Abstract
Latest research shows that SERPINE1 overexpression has an important role in Coronavirus 2019 (COVID-19)-associated coagulopathy leading to acute respiratory distress syndrome (ARDS). However, ways to target this protein remain elusive. In this forum, we discuss recent evidence linking SERPINE1 with COVID-19-related ARDS and summarize the available data on inhibitors of this target.
SERPINE1 Structure and Function
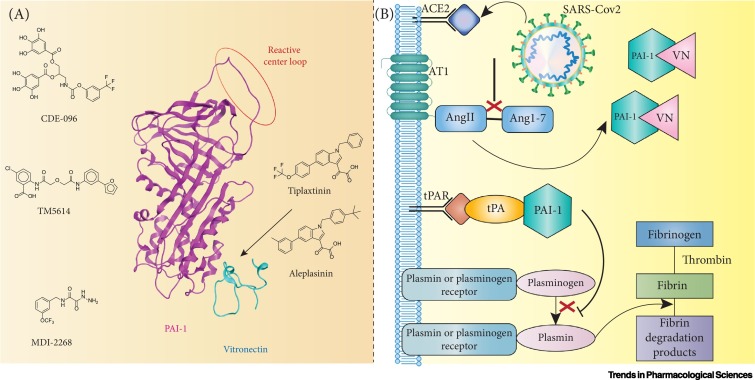
Plasminogen activator inhibitor-1 (PAI-1, aka SERPINE1) is a typical member of the Serpin family of proteins, with a molecular weight of 45 kDa, nine α-helices and three β-sheets [1]. SERPINE1 acts as a ‘suicide inhibitor’ under physiological conditions, binding both tissue type and urokinase plasminogen activator (tPA and uPA) and is regulated by human neutrophil elastase [2., 3., 4.]. As such, SERPINE1 prevents the formation of plasmin and inhibits fibrinolysis and blood clot dissolution. It is synthesized in the active form [with the reactive center loop (RCL) in the position showed in Figure 1A]. This form has a half-life of 1–2 h in the physiological environment, which can increase severalfold when bound to vitronectin (VN). This modulation has attracted attention due to its important role in two vital pathways: a protumorigenic role in cancer due to the proangiogenic and antiapoptotic effects [3]; and a crucial role in maintaining the balance between rates of plasminogen activation and fibrin degradation [4]. It is in this second role as a primary regulator of fibrinolysis in plasma that SERPINE1 is implicated in the symptoms of COVID-19 (Figure 1B).
Figure 1.
SERPINE1 Structure, Inhibitors, and its Role in Fibrinolysis.
(A) SERPINE1 structure and its inhibitors. Tiplaxtinin and aleplasinin are vitronectin (VN) dependent, whereas CDE-096, TM5614, and MDI-2268 are not. However, precise binding pockets for these inhibitors have not yet been identified. (B) Fibrinolysis in Coronavirus 2019 (COVID-19). Angiotensin-converting enzyme 2 (ACE2) converts angiotensin II (AngII) to angiotensin 1–7 (Ang1–7) under normal circumstances. When ACE-2 is hindered by Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-Cov2), this pathway is affected, increasing the levels of Ang2 in the blood. AngII and AngII receptor 1 (AT1) enhance the release of SERPINE1 (PAI-1) by endothelial cells. Most SERPINE1 is likely to be bound to VN, given the high concentration of the latter. SERPINE1 inhibits tPA, limiting the conversion of plasminogen to plasmin and leading to hypofibrinolysis.
SERPINE1 Overexpression in COVID-19
The first evidence that high levels of SERPINE1 in the circulatory system are associated with coronavirus infection started emerging during the Severe Acute Respiratory Syndrome (SARS) epidemic in 2003. A brief article by Wu et al. highlighted that the plasma levels of SERPINE1 in patients with SARS were elevated (355 ng/ml) compared with patients with infectious pneumonias of other causes (88 ng/ml) or healthy controls (61 ng/ml). VN levels in blood plasma were also elevated compared with healthy controls (1538 mg/l versus 310 mg/l) [5]. As a consequence, SERPINE1 is in its active state for longer.
A similar picture is painted for the SARS-coronavirus 2 (Cov2) (COVID-19) pandemic. In a recent single-center study, exploratory analyses of hemostatic factors were performed on 68 patients with COVID-19. There were near-universal elevations in SERPINE1 levels among critically and noncritically ill patients. This is another indication that normal fibrinolysis is prevented in COVID-19-associated coagulopathy [6]. Another study of the fibrinolytic parameters of 78 patients with COVID-19 showed that the observed hypofibrinolysis was immediately related to increased SERPINE1 presence. The level of the protein in patients in intensive care units (ICU) was 96 ng/ml, while for non-ICU patients, the concentration was 77 ng/ml. Both values were elevated compared with its normal range (4-43 ng/ml). A similar increase was observed for tPA [24 ng/ml versus 2–12 ng/ml (normal range)]. The presence of fibrin deposition in the lung parenchyma of patients with COVID-19 suggests that, even though the amount of tPA is elevated, high SERPINE1 levels can overcome local tPA release [7]. This observation is further strengthened by recent work by Cugno et al., who found increased tPA and PAI-1 levels in all patients with COVID-19 regardless of disease severity [8]. The evidence suggests that lethal SARS-Cov2 infection hijacks normal profibrinolytic signaling and leads to overall dysfunction in the system, including increased SERPINE1 expression and severe lung disease [9]. This was further validated by a study that reported that high levels of tPA (in particular) and PAI-1 were associated with mortality and a significant enhancement in spontaneous ex vivo clot lysis [10]. While all this evidence makes a compelling case for the importance of SERPINE1 in the symptoms of COVID-19, the available publications and data on elevated SERPINE1 levels in COVID-19 do not distinguish between active and latent forms.
Inhibition of SERPINE1
Nebulized fibrinolytic agents, such as monoclonal antibodies or small-molecule inhibitors, targeting SERPINE1 considerably enhance the bronchoalveolar fibrinolytic system as well as easing the symptoms of ARDS by elevating the levels of plasmin that effectively removes fibrin [11]. Many inhibitors of SERPINE1 have been developed, both antibodies/nanobodies and small-molecule inhibitors. Nanobodies have been used to both stabilize SERPINE1 and modulate its activity. Recently, VHH-s-a93 was shown to be a potent inhibitor of SERPINE1, with an IC50 of 7 nM [12]. Given that the nanobody binds to the reactive center loop, it also acts independently from the binding of VN to the serpin. MEDI-579 is another antibody that binds to the RCL independently of VN and was found to treat normal human lung fibroblasts with a pIC50 = 9.8 ± 0.14 (10.5 nM) [13]. Small-molecule inhibitors, such as tiplaxtinin and aleplasinin, were the first orally efficacious inhibitors of SERPINE1, developed by Wyeth. They were used extensively in clinical trials on subjects with Alzheimer's disease. The activity of both these drugs depends on whether SERPINE1 is bound to VN, suggesting overlapping binding sites. Given that VN levels are highly elevated during ARDS, this type of inhibitor is unlikely to treat hypofibrinolysis in the lungs efficiently. By contrast, small molecules, such as MDI-2268, CDE-096, and TM5614, are VN-independent inhibitors (Figure 1A) [14,15]. Although preclinical and clinical trials (for indications other than ARDS) are ongoing for these compounds and show promising activity, none of them are yet in advance stages, leaving plenty of room for improvement. Common adverse effects, such as localized uncontrolled bleeding, confirms increased fibrinolysis upon SERPINE1 inhibition. An important aspect to the further development of such compounds would be combining the feasibility of direct administration to the respiratory tract with good permeability to the bloodstream.
One further indication that regulated SERPINE1 production is beneficiary to patients with COVID-19 was shown by Kang et al. [16]. In their study, seven patients with severe COVID-19 exhibited decreased serum SERPINE1 levels and improved clinical features (including lower C-reactive protein levels) after treatment with tocilizumab, an arthritis drug repurposed for SARS-Cov2 [17].
While a procoagulant shift has been observed when the symptoms of COVID-19 become more serious in later stages, plasmin can also promote viral infection by cleaving proteins that play an important part in cell infection, mainly during the earlier stages of the disease. In a recent study by Metcalf et al., it was suggested that the need for enhancement of fibrinolytic activity mainly occurs in stage 3 of the course of COVID-19 [18]. This would mean that the use of SERPINE1 modulators could have a beneficial effect on the later and more serious symptoms of coronavirus infection. However, this point remains controversial because another observational study emphasized that earlier administration of tPA to patients with COVID-19 yielded better results [19].
Concluding Remarks
One of the consequences of SARS-Cov2 interfering with angiotensin-converting enzyme 2 is hypofibrinolysis. Even the smallest blood clots within lungs can cause lasting damage and it is important to bring this COVID-19 symptom under control. In this forum, we have summarized available data indicating SERPINE1 as a promising target for treating severe cases of COVID-19. We have also reviewed the range of SERPINE1 inhibitors available to date and stressed, despite promising results, the need for further development for some existing therapeutics.
Declaration of Interests
All the authors are employees of Evotec (UK) Ltd.
References
- 1.Kellici T.F., et al. Small-molecule modulators of serine protease inhibitor proteins (serpins) Drug Discov. Today. 2021;26:442–454. doi: 10.1016/j.drudis.2020.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Wu K., et al. The cleavage and inactivation of plasminogen activator inhibitor type 1 by neutrophil elastase: the evaluation of its physiologic relevance in fibrinolysis. Blood. 1995;86:1056–1061. [PubMed] [Google Scholar]
- 3.Kubala M.H., DeClerck Y.A. The plasminogen activator inhibitor-1 paradox in cancer: a mechanistic understanding. Cancer Metastasis Rev. 2019;38:483–492. doi: 10.1007/s10555-019-09806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urano T., et al. Recognition of plasminogen activator inhibitor type 1 as the primary regulator of fibrinolysis. Curr. Drug Targets. 2019;20:1695–1701. doi: 10.2174/1389450120666190715102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Y.P., et al. Analysis of thrombotic factors in severe acute respiratory syndrome (SARS) patients. Thromb. Haemost. 2006;96:100–101. doi: 10.1160/TH05-12-0827. [DOI] [PubMed] [Google Scholar]
- 6.Goshua G., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nougier C., et al. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV2 associated thrombosis. J. Thromb. Haemost. 2020;18:2215–2219. doi: 10.1111/jth.15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cugno M., et al. Complement activation and endothelial perturbation parallel COVID-19 severity and activity. J. Autoimmun. 2021;116:102560. doi: 10.1016/j.jaut.2020.102560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Alonzo D., et al. COVID-19 and pneumonia: a role for the uPA/uPAR system. Drug Discov. Today. 2020;25:1528–1534. doi: 10.1016/j.drudis.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuo Y., et al. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Sci. Rep. 2021;11:1580. doi: 10.1038/s41598-020-80010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofstra J.J., et al. Nebulized fibrinolytic agents improve pulmonary fibrinolysis but not inflammation in rat models of direct and indirect acute lung injury. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0055262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sillen M., et al. Structural insights into the mechanism of a nanobody that stabilizes PAI-1 and modulates its activity. Int. J. Mol. Sci. 2020;21:5859. doi: 10.3390/ijms21165859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vousden K.A., et al. Discovery and characterisation of an antibody that selectively modulates the inhibitory activity of plasminogen activator inhibitor-1. Sci. Rep. 2019;9:1605. doi: 10.1038/s41598-019-38842-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rouch A., et al. Small molecules inhibitors of plasminogen activator inhibitor-1 - an overview. Eur. J. Med. Chem. 2015;92:619–636. doi: 10.1016/j.ejmech.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Reinke A.A., et al. Dual-reporter high-throughput screen for small-molecule in vivo inhibitors of plasminogen activator inhibitor type-1 yields a clinical lead candidate. J. Biol. Chem. 2019;294:1464–1477. doi: 10.1074/jbc.RA118.004885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang S., et al. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc. Natl. Acad. Sci. U. S. A. 2020;117:22351–22356. doi: 10.1073/pnas.2010229117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guaraldi G., et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medcalf R.L., et al. Fibrinolysis and COVID-19: a plasmin paradox. J. Thromb. Haemost. 2020;18:2118–2122. doi: 10.1111/jth.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orfanos S., et al. Observational study of the use of recombinant tissue-type plasminogen activator in COVID-19 shows a decrease in physiological dead space. ERJ Open Res. 2020;6:00455–02020. doi: 10.1183/23120541.00455-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]