Abstract
Background
Treatment of blunt splenic trauma (BST) continues to evolve with improved imaging for detection of splenic vascular injuries.
Purpose
To report on treatments for BST from 11 trauma centers, the frequency and clinical impact of splenic vascular injuries, and factors influencing treatment.
Materials and Methods
Patients were retrospectively identified as having BST between January 2011 and December 2018, and clinical, imaging, and outcome data were recorded. Patient data were summarized descriptively, both overall and stratified by initial treatment received (nonoperative management [NOM], angiography, or surgery). Regression analyses were used to examine the primary outcomes of interest, which were initial treatment received and length of stay (LOS).
Results
This study evaluated 1373 patients (mean age, 42 years ± 18; 845 men). Initial treatments included NOM in 849 patients, interventional radiology (IR) in 240 patients, and surgery in 284 patients. Rates from CT reporting were 22% (304 of 1373) for active splenic hemorrhage (ASH) and 20% (276 of 1373) for contained vascular injury (CVI). IR management of high-grade injuries increased 15.6%, from 28.6% (eight of 28) to 44.2% (57 of 129) (2011–2012 vs 2017–2018). Patients who were treated invasively had a higher injury severity score (odds ratio [OR], 1.04; 95% CI: 1.02, 1.05; P < .001), lower temperature (OR, 0.97; 95% CI: 0.97, 1.00; P = .03), and a lower hematocrit (OR, 0.96; 95% CI: 0.93, 0.99; P = .003) and were more likely to show ASH (OR, 8.05; 95% CI: 5.35, 12.26; P < .001) or CVI (OR, 2.70; 95% CI: 1.64, 4.44; P < .001) on CT images, have spleen-only injures (OR, 2.35; 95% CI: 1.45, 3.8; P < .001), and have been administered blood product for fewer than 24 hours (OR, 2.35; 95% CI: 1.58, 3.51; P < .001) compared with those chosen for NOM, after adjusting for key demographic and clinical variables. After adjustment, factors associated with a shorter LOS were female sex (OR, 0.84; 95% CI: 0.73, 0.96; P = .009), spleen-only injury (OR, 0.72; 95% CI: 0.6, 0.86; P < .001), higher admission hematocrit (OR, 0.98; 95% CI: 0.6, 0.86; P < .001), and presence of ASH at CT (OR, 0.74; 95% CI: 0.62, 0.88; P < .001).
Conclusion
Contained vascular injury and active splenic hemorrhage (ASH) were frequently reported, and rates of interventional radiologic management increased during the study period. ASH was associated with a shorter length of stay, and patients with ASH had eight times the odds of undergoing invasive treatment compared with undergoing nonoperative management.
© RSNA, 2021
See also the editorial by Patlas in this issue.
Summary
An increase in interventional radiologic (IR) management of blunt splenic trauma was observed during the study period. IR management of high-grade injuries increased from 29% to 44% during the 7-year study period. Vascular injuries were identified at CT (>20%) more than previously reported and were strongly associated with invasive treatment.
Key Results
■ Contained vascular splenic injuries were present on 20% of blunt-trauma CT images, and active splenic hemorrhage (ASH) was present on 22% of blunt-trauma CT images and was strongly associated with undergoing invasive treatment (odds ratios [ORs], 2.7 and 8.1, respectively).
■ Patients with ASH at CT typically had a shorter hospital stay (OR, 0.74; P < .001), possibly because of the early definitive treatment of splenic injury.
Introduction
The spleen is among the most common solid abdominal organs injured in blunt abdominal trauma, accounting for approximately one-third of all abdominal organ injuries (1,2). Identification and grading of splenic injuries in patients who are symptomatic and asymptomatic guides treatment (3). Splenic injury was described by 1st-century Roman encyclopedist, Aulus Cornelius Celsus in De Medicina, and for centuries splenic trauma was managed nonoperatively. The first description of splenectomy for injury was in the 18th century (4). Undoubtedly, surgeon and Nobel prize winner Dr Emil Kocher influenced the standard of care in the 19th century, stating “No evil effects follow its removal, while the danger of hemorrhage is effectually stopped.” So, for decades, the primary treatment for splenic trauma was splenectomy. Several decades later, a body of literature, including a manuscript by King and Shumacker at Indiana University, reported five cases of fulminant sepsis among 100 children after splenectomy (5). Trauma surgeons then started to consider nonoperative management (NOM) as a treatment option once again (5). The increased accessibility and speed of CT aided in identifying intra-abdominal injuries, including grading splenic injuries. This assisted in identifying patients in need of emergent surgery and those appropriate for NOM (6–8). In addition, endovascular procedures provided by interventional radiologists offer an option beyond just surgical treatment or NOM (9,10).
Treatment of splenic injuries continues to evolve and includes observation, splenic artery embolization, splenorrhaphy, and splenectomy (3,9,11–14). More recently, improved imaging protocols, specifically the addition of arterial phase imaging, allow for the accurate detection of active bleeding or nonbleeding splenic vascular injuries, which also influences treatment pathways for these patients (15–20). The aims of our study are to report trends for treating blunt splenic trauma (BST) from 11 U.S. level 1 trauma centers, evaluate the frequency of reporting of splenic vascular injuries and the impact on treatment and length of stay (LOS), and describe factors influencing the decision to invasively treat.
Materials and Methods
Multi-Institutional Consortium
Financial support from the American Society of Emergency Radiology was provided for direct costs of this study. Data were controlled by the authors. Active members of the American Society of Emergency Radiology voluntarily participated as part of the societal biennial research grant award. Eleven level 1 trauma centers in the United States contributed retrospectively and collected clinical and imaging data. Each institution independently obtained approval from their local institutional review board, with informed consent waived. Data-use agreements were executed between each institution and the host institution (University of Kentucky; Lexington, Ky) before data sharing. Deidentified standardized clinical data were shared by the host institution’s Research Electronic Data Capture (Vanderbilt University; Nashville, Tenn) system (21,22).
Patients
Adult patients age 18 years or older with BST and an admission CT study were eligible for inclusion. Each site was asked to contribute 200 patients or 7 years’ worth of data, whichever came first, to include patients from 2011 to 2018. Clinical (including CT) data were recorded for each patient enrolled. Exclusion criteria included penetrating trauma to the abdomen and/or pelvis, CT images obtained more than 12 hours before or after initial presentation, splenectomy prior to CT imaging, patients with insufficient follow-up or missing key data, CT examination performed on a scanner with fewer than 16 detector rows, and death before definitive treatment of splenic injury.
Data Collection
A potential 96 data fields were collected, including basic demographic information, vital signs, laboratory values, use of blood products, time to imaging, and CT imaging reports. Primary outcome data were choice of initial treatment, LOS, and mortality.
Statistical Analysis
Patient demographics, clinical characteristics, and clinical outcomes were summarized descriptively, both overall and stratified by type of initial treatment received (NOM, interventional radiology [IR], or surgical treatment). Categorical variables were summarized with counts and percentages. Continuous variables were summarized with means and standard deviations or medians and first or third quartiles for those with strong departures from normality. The sample size did not allow for stratified analyses by year or hospital location; therefore, exploratory graphic analyses were performed to examine the relationships of the year and location with the type of initial treatment received.
The primary outcome variables of interest are type of initial treatment received, LOS, and mortality. Separate multivariable regression models were used to examine the effects of the injury severity score, active splenic hemorrhage (ASH) at CT, contained vascular injury (CVI) at CT, and spleen-only versus synchronous major organ injury on the primary outcomes of interest, including adjustment for 12 patient demographic and clinical characteristics. The 12 demographic and clinical variables included in each model are sex, age, systolic blood pressure, heart rate, respiratory rate, oxygen saturation, temperature, hematocrit, hemoglobin, platelets, international normalized ratio, and blood products received within the first 24 hours. Because the sample size is sufficiently large, all adjustment variables, regardless of statistical significance, were retained in each regression model to ensure that all of the models adjusted for important patient characteristics and could be interpreted consistently.
Multinomial logistic regression was used to examine the adjusted associations between these predictors of interest and initial treatment, a three-level categorical variable (NOM vs IR vs surgical treatment), with NOM as the reference category. Separate adjusted odds ratios (ORs) reflecting the relative odds of IR versus NOM as well as surgical management versus NOM for each predictor are reported.
Because of a strong positive skew, LOS was transformed by using the natural logarithm before modeling. Linear regression models were used to model the log-transformed LOS values against injury severity score, ASH at CT, CVI at CT, spleen-only injury, and the 12 patient demographic and clinical variables. Estimated model coefficients were exponentiated so that interpretations could be made on the original LOS scale (days). In the case of log-transformed outcomes, the exponentiated coefficient estimates from linear regression models can be interpreted as multiplicative changes in the (geometric mean of the) outcome. For example, an exponentiated coefficient estimate for a continuous predictor variable of 1.35 can be interpreted in the following way: a 1-unit increase in the predictor variable is associated with a 35% increase in the geometric mean of the outcome, on average, while controlling for all other variables in the model.
Logistic regression was attempted to examine the associations between each of these predictors and mortality. However, the number of deaths observed in our sample was not large enough to fit this multivariable model, and inferential results for the mortality outcome are thus not reported.
The sensitivity and specificity analyses of CT findings of ASH and CVI were performed by using angiographic reports as the diagnostic standard.
All hypothesis tests were performed by using a two-sided significance level of .05. Missing observations were reported and were excluded by available case analysis. All analyses were performed in R statistical software (version 3.6.1; R Foundation for Statistical Computing). Multinomial regression models were fitted by using the multinom function from the nnet R package, version 7.3-12 (Venables et al).
Results
Patient Characteristics
Data from 1633 patients were entered into the Research Electronic Data Capture database. After applying exclusion criteria (age < 18 years [n = 1], penetrating trauma [n = 21], CT performed 12 hours before or after presentation or admission to a study-participating hospital [n = 97], splenectomy prior to undergoing CT [n = 71], CT performed with scanner with <16 detector rows [n = 4], death prior to definitive treatment of splenic injury [n = 21], no CT scan obtained (n = 25), dates outside of study dates [n = 11], and patients who left against medical advice [n = 5]) and removing patients with incomplete data (sex [n = 1] and initial treatment [n = 3]), 1373 patients (mean age, 42 years ± 18 [standard deviation]; 845 men) were evaluated (Fig 1). Of those, 849 patients were treated nonoperatively, 240 underwent IR, and 284 underwent a surgical procedure. Patient characteristics, clinical characteristics, and clinical outcomes are summarized overall and stratified by initial treatment (NOM, IR, or surgical treatment) in Table 1. Without covariate adjustment, on average, patients treated with NOM had a shorter LOS than those who underwent intervention. Regarding original CT reports, the American Association for the Surgery of Trauma grade was reported for 80% (1110 of 1373), ASH was reported in 22% (304 of 1373), and CVI was reported in 20% (276 of 1373) of the patients (Table 1). The average age of patients was not significantly different among those receiving NOM versus those receiving invasive treatment (mean age: NOM, 41 years ± 19; invasive treatment, 43 years ± 18; P = .39).
Figure 1:
Flowchart of the number of patients eligible for analysis.
Table 1:
Patient Characteristics Overall and Stratified by Initial Treatment
Management of BST over Years
Figure 2 displays management by year, subdividing injuries by American Association for the Surgery of Trauma grade into low-grade (grades 1–3) and high-grade injuries (grades 4–5). Treatment of patients with low-grade injuries (grades 1–3) changed little during the study period (2011–2012: NOM, 76% [48 of 63]; IR, 8% [five of 63]; surgical treatment, 16% [10 of 63]; 2017–2018: NOM, 73.3% [311 of 424]; IR, 15.6% [66 of 424]; surgical treatment, 11.1% [47 of 424]). However, in patients with high-grade injuries (grades 4–5), treatment with IR appeared to increase over time (2011–2012: NOM, 18% [five of 28]; IR, 29% [eight of 28]; surgical treatment, 54% [15 of 28]; 2017–2018: NOM, 17.1% [22 of 129]; IR, 44.2% [57 of 129]; surgical treatment, 38.8% [50 of 129]).
Figure 2:
Temporal trends in management of low-grade (grades 1–3) and high-grade (grades 4–5) splenic injuries. Top graph shows treatment of patients with low-grade injuries changed minimally during the study period (2011–2012 vs 2017–2018, respectively: nonoperative management [NOM], 76% [48 of 63] vs 73.3% (311 of 424); interventional radiology [IR], 8% [five of 63] vs 15.6% [66 of 424]; and surgical treatment, 16% [10 of 63] vs 11.1% [47 of 424]). Bottom graph shows that in patients with high-grade injuries IR appears to increase over time (2011–2012 vs 2017–2018, respectively: NOM, 18% [five of 28] vs 17.1% [22 of 129]; IR management, 29% [eight of 28] vs 44.2% [57 of 129]; surgical treatment, 54% [15 of 28] vs 38.8% [50 of 129]).
Management Preference by Institution
Across all institutions, 61.8% (849 of 1373) of patients were provided NOM, 17.5% (240 of 1373) of patients underwent IR, and 20.7% (284 of 1373) of patients were provided surgical treatment. There was some heterogeneity in management preference by institution. Figure 3 shows the distribution of management types by institution for institutions that contributed more than 95 patients. Among these eight institutions, within-institution rates of IR management ranged from 5.1% (10 of 198) to 33% (32 of 98). A few institutions favored IR (institutions A and B) or surgical treatment (institutions D and E; Fig 3). Others showed a more balanced approach (institutions C, F, H, and G; Fig 3). However, among those with a balanced approach, two had lower rates of noninvasive management (institutions C and F; Fig 3).
Figure 3:
Institutional practice patterns based on those institutions submitting greater than 95 patients. A–H, Individual blinded institutions are shown. IR = interventional radiologic, NOM = nonoperative management.
Angiographic Findings
Four hundred twenty-four patients underwent angiographic evaluation, with 46% (193 of 424) of those reporting a vascular injury at the time of the procedure. By using angiographic reports as the standard for assessing CVI and ASH, CT was better in predicting CVI, with a sensitivity of 60% (81 of 136; 95% CI: 51, 68) and a specificity of 79% (57 of 72; 95% CI: 68, 88), than for predicting ASH, with a sensitivity of 52% (71 of 136; 95% CI: 43, 61) and a specificity of 65% (47 of 72; 95% CI: 53, 76).
NOM versus IR versus Surgical Treatment
Complete data were observed in 919 patients. Undergoing invasive treatment (either IR or surgical treatment) versus NOM was associated with a higher injury severity score (OR, 1.04; 95% CI: 1.02, 1.05; P < .001), presence of ASH at CT (OR, 8.05; 95% CI: 5.35, 12.26; P < .001), presence of CVI at CT (OR, 2.70; 95% CI: 1.64, 4.44; P < .001), injury to only the spleen (OR, 2.35; 95% CI: 1.45, 3.8; P < .001), and rate of receiving blood product for fewer than 24 hours (OR, 2.35; 95% CI: 1.58, 3.51; P < .001), after adjustment for key demographic and clinical variables (Table 2).
Table 2:
Invasive Treatment versus NOM Binary Logistic Regression

Adjusting for all other variables in the model, a higher injury severity score (OR, 1.03; 95% CI: 1.01, 1.05; P = .06), presence of ASH at CT (OR, 9.6; 95% CI: 6.0, 15.36; P < .001), presence of CVI at CT (OR, 3.1; 95% CI: 1.72, 5.51; P < .001), and injury to only the spleen (OR, 2.7; 95% CI: 1.54, 4.64; P < .001) were each associated with higher odds of undergoing treatment with IR compared with undergoing NOM for splenic injury (Table 3). After adjusting for the other covariates, having a higher injury severity score (OR, 1.04; 95% CI: 1.02, 1.06; P < .001), presence of ASH at CT (OR, 6.7; 95% CI: 4.05, 11.06; P < .001), and presence of CVI at CT (OR, 2.4; 95% CI: 1.28, 4.51; P = .006) were each associated with higher odds of undergoing surgical treatment compared with receiving NOM for splenic injury (Table 3). Some patient demographic and clinical characteristics also showed associations with the type of initial treatment received (Table 3). Administration of blood products within the first 24 hours of admission was associated with increased odds of undergoing surgical management than of undergoing NOM (OR, 4.6; 95% CI: 2.80, 7.39; P < .001), after adjusting for all other variables in the model (Table 3).
Table 3:
Multinomial Logistic Regression for Initial Treatment
The injury severity score, presence of ASH at CT, presence of CVI at CT, and spleen-only injuries were associated with higher adjusted odds of invasive procedures overall. The presence of ASH at CT was associated with higher odds of undergoing IR (relative to NOM; OR, 9.6) compared with the odds of undergoing surgical management (relative to NOM; OR, 6.7). Not surprisingly, administration of blood products within the first 24 hours of admission was associated with higher adjusted odds of surgical management (relative to NOM) compared with IR management (relative to NOM).
Associations with LOS
Table 4 shows the associations between the same predictor variables on LOS. Higher injury severity score (OR, 1.02; 95% CI: 1.02, 1.03; P < .001), age (OR, 1.01; 95% CI: 0.62, 0.88; P < .001), heart rate (OR, 1.01; 95% CI: 1.01, 1.01; P < .001), respiratory rate (OR, 1.01; 95% CI: 1, 1.02; P = .048), and administration of blood products within the first 24 hours (OR, 1.26; 95% CI: 1.07, 1.48; P = .006), after adjusting for the other variables, were associated with a longer expected hospital stay. Factors associated with a shorter LOS after adjustment were female sex (OR, 1.01; 95% CI: 0.73, 0.96; P = .009), spleen-only injury (OR, 0.72; 95% CI: 0.60, 0.86; P < .001), higher admission hematocrit (OR, 0.98; 95% CI: 0.97, 0.99; P < .001), and active hemorrhage on admission CT scan (OR, 0.74; 95% CI: 0.62, 0.88; P < .001).
Table 4:
Multivariable Linear Regression for Length of Stay
Discussion
Our study engaged members from the American Society of Emergency Radiology to conduct a multi-institutional retrospective study to evaluate factors influencing treatment of patients who sustained blunt splenic trauma (BST) and underwent CT imaging before definitive treatment. Many complex factors are associated with the treatment of patients with BST. Our study aimed to report on practice patterns regarding BST from 11 trauma centers in the United States during the study period and the frequency and clinical impact of CT identification of splenic vascular injuries, among other factors influencing treatment. Two important broad observations were noted. First, a 15.6% increase in treatment with interventional radiology (IR) occurred over the study period, primarily at the expense of surgical treatment (which decreased by 14.8%), for higher-grade injuries. Second, a preference in institutional management of BST was noted (IR management ranged from 5.1% [10 of 198] to 33% [32 of 98] across institutions). Few institutions had higher relative rates of surgical treatment than with IR, whereas others had higher relative rates of IR treatment than surgical. In addition, some institutions had lower rates of patients who were administered only nonoperative management (NOM). CT findings of active splenic hemorrhage (ASH) (adjusted odds ratio [OR], 8.05; 95% CI: 5.35, 12.26; P < .001) and contained vascular injury (adjusted OR, 2.70; 95% CI: 1.64, 4.44; P < .001) were also associated with undergoing invasive treatment (either IR or surgical treatment) versus NOM (Fig 4). CT findings of ASH were also associated with a shorter length of stay in our study (adjusted OR, 0.74; 95% CI: 0.62, 0.88; P < .001).
Figure 4a:
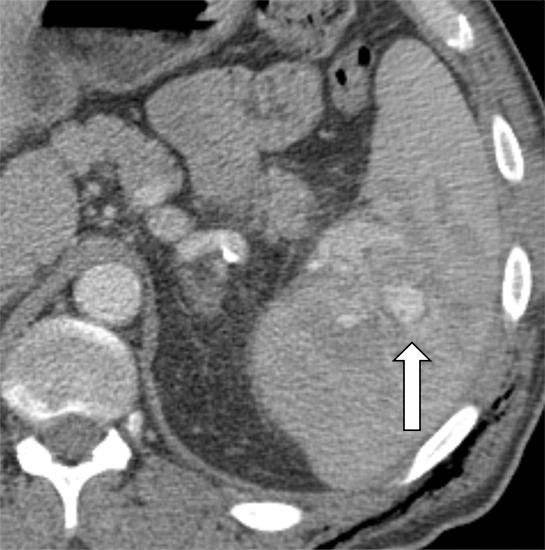
Contrast-enhanced CT images show examples of contained vascular injuries (CVIs) and active splenic hemorrhage (ASH). Axial contrast-enhanced CT scans in the (a) portal venous phase and (b) delayed phase in a 56-year-old man after a motor vehicle collision show a CVI in the portal venous phase (arrow), which does not persist on more delayed imaging. Axial contrast-enhanced CT in the (c) arterial phase and (d) portal venous phase in a 51-year-old female pedestrian struck by a car showing a CVI (arrow). (e) Axial contrast-enhanced CT in the portal venous phase 12 hours later shows interval delayed splenic rupture with an intraparenchymal hematoma (*) and surrounding hemoperitoneum (arrowhead). Axial contrast-enhanced CT scans in the (f) arterial phase and (g) portal venous phase in a 26-year-old man after a motor vehicle collision show a large area of ASH (arrow) medial to the spleen (S). Axial contrast-enhanced CT scans in the (h) arterial phase and (i) portal venous phase in a 43-year-old man after motor vehicle collision show multifocal regions of ASH (arrows) on the lateral aspect of the spleen.
Figure 4b:

Contrast-enhanced CT images show examples of contained vascular injuries (CVIs) and active splenic hemorrhage (ASH). Axial contrast-enhanced CT scans in the (a) portal venous phase and (b) delayed phase in a 56-year-old man after a motor vehicle collision show a CVI in the portal venous phase (arrow), which does not persist on more delayed imaging. Axial contrast-enhanced CT in the (c) arterial phase and (d) portal venous phase in a 51-year-old female pedestrian struck by a car showing a CVI (arrow). (e) Axial contrast-enhanced CT in the portal venous phase 12 hours later shows interval delayed splenic rupture with an intraparenchymal hematoma (*) and surrounding hemoperitoneum (arrowhead). Axial contrast-enhanced CT scans in the (f) arterial phase and (g) portal venous phase in a 26-year-old man after a motor vehicle collision show a large area of ASH (arrow) medial to the spleen (S). Axial contrast-enhanced CT scans in the (h) arterial phase and (i) portal venous phase in a 43-year-old man after motor vehicle collision show multifocal regions of ASH (arrows) on the lateral aspect of the spleen.
Figure 4c:

Contrast-enhanced CT images show examples of contained vascular injuries (CVIs) and active splenic hemorrhage (ASH). Axial contrast-enhanced CT scans in the (a) portal venous phase and (b) delayed phase in a 56-year-old man after a motor vehicle collision show a CVI in the portal venous phase (arrow), which does not persist on more delayed imaging. Axial contrast-enhanced CT in the (c) arterial phase and (d) portal venous phase in a 51-year-old female pedestrian struck by a car showing a CVI (arrow). (e) Axial contrast-enhanced CT in the portal venous phase 12 hours later shows interval delayed splenic rupture with an intraparenchymal hematoma (*) and surrounding hemoperitoneum (arrowhead). Axial contrast-enhanced CT scans in the (f) arterial phase and (g) portal venous phase in a 26-year-old man after a motor vehicle collision show a large area of ASH (arrow) medial to the spleen (S). Axial contrast-enhanced CT scans in the (h) arterial phase and (i) portal venous phase in a 43-year-old man after motor vehicle collision show multifocal regions of ASH (arrows) on the lateral aspect of the spleen.
Figure 4d:

Contrast-enhanced CT images show examples of contained vascular injuries (CVIs) and active splenic hemorrhage (ASH). Axial contrast-enhanced CT scans in the (a) portal venous phase and (b) delayed phase in a 56-year-old man after a motor vehicle collision show a CVI in the portal venous phase (arrow), which does not persist on more delayed imaging. Axial contrast-enhanced CT in the (c) arterial phase and (d) portal venous phase in a 51-year-old female pedestrian struck by a car showing a CVI (arrow). (e) Axial contrast-enhanced CT in the portal venous phase 12 hours later shows interval delayed splenic rupture with an intraparenchymal hematoma (*) and surrounding hemoperitoneum (arrowhead). Axial contrast-enhanced CT scans in the (f) arterial phase and (g) portal venous phase in a 26-year-old man after a motor vehicle collision show a large area of ASH (arrow) medial to the spleen (S). Axial contrast-enhanced CT scans in the (h) arterial phase and (i) portal venous phase in a 43-year-old man after motor vehicle collision show multifocal regions of ASH (arrows) on the lateral aspect of the spleen.
Figure 4e:

Contrast-enhanced CT images show examples of contained vascular injuries (CVIs) and active splenic hemorrhage (ASH). Axial contrast-enhanced CT scans in the (a) portal venous phase and (b) delayed phase in a 56-year-old man after a motor vehicle collision show a CVI in the portal venous phase (arrow), which does not persist on more delayed imaging. Axial contrast-enhanced CT in the (c) arterial phase and (d) portal venous phase in a 51-year-old female pedestrian struck by a car showing a CVI (arrow). (e) Axial contrast-enhanced CT in the portal venous phase 12 hours later shows interval delayed splenic rupture with an intraparenchymal hematoma (*) and surrounding hemoperitoneum (arrowhead). Axial contrast-enhanced CT scans in the (f) arterial phase and (g) portal venous phase in a 26-year-old man after a motor vehicle collision show a large area of ASH (arrow) medial to the spleen (S). Axial contrast-enhanced CT scans in the (h) arterial phase and (i) portal venous phase in a 43-year-old man after motor vehicle collision show multifocal regions of ASH (arrows) on the lateral aspect of the spleen.
Figure 4f:

Contrast-enhanced CT images show examples of contained vascular injuries (CVIs) and active splenic hemorrhage (ASH). Axial contrast-enhanced CT scans in the (a) portal venous phase and (b) delayed phase in a 56-year-old man after a motor vehicle collision show a CVI in the portal venous phase (arrow), which does not persist on more delayed imaging. Axial contrast-enhanced CT in the (c) arterial phase and (d) portal venous phase in a 51-year-old female pedestrian struck by a car showing a CVI (arrow). (e) Axial contrast-enhanced CT in the portal venous phase 12 hours later shows interval delayed splenic rupture with an intraparenchymal hematoma (*) and surrounding hemoperitoneum (arrowhead). Axial contrast-enhanced CT scans in the (f) arterial phase and (g) portal venous phase in a 26-year-old man after a motor vehicle collision show a large area of ASH (arrow) medial to the spleen (S). Axial contrast-enhanced CT scans in the (h) arterial phase and (i) portal venous phase in a 43-year-old man after motor vehicle collision show multifocal regions of ASH (arrows) on the lateral aspect of the spleen.
Figure 4g:

Contrast-enhanced CT images show examples of contained vascular injuries (CVIs) and active splenic hemorrhage (ASH). Axial contrast-enhanced CT scans in the (a) portal venous phase and (b) delayed phase in a 56-year-old man after a motor vehicle collision show a CVI in the portal venous phase (arrow), which does not persist on more delayed imaging. Axial contrast-enhanced CT in the (c) arterial phase and (d) portal venous phase in a 51-year-old female pedestrian struck by a car showing a CVI (arrow). (e) Axial contrast-enhanced CT in the portal venous phase 12 hours later shows interval delayed splenic rupture with an intraparenchymal hematoma (*) and surrounding hemoperitoneum (arrowhead). Axial contrast-enhanced CT scans in the (f) arterial phase and (g) portal venous phase in a 26-year-old man after a motor vehicle collision show a large area of ASH (arrow) medial to the spleen (S). Axial contrast-enhanced CT scans in the (h) arterial phase and (i) portal venous phase in a 43-year-old man after motor vehicle collision show multifocal regions of ASH (arrows) on the lateral aspect of the spleen.
Figure 4h:

Contrast-enhanced CT images show examples of contained vascular injuries (CVIs) and active splenic hemorrhage (ASH). Axial contrast-enhanced CT scans in the (a) portal venous phase and (b) delayed phase in a 56-year-old man after a motor vehicle collision show a CVI in the portal venous phase (arrow), which does not persist on more delayed imaging. Axial contrast-enhanced CT in the (c) arterial phase and (d) portal venous phase in a 51-year-old female pedestrian struck by a car showing a CVI (arrow). (e) Axial contrast-enhanced CT in the portal venous phase 12 hours later shows interval delayed splenic rupture with an intraparenchymal hematoma (*) and surrounding hemoperitoneum (arrowhead). Axial contrast-enhanced CT scans in the (f) arterial phase and (g) portal venous phase in a 26-year-old man after a motor vehicle collision show a large area of ASH (arrow) medial to the spleen (S). Axial contrast-enhanced CT scans in the (h) arterial phase and (i) portal venous phase in a 43-year-old man after motor vehicle collision show multifocal regions of ASH (arrows) on the lateral aspect of the spleen.
Figure 4i:

Contrast-enhanced CT images show examples of contained vascular injuries (CVIs) and active splenic hemorrhage (ASH). Axial contrast-enhanced CT scans in the (a) portal venous phase and (b) delayed phase in a 56-year-old man after a motor vehicle collision show a CVI in the portal venous phase (arrow), which does not persist on more delayed imaging. Axial contrast-enhanced CT in the (c) arterial phase and (d) portal venous phase in a 51-year-old female pedestrian struck by a car showing a CVI (arrow). (e) Axial contrast-enhanced CT in the portal venous phase 12 hours later shows interval delayed splenic rupture with an intraparenchymal hematoma (*) and surrounding hemoperitoneum (arrowhead). Axial contrast-enhanced CT scans in the (f) arterial phase and (g) portal venous phase in a 26-year-old man after a motor vehicle collision show a large area of ASH (arrow) medial to the spleen (S). Axial contrast-enhanced CT scans in the (h) arterial phase and (i) portal venous phase in a 43-year-old man after motor vehicle collision show multifocal regions of ASH (arrows) on the lateral aspect of the spleen.
Increasing rates of IR treatment may have been because of many institutions placing more emphasis on identifying CVI and ASH at CT, resulting in changes in institutional CT protocols, which specifically include arterial phase imaging through the abdomen. Changes in the protocol may have led to a higher rate of detection of splenic vascular injuries (23–25). Another contributing factor may be institutional protocol to send all hemodynamically stable patients with high-grade splenic injuries to undergo diagnostic angiography to reduce the rate of failure of NOM; however, a consensus among institutions is difficult to achieve (26–28). Institutional availability of IR, management algorithms, and surgeon preference all likely contribute to this observed difference. In addition, a general shift toward NOM in the trauma surgery community likely affected the observed changes (29).
Reported CT findings of ASH and CVI were also associated with the treatment of patients with BST. Patients had approximately eight times the odds of undergoing invasive treatment compared with NOM when ASH was reported at CT (OR, 8.05; 95% CI: 5.35, 12.26; P < .001) after adjustment for relevant clinical and demographic characteristics. In addition, the presence of ASH at CT had a stronger association with IR treatment (adjusted OR, 9.6; 95% CI: 6.0, 15.36; P < .001) than with surgical treatment (adjusted OR, 6.7; 95% CI: 4.05, 11.06; P < .001). If standard advanced-trauma life-support protocols were followed, then we hypothesize that this was a result of selection bias because all of these patients would have been hemodynamically stable enough to undergo CT and stable enough to await IR treatment. Interestingly, although ASH at CT is generally thought to represent a higher-grade injury (18,30,31), its presence was associated with a shorter LOS in our study (OR, 0.74; 95% CI: 0.62, 0.88; P < .001), after adjustment for relevant clinical and demographic characteristics. This may correspond to immediate definitive treatment of injuries, often reducing the length of observation. Additionally, patients with isolated spleen injuries underwent IR treatment of the BST more often (OR, 2.76; 95% CI: 1.54, 4.64; P < .001) than they underwent NOM or surgical treatment. Not surprisingly, patients who were administered blood products within the first 24 hours were at higher risk to undergo surgical treatment for BST (adjusted OR, 4.6; 95% CI: 2.80, 7.39; P < .001).
Our study showed high rates of reported CVI and ASH: 20% (276 of 1373) and 22% (304 of 1373), respectively. A study that included arterial phase imaging showed a lower rate near 15% (24). Among our patients, 424 underwent angiography and compared with CT findings of ASH and CVI, the predictive ability of CT was poor for ASH (sensitivity, 52%; specificity, 65%) and fair for CVI (sensitivity, 60%; specificity, 79%). The poor correlation between CT ASH findings and bleeding at angiography were likely because of many factors, but we hypothesize that bleeding stopped or slowed by the time of angiography. Also, patients with clinically important ASH at the time of admission CT who continue to show clinical signs of ongoing bleeding and need for resuscitation are often surgically treated. The higher specificity for CVI at CT suggests that these injuries may persist longer after the injury than does ASH.
Limitations of our study included selection bias because all participating centers are American College of Surgeons–verified level 1 trauma facilities, with transferred patients included. As such, findings likely reflect patients seen at similar centers and should not be generalized to all splenic injuries. Additionally, there was an unequal temporal and absolute contribution of patients from each institution, which may have introduced bias secondary to individual institutional temporal protocol changes and availability of resources. Not all clinical data were successfully retrieved, such as physical examination findings. Of the patients, 67% (919 of 1373) were included in multivariable analysis, which may have introduced selection bias. CT imaging protocols also varied across the entire cohort, which may have impacted the depiction of splenic vascular injury (24). Furthermore, reports were used, rather than blinded reinterpretation. There were not enough deaths to fit a multivariable model, and inferential results for the mortality outcome were therefore not reported.
In conclusion, our multi-institutional study demonstrated an increasing use of interventional radiologic treatment of blunt splenic trauma, most notably reducing surgical treatment during the study period. Preferences in surgical treatment exist between institutions, and these preferences are likely multifactorial and not completely elucidated by our study. Identification of contained vascular injury and active splenic hemorrhage (ASH) at CT was higher than previously reported and was strongly associated with receiving invasive treatment. Patients with ASH at CT typically had a shorter hospital stay, possibly because of early definitive treatment of splenic injury.
Acknowledgments
Acknowledgments
The authors acknowledge the late Dr Kathirkamanthan Shanmuganathan, who provided guidance throughout the study design and implementation phases of our project. In addition, we acknowledge the research support staff, radiology trainees, and students who volunteered time and effort.
Study supported by an American Society of Emergency Radiology Research Grant and the National Institutes of Health National Center for Advancing Translational Sciences (UL1TR001998). The content is solely the responsibility of the authors and does not necessarily represent the official views of the American Society of Emergency Radiology or National Institutes of Health.
Disclosures of Conflicts of Interest: J.T.L. disclosed no relevant relationships. E.S. disclosed no relevant relationships. J.U. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money paid to author for consultancy from Allena Pharmaceuticals; payment for lectures from Siemens Healthineers. Other relationships: disclosed no relevant relationships. S.D.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money to author’s institution for grant from the Department of the Army. Other relationships: disclosed no relevant relationships. S.T.C. disclosed no relevant relationships. R.T. disclosed no relevant relationships. D.R. disclosed no relevant relationships. K.F.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed grants/grants pending from Siemens Healthineers; royalties from Cambridge Press; payment for development of educational presentations from Siemens Healthineers. Other relationships: disclosed no relevant relationships. N.R.C. disclosed no relevant relationships. M.P.D. disclosed no relevant relationships. A.D. disclosed no relevant relationships. Arthur Baghdanian disclosed no relevant relationships. C.F. disclosed no relevant relationships. Armonde Baghdanian disclosed no relevant relationships. C.A.L. disclosed no relevant relationships.
Abbreviations:
- ASH
- active splenic hemorrhage
- BST
- blunt splenic trauma
- CVI
- contained vascular injury
- IR
- interventional radiology
- LOS
- length of stay
- NOM
- nonoperative management
- OR
- odds ratio
References
- 1.Patel N, Alarcon L. Blunt splenic trauma. American Association for the Surgery of Trauma Web site. https://www.aast.org/resources-detail/8764001f-b3b2-425e-89c5-5284d417de1e. Published May 2012. Accessed January 8, 2021.
- 2.Soto JA, Anderson SW. Multidetector CT of blunt abdominal trauma. Radiology 2012;265(3):678–693. [DOI] [PubMed] [Google Scholar]
- 3.Peitzman AB, Heil B, Rivera L, et al. Blunt splenic injury in adults: Multi-institutional Study of the Eastern Association for the Surgery of Trauma. J Trauma 2000;49(2):177–187; discussion 187–189. [DOI] [PubMed] [Google Scholar]
- 4.Forsythe RM, Harbrecht BG, Peitzman AB. Blunt splenic trauma. Scand J Surg 2006;95(3):146–151. [DOI] [PubMed] [Google Scholar]
- 5.King H, Shumacker HB Jr. Splenic studies. I. Susceptibility to infection after splenectomy performed in infancy. Ann Surg 1952;136(2):239–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore EE, Cogbill TH, Jurkovich GJ, Shackford SR, Malangoni MA, Champion HR. Organ injury scaling: spleen and liver (1994 revision). J Trauma 1995;38(3):323–324. [DOI] [PubMed] [Google Scholar]
- 7.Resciniti A, Fink MP, Raptopoulos V, Davidoff A, Silva WE. Nonoperative treatment of adult splenic trauma: development of a computed tomographic scoring system that detects appropriate candidates for expectant management. J Trauma 1988;28(6):828–831. [PubMed] [Google Scholar]
- 8.Buntain WL, Gould HR, Maull KI. Predictability of splenic salvage by computed tomography. J Trauma 1988;28(1):24–34. [DOI] [PubMed] [Google Scholar]
- 9.Haan JM, Bochicchio GV, Kramer N, Scalea TM. Nonoperative management of blunt splenic injury: a 5-year experience. J Trauma 2005;58(3):492–498. [DOI] [PubMed] [Google Scholar]
- 10.Davis KA, Fabian TC, Croce MA, et al. Improved success in nonoperative management of blunt splenic injuries: embolization of splenic artery pseudoaneurysms. J Trauma 1998;44(6):1008–1013; discussion 1013–1015. [DOI] [PubMed] [Google Scholar]
- 11.Zarzaur BL, Dunn JA, Leininger B, et al. Natural history of splenic vascular abnormalities after blunt injury: A Western Trauma Association multicenter trial. J Trauma Acute Care Surg 2017;83(6):999–1005. [DOI] [PubMed] [Google Scholar]
- 12.El-Matbouly M, Jabbour G, El-Menyar A, et al. Blunt splenic trauma: Assessment, management and outcomes. Surgeon 2016;14(1):52–58. [DOI] [PubMed] [Google Scholar]
- 13.Raza M, Abbas Y, Devi V, Prasad KV, Rizk KN, Nair PP. Non operative management of abdominal trauma - a 10 years review. World J Emerg Surg 2013;8(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lauerman MH, Brenner M, Simpson N, Shanmuganathan K, Stein DM, Scalea T. Angioembolization significantly improves vascular injuries in blunt splenic trauma. Eur J Trauma Emerg Surg doi:10.1007/s00068-019-01151-z. Published online June 6, 2019. . [DOI] [PubMed]
- 15.Marmery H, Shanmuganathan K. Multidetector-row computed tomography imaging of splenic trauma. Semin Ultrasound CT MR 2006;27(5):404–419. [DOI] [PubMed] [Google Scholar]
- 16.Marmery H, Shanmuganathan K, Alexander MT, Mirvis SE. Optimization of selection for nonoperative management of blunt splenic injury: comparison of MDCT grading systems. AJR Am J Roentgenol 2007;189(6):1421–1427. [DOI] [PubMed] [Google Scholar]
- 17.Marmery H, Shanmuganathan K, Mirvis SE, et al. Correlation of multidetector CT findings with splenic arteriography and surgery: prospective study in 392 patients. J Am Coll Surg 2008;206(4):685–693. [DOI] [PubMed] [Google Scholar]
- 18.Saksobhavivat N, Shanmuganathan K, Chen HH, et al. Blunt splenic injury: use of a multidetector CT-based splenic injury grading system and clinical parameters for triage of patients at admission. Radiology 2015;274(3):702–711. [DOI] [PubMed] [Google Scholar]
- 19.Shanmuganathan K, Mirvis SE, Boyd-Kranis R, Takada T, Scalea TM. Nonsurgical management of blunt splenic injury: use of CT criteria to select patients for splenic arteriography and potential endovascular therapy. Radiology 2000;217(1):75–82. [DOI] [PubMed] [Google Scholar]
- 20.Anderson SW, Varghese JC, Lucey BC, Burke PA, Hirsch EF, Soto JA. Blunt splenic trauma: delayed-phase CT for differentiation of active hemorrhage from contained vascular injury in patients. Radiology 2007;243(1):88–95. [DOI] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baghdanian AH, Armetta AS, Baghdanian AA, LeBedis CA, Anderson SW, Soto JA. CT of major vascular injury in blunt abdominopelvic trauma. RadioGraphics 2016;36(3):872–890. [DOI] [PubMed] [Google Scholar]
- 24.Uyeda JW, LeBedis CA, Penn DR, Soto JA, Anderson SW. Active hemorrhage and vascular injuries in splenic trauma: utility of the arterial phase in multidetector CT. Radiology 2014;270(1):99–106. [DOI] [PubMed] [Google Scholar]
- 25.Iacobellis F, Ierardi AM, Mazzei MA, et al. Dual-phase CT for the assessment of acute vascular injuries in high-energy blunt trauma: the imaging findings and management implications. Br J Radiol 2016;89(1061):20150952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odedra D, Mellnick V, Patlas M. A 2019 international survey to assess trends in follow-up imaging of blunt splenic trauma. Emerg Radiol 2020;27(1):51–56. [DOI] [PubMed] [Google Scholar]
- 27.Peitzman AB, Ford HR, Harbrecht BG, Potoka DA, Townsend RN. Injury to the spleen. Curr Probl Surg 2001;38(12):932–1008. [DOI] [PubMed] [Google Scholar]
- 28.Coccolini F, Montori G, Catena F, et al. Splenic trauma: WSES classification and guidelines for adult and pediatric patients. World J Emerg Surg 2017;12(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Margari S, Garozzo Velloni F, Tonolini M, et al. Emergency CT for assessment and management of blunt traumatic splenic injuries at a level 1 trauma center: 13-year study. Emerg Radiol 2018;25(5):489–497. [DOI] [PubMed] [Google Scholar]
- 30.Organ injury scaling 2018 update: spleen, liver, and kidney: erratum. J Trauma Acute Care Surg 2019;87(2):512. [DOI] [PubMed] [Google Scholar]
- 31.Kozar RA, Crandall M, Shanmuganathan K, et al. Organ injury scaling 2018 update: Spleen, liver, and kidney. J Trauma Acute Care Surg 2018;85(6):1119–1122. [DOI] [PubMed] [Google Scholar]






![Temporal trends in management of low-grade (grades 1–3) and high-grade (grades 4–5) splenic injuries. Top graph shows treatment of patients with low-grade injuries changed minimally during the study period (2011–2012 vs 2017–2018, respectively: nonoperative management [NOM], 76% [48 of 63] vs 73.3% (311 of 424); interventional radiology [IR], 8% [five of 63] vs 15.6% [66 of 424]; and surgical treatment, 16% [10 of 63] vs 11.1% [47 of 424]). Bottom graph shows that in patients with high-grade injuries IR appears to increase over time (2011–2012 vs 2017–2018, respectively: NOM, 18% [five of 28] vs 17.1% [22 of 129]; IR management, 29% [eight of 28] vs 44.2% [57 of 129]; surgical treatment, 54% [15 of 28] vs 38.8% [50 of 129]).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/98de/7997613/cb727d45c18c/radiol.2021202917.fig2.jpg)


