Abstract
Purpose
Hepatic steatosis has been associated with some cardiovascular risks. Increased alanine aminotransferase (ALT) was suggested to be linked to endothelial dysfunction. We prospectively investigated the joint effect of hepatic steatosis and elevated ALT within the normal range on incident ischemic heart disease (IHD) risk as an extrahepatic complication.
Patients and Methods
We assessed 16,541 participants without diabetes using data from a health risk assessment study (HERAS) and Korean Health Insurance Review and Assessment (HIRA) data. We defined elevated ALT within the normal range as 30–40 IU/L in men and 23–40 IU/L in women, according to previous Korean epidemiological data. We prospectively assessed hazard ratios (HRs) with 95% confidence intervals (CIs) for IHD using multivariate Cox proportional hazards regression models over a 50-month period after the baseline survey.
Results
During the follow-up period, 368 (2.2%) participants developed IHD. Compared to the group with no hepatic steatosis and controlled ALT, the HRs for IHD were 1.68 (95% CI, 1.16–2.42) in the group with hepatic steatosis and elevated ALT after adjusting for confounding variables.
Conclusion
Hepatic steatosis and elevated ALT levels within the normal range may jointly affect the development of IHD among nondiabetic adults. This indicates that lifestyle advice and vascular health management should be recommended among individuals with hepatic steatosis and elevated ALT, even if it falls within the normal range.
Keywords: hepatic steatosis, alanine aminotransferase, prospective cohort study, ischemic heart disease, extrahepatic complication
Introduction
Hepatic steatosis is the marked accumulation of hepatic fat. The prevalence of excess liver fat has been gradually increasing, and it has been estimated that approximately 30% of adults worldwide have hepatic steatosis.1,2 This hepatic manifestation has been considered a benign condition associated with insulin resistance and metabolic syndrome.3,4 However, recent studies have demonstrated that patients with hepatic steatosis are at increased risk for ischemic heart disease (IHD) as an extrahepatic complication.5 Several studies have proposed that hepatic steatosis is also associated with coronary artery disease.6,7
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are circulating liver enzymes that represent liver damage. Of these liver enzymes, ALT is more specific to the liver.8 In addition to hepatic complications, elevated ALT levels are associated with both systemic and hepatic insulin resistance.9,10 Previous studies have reported that elevated ALT is associated with endothelial dysfunction measured as brachial flow-mediated vasodilatation.10 Furthermore, this association was independent of insulin resistance, indicating contributing mechanisms in addition to insulin resistance.4,11
The upper limit of normal for ALT is considered to be approximately 40 IU/L in many laboratories, and elevated ALT is strongly associated with nonalcoholic fatty liver disease (NAFLD).12,13 Liver enzymes are normal in approximately 50% of patients with hepatic steatosis, and slightly increased ALT within the normal range was also shown to be associated with metabolic alterations.14 Although there is a plausible combinatory effect of the two highly correlated hepatic manifestations on IHD, little is known about those joint influences on cardiovascular risks. Therefore, we prospectively investigated the joint effect of hepatic steatosis and elevated ALT within the normal range on incident IHD risk in a large-scale, community-dwelling, nondiabetic adult cohort using National Health Insurance data.
Patients and Methods
Study Population
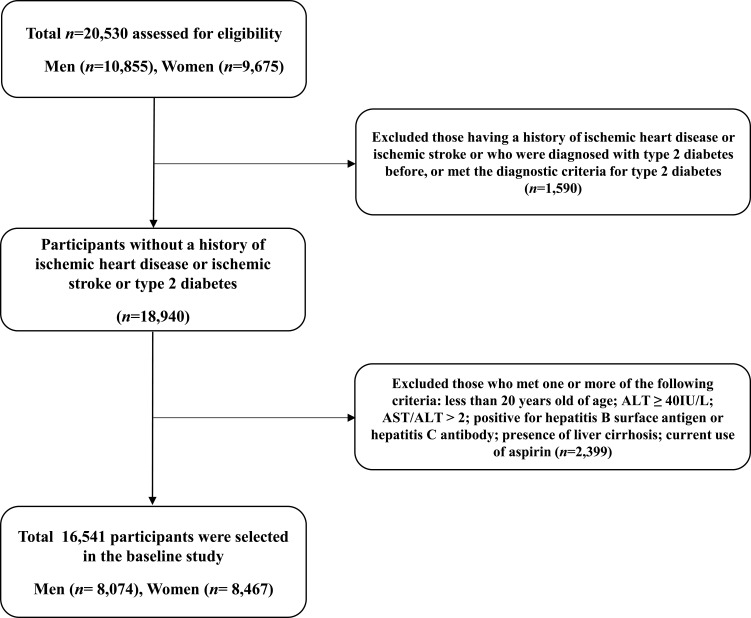
This study is based on health risk assessment study (HERAS) data that aimed to explore surrogate indicators for cardiovascular diseases (CVDs) among Korean adults. The study cohort consisted of 20,530 sequentially enrolled subjects who voluntarily visited the Health Promotion Centre, Gangnam Severance Hospital, Yonsei University College of Medicine, for a health examination between November 2006 and June 2010. Among the participants initially assessed, we excluded 1,590 (7.7%) participants with a history of IHD or ischemic stroke, a previous diagnosis of type 2 diabetes, or a fasting plasma glucose (FPG) level ≥ 126 mg/dL. Participants who met at least one of the following criteria were also excluded: younger than 20 years of age, ALT ≥ 40 IU/L, AST/ALT > 2.0, positive for hepatitis B surface antigen or hepatitis C antibody, presence of liver cirrhosis, and current use of aspirin (n=2,399). After these exclusions, 16,541 participants (8,074 men and 8,467 women) were included in the final analysis (Figure 1). Informed consent was obtained from each participant. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Yonsei University College of Medicine, Seoul, Korea (reference number: 3–2016-0051). The HERAS and HIRA datasets used in the current study are available from the corresponding author on reasonable request.
Figure 1.
Flowchart for the selection of study participants.
Data Collection
All participants completed a questionnaire about lifestyle and past medical history. Self-reported cigarette smoking, alcohol consumption, and physical activity data were obtained from the questionnaires. Smoking status was categorized as nonsmoker or ex-smoker and current smoker. Regular alcohol consumption was defined as alcohol consumption ≥ 140 grams per week. Participants were asked about their physical exercise levels, and regular exercise was defined as physical activity of moderate intensity ≥ three times per week. Body weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, with participants in light indoor clothing without footwear. Body mass index (BMI) was calculated as the weight divided by the height squared (kg/m2). Systolic blood pressure and diastolic blood pressure were measured on the patient’s right arm with a standard mercury sphygmomanometer with the participant in a sitting position after 10 min of rest (Baumanometer, W.A. Baum Co Inc., Copiague, NY, USA). All blood samples were obtained from the antecubital vein after overnight fasting for 12 h. FPG, total cholesterol, triglyceride, high density-lipoprotein cholesterol (HDL-C), ALT, aspartate aminotransferase (AST), and γ-glutamyltransferase (GGT) were measured by enzymatic methods using a Hitachi 7600 automated chemistry analyzer (Hitachi Co., Tokyo, Japan). The hsCRP concentrations were measured with a Roche/Hitachi 912 System (Roche Diagnostics, Indianapolis, IN, USA) using a latex-enhanced immunoturbidimetric method with a lower limit of detection of 0.09 mg/L. Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or current use of hypertension medication. We calculated the hepatic steatosis index (HSI) score as follows: HSI score = 8 x ALT/AST ratio + BMI (kg/m2) (+ 2 if women). The cutoff value of the HSI score for NAFLD was defined as ≥ 36.15
Abdominal Ultrasonography
Liver ultrasonography was performed by experienced radiologists blinded to laboratory and clinical data using a 3.5-MHz transducer (HDI 5000, Philips, Bothell, WA, USA). Fatty liver was assessed semiquantitatively and described as absent (grade 0), mild (grade 1), mild to moderate (grade 2), moderate (grade 3), moderate to severe (grade 4), or severe (grade 5) based on hepatorenal echo contrast, liver brightness, deep attenuation, and vascular blurring. Hepatic steatosis was defined as sonographically detected fatty liver (grade 1 to 5) without liver cirrhosis.
Outcomes
The study outcome assessed was IHD, which consisted of angina pectoris [International Classification of Diseases, 10th revision (ICD-10) code I20] or acute myocardial infarction (ICD-10 code I21) that developed after initial study enrollment. To define baseline and postsurvey outcomes, we linked a personal, 13-digit identification number that was assigned to each subject with Korea Health Insurance Review and Assessment (HIRA) service data, which is a repository of claims data collected in the process of reimbursing healthcare providers, between November 2006 and December 2010.
Statistical Analysis
We defined elevated ALT within the normal range as 30–40 IU/L in men and 23–40 IU/L in women, according to previous Korean epidemiological data.16 To assess the joint effects of hepatic steatosis and serum ALT on incident ischemic heart disease, we divided the study participants into four groups: no hepatic steatosis and controlled ALT (group 1), no hepatic steatosis and elevated ALT (group 2), hepatic steatosis and controlled ALT (group 3), and hepatic steatosis and elevated ALT (group 4). The baseline characteristics of the participants in each group were compared using analysis of variance (ANOVA) for continuous variables and the chi-squared test for categorical variables. Kaplan–Meier curves were used to assess the cumulative incidence of ischemic heart disease. The Log rank test was used to determine whether the distributions of cumulative ischemic heart disease incidence differed among groups. After setting the first group as a reference group, the hazard ratios (HRs) and 95% confidence intervals (CIs) for IHD were calculated using multivariate Cox proportional hazards regression models after adjusting for potential confounding variables. Pairwise comparisons of receiver operating characteristic (ROC) curves were used to compare the area under the ROC curve (AUC) of IHD incidence based on hepatic steatosis with ALT, ALT classification, and NAFLD assessed by the HSI score. To assess the features of the joint effect, we compared the HR according to the presence of each one individually and both together among all participants. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). All statistical tests were two-sided, and statistical significance was set at P < 0.05.
Results
Baseline Characteristics of Study Participants
Table 1 shows the baseline characteristics of the study population (n = 16,541; 8,074 men and 8,467 women) according to hepatic steatosis status and ALT. The mean age and BMI were 45.5 ± 10.7 years and 23.1 ± 2.9 kg/m2, respectively. The prevalence of hepatic steatosis and elevated ALT was 28.7% and 13.7%, respectively.
Table 1.
Baseline Characteristics of the Study Population
| Variables | Overall (n=16,541) | Group 1 | Group 2 | Group 3 | Group 4 | P value* | |||
|---|---|---|---|---|---|---|---|---|---|
| Control of ALT (n = 10,700) | Elevated ALT (n=1,100) | Control of ALT + Hepatic Steatosis (n=3,573) | Elevated ALT + Hepatic Steatosis (n=1,168) | Post Hoc** | |||||
| Age (years) | 45.5 ± 10.7 | 44.3 ± 10.8 | 45.6 ± 10.5 | 48.0 ± 10.2 | 47.9 ± 9.9 | < 0.001 | a,b,c,d,e | ||
| Male sex (%) | 48.8 | 41.6 | 41.1 | 69.1 | 60.5 | < 0.001 | – | ||
| BMI (kg/m2) | 23.1 ± 2.9 | 22.2 ± 2.6 | 23.1 ± 2.9 | 25.0 ± 2.6 | 25.7 ± 2.8 | < 0.001 | a,b,c,d,e,f | ||
| Systolic BP (mmHg) | 121.2 ± 15.4 | 118.8 ± 15.0 | 121.2 ± 15.6 | 126.7 ± 14.7 | 128.3 ± 14.7 | < 0.001 | a,b,c,d,e,f | ||
| Diastolic BP (mmHg) | 75.7 ± 10.1 | 74.0 ± 9.8 | 75.4 ± 10.0 | 79.3 ± 9.6 | 80.3 ± 9.7 | < 0.001 | a,b,c,d,e,f | ||
| AST (IU/L) | 19.6 ± 4.9 | 18.4 ± 4.0 | 26.0 ± 5.9 | 19.5 ± 4.1 | 25.1 ± 5.4 | < 0.001 | a,b,c,d,e,f | ||
| ALT (IU/L) | 18.6 ± 7.4 | 15.5 ± 5.0 | 30.5 ± 4.9 | 19.8 ± 5.4 | 32.3 ± 4.5 | < 0.001 | a,b,c,d,e,f | ||
| GGT (IU/L) | 26.7 ± 23.8 | 21.7 ± 17.3 | 40.7 ± 39.2 | 31.5 ± 22.7 | 45.0 ± 37.9 | < 0.001 | a,b,c,d,e,f | ||
| FPG (mg/dl) | 91.0 ± 9.7 | 89.3 ± 8.9 | 90.1 ± 9.9 | 94.6 ± 9.8 | 96.1 ± 10.8 | < 0.001 | a,b,c,d,e,f | ||
| Total cholesterol (mg/dl) | 188.1 ± 33.0 | 183.5 ± 31.6 | 190.2 ± 33.9 | 196.1 ± 33.2 | 203.9 ± 34.3 | < 0.001 | a,b,c,d,e,f | ||
| Triglyceride (mg/dl) | 117.5 ± 78.0 | 98.6 ± 51.9 | 117.7 ± 77.1 | 156.7 ± 104.1 | 171.4 ± 109.2 | < 0.001 | a,b,c,d,e,f | ||
| HDL-C (mg/dl) | 54.0 ± 12.7 | 56.5 ± 12.7 | 55.3 ± 13.7 | 48.0 ± 10.3 | 47.9 ± 10.3 | < 0.001 | a,b,c,d,e | ||
| hsCRP (mg/L) | 1.3 ± 3.3 | 1.1 ± 2.7 | 1.4 ± 4.3 | 1.6 ± 3.6 | 2.0 ± 5.7 | < 0.001 | a,b,c,e,f | ||
| Current smoker (%) | 23.1 | 20.5 | 25.7 | 28.5 | 28.6 | < 0.001 | - | ||
| Alcohol drinking (%) *** | 42.7 | 41.5 | 39.8 | 46.9 | 43.8 | < 0.001 | - | ||
| Regular exercise (%) # | 32.0 | 32.6 | 30.6 | 31.1 | 30.2 | 0.151 | - | ||
| Hypertension (%) | 20.4 | 15.3 | 20.6 | 30.1 | 37.1 | < 0.001 | - | ||
| Hepatic steatosis severity ## | 0.5 ± 1.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.6 ± 1.0 | 2.2 ± 1.3 | < 0.001 | b,c,d,e,f | ||
| NAFLD by HSI score (%) | 14.0 | 4.2 | 27.3 | 23.8 | 60.5 | < 0.001 | - | ||
Notes: *P values were calculated using 1-way ANOVA test or Pearson’s chi-square test; **Post hoc analysis with Bonferroni method for mean difference between groups: aGroup 1 versus Group 2; bGroup 1 versus Group 3, cGroup 1 versus Group 4; dGroup 2 versus Group 3; eGroup 2 versus Group 4, and fGroup 3 versus Group 4; ***Alcohol intake ≥ 140 g/week; #Moderate intensity physical exercise ≥ three times/week; ##Average grade of hepatic steatosis via ultrasound.
Abbreviations: BMI, body mass index; BP, blood pressure; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ-glutamyltransferase; FPG, fasting plasma glucose; HDL-C, high density-lipoprotein cholesterol; hsCRP, high sensitivity C-reactive protein; NAFLD, nonalcoholic fatty liver disease; HSI, hepatic steatosis index.
The mean values of BMI, systolic blood pressure, diastolic blood pressure, FPG, total cholesterol, triglyceride, and hsCRP were highest in the group with hepatic steatosis and elevated ALT (group 4), whereas the mean HDL-C levels were highest in the group with no hepatic steatosis and controlled ALT (group 1). The proportions of current smoking habit, hypertension, and NAFLD by HSI score were also highest in group 4.
Cumulative Ischemic Heart Disease Incidence
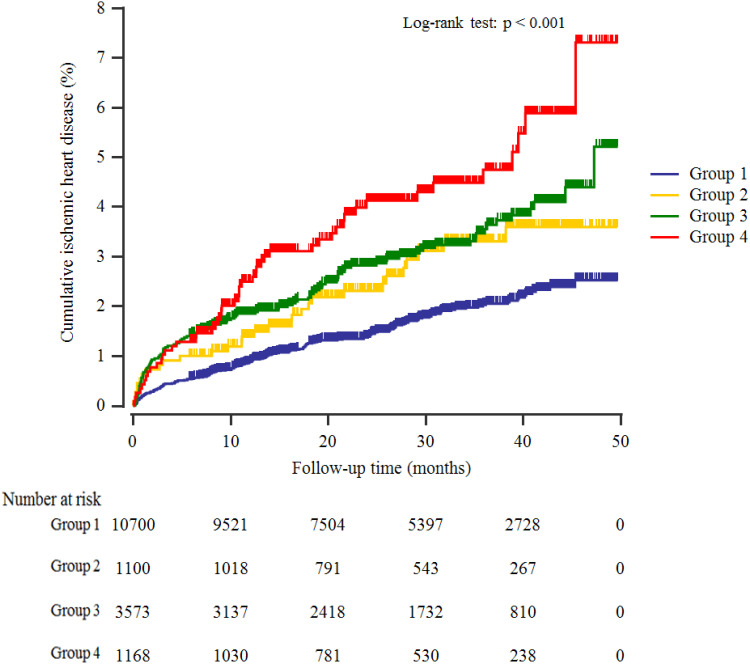
A total of 368 individuals (2.2%, 368/16,541) developed IHD during the follow-up period. Furthermore, group 4 showed the highest cumulative incidences of ischemic heart disease up to 50 months after the baseline survey (Log rank test, P <0.001) (Figure 2). The incidence rates per 1000 person-years were 7.0, 11.4, 13.3, and 18.6 in the four groups.
Figure 2.
Kaplan–Meier plots indicating the cumulative probability of being diagnosed with ischemic heart disease after the baseline survey.
Hazard Ratios for Incident Ischemic Heart Diseases
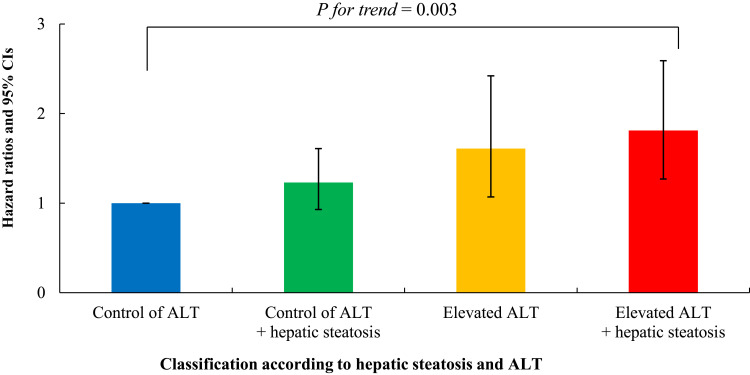
Table 2 shows the results of the multivariate Cox proportional hazards regression analysis for the prediction of IHD according to hepatic steatosis and ALT. Compared with the referent first group, the HRs for IHD were 1.61 (95% CI, 1.07–2.42) in the second group, 1.23 (95% CI, 0.93–1.61) in the third group, and 1.81 (95% CI, 1.27–2.59) in the fourth group after adjusting for age, sex, BMI, smoking status, alcohol intake, and physical activity (Model 2, Figure 3). Similarly, these longitudinal positive associations were found after additionally adjusting for mean arterial pressure, FPG, HDL-C, triglycerides, γ-glutamyltransferase, and hsCRP (Model 3). The corresponding adjusted HR for the fourth group versus the first group was 1.68 (95% CI, 1.16–2.42).
Table 2.
Hazard Ratios and 95% Confidence Intervals for New-Onset Ischemic Heart Diseases
| Group 1 | Group 2 | Group 3 | Group 4 | P for Trend | ||
|---|---|---|---|---|---|---|
| Control of ALT | Elevated ALT | Control of ALT + Hepatic Steatosis | Elevated ALT + Hepatic Steatosis | |||
| New cases of ischemic heart disease, n | 180 | 30 | 109 | 49 | ||
| Mean follow-up, years | 2.4 ± 1.1 | 2.4 ± 1.0 | 2.3 ± 1.1 | 2.3 ± 1.1 | ||
| Pearson-years of follow-up | 25,435 | 2,635 | 8,226 | 2,638 | ||
| Incidence rate/1000 person -years | 7.0 | 11.4 | 13.3 | 18.6 | ||
| Model 1 | HR (95% CI) | 1.00 (reference) | 1.64 (1.11–2.42) | 1.36 (1.07–1.74) | 2.13 (1.55–2.92) | < 0.001 |
| Model 2 | HR (95% CI) | 1.00 (reference) | 1.61 (1.07–2.42) | 1.23 (0.93–1.61) | 1.81 (1.27–2.59) | 0.003 |
| Model 3 | HR (95% CI) | 1.00 (reference) | 1.59 (1.05–2.41) | 1.14 (0.86–1.50) | 1.68 (1.16–2.42) | 0.016 |
Notes: Model 1, adjusted for age and sex; Model 2, adjusted for age, sex, body mass index, smoking status, alcohol intake, and physical activity; Model 3, adjusted for age, sex, body mass index, smoking status, alcohol intake, physical activity, mean arterial blood pressure, fasting plasma glucose, high density-lipoprotein cholesterol, triglycerides, γ-glutamyltransferase, and high sensitivity C-reactive protein level.
Figure 3.
Hazard ratios (95% CIs) for incident ischemic heart disease according to alanine aminotransferase and hepatic steatosis after adjusting for age, sex, body mass index, smoking status, alcohol intake, and physical activity.
Comparison with Other Indicators of Liver Function
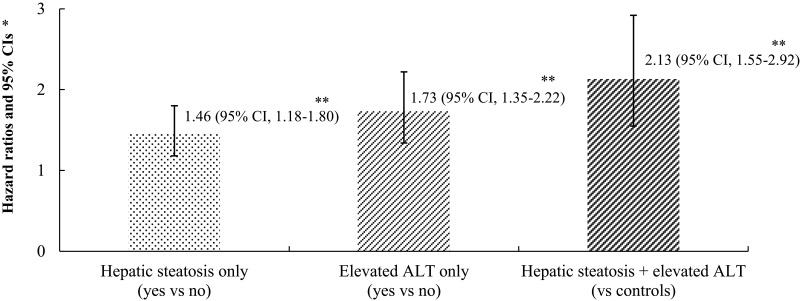
Using pairwise comparison of ROC analyses of incident IHD, the AUC of the groups classified according to hepatic steatosis and ALT was significantly higher than the AUC of the group classified according to ALT and NAFLD assessed by the HSI score (P < 0.001, both). The AUC, sensitivity, and specificity of the groups according to hepatic steatosis and ALT for classifying IHD were 0.587, 51.1%, and 65.1%, respectively (Table 3). Furthermore, Figure 4 shows that the joint effect of hepatic steatosis and ALT has additive features.
Table 3.
Hepatic Steatosis with ALT versus ALT Classification and NAFLD by HSI Score for Predicting Ischemic Heart Disease
| Pairwise Comparison of AUC | Ability to Classify IHD | ||||||
|---|---|---|---|---|---|---|---|
| Difference | 95% CI | P value | Sensitivity (%) | Specificity (%) | AUC | P value | |
| Hepatic steatosis with ALT vs ALT classification | 0.05 | 0.02 to 0.07 | < 0.001 | ||||
| Hepatic steatosis with ALT vs NAFLD by HSI score | 0.07 | 0.04 to 0.09 | < 0.001 | ||||
| ALT classification vs NAFLD by HSI score | 0.02 | −0.01 to 0.04 | 0.201 | ||||
| Hepatic steatosis with ALT | 51.1 | 65.1 | 0.587 | < 0.001 | |||
| ALT classification | 21.5 | 86.5 | 0.540 | < 0.001 | |||
| NAFLD by HSI score | 18.5 | 86.2 | 0.523 | 0.023 | |||
Abbreviations: ALT, alanine aminotransferase; NAFLD, nonalcoholic fatty liver disease; HSI, hepatic steatosis index; AUC, area under the receiver operating characteristic curve.
Figure 4.
Joint effect of hepatic steatosis and elevated alanine aminotransferase for incident ischemic heart disease. *Age- and sex-adjusted Cox proportional hazards regression model analysis. **P value < 0.001.
Discussion
Among community-dwelling Korean adults without diabetes, we found that elevated ALT levels within the normal range were positively and independently associated with IHD incidence in this large-scale, prospective cohort study that included a 50-month follow-up. Our study showed that these associations persisted after further adjustment for lifestyle factors, inflammation and cardiometabolic markers, which was more prominent for individuals with the presence of hepatic steatosis.
We investigated the joint effect of hepatic steatosis and ALT levels within the normal range on the development of IHD among nondiabetic men and women. A previous cross-sectional study showed that hepatic steatosis was independently associated with subclinical coronary arterial calcification, regardless of classical cardiovascular risk factors such as insulin resistance and hypertension.17 In addition, the severity of hepatic steatosis was proportionally associated with the degree of coronary atherosclerosis.18 Schindhelm et al also reported that ALT at baseline was prospectively related to coronary events independent of metabolic risk factors in a large cohort.19 Collectively, these studies suggested that both hepatic steatosis and ALT levels may be associated with a higher risk of CVD.
However, the joint effect of hepatic steatosis and ALT levels on ischemic heart disease has not been investigated in a prospective study. In multivariate Cox proportional hazards regression models, we found that elevation of ALT in response to fat accumulation in the liver was associated with coronary artery disease in a population-based cohort study. To our knowledge, this is the first study showing that an elevated ALT level, even within the normal range, could be a good predictor of IHD in patients with hepatic steatosis. Accordingly, patients with both hepatic steatosis and elevated ALT should be recommended to undergo an assessment of their IHD risk profile, even though their ALT is within the normal range.
NAFLD encompasses a spectrum of liver conditions ranging from simple steatosis to steatohepatitis and cirrhosis.20,21 Steatosis can progress to steatohepatitis and hepatic fibrosis.22 Although steatosis can be diagnosed by using noninvasive techniques such as ultrasound and proton magnetic resonance spectroscopy (H-MRS), assessment of fatty liver severity still requires a liver biopsy.23 In particular, steatohepatitis can only be diagnosed by liver biopsy. However, liver biopsy has limited applicability in clinical studies because of the risk related to the technique and uncertainty of the distribution of fatty infiltration. Therefore, serum ALT levels deserve particular attention because several studies have shown that ALT levels are proportionally correlated with hepatic steatosis.8 Additionally, ALT is the most liver-specific enzyme among all liver enzymes. ALT often rises at some point after initial hepatic steatosis; thus, high-normal ALT may also represent a longer period of hepatic steatosis.24 Overall, serum ALT levels can be a surrogate marker representing fatty infiltration and the severity of hepatic steatosis. In our studies, hepatic steatosis severity was significantly higher in those with high-normal ALT than in controls among individuals with hepatic steatosis. To elucidate the association between the severity of steatosis and the risk of IHD, this could be the best way to investigate the development of IHD in patients with steatosis and elevated serum ALT compared to subjects with neither characteristic or with only one hepatic manifestation. Furthermore, we excluded patients with abnormal ALT levels to investigate whether elevated ALT is a risk factor for IHD even when ALT falls within the normal range. We confirmed that elevated ALT could be a surrogate marker for IHD even when it falls within the normal range.
Some mechanisms may explain the significant relationship between hepatic steatosis and IHD. First, insulin resistance might be the reason hepatic steatosis and elevated ALT are associated with IHD. A recent study showed that elevated hepatic steatosis levels are proportionally correlated with insulin resistance in women.25 Hanley et al reported that serum ALT levels were associated with insulin resistance as measured directly in the Insulin Resistance Atherosclerosis Study.26 In states of insulin resistance, the antilipolytic effect of insulin decreases, causing an increase in free fatty acids (FFAs). The increase in FFAs induces lipotoxicity and liver fat accumulation.27 This relationship between IHD and hepatic manifestations implicated fatty infiltration in the liver, which can lead to the release of proinflammatory adipocytokines. Furthermore, these cytokines derived from adipocytes provide substrates for the formation of toxic metabolites and nonoxidative metabolic pathways, causing mitochondrial dysfunction.28,29 The inflammatory status accompanying reactive oxygen species could link the combination of hepatosteatosis and elevated ALT to the risk of developing IHD. Schindhelm et al found that hepatic steatosis was associated with endothelial dysfunction assessed by brachial artery flow-mediated dilatation, independent of insulin resistance.10 When endothelial cells are damaged, circulating bone marrow-derived endothelial progenitor cells (EPCs) are responsible for regeneration of the endothelial monolayer.12 However, patients with hepatic steatosis have low levels of EPCs, and decreased EPC levels can be correlated with endothelial dysfunction.30
Although there may be variance by laboratory, the upper limit of 40 IU/L is accepted as the normal range.12 However, a recent Korean study has reported that 30 IU/L in men and 23 IU/L in women were the best cutoff values for the prediction of cardiometabolic health.31 In this study, elevated ALT, even in the normal range, could be an early indicator of IHD in patients with hepatic steatosis. Consistent with this finding, several studies have shown that a normal but elevated ALT level is a predictor of metabolic disturbances associated with hepatic steatosis.32
Some strengths and limitations require careful consideration and may affect the interpretation of the results of the present study. A major strength of the work was that we conducted a prospective cohort study using a large number of Korean individuals linked to HIRA data derived from the universal coverage system in Korea. As a result, there was a very low chance that data were missing.33
This study had some limitations that should also be acknowledged. First, although one study reported a day-by-day variation of up to 30% and diurnal variation,34 we evaluated the serum ALT levels only once and did not exclude those with excess alcohol consumption. However, we excluded subjects with viral hepatitis, such as hepatitis B and C, which may interfere with ALT levels, and we collected blood samples in the morning. Also, to minimize the inclusion of alcoholic steatohepatitis, we have excluded those with AST/ALT >2. Second, although biopsy is the gold standard for diagnosis, we did not use liver biopsy to assess NAFLD. However, ultrasound has been validated with reasonable sensitivity (greater than 80%) and specificity (greater than 90%) in the clinical settings.35,36 Third, we may not fully take into consideration possible underlying conditions affecting both ALT levels and IHD development, such as family history, systemic inflammatory processes, or skeletal muscle mass. However, we performed multivariate Cox proportional hazards regression models after adjusting for confounding variables, including hsCRP, a systemic inflammatory marker. Hepatic steatosis combined with ALT, in addition to traditional cardiometabolic markers, may deserve an important consideration for the prediction of IHD, comparable to the HR for ever-smokers. Further studies are necessary to elucidate the role of muscle mass in the effects on incident IHD of hepatic steatosis combined with ALT within the normal range.
Conclusions
Hepatic steatosis and elevated ALT levels in the normal range jointly affect the development of IHD, independent of traditional CVD risk factors. Moreover, these joint effects were found to be a more powerful predictive indicator of IHD than ALT alone or NAFLD assessed by HSI score alone. This implies that patients with steatosis and elevated ALT should be recommended to receive lifestyle advice and a comprehensive assessment of risk factors for IHD. More research is warranted to elucidate the pathophysiological role of hepatic steatosis and ALT in the development of cardiovascular disease.
Acknowledgments
The authors would like to thank the Health Insurance Review and Assessment Services (HIRA) for their cooperation.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 2.Kang SY, Kim YJ, Park HS. Trends in the prevalence of non-alcoholic fatty liver disease and its future predictions in Korean men, 1998–2035. J Clin Med. 2020;9(8):2626. doi: 10.3390/jcm9082626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho HJ, Hwang S, Park JI, et al. Improvement of nonalcoholic fatty liver disease reduces the risk of type 2 diabetes mellitus. Gut Liver. 2019;13(4):440–449. doi: 10.5009/gnl18382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reginato E, Pippi R, Aiello C, et al. Effect of short term intensive lifestyle intervention on hepatic steatosis indexes in adults with obesity and/or type 2 diabetes. J Clin Med. 2019;8(6):851. doi: 10.3390/jcm8060851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li AA, Ahmed A, Kim D. Extrahepatic manifestations of nonalcoholic fatty liver disease. Gut Liver. 2020;14(2):168–178. doi: 10.5009/gnl19069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gastaldelli A, Kozakova M, Højlund K, et al. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology. 2009;49(5):1537–1544. doi: 10.1002/hep.22845 [DOI] [PubMed] [Google Scholar]
- 7.Targher G, Zoppini G, Day CP. Risk of all-cause and cardiovascular mortality in patients with chronic liver disease. Gut. 2011;60(11):1602–1603. doi: 10.1136/gut.2010.230656 [DOI] [PubMed] [Google Scholar]
- 8.Westerbacka J, Corner A, Tiikkainen M, et al. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk. Diabetologia. 2004;47(8):1360–1369. doi: 10.1007/s00125-004-1460-1 [DOI] [PubMed] [Google Scholar]
- 9.Vozarova B, Stefan N, Lindsay RS, et al. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51(6):1889–1895. doi: 10.2337/diabetes.51.6.1889 [DOI] [PubMed] [Google Scholar]
- 10.Schindhelm RK, Diamant M, Bakker SJ, et al. Liver alanine aminotransferase, insulin resistance and endothelial dysfunction in normotriglyceridaemic subjects with type 2 diabetes mellitus. Eur J Clin Invest. 2005;35(6):369–374. doi: 10.1111/j.1365-2362.2005.01502.x [DOI] [PubMed] [Google Scholar]
- 11.Cho EJ, Han K, Lee SP, Shin DW, Yu SJ. Liver enzyme variability and risk of heart disease and mortality: a nationwide population-based study. Liver Int. 2020;40(6):1292–1302. doi: 10.1111/liv.14432 [DOI] [PubMed] [Google Scholar]
- 12.Pacifico L, Ferraro F, Bonci E, Anania C, Romaggioli S, Chiesa C. Upper limit of normal for alanine aminotransferase: Quo vadis? Clin Chim Acta. 2013;422:29–39. doi: 10.1016/j.cca.2013.03.030 [DOI] [PubMed] [Google Scholar]
- 13.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98(5):960–967. doi: 10.1111/j.1572-0241.2003.07486.x [DOI] [PubMed] [Google Scholar]
- 14.Kotronen A, Westerbacka J, Bergholm R, Pietilainen KH, Yki-Jarvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92(9):3490–3497. doi: 10.1210/jc.2007-0482 [DOI] [PubMed] [Google Scholar]
- 15.Jang DK, Lee JK, Lee JK, Kim YH. Independent association of physical activity with nonalcoholic fatty liver disease and alanine aminotransferase levels. J Clin Med. 2019;8(7):1013. doi: 10.3390/jcm8071013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang HS, Um SH, Seo YS, et al. Healthy range for serum ALT and the clinical significance of “unhealthy” normal ALT levels in the Korean population. J Gastroenterol Hepatol. 2011;26(2):292–299. doi: 10.1111/j.1440-1746.2010.06481.x [DOI] [PubMed] [Google Scholar]
- 17.Chen CH, Nien CK, Yang CC, Yeh YH. Association between nonalcoholic fatty liver disease and coronary artery calcification. Dig Dis Sci. 2010;55(6):1752–1760. doi: 10.1007/s10620-009-0935-9 [DOI] [PubMed] [Google Scholar]
- 18.Wang CC, Lin SK, Tseng YF, et al. Elevation of serum aminotransferase activity increases risk of carotid atherosclerosis in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2009;24(8):1411–1416. doi: 10.1111/j.1440-1746.2009.05872.x [DOI] [PubMed] [Google Scholar]
- 19.Schindhelm RK, Dekker JM, Nijpels G, et al. Alanine aminotransferase predicts coronary heart disease events: a 10-year follow-up of the Hoorn Study. Atherosclerosis. 2007;191(2):391–396. doi: 10.1016/j.atherosclerosis.2006.04.006 [DOI] [PubMed] [Google Scholar]
- 20.Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia. 2008;51(11):1947–1953. doi: 10.1007/s00125-008-1135-4 [DOI] [PubMed] [Google Scholar]
- 21.Kim Y, Chang Y, Cho YK, Ahn J, Shin H, Ryu S. Metabolically healthy versus unhealthy obesity and risk of fibrosis progression in non-alcoholic fatty liver disease. Liver Int. 2019;39(10):1884–1894. doi: 10.1111/liv.14184 [DOI] [PubMed] [Google Scholar]
- 22.Tobari M, Hashimoto E. Characteristic features of nonalcoholic fatty liver disease in Japan with a focus on the roles of age, sex and body mass index. Gut Liver. 2020;14(5):537–545. doi: 10.5009/gnl19236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schindhelm RK, Diamant M, Dekker JM, Tushuizen ME, Teerlink T, Heine RJ. Alanine aminotransferase as a marker of non‐alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes Metab Res Rev. 2006;22(6):437–443. doi: 10.1002/dmrr.666 [DOI] [PubMed] [Google Scholar]
- 24.Malakouti M, Kataria A, Ali SK, Schenker S. Elevated liver enzymes in asymptomatic patients - what should i do? J Clin Transl Hepatol. 2017;5(4):394–403. doi: 10.14218/JCTH.2017.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fraser A, Ebrahim S, Smith GD, Lawlor DA. A comparison of associations of alanine aminotransferase and gamma-glutamyltransferase with fasting glucose, fasting insulin, and glycated hemoglobin in women with and without diabetes. Hepatology. 2007;46(1):158–165. doi: 10.1002/hep.21667 [DOI] [PubMed] [Google Scholar]
- 26.Hanley AJ, Wagenknecht LE, Festa A, D’Agostino RB, Haffner SM. Alanine aminotransferase and directly measured insulin sensitivity in a multiethnic cohort: the insulin resistance atherosclerosis study. Diabetes Care. 2007;30(7):1819–1827. doi: 10.2337/dc07-0086 [DOI] [PubMed] [Google Scholar]
- 27.Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5(5):1544–1560. doi: 10.3390/nu5051544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Challa TD, Wueest S, Lucchini FC, et al. Liver ASK1 protects from non‐alcoholic fatty liver disease and fibrosis. EMBO Mol Med. 2019;11(10):e10124. doi: 10.15252/emmm.201810124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yilmaz Y. Is non‐alcoholic fatty liver disease a spectrum, or are steatosis and non‐alcoholic steatohepatitis distinct conditions? Aliment Pharmacol Thera. 2012;36(9):815–823. doi: 10.1111/apt.12046 [DOI] [PubMed] [Google Scholar]
- 30.Wajngot A, Chandramouli V, Schumann WC, et al. Quantitative contributions of gluconeogenesis to glucose production during fasting in type 2 diabetes mellitus. Metab Clin Exp. 2001;50(1):47–52. doi: 10.1053/meta.2001.19422 [DOI] [PubMed] [Google Scholar]
- 31.Kim BK, Han KH, Ahn SH. “Normal” range of alanine aminotransferase levels for Asian population. J Gastroenterol Hepatol. 2011;26(2):219–220. doi: 10.1111/j.1440-1746.2010.06603.x [DOI] [PubMed] [Google Scholar]
- 32.Chang Y, Ryu S, Sung E, Jang Y. Higher concentrations of alanine aminotransferase within the reference interval predict nonalcoholic fatty liver disease. Clin Chem. 2007;53(4):686–692. doi: 10.1373/clinchem.2006.081257 [DOI] [PubMed] [Google Scholar]
- 33.Kim J, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Kor Med Sci. 2017;32(5):718–728. doi: 10.3346/jkms.2017.32.5.718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fraser CG, Cummings ST, Wilkinson SP, et al. Biological variability of 26 clinical chemistry analytes in elderly people. Clin Chem. 1989;35(5):783–786. doi: 10.1093/clinchem/35.5.783 [DOI] [PubMed] [Google Scholar]
- 35.Mathiesen UL, Franzén LE, Aselius H, et al. Increased liver echogenicity at ultrasound examination reflects degree of steatosis but not of fibrosis in asymptomatic patients with mild/moderate abnormalities of liver transaminases. Dig Liver Dis. 2002;34(7):516–522. doi: 10.1016/S1590-8658(02)80111-6 [DOI] [PubMed] [Google Scholar]
- 36.Cheung CL, Lam KS, Wong IC, Cheung BM. Non-invasive score identifies ultrasonography-diagnosed non-alcoholic fatty liver disease and predicts mortality in the USA. BMC Med. 2014;12(1):154. doi: 10.1186/s12916-014-0154-x [DOI] [PMC free article] [PubMed] [Google Scholar]