Graphical Abstract
Keywords: COVID-19, mRNA, Lipid nanoparticles, BNT162b2, mRNA-1273
Abstract
The emergency use authorization (EUA) by the US-FDA for two mRNA-based vaccines BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) has brought hope of addressing the COVID-19 pandemic which has killed more than two million people globally. Nanotechnology has played a significant role in the success of these vaccines. Nanoparticles (NPs) aid in improving stability by protecting the encapsulated mRNA from ribonucleases and facilitate delivery of intact mRNA to the target site. The overwhelming success of these two mRNA based vaccines with ~95% efficacy in phase III clinical trials can be attributed to their unique nanocarrier, the "lipid nanoparticles" (LNPs). LNPs are unique compared with bilayered liposomes and provide improved stability of the cargo, possess rigid morphology, and aid in better cellular penetration. This EUA is a major milestone and showcases the immense potential of nanotechnology for vaccine delivery and for fighting against future pandemics. Currently, these two vaccines are aiding in the alleviation of the COVID-19 health crisis and demonstrate the potential utility of nanomedicine for tackling health problems at the global level.
Introduction
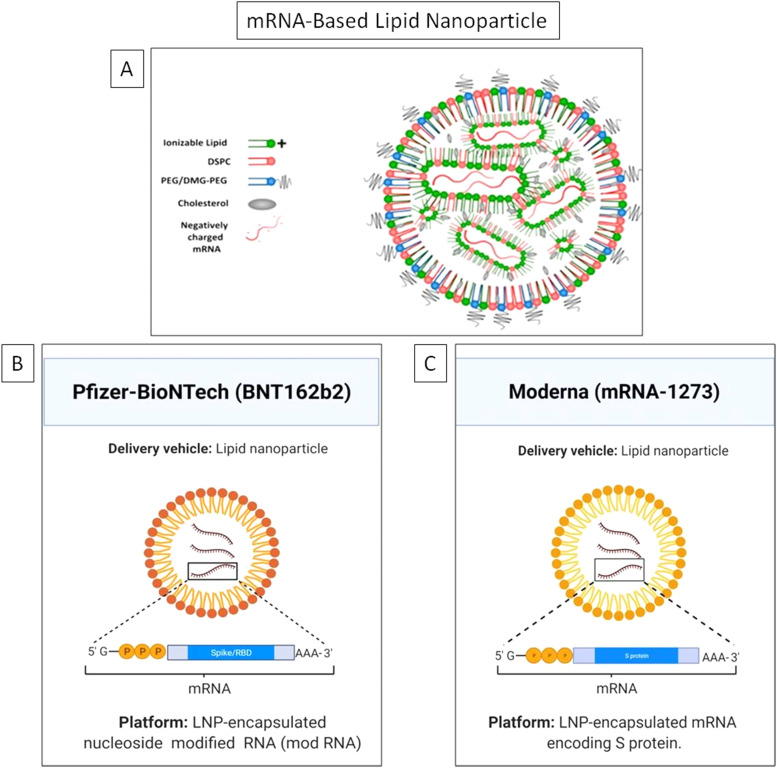
It is the first time in history that two mRNA-based vaccines developed using lipid nanoparticles (LNPs) have been given emergency use authorization (EUA) by the US FDA for clinical therapeutics against the COVID-19 [1]. This undermines the skepticism on the potential of nanotechnology based approaches. Nanoparticles (NPs) offer many unique advantages compared to other conventional drug carriers including tailored drug release profile, enhanced surface area, protection of the cargo from degradation and modulation of drug pharmacokinetics [2], [3], [4], [5], [6], [7], [8], [9]. Nanotechnology has fast tracked the development of mRNA-based COVID-19 vaccines invented by Moderna and Pfizer/BioNTech [10], [11], [12]. On 16th November 2020, Moderna officially shared the preliminary data of the phase III clinical trial of its COVID-19 candidate vaccine mRNA-1273 followed by Pfizer-BioNTech on 18th November, 2020 with the clinical trial outcome of its COVID-19 candidate vaccine BNT162b2. Efficacy is an important parameter for vaccines and is defined as the percentage reduction in disease incidence among the vaccinated group during the clinical trial compared with an unvaccinated control group under similar conditions [13]. The primary data revealed BNT162b2 and mRNA-1273 have an efficacy of 95% and 94.5% against SARS-CoV-2, respectively [14], [15]. The Moderna vaccine is based on a stabilized mRNA of the viral spike protein, [16], [17] and the BNT162b2 is based on a nucleoside modified RNA (modRNA) of the SARS-CoV-2 virus. Table 1 lists the chemical composition of both the vaccines and Fig. 1 depicts the key delivery carrier.
Table 1.
Salient features of Pfizer-BioNTech and Moderna mRNA vaccines.
| Key features | Pfizer-BioNTech (BNT162b2) | Moderna (mRNA-1273) |
|---|---|---|
| Active ingredients: Messenger ribonucleic acid (mRNA) |
|
|
| Fats: Encases & protects the fragile mRNA |
|
|
| Saline solution: Buffer to maintain the pH level close to our body |
|
|
| Storage temperature |
|
|
| Stability |
|
|
| Dosing |
|
|
| Efficacy in phase- III clinical trial |
|
|
Fig. 1.
A) The general structure of lipid nanoparticle (LNP) showing the major components and the unique mRNA cargo of, B) Pfizer-BioNTech, and C) Moderna vaccines.
Figure A) Reprinted (adapted) with permission from [53] and is Copyright (2021) of American Chemical Society. Figure B) and C) were created with BioRender.com.
Over the past one year, some more vaccines have been developed and are now being used clinically on regular a basis. The major new vaccines include inactivated/live attenuated virus, recombinant protein, recombinant viral vector, DNA vaccine, and messenger RNA (mRNA)-based vaccines [7], [18]. COVID-19 vaccines based on conventional technologies include vaccines developed by Oxford/AstraZeneca (UK), Johnson and Johnson (USA), Gamaleya Research Institute (Russia), Bharat Biotech (India), SinoVac (China), Sinopharm (China) and others [19], [20], [21], [22], [23], [24], [25]. These conventional vaccines based on inactivated/live viruses offer advantages like robust immune response, ease of storage and shipping [26], while the disadvantages include difficulty in manufacturing at sufficient titers, cost per dose and the need for multiple doses to achieve immunity [27]. However, mRNA-based vaccines were granted prioritized clinical approval as the technology ensures the stability of the mRNA, together with enhanced delivery efficiency to ferry the mRNA inside the host cell. mRNA vaccines are both non-infectious, and thus safer, and do not require penetration into the nucleus, which is difficult to achieve [28]. Further, these vaccines can be produced rapidly, which is a major advantage in the existing pandemic, where billions of doses are needed in a short time to vaccinate the world population [29], [30], [31], [32]. mRNA uptake by a cell is a very challenging task, firstly, due to presence of RNA degrading enzymes, which debase every RNA molecule they encounter [33], [34], and secondly, being negatively charged, mRNA cannot easily cross the negatively charged cell membrane. Hence, to address this challenge, researchers have designed LNP based carrier molecules to preserve mRNA integrity and foster its uptake inside the cell [35]. LNPs are complex systems, which aid effective delivery of siRNA or mRNA into the host cells. Being a US-FDA approved carrier, LNPs are now extensively used for delivering antigen-encoding mRNA, encapsulating viral antigens against influenza, rabies, human immunodeficiency virus (HIV), cytomegalovirus (CMV) and others [36], [37].
The focus on delivery: key advantages of lipid nanoparticles
In the late 1960s, liposomes were anticipated as a novel drug delivery system (NDDS) and since then have been improved for disease targeting [38]. LNPs present a novel colloidal drug delivery system, and differ from liposomes in that they form micellar structures within the core that can be modified based on formulation and synthesis parameters [39]. The structure of LNPs consists of a solid core made up of lipid, which is composed of triglycerides or any other glyceride mixture. A typical LNP has four parts: (i) an ionizable lipid portion that allows self-assembly, enhances the rate of mRNA encapsulation, and aids endosomal escape, (ii) a stabilizing agent for stability and membrane fusion (cholesterol or a sphingolipid), (iii) a phospholipid that stabilizes the bilayer, encapsulating the lipid structure [40], and (iv) polyethylene glycol (PEG), a lipid-based stabilizing agent that reduces nonspecific binding to proteins, increases half-life, and boosts circulation time by aiding escape from first pass metabolism or reticulo-endothelial system (RES). Their rigid morphology and kinetic stability are key advantages, making LNPs, a carrier of choice over liposomes. Their potential to transport a diverse group of therapeutic cargos from therapeutic drugs to nucleic acids (mRNA, siRNA, DNA) making them an appealing drug delivery system [41], [42], [43]. Once mRNA enters inside the cytoplasm from the endosome, it translates into the encoded immunogenic protein against specific antigens [44], [45]. This recent discovery and integration of nanotechnology have shown various advantages in safer delivery of next generation RNA vaccines.
In addition to their simple synthesis method, small size and serum stability, the efficacy of LNPs in the delivery of nucleic acids into cells makes them superior to other carriers. Since biological membranes and nucleic acids are negatively charged, it is difficult to deliver mRNA across this barrier. LNPs offer an ideal platform for delivering nucleic acid therapies as the ionizable lipids are near-neutrally charged at physiological pH. However, in acidic endosomal compartments (pH-4.5), they become ionized, promoting endosomal escape for effective intracellular delivery [46], [47]. Hence, LNPs achieve high encapsulation rate for nucleic acids with improved transfection efficiency. Furthermore, LNPs have relatively low cytotoxicity and immunogenicity compared to liposomes, thus favoring delivery of nucleic acid based therapeutics [48], [49], [50].
Lipids used in formulating these nanoparticles are biocompatible and are well endured by fatty acids, triglycerides, waxes and steroids. In addition, selecting a good combination of emulsifiers could make the formulation more stable with higher efficiency [52]. In terms of industrial scale up, LNPs offer multiple advantages compared with other carrier systems, such as large scale production using microfluidics or T-junction mixing, stability, and low cost of raw materials [52], [53], [54]. The most significant parameters in LNPs characterization are particle size, their size distribution, degree of polymorphism, zeta potential, crystallinity, drug loading, drug release and entrapment efficiency [55]. Drug release from LNPs mostly relies on the type of matrix used and the position of drug in the matrix formulation. The ingredients of lipid matrix, manufacturing parameters and surfactant concentrations, such as rate of stirring, temperature etc. can modulate the drug release profile [56]. In a nutshell, the most fundamental rationale of using LNPs, as an alternative to polymeric nanoparticles, is the simplicity of large-scale manufacturing and their low toxicity [57], [58].
The path forward
To harness the full potential of mRNA therapeutics in future, the following challenges need particular attention: (i) the intrinsic immunogenicity of mRNA; (ii) the propensity to enzymatic and thermal degradation; and (iii) the inability to cross negatively charged cell membranes. Nucleoside modification, sequence engineering by codon optimization and uridine depletion are some of the methods used to reduce inherent immunogenicity, protect from enzymatic degradation and facilitate cellular uptake of the mRNA platform [59], [60], [61], [62], [63]. The mean half-life of mRNA vaccines decreases with increasing temperature, which is a challenge for their long term storage. However, chemical modification by applying an outer coating of nonionic or an ionic surfactant enhances the thermal stability of mRNA. These chemical alterations change the dimensions of a nanoparticle and aid in the effective carrying of mRNA with higher thermal stability. Some of the reported allergic reactions to LNP nanovaccines can be minimized by using PEG complexed with lipids, which have improved biocompatibility. Hence, in future mRNA-based nanovaccines should possess an improved safety profile, higher stability (for storage at higher temperature), and enhanced cellular penetration. The next generation RNA vaccines will be more personalized and the tools of genetic engineering will play a crucial role in exploiting the full potential of the mRNA platform.
Concluding remarks
The EUA of the Pfizer-BioNTech and Moderna vaccines has undoubtedly brought great hope for the years ahead. However, it remains challenging to decide how to prioritize the allocation of vaccines to the population. While the EUA of these mRNA vaccines brings hope for developed countries, these vaccines remain largely out of reach of developing and underdeveloped nations on economic grounds and need for special storage conditions. At high or at room temperature, mRNA has poor stability, and thus these mRNA-based vaccines need to be stored at such a low temperature. The Pfizer-BioNTech vaccine needs to be stored at −80 °C to −60 °C and the Moderna vaccine at −25 °C to −15 °C to avoid degradation of mRNA encased inside the LNPs. Further, as it is common to all vaccines, mRNA vaccines are not devoid of side effects, including pain at the site of administration, fever, chills, fatigue, headache, muscle pain, and joint pain. In addition, some patients have experienced allergic responses, which has been linked to the presence of PEG in the formulation [64]. The adverse effects, duration of protection and storage at very low temperature are critical issues that require careful consideration for the upcoming COVID-19 nanovaccines [54].
The Oxford-AstraZeneca vaccine has also been approved and is in widespread use in the UK and elsewhere. It is the frontline vaccine candidate for the mass inoculation of global population along with the other conventional vaccines being developed around the globe. Nonetheless, the two mRNA vaccines represent a great success for biotechnology and molecular therapeutics. It motivates materials scientists who have developed and optimized nanoformulations for drug and vaccine delivery over the past two decades and encourages their acceptance in nanomedicines. Until the COVID-19 crisis, oncology had been the major area where nanotechnology based drug carriers had been widely explored. These two mRNA-based vaccine formulations will serve as a stepping stone for future applications of nanomedicine. These nanocarrier based vaccines highlight the importance of the nanoscale and the ability of nanoscale delivery systems to protect payloads from degradation, provide tailored biodistribution and cellular delivery.
CRediT authorship contribution statement
Amit Khurana: Conceptualization, Methodology, Writing - original draft, Writing - review & editing. Prince Allawadhi: Conceptualization, Methodology, Writing - original draft. Isha Khurana: Conceptualization, Methodology, Writing - original draft. Sachin Allwadhi: Conceptualization, Methodology, Writing - original draft. Ralf Weiskirchen: Conceptualization, Methodology, Writing - original draft, Writing - review & editing. Anil Kumar Banothu: Conceptualization, Methodology, Writing - original draft. Deepak Chhabra: Conceptualization, Methodology, Writing - original draft. Kamaldeep Joshi: Conceptualization, Methodology, Writing - original draft. Kala Kumar Bharani: Conceptualization, Methodology, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors sincerely convey their gratitude to Dr. Kannan Govindaraj, the University of Twente, Enschede, The Netherlands for his kind help in proofreading the manuscript. The authors are grateful to the Dean, College of Veterinary Science, PVNRTVU, Hyderabad, Telangana, India.
Biographies

Dr. Amit Khurana is working as a Research Scientist at the Centre for Biomedical Engineering (CBME), IIT-Delhi. His research interests include exploring novel therapeutics for gastroenterological disorders, applications of advanced drug delivery carriers for emerging health problems. He has authored more than forty research and review publications in reputed international journals, has contributed to more than 10 book chapters and is editing a book. He is recipient of multiple prestigious fellowships and awards like the DST-DAAD Doctoral Fellowship, DBT-CNPq Indo-Brazil Fellowship, PC Dandiya Young Scientist Award of the Indian Pharmacological Society and Merit Award from Delhi Pharmacy Council.

Mr. Prince Allawadhi did B.Pharmacy from Vaish Institute of Pharmaceutical Education and Research (VIPER), Rohtak, Haryana, India. He completed his M.Pharmacy (Pharmacology) from Jamia Hamdard (Hamdard University), New Delhi, India. He has multiple publications in international peer reviewed journals including one research paper in Nano Today journal. He has won best paper and presentation awards in national and international conferences. He is recipient of India's prestigious Rajnibhai V Patel Trust PharmInnova Best Master Thesis Award in the Pharmacology category (2019). His research interests are in the field of nanomedicine, acute and chronic pancreatitis, inflammation, immunology and organoids based 3D culture.

Ms. Isha Khurana has qualified GPAT (Graduate Pharmacy Aptitude Test) and currently pursuing Master of Pharmacy in Pharmaceutical Chemistry from the University Institute of Pharmaceutical Sciences (UIPS), Panjab University, Chandigarh, India. She has done her Bachelor of Pharmacy from UIPS, Panjab University, Chandigarh, India. She has co-authored various publications and review articles in various reputed international journals. She has also co-authored multiple book chapters. She has been a recipient of prestigious fellowships such as Jaswant Gill Scholarship and Central Placement Cell Fellowship at University level throughout her studies. Her research interests include drug delivery and therapeutics through nanocarriers and nanotechnology.

Mr. Sachin Allwadhi graduated in Computer Science and Engineering from Kurukshetra University, Kurukshetra, Haryana, India. He holds an M.Tech. degree in Computer Science and Engineering with GATE (Graduate Aptitude Test in Engineering) scholarship from Maharshi Dayanand University (M.D.U.), Rohtak, Haryana, India. He is UGC-NET-JRF fellow and currently, pursuing Ph.D. in Computer Science and Engineering from M.D.U., Rohtak, Haryana, India. He has been a scholar throughout and has also authored multiple publications in internationally reputed journals. He is highly interested in research related to Image Processing, Image Steganography, Artificial Intelligence, Bioinformatics and Machine Learning.

Prof. Ralf Weiskirchen was born on February 2, 1964 in Bergisch Gladbach (Germany). He studied Biology and performed his Ph.D. work at the University of Cologne (Germany). After a five year stay as a post-doctoral fellow at the Institute of Biochemistry at the University of Innsbruck (Austria), he made his habilitation at the RWTH University Hospital Aachen. In 2007 he received a Professor assignment and is currently the head of the Institute of Molecular Pathobiochemistry, Experimental Gene Therapy and Clinical Chemistry (IFMPEGKC) at the RWTH University Aachen. His main research focus is the pathogenesis of hepatic fibrosis and nanomedicine.

Dr. Anil Kumar Banothu is working as an Assistant Professor in the Department of Veterinary Pharmacology and Toxicology, Rajendra Nagar, Hyderabad, India. He has more than nine years experience in academics and research. He has more than 55 research publications in reputed journals and is an associate editorial member of the International Journal of Veterinary Science and Animal Husbandry. He is member of CPCSEA (Committee for the Purpose of Control And Supervision of Experiments on Animals) in various establishments including educational institute, research institute and CRO (Clinical Research Organisation). His area of interests include Molecular Pharmacology, Toxicology, Pharmacokinetics and Nanotechnology.

Dr. Deepak Chhabra is presently working as an Assistant Professor in the Department of Mechanical Engineering, University Institute of Engineering and Technology (UIET) at Maharshi Dayanand University (MDU), Rohtak, Haryana, India. He obtained his Doctorate Degree from National Institute of Technology (NIT) Kurukshetra Haryana, India and Master's Degree in Mechanical Engineering from Maharshi Dayanand University, Rohtak. He has authored more than 40 research papers in international/national journals/book chapter (Elsevier). He holds more than 15 years of teaching experience. His research interests include Active Vibration Control, 3D Printing, Healthcare, Mechanobiology, Bio-informatics etc.

Dr. Kamaldeep Joshi is currently working as an Assistant Professor in Department of Computer Science and Engineering, University Institute of Engineering and Technology, Maharshi Dayanand University, Rohtak, Haryana, India. He has received his B.Tech., M.Tech. and Ph.D. in Computer Science and Engineering degrees from Maharshi Dayanand University, Rohtak, Haryana, India. His research interest includes Image Processing, Neural Network, Machine Learning and Information Security. Dr. Joshi has authored over 25 articles in international peer reviewed journals. He has attended various national and international conferences and contributed research papers in their proceedings. He is also a member of many international societies.

Dr. Kala Kumar Bharani is currently working as Professor and Head, Department of Veterinary Pharmacology and Toxicology, College of Veterinary Science, Mamnoor, Warangal, India. His area of expertize include, novel formulation based approaches for veterinary diseases, production pharmacology, mastitis, and toxicology of insecticides & pesticides. He has successfully completed eight industrially sponsored projects, three government funded (ICAR, ICMR and DBT) extramural projects. He is recipient of the prestigious Dr. S B Pandey Oration Award and Dr. Lalitha Kameswaran Oration Award.
References
- 1.Meo S., et al. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021;25(3):1663–1669. doi: 10.26355/eurrev_202102_24877. [DOI] [PubMed] [Google Scholar]
- 2.Shi J., Votruba A.R., Farokhzad O.C., Langer R. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. 2010;10(9):3223–3230. doi: 10.1021/nl102184c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farokhzad O.C., Langer R. Nanomedicine: developing smarter therapeutic and diagnostic modalities. Adv. Drug Deliv. Rev. 2006;58(14):1456–1459. doi: 10.1016/j.addr.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L., Gu F., Chan J., Wang A., Langer R., Farokhzad O. Nanoparticles in medicine: therapeutic applications and developments. Clin. Pharmacol. Ther. 2008;83(5):761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 5.Khurana A., Tekula S., Saifi M.A., Venkatesh P., Godugu C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019;111:802–812. doi: 10.1016/j.biopha.2018.12.146. [DOI] [PubMed] [Google Scholar]
- 6.Khurana I., Allawadhi P., Khurana A., Srivastava A.K., Navik U., Banothu A.K., Bharani K.K. Can bilirubin nanomedicine become a hope for the management of COVID-19? Med. Hypotheses. 2021;149 doi: 10.1016/j.mehy.2021.110534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang Z., Kong N., Zhang X., Liu Y., Hu P., Mou S., Liljeström P., Shi J., Tan W., Kim J.S., Cao Y., Langer R., Leong K.W., Farokhzad O.C., Tao W. A materials-science perspective on tackling COVID-19. Nat. Rev. Mater. 2020;5(11):847–860. doi: 10.1038/s41578-020-00247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Z., Zhang X., Shu Y., Guo M., Zhang H., Tao W. Insights from nanotechnology in COVID-19 treatment. Nano Today. 2021;36 doi: 10.1016/j.nantod.2020.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allawadhi P., Khurana A., Allwadhi S., Navik U.S., Joshi K., Banothu A.K., Bharani K.K. Potential of electric stimulation for the management of COVID-19. Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanomedicine and the COVID-19 vaccines, Nat. Nanotechnol., 15(12), 2020, pp. 963–963. [DOI] [PMC free article] [PubMed]
- 11.Tang Z., Zhang X., Shu Y., Guo M., Zhang H., Tao W. Insights from nanotechnology in COVID-19 treatment. Nano Today. 2021;36 doi: 10.1016/j.nantod.2020.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allawadhi P., Khurana A., Allwadhi S., Joshi K., Packirisamy G., Bharani K.K. Nanoceria as a possible agent for the management of COVID-19. Nano Today. 2020;35 doi: 10.1016/j.nantod.2020.100982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shim E., Galvani A.P. Distinguishing vaccine efficacy and effectiveness. Vaccine. 2012;30(47):6700–6705. doi: 10.1016/j.vaccine.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh E.E., et al. RNA-based COVID-19 vaccine BNT162b2 selected for a pivotal efficacy study. medRxiv. 2020 [Google Scholar]
- 16.Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.Y., Türeci Ö., Tompkins K.R., Lyke K.E., Raabe V., Dormitzer P.R., Jansen K.U., Şahin U., Gruber W.C. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai L., Gao G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2020:1–10. doi: 10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaur S.P., Gupta V. COVID-19 vaccine: a comprehensive status report. Virus Res. 2020;288 doi: 10.1016/j.virusres.2020.198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohapatra P.R., Mishra B. Regulatory approval of COVID-19 vaccine for restricted use in clinical trial mode. Lancet Infect. Dis. 2021 doi: 10.1016/S1473-3099(21)00045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aleo Luján E., Lopez-Picado A., Rivas A., Joyanes Abancens B., Rodríguez Rojo M.L., Fernández García P., Soto Beauregard C., Rodríguez Alarcón J., González Perrino C., San Pedro de Urquiza B., Arias E., Rodriguez D., Esteban Polonio C., Torrejón M.J. Double-blind, randomized, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of treating healthcare professionals with the adsorbed COVID-19 (inactivated) vaccine manufactured by Sinovac–PROFISCOV: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):1–3. doi: 10.1186/s13063-020-04775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H., Zhang Y., Huang B., Deng W., Quan Y., Wang W., Xu W., Zhao Y., Li N., Zhang J., Liang H., Bao L., Xu Y., Ding L., Zhou W., Gao H., Liu J., Niu P., Zhao L., Zhen W., Fu H., Yu S., Zhang Z., Xu G., Li C., Lou Z., Xu M., Qin C., Wu G., Gao G.F., Tan W., Yang X. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020;182(3):713–721. doi: 10.1016/j.cell.2020.06.008. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Microbe T.L. COVID-19 vaccines: the pandemic will not end overnight. Lancet Microbe. 2021;2(1) doi: 10.1016/S2666-5247(20)30226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knoll M.D., Wonodi C. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397(10269):72–74. doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balakrishnan V.S. The arrival of Sputnik V. Lancet Infect. Dis. 2020;20(10):1128. doi: 10.1016/S1473-3099(20)30709-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livingston E.H., Malani P.N., Creech C.B. The Johnson & Johnson vaccine for COVID-19. JAMA. 2021 doi: 10.1001/jama.2021.2927. [DOI] [PubMed] [Google Scholar]
- 26.Sanders B., Koldijk M., Schuitemaker H. Vaccine Analysis: Strategies, Principles, and Control. Springer; 2015. Inactivated viral vaccines; pp. 45–80. [Google Scholar]
- 27.Bouazzaoui A., Abdellatif A.A.H., Al-Allaf F.A., Bogari N.M., Al-Dehlawi S., Qari S.H. Strategies for vaccination: conventional vaccine approaches versus new-generation strategies in combination with adjuvants. Pharmaceutics. 2021;13(2):140. doi: 10.3390/pharmaceutics13020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines—a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17(4):261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 30.Anderson R.M., Vegvari C., Truscott J., Collyer B.S. Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. Lancet. 2020;396(10263):1614–1616. doi: 10.1016/S0140-6736(20)32318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Islam M.A., Xu Y., Tao W., Ubellacker J.M., Lim M., Aum D., Lee G.Y., Zhou K., Zope H., Yu M., Cao W., Oswald J.T., Dinarvand M., Mahmoudi M., Langer R., Kantoff P.W., Farokhzad O.C., Zetter B.R., Shi J. Restoration of tumour-growth suppression in vivo via systemic nanoparticle-mediated delivery of PTEN mRNA. Nat. Biomed. Eng. 2018;2(11):850–864. doi: 10.1038/s41551-018-0284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong N., Tao W., Ling X., Wang J., Xiao Y., Shi S., Ji X., Shajii A., Gan S.T., Kim N.Y., Duda D.G., Xie T., Farokhzad O.C., Shi J. Synthetic mRNA nanoparticle-mediated restoration of p53 tumor suppressor sensitizes p53-deficient cancers to mTOR inhibition. Sci. Transl. Med. 2019;11(523) doi: 10.1126/scitranslmed.aaw1565. eaaw1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pardi N., Tuyishime S., Muramatsu H., Kariko K., Mui B.L., Tam Y.K., Madden T.D., Hope M.J., Weissman D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release. 2015;217:345–351. doi: 10.1016/j.jconrel.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlake T., Thess A., Fotin-Mleczek M., Kallen K.J. Developing mRNA-vaccine technologies. RNA Biol. 2012;9(11):1319–1330. doi: 10.4161/rna.22269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wadhwa A., Aljabbari A., Lokras A., Foged C., Thakur A. Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics. 2020;12(2):102. doi: 10.3390/pharmaceutics12020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chandler M., Johnson M.B., Panigaj M., Afonin K.A. Innate immune responses triggered by nucleic acids inspire the design of immunomodulatory nucleic acid nanoparticles (NANPs) Curr. Opin. Biotechnol. 2020;63:8–15. doi: 10.1016/j.copbio.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines – a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17(4):261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bozzuto G., Molinari A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015;10:975–999. doi: 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell M.J., et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020:1–24. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guevara M.L., Persano F., Persano S. Advances in lipid nanoparticles for mRNA-based cancer immunotherapy. Front. Chem. 2020;8(963) doi: 10.3389/fchem.2020.589959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Etheridge M.L., Campbell S.A., Erdman A.G., Haynes C.L., Wolf S.M., McCullough J. The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomed. Nanotechnol. Biol. Med. 2013;9(1):1–14. doi: 10.1016/j.nano.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Immordino M.L., Dosio F., Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006;1(3):297–315. [PMC free article] [PubMed] [Google Scholar]
- 43.Kong N., Tao W., Ling X., Wang J., Xiao Y., Shi S., Ji X., Shajii A., Gan S.T., Kim N.Y., Duda D.G., Xie T., Farokhzad O.C., Shi J. Synthetic mRNA nanoparticle-mediated restoration of p53 tumor suppressor sensitizes p53-deficient cancers to mTOR inhibition. Sci. Transl. Med. 2019;11(523) doi: 10.1126/scitranslmed.aaw1565. eaaw1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kauffman K.J., Dorkin J.R., Yang J.H., Heartlein M.W., DeRosa F., Mir F.F., Fenton O.S., Anderson D.G. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Lett. 2015;15(11):7300–7306. doi: 10.1021/acs.nanolett.5b02497. [DOI] [PubMed] [Google Scholar]
- 45.Xue H., Guo P., Wen W.C., Wong H. Lipid-based nanocarriers for RNA delivery. Curr. Pharm. Des. 2015;21(22):3140–3147. doi: 10.2174/1381612821666150531164540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reichmuth A.M., Oberli M.A., Jaklenec A., Langer R., Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016;7(5):319–334. doi: 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hajj K.A., Whitehead K.A. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2017;2(10):1–17. [Google Scholar]
- 48.Cullis P.R., Hope M.J. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 2017;25(7):1467–1475. doi: 10.1016/j.ymthe.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uchida S., Perche F., Pichon C., Cabral H. Nanomedicine-based approaches for mRNA delivery. Mol. Pharm. 2020;17(10):3654–3684. doi: 10.1021/acs.molpharmaceut.0c00618. [DOI] [PubMed] [Google Scholar]
- 50.Billingsley M.M., Singh N., Ravikumar P., Zhang R., June C.H., Mitchell M.J. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 2020;20(3):1578–1589. doi: 10.1021/acs.nanolett.9b04246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo Y., Chen D., Ren L., Zhao X., Qin J. Solid lipid nanoparticles for enhancing vinpocetine’s oral bioavailability. J. Control. Release. 2006;114(1):53–59. doi: 10.1016/j.jconrel.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 52.Silva A.C., González-Mira E., García M.L., Egea M.A., Fonseca J., Silva R., Santos D., Souto E.B., Ferreira D. Preparation, characterization and biocompatibility studies on risperidone-loaded solid lipid nanoparticles (SLN): high pressure homogenization versus ultrasound. Colloids Surf. B Biointerfaces. 2011;86(1):158–165. doi: 10.1016/j.colsurfb.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 53.Elia U., Ramishetti S., Rosenfeld R., Dammes N., Bar-Haim E., Naidu G.S., Makdasi E., Yahalom-Ronen Y., Tamir H., Paran N., Cohen O., Peer D. Design of SARS-CoV-2 hFc-conjugated receptor-binding domain mRNA vaccine delivered via lipid nanoparticles. ACS Nano. 2021 doi: 10.1021/acsnano.0c10180. [DOI] [PubMed] [Google Scholar]
- 54.Buschmann M.D., Carrasco M.J., Alishetty S., Paige M., Alameh M.G., Weissman D. Nanomaterial delivery systems for mRNA vaccines. Vaccines. 2021;9(1):65. doi: 10.3390/vaccines9010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwarz C., Mehnert W., Lucks J.S., Müller R.H. Solid lipid nanoparticles (SLN) for controlled drug delivery. I. Production, characterization and sterilization. J. Control. Release. 1994;30(1):83–96. [Google Scholar]
- 56.zur Mühlen A., Schwarz C., Mehnert W. Solid lipid nanoparticles (SLN) for controlled drug delivery--drug release and release mechanism. Eur. J. Pharm. Biopharm. 1998;45(2):149–155. doi: 10.1016/s0939-6411(97)00150-1. [DOI] [PubMed] [Google Scholar]
- 57.Wang X., Chen H., Luo Z., Fu X. Preparation of starch nanoparticles in water in oil microemulsion system and their drug delivery properties. Carbohydr. Polym. 2016;138:192–200. doi: 10.1016/j.carbpol.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 58.Constantinides P.P. Lipid microemulsions for improving drug dissolution and oral absorption: physical and biopharmaceutical aspects. Pharm. Res. 1995;12(11):1561–1572. doi: 10.1023/a:1016268311867. [DOI] [PubMed] [Google Scholar]
- 59.Karikó K., Buckstein M., Ni H., Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23(2):165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 60.Karikó K., Muramatsu H., Welsh F.A., Ludwig J., Kato H., Akira S., Weissman D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008;16(11):1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thess A., Grund S., Mui B.L., Hope M.J., Baumhof P., Fotin-Mleczek M., Schlake T. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Mol. Ther. 2015;23(9):1456–1464. doi: 10.1038/mt.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heil F. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303(5663):1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 63.Oberli M.A., Reichmuth A.M., Dorkin J.R., Mitchell M.J., Fenton O.S., Jaklenec A., Anderson D.G., Langer R., Blankschtein D. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017;17(3):1326–1335. doi: 10.1021/acs.nanolett.6b03329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Banerji A., et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J. Allergy Clin. Immunol. Pract. 2020 doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]