR, as occurs in patients with AMD. [114].
Aging is the main risk factor for developing AMD. In a study on mouse retinas, autophagy levels were compared at different ages [115]. After lysosomal blockade, aged retinas exhibited lipofuscin-laden lysosomes and ubiquitin aggregates characteristic of autophagic impairment. It was shown that chaperone-mediated up-regulation of autophagy would lead to increased lysosome-dependent proteolysis in aged retinas and that there was uncoordination between autophagic pathways in cone-type photoreceptors. The study proposed that this lack of coordination between the different autophagic pathways could be responsible for the specific pattern of visual loss that occurs in aging (Figure 6).
3.5. Autophagy as a Therapy in Ocular Pathologies
In recent years, the efficacy of certain drugs that utilize autophagy processes in the treatment of different ocular pathological processes has been proven. These drugs include Rapamycin (RAP), AMPK activator, proteasome inhibitor (MG-132), chloroquine, and hydroxychloroquine, among others [116].
In most ocular diseases, proteolytic and autophagic capacity is attenuated. Therefore, some pharmacological activators of autophagy are being studied as potential therapies for ocular pathologies.
A drug that acts as a regulator of the autophagy process is Rapamycin, and it is thought to have a potential therapeutic effect in AMD. It acts through the inhibition of mTOR, which leads to an increase in the autophagy process, protecting RPE cells from A2E toxicity and increasing cell viability [103]. In addition to increasing autophagic flux by inhibiting the mTOR pathway, rapamycin treatment, in conjunction with caloric restriction, has been shown to exert a neuroprotective effect on RGCs following transient IOP increase in experimental models of ischemia-reperfusion [73]. This neuroprotective effect of rapamycin has also been observed in animal models of optic nerve axotomy [65] and in models of chronic glaucoma [117].
Resveratrol (natural phenol) and MG-132 (proteasome inhibitor) provide survival stimuli to RPE cells through autophagic induction, so they could be used to prolong the lifetime of RPE cells in AMD [116]. AMPK (AMP-activated protein kinase) is an inhibitor of mTOR, so AMPK-mTOR could be a good target for treating AMD [118]. Metformin regulates AMP kinase activity, which stimulates the initiation of autophagy [119]. Other substances, such as lithium and valproic acid, stimulate the induction of autophagy [120,121].
The autophagy process could also be stimulated by using microRNAs. These are endogenous small RNAs that regulate gene expression at the post-transcriptional level. Cai et al. showed that the overexpression of micro-RNA-29 (miR-29) in RPE cells could rescue degenerating cells by enhancing autophagy through inhibiting the activity of mTORC1 [122].
Hormones such as 17β-Estradiol and melatonin could promote the autophagy process. Wei et al. 2018 found that 17β-estradiol (βE-2) enhanced autophagy and protected RPE cells from oxidative stress induced by a blue light emitting diode (LED) [123]. Melatonin, a neurohormone derived from tryptophan, which has crucial effects on several systems (circadian rhythm, aging, immune system, and cardiovascular system), as well as being a potent antioxidant, has been shown to positively regulate the autophagy process [95]. It has been shown that in RPE cells exposed to H2O2 (potent inducer of oxidative damage) and treated with melatonin, there was an increase in autophagy mediated by the up-regulation of LC3-II and Beclin-1 and down-regulation of P62 [124].
Some foods with antioxidant properties may help regulate the autophagy process [95]. Intake of fish and nuts can provide n-3 polyunsaturated fatty acids (PUFAs), which may reduce the content of misfolded proteins and inhibit oxidative stress by enhancing autophagy in pathologies such as AMD [125]. In addition, dietary polyphenols found in vegetables, fruits, legumes, or tea can also promote autophagy. This improves the elimination of cellular debris, thereby decreasing oxidative damage, and may help to prevent the development of pathologies such as AMD [126].
On the other hand, excessive autophagic activity may accelerate the development of ocular diseases, so studies should also focus on therapies that inhibit autophagy.
Chloroquine and hydroxychloroquine suppress autophagy by blocking autophagosome fusion and degradation of autophagic contents. Although some studies have shown that these drugs can be effective and even safe, other studies have shown that these agents can bind to the melanin of the RPE increasing the toxic effects of these drugs. Therefore, treatment with chloroquine and hydroxychloroquine may result in retinal toxicity [116].
Sodium trans-hinone IIA sulfonate (STS) has been shown to reduce the expression of the autophagic proteins Atg3, Atg7, and Atg9 by inhibiting vacuole elongation and autophagosome formation. In RPE cells, under oxidative stress conditions, STS activates the mTOR/AKT/PI3K pathway, decreasing autophagy-related cell death [127].
Although the effectiveness of drugs that act on autophagy processes is promising, these drugs nevertheless have some associated problems. They may have undesirable side effects [128,129], as they may have more than one route of action, and they may also have low specificity [128]. Therefore, there is still much to be investigated to eliminate these problems and to obtain safe and effective drugs that can treat ocular pathologies by regulating autophagy processes.
4. Conclusions
Autophagic mechanisms, which are essential for the correct homeostasis and cell viability of ocular tissues, may be altered in pathological processes such as glaucoma, diabetic retinopathy, AMD, and cataracts. The regulation and modulation of autophagy could constitute a potential therapy for the treatment of ocular pathologies.
Author Contributions
Conceptualization, J.A.F.-A., J.J.S. and A.I.R.; data curation, J.A.F.-A., E.d.J.-L., C.S.-D., R.d.H., I.L.-C., E.S.-G., J.M.R., M.D.P.-D., J.J.S. and A.I.R.; formal analysis, J.A.F.-A., E.d.J.-L., C.S.-D., R.d.H., I.L.-C., E.S.-G., J.M.R., M.D.P.-D., J.J.S. and A.I.R.; funding acquisition, J.J.S., R.d.H., J.M.R. and A.I.R.; methodology, J.A.F.-A., E.d.J.-L., C.S.-D., R.d.H., I.L.-C., E.S.-G., J.M.R., M.D.P.-D., J.J.S. and A.I.R.; visualization, J.A.F.-A., J.J.S. and A.I.R.; writing—original draft, J.A.F.-A., E.d.J.-L., C.S.-D., J.J.S. and A.I.R.; writing—review & editing, J.A.F.-A., E.d.J.-L., C.S.-D., R.d.H., I.L.-C., E.S.-G., J.M.R., M.D.P.-D., J.J.S. and A.I.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ophthalmological Network OFTARED (Enfermedades oculares: Prevención, detección precoz, tratamiento y rehabilitación de las patologías oculares) (RD16/0008/0005 and RD16/0008/0026) of the Institute of Health of Carlos III of the Spanish Ministry of Economy and by the European programme FEDER; and Network RETiBRAIN (La retina un modelo para investigar Neuroprotección en patologías del Sistema Nervioso Central) (RED2018-102499-T) of Spanish Ministry of Science and Innovation. And J.A.F.-A. is currently funded by a Predoctoral Fellowship (FPU17/01023) from the Spanish Ministry of Science, Innovation, and Universities; and I.L.-C. is currently funded by a Predoctoral Fellowship (CT42/18-CT43/18) from the Complutense University of Madrid.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Klionsky D.J. Autophagy: From phenomenology to molecular understanding in less than a decade. Nat. Rev. Mol. Cell Biol. 2007;8:931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 2.Shintani T., Klionsky D.J. Autophagy in health and disease: A double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng Y., He D., Yao Z., Klionsky D.J. The machinery of macroautophagy. Cell Res. 2014;24:24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Duve C., Wattiaux R. Functions of Lysosomes. Annu. Rev. Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- 5.Cadwell K. Crosstalk between autophagy and inflammatory signalling pathways: Balancing defence and homeostasis. Nat. Rev. Immunol. 2016;16:661–675. doi: 10.1038/nri.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeshige K., Baba M., Tsuboi S., Noda T., Ohsumi Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J. Cell Biol. 1992;119:301–312. doi: 10.1083/jcb.119.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsukada M., Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993;333:169–174. doi: 10.1016/0014-5793(93)80398-E. [DOI] [PubMed] [Google Scholar]
- 8.Viiri J., Amadio M., Marchesi N., Hyttinen J.M.T., Kivinen N., Sironen R., Rilla K., Akhtar S., Provenzani A., D’Agostino V.G., et al. Autophagy Activation Clears ELAVL1/HuR-Mediated Accumulation of SQSTM1/p62 during Proteasomal Inhibition in Human Retinal Pigment Epithelial Cells. PLoS ONE. 2013;8:e69563. doi: 10.1371/journal.pone.0069563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X., Pan X., Zhao X., Luo J., Xu M., Bai D., Hu Y., Liu X., Yu Q., Gao D. Autophagy and Age-Related Eye Diseases. Biomed. Res. Int. 2019;2019:1–12. doi: 10.1155/2019/5763658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W., Li J., Bao J. Microautophagy: Lesser-known self-eating. Cell. Mol. Life Sci. 2012;69:1125–1136. doi: 10.1007/s00018-011-0865-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooley P.L., Dice P.F. Corneal Dystrophy in the Dog and Cat. Vet. Clin. N. Am. Small Anim. Pract. 1990;20:681–692. doi: 10.1016/S0195-5616(90)50057-1. [DOI] [PubMed] [Google Scholar]
- 12.Kaden T.R., Li W. Autophagy, Mitochondrial Dynamics, and Retinal Diseases. Asia-Pac. J. Ophthalmol. 2013;2:341–348. doi: 10.1097/APO.0b013e31829d3e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin M., Liu X., Klionsky D.J. SnapShot: Selective Autophagy. Cell. 2013;152:368–368.e2. doi: 10.1016/j.cell.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farré J.-C., Subramani S. Mechanistic insights into selective autophagy pathways: Lessons from yeast. Nat. Rev. Mol. Cell Biol. 2016;17:537–552. doi: 10.1038/nrm.2016.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galluzzi L., Baehrecke E.H., Ballabio A., Boya P., Bravo-San Pedro J.M., Cecconi F., Choi A.M., Chu C.T., Codogno P., Colombo M.I., et al. Molecular definitions of autophagy and related processes. EMBO J. 2017;36:1811–1836. doi: 10.15252/embj.201796697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J., Kundu M., Viollet B., Guan K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zoncu R., Efeyan A., Sabatini D.M. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J.W., Park S., Takahashi Y., Wang H.-G. The Association of AMPK with ULK1 Regulates Autophagy. PLoS ONE. 2010;5:e15394. doi: 10.1371/journal.pone.0015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orsi A., Razi M., Dooley H.C., Robinson D., Weston A.E., Collinson L.M., Tooze S.A. Dynamic and transient interactions of Atg9 with autophagosomes, but not membrane integration, are required for autophagy. Mol. Biol. Cell. 2012;23:1860–1873. doi: 10.1091/mbc.e11-09-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kihara A., Noda T., Ishihara N., Ohsumi Y. Two Distinct Vps34 Phosphatidylinositol 3–Kinase Complexes Function in Autophagy and Carboxypeptidase Y Sorting inSaccharomyces cerevisiae. J. Cell Biol. 2001;152:519–530. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimmel M., Backhaus C., Proikas-Cezanne T. WIPI-Mediated Autophagy and Longevity. Cells. 2015;4:202–217. doi: 10.3390/cells4020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zoukhri D., Fix A., Alroy J., Kublin C.L. Mechanisms of Murine Lacrimal Gland Repair after Experimentally Induced Inflammation. Investig. Opthalmol. Vis. Sci. 2008;49:4399–4406. doi: 10.1167/iovs.08-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baird L., Dinkova-Kostova A.T. The cytoprotective role of the Keap1–Nrf2 pathway. Arch. Toxicol. 2011;85:241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 24.Chai P., Ni H., Zhang H., Fan X. The Evolving Functions of Autophagy in Ocular Health: A Double-edged Sword. Int. J. Biol. Sci. 2016;12:1332–1340. doi: 10.7150/ijbs.16245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizushima N., Klionsky D.J. Protein Turnover Via Autophagy: Implications for Metabolism. Annu. Rev. Nutr. 2007;27:19–40. doi: 10.1146/annurev.nutr.27.061406.093749. [DOI] [PubMed] [Google Scholar]
- 26.Pankiv S., Clausen T.H., Lamark T., Brech A., Bruun J.-A., Outzen H., Øvervatn A., Bjørkøy G., Johansen T. p62/SQSTM1 Binds Directly to Atg8/LC3 to Facilitate Degradation of Ubiquitinated Protein Aggregates by Autophagy. J. Biol. Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 27.Hara T., Nakamura K., Matsui M., Yamamoto A., Nakahara Y., Suzuki-Migishima R., Yokoyama M., Mishima K., Saito I., Okano H., et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 28.Komatsu M., Waguri S., Chiba T., Murata S., Iwata J., Tanida I., Ueno T., Koike M., Uchiyama Y., Kominami E., et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 29.Takamura A., Komatsu M., Hara T., Sakamoto A., Kishi C., Waguri S., Eishi Y., Hino O., Tanaka K., Mizushima N. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011;25:795–800. doi: 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuma A., Hatano M., Matsui M., Yamamoto A., Nakaya H., Yoshimori T., Ohsumi Y., Tokuhisa T., Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 31.Harris J., Master S.S., De Haro S.A., Delgado M., Roberts E.A., Hope J.C., Keane J., Deretic V. Th1–Th2 polarisation and autophagy in the control of intracellular mycobacteria by macrophages. Vet. Immunol. Immunopathol. 2009;128:37–43. doi: 10.1016/j.vetimm.2008.10.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitter S.K., Song C., Qi X., Mao H., Rao H., Akin D., Lewin A., Grant M., Dunn W., Ding J., et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy. 2014;10:1989–2005. doi: 10.4161/auto.36184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawashima M., Ozawa Y., Shinmura K., Inaba T., Nakamura S., Kawakita T., Watanabe M., Tsubota K. Calorie restriction (CR) and CR mimetics for the prevention and treatment of age-related eye disorders. Exp. Gerontol. 2013;48:1096–1100. doi: 10.1016/j.exger.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Nishida K., Kyoi S., Yamaguchi O., Sadoshima J., Otsu K. The role of autophagy in the heart. Cell Death Differ. 2009;16:31–38. doi: 10.1038/cdd.2008.163. [DOI] [PubMed] [Google Scholar]
- 35.Rehen S.K., Neves D.D.C., Fragel-Madeira L., Britto L.R.G., Linden R. Selective sensitivity of early postmitotic retinal cells to apoptosis induced by inhibition of protein synthesis. Eur. J. Neurosci. 1999;11:4349–4356. doi: 10.1046/j.1460-9568.1999.00868.x. [DOI] [PubMed] [Google Scholar]
- 36.Kaarniranta K., Sinha D., Blasiak J., Kauppinen A., Veréb Z., Salminen A., Boulton M.E., Petrovski G. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy. 2013;9:973–984. doi: 10.4161/auto.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frost L.S., Mitchell C.H., Boesze-Battaglia K. Autophagy in the eye: Implications for ocular cell health. Exp. Eye Res. 2014;124:56–66. doi: 10.1016/j.exer.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McWilliams T.G., Prescott A.R., Villarejo-Zori B., Ball G., Boya P., Ganley I.G. A comparative map of macroautophagy and mitophagy in the vertebrate eye. Autophagy. 2019;15:1296–1308. doi: 10.1080/15548627.2019.1580509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Remé C., Wirz-Justice A., Rhyner A., Hofmann S. Circadian rhythm in the light response of rat retinal disk-shedding and autophagy. Brain Res. 1986;369:356–360. doi: 10.1016/0006-8993(86)90550-0. [DOI] [PubMed] [Google Scholar]
- 40.Yao J., Jia L., Shelby S.J., Ganios A.M., Feathers K., Thompson D.A., Zacks D.N. Circadian and Noncircadian Modulation of Autophagy in Photoreceptors and Retinal Pigment Epithelium. Investig. Opthalmol. Vis. Sci. 2014;55:3237–3246. doi: 10.1167/iovs.13-13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hornung J.P., Koppel H., Clarke P.G.H. Endocytosis and autophagy in dying neurons: An ultrastructural study in chick embryos. J. Comp. Neurol. 1989;283:425–437. doi: 10.1002/cne.902830310. [DOI] [PubMed] [Google Scholar]
- 42.Karantza V., White E. Role of Autophagy in Breast Cancer. Autophagy. 2007;3:610–613. doi: 10.4161/auto.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Degenhardt K., Mathew R., Beaudoin B., Bray K., Anderson D., Chen G., Mukherjee C., Shi Y., Gélinas C., Fan Y., et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang J., Shao S.H., Xu Z.-X., Hennessy B., Ding Z., Larrea M., Kondo S., Dumont D.J., Gutterman J.U., Walker C.L., et al. The energy sensing LKB1–AMPK pathway regulates p27kip1 phosphorylation mediating the decision to enter autophagy or apoptosis. Nat. Cell Biol. 2007;9:218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 45.Maiuri M.C., Zalckvar E., Kimchi A., Kroemer G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 46.Wang A.L., Lukas T.J., Yuan M., Du N., Tso M.O., Neufeld A.H. Autophagy, exosomes and drusen formation in age-related macular degeneration. Autophagy. 2009;5:563–564. doi: 10.4161/auto.5.4.8163. [DOI] [PubMed] [Google Scholar]
- 47.Kim S.H., Munemasa Y., Kwong J.M.K., Ahn J.H., Mareninov S., Gordon L.K., Caprioli J., Piri N. Activation of autophagy in retinal ganglion cells. J. Neurosci. Res. 2008;86:2943–2951. doi: 10.1002/jnr.21738. [DOI] [PubMed] [Google Scholar]
- 48.Fu C.T., Sretavan D. Involvement of EphB/ephrin-B signaling in axonal survival in mouse experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2012;53:76–84. doi: 10.1167/iovs.11-8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y., Sawada O., Kohno H., Le Y.Z., Subauste C., Maeda T., Maeda A. Autophagy protects the retina from light-induced degeneration. J. Biol. Chem. 2013;288:7506–7518. doi: 10.1074/jbc.M112.439935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McMonnies C.W. Hyperbaric oxygen therapy and the possibility of ocular complications or contraindications. Clin. Exp. Optom. 2015;98:122–125. doi: 10.1111/cxo.12203. [DOI] [PubMed] [Google Scholar]
- 51.Pinazo-Duran M.D., Shoaie-Nia K., Zanon-Moreno V., Sanz-Gonzalez S.M., del Castillo J.B., Garcia-Medina J.J. Strategies to Reduce Oxidative Stress in Glaucoma Patients. Curr. Neuropharmacol. 2018;16:903–918. doi: 10.2174/1570159X15666170705101910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Porter K., Nallathambi J., Lin Y., Liton P.B. Lysosomal basification and decreased autophagic flux in oxidatively stressed trabecular meshwork cells. Autophagy. 2013;9:581–594. doi: 10.4161/auto.23568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Porter K.M., Jeyabalan N., Liton P.B. MTOR-independent induction of autophagy in trabecular meshwork cells subjected to biaxial stretch. Biochim. Biophys. Acta Mol. Cell Res. 2014;1843:1054–1062. doi: 10.1016/j.bbamcr.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pulliero A., Seydel A., Camoirano A., Saccà S.C., Sandri M., Izzotti A. Oxidative Damage and Autophagy in the Human Trabecular Meshwork as Related with Ageing. PLoS ONE. 2014;9:e98106. doi: 10.1371/journal.pone.0098106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Porter K., Hirt J., Stamer W.D., Liton P.B. Autophagic dysregulation in glaucomatous trabecular meshwork cells. Biochim. Biophys. Acta Mol. Basis Dis. 2015;1852:379–385. doi: 10.1016/j.bbadis.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wild P., Farhan H., McEwan D.G., Wagner S., Rogov V.V., Brady N.R., Richter B., Korac J., Waidmann O., Choudhary C., et al. Phosphorylation of the Autophagy Receptor Optineurin Restricts Salmonella Growth. Science. 2011;333:228–233. doi: 10.1126/science.1205405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davis L.K., Meyer K.J., Schindler E.I., Beck J.S., Rudd D.S., Grundstad A.J., Scheetz T.E., Braun T.A., Fingert J.H., Alward W.L.M., et al. Copy Number Variations and Primary Open-Angle Glaucoma. Investig. Opthalmol. Vis. Sci. 2011;52:7122–7133. doi: 10.1167/iovs.10-5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Awadalla M.S., Fingert J.H., Roos B.E., Chen S., Holmes R., Graham S.L., Chehade M., Galanopolous A., Ridge B., Souzeau E., et al. Copy Number Variations of TBK1 in Australian Patients With Primary Open-Angle Glaucoma. Am. J. Ophthalmol. 2015;159:124–130.e1. doi: 10.1016/j.ajo.2014.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sirohi K., Kumari A., Radha V., Swarup G. A Glaucoma-Associated Variant of Optineurin, M98K, Activates Tbk1 to Enhance Autophagosome Formation and Retinal Cell Death Dependent on Ser177 Phosphorylation of Optineurin. PLoS ONE. 2015;10:e0138289. doi: 10.1371/journal.pone.0138289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ryan T.A., Tumbarello D.A. Optineurin: A Coordinator of Membrane-Associated Cargo Trafficking and Autophagy. Front. Immunol. 2018;9:1024. doi: 10.3389/fimmu.2018.01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rezaie T. Adult-Onset Primary Open-Angle Glaucoma Caused by Mutations in Optineurin. Science. 2002;295:1077–1079. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- 62.Chi Z.-L., Akahori M., Obazawa M., Minami M., Noda T., Nakaya N., Tomarev S., Kawase K., Yamamoto T., Noda S., et al. Overexpression of optineurin E50K disrupts Rab8 interaction and leads to a progressive retinal degeneration in mice. Hum. Mol. Genet. 2010;19:2606–2615. doi: 10.1093/hmg/ddq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chalasani M.L., Radha V., Gupta V., Agarwal N., Balasubramanian D., Swarup G. A Glaucoma-Associated Mutant of Optineurin Selectively Induces Death of Retinal Ganglion Cells Which Is Inhibited by Antioxidants. Investig. Opthalmol. Vis. Sci. 2007;48:1607–1614. doi: 10.1167/iovs.06-0834. [DOI] [PubMed] [Google Scholar]
- 64.Boya P. Why autophagy is good for retinal ganglion cells? Eye. 2017;31:185–190. doi: 10.1038/eye.2016.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodríguez-Muela N., Germain F., Mariño G., Fitze P.S., Boya P. Autophagy promotes survival of retinal ganglion cells after optic nerve axotomy in mice. Cell Death Differ. 2012;19:162–169. doi: 10.1038/cdd.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin W., Kuang H. Oxidative stress induces autophagy in response to multiple noxious stimuli in retinal ganglion cells. Autophagy. 2014;10:1692–1701. doi: 10.4161/auto.36076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bell K., Rosignol I., Sierra-Filardi E., Rodriguez-Muela N., Schmelter C., Cecconi F., Grus F., Boya P. Age related retinal Ganglion cell susceptibility in context of autophagy deficiency. Cell Death Discov. 2020;6:21. doi: 10.1038/s41420-020-0257-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park H.Y.L., Kim J.H., Park C.K. Activation of autophagy induces retinal ganglion cell death in a chronic hypertensive glaucoma model. Cell Death Dis. 2012;3:e290–e299. doi: 10.1038/cddis.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Knoferle J., Koch J.C., Ostendorf T., Michel U., Planchamp V., Vutova P., Tonges L., Stadelmann C., Bruck W., Bahr M., et al. Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc. Natl. Acad. Sci. USA. 2010;107:6064–6069. doi: 10.1073/pnas.0909794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei T., Kang Q., Ma B., Gao S., Li X., Liu Y. Activation of autophagy and paraptosis in retinal ganglion cells after retinal ischemia and reperfusion injury in rats. Exp. Ther. Med. 2015;9:476–482. doi: 10.3892/etm.2014.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Piras A., Gianetto D., Conte D., Bosone A., Vercelli A. Activation of Autophagy in a Rat Model of Retinal Ischemia following High Intraocular Pressure. PLoS ONE. 2011;6:e22514. doi: 10.1371/journal.pone.0022514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Russo R., Berliocchi L., Adornetto A., Varano G.P., Cavaliere F., Nucci C., Rotiroti D., Morrone L.A., Bagetta G., Corasaniti M.T. Calpain-mediated cleavage of Beclin-1 and autophagy deregulation following retinal ischemic injury in vivo. Cell Death Dis. 2011;2:e144. doi: 10.1038/cddis.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Russo R., Varano G.P., Adornetto A., Nazio F., Tettamanti G., Girardello R., Cianfanelli V., Cavaliere F., Morrone L.A., Corasaniti M.T., et al. Rapamycin and fasting sustain autophagy response activated by ischemia/reperfusion injury and promote retinal ganglion cell survival. Cell Death Dis. 2018;9:1–18. doi: 10.1038/s41419-018-1044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hirt J., Porter K., Dixon A., McKinnon S., Liton P.B. Contribution of autophagy to ocular hypertension and neurodegeneration in the DBA/2J spontaneous glaucoma mouse model. Cell Death Discov. 2018;4:75. doi: 10.1038/s41420-018-0077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Russo R., Nucci C., Corasaniti M.T., Bagetta G., Morrone L.A. Autophagy dysregulation and the fate of retinal ganglion cells in glaucomatous optic neuropathy. Prog. Brain Res. 2015;220:87–105. doi: 10.1016/bs.pbr.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 76.Costello M.J., Brennan L.A., Basu S., Chauss D., Mohamed A., Gilliland K.O., Johnsen S., Menko A.S., Kantorow M. Autophagy and mitophagy participate in ocular lens organelle degradation. Exp. Eye Res. 2013;116:141–150. doi: 10.1016/j.exer.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou J., Yao K., Zhang Y., Chen G., Lai K., Yin H., Yu Y. Thioredoxin Binding Protein-2 Regulates Autophagy of Human Lens Epithelial Cells under Oxidative Stress via Inhibition of Akt Phosphorylation. Oxid. Med. Cell. Longev. 2016;2016:1–17. doi: 10.1155/2016/4856431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sidjanin D.J., Park A.K., Ronchetti A., Martins J., Jackson W.T. TBC1D20 mediates autophagy as a key regulator of autophagosome maturation. Autophagy. 2016;12:1759–1775. doi: 10.1080/15548627.2016.1199300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morishita H., Eguchi S., Kimura H., Sasaki J., Sakamaki Y., Robinson M.L., Sasaki T., Mizushima N. Deletion of Autophagy-related 5 (Atg5) and Pik3c3 Genes in the Lens Causes Cataract Independent of Programmed Organelle Degradation. J. Biol. Chem. 2013;288:11436–11447. doi: 10.1074/jbc.M112.437103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen J., Ma Z., Jiao X., Fariss R., Kantorow W.L., Kantorow M., Pras E., Frydman M., Pras E., Riazuddin S., et al. Mutations in FYCO1 Cause Autosomal-Recessive Congenital Cataracts. Am. J. Hum. Genet. 2011;88:827–838. doi: 10.1016/j.ajhg.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wignes J.A., Goldman J.W., Weihl C.C., Bartley M.G., Andley U.P. p62 expression and autophagy in αB-crystallin R120G mutant knock-in mouse model of hereditary cataract. Exp. Eye Res. 2013;115:263–273. doi: 10.1016/j.exer.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ge X.-L., Zhang Y., Wu Y., LV J., Zhang W., Jin Z.-B., Qu J., Gu F. Identification of a Novel GJA8 (Cx50) Point Mutation Causes Human Dominant Congenital Cataracts. Sci. Rep. 2015;4:4121. doi: 10.1038/srep04121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tian Y., Li Z., Hu W., Ren H., Tian E., Zhao Y., Lu Q., Huang X., Yang P., Li X., et al. C. elegans Screen Identifies Autophagy Genes Specific to Multicellular Organisms. Cell. 2010;141:1042–1055. doi: 10.1016/j.cell.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 84.Sagona A.P., Nezis I.P., Stenmark H. Association of CHMP4B and Autophagy with Micronuclei: Implications for Cataract Formation. Biomed. Res. Int. 2014;2014:1–10. doi: 10.1155/2014/974393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Volpe C.M.O., Villar-Delfino P.H., dos Anjos P.M.F., Nogueira-Machado J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018;9:119. doi: 10.1038/s41419-017-0135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fanjul-Moles M.L., López-Riquelme G.O. Relationship between Oxidative Stress, Circadian Rhythms, and AMD. Oxid. Med. Cell. Longev. 2016;2016:1–18. doi: 10.1155/2016/7420637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Piano I., Novelli E., Della Santina L., Strettoi E., Cervetto L., Gargini C. Involvement of autophagic pathway in the progression of retinal degeneration in a mouse model of diabetes. Front. Cell. Neurosci. 2016;10:1–12. doi: 10.3389/fncel.2016.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yao J., Tao Z.F., Li C.P., Li X.M., Cao G.F., Jiang Q., Yan B. Regulation of autophagy by high glucose in human retinal pigment epithelium. Cell. Physiol. Biochem. 2014;33:107–116. doi: 10.1159/000356654. [DOI] [PubMed] [Google Scholar]
- 89.Fu D., Wu M., Zhang J., Du M., Yang S., Hammad S.M., Wilson K., Chen J., Lyons T.J. Mechanisms of modified LDL-induced pericyte loss and retinal injury in diabetic retinopathy. Diabetologia. 2012;55:3128–3140. doi: 10.1007/s00125-012-2692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Q., Tikhonenko M., Bozack S.N., Lydic T.A., Yan L., Panchy N.L., Mcsorley K.M., Faber M.S., Yan Y., Boulton M.E., et al. Changes in the Daily Rhythm of Lipid Metabolism in the Diabetic Retina. PLoS ONE. 2014;9:e95028. doi: 10.1371/journal.pone.0095028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Q., Bozack S.N., Yan Y., Boulton M.E., Grant M.B., Busik J.V. Regulation of Retinal Inflammation by Rhythmic Expression of MiR-146a in Diabetic Retina. Investig. Opthalmol. Vis. Sci. 2014;55:3986–3994. doi: 10.1167/iovs.13-13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O’Brien I.A.D., Lewin I.G., O’Hare J.P., Arendt J., Corrall R.J.M. Abnormal circadian rhythm of melatonin i ndiabetic autonomic neuropathy. Clin. Endocrinol. 1986;24:359–364. doi: 10.1111/j.1365-2265.1986.tb01639.x. [DOI] [PubMed] [Google Scholar]
- 93.Kaarniranta K., Tokarz P., Koskela A., Paterno J., Blasiak J. Autophagy regulates death of retinal pigment epithelium cells in age-related macular degeneration. Cell Biol. Toxicol. 2017;33:113–128. doi: 10.1007/s10565-016-9371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Golestaneh N., Chu Y., Xiao Y.Y., Stoleru G.L., Theos A.C. Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death Dis. 2017;8:e2537. doi: 10.1038/cddis.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Z.Y., Bao X.L., Cong Y.Y., Fan B., Li G.Y., Žerovnik E. Autophagy in Age-Related Macular Degeneration: A Regulatory Mechanism of Oxidative Stress. Oxid. Med. Cell. Longev. 2020;2020:2896036. doi: 10.1155/2020/2896036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Godley B.F., Shamsi F.A., Liang F.-Q., Jarrett S.G., Davies S., Boulton M. Blue Light Induces Mitochondrial DNA Damage and Free Radical Production in Epithelial Cells. J. Biol. Chem. 2005;280:21061–21066. doi: 10.1074/jbc.M502194200. [DOI] [PubMed] [Google Scholar]
- 97.Lin H., Xu H., Liang F.-Q., Liang H., Gupta P., Havey A.N., Boulton M.E., Godley B.F. Mitochondrial DNA Damage and Repair in RPE Associated with Aging and Age-Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2011;52:3521–3529. doi: 10.1167/iovs.10-6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Szatmári-Tóth M., Kristóf E., Veréb Z., Akhtar S., Facskó A., Fésüs L., Kauppinen A., Kaarniranta K., Petrovski G. Clearance of autophagy-associated dying retinal pigment epithelial cells—A possible source for inflammation in age-related macular degeneration. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Szatmári-Tóth M., Ilmarinen T., Mikhailova A., Skottman H., Kauppinen A., Kaarniranta K., Kristóf E., Lytvynchuk L., Veréb Z., Fésüs L., et al. Human embryonic stem cell-derived retinal pigment epithelium-role in dead cell clearance and inflammation. Int. J. Mol. Sci. 2019;20:926. doi: 10.3390/ijms20040926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang S., Ji L., Li L., Li J. Oxidative stress, autophagy and pyroptosis in the neovascularization of oxygen‑induced retinopathy in mice. Mol. Med. Rep. 2018;19:927–934. doi: 10.3892/mmr.2018.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Holz F., Schütt F., Kopitz J., Eldred G., Kruse F., Völcker H., Cantz M. Inhibition of Lysosomal Degradative Functions in RPE Cells by a Retinoid Component of Lipofuscin. Investig. Ophthalmol. Vis. Sci. 1999;40:737–743. [PubMed] [Google Scholar]
- 102.De Jong P.T.V.M. Drusen, AMD, and history. Graefes Arch. Clin. Exp. Ophthalmol. 2015;253:2061–2062. doi: 10.1007/s00417-015-3180-2. [DOI] [PubMed] [Google Scholar]
- 103.Zhang J., Bai Y., Huang L., Qi Y., Zhang Q., Li S., Wu Y., Li X. Protective effect of autophagy on human retinal pigment epithelial cells against lipofuscin fluorophore A2E: Implications for age-related macular degeneration. Cell Death Dis. 2015;6:e1972. doi: 10.1038/cddis.2015.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaarniranta K., Salminen A., Eskelinen E.L., Kopitz J. Heat shock proteins as gatekeepers of proteolytic pathways-Implications for age-related macular degeneration (AMD) Ageing Res. Rev. 2009;8:128–139. doi: 10.1016/j.arr.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 105.Boya P., González-Polo R.-A., Casares N., Perfettini J.-L., Dessen P., Larochette N., Métivier D., Meley D., Souquere S., Yoshimori T., et al. Inhibition of Macroautophagy Triggers Apoptosis. Mol. Cell. Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang A.L., Lukas T.J., Yuan M., Du N., Tso M.O., Neufeld A.H. Autophagy and Exosomes in the Aged Retinal Pigment Epithelium: Possible Relevance to Drusen Formation and Age-Related Macular Degeneration. PLoS ONE. 2009;4:e4160. doi: 10.1371/journal.pone.0004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kauppinen A., Piippo N., Hytti M., Kinnunen K., Salminen A., Kaarniranta K. Prevention of autophagy activates inflammasome signaling in ARPE-19 cells treated with a proteasome inhibitor. Acta Ophthalmol. 2013;91 doi: 10.1111/j.1755-3768.2013.4661.x. [DOI] [Google Scholar]
- 108.Liu J., Copland D.A., Theodoropoulou S., Chiu H.A.A., Barba M.D., Mak K.W., Mack M., Nicholson L.B., Dick A.D. Impairing autophagy in retinal pigment epithelium leads to inflammasome activation and enhanced macrophage-mediated angiogenesis. Sci. Rep. 2016;6:20639. doi: 10.1038/srep20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang L., Cano M., Handa J.T. p62 provides dual cytoprotection against oxidative stress in the retinal pigment epithelium. Biochim. Biophys. Acta Mol. Cell Res. 2014;1843:1248–1258. doi: 10.1016/j.bbamcr.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Korolchuk V.I., Miwa S., Carroll B., von Zglinicki T. Mitochondria in Cell Senescence: Is Mitophagy the Weakest Link? EBioMedicine. 2017;21:7–13. doi: 10.1016/j.ebiom.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yao J., Jia L., Khan N., Lin C., Mitter S.K., Boulton M.E., Dunaief J.L., Klionsky D.J., Guan J.-L., Thompson D.A., et al. Deletion of autophagy inducer RB1CC1 results in degeneration of the retinal pigment epithelium. Autophagy. 2015;11:939–953. doi: 10.1080/15548627.2015.1041699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Valapala M., Edwards M., Hose S., Grebe R., Bhutto I.A., Cano M., Berger T., Mak T.W., Wawrousek E., Handa J.T., et al. Increased Lipocalin-2 in the retinal pigment epithelium of Cryba1 cKO mice is associated with a chronic inflammatory response. Aging Cell. 2014;13:1091–1094. doi: 10.1111/acel.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Valapala M., Wilson C., Hose S., Bhutto I.A., Grebe R., Dong A., Greenbaum S., Gu L., Sengupta S., Cano M., et al. Lysosomal-mediated waste clearance in retinal pigment epithelial cells is regulated by CRYBA1/βA3/A1-crystallin via V-ATPase-MTORC1 signaling. Autophagy. 2014;10:480–496. doi: 10.4161/auto.27292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sinha D., Klise A., Sergeev Y., Hose S., Bhutto I.A., Hackler L., Malpic-llanos T., Samtani S., Grebe R., Goldberg M.F., et al. βA3/A1-crystallin in astroglial cells regulates retinal vascular remodeling during development. Mol. Cell. Neurosci. 2008;37:85–95. doi: 10.1016/j.mcn.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rodríguez-Muela N., Koga H., García-Ledo L., de la Villa P., de la Rosa E.J., Cuervo A.M., Boya P. Balance between autophagic pathways preserves retinal homeostasis. Aging Cell. 2013;12:478–488. doi: 10.1111/acel.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li Y., Jiang Q., Cao G., Yao J., Yan B. Repertoires of Autophagy in the Pathogenesis of Ocular Diseases. Cell. Physiol. Biochem. 2015;35:1663–1676. doi: 10.1159/000373980. [DOI] [PubMed] [Google Scholar]
- 117.Su W., Li Z., Jia Y., Zhuo Y. Rapamycin Is Neuroprotective in a Rat Chronic Hypertensive Glaucoma Model. PLoS ONE. 2014;9:e99719. doi: 10.1371/journal.pone.0099719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hyttinen J.M.T., Petrovski G., Salminen A., Kaarniranta K. 5′-Adenosine Monophosphate-Activated Protein Kinase–Mammalian Target of Rapamycin Axis As Therapeutic Target for Age-Related Macular Degeneration. Rejuvenation Res. 2011;14:651–660. doi: 10.1089/rej.2011.1220. [DOI] [PubMed] [Google Scholar]
- 119.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Motoi Y., Shimada K., Ishiguro K., Hattori N. Lithium and Autophagy. ACS Chem. Neurosci. 2014;5:434–442. doi: 10.1021/cn500056q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fu J., Shao C.-J., Chen F.-R., Ng H.-K., Chen Z.-P. Autophagy induced by valproic acid is associated with oxidative stress in glioma cell lines. Neuro. Oncol. 2010;12:328–340. doi: 10.1093/neuonc/nop005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cai J., Zhang H., Zhang Y., Zhou Z., Wu S. MicroRNA-29 enhances autophagy and cleanses exogenous mutant αB-crystallin in retinal pigment epithelial cells. Exp. Cell Res. 2019;374:231–248. doi: 10.1016/j.yexcr.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 123.Wei Q., Liang X., Peng Y., Yu D., Zhang R., Jin H., Fan J., Cai W., Ren C., Yu J. 17β-estradiol ameliorates oxidative stress and blue light-emitting diode-induced retinal degeneration by decreasing apoptosis and enhancing autophagy. Drug Des. Devel. Ther. 2018;12:2715–2730. doi: 10.2147/DDDT.S176349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chang C.C., Huang T.Y., Chen H.Y., Huang T.C., Lin L.C., Chang Y.J., Hsia S.M. Protective Effect of Melatonin against Oxidative Stress-Induced Apoptosis and Enhanced Autophagy in Human Retinal Pigment Epithelium Cells. Oxid. Med. Cell. Longev. 2018;2018:9015765. doi: 10.1155/2018/9015765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Johansson I., Monsen V.T., Pettersen K., Mildenberger J., Misund K., Kaarniranta K., Schønberg S., Bjørkøy G. The marine n-3 PUFA DHA evokes cytoprotection against oxidative stress and protein misfolding by inducing autophagy and NFE2L2 in human retinal pigment epithelial cells. Autophagy. 2015;11:1636–1651. doi: 10.1080/15548627.2015.1061170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pawlowska E., Szczepanska J., Koskela A., Kaarniranta K., Blasiak J. Dietary polyphenols in age-related macular degeneration: Protection against oxidative stress and beyond. Oxid. Med. Cell. Longev. 2019;2019:9682318. doi: 10.1155/2019/9682318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Han D., Wu X., Liu L., Shu W., Huang Z. Sodium tanshinone IIA sulfonate protects ARPE-19 cells against oxidative stress by inhibiting autophagy and apoptosis. Sci. Rep. 2018;8:15137. doi: 10.1038/s41598-018-33552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Levine B., Packer M., Codogno P. Development of autophagy inducers in clinical medicine. J. Clin. Investig. 2015;125:14–24. doi: 10.1172/JCI73938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Laplante M., Sabatini D.M. mTOR Signaling in Growth Control and Disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.