Abstract
Background
COVID-19 has impacted acute stroke care with several reports showing worldwide drops in stroke caseload during the pandemic. We studied the impact of COVID-19 on acute stroke care in our health system serving Southeast Michigan as we rolled out a policy to limit admissions and transfers.
Methods
in this retrospective study conducted at two stroke centers, we included consecutive patients presenting to the ED for whom a stroke alert was activated during the period extending from 3/20/20 to 5/20/20 and a similar period in 2019. We compared demographics, time metrics, and discharge outcomes between the two groups.
Results
of 385 patients presented to the ED during the two time periods, 58% were African American. There was a significant decrease in the number of stroke patients presenting to the ED and admitted to the hospital between the two periods (p <0.001). In 2020, patients had higher presenting NIHSS (median: 2 vs 5, p = 0.012), discharge NIHSS (median: 2 vs 3, p = 0.004), and longer times from LKW to ED arrival (4.8 vs 9.4 h, p = 0.031) and stroke team activation (median: 10 vs 15 min, p = 0.006). In 2020, stroke mimics rates were lower among African Americans. There were fewer hospitalizations (p <0.001), and transfers from outside facilities (p = 0.015).
Conclusion
a trend toward faster stroke care in the ED was observed during the pandemic along with dramatically reduced numbers of ED visits, hospitalizations and stroke mimics. Delayed ED presentations and higher stroke severity characterized the African American population, highlighting deepening of racial disparities during the pandemic.
Key Words: COVID, Metrics, Racial disparities, Outcomes, Race, Ethnicity, Stroke volume
Abbreviations: COVID19, Coronavirus Disease 2019; AA, African American; SA, stroke alert; HCP, health care providers; AS, acute stroke; AST, acute stroke team; AIS, acute ischemic stroke; TIA, Transient ischemic attack; ED, emergency department; IVT, Intravenous thrombolysis; MT, mechanical thrombectomy; LKW, last known well; STA, stoke team activation; NIHSS, National Institute of health stroke scale; ICH, intracerebral hemorrhage; SAH, subarachnoid hemorrhage; EMS, emergency medical service; AMA, against medical advice; LWCS, left without completion of service; CAD, coronary artery disease; MI, myocardial infarction; PVD, peripheral vascular disease; DVT, deep vein thrombosis; PE, pulmonary embolism; CTA, computed tomography angiogram; DSA, digital subtraction angiography; MRA, magnetic resonance angiogram
Introduction
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV2) pandemic has had drastic social, economic, and public health repercussions worldwide.1 At the various peaks of the pandemic around the world, hospitals ran short on personnel, medical supplies, protective personal equipment (PPE), intensive care unit and general floor beds, and ventilators, shunting most of the resources towards the care of patients suspected or confirmed to have Coronavirus Disease 2019 (COVID-19). As such, medical centers have developed and rolled out various emergency protocols aimed at optimizing resource utilization by guiding the triaging and management of acute medical conditions to protect patients and healthcare providers (HCP) from exposure and accommodate the sudden surge in COVID-19 patient volumes.2 Beds have been turned into isolation rooms, HCP of various specialties have been deployed to cover COVID-19 units, diagnostics and imaging modalities have been restricted to the bare essentials, and most outpatients services have migrated to virtual platforms.3 The care of acute medical conditions requiring urgent evaluation and management, such as acute ischemic strokes (AIS) and transient ischemic attacks (TIA), has consequently been impacted.
In fact, many reports from around the world point at a reduction in the caseload of strokes presenting to the emergency department (ED) and admitted to the hospital. Intravenous thrombolysis (IVT) and mechanical thrombectomy (MT) therapies have decreased by 18–60% compared to the same period a year ago, based on reports emanating from China, Italy, and Spain.4 , 5 Notably, patients have been presenting later and with worse stroke severity indices, mostly concerned about their risk of getting exposed to the virus within the hospital environment.6, 7, 8 Fear of contracting the disease, enforcement of social distancing measures and quarantines may have contributed to stroke symptoms being discovered late, reducing the usability of time-sensitive therapies.9
Anecdotally, these factors appear to have disproportionately impacted minorities and in particular African Americans (AAs). Reports that came early in the pandemic alerted to AAs being at risk of more severe disease after contracting COVID-19. Nonetheless, thus far, there is only a scant account of the differential impact the COVID-19 pandemic has had on the care of AIS and TIA among AAs and non-AAs.
In this study, we aim to showcase our experience with ED stroke alerts and inpatient stroke admissions, comparing a time period before (referred to as 2019) and a time period during (referred to as 2020) the pandemic in a comprehensive stroke and thrombectomy capable centers of a large tertiary health system in southeast Michigan, and serving a large AA population.
Methods
Study setting
This is a retrospective study conducted at two teaching hospitals within Henry Ford Health System (HFHS) serving southeast Michigan. Henry Ford Hospital (HFH) is a designated comprehensive stroke center located in Detroit (Wayne County) and Henry Ford West Bloomfield (HFWB) is a Joint Commission-certified thrombectomy-capable center in West Bloomfield (Oakland County). Both hospitals share the same acute stroke management pathways and are covered by common stroke and neuro-interventional teams.
Acute stroke activation process
The acute stroke team (AST) is a multidisciplinary team composed of vascular neurology and neuro-interventional faculty and fellows, neurology residents, rapid response nursing team, nursing staff and pharmacists. The AST is available on call 24/7. A Stroke Alert (SA) is activated if symptom onset or time of last known well (LKW) is within 24 h of presentation to a HFHS ED or hospital.
The interventional and vascular neurology teams were of the same composition during the two time periods.
Study population and timeline
This study was approved by the HFHS Institutional Review Board. Our prospectively collected data from the Get With The Guidelines database as well as our SA logs were mined for eligible patients. We analyzed two cohorts of patients. The first cohort was consecutive patients presenting to the EDs of HFH and HFWB on whom a SA was activated from 3/20/2020 to 5/20/2020 (COVID period, or “2020”) and the same epoch in 2019 (pre-COVID period, or “2019”) for comparison. 3/20/2020 corresponds to the date the amended HFHS tier 1 policy on Emergency Care and Admissions of patients with AIS and TIA went into effect system-wide to address resource use optimization and exposure risk reduction during the pandemic. The second cohort included patients who were admitted to our stroke units from HFHS EDs or directly transferred from outside hospitals or EDs with a diagnosis of AIS, TIA, intracerebral hemorrhage (ICH) or subarachnoid hemorrhage (SAH).
Clinical and demographic variables
The following demographic and clinical variables were abstracted from review of electronic records. Demographic variables: age, sex, race, ethnicity. Vascular risk factors: hypertension, diabetes, hyperlipidemia, atrial fibrillation/atrial flutter, tobacco smoking, coronary artery disease, peripheral artery disease, history of strokes/TIA, heart failure and substance abuse. Clinical variables: mode of arrival to initial ED, initial and discharge NIHSS, discharge after hospitalization modified Rankin score (mRS), COVID-19 status of patient (negative or positive/suspected), received IVT, received MT, final diagnosis of stroke or stroke mimics (seizures, conversion disorder, toxic and metabolic disorders), stroke etiology based on the TOAST classification criteria,10 and final disposition.
Time metrics for all patients
The following time metrics were collected for all patients: LKW date/time, ED arrival date/time (door time), door-to-physician contact time, door-to-stroke team activation (STA) date/times, door-to-first brain imaging time, and door-to-discharge time for patients discharged from the ED and from the hospital for admitted patients.
Additional time metrics for IVT
LKW-to-needle time and door-to-needle (DTN) time.
Additional time metrics for MT
Door-to-arterial puncture time.
Statistical methods
For the categorical and binary variables, the number and percentages were presented. For age, the mean and standard deviation were presented. For the presenting NIHSS and time variables, the median and interquartile range were presented. All variables were compared between 2019 and 2020 using univariate Wilcoxon rank-sum tests for continuous and ordinal variables and using Fisher's exact tests for categorical variables. To test whether the number of ED patients or inpatient admissions were similar between the 2019 and 2020 time periods a binomial test was done with a null hypothesis of 50/50 split between the two years. The above analyses were done for all patients and then for AAs and non-AAs separately. Patients with unknown race were not included in the analyses by race. Two-way analysis of variance (ANOVA) were done for the continuous variables to test whether the differences between 2019 and 2020 were similar for AAs and non-AAs. For the time variables, log (x+1) transformations were used in the ANOVA to reduce the impact of outliers. In addition, multiple linear or ordinal logistic regression analyses were done to adjust for patient demographics and hospital for select outcomes in the ED cohort and to adjust for patient demographics, hospital and NIHSS at admission for select outcomes in the inpatient admission cohort. The testing level for all comparisons was 0.05. All analyses are performed using SAS 9.4 (SAS Institute Inc, Cary, NC, USA).
Results
ED visits
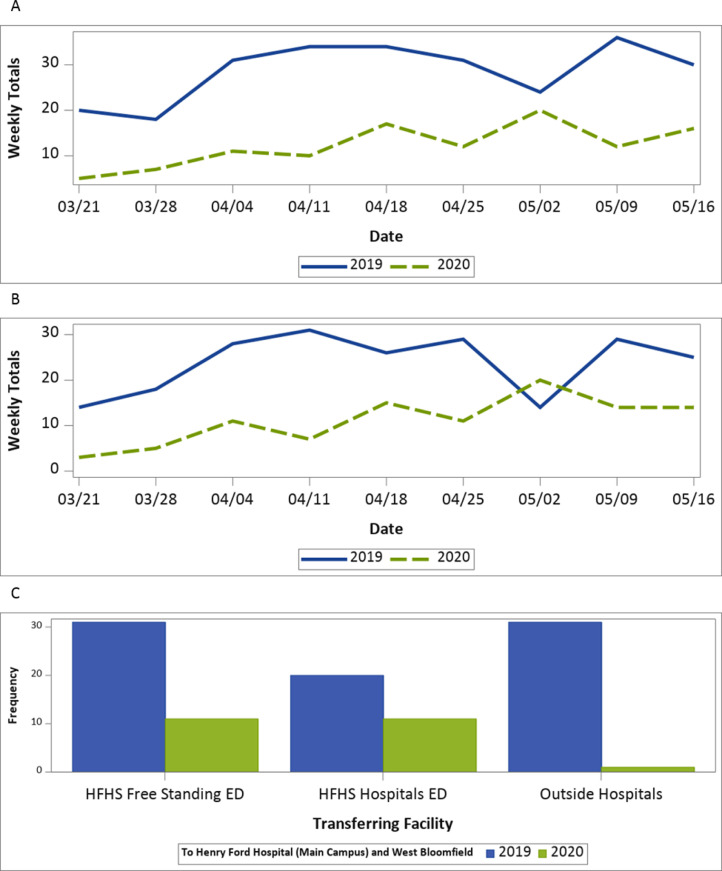
All patients: There were a total of 394 ED visits for 385 patients. Seven patients had two ED visits during these time intervals and one person had three visits. For the following analysis, only the first ED visits were included. For the 385 patients, the mean age was 64 years (s.d.=15.5) with a range from 20 to 97. Two hundred and two (52%) were males, 224 (58%) were AAs, 128 (33%) were Caucasian, 17 (4%) were other races and 16 (4%) had unknown/not documented race. In 2019; 264 (69%) patients were seen and 121 (31%) in 2020. The decrease in the number of patients seen between 2019 and 2020 was significant (p <0.001) ( Fig.1A). The reduction between 2019 and 2020 was 54%, which was 10% larger than the reduction in all ED visits during the same time periods (2019: 24,729 visits vs 2020: 13,779, 44% reduction). The patients seen in 2020 had significantly higher presenting NIHSS when compared to the 2019 patients (median: 5 vs 2, p = 0.012). They also had significantly longer times since LKW to ED arrival (median: 9.4 vs 4.8 h, p = 0.031). For the patients with STA times available, the 2020 patients had significantly lower times from arrival to STA (median: 10 vs 15 min, p=0.006). This difference remained significant after adjusting for age, gender, race and hospital (p = 0.026). They also had significantly lower times from arrival to brain imaging (median: 26 vs 35 min, p = 0.042). However, after adjusting for age, gender, race and hospital this difference was no longer significant (p =0.676). The difference in final diagnosis was significant between the two groups of patients (p <0.001), with the 2020 patients having higher rates of AIS/TIA and ICH/SAH and lower rates of stroke mimics compared to the 2019 patients (Table 1 ).
Fig. 1.
A: Weekly trends of ED visits comparing 2019 to 2020. B: Weekly trends of inpatient admissions comparing 2019 to 2020. C: Comparison between transfer rates to HFH and HFWB from HFHS freestanding EDs, HFHS Hospitals EDs and outside hospitals.
Table 1.
Comparing 2019 to 2020 for ED visits.
| Variable | Response | Total |
African American |
Non-African American |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2019 (N= 264) | 2020 (N= 121) | p-value | 2019 (N= 154) | 2020 (N= 70) | p-value | 2019 (N= 102) | 2020 (N= 43) | p-value | ||
| Age | N Mean ± Std Dev | 264 63.6 ± 16.0 | 121 65.1 ± 14.3 | 0.558 | 154 62.3 ± 14.1 | 70 62.1 ± 13.1 | 0.860 | 102 65.2 ± 18.4 | 43 70.9 ± 13.3 | 0.192 |
| Gender | Female | 129 (49%) | 54 (45%) | 0.444 | 80 (52%) | 31 (44%) | 0.315 | 44 (43%) | 18 (42%) | >.99 |
| Male | 135 (51%) | 67 (55%) | 74 (48%) | 39 (56%) | 58 (57%) | 25 (58%) | ||||
| Race | African American | 154 (58%) | 70 (58%) | 0.133 | ||||||
| Caucasian | 87 (33%) | 41 (34%) | ||||||||
| Other | 15 (6%) | 2 (2%) | ||||||||
| Unknown | 8 (3%) | 8 (6%) | ||||||||
| African American | Yes | 154 (60%) | 70 (62%) | 0.817 | ||||||
| No | 102 (40%) | 43 (38%) | ||||||||
| Hospital | HFHMC | 214 (81%) | 89 (74%) | 0.108 | 139 (90%) | 63 (90%) | >.99 | 67 (66%) | 19 (44%) | 0.026 |
| WBH | 50 (19%) | 32 (26%) | 15 (10%) | 7 (10%) | 35 (34%) | 24 (56%) | ||||
| NIHSS at Admission | N Median (IQR) | 244 2 (1,6) | 109 5 (1, 9) | 0.012 | 143 3 (1, 6) | 60 5 (2, 9) | 0.004 | 95 2 (1, 7) | 41 4 (1, 8) | 0.631 |
| Time Since Last Known Well to arrival (Hours) | N Median (IQR) | 230 4.8 (1.4, 12.3) | 105 9.4 (2.0, 21.2) | 0.031 | 142 5.2 (1.6, 12.8) | 59 10.4 (2, 21.7) | 0.038 | 80 4.4 (1.2, 12.3) | 38 4.6 (1.6, 28.7) | 0.466 |
| Time from Arrival to Stroke Team Activation (Minutes) | N Median (IQR) | 120 15 (10, 25) | 38 10 (6, 19) | 0.0061 | 80 15 (10, 25.5) | 21 10 (7, 19) | 0.035 | 35 15 (10, 23) | 11 9 (6, 24) | 0.308 |
| Time from Arrival to Initial Brain Imaging (Minutes) | N Median (IQR) | 224 35 (24, 62) | 110 26 (17, 101) | 0.0422 | 144 35 (24, 64.5) | 67 35 (18, 124) | 0.266 | 73 33 (24, 61) | 35 24 (15, 97) | 0.281 |
| Time from Arrival to Discharge (Hours) (Just patients discharged from ED) | N Median (IQR) | 42 6.9 (4.7, 9) | 15 5 (3.1, 9.7) | 0.244 | 25 7.7 (5, 9.2) | 8 7.4 (2.7, 12.1) | 0.677 | 15 5.6 (3.0, 8.4) | 3 5.1 (4.8, 6.8) | >.99 |
| ED Disposition | Left AMA/LWCS | 4 (2%) | 0 (0%) | 0.448 | 2 (1%) | 0 (0%) | 0.639 | 1 (1%) | 0 (0%) | 0.488 |
| Admit | 217 (82%) | 106 (88%) | 126 (82%) | 62 (89%) | 86 (84%) | 40 (93%) | ||||
| Discharge | 42 (16%) | 15 (12%) | 25 (16%) | 8 (11%) | 15 (15%) | 3 (7%) | ||||
| Transfer to Another Facility | 1 (0%) | 0 (0%) | 1 (1%) | 0 (0%) | ||||||
| Final Diagnosis | ICH/SAH | 23 (9%) | 17 (14%) | <.001 | 11 (7%) | 15 (21%) | <.001 | 11 (11%) | 2 (5%) | 0.065 |
| AIS/TIA | 144 (55%) | 83 (69%) | 80 (52%) | 46 (66%) | 65 (64%) | 36 (84%) | ||||
| Stroke Mimic | 96 (37%) | 21 (17%) | 63 (41%) | 9 (13%) | 26 (25%) | 5 (12%) | ||||
| Discharge Disposition | Left AMA/LWCS | 7 (3%) | 1 (1%) | 0.390 | 4 (3%) | 1 (1%) | 0.498 | 2 (2%) | 0 (0%) | 0.616 |
| Acute Care Facility | 2 (1%) | 0 (0%) | 1 (1%) | 0 (0%) | 1 (1%) | 0 (0%) | ||||
| Expired | 9 (3%) | 8 (7%) | 4 (3%) | 3 (4%) | 3 (3%) | 4 (9%) | ||||
| Home | 175 (66%) | 71 (59%) | 103 (67%) | 38 (54%) | 69 (68%) | 27 (63%) | ||||
| Hospice | 5 (2%) | 4 (3%) | 3 (2%) | 3 (4%) | 2 (2%) | 1 (2%) | ||||
| Nursing Home | 44 (17%) | 23 (19%) | 24 (16%) | 15 (21%) | 19 (19%) | 7 (16%) | ||||
| Rehab | 21 (8%) | 14 (12%) | 14 (9%) | 10 (14%) | 6 (6%) | 4 (9%) | ||||
| Transfer to Another Hospital | 1 (0%) | 0 (0%) | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | ||||
p =0.026 after adjusting for age, sex, race and hospital.
p =0.676 after adjusting for age, sex, race and hospital.
AA patients: AA patients seen in 2020 had significantly higher presenting NIHSS when compared to 2019 (median: 5 vs 3, p =0.004). They also had significantly longer times since LKW to ED arrival (median: 10.4 vs 5.2 h, p=0.038). For the AA patients with STA times available, the 2020 patients had significantly lower times from arrival to STA (median: 10 vs 15 min, p =0.035). The difference in final diagnosis was significant between the two years (p <0.001), with the 2020 patients having higher rates of AIS/TIA and ICH/SAH and lower rates of stroke mimics compared to the 2019 AA patients (Table 1).
Non-AA patients: Although it did not reach statistical significance, the difference in final diagnosis did show a trend similar to the AA patients with a higher rate of AIS/TIA and a lower rate of stroke mimics in 2020 (p =0.065) (Table 1).
For all time variables as well as NIHSS, the differences between 2019 and 2020 for the AA and non-AA patients were not significant (p>0.07 for all).
Inpatient admissions
All patients: There were a total of 336 inpatient admissions for 327 patients. Nine patients had two admissions during these time intervals. For the following analysis, only the first admissions were included. For the 327 patients, the mean age was 66.2 (s.d.=14.1) with a range from 27 to 97, 186 (57%) were males, 167 (51%) were AAs, 148 (45%) were Caucasian, 9 (3%) were other race and 3 (1%) had unknown/not documented race, and 219 (67%) were seen in 2019 and 108 (33%) in 2020. The decrease in the number of patients seen between 2019 and 2020 was significant (p<0.001) (Fig. 1B). The racial distribution between the two years differed with a significantly higher rate of AAs seen in 2020 compared to 2019 (62% vs 47%, p =0.012). The difference between the two years for the method of arrival was significant (p<0.001) with more 2020 patients directly transported by EMS to our EDs (51% vs 28%, p<0.001) and fewer transferred from other hospitals (28% vs 42%, p =0.015) (Fig. 1C). The patients seen in 2020 had significantly higher presenting NIHSS when compared to the 2019 patients (median: 5 vs 3, p =0.002). For the patients with times available, the shift toward shorter times for the 2020 patients was significant for times to STA (median: 8 vs 14 min, p =0.049). Patients with ischemic strokes or TIAs admitted in 2020 had significantly higher NIHSS on discharge (median: 3 vs 2, p =0.004) (Table 2 ).
Table 2.
Comparing 2019 vs 2020 – inpatient.
| Variable | Response | Total |
African American |
Non-African American |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2019 (N= 219) | 2020 (N= 108) | p-value | 2019 (N= 101) | 2020 (N= 66) | p-value | 2019 (N= 116) | 2020 (N= 41) | p-value | ||
| Demographic information | ||||||||||
| Age | N Mean ± Std Dev | 219 66.5 ± 14.0 | 108 65.6 ± 14.2 | 0.473 | 101 64.3 ± 12.9 | 66 63.2 ± 12.8 | 0.594 | 116 68.2 ± 14.7 | 41 69.5 ± 15.7 | 0.629 |
| Gender | Female | 94 (43%) | 47 (44%) | >.99 | 51 (50%) | 27 (41%) | 0.268 | 42 (36%) | 20 (49%) | 0.194 |
| Male | 125 (57%) | 61 (56%) | 50 (50%) | 39 (59%) | 74 (64%) | 21 (51%) | ||||
| Race | African American | 101 (46%) | 66 (61%) | 0.011 | ||||||
| Caucasian | 107 (49%) | 41 (38%) | ||||||||
| Other | 9 (4%) | 0 (0%) | ||||||||
| Unknown | 2 (1%) | 1 (1%) | ||||||||
| African American | Yes | 101 (47%) | 66 (62%) | 0.012 | ||||||
| No | 116 (54%) | 41 (38%) | ||||||||
| Campus | HFHMC | 177 (81%) | 81 (75%) | 0.250 | 90 (89%) | 59 (89%) | >.99 | 85 (73%) | 22 (54%) | 0.031 |
| WBH | 42 (19%) | 27 (25%) | 11 (11%) | 7 (11%) | 31 (27%) | 19 (46%) | ||||
| Insurance type | Medicare plus others | 137 (63%) | 68 (63%) | 0.982 | 59 (58%) | 41 (62%) | 0.864 | 77 (66%) | 27 (66%) | 0.852 |
| Private | 37 (17%) | 17 (16%) | 13 (13%) | 8 (12%) | 23 (20%) | 8 (20%) | ||||
| Medicaid only | 42 (19%) | 22 (20%) | 27 (27%) | 17 (26%) | 15 (13%) | 5 (12%) | ||||
| None | 3 (1%) | 1 (1%) | 2 (2%) | 0 (0%) | 1 (1%) | 1 (2%) | ||||
| Past Medical History/Comorbidities | ||||||||||
| Atrial Fibrillation/Flutter | 34 (16%) | 9 (8%) | 0.082 | 11 (11%) | 4 (6%) | 0.408 | 23 (20%) | 5 (12%) | 0.347 | |
| CAD/prior MI | 36 (16%) | 20 (19%) | 0.642 | 17 (17%) | 10 (15%) | 0.833 | 18 (16%) | 10 (24%) | 0.237 | |
| Carotid Stenosis | 5 (2%) | 1 (1%) | 0.668 | 2 (2%) | 0 (0%) | 0.519 | 3 (3%) | 1 (2%) | >.99 | |
| Dyslipidemia | 100 (46%) | 44 (41%) | 0.410 | 46 (46%) | 25 (38%) | 0.342 | 53 (46%) | 19 (46%) | >.99 | |
| Hypertension | 171 (78%) | 87 (81%) | 0.667 | 86 (85%) | 55 (83%) | 0.828 | 83 (72%) | 32 (78%) | 0.539 | |
| Previous Stroke | 61 (28%) | 23 (21%) | 0.227 | 29 (29%) | 13 (20%) | 0.207 | 32 (28%) | 10 (24%) | 0.838 | |
| Previous TIA | 13 (6%) | 5 (5%) | 0.798 | 5 (5%) | 2 (3%) | 0.705 | 8 (7%) | 3 (7%) | >.99 | |
| Depression | 27 (12%) | 13 (12%) | >.99 | 12 (12%) | 6 (9%) | 0.620 | 15 (13%) | 7 (17%) | 0.601 | |
| Heart Failure | 24 (11%) | 7 (6%) | 0.232 | 12 (12%) | 6 (9%) | 0.620 | 11 (9%) | 1 (2%) | 0.187 | |
| Obesity/Overweight | 140 (64%) | 63 (58%) | 0.335 | 66 (65%) | 40 (61%) | 0.622 | 73 (63%) | 22 (54%) | 0.354 | |
| Smoker | 58 (26%) | 34 (31%) | 0.362 | 36 (36%) | 23 (35%) | >.99 | 22 (19%) | 11 (27%) | 0.372 | |
| Family History of Stroke | 29 (13%) | 14 (13%) | >.99 | 14 (14%) | 8 (12%) | 0.818 | 15 (13%) | 6 (15%) | 0.792 | |
| PVD | 10 (5%) | 3 (3%) | 0.556 | 8 (8%) | 1 (2%) | 0.089 | 2 (2%) | 2 (5%) | 0.279 | |
| Sleep Apnea | 14 (6%) | 6 (6%) | >.99 | 8 (8%) | 3 (5%) | 0.530 | 6 (5%) | 3 (7%) | 0.698 | |
| DVT/PE | 4 (2%) | 6 (6%) | 0.087 | 1 (1%) | 3 (5%) | 0.302 | 3 (3%) | 3 (7%) | 0.184 | |
| Drugs/Alcohol Abuse | 16 (7%) | 14 (13%) | 0.106 | 8 (8%) | 10 (15%) | 0.201 | 8 (7%) | 4 (10%) | 0.513 | |
| In-hospital information | ||||||||||
| Place of first care at HFH | Direct Admit, not through ED | 57 (26%) | 19 (18%) | 0.095 | 13 (13%) | 11 (17%) | 0.507 | 43 (38%) | 8 (20%) | 0.035 |
| Emergency Department/Urgent Care | 160 (74%) | 89 (82%) | 88 (87%) | 55 (83%) | 71 (62%) | 33 (80%) | ||||
| Method of Arrival | EMS from home/scene | 61 (28%) | 55 (51%) | <.001 | 40 (40%) | 36 (55%) | 0.124 | 21 (18%) | 18 (44%) | 0.008 |
| Private transport/taxi/other from home/scene | 66 (30%) | 23 (21%) | 38 (38%) | 16 (24%) | 28 (24%) | 7 (17%) | ||||
| Transfer from other hospital | 91 (42%) | 30 (28%) | 23 (23%) | 14 (21%) | 66 (57%) | 16 (39%) | ||||
| NIHSS at Admission | N Median (IQR) | 195 3 (1, 6) | 91 5 (3, 10) | 0.002 | 92 3 (1, 6) | 53 5 (3, 10) | 0.005 | 102 2 (0, 7) | 37 5 (1, 11) | 0.165 |
| Stroke type | ICH/SAH | 29 (13%) | 23 (21%) | 0.076 | 12 (12%) | 18 (27%) | 0.014 | 15 (13%) | 5 (12%) | >.99 |
| AIS/TIA | 190 (87%) | 85 (79%) | 89 (88%) | 48 (73%) | 101 (87%) | 36 (88%) | ||||
| AIS Etiology | Large-artery atherosclerosis | 33 (20%) | 22 (28%) | 0.216 | 14 (18%) | 12 (27%) | 0.303 | 19 (22%) | 10 (31%) | 0.585 |
| Cardio-embolism | 40 (24%) | 18 (23%) | 18 (23%) | 9 (20%) | 22 (25%) | 9 (28%) | ||||
| Small-vessel disease | 28 (17%) | 6 (8%) | 18 (23%) | 5 (11%) | 10 (11%) | 1 (3%) | ||||
| Stroke of other determined etiology | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | 0 (0%) | ||||
| Cryptogenic Stroke | 63 (38%) | 32 (41%) | 28 (36%) | 19 (42%) | 35 (40%) | 12 (38%) | ||||
| Time Since Last Known Well to arrival (Hours)1 | N Median (IQR) | 102 6.3 (1.4, 24) | 64 13.4 (2.7, 35.9) | 0.089 | 66 6.2 (1.7, 23.7) | 41 13.6 (3.9, 38.8) | 0.062 | 36 6.4 (0.9, 30.7) | 22 12.1 (1.7, 34.1) | 0.633 |
| Time from Arrival to Initial Brain Imaging (Minutes)1 | N Median (IQR) | 122 41 (23, 159) | 78 40.5 (18, 144) | 0.145 | 78 42 (23, 166) | 52 54.5 (19, 149) | 0.284 | 44 37 (22.5, 129.5) | 25 24 (15, 100) | 0.198 |
| Time from Arrival to Stroke Team Activation (Minutes)1 | N Median (IQR) | 47 14 (9, 23) | 17 8 (7, 14) | 0.049 | 34 14.5 (9, 23) | 11 8 (6, 12) | 0.020 | 13 14 (9, 22) | 5 8 (7, 17) | 0.628 |
| Time from Arrival to ED Physician Assessment (Minutes)1 | N Median (IQR) | 116 14 (6.5, 23) | 77 13 (5, 23) | 0.122 | 76 14.5 (4.5, 66) | 52 17 (9, 30) | 0.608 | 40 13.5 (7, 31) | 24 6.5 (1.5, 15.1) | 0.015 |
| CT Perfusion | 36 (16%) | 21 (19%) | 0.536 | 20 (20%) | 14 (21%) | 0.846 | 16 (14%) | 7 (17%) | 0.613 | |
| DSA | 5 (2%) | 6 (6%) | 0.188 | 1 (1%) | 1 (2%) | >.99 | 4 (3%) | 5 (12%) | 0.053 | |
| CTA | 101 (46%) | 59 (55%) | 0.159 | 58 (57%) | 37 (56%) | 0.874 | 43 (37%) | 21 (51%) | 0.140 | |
| MRA | 20 (9%) | 10 (9%) | >.99 | 9 (9%) | 7 (11%) | 0.790 | 11 (9%) | 3 (7%) | >.99 | |
| Discharge information | ||||||||||
| Discharge NIHSS for stroke and TIA patients | N Median (IQR) | 137 2 (0, 3) | 53 3 (1, 6) | 0.004 | 741 (0, 3) | 36 3.5 (2, 7) | <.001 | 63 2 (0, 3) | 17 1 (0, 5) | 0.538 |
| mRS at Discharge (0-2 vs 3-5 vs died) for AIS and TIA patients | 0–2 (Favorable) | 109 (59%) | 41 (50%) | 0.1542 | 55 (64%) | 23 (49%) | 0.173 | 54 (55%) | 17 (50%) | 0.036 |
| 3–5 (Unfavorable) | 71 (39%) | 36 (44%) | 28 (33%) | 23 (49%) | 43 (44%) | 13 (38%) | ||||
| Died | 4 (2%) | 5 (6%) | 3 (3%) | 1 (2%) | 1 (1%) | 4 (12%) | ||||
| Length of stay (Hours) | N Median (IQR) | 219 91.1 (51.1, 188.2) | 108 96.5 (51.1, 194.8) | 0.748 | 101 109.1 (53.8, 183.6) | 66 101.6 (58.7, 211.6) | 0.999 | 116 85.4 (47.8, 193.8) | 41 84.1 (41.5, 148.8) | 0.356 |
| Disposition | Home | 130 (59%) | 56 (52%) | 0.0553 | 60 (59%) | 31 (47%) | 0.329 | 69 (59%) | 24 (59%) | 0.087 |
| Hospice - Home | 2 (1%) | 4 (4%) | 1 (1%) | 3 (5%) | 1 (1%) | 1 (2%) | ||||
| Hospice - Health Care Facility | 4 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (3%) | 0 (0%) | ||||
| Acute Care Facility | 2 (1%) | 0 (0%) | 1 (1%) | 0 (0%) | 1 (1%) | 0 (0%) | ||||
| Other Health Care Facility | 69 (32%) | 36 (33%) | 33 (33%) | 26 (39%) | 36 (31%) | 10 (24%) | ||||
| Expired | 8 (4%) | 11 (10%) | 4 (4%) | 5 (8%) | 3 (3%) | 6 (15%) | ||||
| Left AMA | 4 (2%) | 1 (1%) | 2 (2%) | 1 (2%) | 2 (2%) | 0 (0%) | ||||
| Type of Rehabilitation | Inpatient Rehabilitation Facility (IRF) | 24 (35%) | 15 (42%) | 0.728 | 12 (36%) | 11 (42%) | 0.893 | 12 (33%) | 4 (40%) | >.99 |
| Long Term Care Hospital (LTCH) | 4 (6%) | 1 (3%) | 1 (3%) | 1 (4%) | 3 (8%) | 0 (0%) | ||||
| Skilled Nursing Facility (SNF) | 41 (59%) | 20 (56%) | 20 (61%) | 14 (54%) | 21 (58%) | 6 (60%) | ||||
Does not include patients transferred from other hospitals.
p =0.958 for favorable (mRS 0-2) vs unfavorable (mRS 3-6) after adjusting for age, sex, race, hospital and NIHSS at admission.
p =0.301 for home/left AMA vs care facility vs hospice/death after adjusting for age, sex, race, hospital and NIHSS at admission.
AA patients: Patients seen in 2020 had higher NIHSS at admission compared to patients seen in 2019 (median: 5 vs 3, p =0.005). They also had higher rates of ICH/SAH (27% vs 12%, p =0.014). For patients with STA, patients seen in 2020 had short times from arrival to STA (median: 8 vs 14.5 min, p =0.02). Patients seen in 2020 had higher NIHSS at discharge (median: 3.5 vs 1, p<0.001) (Table 2).
Non-AA patients: Patients seen in 2020 were more likely to have been seen first in the ED (80% vs 62%, p =0.035) and had higher rates of EMS for the method of arrival (44% vs 18%, p =0.002) and lower rates of transfer from other hospitals (39% vs 57%, p =0.047). For patients with available time information for ED physician assessment, patients seen in 2020 had shorter times from arrival to physician assessment (median: 6.5 vs 13.5 min, p =0.015). Patients seen in 2020 with AIS/TIA had a higher rate of death compared to patients seen in 2019 (12% vs 1%, p =0.015) (Table 2).
For all time variables as well as NIHSS, the differences between 2019 and 2020 for the AA and non-AA patients were not significant (p>0.29 for all).
Intervention group
IVT: For all patients receiving IVT, there were no differences detected between the two years for IVT administration rates, reasons for not receiving IVT, and times between LKW and IVT initiation. There was, however, a trend toward shorter times from ED arrival to IVT initiation in 2020 compared to 2019 (median: 38 vs 51 min, p =0.051) (Table 3 ).
Table 3.
IVT and MT information.
| Variable | Response | Total |
African American |
Non-African American |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | p-value | 2019 | 2020 | p-value | 2019 | 2020 | p-value | ||
| IVT information for eligible patients (African American n=125 with 78 in 2019 and 47 in 2020; Non-African American=121 with 89 in 2019 and 32 in 2020) | ||||||||||
| IVT given at HFH | Yes | 17 (12%) | 13 (20%) | 0.143 | 12 (17%) | 7 (16%) | >.99 | 5 (7%) | 5 (23%) | 0.048 |
| No1 | 126 (88%) | 53 (80%) | 58 (83%) | 36 (84%) | 68 (93%) | 17 (77%) | ||||
| IVT given at HFH or outside HFH | Yes | 26 (17%) | 19 (26%) | 0.112 | 16 (22%) | 9 (20%) | >.99 | 10 (13%) | 9 (35%) | 0.019 |
| No2 | 126 (83%) | 53 (74%) | 58 (78%) | 36 (80%) | 68 (87%) | 17 (65%) | ||||
| Reasons for not receiving IVT3 | Absolute exclusions4 | 15 (11%) | 8 (13%) | 0.075 | 4 (6%) | 2 (5%) | 0.626 | 11 (14%) | 6 (26%) | 0.106 |
| Rapid improvement | 10 (7%) | 0 (0%) | 3 (5%) | 0 (0%) | 7 (9%) | 0 (0%) | ||||
| Delay in patient arrival | 108 (77%) | 52 (85%) | 51 (82%) | 36 (95%) | 57 (72%) | 16 (70%) | ||||
| Stroke severity too mild | 6 (4%) | 0 (0%) | 2 (3%) | 0 (0%) | 4 (5%) | 0 (0%) | ||||
| Care team unable to determine eligibility | 1 (1%) | 1 (2%) | 1 (2%) | 0 (0%) | 0 (0%) | 1 (4%) | ||||
| Patient/family refused | 1 (1%) | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | ||||
| Time from Arrival to IVT Initiation (Minutes)5 | N Median (IQR) | 17 51 (45, 76) | 13 38 (34, 50) | 0.051 | 12 58.5 (44, 76.5) | 7 38 (29, 42) | 0.029 | 5 47 (45, 48) | 5 38 (38, 59) | 0.424 |
| Time from LKW to IVT initiation (Hours)5 | N Median (IQR) | 17 2 (1.67, 2.45) | 13 1.98 (1.27, 2.87) | 0.901 | 12 2.2 (1.8, 3) | 7 1.8 (1.2, 2.7) | 0.286 | 5 1.7 (1.5, 1.9) | 5 2 (2, 2.9) | 0.324 |
| MT information for eligible patients (African American=28 with 17 in 2019 and 11 in 2020; Non-African American=40 with 25 in 2019 and 15 in 2020) | ||||||||||
| MT received | Yes | 16 (38%) | 14 (52%) | 0.323 | 6 (35%) | 3 (27%) | >.99 | 10 (40%) | 11 (73%) | 0.055 |
| No | 26 (62%) | 13 (48%) | 11 (65%) | 8 (73%) | 15 (60%) | 4 (27%) | ||||
| Reasons for not receiving MT6 | NIHSS<6 | 12 (29%) | 7 (26%) | 0.821 | 5 (45%) | 4 (50%) | 0.592 | 7 (47%) | 2 (50%) | >0.99 |
| Established Stroke | 5 (12%) | 3 (11%) | 1 (9%) | 2 (25%) | 4 (27%) | 1 (25%) | ||||
| Other reason for no MT7 | 9 (21%) | 3 (11%) | 5 (45%) | 2 (25%) | 4 (27%) | 1 (25%) | ||||
| Time from Arrival to Arterial Puncture (Hours) | N Median (IQR) | 16 1.33 (0.79, 1.86) | 14 1.24 (0.63, 2) | 0.579 | 6 1.72 (1.27, 6.63) | 3 1.43 (1.28, 4.75) | 0.900 | 101.01 (0.7, 1.5) | 110.82 (0.33, 2) | 0.652 |
Excludes IVT given outside HFH and absolute exclusions.
Excludes absolute exclusions.
Only patients not receiving IVT.
One patient with elevated blood pressure despite treatment, 4 patients with recent intracranial or spinal surgery or significant head trauma, or prior stroke in previous 3 months, 5 patients with history of previous ICH, intracranial neoplasm, arteriovenous malformation, or aneurysm, 3 patients with active internal bleeding including presence of SDH or ICH and 10 patients with acute bleeding diathesis.
Only patients receiving IVT at HFH.
Only MT eligible patients not receiving MT.
Three patients with brain hemorrhage, 2 patients with anatomical exclusion, 3 patients with chronic occlusion, 2 patients with stenosis no occlusion, 1 patient with non-occlusive thrombus and 1 patient with significant pre-disability.
In AAs, there was a significant shift toward shorter times from ED arrival to IVT initiation in 2020 compared to 2019 (median: 38 vs 58.5 min, p =0.029). In the non-AA patients, patients seen in 2020 had higher IVT administration rates at HFH (23% vs 7%, p =0.048), as well as at any hospital facility (35% vs 13%, p =0.019) (Table 3).
MT: For all patients, no differences were detected between the patients seen in 2019 and 2020 for MT rates, reasons for not receiving MT, and time from arrival to arterial puncture (Table 3). The same was true for AA patients. For the non-AA patients, there was a trend toward higher MT rates in 2020 vs 2019 (73% vs 40%, p =0.055) (Table 3).
Discussion
In the US, Michigan experienced the first surge of COVID-19 cases in the early days of the pandemic, from March 10 to May 2020.11 Wayne County (Detroit) accounted for most of the cases in the state and was in the top 5 most affected counties early in the pandemic.12 , 13 HFHS and its 6 hospitals across southeast and central Michigan adopted a system-wide amendment of its Emergency Care and Admission of patients with AIS/TIA policy. The policy addressed the following key points: (1) increase the response to stroke alerts via telephone or video consults, (2) limit the number of transfers from outside facilities, (3) provide guidance for the management and triaging of patients with AIS/TIA for safe discharge from the ED, and (4) intubate all patients requiring MT in an effort to reduce the risk of aerosolization. The policy went into effect on March 20 and was still active on May 20, last day of patient inclusion into the study.
Our study echoes several others’ findings linked to the pandemic's impact on AIS/TIA and ICH/SAH care. First, consistent with prior reports,14 we found that the rates of STA in our EDs dramatically decreased during the pandemic by 54%. Reports around the world vary in the magnitude of this effect based on geographical location and timing of reporting: 9–40% in France,9 , 15 23–28% in Spain,4 24–31% in Scandinavia,16 25% in California,17 32% in neighboring Ontario (Canada),18 40% in China,5 , 14 and 30–46% in New York.19 These observations are a direct consequence of the implementation of stay-at-home orders and lockdowns enforced by local and federal governments.20 Compared to New England which experienced the surge in COVID-19 cases at around the same time as Michigan, our health system, however, registered much higher declines in stroke presentations and admissions for reasons that are not entirely clear. Second, transfers from outside facilities into HFHS were found to be significantly reduced, congruent with the tier 1 policy delineated above and in line with other reports. Similarly, both IVT and MT rates declined during the COVID-19 period, as has been previously described.20, 21, 22
While confirming previously reported findings, our study distinguishes itself in the fact that our health system serves a large AA population. For instance, while we found that times from LKW to ED presentation were significantly increased during the pandemic, when dichotomized on the basis of race, this finding held true only for AAs (10.4 vs 5.2 h). In fact, delayed presentation was more frequently a reason to exclude AAs than other groups from receiving IVT in 2020. Nonetheless, the rate of IVT administration did not differ between the two groups in 2020. The only other report we found in the literature addressing racial disparity in stroke presentation during the pandemic is a study out of South Carolina that reported lower rates of AAs seeking medical care for AIS compared to the prior year (13.9% vs 29%, P ≤0.002).23 While our data does not support this observation (i.e., the percentage of AAs presenting for stroke symptoms to the ED pre-COVID and during the pandemic were the same), it does however show that AAs had on average higher NIHSS on admission and discharge during the pandemic compared to the year prior. A likely explanation for this finding is that AAs were either reluctant to seek medical attention early but eventually did when symptoms were not improving, or patients were more frequently by themselves and unattended due to the observation of social distancing. This may have contributed to the poorer outcomes of AAs compared to other groups in 2020. This disparity can find its root in the fact that, early in the pandemic, it became widely recognized that AAs were disproportionately more vulnerable to COVID-19’s complications and endured higher mortality rates than other races, presumably due to more severe comorbidities, lower socioeconomic status, and decreased access to healthcare.24, 25, 26
Despite the longer times to ED arrival in 2020, metrics related to processes occurring within the ED, such as times from arrival to STA, were remarkably improved and had no racial differential. Additionally, average DTN for IVT administration was faster, particularly among AAs (38 vs 58.5 min), despite the requirement to don and doff PPE for all patients, regardless of their COVID-19 status. Similarly and perhaps counterintuitively, the requirement to intubate all patients undergoing MT during the pandemic did not appear to lengthen the door-to-arterial puncture time. This is in stark contrast with previous data reporting either no significant difference or longer treatment times for either or both IVT and MT.27, 28, 29 Taken together, these findings illustrate the greater efficiency of our ED, stroke, neurointerventional and anesthesia teams in triaging and managing AIS during the pandemic. This can be explained in part by the unusually small numbers of stroke patients presenting to two busy centers that ordinarily manage multiple stroke codes simultaneously. A likely explanation is the significantly lower rates of stroke mimics (17% vs 37%) and minor/rapidly improving strokes seen during the pandemic (0% vs 7%), thereby offloading the ED. The reluctance of patients to seek care in EDs for mild symptomatology during COVID has been described in prior studies.16 It is worth adding that the lower incidence of stroke mimics may account for the artificially increased rates of other diagnoses such as AIS, ICH and SAH in 2020.
Our study has several limitations. First, a number of data points could not be obtained from charts, thereby potentially diluting the effects of race and time period on some of the variables such as time metrics, NIHSS or mRS. Most of the unavailable data comes from incomplete records from outside facilities transferring patients to our hospitals. We believe that additional and more robust associations may have been possible had the dataset been more complete. Second, reasons for delayed presentations to the hospital were usually not documented, making them subject to interpretation. We believe, however, that these interpretations are sound, logical and legitimate. Third, having 2 comparison periods (COVID and Pre-COVID periods) did not allow us to account for the temporal trends over a year. Fourth, our data is confined to a single, albeit large, health system in Southeast Michigan and may therefore not be representative of other areas across the state or the country, limiting therefore the generalizability of our data. Nonetheless, we believe that the large AA population the data is derived from can be utilized to understand the impact of the pandemic on stroke care in other large urban areas across the US with a similar racial and ethnic makeup. Fifth, we chose not to include teleneurology data since this novel technology was only rolled out during the pandemic and did not make up a significant proportion of stroke code evaluations. We also decided not to include in-house SAs in our analysis in order not to introduce bias since we only keep records of those SAs that result in true ischemic or hemorrhagic strokes. Lastly, although we found a statistically significant increase in mortality in non-AAs between 2019 and 2020 (1% vs 12%, p =0.036), we believe that this increase was unlikely to be related to COVID-19 since only one patient of those who died in 2020 tested positive for the virus. The cause for this observation remains unclear to us. The small sample size may have played a role and statistical significance may not hold in a larger sample.
Assessing data from outpatient visits of patients whose strokes occurred during the pandemic but chose not to seek medical attention at the time, understanding the reasons that motivated patients to not seek care early, and expanding data collection to other centers in the area would be useful to refine our understanding of the multi-layered impact COVID-19 has had on our specific stroke patient population. This knowledge may be instrumental in preparing for future waves of COVID-19 infections.
Conclusion
Our study demonstrates that the COVID-19 pandemic has caused a reduction in the number of patients seeking medical attention for stroke symptoms and in those receiving acute therapies such as IVT and MT. It has also resulted in fewer stroke mimics and minor/rapidly improving strokes presenting to the ED. Furthermore, ED processes were markedly improved during the pandemic such as time from patient arrival to stroke code activation, imaging and IVT but it did not significantly alter MT metrics. Finally, among our predominantly AA population, patients’ ED presentation was significantly delayed. They also had more severe strokes and more disability on discharge.
Grant support
None.
Source of funding
None.
Declaration of Competing Interest
None.
Acknowledgments
Acknowledgments
None.
References
- 1.Markus H.S., Brainin M. COVID-19, and stroke—a global World Stroke Organization perspective. Int J Stroke. 2020;15(4):361–364. doi: 10.1177/1747493020923472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leira E.C., Russman A.N., Biller J., et al. Preserving stroke care during the COVID-19 pandemic: potential issues and solutions. Neurology. 2020;95(3):124–133. doi: 10.1212/WNL.0000000000009713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao J., Rudd A., Liu R. Challenges and potential solutions of stroke care during the coronavirus disease 2019 (COVID-19) outbreak. Stroke. 2020;51(5):1356–1357. doi: 10.1161/STROKEAHA.120.029701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudilosso S., Laredo C., Vera V., et al. Acute stroke care is at risk in the era of COVID-19: experience at a Comprehensive Stroke Center in Barcelona. Stroke. 2020;51(7):1991–1995. doi: 10.1161/STROKEAHA.120.030329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao J., Li H., Kung D., Fisher M., Shen Y., Liu R. Impact of the COVID-19 epidemic on Stroke Care and potential solutions. Stroke. 2020;51(7):1996–2001. doi: 10.1161/STROKEAHA.120.030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baracchini C., Pieroni A., Viaro F., et al. Acute stroke management pathway during Coronavirus-19 pandemic. Neurol Sci. 2020;41:1003–1005. doi: 10.1007/s10072-020-04375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morelli N., Rota E., Terracciano C., et al. The baffling case of ischemic stroke disappearance from the casualty department in the COVID-19 era. Eur Neurol. 2020:1–3. doi: 10.1159/000507666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brainin M. Stroke care and the COVID19 pandemic words from our President, www.world-stroke.org/news-andblog/news/stroke-care-and-the-covid19-pandemic
- 9.Pop R., Quenardelle V., Hasiu A., et al. Impact of the COVID-19 outbreak on acute stroke pathways – insights from the Alsace region in France. Eur J Neurol. 2020;27(9):1783–1787. doi: 10.1111/ene.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams H.P., Bendixen B.H., Kappelle L.J., Biller J., Love B.B., Gordon D.L., Marsh E.E. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. Jr. [DOI] [PubMed] [Google Scholar]
- 11.Haddad K. Michigan coronavirus timeline: key dates, COVID-19 case tracking, state orders. WDIV. https://www.clickondetroit.com/health/2020/03/24/michigan-coronavirus-timeline-key-dates-covid-19-case-tracking-state-orders/. Published 2020. Accessed September 22, 2020.
- 12.COVID-19 Map - Johns Hopkins Coronavirus Resource Center. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. Published 2020. Accessed September 22, 2020.
- 13.SA. Wayne County's 1,148 COVID deaths are 5th among U.S. counties; state cases rise just 1.8%. Deadlinedetroit.com. https://www.deadlinedetroit.com/articles/25027/wayne_county_s_1_148_covid_deaths_are_5th_among_u_s_counties_state_cases_rise_just_1_8. Published 2020. Accessed September 22, 2020.
- 14.Aguiar de Sousa D., Sandset E.C., Elkind M.S.V. The curious case of the missing strokes during the COVID-19 pandemic. Stroke. 2020;51(7):1921–1923. doi: 10.1161/STROKEAHA.120.030792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plumereau C., Cho T.H., Buisson M., et al. Effect of the COVID-19 pandemic on acute stroke reperfusion therapy: data from the Lyon Stroke Center Network [published online ahead of print, 2020 Sep 9] J Neurol. 2020 doi: 10.1007/s00415-020-10199-6. 10.1007/s00415-020-10199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butt J.H., Fosbøl E.L., Østergaard L., et al. Effect of COVID-19 on first-time acute stroke and transient ischemic attack admission rates and prognosis in Denmark: a Nationwide cohort study. Circulation. 2020;142(12):1227–1229. doi: 10.1161/CIRCULATIONAHA.120.050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen-Huynh M.N., Tang X.N., Vinson D.R., et al. Acute stroke presentation, care, and outcomes in community hospitals in Northern California during the COVID-19 pandemic. Stroke. 2020 doi: 10.1161/STROKEAHA.120.031099. [published online ahead of print, 2020 Aug 7] STROKEAHA120031099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowlatshahi D., Stotts G., Bourgoin A., et al. Decreased stroke presentation rates at a Comprehensive Stroke Center during COVID-19. Can J Neurol Sci. 2020:1–12. doi: 10.1017/cjn.2020.193. [published online ahead of print, 2020 Sep 3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esenwa C., Parides M.K., Labovitz D.L. The effect of COVID-19 on stroke hospitalizations in New York City. J Stroke Cerebrovasc Dis. 2020;29(10) doi: 10.1016/j.jstrokecerebrovasdis.2020.105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlachetzki F., Theek C., Hubert N.D., et al. Low stroke incidence in the TEMPiS telestroke network during COVID-19 pandemic - effect of lockdown on thrombolysis and thrombectomy. J Telemed Telecare. 2020 doi: 10.1177/1357633X20943327. [published online ahead of print, 2020 Aug 18] 1357633X20943327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qureshi A.I., Siddiq F., French B.R., et al. Effect of COVID-19 pandemic on mechanical thrombectomy for acute ischemic stroke treatment in the United States. J Stroke Cerebrovasc Dis. 2020;29(10) doi: 10.1016/j.jstrokecerebrovasdis.2020.105140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajdu S.D., Pittet V., Puccinelli F., et al. Acute stroke management during the COVID-19 pandemic: does confinement impact eligibility for endovascular therapy? Stroke. 2020;51(8):2593–2596. doi: 10.1161/STROKEAHA.120.030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cummings C., Almallouhi E., Al Kasab S., Spiotta A.M., Holmstedt C.A. Blacks are less likely to present with strokes during the COVID-19 pandemic: observations from the buckle of the stroke belt. Stroke. 2020 doi: 10.1161/STROKEAHA.120.031121. [published online ahead of print, 2020 Aug 5] STROKEAHA120031121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abedi V., Olulana O., Avula V., et al. Economic, and health inequality and COVID-19 infection in the United States. J Racial Ethn Health Dispar. 2020:1–11. doi: 10.1007/s40615-020-00833-4. [published online ahead of print, 2020 Sep 1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vahidy F.S., Nicolas J.C., Meeks J.R., et al. Racial and ethnic disparities in SARS-CoV-2 pandemic: analysis of a COVID-19 observational registry for a diverse US metropolitan population. BMJ Open. 2020;10(8) doi: 10.1136/bmjopen-2020-039849. e039849. Published 2020 Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holtgrave D.R., Barranco M.A., Tesoriero J.M., Blog D.S., Rosenberg E.S. Assessing racial and ethnic disparities using a COVID-19 outcomes continuum for New York State. Ann Epidemiol. 2020;48:9–14. doi: 10.1016/j.annepidem.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neves Briard J., Ducroux C., Jacquin G., et al. Early impact of the COVID-19 pandemic on acute stroke treatment delays. Can J Neurol Sci. 2020:1–15. doi: 10.1017/cjn.2020.160. [published online ahead of print, 2020 Jul 23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rinkel L.A., Prick J.C.M., Slot R.E.R., et al. Impact of the COVID-19 outbreak on acute stroke care. J Neurol. 2020:1–6. doi: 10.1007/s00415-020-10069-1. [published online ahead of print, 2020 Jul 20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal S., Scher E., Rossan-Raghunath N., et al. Acute stroke care in a New York City comprehensive stroke center during the COVID-19 pandemic. J Stroke Cerebrovasc Dis. 2020;29(9) doi: 10.1016/j.jstrokecerebrovasdis.2020.105068. [DOI] [PMC free article] [PubMed] [Google Scholar]