Abstract
Malnutrition negatively affects the quality of life of patients with dysphagia. Despite the need for nutritional status assessment in patients with dysphagia, standard, effective nutritional assessments are not yet available, and the identification of optimal nutritional assessment items for patients with dysphagia is inadequate. We conducted a scoping review of the use of nutritional assessment items in adult patients with oropharyngeal and esophageal dysphagia. The MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials databases were searched to identify articles published in English within the last 30 years. Twenty-two studies met the inclusion criteria. Seven nutritional assessment categories were identified: body mass index (BMI), nutritional screening tool, anthropometric measurements, body composition, dietary assessment, blood biomarkers, and other. BMI and albumin were more commonly assessed in adults. The Global Leadership Initiative on Malnutrition (GLIM), defining new diagnostic criteria for malnutrition, includes the categories of BMI, nutritional screening tool, anthropometric measurements, body composition, and dietary assessment as its required components, but not the blood biomarkers and the “other” categories. We recommend assessing nutritional status, including GLIM criteria, in adult patients with dysphagia. This would standardize nutritional assessments in patients with dysphagia and allow future global comparisons of the prevalence and outcomes of malnutrition, as well as of appropriate interventions.
Keywords: swallowing, malnutrition, nutritional status, GLIM, adults
1. Introduction
Dysphagia is a global health problem estimated to affect 8% of the world’s population [1]. Dysphagia diminishes the quality of life of individuals [2,3], and dysphagia patients who are malnourished and who do not have access to appropriate treatment and interventions sustain a longer hospital stay, higher risk of complications, and higher mortality rate than those who are properly nourished [4,5]. Therefore, dysphagia and malnutrition are closely associated [6,7]. It is reported that 39.2% of dysphagic patients are at risk for malnutrition and that 13.6% of individuals at risk for malnutrition have dysphagia [8]. Besides, the prevalence of concurrent malnutrition and dysphagia has been estimated between 3% and 29% [9,10]. Patients with oropharyngeal dysphagia (OD) are prone to receiving inadequate food intake and presenting malnutrition because of fear of choking, anorexia, and decreased food preference related to food texture [11]. In addition, texture-modified diets are lower in nutrients than a regular diet and are more likely to induce malnutrition and sarcopenia than a regular diet [12,13,14]. Malnutrition leads to systemic muscle mass loss and atrophy of the muscles used to swallow, and this ultimately leads to dysphagia [15,16]. Therefore, it is recommended that the nutritional status of all dysphagic patients should be assessed [17,18].
Nutritional assessment is the process of determining if there is a problem with an individual’s nutritional status, identifying it, and performing a detailed examination to determine the severity of malnutrition [19]. A nutritional assessment must also include variables that will help in the appropriate follow-up of the patient after nutritional therapy has been implemented [20]. Specifically, it includes the evaluation of subjective and objective parameters, such as medical history, dietary intake, physical examination, anthropometric measurements, physical function, mental function, quality of life, medications, and laboratory data [21,22]. Namasivayam et al. [9] conducted a systematic review of the impact of dysphagia on malnutrition in patients in long-term care. Body mass index (BMI), weight loss, Mini Nutritional Assessment (MNA), and laboratory data (serum and urinary tests) were identified as indicators in a nutritional assessment, but there was no uniformity in their review. In addition, for BMI, which was the most commonly used measure in the studies reviewed, different cutoff values were chosen. Namasivayam et al. concluded that it was difficult to accurately ascertain the prevalence of malnutrition due to discrepancies in the measurement methods used. The Global Leadership Initiative on Malnutrition (GLIM) was advocated by several of the global clinical nutrition societies in 2018, with the aim of enabling global comparisons of the prevalence of malnutrition and related interventions and outcomes [23,24]. However, the optimal nutritional assessment items for dysphagia patients have not yet been identified. Deeper knowledge in this area will facilitate the identification of the optimal nutritional assessment items for patients with dysphagia and help us to understand the actual prevalence of malnutrition in these patients. This may allow us to spread awareness on the issue of malnutrition and facilitate early nutritional interventions in dysphagia patients. As a result, it may be possible to prevent a reduction in the quality of life caused by malnutrition in dysphagia patients. The aim of this scoping review was to identify the most important items to include in the nutritional assessment for patients with dysphagia.
2. Materials and Methods
We conducted a scoping review to answer the following research question: “What are the appropriate nutritional assessment items for adult patients with oropharyngeal and esophageal dysphagia?” This scoping review protocol was registered in advance [25]. Scoping reviews are conducted to map out key concepts underlying a research area, the main sources of information, types of study, and evidence available and to clarify the definitions and conceptual boundaries of a topic [26]. In other words, they aim at (i) identifying types of available evidence in a given field, (ii) identifying and analyzing knowledge gaps, (iii) clarifying key concepts/definitions in the literature, (iv) examining how research is conducted in a certain topic or field, and (v) identifying key characteristics or factors associated with a concept [27]. The most common reasons for conducting scope reviews are to explore the breadth and scope of the literature, map and summarize the evidence, and inform future research [28]. Scope reviews can be conducted as a preliminary exercise before conducting systematic reviews [29], and unlike systematic reviews, the process of assessing the risk of bias and synthesizing findings from individual studies to generate “summary” findings is not mandatory [27]. We used a scoping review methodology consistent with the Joanna Briggs Institute’s guidance [30] to ensure clarity and rigor in the review process. Additionally, our review was performed in accordance with the scoping review reporting guidelines of the PRISMA Extension for Scoping Reviews (PRISMA-ScR) [31].
2.1. Eligibility Criteria
This scope review included studies in which nutritional assessment was performed on adult patients with dysphagia. The concepts examined in this scoping review included components of nutritional assessment in the adult population with dysphagia. Components of the nutritional assessment were “Nutritional Assessment,” “Nutritional Status,” “Body Composition,” and “Dietary Assessment” [21,32,33]. Individuals with dysphagia were defined as those clinically diagnosed with dysphagia by videofluoroscopy swallowing studies or fiberoptic endoscopic swallowing assessment [34]. The inclusion criteria were observational and intervention studies in which nutritional assessment was performed in adult patients with oropharyngeal and esophageal dysphagia. In addition, articles and literature in textbooks and peer-reviewed journals written in English and published between January 1991 and May 2020 were eligible for inclusion. Review articles, studies not including 100% of patients with dysphagia, studies using animal models, qualitative studies, case reports, and conference abstracts were excluded.
2.2. Search Strategy
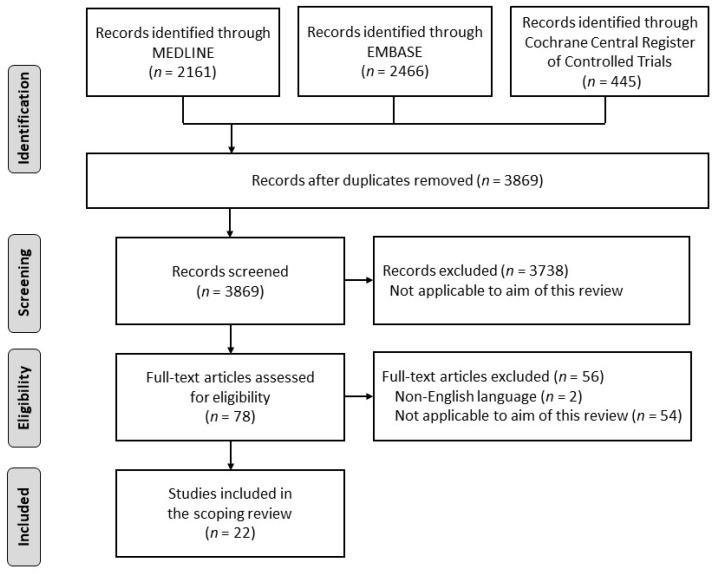
The first step in the search strategy consisted in conducting a pilot search in MEDLINE to identify articles on this topic. Text words in the titles and abstracts and the index terms used to describe these articles were used to develop the search strategy. Next, the search formula developed was used to search three databases (MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials). The most recent search was performed on 12 May 2020. The search strategy was presented in the protocol registration in advance [25]. The references searched were imported into Rayyan version 0.1.0 (Qatar Computing Research Institute, Doha, Qatar; https://rayyan.qcri.org/ (accessed on 12 May 2020)). Twelve reviewers used Rayyan to screen the titles and abstracts of articles for eligibility and to assess their full text. Disagreements between reviewers were resolved through discussion, and an independent reviewer was consulted when necessary to resolve any disagreements. An overview of the scoping review process is shown in Figure 1.
Figure 1.
Flowchart of the search strategy. An initial search of studies was performed using the MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials databases (n = 5072). Screening for eligibility and full-text assessments were performed. Twenty-two articles were included in the final analysis.
2.3. Data Extraction
A single reviewer extracted data from the articles that satisfied the eligibility criteria. Narrative synthesis was used in the data analysis to characterize the studies analyzing nutritional assessments in patients with dysphagia. The extracted data were analyzed according to the following variables: setting, characteristics of the patients with dysphagia (age and comorbidities), nutritional assessor, nutritional assessment items, and author(s), year, and country of the publication (Table S1).
3. Results
In the initial search, we identified 5072 articles using the MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials databases. Duplicates were removed, the titles and abstracts of these articles were screened for eligibility, and their full texts were assessed. After applying the exclusion criteria (Table S2), twenty-two articles were included in the analysis (Figure 1). The nutritional assessment items from the included articles were categorized into seven categories: BMI, nutritional screening tool, anthropometric measurements, body composition, dietary assessment, blood biomarkers, and other. The assessors included professionals, dietitians, multidisciplinary teams (Table S1).
3.1. Nutritional Assessment in Adult Patients with Dysphagia
Table 1 shows the nutritional assessment items for adult patients with oropharyngeal and esophageal dysphagia identified in this review. The most frequently measured nutritional assessment in adults was BMI, which was recognized in nine articles. It was assessed with major diseases that cause dysphagia. The MNA short form (MNA-SF) was recognized as a nutritional screening tool in four articles. The most common anthropometric measurement was body weight, recognized in five articles, followed by triceps skinfold thickness (TSFT) and midarm muscle circumference (MAMC). Body composition was measured using the bioelectric impedance analysis (BIA) method; percent body fat was measured in one article. No articles measured body composition using dual-energy X-ray absorptiometry. For dietary assessment, two articles assessed the food intake level through the Food Frequency Questionnaire, evaluating energy, daily food intake, and dietary form. MNA and Onodera’s Prognostic Nutritional Index were included in the “other” category. Several articles used only one indicator to assess the nutritional status. Two articles used only BMI (da Silva et al. [35] and Ikenaga et al. [36]), two articles used only weight (Kim et al. [37] and Wang et al. [38]), one article assessed nutrition using only the nutrition screening tool (Vilardell et al. [39]), and one article (Masiero et al. [40]) reported data on daily food intake. The majority of the participants in the studies included in the present scope review were older adults (Table S1).
Table 1.
Nutritional assessment items used for adult patients with dysphagia.
| Broad Category | Subcategory | Number of Articles | Disease, (n) | First Author | Year |
|---|---|---|---|---|---|
| Body mass index | Body mass index | 9 [35,36,41,42,43,44,45,46,47] | Stroke (5), HNC (2), Amyotrophic lateral sclerosis (2), Pulmonary disease (2), Cardiovascular disease (2), Firearm injury (2), Cervical trauma (2), Diabetes mellitus (2), Dyslipidemia (2), Hypertension (2), Machado–Joseph disease (2), Meyge’s syndrome (2), Rubinstein–Taybi syndrome (2), Parkinson’s disease (2), Alzheimer’s disease (2), Dementia, Esophageal cancer, Brain tumor, Myelitis, Huntington’s disease, Progressive supranuclear palsy, Trigeminal neuropathy, Traumatic brain injury, Presbyphagia |
da Silva AF, Barni GC | 2020 |
| Maeda K, Ikenaga Y | 2017 | ||||
| Toh Yoon EW, Nakadate A | 2016 | ||||
| Ortega O | 2015 | ||||
| Lecleire S | 2006 | ||||
| Jacobsson C | 1997 | ||||
| Nutritional screening tool | MNA-SF | 4 [39,42,45,48] | Stroke | Nakazawa Y | 2020 |
| Vilardell N, Maeda K | 2017 | ||||
| Ortega O | 2015 | ||||
| Anthropometric measurements | Weight | 5 [37,38,46,49,50] | Esophageal cancer (2), Stroke, HNC, Lung cancer | Kim J | 2018 |
| Smith ZL | 2017 | ||||
| Wang YJ | 2014 | ||||
| Lecleire S | 2006 | ||||
| Elmståhl S | 1999 | ||||
| TSFT | 1 [47] | Stroke, brain tumor | Jacobsson C | 1997 | |
| MAMC | 1 [47] | Stroke, brain tumor | Jacobsson C | 1997 | |
| Body composition | SMM (BIA) | 1 [48] | Nakazawa Y | 2020 | |
| Percent body fat | 1 [50] | Stroke | Elmståhl S | 1999 | |
| Lean body mass | 1 [50] | Stroke | Elmståhl S | 1999 | |
| Dietary assessment | Food intake level | 2 [51,52] | Stroke, Brain trauma, Encephalitis, Central pontine myelinolysis, Neoplasm | Bülow M | 2008 |
| Bartolome G | 1997 | ||||
| Food Frequency Questionnaire | 1 [41] | Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, Amyotrophic lateral sclerosis, Machado–Joseph disease, Meyge’s syndrome, Progressive supranuclear palsy, Stroke, Trigeminal neuropathy, Myelitis, Rubinstein–Taybi syndrome, Firearm injury, Cervical trauma, Presbyphagia, Diabetes mellitus, Hypertension, Dyslipidemia, Cardiovascular disease |
Barni GC | 2020 | |
| Energy intake | 1 [42] | Maeda K | 2017 | ||
| Period to meal resumption and dietary form | 1 [53] | Kishimoto N | 2016 | ||
| Daily food intake | 1 [40] | Stroke | Masiero, S | 2008 | |
| Others | MNA | 1 [54] | Alzheimer’s Disease | Tang Y | 2017 |
| O-PNI | 1 [43] | Toh Yoon EW | 2016 |
Abbreviations: HNC, head and neck cancer; MNA-SF, Mini Nutritional Assessment-Short Form; MNA, Mini Nutritional Assessment; TSFT, Triceps skinfolds thickness; MAMC, mid-arm muscle circumference (MAMC = mid-upper arm circumference − π × TSFT); SMM, skeletal muscle mass; BIA, bioelectric impedance analysis; O-PNI, Onodera’s Prognostic Nutritional Index.
3.2. Blood Biomarkers from Nutritional Assessment in Adult Patients with Dysphagia
Blood biomarkers included in the nutritional assessment of adults are shown in Table 2. The most common item was albumin (nine articles). Serum visceral proteins, such as pre-albumin, transferrin, and retinol-binding protein, were also measured. Albumin was measured mostly in patients with stroke dysphagia. Two articles assessed nutrition based on blood biomarkers alone: Miyake et al. [55] assessed total protein and albumin levels, and Kimura et al. [56] assessed albumin and lymphocytes levels.
Table 2.
Nutritional assessment items related to blood biomarkers for adult patients with dysphagia.
| Broad Category | Subcategory | Number of Articles | Disease, (n) | First Author | Year |
|---|---|---|---|---|---|
| Blood biomarkers | Albumin | 9 [43,44,46,47,49,50,54,55,56] | Stroke (3), Esophageal cancer (2), Oropharyngeal cancer, Alzheimer’s disease, Gaucher disease, Niemann–Pick disease, High cervical spinal cord injury, Brain tumor |
Kimura Y | 2019 |
| Smith ZL, Tang, Y | 2017 | ||||
| Toh Yoon EW, Nakadate A |
2016 | ||||
| Miyake N | 2013 | ||||
| Lecleire S | 2006 | ||||
| Elmståhl S | 1999 | ||||
| Jacobsson C | 1997 | ||||
| Hemoglobin | 1 [54] | Alzheimer’s disease | Tang Y | 2017 | |
| Total protein | 1 [55] | Gaucher disease, Niemann–Pick disease, High cervical spinal cord injury, Oropharyngeal cancer | Miyake N | 2013 | |
| Transferrin | 1 [47] | Stroke, Brain tumor | Jacobsson C | 1997 | |
| Lymphocytes | 1 [56] | Kimura Y | 2019 | ||
| Pre-albumin | 1 [47] | Stroke, Brain tumor | Jacobsson C | 1997 | |
| C-reactive protein | 1 [50] | Stroke | Elmståhl S | 1999 | |
| Ceruloplasmin | 1 [50] | Stroke | Elmståhl S | 1999 | |
| Transthyretin | 1 [50] | Stroke | Elmståhl S | 1999 | |
| Retinol-binding protein | 1 [50] | Stroke | Elmståhl S | 1999 | |
| Total iron-binding capacity | 1 [50] | Stroke | Elmståhl S | 1999 | |
| Orosomucoid | 1 [50] | Stroke | Elmståhl S | 1999 |
3.3. Comparison of Nutritional Assessment Items for Patients with Dysphagia in Acute and Postacute Settings
Nutritional assessment items were categorized by setting (Table 3). We classified and reviewed the clinical situations as acute settings (e.g., acute care hospitals) and post-acute settings (nonacute settings such as rehabilitation hospitals and clinics) because a previous report [57] exists on the nutritional status of OD patients in different clinical situations (chronic vs. acute). The acute setting included six of the seven categories identified in this study, except for “other”. Moreover, the dietary assessment, in particular, was an item that allowed a detailed assessment of the daily nutritional intake and form. Blood biomarkers with short half-lives (e.g., transthyretin, pre-albumin, and transferrin) were often used. In contrast, the post-acute setting included categories other than anthropometric measurements among the seven categories. Consequently, the number of items used in each nutritional assessment category was fewer. BMI, MNA-SF, and albumin were used in both settings. The diseases in each setting showed a mixture of acute and chronic dysphagia.
Table 3.
Nutritional assessment items used for adult patients with dysphagia in acute and post-acute settings.
| Acute Setting, (n) | Post-Acute Setting, (n) | |
|---|---|---|
| Disease | Parkinson’s Disease, Alzheimer’s Disease, Huntington’s Disease, Amyotrophic Lateral Sclerosis, Stroke, Machado–Joseph Disease, Meige Syndrome, Rubinstein–Taybi Syndrome, Progressive supranuclear palsy, Trigeminal neuropathy, Traumatic brain injury, Firearm Injury, Myelitis, Cervical Trauma, Systemic arterial hypertension, Pneumonia, Diabetes mellitus, Dyslipidemia, Cardiovascular disease, Chronicobstructive pulmonary disease, Presbyphagia, Gaucher disease, Niemann-Pick disease, High cervical spinal cord injury, Oropharyngeal cancer, Head and Neck cancer, Esophageal cancer, Brain tumor, Lung cancer, Nasopharyngeal carcinoma | Stroke, Brain trauma, Encephalitis, Central pontine myelinolysis, Neoplasm, Alzheimer’s Disease |
| Body mass index | Body mass index (6) | Body mass index (3) |
| Nutritional screening tool | MNA-SF (2) | MNA-SF (2) |
| Anthropometric measurements | Weight (5), TSFT (1), MAMC (1) | none |
| Body composition | Percentage body fat (1), Lean body mass (1) | SMM (BIA) (1) |
| Dietary assessment | Food Frequency Questionnaire (1), Energy intake (1), Period to meal resumption and dietary form (1), Daily food intake (1) |
Food intake level (2) |
| Blood biomarkers | Albumin (6), Total protein (1), Transferrin (1), Lymphocytes (1), Pre-albumin (1), C-reactive protein (1), Ceruloplasmin (1), Transthyretin (1), Retinol-binding protein (1), TIBC (1), Orosomucoid (1) | Albumin (3), Hemoglobin (1) |
| Others | none | MNA (1), O-PNI (1) |
Abbreviations: MNA-SF, Mini Nutritional Assessment-Short Form; TSFT, Triceps skinfolds thickness; MAMC, mid-arm muscle circumference (MAMC = mid-upper arm circumference − π × TSFT); SMM, skeletal muscle mass; BIA, bioelectric impedance analysis; TIBC, total iron binding capacity; MNA, Mini Nutritional Assessment; O-PNI, Onodera’s Prognostic Nutritional Index.
4. Discussion
Three conclusions were achieved with this scoping review. First, the nutritional assessment items for patients with dysphagia were categorized into seven categories, and BMI was one of the most commonly used nutritional assessment item. Second, serum visceral proteins were commonly used as blood biomarkers items, with albumin being the most frequently used. Third, BMI, MNA-SF, and albumin were items that could be used regardless of the setting. Consequently, this study was able to identify several additional nutritional assessment items that were characteristic of the study setting.
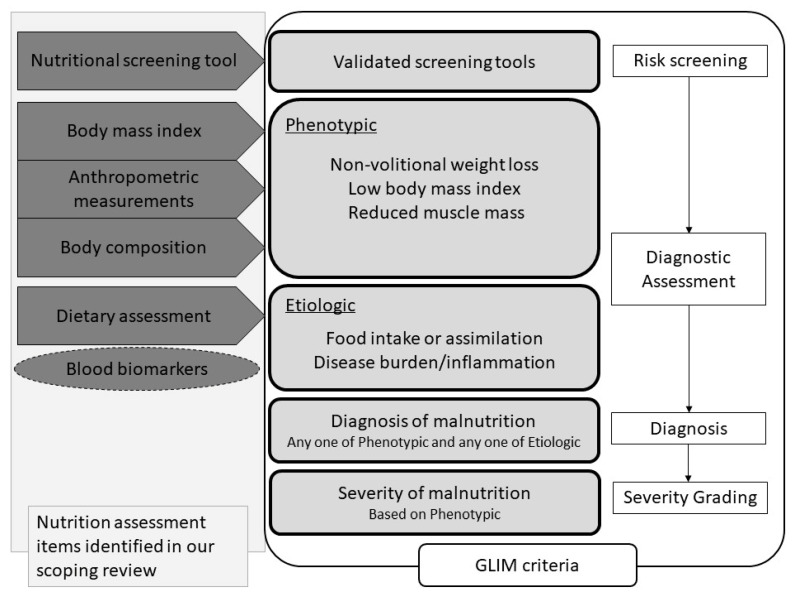
BMI was one of the most commonly used items in nutritional assessment. BMI is generally used as a common indicator of malnutrition [58]. Although many global regions use BMI as a criterion for determining malnutrition [59,60,61,62], overweightedness and obesity are more of a problem in North America, including in the United States, than a low BMI [23]. Therefore, BMI is not used necessarily as a marker of clinical malnutrition [23]. In addition, the percentages of lean fat mass and fat mass in the body are not determined by the BMI. Sarcopenia is found in obese and nonobese individuals and is an important health problem for the older population, leading to poor prognosis in terms of physical dysfunction, poor quality of life, and increased mortality [63]. Therefore, in older adults, not only BMI but also muscle mass and muscle function should be assessed [64]. Sarcopenia also causes dysphagia [16,65]; therefore, muscle mass, muscle strength, and physical function should also be assessed in addition to BMI. The GLIM criteria [23,24], which are new malnutrition diagnostic criteria, may be suitable for assessing nutrition in adults with dysphagia, because they can assess both muscle mass and BMI. The components of the GLIM criteria include the nutritional screening tool, BMI, anthropometric measurements, body composition, dietary assessment, and impact of disease, and these criteria contain five of the seven categories identified in this review (Figure 2). Various diagnostic criteria for malnutrition exist (e.g., Subjective Global Assessment [66], American Society for Parenteral and Enteral Nutrition/Academy of Nutrition and Dietetics 2012 [67], and European Society for Clinical Nutrition and Metabolism 2015 [59]). However, none of them include all items of the nutritional screening tool, BMI, anthropometric measurements, body composition, dietary assessment, and impact of the disease. MNA was recognized in this study to consider nutritional screening tools, BMI, anthropometric measurements, body composition, dietary assessment, and impact of the disease, but its indications are for older adults. The use of MNA may be limited for patients with dysphagia in a wide age group.
Figure 2.
Flowchart for nutritional diagnosis using the GLIM criteria and nutritional assessment components. The nutritional assessment items identified in this review are listed on the far left. Among the items identified in this study, the components of the GLIM criteria are nutritional screening tools, BMI, anthropometric measurements, body composition, and dietary assessment. Blood biomarkers are surrounded by a dotted line because the GLIM criteria recommend their use as adjunct indicators of disease burden/inflammation. Abbreviations: GLIM, Global Leadership Initiative on Malnutrition.
Serum visceral proteins were commonly used as blood biomarkers items. Of these, albumin was the most frequently used. Albumin is one of the biochemical indices that decrease during malnutrition. Still, in periods of acute illness, the hepatic production of proteins such as albumin, prealbumin, and transferrin is downregulated, resulting in lower levels in the serum [68,69]. Therefore, these proteins can have a low serum concentration independent of the actual nutritional status [11] and should be interpreted with caution in patients with infections, acute inflammation, and trauma [70]. Dysphagic patients are at high risk for developing pneumonia, which is often an acute inflammatory condition. Evans et al. [71] propose that visceral proteins should not be used as nutrition markers because they characterize inflammation rather than describe the nutrition status. In the GLIM criteria, the albumin level is also a useful reference for a patient’s inflammatory status, but it is not included as a component of the diagnosis (Figure 2). For these reasons, nutritional assessments using only albumin, prealbumin, or transthyretin are not appropriate for dysphagia patients who are prone to acute inflammation such as pneumonia. We suggest that blood biomarkers should not be used as nutritional assessments by themselves, but they should rather be employed as an adjunct or additional indicator to the nutritional assessment.
In the list of nutritional assessment items by setting (Table 3), BMI, MNA-SF, and albumin were used in acute and post-acute settings. Therefore, on the one hand, BMI and MNA-SF can be used as nutritional assessment items for patients with dysphagia. On the other hand, albumin can be used as an adjunct indicator for nutritional assessment, regardless of the setting. Moreover, several unique nutritional assessment items have been identified depending on the study setting. In the acute setting, items that can be used to assess nutritional intake and form in detail were used as dietary assessment items, in contrast to the post-acute setting, suggesting that daily food intake, period to meal resumption, and dietary form may be used as short-term nutritional indicators. In addition, the impact of the inflammatory response may need to be more strongly considered in the acute setting. In a previous study [57] that examined the differences in the nutritional status of OD patients in acute and chronic situations, OD patients in chronic situations presented with malnutrition, sarcopenia, reduced visceral and muscular protein compartments and fat compartments, muscle weakness, intracellular water depletion, and weight loss. Patients in acute situations also presented with malnutrition and sarcopenia, but also showed more severe reductions in serum visceral protein and muscle mass due to the inflammatory response to pneumonia. The current scoping review also assessed visceral proteins with a short half-life and C-reactive protein (CRP) in the acute setting. This suggests that the evaluation of visceral protein and CRP is essential in addition to the evaluation of malnutrition in patients with OD in the acute setting. Furthermore, assessing sarcopenia and dehydration in addition to malnutrition may be necessary in the chronic setting, as reported by Carrión et al. [57], although sarcopenia and dehydration were not assessed in the post-acute setting in this scoping review.
Although none of the articles in this review used the GLIM criteria to diagnose malnutrition, we recommend using the GLIM criteria initially for adult patients with dysphagia. The reasons are that the GLIM criteria can assess both muscle mass and BMI and can determine the effects of a disease [23,24]. It is essential to consider the impact of acute or chronic diseases in the nutritional assessment of dysphagia patients, such as post-acute stroke and neuromuscular diseases. One of the advantages of the GLIM criteria is that they consider the impact of disease, such as whether an inflammatory condition is acute disease-, injury-, or chronic disease-related. An association between GLIM-defined malnutrition and post-stroke dysphagia has already been reported [72]; however, the association between GLIM-defined malnutrition and dysphagia caused by other diseases has not been analyzed. As mentioned above, malnutrition in patients with dysphagia is influenced not only by the disease itself but also by background diseases. Therefore, the prevalence of malnutrition may vary for each disease that causes dysphagia. However, due to differences in the nutritional assessment methods used, the current actual prevalence of malnutrition is difficult to determine [9,11]. This makes it difficult to develop and compare effective intervention methods. The GLIM criteria were developed to disseminate the use of standardized assessment items for comparing the prevalence of malnutrition and intervention methods globally. However, the GLIM criteria can be used for risk screening and malnutrition diagnosis, but not for a detailed comprehensive nutritional assessment [23,24]. Therefore, the nutritional assessment of adults should be carried out using the GLIM criteria at a minimum, and additional comprehensive nutritional assessments should be conducted in the presence of malnutrition. Based on the results of this review, patients with OD need to be assessed for visceral protein and CRP in addition to the assessment of malnutrition in the acute setting. However, sarcopenia and dehydration may need to be assessed in addition to malnutrition in the chronic setting. This will enable us to identify the real prevalence of malnutrition in adult patients with dysphagia and to develop and compare effective interventions.
There are several limitations to this review. First, because it includes only articles with 100% of the participants being dysphagia patients, there is no recognition of the nutritional assessments used in studies comparing these patients with individuals without dysphagia. The results of the review may change when this is accounted for. Second, only three databases were used for the literature search. Although we implemented a rigorous search and review process, some relevant manuscripts may not have been considered because of the database selection, search strategy, and article selection method. Third, this study was not able to strictly distinguish between acute and chronic situations in patients with dysphagia, as Carrión et al. did [57]. However, the study setting (acute and post-acute) was used as a reference to classify the patients. Fourth, this study focused on patients with oropharyngeal dysphagia and esophageal dysphagia. However, the current study was not able to sufficiently examine the nutritional assessment items in relation to these two different types of dysphagia because only two papers on esophageal dysphagia were found. (References [73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126] are cited in the “Supplementary Materials”).
5. Conclusions
We identified nutritional assessment items for adult patients with oropharyngeal and esophageal dysphagia in this scope review. We found that various nutritional assessment items were used, making it difficult to confirm the real nutritional status of patients with dysphagia. Therefore, we recommend that the GLIM criteria be used as minimum nutritional assessment items for adults, also including a detailed comprehensive nutritional assessment in the presence of malnutrition. The use of this additional assessment may be beneficial for developing effective nutritional interventions. Future studies should provide information on how the nutritional status of patients with dysphagia is influenced by the implementation of a qualified nutritional assessment worldwide.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/13/3/778/s1, Table S1: Nutritional assessment used for adult patients with dysphagia, Table S2: Sources excluded following full-text review.
Author Contributions
Conceptualization, J.U., R.M., K.M., and M.S. (Mika Sonoi) and A.S.; investigation, J.U., R.M., K.M., M.S. (Mika Sonoi), A.S., and Y.S.; methodology, J.U., R.M., K.M., M.S. (Mika Sonoi), A.S., and Y.S.; literature search and selection, J.U., R.M., K.M., M.S. (Mika Sonoi), A.S., Y.S., C.U., Y.K., M.S. (Midori Shimizu), A.N., D.M., K.Y., and K.S.; consultation on literature inclusion, J.U., R.M., K.M., M.S. (Mika Sonoi), A.S., Y.S., C.U., Y.K., M.S. (Midori Shimizu), A.N., D.M., K.Y., and K.S.; writing—original draft preparation, J.U.; writing—review and editing, R.M., K.M., M.S. (Mika Sonoi), A.S., Y.S., C.U., Y.K., M.S. (Midori Shimizu), A.N., D.M., K.Y., and K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cichero J.A., Lam P., Steele C.M., Hanson B., Chen J., Dantas R.O., Duivestein J., Kayashita J., Lecko C., Murray J., et al. Development of International Terminology and Definitions for Texture-Modified Foods and Thickened Fluids Used in Dysphagia Management: The IDDSI Framework. Dysphagia. 2017;32:293–314. doi: 10.1007/s00455-016-9758-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pontes É.S., Amaral A.K., Rêgo F.L., Azevedo E.H., Silva P.O. Quality of life in swallowing of the elderly patients affected by stroke. Arq. Gastroenterol. 2017;54:27–32. doi: 10.1590/s0004-2803.2017v54n1-05. [DOI] [PubMed] [Google Scholar]
- 3.Kim J.Y., Lee Y.W., Kim H.S., Lee E.H. The mediating and moderating effects of meaning in life on the relationship between depression and quality of life in patients with dysphagia. J. Clin. Nurs. 2019;28:2782–2789. doi: 10.1111/jocn.14907. [DOI] [PubMed] [Google Scholar]
- 4.Thomas M.N., Kufeldt J., Kisser U., Hornung H.M., Hoffmann J., Andraschko M., Werner J., Rittler P. Effects of malnutrition on complication rates, length of hospital stay, and revenue in elective surgical patients in the G-DRG-system. Nutrition. 2016;32:249–254. doi: 10.1016/j.nut.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Allard J.P., Keller H., Jeejeebhoy K.N., Laporte M., Duerksen D.R., Gramlich L., Payette H., Bernier P., Vesnaver E., Davidson B., et al. Malnutrition at Hospital Admission-Contributors and Effect on Length of Stay: A Prospective Cohort Study From the Canadian Malnutrition Task Force. JPEN J. Parenter. Enter. Nutr. 2016;40:487–497. doi: 10.1177/0148607114567902. [DOI] [PubMed] [Google Scholar]
- 6.Gallegos C., Brito-de la Fuente E., Clave P., Costa A., Assegehegn G. Nutritional Aspects of Dysphagia Management. Adv. Food Nutr. Res. 2017;81:271–318. doi: 10.1016/bs.afnr.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Popman A., Richter M., Allen J., Wham C. High nutrition risk is associated with higher risk of dysphagia in advanced age adults newly admitted to hospital. Nutr. Diet. 2018;75:52–58. doi: 10.1111/1747-0080.12385. [DOI] [PubMed] [Google Scholar]
- 8.Blanar V., Hodl M., Lohrmann C., Amir Y., Eglseer D. Dysphagia and factors associated with malnutrition risk: A 5-year multicentre study. J. Adv. Nurs. 2019;75:3566–3576. doi: 10.1111/jan.14188. [DOI] [PubMed] [Google Scholar]
- 9.Namasivayam A.M., Steele C.M. Malnutrition and Dysphagia in long-term care: A systematic review. J. Nutr. Gerontol. Geriatr. 2015;34:1–21. doi: 10.1080/21551197.2014.1002656. [DOI] [PubMed] [Google Scholar]
- 10.Namasivayam-MacDonald A.M., Morrison J.M., Steele C.M., Keller H. How Swallow Pressures and Dysphagia Affect Malnutrition and Mealtime Outcomes in Long-Term Care. Dysphagia. 2017;32:785–796. doi: 10.1007/s00455-017-9825-z. [DOI] [PubMed] [Google Scholar]
- 11.Foley N.C., Martin R.E., Salter K.L., Teasell R.W. A review of the relationship between dysphagia and malnutrition following stroke. J. Rehabil. Med. 2009;41:707–713. doi: 10.2340/16501977-0415. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu A., Momosaki R., Kayashita J., Fujishima I. Impact of Multiple Texture-Modified Diets on Oral Intake and Nutritional Status in Older Patients with Pneumonia: A Retrospective Cohort Study. Dysphagia. 2020;35:574–582. doi: 10.1007/s00455-019-10063-4. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu A., Maeda K., Tanaka K., Ogawa M., Kayashita J. Texture-modified diets are associated with decreased muscle mass in older adults admitted to a rehabilitation ward. Geriatr. Gerontol. Int. 2018;18:698–704. doi: 10.1111/ggi.13233. [DOI] [PubMed] [Google Scholar]
- 14.Wright L., Cotter D., Hickson M., Frost G. Comparison of energy and protein intakes of older people consuming a texture modified diet with a normal hospital diet. J. Hum. Nutr. Diet. 2005;18:213–219. doi: 10.1111/j.1365-277X.2005.00605.x. [DOI] [PubMed] [Google Scholar]
- 15.Hudson H.M., Daubert C.R., Mills R.H. The interdependency of protein-energy malnutrition, aging, and dysphagia. Dysphagia. 2000;15:31–38. doi: 10.1007/s004559910007. [DOI] [PubMed] [Google Scholar]
- 16.Fujishima I., Fujiu-Kurachi M., Arai H., Hyodo M., Kagaya H., Maeda K., Mori T., Nishioka S., Oshima F., Ogawa S., et al. Sarcopenia and dysphagia: Position paper by four professional organizations. Geriatr. Gerontol. Int. 2019;19:91–97. doi: 10.1111/ggi.13591. [DOI] [PubMed] [Google Scholar]
- 17.Wakabayashi H., Matsushima M. Dysphagia Assessed by the 10-Item Eating Assessment Tool Is Associated with Nutritional Status and Activities of Daily Living in Elderly Individuals Requiring Long-Term Care. J. Nutr. Health Aging. 2016;20:22–27. doi: 10.1007/s12603-016-0671-8. [DOI] [PubMed] [Google Scholar]
- 18.Andrade P.A., Santos C.A.D., Firmino H.H., Rosa C.O.B. The importance of dysphagia screening and nutritional assessment in hospitalized patients. Einstein. 2018;16:eAO4189. doi: 10.1590/s1679-45082018ao4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charney P. Nutrition screening vs nutrition assessment: How do they differ? Nutr. Clin. Pract. 2008;23:366–372. doi: 10.1177/0884533608321131. [DOI] [PubMed] [Google Scholar]
- 20.Correia M. Nutrition Screening vs Nutrition Assessment: What's the Difference? Nutr. Clin. Pract. 2018;33:62–72. doi: 10.1002/ncp.10010. [DOI] [PubMed] [Google Scholar]
- 21.Reber E., Gomes F., Vasiloglou M.F., Schuetz P., Stanga Z. Nutritional Risk Screening and Assessment. J. Clin. Med. 2019;8:65. doi: 10.3390/jcm8071065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dent E., Hoogendijk E.O., Visvanathan R., Wright O.R.L. Malnutrition Screening and Assessment in Hospitalised Older People: A Review. J. Nutr. Health Aging. 2019;23:431–441. doi: 10.1007/s12603-019-1176-z. [DOI] [PubMed] [Google Scholar]
- 23.Cederholm T., Jensen G.L., Correia M., Gonzalez M.C., Fukushima R., Higashiguchi T., Baptista G., Barazzoni R., Blaauw R., Coats A., et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019;38:1–9. doi: 10.1016/j.clnu.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Jensen G.L., Cederholm T., Correia M., Gonzalez M.C., Fukushima R., Higashiguchi T., de Baptista G.A., Barazzoni R., Blaauw R., Coats A.J.S., et al. GLIM Criteria for the Diagnosis of Malnutrition: A Consensus Report From the Global Clinical Nutrition Community. JPEN J. Parenter. Enter. Nutr. 2019;43:32–40. doi: 10.1002/jpen.1440. [DOI] [PubMed] [Google Scholar]
- 25.Ueshima J., Momosaki R., Motokawa K., Sonoi M., Shimizu A., Shirai Y., Uno C., Kokura Y., Shimizu M., Nishiyama A., et al. Exploring effective nutritional assessment for patients with dysphagia: A scoping review protocol. [(accessed on 6 October 2020)]; doi: 10.6084/m9.figshare.12627416.v1. Available online: [DOI]
- 26.Arksey H., O’Malley L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005;8:19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 27.Munn Z., Peters M.D.J., Stern C., Tufanaru C., McArthur A., Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018;18:143. doi: 10.1186/s12874-018-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tricco A.C., Lillie E., Zarin W., O’Brien K., Colquhoun H., Kastner M., Levac D., Ng C., Sharpe J.P., Wilson K., et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med. Res. Methodol. 2016;16:15. doi: 10.1186/s12874-016-0116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson S., Allen P., Peckham S., Goodwin N. Asking the right questions: Scoping studies in the commissioning of research on the organisation and delivery of health services. Health Res. Policy Syst. 2008;6:7. doi: 10.1186/1478-4505-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters M.D.J., Godfrey C., McInerney P., Munn Z., Tricco A.C., Khalil H. Chapter 11: Scoping Reviews (2020 Version) [(accessed on 12 June 2020)]; Available online: https://synthesismanual.jbi.global.
- 31.Tricco A.C., Lillie E., Zarin W., O’Brien K.K., Colquhoun H., Levac D., Moher D., Peters M.D.J., Horsley T., Weeks L., et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 32.Sampaio A.D.S., Epifanio M., Costa C.A.D., Bosa V.L., Benedetti F.J., Sarria E.E., Oliveira S.G., Mundstock E., Mattiello R. Evidence on nutritional assessment techniques and parameters used to determine the nutritional status of children and adolescents: Systematic review. Cien Saude Colet. 2018;23:4209–4219. doi: 10.1590/1413-812320182312.31502016. [DOI] [PubMed] [Google Scholar]
- 33.Cederholm T., Jensen G.L., Correia M., Gonzalez M.C., Fukushima R., Higashiguchi T., Baptista G., Barazzoni R., Blaauw R., Coats A.J.S., et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle. 2019;10:207–217. doi: 10.1002/jcsm.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bath P.M., Lee H.S., Everton L.F. Swallowing therapy for dysphagia in acute and subacute stroke. Cochrane Database Syst. Rev. 2018;10:CD000323. doi: 10.1002/14651858.CD000323.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.da Silva A.F., Moreira E.A.M., Barni G.C., Panza V.S.P., Furkim A.M., Moreno Y.M.F. Relationships between high comorbidity index and nutritional parameters in patients with Oropharyngeal Dysphagia. Clin. Nutr. ESPEN. 2020;38:218–222. doi: 10.1016/j.clnesp.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Ikenaga Y., Nakayama S., Taniguchi H., Ohori I., Komatsu N., Nishimura H., Katsuki Y. Factors Predicting Recovery of Oral Intake in Stroke Survivors with Dysphagia in a Convalescent Rehabilitation Ward. J. Stroke Cerebrovasc. Dis. 2017;26:1013–1019. doi: 10.1016/j.jstrokecerebrovasdis.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Kim J., Min Y.W., Lee H., Min B.H., Lee J.H., Rhee P.L., Kim J.J. Comparative Study of Esophageal Self-expandable Metallic Stent Insertion and Gastrostomy Feeding for Dysphagia Caused by Lung Cancer. Korean J. Gastroenterol. 2018;71:124–131. doi: 10.4166/kjg.2018.71.3.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y.J., Chen W.X., Zhang J.L., He F.Y., Zhu Z.F., Zeng Y., Yang F., Tang S.C. Cervical oesophagostomy in patients with severe dysphagia following radiotherapy for nasopharyngeal carcinoma. J. Laryngol. Otol. 2014;128:142–146. doi: 10.1017/S0022215113003423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vilardell N., Rofes L., Arreola V., Martin A., Muriana D., Palomeras E., Ortega O., Clavé P. Videofluoroscopic assessment of the pathophysiology of chronic poststroke oropharyngeal dysphagia. Neurogastroenterol. Motil. 2017;29:1–8. doi: 10.1111/nmo.13111. [DOI] [PubMed] [Google Scholar]
- 40.Masiero S., Pierobon R., Previato C., Gomiero E. Pneumonia in stroke patients with oropharyngeal dysphagia: A six-month follow-up study. Neurol. Sci. 2008;29:139–145. doi: 10.1007/s10072-008-0925-2. [DOI] [PubMed] [Google Scholar]
- 41.Barni G.C., Moreira E.A.M., da Silva A.F., Panza V.S.P., de Lima Oliveira D., Moreno Y.M.F., Furkim A.M. The relationship of serum 25-hydroxyvitamin D concentration with clinical variables in patients with oropharyngeal dysphagia. Clin. Nutr. ESPEN. 2020;38:229–235. doi: 10.1016/j.clnesp.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Maeda K., Koga T., Akagi J. Interferential current sensory stimulation, through the neck skin, improves airway defense and oral nutrition intake in patients with dysphagia: A double-blind randomized controlled trial. Clin. Interv. Aging. 2017;12:1879–1886. doi: 10.2147/CIA.S140746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toh Yoon E.W., Hirao J., Minoda N. Outcome of Rehabilitation and Swallowing Therapy after Percutaneous Endoscopic Gastrostomy in Dysphagia Patients. Dysphagia. 2016;31:730–736. doi: 10.1007/s00455-016-9717-7. [DOI] [PubMed] [Google Scholar]
- 44.Nakadate A., Otaka Y., Kondo K., Yamamoto R., Matsuura D., Honaga K., Muraoka K., Akaboshi K., Liu M. Age, Body Mass Index, and White Blood Cell Count Predict the Resumption of Oral Intake in Subacute Stroke Patients. J. Stroke Cerebrovasc. Dis. 2016;25:2801–2808. doi: 10.1016/j.jstrokecerebrovasdis.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 45.Ortega O., Sakwinska O., Combremont S., Berger B., Sauser J., Parra C., Zarcero S., Nart J., Carrión S., Clavé P. High prevalence of colonization of oral cavity by respiratory pathogens in frail older patients with oropharyngeal dysphagia. Neurogastroenterol. Motil. 2015;27:1804–1816. doi: 10.1111/nmo.12690. [DOI] [PubMed] [Google Scholar]
- 46.Lecleire S., Di Fiore F., Antonietti M., Ben Soussan E., Hellot M.F., Grigioni S., Déchelotte P., Lerebours E., Michel P., Ducrotté P. Undernutrition is predictive of early mortality after palliative self-expanding metal stent insertion in patients with inoperable or recurrent esophageal cancer. Gastrointest. Endosc. 2006;64:479–484. doi: 10.1016/j.gie.2006.03.930. [DOI] [PubMed] [Google Scholar]
- 47.Jacobsson C., Axelsson K., Norberg A., Asplund K., Wenngren B.I. Outcomes of individualized interventions in patients with severe eating difficulties. Clin. Nurs. Res. 1997;6:25–44. doi: 10.1177/105477389700600104. [DOI] [PubMed] [Google Scholar]
- 48.Nakazawa Y., Kikutani T., Igarashi K., Yajima Y., Tamura F. Associations between tongue strength and skeletal muscle mass under dysphagia rehabilitation for geriatric out patients. J. Prosthodont. Res. 2020;64:188–192. doi: 10.1016/j.jpor.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Smith Z.L., Gonzaga J.E., Haasler G.B., Gore E.M., Dua K.S. Self-Expanding Metal Stents Improve Swallowing and Maintain Nutrition During Neoadjuvant Therapy for Esophageal Cancer. Dig. Dis. Sci. 2017;62:1647–1656. doi: 10.1007/s10620-017-4562-6. [DOI] [PubMed] [Google Scholar]
- 50.Elmståhl S., Bülow M., Ekberg O., Petersson M., Tegner H. Treatment of dysphagia improves nutritional conditions in stroke patients. Dysphagia. 1999;14:61–66. doi: 10.1007/PL00009588. [DOI] [PubMed] [Google Scholar]
- 51.Bülow M., Speyer R., Baijens L., Woisard V., Ekberg O. Neuromuscular electrical stimulation (NMES) in stroke patients with oral and pharyngeal dysfunction. Dysphagia. 2008;23:302–309. doi: 10.1007/s00455-007-9145-9. [DOI] [PubMed] [Google Scholar]
- 52.Bartolome G., Prosiegel M., Yassouridis A. Long-term functional outcome in patients with neurogenic dysphagia. NeuroRehabilitation. 1997;9:195–204. doi: 10.3233/NRE-1997-9304. [DOI] [PubMed] [Google Scholar]
- 53.Kishimoto N., Stegaroiu R., Shibata S., Ito K., Inoue M., Ohuchi A. Changes in the Oral Moisture and the Amount of Microorganisms in Saliva and Tongue Coating after Oral Ingestion Resumption: A Pilot Study. Open Dent. J. 2016;10:79–88. doi: 10.2174/1874210601610010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang Y., Lin X., Lin X.J., Zheng W., Zheng Z.K., Lin Z.M., Chen J.H. Therapeutic efficacy of neuromuscular electrical stimulation and electromyographic biofeedback on Alzheimer's disease patients with dysphagia. Medicine. 2017;96:e8008. doi: 10.1097/MD.0000000000008008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyake N., Kawamoto K., Fujiwara K., Hasegawa Y., Kitano H. Subglottic laryngeal closure: A unique modified method of laryngotracheal separation to prevent aspiration. Ann. Otol. Rhinol. Laryngol. 2013;122:427–434. doi: 10.1177/000348941312200703. [DOI] [PubMed] [Google Scholar]
- 56.Kimura Y., Kishimoto S., Sumi T., Uchiyama M., Ohno K., Kobayashi H., Kano M. Improving the Quality of Life of Patients With Severe Dysphagia by Surgically Closing the Larynx. Ann. Otol. Rhinol. Laryngol. 2019;128:96–103. doi: 10.1177/0003489418808300. [DOI] [PubMed] [Google Scholar]
- 57.Carrión S., Roca M., Costa A., Arreola V., Ortega O., Palomera E., Serra-Prat M., Cabré M., Clavé P. Nutritional status of older patients with oropharyngeal dysphagia in a chronic versus an acute clinical situation. Clin. Nutr. 2017;36:1110–1116. doi: 10.1016/j.clnu.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 58.Bahat G., Tufan F., Saka B., Akin S., Ozkaya H., Yucel N., Erten N., Karan M.A. Which body mass index (BMI) is better in the elderly for functional status? Arch. Gerontol. Geriatr. 2012;54:78–81. doi: 10.1016/j.archger.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 59.Cederholm T., Bosaeus I., Barazzoni R., Bauer J., Van Gossum A., Klek S., Muscaritoli M., Nyulasi I., Ockenga J., Schneider S.M., et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015;34:335–340. doi: 10.1016/j.clnu.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Kondrup J., Allison S.P., Elia M., Vellas B., Plauth M. ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003;22:415–421. doi: 10.1016/S0261-5614(03)00098-0. [DOI] [PubMed] [Google Scholar]
- 61.Rubenstein L.Z., Harker J.O., Salvà A., Guigoz Y., Vellas B. Screening for undernutrition in geriatric practice: Developing the short-form mini-nutritional assessment (MNA-SF) J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001;56:M366–M372. doi: 10.1093/gerona/56.6.M366. [DOI] [PubMed] [Google Scholar]
- 62.Stratton R.J., Hackston A., Longmore D., Dixon R., Price S., Stroud M., King C., Elia M. Malnutrition in hospital outpatients and inpatients: Prevalence, concurrent validity and ease of use of the 'malnutrition universal screening tool' ('MUST') for adults. Br. J. Nutr. 2004;92:799–808. doi: 10.1079/BJN20041258. [DOI] [PubMed] [Google Scholar]
- 63.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., Cooper C., Landi F., Rolland Y., Sayer A.A., et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Riobó Serván P., Sierra Poyatos R., Soldo Rodríguez J., Gómez-Candela C., García Luna P.P., Serra-Majem L. Special considerations for nutritional studies in elderly. Nutr. Hosp. 2015;31(Suppl. S3):84–90. doi: 10.3305/nh.2015.31.sup3.8756. [DOI] [PubMed] [Google Scholar]
- 65.Zhao W.T., Yang M., Wu H.M., Yang L., Zhang X.M., Huang Y. Systematic Review and Meta-Analysis of the Association between Sarcopenia and Dysphagia. J. Nutr. Health Aging. 2018;22:1003–1009. doi: 10.1007/s12603-018-1055-z. [DOI] [PubMed] [Google Scholar]
- 66.Detsky A.S., McLaughlin J.R., Baker J.P., Johnston N., Whittaker S., Mendelson R.A., Jeejeebhoy K.N. What is subjective global assessment of nutritional status? JPEN J. Parenter. Enter. Nutr. 1987;11:8–13. doi: 10.1177/014860718701100108. [DOI] [PubMed] [Google Scholar]
- 67.White J.V., Guenter P., Jensen G., Malone A., Schofield M. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: Characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) JPEN J. Parenter. Enter. Nutr. 2012;36:275–283. doi: 10.1177/0148607112440285. [DOI] [PubMed] [Google Scholar]
- 68.Gabay C., Kushner I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 69.Fleck A. Clinical and nutritional aspects of changes in acute-phase proteins during inflammation. Proc. Nutr. Soc. 1989;48:347–354. doi: 10.1079/PNS19890050. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Z., Pereira S.L., Luo M., Matheson E.M. Evaluation of Blood Biomarkers Associated with Risk of Malnutrition in Older Adults: A Systematic Review and Meta-Analysis. Nutrients. 2017;9:829. doi: 10.3390/nu9080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Evans D.C., Corkins M.R., Malone A., Miller S., Mogensen K.M., Guenter P., Jensen G.L. The Use of Visceral Proteins as Nutrition Markers: An ASPEN Position Paper. Nutr. Clin. Pract. 2020 doi: 10.1002/ncp.10588. [DOI] [PubMed] [Google Scholar]
- 72.Shimizu A., Maeda K., Koyanagi Y., Kayashita J., Fujishima I., Mori N. The Global Leadership Initiative on Malnutrition-Defined Malnutrition Predicts Prognosis in Persons With Stroke-Related Dysphagia. J. Am. Med. Dir. Assoc. 2019;20:1628–1633. doi: 10.1016/j.jamda.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 73.Jaafar M.H., Mahadeva S., Tan K.M., Chin A.V., Kamaruzzaman S.B., Khor H.M., Saedon N.I., Tan M.P. Long-Term Nasogastric Versus Percutaneous Endoscopic Gastrostomy Tube Feeding in Older Asians With Dysphagia: A Pragmatic Study. Nutr. Clin. Pract. 2019;34:280–289. doi: 10.1002/ncp.10195. [DOI] [PubMed] [Google Scholar]
- 74.Furuta M., Yokota T., Tsushima T., Todaka A., Machida N., Hamauchi S., Yamazaki K., Fukutomi A., Kawai S., Kawabata T., et al. Comparison of enteral nutrition with total parenteral nutrition for patients with locally advanced unresectable esophageal cancer harboring dysphagia in definitive chemoradiotherapy. Jpn. J. Clin. Oncol. 2019;49:910–918. doi: 10.1093/jjco/hyz089. [DOI] [PubMed] [Google Scholar]
- 75.Wakabayashi H., Takahashi R., Murakami T. The Prevalence and Prognosis of Sarcopenic Dysphagia in Patients Who Require Dysphagia Rehabilitation. J. Nutr. Health Aging. 2019;23:84–88. doi: 10.1007/s12603-018-1117-2. [DOI] [PubMed] [Google Scholar]
- 76.Chen H.J., Chen J.L., Chen C.Y., Lee M., Chang W.H., Huang T.T. Effect of an Oral Health Programme on Oral Health, Oral Intake, and Nutrition in Patients with Stroke and Dysphagia in Taiwan: A Randomised Controlled Trial. Int. J Environ. Res. Public Health. 2019;16:2228. doi: 10.3390/ijerph16122228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reyes-Torres C.A., Castillo-Martínez L., Reyes-Guerrero R., Ramos-Vázquez A.G., Zavala-Solares M., Cassis-Nosthas L., Serralde-Zúñiga A.E. Design and implementation of modified-texture diet in older adults with oropharyngeal dysphagia: A randomized controlled trial. Eur. J. Clin. Nutr. 2019;73:989–996. doi: 10.1038/s41430-019-0389-x. [DOI] [PubMed] [Google Scholar]
- 78.Masaki S., Kawamoto T. Comparison of long-term outcomes between enteral nutrition via gastrostomy and total parenteral nutrition in older persons with dysphagia: A propensity-matched cohort study. PLoS ONE. 2019;14:e0217120. doi: 10.1371/journal.pone.0217120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wakabayashi H., Matsushima M., Momosaki R., Yoshida S., Mutai R., Yodoshi T., Murayama S., Hayashi T., Horiguchi R., Ichikawa H. The effects of resistance training of swallowing muscles on dysphagia in older people: A cluster, randomized, controlled trial. Nutrition. 2018;48:111–116. doi: 10.1016/j.nut.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 80.Martín A., Ortega O., Roca M., Arús M., Clavé P. Respond to Letter to the editor: Effect of a Minimal-Massive Intervention in Hospitalized Older Patients with Oropharyngeal Dysphagia: A Proof of Concept Study. J. Nutr. Health Aging. 2018;22:1019–1020. doi: 10.1007/s12603-018-1091-8. [DOI] [PubMed] [Google Scholar]
- 81.Maeda K., Wakabayashi H., Shamoto H., Akagi J. Cognitive impairment has no impact on hospital-associated dysphagia in aspiration pneumonia patients. Geriatr. Gerontol. Int. 2018;18:233–239. doi: 10.1111/ggi.13164. [DOI] [PubMed] [Google Scholar]
- 82.Nishioka S., Okamoto T., Takayama M., Urushihara M., Watanabe M., Kiriya Y., Shintani K., Nakagomi H., Kageyama N. Malnutrition risk predicts recovery of full oral intake among older adult stroke patients undergoing enteral nutrition: Secondary analysis of a multicentre survey (the APPLE study) Clin. Nutr. 2017;36:1089–1096. doi: 10.1016/j.clnu.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 83.Santos C.A., Fonseca J., Carolino E., Guerreiro A.S. Low Serum Chromium Is Rare In Patients That Underwent Endoscopic Gastrostomy For Long Term Enteral Feeding. Arq. Gastroenterol. 2017;54:211–216. doi: 10.1590/s0004-2803.201700000-25. [DOI] [PubMed] [Google Scholar]
- 84.Zanini M., Bagnasco A., Catania G., Aleo G., Sartini M., Cristina M.L., Ripamonti S., Monacelli F., Odetti P., Sasso L. A Dedicated Nutritional Care Program (NUTRICARE) to reduce malnutrition in institutionalised dysphagic older people: A quasi-experimental study. J. Clin. Nurs. 2017;26:4446–4455. doi: 10.1111/jocn.13774. [DOI] [PubMed] [Google Scholar]
- 85.Kuroda Y. Factors associated with the level of oral intake in hospitalized older adults with dysphagia: The importance of mental activity. Clin. Nutr. ESPEN. 2016;13:e52–e54. doi: 10.1016/j.clnesp.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 86.van den Berg M.G., Kalf J.G., Hendriks J.C., Takes R.P., van Herpen C.M., Wanten G.J., Drenth J.P., Kaanders J.H., Merkx M.A. Normalcy of food intake in patients with head and neck cancer supported by combined dietary counseling and swallowing therapy: A randomized clinical trial. Head Neck. 2016;38(Suppl. 1):E198–E206. doi: 10.1002/hed.23970. [DOI] [PubMed] [Google Scholar]
- 87.Fonseca J., Santos C.A., Brito J. Malnutrition and Clinical Outcome of 234 Head and Neck Cancer Patients who Underwent Percutaneous Endoscopic Gastrostomy. Nutr. Cancer. 2016;68:589–597. doi: 10.1080/01635581.2016.1158297. [DOI] [PubMed] [Google Scholar]
- 88.Chen L.L., Li H., Lin R., Zheng J.H., Wei Y.P., Li J., Chen P., Chen H.Y. Effects of a feeding intervention in patients with Alzheimer's disease and dysphagia. J. Clin. Nurs. 2016;25:699–707. doi: 10.1111/jocn.13013. [DOI] [PubMed] [Google Scholar]
- 89.Crary M.A., Carnaby G.D., Shabbir Y., Miller L., Silliman S. Clinical Variables Associated with Hydration Status in Acute Ischemic Stroke Patients with Dysphagia. Dysphagia. 2016;31:60–65. doi: 10.1007/s00455-015-9658-6. [DOI] [PubMed] [Google Scholar]
- 90.Santos C.A., Fonseca J., Carolino E., Guerreiro A.S. Serum trace elements in dysphagic gastrostomy candidates before endoscopic gastrostomy for long term enteral feeding. Clin. Nutr. 2016;35:718–723. doi: 10.1016/j.clnu.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 91.Iwamoto M., Higashibeppu N., Arioka Y., Nakaya Y. Swallowing rehabilitation with nutrition therapy improves clinical outcome in patients with dysphagia at an acute care hospital. J. Med. Investig. 2014;61:353–360. doi: 10.2152/jmi.61.353. [DOI] [PubMed] [Google Scholar]
- 92.Rio A., Ellis C., Shaw C., Willey E., Ampong M.A., Wijesekera L., Rittman T., Nigel Leigh P., Sidhu P.S., Al-Chalabi A. Nutritional factors associated with survival following enteral tube feeding in patients with motor neurone disease. J. Hum. Nutr. Diet. 2010;23:408–415. doi: 10.1111/j.1365-277X.2010.01057.x. [DOI] [PubMed] [Google Scholar]
- 93.Obara H., Tomite Y., Doi M. Serum trace elements in tube-fed neurological dysphagia patients correlate with nutritional indices but do not correlate with trace element intakes: Case of patients receiving enough trace elements intake. Clin. Nutr. 2008;27:587–593. doi: 10.1016/j.clnu.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 94.Wright L., Cotter D., Hickson M. The effectiveness of targeted feeding assistance to improve the nutritional intake of elderly dysphagic patients in hospital. J. Hum. Nutr. Diet. 2008;21:555–562. doi: 10.1111/j.1365-277X.2008.00915.x. [DOI] [PubMed] [Google Scholar]
- 95.Hamidon B.B., Abdullah S.A., Zawawi M.F., Sukumar N., Aminuddin A., Raymond A.A. A prospective comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding in patients with acute dysphagic stroke. Med. J. Malaysia. 2006;61:59–66. [PubMed] [Google Scholar]
- 96.Klose J., Heldwein W., Rafferzeder M., Sernetz F., Gross M., Loeschke K. Nutritional status and quality of life in patients with percutaneous endoscopic gastrostomy (PEG) in practice: Prospective one-year follow-up. Dig. Dis. Sci. 2003;48:2057–2063. doi: 10.1023/A:1026199110891. [DOI] [PubMed] [Google Scholar]
- 97.Westergren A., Karlsson S., Andersson P., Ohlsson O., Hallberg I.R. Eating difficulties, need for assisted eating, nutritional status and pressure ulcers in patients admitted for stroke rehabilitation. J. Clin. Nurs. 2001;10:257–269. doi: 10.1046/j.1365-2702.2001.00479.x. [DOI] [PubMed] [Google Scholar]
- 98.Thomas F.J., Wiles C.M. Dysphagia and nutritional status in multiple sclerosis. J. Neurol. 1999;246:677–682. doi: 10.1007/s004150050431. [DOI] [PubMed] [Google Scholar]
- 99.Norton B., Homer-Ward M., Donnelly M.T., Long R.G., Holmes G.K. A randomised prospective comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding after acute dysphagic stroke. Bmj. 1996;312:13–16. doi: 10.1136/bmj.312.7022.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smithard D.G., O’Neill P.A., Parks C., Morris J. Complications and outcome after acute stroke. Does dysphagia matter? Stroke. 1996;27:1200–1204. doi: 10.1161/01.STR.27.7.1200. [DOI] [PubMed] [Google Scholar]
- 101.Håglin L., Edström M., Bäckman L., Forsgren L. Low level of phosphate in male patients reporting swallowing disturbances in early Parkinson's disease. Clin. Nutr. Exp. 2020;29:18–29. doi: 10.1016/j.yclnex.2019.11.005. [DOI] [Google Scholar]
- 102.Morone G., Iosa M., Paolucci T., Muzzioli L., Paolucci S. Relationship Between Body Mass Index and Rehabilitation Outcomes in Subacute Stroke With Dysphagia. Am. J. Phys. Med. Rehabil. 2019;98:608–612. doi: 10.1097/PHM.0000000000001159. [DOI] [PubMed] [Google Scholar]
- 103.Nishida T., Yamabe K., Honda S. Dysphagia is associated with oral, physical, cognitive and psychological frailty in Japanese community-dwelling elderly persons. Gerodontology. 2020;37:185–190. doi: 10.1111/ger.12455. [DOI] [PubMed] [Google Scholar]
- 104.Akazawa N., Okawa N., Hino T., Tsuji R., Tamura K., Moriyama H. Dysphagia is more strongly associated with increased intramuscular adipose tissue of the quadriceps than with loss of muscle mass in older inpatients. Nutr. Res. 2019;65:71–78. doi: 10.1016/j.nutres.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 105.Saito T., Hayashi K., Nakazawa H., Yagihashi F., Oikawa L.O., Ota T. A Significant Association of Malnutrition with Dysphagia in Acute Patients. Dysphagia. 2018;33:258–265. doi: 10.1007/s00455-017-9855-6. [DOI] [PubMed] [Google Scholar]
- 106.Ticinesi A., Nouvenne A., Lauretani F., Prati B., Cerundolo N., Maggio M., Meschi T. Survival in older adults with dementia and eating problems: To PEG or not to PEG? Clin. Nutr. 2016;35:1512–1516. doi: 10.1016/j.clnu.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 107.Wakabayashi H., Matsushima M. Neck Circumference Is Not Associated with Dysphagia but with Undernutrition in Elderly Individuals Requiring Long-term Care. J. Nutr. Health Aging. 2016;20:355–360. doi: 10.1007/s12603-015-0587-8. [DOI] [PubMed] [Google Scholar]
- 108.Wakabayashi H., Sashika H., Matsushima M. Head lifting strength is associated with dysphagia and malnutrition in frail older adults. Geriatr. Gerontol. Int. 2015;15:410–416. doi: 10.1111/ggi.12283. [DOI] [PubMed] [Google Scholar]
- 109.Gellrich N.C., Handschel J., Holtmann H., Krüskemper G. Oral cancer malnutrition impacts weight and quality of life. Nutrients. 2015;7:2145–2160. doi: 10.3390/nu7042145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mittal B.B., Pauloski B.R., Rademaker A.W., Discekici-Harris M., Helenowski I.B., Mellot A., Agulnik M., Logemann J.A. Effect of induction chemotherapy on swallow physiology and saliva production in patients with head and neck cancer: A pilot study. Head Neck. 2015;37:567–572. doi: 10.1002/hed.23635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zheng T., Zhu X., Liang H., Huang H., Yang J., Wang S. Impact of early enteral nutrition on short term prognosis after acute stroke. J. Clin. Neurosci. 2015;22:1473–1476. doi: 10.1016/j.jocn.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 112.Medin J., Windahl J., von Arbin M., Tham K., Wredling R. Eating difficulties among patients 3 months after stroke in relation to the acute phase. J. Adv. Nurs. 2012;68:580–589. doi: 10.1111/j.1365-2648.2011.05759.x. [DOI] [PubMed] [Google Scholar]
- 113.Terré R., Mearin F. Prospective evaluation of oro-pharyngeal dysphagia after severe traumatic brain injury. Brain Inj. 2007;21:1411–1417. doi: 10.1080/02699050701785096. [DOI] [PubMed] [Google Scholar]
- 114.Bertoli S., Cardinali S., Veggiotti P., Trentani C., Testolin G., Tagliabue A. Evaluation of nutritional status in children with refractory epilepsy. Nutr. J. 2006;5:14. doi: 10.1186/1475-2891-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Penhavel F.A., Waitzberg D.L., Trevenzol H.P., Alves L., Zilberstein B., Gama-Rodrigues J. Pre-and postoperative nutritional evaluation in patients with chagasic megaesophagus. Nutr. Hosp. 2004;19:89–94. [PubMed] [Google Scholar]
- 116.Reyes A.L., Cash A.J., Green S.H., Booth I.W. Gastrooesophageal reflux in children with cerebral palsy. Child Care Health Dev. 1993;19:109–118. doi: 10.1111/j.1365-2214.1993.tb00718.x. [DOI] [PubMed] [Google Scholar]
- 117.Topf M.C., Magaña L.C., Salmon K., Hamilton J., Keane W.M., Luginbuhl A., Curry J.M., Cognetti D.M., Boon M., Spiegel J.R. Safety and efficacy of functional laryngectomy for end-stage dysphagia. Laryngoscope. 2018;128:597–602. doi: 10.1002/lary.26760. [DOI] [PubMed] [Google Scholar]
- 118.Rogus-Pulia N., Rusche N., Hind J.A., Zielinski J., Gangnon R., Safdar N., Robbins J. Effects of Device-Facilitated Isometric Progressive Resistance Oropharyngeal Therapy on Swallowing and Health-Related Outcomes in Older Adults with Dysphagia. J. Am. Geriatr. Soc. 2016;64:417–424. doi: 10.1111/jgs.13933. [DOI] [PubMed] [Google Scholar]
- 119.Ikeda E., Kojima T., Kaneko K., Minashi K., Onozawa M., Nihei K., Fuse N., Yano T., Yoshino T., Tahara M., et al. Efficacy of concurrent chemoradiotherapy as a palliative treatment in stage IVB esophageal cancer patients with dysphagia. Jpn. J. Clin. Oncol. 2011;41:964–972. doi: 10.1093/jjco/hyr088. [DOI] [PubMed] [Google Scholar]
- 120.Martino R., Beaton D., Diamant N.E. Using different perspectives to generate items for a new scale measuring medical outcomes of dysphagia (MOD) J. Clin. Epidemiol. 2009;62:518–526. doi: 10.1016/j.jclinepi.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 121.Benfer K.A., Weir K.A., Ware R.S., Davies P.S.W., Arvedson J., Boyd R.N., Bell K.L. Parent-reported indicators for detecting feeding and swallowing difficulties and undernutrition in preschool-aged children with cerebral palsy. Dev. Med. Child Neurol. 2017;59:1181–1187. doi: 10.1111/dmcn.13498. [DOI] [PubMed] [Google Scholar]
- 122.Schwarz S.M., Corredor J., Fisher-Medina J., Cohen J., Rabinowitz S. Diagnosis and treatment of feeding disorders in children with developmental disabilities. Pediatrics. 2001;108:671–676. doi: 10.1542/peds.108.3.671. [DOI] [PubMed] [Google Scholar]
- 123.Motil K.J., Schultz R.J., Browning K., Trautwein L., Glaze D.G. Oropharyngeal dysfunction and gastroesophageal dysmotility are present in girls and women with Rett syndrome. J. Pediatr. Gastroenterol. Nutr. 1999;29:31–37. doi: 10.1097/00005176-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 124.Ferrero López M.I., De la Rubia Ortí J.E., Castellano Vela E., González Monte C., Sanchis-Bayarri Bernal V., Navarro Sanz R. Factors associated with mortality in patients with dysphagia help in making dietary and nutritional choices. Nutr. Hosp. 2014;31:820–828. doi: 10.3305/nh.2015.31.2.7766. [DOI] [PubMed] [Google Scholar]
- 125.Motsch C., Hackelsberger A., Nebelung K. Percutaneous endoscopic gastrostomy in patients with ENT tumors. Hno. 1998;46:925–931. doi: 10.1007/s001060050337. [DOI] [PubMed] [Google Scholar]
- 126.Fujishima I. Nutritional Management in Home Care: Including Eating Disorder and Dysphagia Assessments. Jpn. Med. Assoc. J. 2015;58:31–35. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.