Abstract
This study was aimed at identifying Alternaria species associated with heart rot disease of pomegranate fruit in southern Italy and characterizing their mycotoxigenic profile. A total of 42 Alternaria isolates were characterized. They were obtained from pomegranate fruits with symptoms of heart rot sampled in Apulia and Sicily and grouped into six distinct morphotypes based on macro- and microscopic features. According to multigene phylogenetic analysis, including internal transcribed spacer (ITS), translation elongation factor 1-α (EF-1α), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and a SCAR marker (OPA10-2), 38 isolates of morphotypes 1 to 5 were identified as Alternaria alternata, while isolates of morphotype 6, all from Sicily, clustered within the Alternaria arborescens species complex. In particular, isolates of morphotype 1, the most numerous, clustered with the ex-type isolate of A. alternata, proving to belong to A. alternata. No difference in pathogenicity on pomegranate fruits was found between isolates of A. alternata and A. arborescens and among A. alternata isolates of different morphotypes. The toxigenic profile of isolates varied greatly: in vitro, all 42 isolates produced tenuazonic acid and most of them other mycotoxins, including alternariol, alternariol monomethyl ether, altenuene and tentoxin.
Keywords: Alternaria alternata, Alternaria arborescens, morphotypes, four-gene phylogeny, mycotoxins
1. Introduction
In Italy, the commercial cultivation of pomegranate is rapidly expanding and has grown from around 130 ha in 2013 to 1234 ha in 2019 [1,2]. The most successful cultivar is Wonderful, originating from California and then introduced by Israeli in Italy, which is suitable for consumption as fresh fruit and fresh-cut products as well as for processing to produce juice. Moreover, several industrial and medical applications of pomegranate peel extracts, e.g., as food preservatives, are being envisaged, as pomegranate peel is rich in phenolic compounds, which are responsible for strong antioxidant and antimicrobial activity [3,4,5,6,7,8,9,10].
Currently, a major constraint of pomegranate commercial production is constituted by heart rot, an emerging disease caused by Alternaria spp. and reported from California, India and several Mediterranean countries, including Cyprus, Greece, Egypt, Israel and Italy [1,11,12,13,14,15]. The disease is also named Alternaria heart rot or black heart [16,17]. No precise estimation of losses caused yearly by heart rot to pomegranate production in Italy is available. According to the first report of the disease in Italy, its incidence in commercial orchards varies from 1 to 9% of fruits [1]. However, due to the difficulty in screening infected fruits on the basis of external symptoms, there are few chances to detect the presence of infected fruits, thus causing a serious value loss of the whole fruit stock.
Different Alternaria species were identified as causative agents of pomegranate heart rot on the basis of morphological characteristics and multilocus phylogenetic analyses [1,13,18,19]. Airborne spores of the pathogen are thought to cause flower infections [13,18]. Following fruit onset, infections can remain latent for most of the growing season until the establishment of favorable conditions [13]. Since the affected fruits are more prone to fall, one of the preventive agronomic practices is shaking the trees before harvesting [17]. Precise identification of the causative agent of heart rot is crucial for all aspects concerning the epidemiology and management of the disease. Unfortunately, the taxonomy of Alternaria is problematic and has undergone several revisions [20,21,22]. The difficulties in the identification of this genus at species level are related to the considerable morphological plasticity of most of the recognized Alternaria species. Historically, the identification of Alternaria spp. was based on morphological characteristics, including cultural features, size and shape of conidia and branching patterns of conidial chains [23,24]. Although the main sections of Alternaria can be differentiated using these features, this approach is not sufficient for distinguishing closely related species [25,26]. In the past, the production of host-specific toxins (HSTs) has been used to distinguish among species [27], but this criterion proved unreliable since HST biosynthetic gene clusters are located on small conditionally dispensable chromosomes, which can be lost or gained [28]. Molecular identification based on ribosomal DNA (rDNA), a genomic region typically used in fungal systematics, failed to differentiate small-spored Alternaria species [29,30,31]. Furthermore, the poor resolution obtained even using more variable genetic loci generated debate on which species should be kept within the Alternaria section Alternaria (i.e., A. alternata, A. tenuissima, A. arborescens, A. mali and A. gaisen), suggesting to combine A. alternata and A. tenuissima or to merge the latter two species with A. arborescens [20,25,26].
The analysis of secondary metabolites has also been proposed as a means to support species identification within the genus [22,32]. Alternaria is one of the major mycotoxigenic fungal genera [22,32,33,34,35,36,37,38,39]. Furthermore, it produces more than 70 phytotoxic metabolites, including host-specific toxins and non-host-specific toxins [40]. However, only a few mycotoxins (e.g., tenuazonic acid, alternariol, alternariol monomethyl ether) may be found in food and are of major toxicological concern. These toxins are suspected to exert both acute and chronic detrimental effects [41]. As a consequence, their presence as contaminants in pomegranate fruits and juice may represent a threat to human health.
This study aimed at the characterization of Alternaria isolates obtained from pomegranate fruit with symptoms of heart rot in southern Italy and at determining their mycotoxigenic profile.
2. Materials and Methods
2.1. Alternaria Isolates
Overall, 42 Alternaria isolates obtained from pomegranate fruit with symptoms of heart rot were included in this study (Table 1).
Table 1.
Alternaria isolates from pomegranate fruits characterized in this study, their geographical origins and accession numbers of their internal transcribed spacer (ITS), translation elongation factor 1-α (EF-1α), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and a SCAR marker (OPA 10-2) sequences in GenBank.
| Isolate | Morphotype | Location | Host, Cultivar | Accession Numbers | |||
|---|---|---|---|---|---|---|---|
| ITS | EF-1α | GAPDH | OPA 10-2 | ||||
| AaMR7 | 1 | Italy, Sicily | Punica granatum cv. Wonderful | MW580732 | MW585113 | MW590491 | MW590533 |
| AaMP7a | 1 | Italy, Sicily | Punica granatum cv. Wonderful | MW580733 | MW585114 | MW590492 | MW590534 |
| AaMP10 | 1 | Italy, Sicily | Punica granatum cv. Wonderful | MW580734 | MW585115 | MW590493 | MW590535 |
| AaMR11 | 1 | Italy, Sicily | Punica granatum cv. Wonderful | MW580735 | MW585116 | MW590494 | MW590536 |
| AaMMH6e | 1 | Italy, Sicily | Punica granatum cv. Mollar de Elche | MW580743 | MW585121 | MW590502 | MW590544 |
| AaMP3 | 1 | Italy, Sicily | Punica granatum cv. Wonderful | MW580745 | MW585123 | MW590504 | MW590546 |
| AaMR9a | 1 | Italy, Sicily | Punica granatum cv. Wonderful | MW580747 | MW585125 | MW590506 | MW590548 |
| AaMP9 | 1 | Italy, Sicily | Punica granatum cv. Wonderful | MW580748 | MW585126 | MW590507 | MW590549 |
| AaMDc5b | 1 | Italy, Sicily | Punica granatum cv. Dente di cavallo | MW580754 | MW585132 | MW590513 | MW590555 |
| AaMDc5d | 1 | Italy, Sicily | Punica granatum cv. Dente di cavallo | MW580755 | MW585133 | MW590514 | MW590556 |
| AaMMH6b | 1 | Italy, Sicily | Punica granatum cv. Mollar de Elche | MW580756 | MW585134 | MW590515 | MW590557 |
| AaMMH7a | 1 | Italy, Sicily | Punica granatum cv. Mollar de Elche | MW580757 | MW585135 | MW590516 | MW590558 |
| AaMMH7d | 1 | Italy, Sicily | Punica granatum cv. Mollar de Elche | MW580758 | MW585136 | MW590517 | MW590559 |
| AaMMH6a | 1 | Italy, Sicily | Punica granatum cv. Dente di cavallo | MW580763 | MW585140 | MW590522 | MW590564 |
| AaMMH6d | 1 | Italy, Sicily | Punica granatum cv. Mollar de Elche | MW580764 | MW585141 | MW590523 | MW590565 |
| AaMMH7b | 1 | Italy, Sicily | Punica granatum cv. Mollar de Elche | MW580765 | MW585142 | MW590524 | MW590566 |
| M24-BB1 | 1 | Italy, Apulia | Punica granatum cv. Dente di cavallo | MW580768 | MW585145 | MW590527 | MW590569 |
| M95 A2 | 1 | Italy, Apulia | Punica granatum cv. Wonderful | MW580770 | MW585147 | MW590529 | MW590571 |
| M103 A2-1 | 1 | Italy, Apulia | Punica granatum cv. Wonderful | MW580771 | MW585148 | MW590530 | MW590572 |
| M109 3 | 1 | Italy, Apulia | Punica granatum cv. Wonderful | MW580772 | MW585149 | MW590531 | MW590573 |
| AaMR4 | 2 | Italy, Sicily | Punica granatum cv. Wonderful | MW580739 | MW585117 | MW590498 | MW590540 |
| AaMR12 | 2 | Italy, Sicily | Punica granatum cv. Wonderful | MW580742 | MW585120 | MW590501 | MW590543 |
| AaMR2b | 2 | Italy, Sicily | Punica granatum cv. Wonderful | MW580744 | MW585122 | MW590503 | MW590545 |
| AaMP4 | 2 | Italy, Sicily | Punica granatum cv. Wonderful | MW580746 | MW585124 | MW590505 | MW590547 |
| AaMR14b | 2 | Italy, Sicily | Punica granatum cv. Wonderful | MW580760 | MW585137 | MW590519 | MW590561 |
| AaMP14a | 2 | Italy, Sicily | Punica granatum cv. Wonderful | MW580761 | MW585138 | MW590520 | MW590562 |
| AaMR14a | 2 | Italy, Sicily | Punica granatum cv. Wonderful | MW580766 | MW585143 | MW590525 | MW590567 |
| AaMP14b | 2 | Italy, Sicily | Punica granatum cv. Wonderful | MW580767 | MW585144 | MW590526 | MW590568 |
| M80 B5 | 2 | Italy, Apulia | Punica granatum cv. Wonderful | MW580769 | MW585146 | MW590528 | MW590570 |
| AaMR5b | 3 | Italy, Sicily | Punica granatum cv. Wonderful | MW580731 | MW585112 | MW590490 | MW590532 |
| AaMDc3a | 4 | Italy, Sicily | Punica granatum cv. Dente di cavallo | MW580751 | MW585129 | MW590510 | MW590552 |
| AaMDc3b | 4 | Italy, Sicily | Punica granatum cv. Dente di cavallo | MW580752 | MW585130 | MW590511 | MW590553 |
| AaMDc3c | 4 | Italy, Sicily | Punica granatum cv. Dente di cavallo | MW580753 | MW585131 | MW590512 | MW590554 |
| AaMDc3d | 4 | Italy, Sicily | Punica granatum cv. Wonderful | MW580762 | MW585139 | MW590521 | MW590563 |
| AaMR6b | 5 | Italy, Sicily | Punica granatum cv. Wonderful | MW580740 | MW585118 | MW590499 | MW590541 |
| AaMP6b | 5 | Italy, Sicily | Punica granatum cv. Wonderful | MW580741 | MW585119 | MW590500 | MW590542 |
| AaMDc2a | 5 | Italy, Sicily | Punica granatum cv. Dente di cavallo | MW580749 | MW585127 | MW590508 | MW590550 |
| AaMDc2b | 5 | Italy, Sicily | Punica granatum cv. Dente di cavallo | MW580750 | MW585128 | MW590509 | MW590551 |
| AaMDc1a | 6 | Italy, Sicily | Punica granatum cv. Dente di cavallo | MW580736 | MW585150 | MW590495 | MW590537 |
| AaMDc1b | 6 | Italy, Sicily | Punica granatum cv. Dente di cavallo | MW580737 | MW585151 | MW590496 | MW590538 |
| AaMDc1d | 6 | Italy, Sicily | Punica granatum cv. Dente di cavallo | MW580738 | MW585152 | MW590497 | MW590539 |
| AaMRa1 | 6 | Italy, Sicily | Punica granatum cv. Mollar de Elche | MW580759 | MW585153 | MW590518 | MW590560 |
They were sourced from fruits of the three pomegranate cultivars Wonderful (Californian/Israeli origin), Mollar de Elche (Spanish origin) and Dente di Cavallo (Italian origin) [42,43], picked up in commercial pomegranate orchards in Apulia and Sicily from 2015 to 2016.
Isolates were preserved in the collection of the laboratory of Molecular Plant Pathology at the Department of Agriculture, Food and Environment (Di3A) of the University of Catania, Italy. Reference strains of A. alternata and A. arborescens from CBS-KNAW were included for comparison (Table 2).
Table 2.
GenBank accession numbers of sequences of the Alternaria spp. isolates of different country and host origins used as references in phylogenetic analyses.
| Species | Isolate | Country | Host | Accession Numbers a | |||
|---|---|---|---|---|---|---|---|
| ITS | EF-1α | GPDH | OPA 10-2 | ||||
| Alternaria alternata (ex A. citri) | CBS 102.47 | USA | Citrus sinensis | KP124304 | KP125080 | KP124161 | KP124610 |
| Alternaria alternata (ex-type) | CBS 916.96 | India | Ara chis hypogaea | AF347031 | KC584634 | AY278808 | KP124632 |
|
Alternaria alternata (ex A. limoniasperae) |
CBS 102595 | USA | Citrus jambhiri | FJ266476 | KC584666 | AY562411 | KP124636 |
|
Alternaria alternata (ex A. tenuissima) |
CBS 112252 | - | - | KP124340 | KP125116 | KP124194 | KP124650 |
|
Alternaria alternata (ex A. godetiae) |
CBS 117.44 | Denmark | Godetia sp. | KP124303 | KP125079 | KP124160 | KP124609 |
| Alternaria gaisen | CBS 118488 | Japan | Pyrus pyrifolia | KP124427 | KP124278 | KP125206 | KP124743 |
| Alternaria alstroemeriae | CBS 118808 | USA | Alstroemeria sp. | KP124296 | KP125071 | KP124153 | KP124601 |
| Alternaria iridiaustralis | CBS 118487 | Australia | Iris sp. | KP124436 | KP125215 | KP124285 | KP124752 |
| Alternaria jacinthicola | CBS 878.95 | Mauritius | Arachis hypogaea | KP124437 | KP125216 | KP124286 | KP124753 |
| Alternaria tomato | CBS 103.30 | - | Solanum lycopersicum | KP124445 | KP125224 | KP124294 | KP124762 |
| Alternaria burnsii | CBS 107.38 | India | Cuminum cyminum | KP124420 | JQ646305 | KP125198 | KP124734 |
|
Alternaria alternata (ex A. toxicogenica) |
CBS 102600 | USA | Citrus reticulata | KP124331 | KP125107 | KP124186 | KP124640 |
| Alternaria longipes | CBS 540.94 | USA | Nicotiana tabacum | AY278835 | KC584667 | AY278811 | KP124758 |
| Alternaria alternata | CBS 109803 | Germany | human skin | KP124336 | KP125112 | KP124190 | KP124645 |
| Alternaria betae-kenyensis | CBS 118810 | Kenya | Beta vulgaris var. cicla | KP124419 | KP125197 | KP124270 | KP124733 |
| Alternaria eichhorniae | CBS 119778 | Indonesia | Eichhornia crassipes | KP124426 | KP125205 | KP124277 | KP124741 |
| Alternaria arborescens | CBS 109730 | USA | Solanum lycopersicum | KP124399 | KP125177 | KP124251 | KP124713 |
| Alternaria arborescens | CBS 105.24 | - | Solanum tuberosum | KP124393 | KP125171 | KP124245 | KP124706 |
| Alternaria arborescens | CBS 108.41 | - | wood | KP124394 | KP125172 | KP124246 | KP124707 |
| Alternaria arborescens | CBS 112749 | South Africa | Malus domestica | KP124401 | KP125179 | KP124253 | KP124715 |
| Alternaria arborescens | CBS 118389 | Japan | Pyrus pyrifolia | KP124407 | KP125185 | KP124259 | KP124721 |
| Alternaria arborescens | CBS 115517 | South Africa | Malus domestica | KP124404 | KP125182 | KP124256 | KP124718 |
a source [20].
2.2. Symptoms, Distribution and Incidence of the Disease
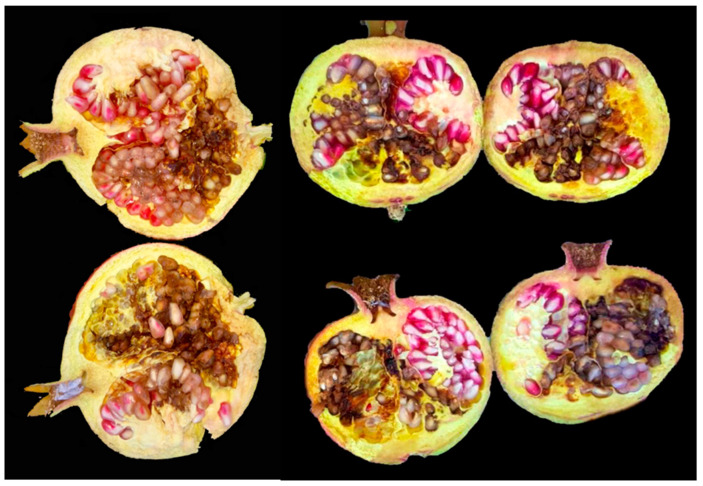
Infected fruits were characterized by a brown to black, soft to dry rot of the arils, visible when the fruit was cut open. Typically, the rot was confined to some aril compartments (Figure 1C) and did not affect the peel and compartment membranes (septa). The outer peel (epicarp), in correspondence of the internal rot, showed symptoms difficult to recognize, such as a dark-red discoloration and wrinkling of the peel (Figure 1A,B).
Figure 1.
(A,B) External symptoms of heart rot in pomegranate fruit: dark-red discoloration and wrinkling of the peel. (C) Cut-open fruit of pomegranate with typical symptoms of heart rot. Note the dark brown mass of conidia produced by Alternaria sporulating on rotten arils within the fruit. Symptoms were restricted by membranes that separate the fruit compartments.
Heavily affected fruits were asymmetric and lighter in weight. The incidence of pomegranate fruits affected by heart rot in southern Italy was estimated to vary from 1 to 9% [1], although it might have been higher under favorable conditions, such as rainy and warm weather during flowering and early fruit development [44]. Heart rot is considered a serious postharvest problem due to the difficulty in recognizing affected fruits from external symptoms [1,13,17,44].
2.3. Morphological Characterization
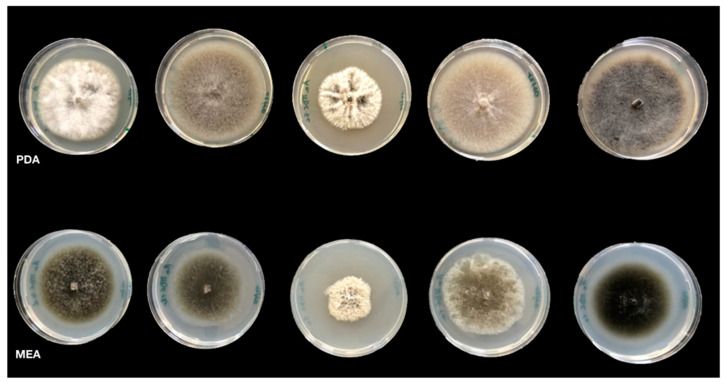
Isolates were grown in Petri dishes on Potato Dextrose Agar (PDA; Oxoid Ltd., Basingstoke, UK) and Malt Extract Agar (MEA; Sigma-Aldrich, Burlington, MA, USA), prepared according to CBS-KNAW Fungal Biodiversity Centre (Utrecht, The Netherlands) [45]. Dishes were incubated for 7 days at 25 ± 1 °C in the dark (Figure 2).
Figure 2.
Growth pattern and colony morphology of isolates of Alternaria spp. obtained from pomegranate fruits with symptoms of heart rot collected from different Sicilian and Apulian orchards. From left to right: AaMDc1a, AaMP4, AaMRa1, AaMR4 and AaMDc5d. All the Alternaria isolated were grown on Potato Dextrose Agar (PDA) and Malt Extract Agar (MEA) culture media and incubated at 25 ± 1 °C for 7 days in the dark.
The macroscopic characteristics (color, margin, diameter and texture) of colonies were examined according to Pryor and Michailides [46], whereas microscopic features (conidium and conidiophore branch morphology) according to Simmons [24].
2.4. Molecular Characterization
Isolates were grown on PDA for 7 days at 25 ± 1 °C. Mycelium of each isolate was harvested with a sterile scalpel, and the genomic DNA was extracted using a PowerPlant® Pro DNA isolation Kit (MO BIO Laboratories, Inc., Carlsbad, CA, USA), following the manufacturer’s protocol. The DNA was preserved at −20 °C. A multilocus approach was adopted to characterize and determine the phylogenetic allocation of the 42 isolates from pomegranate fruit.
Portions of four Alternaria barcoding genes/regions, i.e., internal transcribed spacer (ITS), translation elongation factor 1-α (EF-1α), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and one SCAR marker (OPA10–2), were sequenced [20]. The primers used for amplifying these genes/regions were ITS1/ITS4 for ITS [47], EF1-728F/EF1-986R for EF-1α [48], GPD1/GPD2 for GAPDH [49] and OPA10-2R/OPA10-2L for OPA10-2 [25] (Table 3).
Table 3.
Primers used in this study and PCR conditions.
| Primer | Primer DNA Sequence | PCR Conditions | Reference |
|---|---|---|---|
| ITS1 | 5’ TCC GTA GGT GAA CCT GCG G 3′ | 94 °C for 3 min; 94 °C for 30 s, 55 °C for 30 s, 72 °C for 30 s for 35 cycles and final extension at 72 °C for 10 min | [47] |
| ITS4 | 5’ GCT GCG TTC TTC ATC GAT GC 3′ | ||
| EF1-728F | 5’ CAT CGA GAA GTT CGA GAA GG 3′ | 94 °C for 3 min; 94 °C for 30 s, 58 °C for 30 s, 72 °C for 30 s for 35 cycles and final extension at 72 °C for 10 min | [48] |
| EF1-986R | 5’ TAC TTG AAG GAA CCC TTA CC 3′ | ||
| GPD1 | 5’ CAA CGG CTT CGG TCG CAT TG 3′ | 94 °C for 3 min; 94 °C for 30 s, 54 °C for 30 s, 72 °C for 30 s for 35 cycles and final extension at 72 °C for 10 min | [49] |
| GPD2 | 5’ GCC AAG CAG TTG GTT GTG C 3′ | ||
| OPA 10-2R | 5’ GAT TCG CAG CAG GGA AAC TA 3′ | 94 °C for 3 min; 94 °C for 30 s, 62 °C for 30 s, 72 °C for 30 s for 35 cycles and final extension at 72 °C for 10 min | [25] |
| OPA 10-2L | 5’ TCG CAG TAA GAC ACA TTC TAC G 3′ |
PCR amplifications were performed on a GeneAmp PCR System 9700 (Applied Biosystems, Monza-Brianza, Italy). All PCR reactions were carried out by using Taq DNA polymerase recombinant (Invitrogen™) in a total volume of 25 μL containing PCR Buffer (1×), dNTP mix (0.2 mM), MgCl2 (1.5 mM), forward and reverse primers (0.5 μM each), Taq DNA Polymerase (1 U) and 1 μL of genomic DNA. Reaction conditions were 94 °C for 3 min followed by 35 cycles of 94 °C for 30 s, 55 °C (ITS region)/58 °C (EF-1α)/54 °C (GAPDH)/62 °C (OPA10-2) for 30 s, and 72 °C for 30 s, followed by an additional 10-min extension at 72 °C.
The amplicons were detected on 1% agarose gel, and purified products were sequenced with both forward and reverse primers by Macrogen Europe (Amsterdam, The Netherlands).
Sequences were analyzed by using FinchTV v.1.4.0 [50], and the consensus sequences were deposited in GenBank (Table 2).
For molecular identification, sequences obtained in the present study and validated sequences of CBS representative strains of species within Alternaria sect. Alternaria, were phylogenetically analyzed. Before analyses, the complete panel of reference sequences was tested utilizing Elim Dupes software [51] to delete multiple identical sequences. Identical reference sequences were included in the panel when representative of different Alternaria species [20]. Sequences were aligned using MUSCLE and introduced to MEGA6 for phylogenetic analysis with the Maximum Likelihood method using the Tamura–Nei model [52]. Analyses were performed with 1000 bootstrap replications. In order to maximize the effectiveness of the investigation into the genetic diversity among isolates obtained in the present study, the phylogenetic analysis was conducted using a combined dataset of all sequenced markers (ITS, EF-1α, GPDH and OPA10-2).
2.5. Pathogenicity Tests
The pathogenicity of two A. arborescens isolates (AaMDc1A and AaMDc1d) and six A. alternata isolates, one for each morphotype from 2 to 5 (AaMR2b, AaMR5b, AaMDc3a and AaMDc1a) plus two for morphotype 1 (M95 A2 and AaMP3, from Apulia and Sicily, respectively), was tested in a commercial farm at Lentini (Syracuse, Sicily) on pomegranate fruits ‘Wonderful’, using the injection method described by Luo et al. [14]. In detail, on 15 August 2020, a conidial suspension (5 µL of 2 × 105 conidia·mL−1) was injected with a syringe into one side of pomegranate fruits (10 fruits per each isolate). Control fruits were injected with sterile distilled water. Ten weeks later, fruits were cut open longitudinally into two halves to observe heart rot symptoms.
2.6. Extraction and Analyses of Secondary Metabolites
Isolates were tested for production of secondary metabolites using a modified Czapek-Dox liquid medium: 10 g/L glucose, 0.162 g/L NH4NO3, 1.7 g/L KH2PO4, 0.85 g/L MgSO4, 0.425 g/L NaCl, 0.425 g/L KCl, 0.017 g/L FeSO4, 0.017 g/L ZnSO4 and 1.7 g/L yeast extract, pH 5.5. Cultures were inoculated with three mycelial plugs in 50 mL of medium. All cultures were performed in triplicate and incubated in the dark at 28 °C. After 8 days, cultures were filtered, and Alternaria toxins in the clear medium were extracted by liquid-liquid extraction. Each sample was adjusted to pH 2 with HCl, and an aliquot (5 mL) was transferred in a separating funnel. Ten milliliters of dichloromethane were added three times, and the mixture was shaken for 1 min, then, the dichloromethane extracts were collected in a flask. The final extract was evaporated to dryness in a rotary evaporator at 35 °C. The residue was dissolved in 1 mL of H2O/CH3OH 1:1 for the HPLC-MS/MS simultaneous detection of the main five Alternaria toxins. Analyses were carried out according to a previously validated method [35].
Standards of tenuazonic acid (TeA) copper salt from A. alternata (purity ≥ 98%), alternariol (AOH) from Alternaria spp. (purity ≥ 94%), alternariol monomethyl ether (AME) from Alternaria alternata (purity ≥ 98%), altenuene (ALT) from Alternaria spp. (purity ≥ 98%) and tentoxin (TEN) from Alternaria tenuis (purity 99%) were purchased from Sigma-Aldrich in crystallized form. A stock solution of 1 mg/mL and a working solution of 10 μg/mL were prepared in methanol for each molecule and kept at −20 °C. Standards for HPLC calibration and standards for addition experiment were prepared by diluting the working solution, and a calibration curve was built for each analyte. Good linearity was obtained for all analytes (R2 > 0.999). Recovery experiments were done spiking the matrix before extractions with a standard solution and the calculated recovery ranged between 80 and 100%.
3. Results
3.1. Morphological Characterization of Isolates
All 42 isolates were grouped according to macro- and microscopic features on PDA and MEA, along with reference strains of A. alternata and A. arborescens from CBS-KNAW. Higher variability in colony morphology was observed on PDA, as compared to MEA (Figure 2), allowing the differentiation of six morphotypes.
In particular, on PDA, twenty isolates (AaMMH7d, AaMDc5d, AaMP3, M24-BB1, AaMMH6b, AaMMH6a, AaMR9a, AaMR7, AaMR11, AaMDc5b, AaMMH7a, AaMMH7b, M103 A2-1, M109 3, M95 A2, AaMMH6d, AaMP7a, AaMP10, AaMMH6e and AaMP9) showed colonies that were flat, woolly, with colors ranging from brown to black and an average diameter of 65 mm. They produced dark brown conidia arranged in branched chains. Conidia appeared oval-ellipsoidal with 3–5 transverse septa. These features matched those of A. alternata ex-type reference strain CBS 916.96 (morphotype 1).
Nine isolates (AaMR2b, M80 B5, AaMP4, AaMR4, AaMR14a, AaMR14b, AaMP14b, AaMR12 and AaMP14a) showed greenish colonies with white margins. Conidia appeared elongated with a long-tapered beak. The characteristic sporulation pattern matched that of the reference strain of A. alternata (ex A. tenuissima) CBS 112252 (morphotype 2).
One isolate (AaMR5b) exhibited a colony that was pale brown, flat, granulated with undulating edges. Conidia appeared long and ellipsoidal with 1–3 transverse septa. The sporulation pattern resembled that of A. alternata (ex A. limoniasperae) CBS 102595 (morphotype 3).
Four isolates (AaMDc3a, AaMDc3b, AaMDc3c and AaMDc3d) exhibited a sporulation pattern resembling that of the reference strain of A. alternata (ex A. citri) CBS 102.47, with elliptical and subglobose conidia (morphotype 4).
Four isolates (AaMDc2a, AaMDc2b, AaMP6b and AaMR6b) exhibited wide and long conidia, with a sporulation pattern resembling that of A. alternata (ex A. toxicogenica) CBS 102600 (morphotype 5).
Four isolates (AaMDc1a, AaMRa1, AaMDc1d and AaMDc1b) showed colonies varying from greenish grey to brown, characterized by a lower growth rate (average diameter 45 mm after 7 days on PDA). Conidia appeared oval or ellipsoidal with 1–4 transverse septa and 1 or 2 longitudinal septa. They were borne by long primary conidiophores, occasionally presenting subterminal branches. These characteristics matched those of the reference strain for A. arborescens CBS 109730 (morphotype 6).
3.2. Molecular Characterization
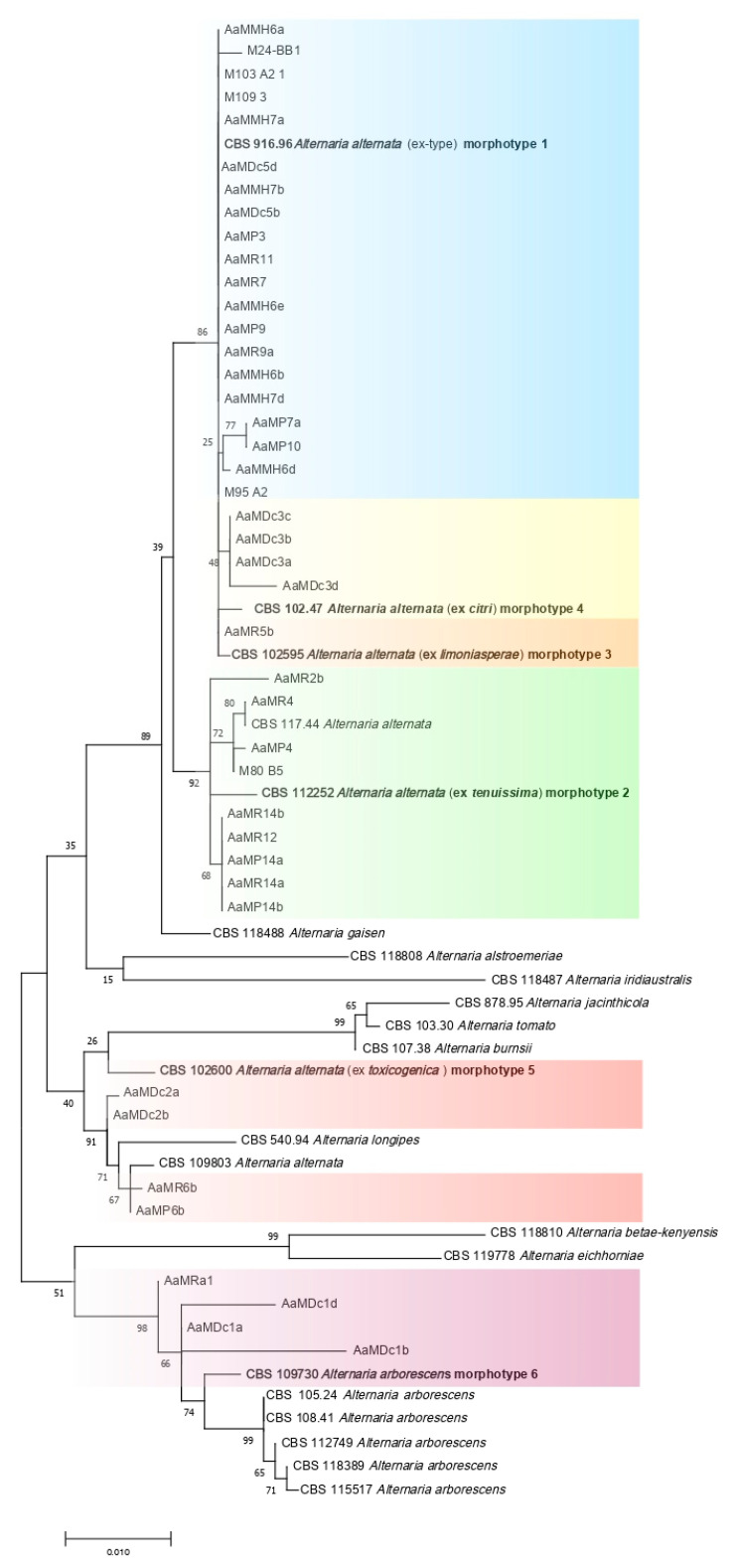
Alternaria isolates from pomegranate sourced in southern Italy and reference isolates from CBS [20] were grouped on the basis of four-gene phylogeny, including ITS, EF-1α, GAPDH and OPA 10-2 sequences. According to this analysis, 38 out of the 42 isolates were associated with A. alternata. They were from both geographical sampling areas, Sicily and Apulia, and clustered with reference isolates of A. alternata, including the isolates CBS 916.96 (ex-type), and CBS 112252 (Figure 3). The A. alternata phylogentic group included morphotypes from 1 to 5. The most numerous phylogenetic subgroup, corresponding to morphotype 1, included the ex-type isolate of A. alternata CBS 916.96.
Figure 3.
Internal transcribed spacer (ITS), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), translation elongation factor 1-α (EF-1α) and one SCAR marker (OPA 10-2) multilocus phylogenetic tree developed using the Maximum Likelihood Method, based on the Tamura–Nei model. The tree with the greatest log likelihood (-3746.14) is shown. Relationships between the 42 isolates from pomegranate sourced in southern Italy and the CBS reference isolates of Alternaria alternata, A. arborescens (in bold CBS isolates used as reference for diverse morphotypes) and other Alternaria spp. The six morphotypes are highlighted in different colors.
Conversely, four isolates, namely AaMDc1a, AaMRa1, AaMDc1d and AaMDc1b, all from Sicily, albeit from two different pomegranate cultivars, clustered within the A. arborescens species complex along with strains CBS 109730 from Solanum lycopersicum, CBS 105.24 from S. tuberosum, CBS 108.41 from wood, CBS 112749 from Malus domestica, CBS 118389 from Pyrus pyrifolia and CBS 115517 from M. domestica. This group of isolates corresponded to morphotype 6.
3.3. Pathogenicity Tests
All eight tested isolates of A. alternata and A. arborescens induced typical symptoms of heart rot on artificially inoculated pomegranate fruits and were reisolated from rotten arils. The proportion of symptomatic fruits for each isolate ranged from 88 to 100%, not to count inoculated fruits that dropped before harvesting (from one to two per each isolate). In each symptomatic fruit the rot extended to about half of the entire longitudinal section (Figure 4). No symptoms were observed on control fruit.
Figure 4.
Symptoms of heart rot in pomegranate fruits artificially inoculated by injecting a suspension of Alternaria conidia, 10 weeks after inoculation.
3.4. Analyses of Alternaria Mycotoxins
The toxigenic potential of the 42 Alternaria spp. isolates was investigated in vitro on liquid culture medium, and the concentration levels of the five mycotoxins extracted from culture filtrates are reported in Table 4. All isolates produced TeA, which was the prevalent mycotoxin found in culture extracts, with the only exception of isolates AaMR9a and AaMP9 of morphotype 1. Benzopyrone derivatives, AOH and AME, were produced by about 70% of the isolates. However, the amount of these two mycotoxins varied greatly among isolates and within species and morphotypes. The isolates that produced AOH also produced AME, but the concentrations of the two toxins in culture filtrates were not correlated. ALT, a third toxin in the class of dibenzo-α-pyrones, was quantified in less than 50% of samples; it was not detected in culture filtrates of isolates that did not produce AOH and AME. TEN, a cyclic tetrapeptide, was produced by 34 out of 42 A. alternata isolates, but its amount varied greatly, ranging from 3.09 to 2434 μg/L. None of the four strains of morphotype 6 (A. arborescens) tested produced TEN. Two isolates, AaMR14b and AaMP14a, both of morphotype 2 (tenuissima), produced only TeA. Overall, however, no correlation was found between the toxigenic profile of the isolates and their specific phylogenetic identity, morphotype and geographical origin.
Table 4.
In vitro mycotoxin production [µg/mL] by Alternaria isolates after eight days of incubation at 28 ± 1 °C in the dark.
| Isolate | Morphotype | Origin | Mycotoxin Concentration (μg/mL) a | ||||
|---|---|---|---|---|---|---|---|
| TeA | AOH | AME | ALT | TEN | |||
| AaMR7 | 1 | Italy, Sicily | 5133 ± 561 | 9.141 ± 1.46 | 2.094 ± 0.22 | 9.177 ± 1.32 | 167.5 ± 31.9 |
| AaMP7a | 1 | Italy, Sicily | 1555 ± 139 | 41.44 ± 7.22 | 26.56 ± 4.01 | 307.8 ± 34.7 | 303.2 ± 13.6 |
| AaMP10 | 1 | Italy, Sicily | 1066 ± 125 | n.d. b | n.d. | n.d. | 91.85 ± 1.76 |
| AaMR11 | 1 | Italy, Sicily | 1889 ± 146 | 38.73 ± 3.95 | 23.98 ± 8.38 | 131.41 ± 13.5 | 214.4 ± 13.1 |
| AaMMH6e | 1 | Italy, Sicily | 279.4 ± 44.6 | 5.619 ± 0.44 | 4.735 ± 0.42 | n.d. | 1.378 ± 0.12 |
| AaMP3 | 1 | Italy, Sicily | 495.6 ± 50.3 | n.d. | n.d. | n.d. | 3.16 ± 0.25 |
| AaMR9a | 1 | Italy, Sicily | 25.61 ± 0.48 | n.d. | n.d. | n.d. | 30.77 ± 2.28 |
| AaMP9 | 1 | Italy, Sicily | 24.13 ± 2.02 | 7.734 ± 0.17 | 4.462 ± 0.18 | 17.98 ± 0.79 | 34.05 ± 4.67 |
| AaMDc5b | 1 | Italy, Sicily | 839.2 ± 65.7 | n.d. | n.d. | n.d. | 12.99 ± 2.02 |
| AaMDc5d | 1 | Italy, Sicily | 3146 ± 256 | 26.50 ± 0.32 | 12.34 ± 1.65 | 83.59 ± 2.33 | 40.80 ± 4.32 |
| AaMMH6b | 1 | Italy, Sicily | 786.8 ± 111 | 6.604 ± 0.29 | 2.451 ± 0.40 | 8.236 ± 1.31 | n.d. |
| AaMMH7a | 1 | Italy, Sicily | 2799 ± 132 | 55.49 ± 3.16 | 14.41 ± 1.21 | 36.82 ± 1.57 | 13.77 ± 0.72 |
| AaMMH7d | 1 | Italy, Sicily | 1004 ± 67.9 | 4.010 ± 1.14 | 3.978 ± 0.72 | 3.464 ± 0.32 | 1.295 ± 0.01 |
| AaMMH6a | 1 | Italy, Sicily | 1606 ± 168 | 57.24 ± 5.39 | 24.06 ± 0.35 | 44.33 ± 4.31 | 19.37 ± 2.13 |
| AaMMH6d | 1 | Italy, Sicily | 1553 ± 70.8 | 83.28 ± 4.30 | 22.71 ± 2.29 | 17.08 ± 0.54 | 3.455 ± 0.58 |
| AaMMH7b | 1 | Italy, Sicily | 2189 ± 149 | 11.64 ± 0.20 | 4.932 ± 0.05 | n.d. | 31.82 ± 0.25 |
| M24-BB1 | 1 | Italy, Apulia | 4204 ± 650 | 222.3 ± 0.14 | 472.7 ± 1.21 | n.d. | 1957 ± 2.67 |
| M95 A2 | 1 | Italy, Apulia | 5204 ± 233 | 161.2 ± 0.25 | 407.3 ± 1.97 | n.d. | 2434 ± 2.78 |
| M103 A2-1 | 1 | Italy, Apulia | 1267 ± 198 | 547.7 ± 0.73 | 604.2 ± 0.60 | n.d. | 1655 ± 28.8 |
| M109 3 | 1 | Italy, Apulia | 3258 ± 7.78 | 384.9 ± 0.09 | 196.5 ± 0.36 | n.d. | n.d. |
| AaMR4 | 2 | Italy, Sicily | 1950 ± 56.5 | 23.82 ± 1.77 | 24.12 ± 4.32 | n.d. | 87.62 ± 4.76 |
| AaMR12 | 2 | Italy, Sicily | 1117 ± 20.9 | n.d. | n.d. | n.d. | 20.80 ± 0.31 |
| AaMR2b | 2 | Italy, Sicily | 1177 ± 44.6 | 3.922 ± 0.64 | 2.209 ± 0.38 | 6.019 ± 0.27 | 3.09 ± 0.86 |
| AaMP4 | 2 | Italy, Sicily | 789.3 ± 113 | n.d. | n.d. | n.d. | 15.19 ± 1.13 |
| AaMR14b | 2 | Italy, Sicily | 952.9 ± 0.34 | n.d. | n.d. | n.d. | n.d. |
| AaMP14a | 2 | Italy, Sicily | 488.2 ± 22.3 | n.d. | n.d. | n.d. | n.d. |
| AaMR14a | 2 | Italy, Sicily | 1169 ± 33.8 | n.d. | n.d. | n.d. | 3.542 ± 0.66 |
| AaMP14b | 2 | Italy, Sicily | 9227 ± 904 | 44.87 ± 2.87 | 6.603 ± 0.27 | n.d. | 5.986 ± 0.59 |
| M80 B5 | 2 | Italy, Apulia | 2987 ± 1.09 | 39.15 ± 0.55 | 73.27 ± 0.68 | n.d. | 8621 ± 37.1 |
| AaMR5b | 3 | Italy, Sicily | 1264 ± 73.0 | 18.29 ± 1.63 | 23.59 ± 1.36 | 112.5 ± 2.51 | 153.2 ± 15.4 |
| AaMDc3a | 4 | Italy, Sicily | 1762 ± 38.6 | 37.64 ± 1.16 | 4.853 ± 0.01 | 17.97 ± 1.06 | 51.69 ± 5.91 |
| AaMDc3b | 4 | Italy, Sicily | 2887 ± 195 | 89.64 ± 2.97 | 10.77 ± 0.10 | 7.465 ± 0.28 | 27.94 ± 2.72 |
| AaMDc3c | 4 | Italy, Sicily | 2336 ± 154 | n.d. | n.d. | n.d. | 64.26 ± 2.55 |
| AaMDc3d | 4 | Italy, Sicily | 2532 ± 214 | 32.55 ± 0.60 | 4.905 ± 0.28 | 9.409 ± 0.40 | 77.48 ± 7.63 |
| AaMR6b | 5 | Italy, Sicily | 1460 ± 83.6 | n.d. | n.d. | n.d. | 235.7 ± 38.1 |
| AaMP6b | 5 | Italy, Sicily | 1115 ± 24.9 | 1.848 ± 0.01 | 4.928 ± 0.43 | 94.28 ± 8.51 | 201.4 ± 20.3 |
| AaMDc2a | 5 | Italy, Sicily | 2028 ± 92.3 | n.d. | n.d. | n.d. | 116.1 ± 6.53 |
| AaMDc2b | 5 | Italy, Sicily | 1840 ± 159 | n.d. | n.d. | n.d. | 51.42 ± 0.19 |
| AaMDc1a | 6 | Italy, Sicily | 2769 ± 207 | 56.18 ± 6.82 | 7.050 ± 1.71 | 425.97 ± 33.2 | n.d. |
| AaMDc1b | 6 | Italy, Sicily | 3918 ± 473 | 18.04 ± 2.17 | 4.074 ± 0.04 | 43.23 ± 0.92 | n.d. |
| AaMDc1d | 6 | Italy, Sicily | 1680 ± 171 | 33.12 ± 4.62 | 2.984 ± 1.26 | 67.54 ± 2.91 | n.d. |
| AaMRa1 | 6 | Italy, Sicily | 2666 ± 19.5 | 76.98 ± 1.37 | 9.974 ± 1.29 | 384.92 ± 42.3 | n.d. |
a Means ± standard error of three independent biological experiments consisting of three technical replicates each. b n.d.= not detected.
4. Discussion
Results evidenced the complexity of Alternaria populations associated to heart rot of pomegranate fruits. According to the classification system proposed recently by Woudenberg et al. [20] for the small-spored Alternaria species in the Alternaria section Alternaria, the isolates recovered from symptomatic pomegranate fruit sampled in two major producing regions of southern Italy, Apulia and Sicily, were referred to A. alternata and A. arborescens species complex (AASC) [20], the former being by far the prevalent species. Despite the morphological and genetic variability, all tested isolates of both A. alternata and A. arborescens were pathogenic and induced typical symptoms of heart rot in pomegranate fruit artificially inoculated by injecting separately a conidial suspension of these two fungi into the fruit. These results indicate that A. alternata and A. arborescens are responsible for pomegranate heart rot disease in southern Italy. According to the available information, this is the first report of A. arborescens as a pathogen of pomegranate in Italy. Both species are known as plant pathogens and mycotoxin producers on a wide range of host plants [32,53]. Due to their growth even at low temperature, these Alternaria species are also responsible for spoilage of fruits and processed plant products during refrigerated transport and storage [54].
In agreement with a previous study aimed at identifying Alternaria species associated to brown spot of tangerines in southern Italy [19], A. alternata isolates recovered from pomegranate fruits showed great morphological and molecular variability and were separated into six morphotypes corresponding to distinct albeit phylogenetically related clusters. Our results confirmed previous findings of other authors in California showing that diverse species of Alternaria are associated to heart rot of pomegranate [14,18]. Although in California heart rot is regarded as a minor disease of pomegranate, the correct identification of Alternaria species associated to this disease is considered a crucial aspect, as A. gaisen, included in the section Alternaria and closely related to A. arborescens, is a quarantine pathogen, and its presence might impose export restrictions [14]. In the present study, multilocus phylogenetic analysis based on four gene regions ITS, EF-1α, GPDH and OPA 10-2, clearly separated A. alternata, A. arborescens and A. gaisen. Whereas, neither ITS nor EF-1α and GPDH, alone or in combination, were able to discriminate these three species. Consistently with the results of this study aimed at characterizing the diversity of Alternaria species associated to heart rot of pomegranate, A. alternata and A. arborescens were the species associated to the brown fruit rot of tangerines and mandarins in southern Italy and California, and also in these cases, A. alternata was the prevalent species [19,55]. It was demonstrated that the virulence of the Alternaria isolates recovered from citrus was positively correlated with the expression level of the ACTT1 and ACTT1 genes encoding for phytotoxins ACTT1 and ACTT2, respectively [19]. Several formae speciales of A. alternata infecting fruit and vegetable crops and producing host-specific toxins are currently recognized, including A. alternata f. sp. lycopersici producing AAL-toxins and causing necrotic lesions on tomato, A. alternata f. sp. mali producing the AM toxin, A. alternata f. sp. fragariae producing the AF-toxin, and A. alternata f. sp. citri with two pathotypes, i.e., pathotype rough lemon for isolates producing the ACR-toxin, and pathotype tangerine for isolates producing the ACT-toxins [39,56]. No f. sp. or pathotype of A. alternata have been identified so far within the A. alternata populations associated to pomegranate fruits.
In standard laboratory conditions, isolates of both A. alternata and A. arborescens recovered from pomegranate fruits with symptoms of heart rot were able to produce mycotoxins, mainly tenuazonic acid (TeA), a tetramic acid derivative, and to a lesser extent alternariol (AOH), alternariol monomethyl ether (AME) and altenuene (ALT), in the structural group of dibenzopyrone derivatives. The majority of A. alternata also produced the cyclic tetrapeptide tentoxin (TEN), whereas none of the tested A. arborescens isolates produced this metabolite. The results of the present study are consistent with previous reports indicating that small-spored species in the section Alternaria (A. tenuissima, A. arborescens and A. alternata species groups) share a common secondary metabolite profile, but only a small proportion of A. arboresecns isolates are able to produce TEN [57,58]. TeA, AOH, AME and TEN have a broad host range [40] and in pomegranate heart rot disease they might act as virulence factors [59]. Some of these non-host-specific toxins, such as TeA, AOH and AME, have been reported to induce harmful effects in mammals [60]. In vitro tests provided clear evidence of genotoxicity or acute toxicity in animals or rodent cells of AOH, AME, ALT and TeA [41]. Moldy arils of fresh pomegranate fruits affected by heart rot are usually removed by the consumer, as they are not appropriate for human consumption. However, toxins can move from the rotten part to the surrounding tissues or processed fruit products. This may occur more frequently in Alternaria diseases that go unnoticed, as symptoms are internal, such as pomegranate heart rot. Moreover, current industrial processing methods do not exclude the risk of juice contamination by Alternaria toxins [33,60,61]. As a consequence, the occurrence of heart rot on pomegranate fruits and of Alternaria toxins as contaminants in juice may be a risk factor for consumer health. In a comprehensive study of EFSA (European Food Safety Authority) aimed at investigating the distribution of Alternaria toxins across the food categories and groups, AOH, AME, ALT, TeA and TEN were the toxins occurring most commonly in fruit and vegetable juices [41]. In this study the ability of Alternaria species associated to heart rot of pomegranate to produce toxins has been investigated for the first time in Europe.
5. Conclusions
The diversity of Alternaria responsible for heart rot of pomegranate in southern Italy, encompassing ubiquitous and polyphagous species with both a saprophytic and pathogenic lifestyle, like A. alternata and A. arborescens, and the ability of all identified Alternaria genotypes to induce disease in artificially wound inoculated fruits would suggest this is a complex disease. Generally speaking, environmental factors and host plant susceptibility usually have a key role as disease determinants of this type of diseases. The factors favoring the susceptibility of pomegranate fruits to infection by A. alternata and the onset of Alternaria heart rot in commercial pomegranate orchards in Israel have been investigated, but results are not yet conclusive [62]. Our study also highlights another aspect of Alternaria heart rot of pomegranate, namely the toxigenic potential of Alternaria species associated with the disease, which has practical implications for the juice industry and the EU regulations of the limits of mycotoxins in foods. The actual risk and level of contamination of pomegranate juice by Alternaria toxins during the industrial processing have not yet been quantified and deserve to be thoroughly investigated. Tools to rapidly screen internally rotten pomegranate fruits during postharvest processing, thus preventing the risk of contamination by mycotoxins, are being envisaged [17]. A practical method to identify fruits affected by heart rot might be to shake the trees before harvest, as internally rotten fruits are more prone to drop, whereas fungicide treatments proved to be scarcely effective in preventing the disease [13].
Acknowledgments
Authors wish to thank Sebastiano Alba and Alessandro Alba for hosting the authors at Carmito estate in Lentini (SR, Italy) and Giovanni Audoly for the invaluable collaboration during the surveys and Vittorio Lo Giudice for the encouragement to focus on this emerging phytopathological problem. Authors are grateful to Ann Davies for the English revision of the text.
Author Contributions
Conceptualization, S.O.C., A.P., M.L.G., and A.I.; methodology, M.R., F.A., S.M.S., A.P., S.O.C., and I.S.; software, F.A. and M.R.; validation, S.O.C., S.M.S., A.I., A.P., and M.L.G.; formal analysis, F.A and M.R.; investigation, F.A., A.M., M.R., and I.S.; resources, S.O.C., A.P., A.I., and M.L.G.; data curation, F.A., A.M., S.M.S., and M.R.; writing—original draft preparation, F.A. and M.R.; writing—review and editing, S.O.C., A.P., S.M.S., A.M., A.I., and M.L.G.; visualization, S.O.C.; supervision, S.O.C., A.I., and M.L.G.; project administration, S.O.C. and A.P.; funding acquisition, S.O.C., A.P., A.I. and M.L.G. All authors have read and agreed to the published version of the manuscript.
Funding
Research of F.A., M.R., A.P. and S.O.C. was funded by the University of Catania, Italy (UNICT), Italy, Grant “5A722192155” “Investigation of phytopathological problems of the main Sicilian productive contexts and eco-sustainable defense strategies (MEDIT-ECO)” PiaCeRi–PIAno di inCEntivi per la Ricerca di Ateneo 2020-22 linea 2”; by the project “PROMETEO”, Strategic project ENI Italy-Tunisia 2014-2020 (UNICT), and by the project “Smart and innovative packaging, postharvest rot management and shipping of organic citrus fruit (BiOrangePack)” Partnership for Research and Innovation in the Mediterranean Area (PRIMA- S2-2019) (E69C20000130001) (UNICT). F.A. has been granted a Ph.D. fellowship “Scienze Agrarie, Alimentari, Forestali e Ambientali–XXXIII cycle”, University of Palermo; M.R. has been granted a fellowship by CREA-OFA (Rende, Italy), this study is part of his activity as PhD, in “Agricultural, Food, and Forestry Science”, University Mediterranea of Reggio Calabria, XXXV cycle.” Research of A.M. and A.I. was funded by the Department of Soil, Plant, and Food Sciences (University of Bari Aldo Moro, Italy) as preliminary study within the framework of the PRIMA Project “Innovative sustainable technologies to extend the shelf-life of perishable Mediterranean fresh fruit, vegetables and aromatic plants and to reduce waste (StopMedWaste).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Faedda R., Granata G., Cocuzza G.E.M., Giudice V.L., Audoly G., Pane A., Cacciola S.O. First report of heart rot of pomegranate (Punica granatum) caused by Alternaria alternata in Italy. Plant Dis. 2015;99:1446. doi: 10.1094/PDIS-02-15-0238-PDN. [DOI] [Google Scholar]
- 2.Istat.it. [(accessed on 18 September 2020)]; Available online: http://dati.istat.it/Index.aspx?QueryId=33705.
- 3.Wang R., Ding Y., Liu R., Xiang L., Du L. Pomegranate: Constituents, bioactivities and fharmacokinetics. In: Chandra R., editor. Pomegranate, Fruit, Vegetable and Cereal Science and Biotechnology. National Research Center on Pomegranate; Solapur, India: 2010. pp. 77–87. [Google Scholar]
- 4.Tehranifar A., Selahvarzi Y., Kharrazi M., Bakhsh V.J. High potential of agro-industrial by-products of pomegranate (Punica granatum L.) as the powerful antifungal and antioxidant substances. Ind. Crop. Prod. 2011;34:1523–1527. doi: 10.1016/j.indcrop.2011.05.007. [DOI] [Google Scholar]
- 5.Pangallo S., Li Destri Nicosia M.G., Agosteo G.E., Abdelfattah A., Romeo F.V., Cacciola S.O., Rapisarda P., Schena L. Evaluation of a pomegranate peel extract as an alternative means to control olive anthracnose. Phytopathology. 2017;107:1462–1467. doi: 10.1094/PHYTO-04-17-0133-R. [DOI] [PubMed] [Google Scholar]
- 6.Pangallo S., Nicosia M.G.L.D., Raphael G., Levin E., Ballistreri G., Cacciola S.O., Rapisarda P., Droby S., Schena L. Elicitation of resistance responses in grapefruit and lemon fruits treated with a pomegranate peel extract. Plant Pathol. 2017;66:633–640. doi: 10.1111/ppa.12594. [DOI] [Google Scholar]
- 7.Belgacem I., Pangallo S., Abdelfattah A., Romeo F.V., Cacciola S.O., Li Destri Nicosia M.G., Ballistreri G., Schena L. Transcriptomic analysis of orange fruit treated with pomegranate peel extract (PGE) Plants. 2019;8:101. doi: 10.3390/plants8040101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J., Liao C., Ouyang X., Kahramanoǧlu I., Gan Y., Li M. Antimicrobial activity of pomegranate peel and its applications on food preservation. J. Food Qual. 2020:8850339. doi: 10.1155/2020/8850339. [DOI] [Google Scholar]
- 9.Magangana T.P., Makunga N.P., Fawole O.A., Opara U.L. Processing factors affecting the phytochemical and nutritional properties of pomegranate (Punica granatum L.) peel waste: A review. Molecules. 2020;25:4690. doi: 10.3390/molecules25204690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pangallo S., Nicosia M.G.L.D., Scibetta S., Strano M.C., Cacciola S.O., Agosteo G.E., Belgacem I., Schena L. Pre- and postharvest applications of a pomegranate peel extract to control citrus fruit decay during storage and shelf life. Plant Dis. 2020 doi: 10.1094/PDIS-01-20-0178-RE. [DOI] [PubMed] [Google Scholar]
- 11.Tziros G.T., Lagopodi A.L., Tzavella-Klonari K. Alternaria alternata fruit rot of pomegranate (Punica granatum) in Greece. Plant Pathol. 2008;57:379. doi: 10.1111/j.1365-3059.2007.01668.x. [DOI] [Google Scholar]
- 12.Thomidis T. Fruit rots of pomegranate (cv. Wonderful) in Greece. Australas. Plant Pathol. 2014;43:583–588. doi: 10.1007/s13313-014-0300-0. [DOI] [Google Scholar]
- 13.Ezra D., Kirshner B., Hershcovich M., Shtienberg D., Kosto I. Heart rot of pomegranate: Disease etiology and the events leading to development of symptoms. Plant Dis. 2015;99:496–501. doi: 10.1094/PDIS-07-14-0707-RE. [DOI] [PubMed] [Google Scholar]
- 14.Luo Y., Hou L., Förster H., Pryor B., Adaskaveg J.E. Identification of Alternaria species causing heart rot of pomegranates in California. Plant Dis. 2017;101:421–427. doi: 10.1094/PDIS-08-16-1176-RE. [DOI] [PubMed] [Google Scholar]
- 15.Benagi V.I., Ravi Kumar M.R., Gowdar S.B., Pawar B.B. Survey on diseases of pomegranate in Northern Karnataka, India. Acta Hortic. 2011;890:509–511. doi: 10.17660/ActaHortic.2011.890.71. [DOI] [Google Scholar]
- 16.Day K.R., Wilkins E.D. Commercial pomegranate (Punica granatum L.) production in California. Acta Hortic. 2011;890:275–285. doi: 10.17660/ActaHortic.2011.890.39. [DOI] [Google Scholar]
- 17.Zhang L., McCarthy M.J. Black heart characterization and detection in pomegranate using NMR relaxometry and MR imaging. Postharvest Biol. Technol. 2012;67:96–101. doi: 10.1016/j.postharvbio.2011.12.018. [DOI] [Google Scholar]
- 18.Michailides T., Morgan D., Quist M., Reyes H. Infection of pomegranate by Alternaria spp. causing black heart. Phytopathology. 2008;98:S105. [Google Scholar]
- 19.Garganese F., Schena L., Siciliano I., Prigigallo M.I., Spadaro D., dD Grassi A., Ippolito A., Sanzani S.M. Characterization of citrus-associated Alternaria species in Mediterranean areas. PLoS ONE. 2016;11:e0163255. doi: 10.1371/journal.pone.0163255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woudenberg J.H.C., Seidl M.F., Groenewald J.Z., de Vries M., Stielow J.B., Thomma B.P.H.J., Crous P.W. Alternaria section Alternaria: Species, formae speciales or pathotypes? Stud. Mycol. 2015;82 doi: 10.1016/j.simyco.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawrence D.P., Rotondo F., Gannibal P.B. Biodiversity and taxonomy of the pleomorphic genus Alternaria. Mycol. Prog. 2016;15 doi: 10.1007/s11557-015-1144-x. [DOI] [Google Scholar]
- 22.Patriarca A. Alternaria in food products. Curr. Opin. Food Sci. 2016;11 doi: 10.1016/j.cofs.2016.08.007. [DOI] [Google Scholar]
- 23.Simmons E.G. Alternaria themes and variations (236–243) host-specific toxin producers. Mycotaxon. 1999;70:325–369. [Google Scholar]
- 24.Simmons E.G. Alternaria. In: Simmons E.G., editor. An Identification Manual: Fully Illustrated and with Catalogue Raisonné 1796–2007. CBS Fungal Biodiversity Centre; Utrecht, The Netherlands: 2007. [Google Scholar]
- 25.Andrew M., Peever T.L., Pryor B.M. An expanded multilocus phylogeny does not resolve morphological species within the small-spored Alternaria species complex. Mycologia. 2009;101:95–109. doi: 10.3852/08-135. [DOI] [PubMed] [Google Scholar]
- 26.Armitage A.D., Barbara D.J., Harrison R.J., Lane C.R., Sreenivasaprasad S., Woodhall J.W., Clarkson J.P. Discrete lineages within Alternaria alternata species group: Identification using new highly variable loci and support from morphological characters. Fungal Biol. 2015;119:994–1006. doi: 10.1016/j.funbio.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Kusaba M., Tsuge T. Philogeny of Alternaria fungi known to produce host-specific toxins on the basis of variation in internal transcribed spacers of ribosomal DNA. Curr. Genet. 1995;28:491–498. doi: 10.1007/BF00310821. [DOI] [PubMed] [Google Scholar]
- 28.Johnson L.J., Johnson R.D., Akamatsu H., Salamiah A., Otani H., Kohmoto K., Kodama M. Spontaneous loss of a conditionally dispensable chromosome from the Alternaria alternata apple pathotype leads to loss of toxin production and pathogenicity. Curr. Genet. 2001;40:65–72. doi: 10.1007/s002940100233. [DOI] [PubMed] [Google Scholar]
- 29.Peever T.L., Su G., Carpenter-Boggs L., Timmer L.W. Molecular systematics of citrus-associated Alternaria species. Mycologia. 2004;96:119–134. doi: 10.1080/15572536.2005.11833002. [DOI] [PubMed] [Google Scholar]
- 30.Pryor B.M., Bigelow D.M. Molecular characterization of Embellisia and Nimbya species and their relationship to Alternaria, Ulocladium and Stemphylium. Mycologia. 2003;95:1141–1154. doi: 10.1080/15572536.2004.11833024. [DOI] [PubMed] [Google Scholar]
- 31.Pryor B.M., Gilbertson R.L. Molecular phylogenetic relationships amongst Alternaria species and related fungi based upon analysis of nuclear ITS and mt SSU rDNA sequences. Mycol. Res. 2000;104:1312–1321. doi: 10.1017/S0953756200003002. [DOI] [Google Scholar]
- 32.Andersen B., Nielsen K.F., Pinto V.F., Patriarca A. Characterization of Alternaria strains from Argentinean blueberry, tomato, walnut and wheat. Int. J. Food Microbiol. 2015;196 doi: 10.1016/j.ijfoodmicro.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 33.Logrieco A., Moretti A., Solfrizzo M. Alternaria toxins and plant diseases: An overview of origin, occurrence and risks. World Mycotoxin J. 2009;2:129–140. doi: 10.3920/WMJ2009.1145. [DOI] [Google Scholar]
- 34.Prelle A., Spadaro D., Garibaldi A., Gullino M.L. A new method for detection of five Alternaria toxins in food matrices based on LC-APCI-MS. Food Chem. 2013;140:161–167. doi: 10.1016/j.foodchem.2012.12.065. [DOI] [PubMed] [Google Scholar]
- 35.Siciliano I., Ortu G., Gilardi G., Gullino M.L., Garibaldi A. Mycotoxin production in liquid culture and on plants infected with Alternaria spp. isolated from rocket and cabbage. Toxins. 2015;7:743–754. doi: 10.3390/toxins7030743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Escrivá L., Oueslati S., Font G., Manyes L. Alternaria Mycotoxins in food and feed: An Overview. J. Food Qual. 2017;5 doi: 10.1155/2017/1569748. [DOI] [Google Scholar]
- 37.Fraeyman S., Croubels S., Devreese M., Antonissen G. Emerging Fusarium and Alternaria mycotoxins: Occurrence, toxicity and toxicokinetics. Toxins. 2017;9:228. doi: 10.3390/toxins9070228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masiello M., Somma S., Susca A., Ghionna V., Logrieco A.F., Franzoni M., Ravaglia S., Meca G., Moretti A. Molecular identification and mycotoxin production by Alternaria species occurring on durum wheat, showing black point symptoms. Toxins. 2020;12:275. doi: 10.3390/toxins12040275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hickert S., Bergmann M., Ersen S., Cramer B., Humpf H.U. Survey of Alternaria toxin contamination in food from the German market, using a rapid HPLC-MS/MS approach. Mycotoxin Res. 2016;32:7–18. doi: 10.1007/s12550-015-0233-7. [DOI] [PubMed] [Google Scholar]
- 40.Thomma B.P.H.J. Alternaria spp.: From general saprophyte to specific parasite. Mol. Plant Pathol. 2003;4:225–236. doi: 10.1046/j.1364-3703.2003.00173.x. [DOI] [PubMed] [Google Scholar]
- 41.EFSA Panel on Contaminants in the Food Chain Scientific opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J. 2011;9:2407. doi: 10.2903/j.efsa.2011.2407. [DOI] [Google Scholar]
- 42.Holland D., Hatib K., Bar-Ya’akov I. Pomegranate: Botany, horticulture, breeding. Hortic. Rev. 2009;35:127–191. [Google Scholar]
- 43.Pareek S., Valero D., Serrano M. Postharvest biology and technology of pomegranate. J. Sci. Food Agric. 2015;95:2360–2379. doi: 10.1002/jsfa.7069. [DOI] [PubMed] [Google Scholar]
- 44.Prasad R.N., Chandra R., da Silva J.A.T. Postharvest handling and processing of pomegranate. In: Chandra R., editor. Pomegranate, Fruit, Vegetable and Cereal Science and Biotechnology. Global Science Books; Solapur, India: 2010. pp. 88–95. [Google Scholar]
- 45.Crous P.W., Verkley G.J.M., Groenwald E., Houbraken J. Fungal Biodiversity, Westerdijk Laboratory Manual Series Vol.1. Westerdijk Fungal Biodiversity Institute; Utrecht, The Netherlands: 2009. [Google Scholar]
- 46.Pryor B.M., Michailides T.J. Morphological, pathogenic, and molecular characterization of Alternaria isolates associated with alternaria late blight of pistachio. Phytopathology. 2002;92:406–416. doi: 10.1094/PHYTO.2002.92.4.406. [DOI] [PubMed] [Google Scholar]
- 47.White T.J., Bruns T., Lee S., Taylor J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR Protocols: A guide to Methods and Applications. Volume 18. Academic Press, Inc.; San Diego, CA, USA: 1990. pp. 315–322. [Google Scholar]
- 48.Carbone I., Kohn L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. doi: 10.1080/00275514.1999.12061051. [DOI] [Google Scholar]
- 49.Berbee M.L., Pirseyedi M., Hubbard S. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia. 1999;91:964–977. doi: 10.1080/00275514.1999.12061106. [DOI] [Google Scholar]
- 50.FinchTV v.1.4.0. [(accessed on 18 May 2020)]; Available online: https://digitalworldbiology.com/FinchTV.
- 51.Elim Dupes Software. [(accessed on 15 June 2020)]; Available online: http://hcv.lanl.gov/content/sequence/ELIMDUPES/elimdupes.html.
- 52.Hall M.P., Nagel R.J., Fagg W.S., Shiue L., Cline M.S., Perriman R.J., Donohue J.P., Ares M. Quaking and PTB control overlapping splicing regulatory networks during muscle cell differentiation. RNA. 2013;19:627–638. doi: 10.1261/rna.038422.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Troncoso-Rojas R., Tiznado-Hernández M.E. Postharvest Decay: Control Strategies. Academic Press; Cambridge, MA, USA: 2014. Alternaria alternata (Black Rot, Black Spot) pp. 147–187. [Google Scholar]
- 54.Faedda R., D’Aquino S., Granata G., Pane A., Palma A., Sanzani S.M., Schena L., Cacciola S.O. Postharvest fungal diseases of cactus pear fruit in southern Italy. Acta Hortic. 2016;1144:215–218. doi: 10.17660/ActaHortic.2016.1144.31. [DOI] [Google Scholar]
- 55.Wang F., Saito S., Michailides T., Xiao C.-L. Phylogenetic, morphological, and pathogenic characterization of Alternaria species associated with fruit rot of mandarin in California. Plant Dis. 2020 doi: 10.1094/PDIS-10-20-2145-RE. [DOI] [PubMed] [Google Scholar]
- 56.Masunaka A., Ohtani K., Peever T.L., Timmer L.W., Tsuge T., Yamamoto M., Yamamoto H., Akimitsu K. An isolate of Alternaria alternata that is pathogenic to both tangerines and rough lemon and produces two host-selective toxins, ACT- and ACR-toxins. Phytopathology. 2005;95:241–247. doi: 10.1094/PHYTO-95-0241. [DOI] [PubMed] [Google Scholar]
- 57.Pinto V.E.F., Patriarca A. Alternaria species and their associated mycotoxins. In: Moretti A., Susca A., editors. Mycotoxigenic Fungi, Methods and Protocols. Humana Press; New York, NY, USA: 2017. pp. 13–32. (Volume 1542 of Methods in Molecular Biology series). [DOI] [PubMed] [Google Scholar]
- 58.Patriarca A., da Cabral L.C., Pavicich M.A., Nielsen K.F., Andersen B. Secondary metabolite profiles of small-spored Alternaria support the new phylogenetic organization of the genus. Int. J. Food Microbiol. 2019;291:135–143. doi: 10.1016/j.ijfoodmicro.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 59.Wenderoth M., Garganese F., Schmidt-Heydt M., Soukup S.T., Ippolito A., Sanzani S.M., Fischer R. Alternariol as virulence and colonization factor of Alternaria alternata during plant infection. Mol. Microbiol. 2019;112:131–146. doi: 10.1111/mmi.14258. [DOI] [PubMed] [Google Scholar]
- 60.Barkai-Golan R., Paster N. In: Mycotoxins in Fruits and Vegetables. Barkai-Golan R., Nachman P., editors. Academic Press; San Diego, CA, USA: 2008. [Google Scholar]
- 61.Elhariry H.M., Khiralla G.M., Gherbawy Y., Elrahman H.A. Natural occurrence of Alternaria toxins in pomegranate fruit and the influence of some technological processing on their levels in juice. Acta Aliment. 2016;45:380–389. doi: 10.1556/066.2016.45.3.9. [DOI] [Google Scholar]
- 62.Ezra D., Shulhani R., Ya’Akov I.B., Harel-Beja R., Holland D., Shtienberg D. Factors affecting the response of pomegranate fruit to Alternaria alternata, the causal agent of heart rot. Plant Dis. 2019;103:315–323. doi: 10.1094/PDIS-01-18-0147-RE. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.