Abstract
Introduction
Evidence indicate that adiponectin may exert pro-inflammatory effects on inflammatory cells. We have found that adiponectin knockout decreased inflammatory reaction and tubular damage in the ischemia-reperfusion kidney in APN-knockout mice. Globular adiponectin and full-length adiponectin (g-APN and f-APN) were used in this study to investigate the effects of adiponectin on proinflammatory cytokines production and migration in Raw 264.7 cells.
Methods
Proinflammatory cytokines production was detected by real-time RT-PCR. NF-kappaB activation was interrupted through Ad-DN-IκBα or SN-50 to confirm how g-APN induces proinflammatory cytokines production. The siRNA against AdipoR1 and AdipoR2 was investigated to uncover the signaling pathway that may involve in NF-kappaB activation and migration in Raw 264.7 cells.
Results
g-APN, not f-APN, was found triggering the production of inflammatory cytokine MIP-2, IL-6, TNFα, and MCP-1 in Raw 264.7 cells. NF-kappaB Inhibition by Ad-DN-IκBα expression or cell-permeable NF-κB inhibitor SN-50 could decrease NF-kappaB nuclear translocation and subsequently decrease inflammatory cytokine expression triggered by globular ANP. However, AdipoR1 and AdipoR2 were not found involved in NF-kappaB activation in this study. Full-length APN, not g-APN, was involved in the promotion of macrophage migration. The migration induced by full-length APN could be inhibited by knockdown of AdipoR1 expression with siRNA. The migration effect could also be inhibited by PI3Kγ inhibitor, AS-605240.
Discussion
These results suggested that full-length adiponectin increases macrophage migration through Adiponectin-AdipoR1-PI3Kgamma signaling pathway. However, NF-κB activation induced by g-APN in this study was independent of AdipoR1 or AdipoR2. The exact signaling pathway of NF-κB activation by adiponectin should be further studied to find a new anti-inflammatory target.
Keywords: adiponectin, macrophages, inflammation, migration, cytokine
Introduction
Adiponectin is well known as a beneficial adipokine that could increase insulin sensitization and display anti-inflammatory, anti-atherogenic, and anti-diabetic effects.1–3 The metabolism effects may involve in the activation of the cell surface receptors, AdipoR1 and AdipoR2, to enhance oxidation of fatty acid and uptake of glucose in muscle and decrease gluconeogenesis in the liver.4–6 Adiponectin’s anti-atherogenic effects are associated with its anti-inflammation.7,8 Piccio et al reported that adiponectin deficient mice displayed worse myelin-immunized inflammation and damage in the central nerve system than wild-type mice. Another study showed the multiple sclerosis model could be improved by globular adiponectin treatment.9 Adiponectin administration increased expression of anti-inflammatory factor eNOS and IL-10, reduced mRNA expression of pro-inflammatory cytokines, such as TNFα, vascular cell adhesion molecule-1 (VCAM-1), and IL-6.2 It was reported that adiponectin could effectively inhibit NF-κB activation including translocation of NF-κB from the cytoplasm to nucleus.1 Furthermore, it was found that adiponectin enhanced the clearance of apoptotic debris to decrease inflammation.10 Recent studies also demonstrated that APN decreased macrophage transformation from M1 to M2 to reduce inflammation reaction.11,12
However, several studies demonstrated that adiponectin may play pro-inflammatory roles in the patients who have autoimmune diseases.1,2,13–15 In the patients with chronic kidney disease, rheumatoid arthritis, and inflammatory bowel disease, the increased adiponectin serum level was accompanied by worse inflammation severity and pathological progression.2,16,17 From an in vitro experiment, T cells incubated with adiponectin could increase production of interferon gamma and IL-6 through activation of the p38-STAT4-T-bet signaling pathway, and adiponectin treatment promoted a pro-inflammatory response in macrophage.13 We had also discovered that APN participated in kidney damage after ischemia and reperfusion by enhancing the infiltration and activation of inflammatory cells.18 We found that adiponectin increased the pro-inflammatory factor by activation of the inflammatory cells through NF-κB during kidney ischemia and reperfusion damage. And adiponectin increased the migration of macrophages by activation of PI3K. Therefore, adiponectin may have both pro-inflammatory and anti-inflammatory effects.
Some papers contributed the dual effects of adiponectin on inflammation to its different isoforms or concentrations or timing effects.19–22 Adiponectin isoforms including different oligomer isoforms (containing three major isoforms: LMW trimer, MMW hexamer, and HMW), full-length, and proteolytic production-globular adiponectin.1 The full-length APN was found to decrease inflammation reaction in macrophage, but globular adiponectin showed increasing inflammation reaction in macrophage by activation of NF-κB.1,23–25
At present, it is still unknown how full-length adiponectin and globular adiponectin affect migration and inflammatory reaction in inflammatory cells. So, we used full-length and globular adiponectin (f-APN and g-APN) to investigate how adiponectin affects the production of cytokines and migration in macrophage, the center point in the innate immune system. The mRNA expression of pro-inflammation cytokines and migration will be measured in vitro in Raw cells to uncover the underlying mechanism involving in the AdipoR1 and AdipoR2.
Materials and Methods
Cell Culture
Mouse macrophage Raw 264.7 cells were obtained commercially from the American Type Culture Collection and cultured in a 75-cm2 tissue culture flask in Dulbecco’s Modified Eagle’s medium (DMEM) (GibcoBRL, USA) containing 10% fetal bovine serum (FBS, Invitrogen, USA) and 100 Units/mL penicillin G sodium and 100 mg/mL streptomycin sulfate. Raw 264.7 cells were used for treatment or transfer for culture until growing to 80% confluence.
Adiponectin Treatment
Both full-length and globular adiponectin were purchased from R&D Systems (Minneapolis, MN, USA). Cell suspension was ready by a concentration of 1×105 cells/mL in culture medium contained 10% FBS. 1 mL cell suspension was added to each well of 12-well plate. The Raw cells were ready for study until confluence to 70%. The Raw cells were starved with 1% FBS medium for 12 hours before treatment. Then, full-length or global Adiponectin was added in the corresponding well and incubate for desired time.
Quantitative Real-Time Reverse Transcriptase PCR
Total RNA from the cells was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA). The RNA (1 μg) from each sample was reverse transcribed with SuperScript II reverse transcriptase. IQ SYBR green supermix reagent (Bio-Rad, Hercules, CA) was used to run Realtime PCR with a Bio-Rad real-time PCR. 2−DDCt was calculated to quantify gene expression. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) level was used to normalize the expression levels of the target genes. The primer sequences were listed here (Table 1).
Table 1.
The Primers of the Target Genes
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| Adiponectin | 5ʹ-GCAGAGATGGCACTCCTGGA-3’ | 5ʹ-CCCTTCAGCTCCTGTCATTCC-3’ |
| IL-6 | 5ʹ-AGGATACCACTCCCAACAGACCTG-3’ | 5ʹ-CTGCAAGTGCATCATCGTTGTTCA-3’ |
| TNFa | 5ʹ-CATGAGCACAGAAAGCATGATCCG-3’ | 5ʹ-AAGCAGGAATGAGAAGAGGCTGAG-3’ |
| MCP-1 | 5ʹ-TCACCTGCTGCTACTCATTCACCA-3’ | 5ʹ-TACAGCTTCTTTGGGACACCTGCT-3’ |
| MIP-2 | 5ʹ-AAAGTTTGCCTTGACCCTGAAGCC-3’ | 5ʹ-TCCAGGTCAGTTAGCCTTGCCTTT-3’ |
| GAPDH | 5ʹ-CCAATGTGTCCGTCGCGTGGATCT-3’ | 5ʹ-GTTGAAGTCGCAGGAGACAACC-3’ |
Immunohistochemistry
Immunohistochemical staining was done on cells. At first, the slides were blocked with 5% normal serum, and then the primary antibodies were incubated with the slides overnight. After washing three times, slides were incubated with the secondary antibodies and ABC solution using the ABC kit (Vector Laboratories). Slides were then stained with a diaminobenzidine solution. Nuclei were stained by hematoxylin. The images from these slides were analyzed by NIS Element software (Nikon Instruments, Melville, NY) in the Nikon microscope image system (Nikon Instruments).
Protein Extraction from Nuclear and Cytoplasm
Buffer A (10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, 0.05% NP40 (or 0.05% Igepal or Tergitol) pH 7.9) 500 μL containing the cocktail of usual inhibitors was combined with the Raw cell dish on ice. Then, the cells lysis was put on ice for 10 min. The cell lysis was centrifuged at 4°C by 3000 rpm for 10 min. The supernatant of the cell lysis was sucked as cytoplasm extraction and the precipitation was kept as nuclear extraction for further study (which did not have large plasma membrane pieces, DNA, nucleoli). The precipitation was resuspended in 374 μL of buffer B (5 mM HEPES, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 26% glycerol (v/v), pH 7.9) and 26 μL of 4.6 M NaCl to give 300mM NaCl (high salt helps lyse membranes and forces DNA into solution). The Homogenized sample was put on ice for 30 min. The sample was Centrifuged at 24,000 g for 20 min at 4°C. The supernatant of the sample containing nuclear protein was aliquoted for the next study. The supernatant 10 μL was used for Bradford assay and stored at −70°C.
Cell-Permeable NF-Kappa B Inhibitor SN-50 Treatment
Raw cells with a concentration of 1×106/L was seeded on a 6-well plate and incubated in a culture incubator until 70% confluence. Raw cells were starved for 12 hours before the treatment. SN-50 or SN-50M (used as a control) was added to each well by a final concentration of 25 μg/mL. And Raw cells were incubated for 20–30 min. Next, g-APN was added by a final concentration of 5 μg/mL and incubated with the Raw cells for 6 hours. The medium was removed and Trazol was added to extract total RNA .
Transfection of Raw Cells with Adenovirus
Raw cells with a concentration of 1×106/L were seeded on a 6-well plate and incubated in a culture incubator until 70% confluence. The vector was thawed on ice and kept on ice during the duration of the experiment. The culture medium was replaced with a 1 mL medium containing 1% FBS, and then the virus was added by an MOI of 50. The media was replaced after 8–12 hours infection with a new medium with 1% FBS. The transfected Raw cells were continuing to culture another 24 h until 80% transfection rate through watching the fluorescence GFP. And then full-long APN or g-APN was added to the wells at the final concentration of 5μg for 6 hours. Finally, the cells were harvested for extraction of total RNA for RT and Real-time PCR.
Western Blot Analysis
Proteins were extracted using RIPA buffer containing cocktail proteinase inhibitors. The protein concentration was quantified with a Bio-Rad protein assay. The same amounts of protein were run on SDS-polyacrylamide gels in Tris/SDS buffer system, and then transferred into nitrocellulose membranes. The membranes and the primary antibodies were incubated together overnight, and then incubated with a fluorescence-conjugated secondary antibody. The proteins expression was analyzed using an Odyssey (LI-COR Bioscience, Lincoln, NE) IR scanner, and signal intensities of the protein expression were quantified using NIH Image/J software (National Institutes of Health, Bethesda, MD).
Transfection of SiRNA
For each transfection, siRNA 3 μL was diluted into 100 μL siRNA Transfection Medium (sc-36868). And then siRNA Transfection Reagent 6 μL (sc-29528) was added into 100 μL siRNA Transfection Medium. The combined solution in the tube was mixed gently and incubated for 35 minutes at room temperature. 0.8 mL siRNA Transfection Medium was added to each tube containing the siRNA. Finally, the transfection reagent mixture was prepared for transfection.
The Raw cells in a 6-well plate with 70% confluence were washed one time using 2 mL of siRNA transfection medium (sc-36868). And then the medium was aspirated immediately for the next step. For each well, a 1-mL transfection reagent mixture was added for transfection. The cells were incubated for 5–7 hours at 37°C in a CO2 incubator. Next, a normal growth medium 1 mL containing 20% FBS and antibiotics were put into each well. If there is a toxicity sign showing in the Raw cells, the transfection mixture can be removed and replaced with a normal growth medium. The cells will be incubated for additional 18–24 hours for the next experiment.
Migration Assay
The migration assay was detected by CytoSelect™ 96-Well Cell Migration Assay (3 µm, Fluorometric Format) (Cell Biolabs, Inc. San Diego, USA). A cell suspension containing 1 x 106 cells/mL in DMEM with 0.1% BSA were prepared for the membrane chamber. The inhibitor was added directly to the cell suspension. The membrane chamber was placed back into the feeder tray containing adiponectin and incubated for 6 hours. The cells migrated from the top side of the membrane chamber were lysed using cell detachment solution. The detachment solution was incubated with Lysis Buffer/CyQuant® GR dye solution. The fluorescence was read with a fluorescence plate reader at 485 nm/520 nm.
Statistical Analysis
Data presented in this study are described as mean ± SEM. Group analysis was performed using one-way ANOVA followed by Turkey’s post hoc test. P < 0.05 was considered as significant difference.
Results
g-APN, Not f-APN, Increased Pro-Inflammatory Cytokines Expression in Macrophages
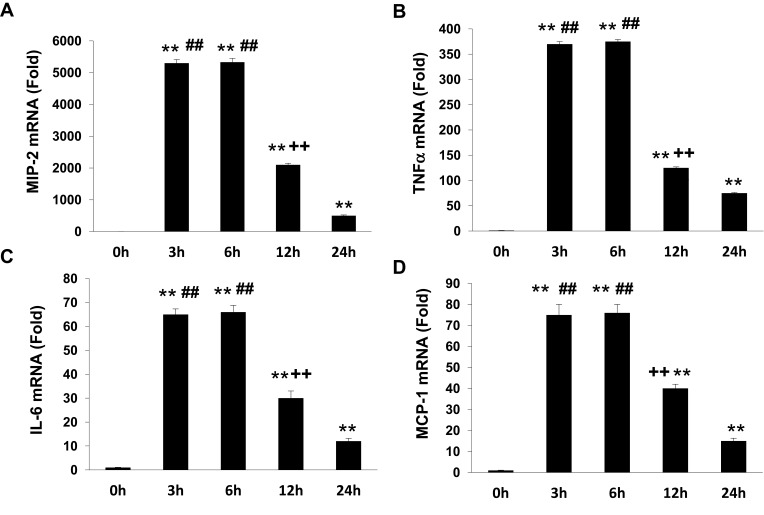
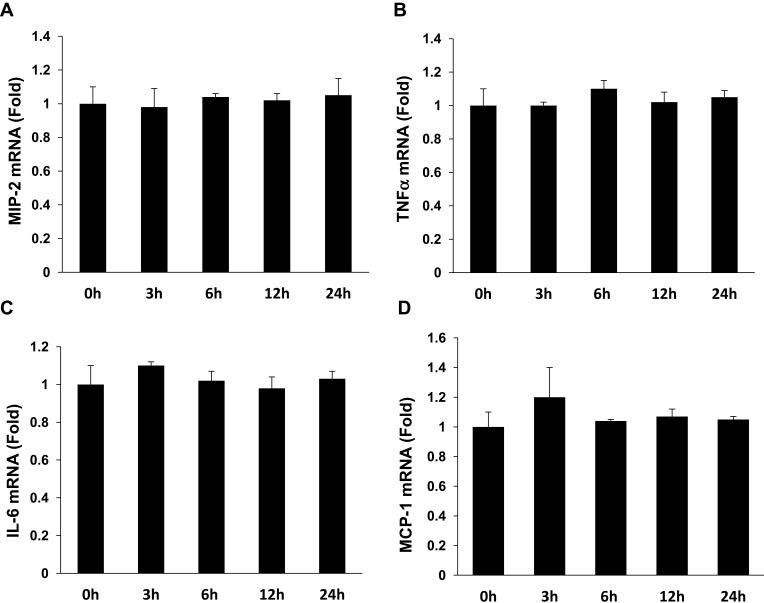
To determine if APN induces pro-inflammatory cytokine production, Raw 264.7 cells were incubated with g-APN or f-APN at 5μg/mL for 0, 3, 6, 12 and 24 hours. The real-time RT-PCR results showed that g-APN significantly increased mRNA expression of MIP-2, TNFα, IL-6, and MCP-1 (Figure 1A–D). But f-APN showed no effect on the production of these pro-inflammatory cytokines (Figure 2A–D).
Figure 1.
G-APN induced the mRNA expression inflammatory cytokines MIP-2, TNFα, MCP-1, and IL-6in Raw 264.7 cells (A-D). **Compared with 0 h, P<0.01; ##Compared with 24 h, P<0.01; ++Compared to 3 and 6 h, P<0.01, each group n=6.
Figure 2.
F-APN showed no effect on the production of inflammatory cytokine MIP-2, TNFα, MCP-1, and IL-6 (A–D) in Raw 264.7 cells, each group n=6.
g-APN Promoted NF-κB Nuclear Translocation in Macrophages
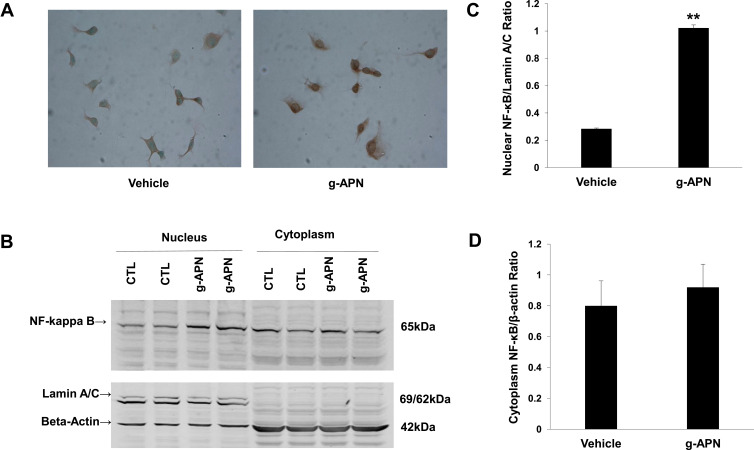
We explored the possible mechanisms through which g-APN increases the production of inflammatory cytokines. Because NF-κB plays as a critic regulator on inflammation, we try to figure out if NF-κB mediates the effect of g-APN on inflammatory cytokine production. Immunohistochemistry results revealed that g-APN enhanced NF-κB (p65) translocation from cytoplasm to nucleus in Raw 264.7 cells (Figure 3A). Western Blot results showed that g-APN increased NF-kappa B (P65) presentation in the nucleus (Lamin A/C, nuclear mark) (Figure 3B and C). There is no difference in NF-kappa B expression in the cytoplasm between vehicle and g-APN (Figure 3B and D). These data suggested that g-APN could promote NF-kappa B translocation from cytoplasm to nucleus and then activate NF-κB.
Figure 3.
G-APN enhanced NF-kappa B nuclear translocation of in Raw 246.7 cells. A, the representative figures indicated the expression of NF-kappa B P65 in the Raw 246.7 treated by 5 μg/mL g-APN for 30 min. B, Western blot results show NF-kappa B in nuclear (Lamin A/C, Nuclear Mark as internal control) and cytoplasm (beta-actin as internal control in cytoplasm) in Raw 246.7 cells treated with g-APN. C, group analysis of the NF-kappa B expression in the nucleus. D, group analysis of the NF-kappa B expression in the cytoplasm. **Compared to vehicle, P<0.01, each group n=6.
Interruption of NF-Kappa B Activation Decreased the g-APN-Induced NF-κB Translocation and Pro-Inflammatory Cytokines Production in Raw 264.7 Cells
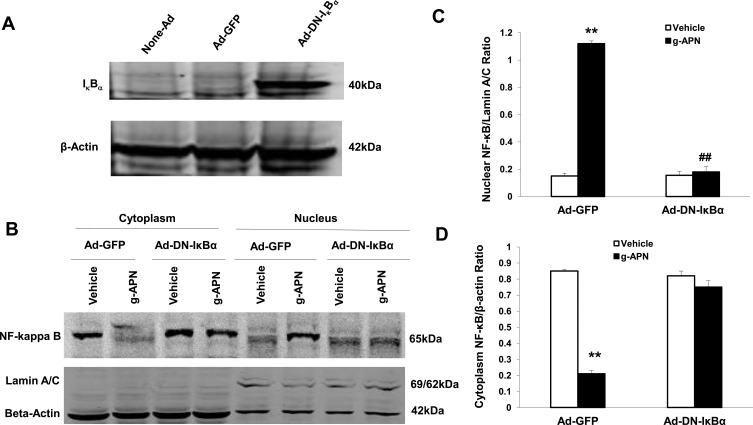
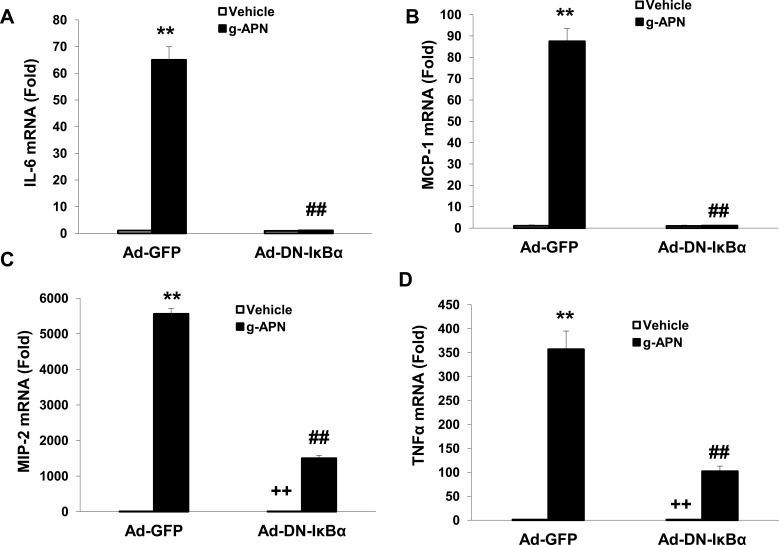
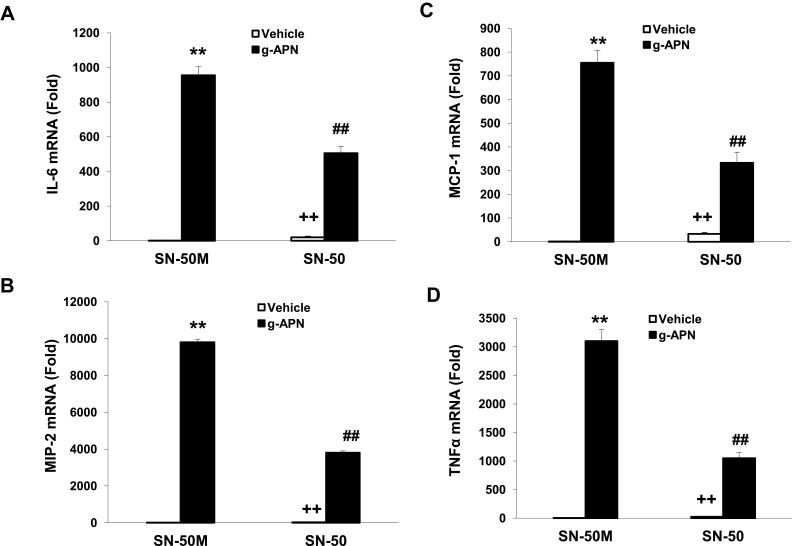
To confirm that the NF-κB is involved in the effects of g-APN on Raw 264.7 cells, the adenovirus carrying domain negative IκBα (Ad-DN-IκBα) was used to block the nuclear translocation of NF-κB. Figure 4A indicates that Ad-DN-IκBα was successfully introduced into the Raw 264.7 cells. Western Blot results showed the NF-κB translocation from cytoplasm to nucleus was blocked after the transfection (Figure 4B–D). The pro-inflammatory cytokines productions were also blocked after transfection of Ad-DN-IκBα into Raw cells (Figure 5A–D). NF-κB inhibitor SN-50 was also used to decrease NF-kappa B activity in Raw cells. The productions of IL-6, TNFα, MIP-2, and MCP-1 were also decreased, but not blocked completely, after SN-50 administration (Figure 6A–D). The results demonstrated that g-APN induced production of the pro-inflammatory cytokines through NF-κB activation in Raw 264.7 cells.
Figure 4.
Ad-DN-IκBα blocked g-APN-induced NF-kappa B activation in Raw 264.7 cells. A showed Ad-DN- IκBα was successfully infected into the cells. B, Western Blot showed that Ad-DN-IκBα inhibited NF-kappa B activation which triggered by g-APN. C. Group analysis indicated that the quantity of NF-kappa B presented in nucleus. D, Group analysis indicated that the quantity of NF-kappa B presented in cytoplasm. **Compared with vehicle in Ad-GFP cells, P<0.01; ##Compared with vehicle in Ad-DN-IκBα cells; each group n=6.
Figure 5.
Ad-DN- IκBα decreased the expression of IL-6, MCP-1, MIP-2 and TNFα (A–D) in presence of g-APN in Raw 264.7 cells. **Compared with vehicle in Ad-GFP cells, P<0.01; ##Compared with vehicle in Ad-DN-IκBα cells; ++Compared with g-APN in Ad-DN-IκBα cells, P<0.01, each group n=6.
Figure 6.
SN-50 inhibited g-APN-induced cytokines production in Raw 264.7 cells. Figures showed that SN-50 inhibited the expression of IL-6, MIP-2, MCP-1, and TNFα (A–D) in the presence of g-APN in Raw 264.7 cells. **Compared with vehicle in the presence of SN-50M, P<0.01; ##Compared with vehicle in the presence of SN-50M; ++Compared with g-APN in the presence of SN-50, P<0.01, each group n=6.
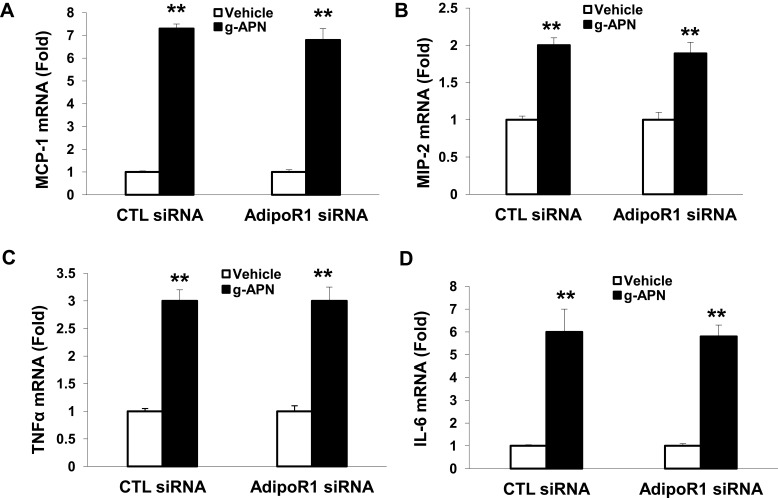
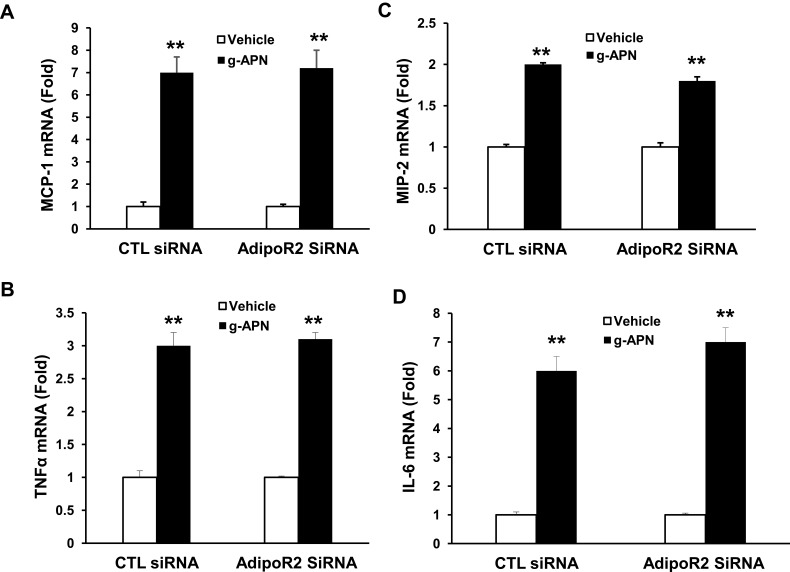
g-APN Induced Cytokines Production without Depending on the AdipoR1 and AdipoR2 in Raw 264.7 Cells
To determine if g-APN acted on AdipoR1 or AdipoR2 to induce production of pro-inflammatory cytokines in vitro, the siRNA specific to AdipoR1 or AdipoR2 was used to knock down expression of AdipoR1 or R2 on the membrane of Raw 264.7 cells. Knock-down AdipoR1 or R2 did not affect the expression of IL-6, MIP-2, TNFα and MCP-1 mRNA after g-APN treatment (Figures 7 and 8).
Figure 7.
Knock-down AdipoR1 did not affected the expression of cytokines in presence of g-APN. Knockdown of AdipoR1 had no effect on the expression of MCP-1, MIP-2,TNFα, and IL-6(A–D) in presence of g-APN in Raw 264.7 cells. **Compared with vehicle of CTL siRNA and AdipoR1siRNA, P<0.01, each group n=6.
Figure 8.
Knock-down AdipoR2 had no effect on the expression of cytokines in presence of g-APN. Knockdown of AdipoR1 had no effect on the expression ofMCP-1, MIP-2, TNFα, and IL-6 (A–D) in presence of g-APN in Raw 264.7 cells. **Compared with vehicle of CTL siRNA and AdipoR2siRNA, P<0.01, each group n=6.
f-APN, Not g-APN, Promoted the Migration of Raw 264.7 Cells Through AdipoR1-PI3kγ
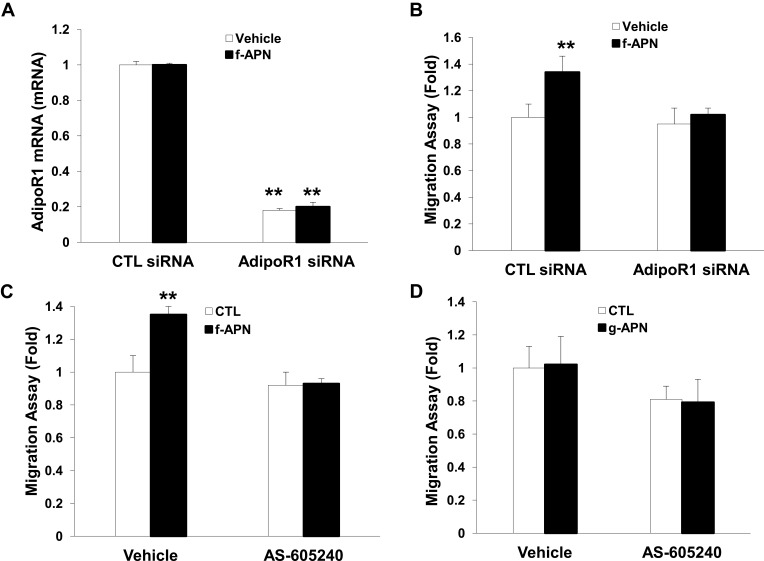
We have found that f-APN was incubated with Raw 264.7 cells for 6 hours and promoted their migration, which could be blocked by knocking down Adiponectin Receptor1 (Figure 9A–B). The PI3Kγ inhibitor, AS-605240, could block Raw 264.7 cells migration in presence of f-APN (Figure 9C). g-APN had no effect on Raw 264.7 cell migration (Figure 9D). These data suggested that f-APN induced macrophages migration through activation AdipoR1-PI3Kγ signaling pathway. Decreasing expression of AdipoR2 by siRNA has no effects on the f-APN-induced migration of Raw cells (Figure 10).
Figure 9.
F-APN-induced migration was mediated by AdipoR1-PI3Kgamma signaling pathway. A showed AdipoR1 mRNA was knocked down by the siRNA specific to AdipoR1. B indicated knocking down of AdipoR1 blocked the migration induced by f-APN. C showed that PI3Kgamma inhibitor AS-605240 blocked the migration in presence of f-APN. D indicated that g-APN had no effect on the migration in vitro. **Compared with vehicle of control (CTL) siRNA and AdipoR1 siRNA, P<0.01, each group n=6.
Figure 10.
Knock-down AdipoR2 had no effect on f-APN induced migration in Raw cells. A showed that AdipoR2 mRNA was decreased by siRNA. B showing knocking down of AdipoR2 had no effect on the migration in presence of f-APN in Raw 264.7 cells. **Compared with vehicle of control (CTL) siRNA and AdipoR2 siRNA, P<0.01 each group n=6.
Discussion
The aim of our study was to determine the effects of adiponectin on proinflammatory cytokine production and migration in Raw 264.7 cells and dissect out the underlying mechanisms. We have found that pro-inflammatory cytokine production decreased in the ischemia-reperfusion kidney in adiponectin-knockout mice.18 In this study, a mouse macrophage cell line, Raw 264.7 cell, was used to test the effects of adiponectin on proinflammatory cytokine production and migrtion. We found that pro-inflammatory cytokine mRNA expression, including IL-6, TNFα, MIP-2, and MCP-1, increased and reached a peak about 3–6 h after administration of globular APN. The pro-inflammatory effects lasted for 24 hours after incubating with globular APN for 3 h. However, full-length adiponectin showed no effect on inflammatory cytokine production in Raw 264.7 cells.
To study the mechanism of how g-APN stimulated inflammatory cytokines mRNA expression, NF-kappa B, the master regulator of inflammation, was chosen in this study. We found that NF-κB unit P65 was translocated from the cytoplasm to the nucleus in Raw cells after g-APN administration. Western Blot was used to detect the levels of P65 in nucleus and cytoplasm to confirm if the NF-κB was activated by g-APN. Nuclear protein extraction was confirmed by the nuclear protein marker Lamin A/C. Our results indicated that nuclear P65 increased significantly in the Raw cells after g-APN treatment. The results suggested that g-APN could activate NF-κB by translocation of NF-κB from the cytoplasm to the nucleus.
To further determine if NF-kappa B activation is - responsible for the expression of inflammatory cytokines, the adenovirus carrying domain negative IκBα (Ad-DN-IκBα) and NF-kappa B inhibitor SN-50 were used in our study. Raw cell was transfected by adenovirus carrying Ad-DN-IκBα or Ad-GFP. The protein expression level of DN-IκBα increased significantly comparing to Ad-GFP, which indicate that Ad-DN-IκBα was successfully transfected in Raw cells. Our results showed that NF-kappa B was accumulated in the cytoplasm and could not enter the nucleus after blockage of NF-κB translocation by Ad-DN-IκBα. Consequently, the inflammatory cytokine expression induced by g-APN was blocked after blockage of NF-κB activation. However, NF-κB inhibitor SN-50 could only partly inhibit g-APN-induced NF-κB activation. Our findings indicated that g-ANP stimulated pro-inflammatory cytokines expression in Raw cells through NF-κB activation.
Interestingly, we found that g-APN still activated NF-kappa B after interrupting AdipoR1 or AdipoR2 expression by siRNA. These results indicate that the effect of g-APN on proinflammatory cytokine production in Raw 264.7 macrophages is not mediated by AdipoR1 or AdipoR2. it is not clear how NF-kappa B was activated by g-APN in this study. According to the published studies, we speculate three possibilities. First, g-APN should still activate NF-κB through adipoR1 or AdipoR2. We might get a negative result because AdipoR1 and AdipoR2 were not decreased enough by the low-efficiency siRNA interruption to block the effect of g-APN on NF-kappaB activation in this study. Second, g-APN could activate NF-kappa B through T-cadherin other than AdipoR1 or AdipoR2.1 It was reported that cellular migration and proliferation were influenced by T-cadherin deficiency through inhibition of adiponectin.26,27 Third, g-APN could activate NF-kappaB through other signaling pathway. For example, acid-sensing ion channels and Na+/Ca2+ exchangers were reported involving in the activation of macrophages in a chronic low adiponectin concentration.21,29 Therefore, further studies should be done to figure out how NF-kappa B was activated by g-APN in Raw 264.7 cells.
Adiponectin is the richest adipokines produced by adipose tissue. It amounts to around 0.1% total serum proteins. Most adiponectin presents in the serum as an oligomer, including low molecular weight (LMW), medium molecular weight (MMW), and high molecular weight (HMW).3 Among them, HMW is considered as the most biologically active. Serum adiponectin concentration ranges widely in healthy adults. McEntegart et al had reported that the median of adiponectin in healthy adults was 9.8 μg/mL with a range from 2.5 to 16.8 μg/mL (9.8 (2.5–16.8)).30 However, the adiponectin concentration in the patients with coronary artery disease was 22.5 (10.2–37.2) μg/mL in heart failure and cachexia patients, 7.1 (0.5–16.6) μg/mL in the heart failure patients with no-cachexia, and 7.0 (0.4–10.7) in the heart failure patients under control.30 It suggested that high adiponectin concentration was coming with cachexia in coronary artery disease. Monda et al found that adiponectin concentration mean in people with obesity was 10.8 μg/mL with a standard deviation of 1.2 (10.8±1.2), which could be increased to 25.55±1.3 μg/mL by a very low-calorie ketogenic diet.3 From the above evidences, it is suggested that the functions of adiponectin are closely associated with its concentrations. Moreover, Negoro et al found that low adiponectin concentration is responsible for the systemic inflammation in adiponectin anti-sense transgenic mice.21 They found that low concentration adiponectin could facilitate the production of proinflammatory, such as IL-6 and TNFα from macrophage by increasing intracellular calcium concentration through expression of acid-sensing ion channels and Na+/Ca2+ exchangers. The dose of 5 μg/mL in this study may be a low concentration in mice according to the published evidence.22 A higher dose may get a totally different result in macrophage activation. So, one dose of g-APN and f-APN (5 μg/mL) is not enough to display the functions range of adiponectin. Considering the concentration varied widely in physiological and pathological situations, a further study is necessary to clarify the effects of different adiponectin concentrations on proinflammatory cytokine production and migration in macrophages.
In summary, our results indicated that globular adiponectin could stimulate proinflammatory cytokine production in macrophages through NF-kappaB activation which was independent of AdipoR1 or AdipoR2, and full-length Adiponectin could promote macrophage migration through AdipoR1-PI3Kγ signaling pathway. The exact signaling pathway for NF-kappaB activation should be further investigated to find a new therapeutic target against inflammation in the future.
Funding Statement
This work is funded in part by a grant from NIDDK (R01DK95835).
Disclosure
The authors declare no conflict of interest.
References
- 1.Fang H, Judd RL. Adiponectin regulation and function. Compr Physiol. 2018;8(3):1031–1063. [DOI] [PubMed] [Google Scholar]
- 2.Choi HM, Doss HM, Kim KS. Multifaceted physiological roles of adiponectin in inflammation and diseases. Int J Mol Sci. 2020;21(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monda V, Polito R, Lovino A, et al. Short-term physiological effects of a very low-calorie ketogenic diet: effects on adiponectin levels and inflammatory states. Int J Mol Sci. 2020;21(9):3228. doi: 10.3390/ijms21093228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stern JH, Rutkowski JM, Scherer PE. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016;23(5):770–784. doi: 10.1016/j.cmet.2016.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diniz TA, Aquino Junior JCJ, Mosele FC, et al. Exercise-induced AMPK activation and IL-6 muscle production are disturbed in adiponectin knockout mice. Cytokine. 2019;119:71–80. doi: 10.1016/j.cyto.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 6.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288–1295. doi: 10.1038/nm788 [DOI] [PubMed] [Google Scholar]
- 7.Fiaschi T. Mechanisms of adiponectin action. Int J Mol Sci. 2019;20(12):2894. doi: 10.3390/ijms20122894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villarreal-Molina MT, Antuna-Puente B. Adiponectin: anti-inflammatory and cardioprotective effects. Biochimie. 2012;94(10):2143–2149. doi: 10.1016/j.biochi.2012.06.030 [DOI] [PubMed] [Google Scholar]
- 9.Piccio L, Cantoni C, Henderson JG, et al. Lack of adiponectin leads to increased lymphocyte activation and increased disease severity in a mouse model of multiple sclerosis. Eur J Immunol. 2013;43(8):2089–2100. doi: 10.1002/eji.201242836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takemura Y, Ouchi N, Shibata R, et al. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest. 2007;117(2):375–386. doi: 10.1172/JCI29709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohashi K, Parker JL, Ouchi N, et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285(9):6153–6160. doi: 10.1074/jbc.M109.088708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lovren F, Pan Y, Quan A, et al. Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. Am J Physiol Heart Circ Physiol. 2010;299(3):H656–663. doi: 10.1152/ajpheart.00115.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng X, Folco EJ, Shimizu K, Libby P. Adiponectin induces pro-inflammatory programs in human macrophages and CD4+ T cells. J Biol Chem. 2012;287(44):36896–36904. doi: 10.1074/jbc.M112.409516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng YJ, Shen TL, Chen YS, Mersmann HJ, Liu BH, Ding ST. Adiponectin and adiponectin receptor 1 overexpression enhance inflammatory bowel disease. J Biomed Sci. 2018;25(1):018–0419. doi: 10.1186/s12929-018-0419-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang R, Yin X, Shi H, et al. Adiponectin modulates DCA-induced inflammation via the ROS/NF-κ B signaling pathway in esophageal adenocarcinoma cells. Dig Dis Sci. 2014;59(1):89–97. doi: 10.1007/s10620-013-2877-5 [DOI] [PubMed] [Google Scholar]
- 16.Woodward L, Akoumianakis I, Antoniades C. Unravelling the adiponectin paradox: novel roles of adiponectin in the regulation of cardiovascular disease. Br J Pharmacol. 2017;174(22):4007–4020. doi: 10.1111/bph.13619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markaki A, Psylinakis E, Spyridaki A. Adiponectin and end-stage renal disease. Hormones (Athens). 2016;15(3):345–354. doi: 10.14310/horm.2002.1698 [DOI] [PubMed] [Google Scholar]
- 18.Jin X, Chen J, Hu Z, Chan L, Wang Y. Genetic deficiency of adiponectin protects against acute kidney injury. Kidney Int. 2013;83(4):604–614. doi: 10.1038/ki.2012.408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H, Lu XJ, Chen J. Full-length and a smaller globular fragment of adiponectin have opposite roles in regulating monocyte/macrophage functions in ayu, Plecoglossus altivelis. Fish Shellfish Immunol. 2018;82:319–329. doi: 10.1016/j.fsi.2018.08.041 [DOI] [PubMed] [Google Scholar]
- 20.Polito R, Nigro E, Messina A, et al. Adiponectin and orexin-A as a potential immunity link between adipose tissue and central nervous system. Front Physiol. 2018;9:982. doi: 10.3389/fphys.2018.00982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Negoro T, Kin M, Takuma A, Saito K, Shimizu S, Nakano Y. Potentiated macrophage activation by acid sensing under low adiponectin levels. Mol Immunol. 2014;57(2):141–150. doi: 10.1016/j.molimm.2013.08.015 [DOI] [PubMed] [Google Scholar]
- 22.Okamoto M, Ohara-Imaizumi M, Kubota N, et al. Adiponectin induces insulin secretion in vitro and in vivo at a low glucose concentration. Diabetologia. 2008;51(5):827–835. doi: 10.1007/s00125-008-0944-9 [DOI] [PubMed] [Google Scholar]
- 23.Zhang R, Wu J, Liu D, Shan H, Zhang J. Anti-inflammatory effect of full-length adiponectin and proinflammatory effect of globular adiponectin in esophageal adenocarcinoma cells. Oncol Res. 2013;21(1):15–21. doi: 10.3727/096504013X13786659070235 [DOI] [PubMed] [Google Scholar]
- 24.Wan Z, Mah D, Simtchouk S, Klegeris A, Little JP. Globular adiponectin induces a pro-inflammatory response in human astrocytic cells. Biochem Biophys Res Commun. 2014;446(1):37–42. doi: 10.1016/j.bbrc.2014.02.077 [DOI] [PubMed] [Google Scholar]
- 25.Zhang K, Tai Z, Han Q, Pang Y, Li Q. Adiponectin as inducer of inflammatory and apoptosis involving in immune defense in lamprey. Fish Shellfish Immunol. 2019;90:446–455. doi: 10.1016/j.fsi.2019.04.045 [DOI] [PubMed] [Google Scholar]
- 26.Parker-Duffen JL, Nakamura K, Silver M, et al. T-cadherin is essential for adiponectin-mediated revascularization. J Biol Chem. 2013;288(34):24886–24897. doi: 10.1074/jbc.M113.454835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka Y, Kita S, Nishizawa H, et al. Adiponectin promotes muscle regeneration through binding to T-cadherin. Sci Rep. 2019;9(1):16. doi: 10.1038/s41598-018-37115-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren J, Su D, Li L, et al. Anti-inflammatory effects of aureusidin in LPS-stimulated RAW264.7 macrophages via suppressing NF-kappaB and activating ROS- and MAPKs-dependent Nrf2/HO-1 signaling pathways. Toxicol Appl Pharmacol. 2020;387:114846. doi: 10.1016/j.taap.2019.114846 [DOI] [PubMed] [Google Scholar]
- 29.Yu XW, Hu ZL, Ni M, et al. Acid-sensing ion channels promote the inflammation and migration of cultured rat microglia. Glia. 2015;63(3):483–496. doi: 10.1002/glia.22766 [DOI] [PubMed] [Google Scholar]
- 30.McEntegart MB, Awede B, Petrie MC, et al. Increase in serum adiponectin concentration in patients with heart failure and cachexia: relationship with leptin, other cytokines, and B-type natriuretic peptide. Eur Heart J. 2007;28(7):829–835. doi: 10.1093/eurheartj/ehm033 [DOI] [PubMed] [Google Scholar]