Abstract
Contrast-enhanced mammography (CEM) has emerged as a viable alternative to contrast-enhanced breast MRI, and it may increase access to vascular imaging while reducing examination cost. Intravenous iodinated contrast materials are used in CEM to enhance the visualization of tumor neovascularity. After injection, imaging is performed with dual-energy digital mammography, which helps provide a low-energy image and a recombined or iodine image that depict enhancing lesions in the breast. CEM has been demonstrated to help improve accuracy compared with digital mammography and US in women with abnormal screening mammographic findings or symptoms of breast cancer. It has also been demonstrated to approach the accuracy of breast MRI in preoperative staging of patients with breast cancer and in monitoring response after neoadjuvant chemotherapy. There are early encouraging results from trials evaluating CEM in the screening of women who are at an increased risk of breast cancer. Although CEM is a promising tool, it slightly increases radiation dose and carries a small risk of adverse reactions to contrast materials. This review details the CEM technique, diagnostic and screening uses, and future applications, including artificial intelligence and radiomics.
© RSNA, 2021
Summary
Contrast-enhanced mammography is an emerging breast imaging modality that helps improve diagnostic accuracy when routine breast imaging produces inconclusive findings and that may serve as an alternative to breast MRI.
Essentials
■ Contrast-enhanced mammography (CEM) is helpful in resolving equivocal findings detected at conventional breast imaging examinations.
■ CEM can be used for the preoperative staging of breast cancer to evaluate the extent of disease.
■ Screening CEM could be performed as an alternative to breast MRI in women who are at an increased risk of developing breast cancer.
■ CEM can accurately monitor response to neoadjuvant chemotherapy in patients with breast cancer.
■ Areas of active investigation of CEM developments include CEM-guided biopsy systems and artificial intelligence applications.
Introduction
Contrast-enhanced mammography (CEM) is an emerging technique that uses iodinated contrast materials for the visualization of breast neovascularity in a fashion similar to MRI (1). Vessels formed through the process of angiogenesis often leak the contrast material (2), and contrast diffuses within tumor tissue, resulting in an iodine-enhanced image. This allows a malignant tumor to be seen despite overlying dense breast tissue. Other names for CEM include contrast-enhanced spectral mammography, contrast-enhanced digital mammography, and contrast-enhanced dual-energy mammography.
Full-field digital mammography (FFDM) and digital breast tomosynthesis (DBT) rely on anatomic changes in the breast caused by breast cancer. Currently, they are pivotal in breast cancer screening and in assessment of symptomatic patients, although they have limited accuracy in women with dense breasts (1,2). For example, one study (3) reported that mammography sensitivity is 87.0% in women with entirely fatty breasts, but it decreases to 62.9% in women with extremely dense breasts (with specificity decreasing from 96.9% to 89.1%). A more recent study (4) using digital mammography also demonstrated a reduction of sensitivity with increasing breast density. Sensitivity was 79.9% overall, 100% for fatty breasts, 83.9% for breasts with scattered fibroglandular tissue, 72.9% for heterogeneously dense breasts, and 50% for extremely dense breasts. Although other studies (5) have demonstrated US to be superior to DBT as a supplemental examination, Kim et al (6) reported that DBT is comparable to US as an adjunct to FFDM for helping detect breast cancer, except in women with extremely dense breasts. Meanwhile, although supplemental imaging with MRI can be used to better evaluate women with dense breasts and those at an intermediate risk of breast cancer, CEM is less expensive and may be accessible to larger numbers of women.
In CEM, a dual-energy mammogram is acquired approximately 2 minutes after the intravenous injection of iodinated contrast material. The radiologist is provided with two images per breast and per view—one like a regular mammogram and the other highlighting areas of contrast uptake. CEM has the advantage of demonstrating both anatomic changes and local changes in breast perfusion, presumably caused by tumor angiogenesis. In a study involving 89 women with dense breasts and 100 lesions (7), CEM in addition to mammography improved sensitivity from 71.5% to 92.7% and specificity from 51.8% to 67.9%. Another study (8) reported that 13 of 14 cancers (93%) detected at CEM that were not seen at FFDM were seen in women with dense breasts.
The purpose of this review is to discuss the CEM technique, its use in diagnostic and screening settings, and its potential for future applications, including early data on artificial intelligence and radiomics. Where CEM is compared with mammography, the review compares CEM with two-dimensional mammography and does not consider tomosynthesis unless otherwise specified.
Imaging Protocol and Acquisition
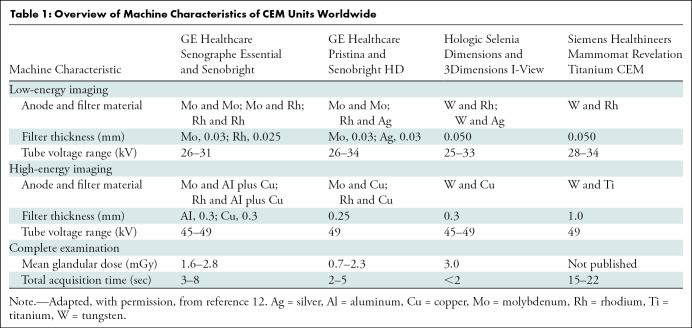
CEM is currently available in four commercial systems offered by three vendors, as follows: (a) contrast-enhanced spectral mammography (GE Healthcare), (b) contrast-enhanced two-dimensional imaging (Hologic), and (c) Titanium CEM, or TiCEM (Siemens Healthineers). Table 1 outlines current systems that are commercially available.
Table 1:
Overview of Machine Characteristics of CEM Units Worldwide
Although no studies have been published that document optimal imaging parameters for CEM (including contrast material concentration, dose, flow rate, and time interval between administration and image acquisition), generally accepted guidelines exist. A low-osmolarity iodine-based contrast material is intravenously injected before image acquisition. The intravenous line is placed by either a nurse or a technologist, depending on the institution. The iodine concentration of the contrast material can vary from 300 mg/mL to 370 mg/mL. A total volume of 1.5 mL/kg of body weight (with a maximum of 150 mL) is administered, preferably using an injector (injection rate, 2–3 mL/sec), followed by a saline flush.
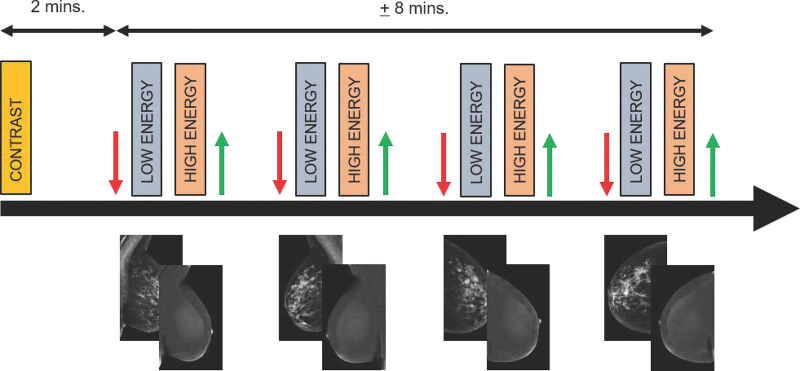
Image acquisition begins 2−2.5 minutes after contrast material injection. Standard craniocaudal and mediolateral oblique views of each breast are acquired (Fig 1). As contrast material may remain present in tissue for up to 10 minutes, there is often enough time for additional views (eg, rolled or compression views) if needed. Although some radiologists may prefer to image the symptomatic breast first, there is currently no scientific evidence to support this approach. It is our expert opinion and experience that image acquisition is best performed in the identical order that routine mammography is performed. To prevent contrast material splatter or contamination on the detector or breast, technologists should wear gloves and then remove them between contrast material administration and patient positioning. Alternatively, one technologist could administer the contrast material, while the other positions the patient.
Figure 1:
Diagram of imaging protocol for contrast-enhanced mammography. Two minutes before image acquisition, iodine-based contrast material is injected. Next, at minimum, both breasts are imaged in craniocaudal and mediolateral oblique views. In each step, compression is applied (red arrow), followed by rapid acquisition of low- and high-energy images. These images are processed to generate low-energy and recombined images. After each exposure, compression is released (green arrow). Images are considered to be of diagnostic value if they are acquired within 10 minutes after contrast material administration. mins. = minutes.
During CEM, low-energy (LE) and high-energy images are obtained in quick succession while the breast remains compressed. The x-ray spectrum of the LE exposure is that of FFDM using a tube voltage of 25–34 kV. Molybdenum, rhodium, or tungsten are typically used as anode material, whereas molybdenum, rhodium, or silver are typically used as filter material. Considering these settings, the LE image may replace the FFDM image with a similar performance (9,10). The high-energy image is obtained by using a higher tube voltage of 45–49 kV, and it uses similar anode materials with titanium or copper as filter material. The high-energy image is not suitable for diagnostic purposes but is used in postprocessing to generate the recombined or iodine image that shows areas of contrast enhancement. The total breast compression time of a single exposure depends on breast composition and thickness, and the time may vary from 2 seconds to 20 seconds (Table 1) (11).
When the energy of a photon is high enough to match the energy needed to eject an electron of iodine’s innermost K shell (during the high-energy exposure), the x-ray photon absorption probability rises by a factor of five, subsequently decreasing again with increasing photon energy. This is called the k-edge of iodine (33.2 keV). By acquiring the high energy above the level of this k-edge and by using this image for postprocessing of the recombined image, areas of iodine enhancement can be identified while unenhanced background tissue is eliminated (12). In clinical practice, the radiologist reads the LE and recombined (or iodine-enhanced) images. It should be stressed that the results of CEM examination are provided in a single report that includes the results of the LE images (using the Breast Imaging Reporting and Data System mammography lexicon) and the iodine-enhanced images (using part of the Breast Imaging Reporting and Data System MRI lexicon) (13). Kinetic curves are not used with CEM because there is only a single time point for each image, whereas other enhancement characteristics are specific to CEM. The American College of Radiology Breast Imaging Reporting and Data System group is in the process of developing a specific lexicon for CEM.
Evaluation of Abnormalities Found at Screening Mammography
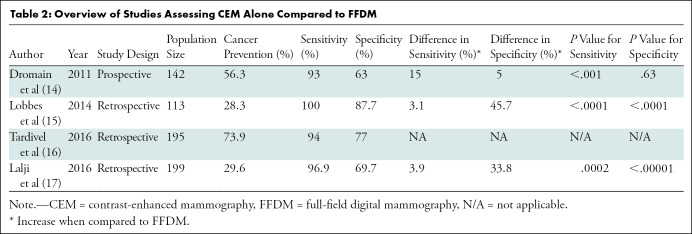
One of the earlier studied uses of CEM and still one of its most common indications is the assessment of abnormalities detected at screening mammography (Fig 2). Four initial studies (14–17) including 649 women (Table 2) demonstrated that the sensitivity of CEM ranged from 93% to 100% and specificity ranged from 63% to 88%. Three of these studies compared CEM with FFDM and showed significant improvement in specificity and sensitivity with CEM compared with FFDM, with increases in the ranges of 5%–46% and 3%–15%, respectively (14,15,17). One study (16) did not directly compare CEM to FFDM but used CEM as an adjunct to FFDM and US in postscreening evaluation; CEM sensitivity and specificity were 94% and 77%, respectively. Additionally, the study by Lalji et al (17) found that diagnostic performance was similar among radiologists with and without experience reading images from CEM, which suggests that the learning curve for CEM is not very steep, particularly for radiologists already experienced in reading images from FFDM and breast MRI examinations.
Figure 2:
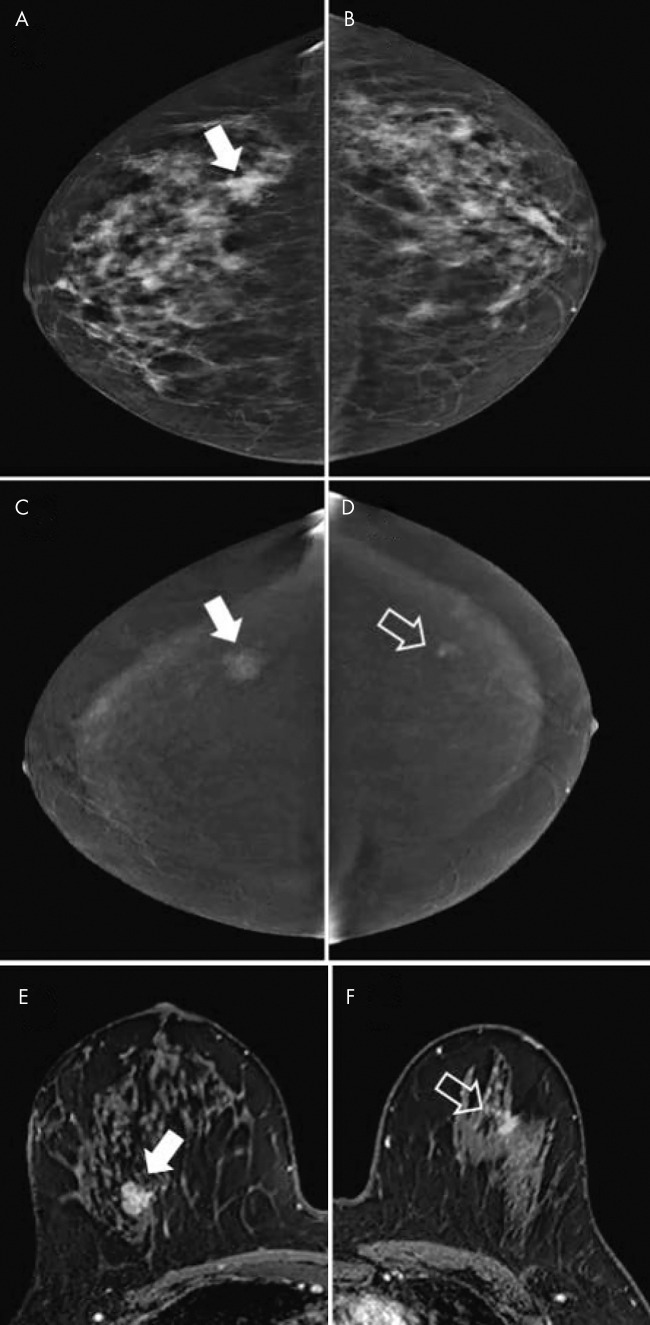
A, B, Contrast-enhanced mammographic (CEM) images in 52-year-old woman recalled from screening for new ill-defined mass (arrow in A) visible on, A, low-energy craniocaudal view but not visible on, B, low-energy mediolateral oblique view. C, D, Subsequent evaluation of recombined CEM images revealed no suspicious enhancement. Targeted US showed no lesions, but because of small lesion size, patient was categorized as having Breast Imaging Reporting and Data System category 3 lesion (follow-up after 6 months showed no breast cancer). Two subsequent rounds of screening (up to 4 years after primary evaluation) revealed no breast cancer.
Table 2:
Overview of Studies Assessing CEM Alone Compared to FFDM
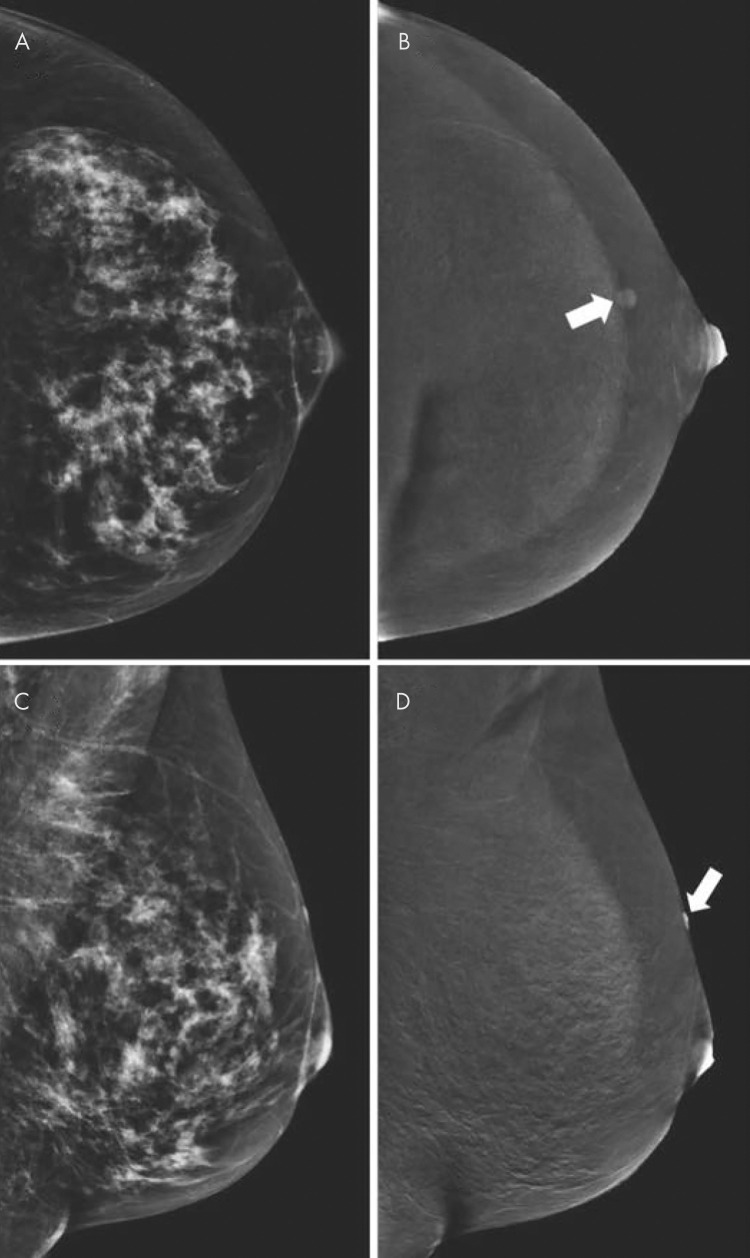
Several issues should be considered when using CEM to evaluate equivocal mammographic findings. First, benign lesions can show enhancement (Fig 3); the most commonly observed benign enhancing lesions in one study (18) were fibroadenomas, papillomas, and hyperplasia. Second, cysts have a classic appearance at CEM. On LE images, cysts are commonly observed as well-defined round or oval masses, but the assessment of margins may be partly obscured by fibroglandular tissue. On recombined images, cysts show no enhancement except for a thin enhancing rim. As in breast MRI, an inflamed cyst may have a thicker enhancing wall (Fig 4).
Figure 3:
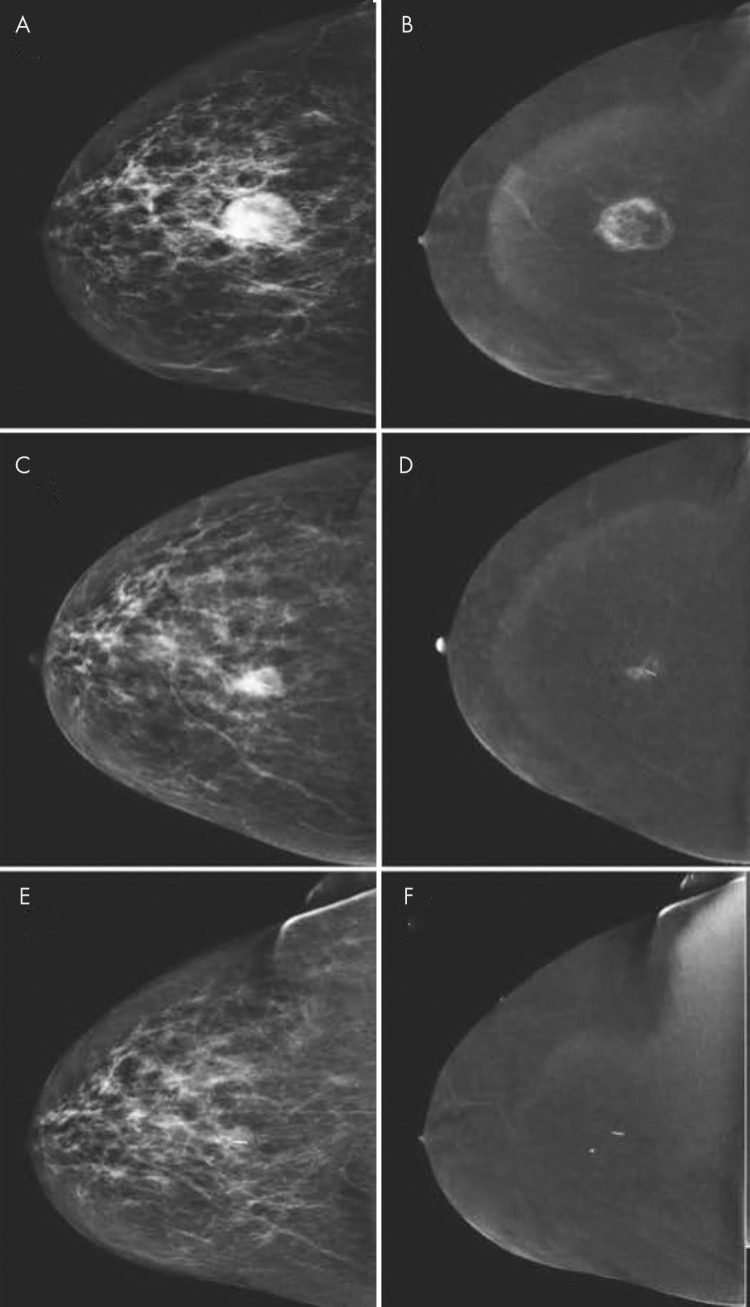
Contrast-enhanced mammographic images of enhancing benign lesion in 50-year-old woman. A, Low-energy craniocaudal image. B, Contrast-enhanced recombined craniocaudal image. C, Low-energy mediolateral oblique image. D, Contrast-enhanced recombined mediolateral oblique image. Contrast-enhanced craniocaudal view of left breast demonstrates a small, well-defined enhancing mass (arrow in B). Mediolateral oblique view of same breast demonstrates that lesion is located on skin (arrow in D). Visual inspection revealed skin hemangioma.
Figure 4:
Images in 44-year-old woman presenting with palpable abnormality in left breast illustrate that cysts have different appearance at contrast-enhanced mammography (CEM) and contrast-enhanced breast MRI. A, Recombined CEM image of left breast in mediolateral oblique view. B, Corresponding contrast-enhanced fat-suppressed T1-weighted breast MRI scan reconstructed in sagittal view. On both images, simple cysts (arrowheads) can be identified as well-defined masses showing no or negative enhancement. Inflamed cysts (arrow) may show thickened and often slightly irregular wall, which enhances after contrast material administration.
With respect to the assessment of suspicious microcalcifications, CEM might be beneficial. In one study (19), the enhancement of calcifications suggested underlying breast cancer or ductal carcinoma in situ (DCIS) (sensitivity and specificity of CEM were 88.9% and 86.6%, respectively). However, in another study with a comparable population (20), differences in diagnostic performance between CEM and conventional mammography were negligible (sensitivity of 93.8% and specificity of 36.6% for CEM vs sensitivity of 90.8% and specificity of 39.1% for conventional mammography). Hence, it was concluded that there was no clear benefit in using CEM to evaluate suspicious breast calcifications. Therefore, as with MRI, suspicious calcifications at mammography should be biopsied whether or not they show enhancement on CEM images.
Despite the success of CEM for the evaluation of abnormalities found at screening, most studies cited within this section are retrospective and considered only scenarios with a high prevalence of breast cancer. Prospective randomized clinical trials are underway to compare the work-up of women recalled with either conventional imaging or CEM (21).
Evaluation of Symptomatic Patients
One of the most common symptoms among women presenting for breast imaging is a palpable mass. In a multireader study involving 100 women with palpable abnormalities (22), 73% of whom had a malignancy, CEM outperformed mammography in sensitivity (95% vs 84%; P < .025) and specificity (81% vs 63; P < .025). However, when CEM was compared with US in 115 symptomatic women, 89% of whom had palpable masses, there were no significant differences in performance between the two modalities (23). A third study (24) demonstrated the superior sensitivity of CEM compared with LE images. To our knowledge, no studies are dedicated to CEM in women experiencing other symptoms.
Preoperative Assessment of Extent of Local Disease Using CCEM
Another setting in which CEM was first studied was for the preoperative staging of women with newly diagnosed breast cancer. This imaging indication for breast MRI has been used worldwide for many years, albeit with some controversy because of its high cost, limited availability, and false-positive findings that can lead to additional biopsies, patient anxiety, and in some cases, mastectomy. CEM can be an alternative to MRI, although CEM does not enable evaluation of the axilla or other local nodal groups. As CEM contains information of both an FFDM examination (in the LE image) and a vascular image in a single appointment, those women who undergo CEM as part of their initial diagnostic work-up and are subsequently diagnosed with breast cancer will have already had preoperative (in-breast) staging.
To ensure a reliable staging test, it is important to accurately assess the size and extent of the index tumor. A few studies (25–27) have shown that tumor sizes measured with CEM ranged from 0.03 mm to 5 mm of the actual tumor size measured at the time of surgery. Although one study (8) showed that CEM was most highly correlated with surgical specimens (Pearson correlation coefficients were 0.73 for CEM, 0.65 for MRI, and 0.6 for LE images), another study (28) showed better accuracy with MRI than with LE or CEM for size at microscopy (Pearson correlation coefficients were 0.84 for MRI, 0.44 for LE, and 0.77 for CEM, with P < .001 for all). Once the initial size of the index tumor is assessed, CEM can be used to accurately determine if the disease is unifocal, multifocal, multicentric, or bilateral (Fig 5). These determinations may lead to valuable improvements in treatment.
Figure 5:
Images obtained for disease extent assessment in 64-year-old woman recalled from screening for irregular mass in right breast. A, Low-energy image of right breast (craniocaudal view) shows irregular mass (arrow). B, No lesion is seen on low-energy image in contralateral breast. C, Image from contrast-enhanced mammography (CEM) (right craniocaudal view) shows enhancement of mass (arrow). D, CEM image shows contralateral irregular enhancing mass (arrow), which was not visible on low-energy image. E, F, Contrast-enhanced MRI scans of, E, right breast and, F, left breast show both lesions (arrow). Lesions were diagnosed as invasive breast cancer of no special type (estrogen receptor positive, progesterone receptor positive, and human epidermal growth factor receptor type 2 negative).
As to the value of CEM in assessing disease extent of the index tumor, the first study to evaluate this (25) demonstrated that CEM was significantly better than FFDM in depicting the index tumor and was equal in sensitivity to MRI. In the ipsilateral breast, MRI depicted more additional sites of cancer than did CEM (22 sites with MRI vs 14 sites with CEM). In the contralateral breast, a single cancer was missed with both CEM and MRI (Paget disease). The additional sensitivity of MRI was at the expense of a much higher false-positive rate compared with CEM (13 false-positive findings with MRI vs two false-positive findings with CEM). Additional findings appropriately altered treatment in 11 patients who underwent MRI compared with eight patients who underwent CEM. A later study in 80 women (8) demonstrated equal sensitivity of CEM and MRI for both the index tumor and additional lesions. A third study comparing the diagnostic performance of CEM and MRI in 84 women with newly diagnosed invasive breast cancer and DCIS (29) found no significant difference in sensitivity to detect index cancers or additional lesions in either the ipsilateral or contralateral breasts, with similar changes in surgical management.
In a study evaluating change in surgical management based on CEM findings (30), CEM depicted 98% of index lesions among 128 women with breast cancer, missing one finding of Paget disease and a parasternal lesion not included in the field of view. Twelve percent of patients required additional biopsies based on CEM findings, 67% of which were cancers. CEM changed surgical management in 20% of patients, leading to mastectomy in 4%.
Altogether, studies of CEM in preoperative staging show that CEM can be a viable alternative to MRI, with accurate assessment of index tumor size, similar or possibly slightly inferior detection of additional sites of disease, and superior positive predictive value for detected additional lesions.
CEM in Monitoring Response to Neoadjuvant Chemotherapy
Neoadjuvant chemotherapy (NAC) is increasingly used before definitive breast cancer surgery to improve the likelihood of candidacy for breast conservation or to improve cosmetic outcomes in women with locally advanced tumors. The use of chemotherapy to treat the intact tumor is informed by the effectiveness of the drug combination for a specific breast cancer. Accurate delineation of disease extent is critical in planning breast conservation in the neoadjuvant setting. Multiple studies (31,32) have demonstrated the superiority of MRI to physical examination, mammography, and US in evaluating response to NAC. Early data suggest that the same may be true of CEM (Fig 6).
Figure 6:
Images of response monitoring using contrast-enhanced mammography in a 54-year-old woman diagnosed with 3.0-cm invasive breast cancer of no special type (grade 3, estrogen receptor negative, progesterone receptor negative, and human epidermal growth factor receptor type 2 positive). A, Low-energy image in craniocaudal view shows cancer. B, Recombined image shows peripheral enhancement, which is suggestive of central necrosis. The patient underwent neoadjuvant systemic therapy. C, Low-energy, and, D, recombined craniocaudal views obtained during treatment show decrease in index tumor size (C) with decrease in enhancement (D). E, Low-energy and, F, recombined images obtained after completion of therapy. There was no residual mass on low-energy image. Resolution of enhancement on recombined image was consistent with complete radiologic response. Surgical specimen showed pathologic complete response without any residual ductal carcinoma in situ.
In the first study to compare the ability of CEM and MRI to assess response to NAC(33), 54 women prospectively underwent both imaging examinations before and after completion of NAC. The examinations were read independently, and readers were blinded to the results of the other examination. Forty-six women (85%) completed the study. CEM was superior to MRI in the prediction of pathologic complete response (the Lin coefficient was 0.81 for CEM vs 0.59 for MRI). The extent of residual disease was slightly underestimated with both imaging examinations, with CEM estimating more accurately than MRI (mean underestimation, 4.1 mm with CEM vs 7.5 mm with breast MRI). In another prospective study involving 21 women, CEM was also shown to be valuable in the prediction of tumor response after neoadjuvant chemotherapy (34), with a negative predictive value of 80.0% and a positive predictive value of 62.5%.
A third study (35), which was retrospective, compared the results of CEM and MRI in 65 patients who underwent NAC, using surgical pathologic findings as the reference standard. Both modalities indicated equivalence to the histopathologic results within 1 cm. Sensitivity, specificity, positive predictive value, and negative predictive value in predicting complete response were also equivalent. In comparison, a prospective study comparing the results of CEM to MRI among 33 women undergoing NAC (36) found that performance metrics varied between MRI and CEM for sensitivity (92% vs 76%), specificity (75% vs 87.5%), positive predictive value (92% vs 95%), and negative predictive value (75% vs 86.4%), and the mean differences in residual tumor size assessment using CEM or MRI (compared with histopathologic results) were 0.8 cm and 1.8 cm, respectively.
It is important to clarify the definition of pathologic complete response when evaluating the results of neoadjuvant imaging trials. Although the presence or absence of residual DCIS must be accurately defined for surgical purposes, the absence of residual invasive cancer is defined as a pathologic complete response in some studies (ie, residual DCIS is allowable). In other studies, complete absence of any residual tumor (both in situ and invasive) is required to be considered a pathologic complete response. Only the study by Barra et al (36) defined pathologic complete response as the absence of any invasive residual breast cancer regardless of the presence of DCIS.
Thus far, early data regarding the use of CEM to assess response to NAC are promising. Although the number of patients studied after receiving NAC is small, the results appear consistent. Additionally, the LE image replaces and obviates the need for an additional FFDM examination so that the patient needs only one appointment after therapy.
CEM as a Screening Tool
Contrast-enhanced breast MRI is recommended for women at high risk of developing breast cancer (ie, >20% lifetime risk of developing breast cancer); it is the most sensitive breast cancer screening tool, reported to have sensitivity of up to 97% (37,38), largely because of its ability to help detect neovascularity. However, in addition to women at high risk of developing breast cancer, many women at intermediate risk (15%–20% lifetime risk) and many with dense breasts may also benefit from supplemental screening. This includes women with a family history of breast cancer, women with certain high-risk lesions, as well as 3.8 million survivors of breast cancer and 25 million women with dense breasts in the United States alone (39). Currently, it is difficult to accommodate such a large number of patients requiring MRI or even abbreviated breast MRI. As extensive data demonstrate that CEM may help improve sensitivity and specificity when compared with digital mammography alone or to mammography plus US, particularly in patients with dense breasts (6,7,16,19,25,27,36), CEM may provide additional vascular imaging capacity to better screen patients at intermediate risk of breast cancer and patients with dense breasts.
The first prospective study comparing CEM to MRI (using the LE image as a substitute for the FFDM image) (40) involved 307 women at increased risk of breast cancer who had undergone yearly screening with MRI and mammography. No cancers were detected on the LE image. MRI and CEM depicted the same two invasive lobular cancers. A third patient with a focus of enhancement at MRI that was occult at CEM demonstrated atypical ductal hyperplasia at biopsy 1 year later (the patient delayed follow-up examination), and the lesion was upgraded to DCIS at surgery. There were no symptomatic interval cancers, but two screening-detected cancers were detected at MRI at 1-year follow-up. Specificity of CEM was equivalent to that of MRI in this study. Although the specificity of CEM was expected to be better than that of MRI according to previous data, it is possible that it was lower in this study as all CEM examinations were prevalence studies (as opposed to MRI, where multiple previous examinations were available for comparison), leading to a higher false-positive rate and therefore lower specificity.
Three other studies have addressed the potential of CEM to be used in screening in different ways. One retrospective study (41) compared CEM to US in a cohort of 953 patients, 725 (76%) of whom were asymptomatic screening patients, to determine the value of US after CEM. Because CEM outperformed US in sensitivity (97% vs 92%) and specificity (40% vs 8%), the investigators concluded that the use of US was questionable in the setting of a negative CEM finding, as it could lead to unnecessary biopsies. Another retrospective study (24) compared CEM and US to screening mammography in 611 women with dense breasts at an increased risk of breast cancer. CEM outperformed mammography in sensitivity (90.5% vs 52.4%; P = .0008) but had reduced specificity (76.1% vs 90.5%; P = .001). This study also showed the potential disadvantage of screening US in women undergoing CEM, as 74 lesions were detected at US that did not enhance, leading to additional biopsies with no added cancers. Last, a retrospective study comparing 904 baseline screening CEM examinations with conventional mammography (77% of participants had dense breasts, and more than 90% had other risk factors) (42) showed that CEM depicted 15 cancers in 14 patients, corresponding to a cancer detection rate of 15.5 per 1000 examinations, similar to that reported for MRI. Six of the 16 cancers (38%) were detected on contrast-enhanced images alone.
CEM may be a viable tool for imaging women at an increased risk of breast cancer, and it is important to determine if specific subgroups of women may benefit from CEM. The number of patients in the current literature is too small for adequate subgroup analysis, but researchers have used early data to begin to define those populations. A recent study among 132 women with a history of lobular neoplasia (43) demonstrated the value of CEM in detecting six cancers (sensitivity, 100%; specificity, 88%; negative predictive value, 100%; and accuracy, 88%).
Thus, preliminary data on the use of CEM in the screening setting are promising. Results suggest that CEM is more sensitive than FFDM alone, more sensitive than FFDM plus US, and more specific than FFDM plus US (24,41,42). The sensitivity of CEM approaches that of breast MRI, and CEM is likely more specific (44). Currently, the total number of patients studied thus far is small. Prospective multicenter trials that compare cancer detection rates and other metrics of CEM with those of standard screening studies, such as FFDM or DBT, with screening US or breast MRI (complete or abbreviated) are needed. The forthcoming American College of Radiology Contrast-enhanced Mammography Imaging Screening Trial will determine if CEM can help improve breast cancer detection compared with that of DBT plus screening US in patients with dense breasts and with an average to intermediate risk of breast cancer. Secondary end points will compare the tumor biologic characteristics of invasive cancers and DCIS detected at CEM and DBT plus screening US. The study is intended to have 2500 enrolled patients who will undergo two rounds of screening and 1-year follow-up.
In addition to favorable performance metrics, CEM is well tolerated by patients. When 49 women were interviewed regarding their preference of CEM compared with MRI, the women indicated that they preferred CEM because it was faster, more comfortable, and less noisy (P < .001) (45). In a different prospective screening trial (46), 79% of women preferred CEM to MRI if the examinations had equal sensitivity. Therefore, CEM can be considered a good alternative for women who are unable or unwilling to undergo MRI.
Limitations of CEM
The most substantial limitation of CEM is the possibility of contrast material reactions, which can range from mild to fatal hypersensitivity reactions. In a systematic review of 84 articles involving 14,012 patients, Zanardo et al (47) reported that the pooled reaction rate in 17 of 84 of the studies was 0.82% (of 30 reactions, 26 were mild, three were moderate, and one was a severe nonfatal reaction). Nevertheless, patients should always be screened for contrast material allergy history, and patients with such a history, even if mild, should be considered for MRI rather than CEM.
Contrast material–induced renal insufficiency is extremely rare. Older patients or those with potential renal insufficiency are screened in advance, and anyone with abnormal renal function does not receive iodinated contrast material.
CEM requires an increased radiation dose varying from 20% to 80% depending on system settings, breast thickness, and type of mammographic device (48,49). This includes using the LE images as the routine mammogram (9,10,50). Although minimizing radiation exposure is important, the radiation dose of CEM is less than that of FFDM plus DBT, falls within the Mammography Quality Standards Act guidelines, and does not result in an important increase of lifetime attributable risk factors (37). Some breast lesions cannot be visualized because of their position within the breast. Lesions close to the chest wall or in the medial part of the breast may be overlooked (17), and breast MRI should be considered if lesions are suspected in these locations.
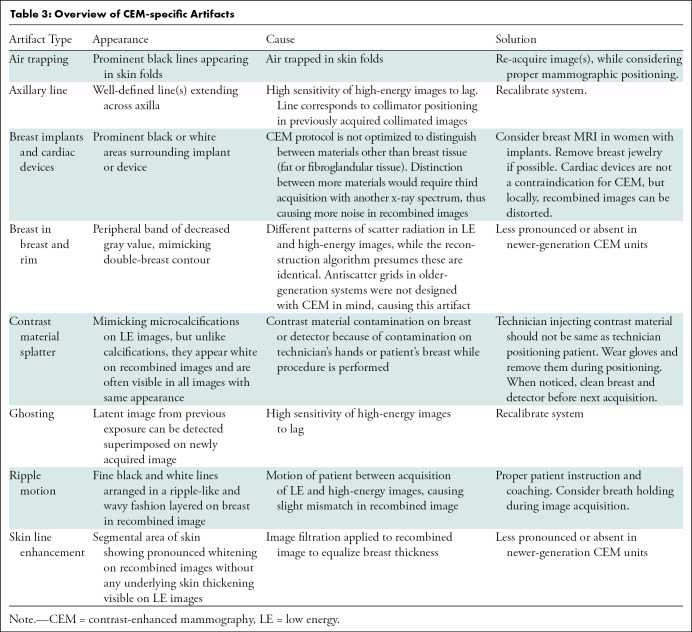
Artifacts observed at FFDM, such as hair or antiperspirant, may also be seen on CEM images (51). Additionally, some breast lesions cannot be visualized because of their position within the breast. Lesions close to the chest wall or in the medial part of the breast may be overlooked (17), and supplemental breast MRI should be considered if lesions are suspected in these locations. Artifacts specific to CEM, such as those caused by contrast material contamination, are seen on the recombined image (52,53). A summary of the appearance, cause, and solutions to frequently observed artifacts is presented in Table 3. Most artifacts occur in older CEM systems, and image quality has improved in newer systems.
Table 3:
Overview of CEM-specific Artifacts
Although the negative predictive value of CEM varies from 94% to 100% (15,24), some breast cancer phenotypes (eg, mucinous carcinoma or invasive lobular carcinoma) may show weak or even no enhancement at CEM and may also be overlooked with breast MRI (17,54). As these studies demonstrate, lack of enhancement may be a false-negative finding, but faintly enhancing lesions may be malignant and should be followed up. Noncalcified DCIS is known to be missed at mammography. If there is also no enhancement, then this could be another potential false-negative lesion.
Finally, it should be emphasized that scientific evidence on the use of CEM is largely based on single-center studies with relatively small study cohorts. The results of these studies should be interpreted with this limitation in mind. It also confirms the need for larger, multicenter prospective trials using CEM to further confirm its potential as a breast imaging tool.
The Future of CEM
CEM is becoming more commonly used, with an increasing number of units installed, examinations performed, and studies published (47). Continuous developments in this field are to be expected.
Since the advent of CEM, DBT has been increasingly used for routine mammography, particularly in the United States, where it may eventually replace two-dimensional mammography to some extent. Data evaluating contrast-enhanced DBT are limited. Although there has not yet been a direct comparison of CEM to DBT in the screening setting, vascular imaging has been compared with nonvascular imaging in 185 patients with Breast Imaging Reporting and Data System category 4 or 5 lesions (55). Not surprisingly, MRI, CEM, and contrast-enhanced tomosynthesis were more sensitive than unenhanced DBT. In another study (56), contrast-enhanced DBT in 21 patients enabled improved lesion margin assessment compared with CEM, without reduced contrast enhancement of the lesions. Clearly, contrast-enhanced DBT is a promising area for future research.
Although most lesions detected at CEM have a correlate on the LE images or at US scans, some enhancing lesions may be observed without a mammographic or sonographic correlate for image-guided biopsy. Currently, MRI is performed to enable an image-guided biopsy, and some institutions might even consider CT-guided biopsies. The U.S. Food and Drug Administration has approved a CEM biopsy device developed by GE Healthcare (57). Once it becomes more available, this will obviate the need for MRI-guided biopsies.
In line with current developments in artificial intelligence and radiomics in breast imaging research, investigators have begun to evaluate artificial intelligence with CEM. By using machine learning algorithms based on morphologic and textural features of 50 breast lesions derived from both LE and recombined images, Patel et al (58) correctly classified 45 of 50 breast lesions (90% accuracy). By applying radiomics to CEM images, Marino et al (59) were able to distinguish between invasive and noninvasive breast cancers, hormone receptor–positive and hormone receptor–negative cancers, human epidermal growth factor receptor 2–positive and/or hormone receptor–negative and human epidermal growth factor receptor 2–negative and/or hormone receptor–positive breast cancers, triple-negative and triple-positive breast cancers, and triple-negative and hormone receptor–positive breast cancers, with accuracies from 78.4% to 100%. These preliminary results establish the promise of these techniques in CEM.
Conclusion
Contrast-enhanced mammography (CEM) was commercially introduced in 2011 and is an emerging breast imaging tool. CEM unit installations, examinations, and scientific publications have all increased. CEM is easy to perform in everyday clinical practice and is useful in indications including abnormal screenings, symptomatic patients, preoperative staging of breast cancer, evaluation of response to neoadjuvant chemotherapy, screening women with dense breasts, and screening women at an increased risk of developing breast cancer. As CEM vendors increase and expected improvements in the technique evolve, the use of CEM will further expand, especially where breast MRI availability is limited.
Acknowledgments
Acknowledgments
Joanne Chin, MFA, ELS, and Alyssa Duck, PhD, provided assistance in editing this manuscript.
M.S.J. supported in part through the National Institutes of Health and National Cancer Institute Cancer Center Support Grant (P30 CA008748).
Disclosures of Conflicts of Interest: M.S.J. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: has consulted for Bayer; receives payment for lectures, including service on speakers bureaus, from GE Healthcare. Other relationships: disclosed no relevant relationships. M.B.I.L. Activities related to the present article: institution received grant from GE Healthcare; institution received consulting fee or honorarium from GE Healthcare. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships.
Abbreviations:
- CEM
- contrast-enhanced mammography
- DBT
- digital breast tomography
- DCIS
- ductal carcinoma in situ
- FFDM
- full-field digital mammography
- LE
- low energy
- NAC
- neoadjuvant chemotherapy
References
- 1.Patel BK, Lobbes MBI, Lewin J. Contrast Enhanced Spectral Mammography: A Review. Semin Ultrasound CT MR 2018;39(1):70–79. [DOI] [PubMed] [Google Scholar]
- 2.Kuhl C. The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology 2007;244(2):356–378. [DOI] [PubMed] [Google Scholar]
- 3.Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med 2003;138(3):168–175. [DOI] [PubMed] [Google Scholar]
- 4.Weigel S, Heindel W, Heidrich J, Hense HW, Heidinger O. Digital mammography screening: sensitivity of the programme dependent on breast density. Eur Radiol 2017;27(7):2744–2751. [DOI] [PubMed] [Google Scholar]
- 5.Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct Screening With Tomosynthesis or Ultrasound in Women With Mammography-Negative Dense Breasts: Interim Report of a Prospective Comparative Trial. J Clin Oncol 2016;34(16):1882–1888. [DOI] [PubMed] [Google Scholar]
- 6.Kim WH, Chang JM, Lee J, et al. Diagnostic performance of tomosynthesis and breast ultrasonography in women with dense breasts: a prospective comparison study. Breast Cancer Res Treat 2017;162(1):85–94 [Published correction appears in Breast Cancer Res Treat 2017;163(1):197.]. [DOI] [PubMed] [Google Scholar]
- 7.Cheung YC, Lin YC, Wan YL, et al. Diagnostic performance of dual-energy contrast-enhanced subtracted mammography in dense breasts compared to mammography alone: interobserver blind-reading analysis. Eur Radiol 2014;24(10):2394–2403. [DOI] [PubMed] [Google Scholar]
- 8.Fallenberg EM, Dromain C, Diekmann F, et al. Contrast-enhanced spectral mammography versus MRI: Initial results in the detection of breast cancer and assessment of tumour size. Eur Radiol 2014;24(1):256–264. [DOI] [PubMed] [Google Scholar]
- 9.Francescone MA, Jochelson MS, Dershaw DD, et al. Low energy mammogram obtained in contrast-enhanced digital mammography (CEDM) is comparable to routine full-field digital mammography (FFDM). Eur J Radiol 2014;83(8):1350–1355. [DOI] [PubMed] [Google Scholar]
- 10.Lalji UC, Jeukens CR, Houben I, et al. Evaluation of low-energy contrast-enhanced spectral mammography images by comparing them to full-field digital mammography using EUREF image quality criteria. Eur Radiol 2015;25(10):2813–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lobbes M, Jochelson MS, eds. Contrast-Enhanced Mammography. New York, NY: Springer, 2019. [Google Scholar]
- 12.Jeukens CRLPN. Physics of Contrast-Enhanced Mammography. In: Lobbes M, Jochelson MS, eds. Contrast-Enhanced Mammography. Cham, Switzerland: Springer, 2019; 23–39. [Google Scholar]
- 13.D’Orsi CJ, Sickles EA, Mendelson EB, et al. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, Va: American College of Radiology, 2013. [Google Scholar]
- 14.Dromain C, Thibault F, Muller S, et al. Dual-energy contrast-enhanced digital mammography: initial clinical results. Eur Radiol 2011;21(3):565–574. [DOI] [PubMed] [Google Scholar]
- 15.Lobbes MB, Lalji U, Houwers J, et al. Contrast-enhanced spectral mammography in patients referred from the breast cancer screening programme. Eur Radiol 2014;24(7):1668–1676. [DOI] [PubMed] [Google Scholar]
- 16.Tardivel AM, Balleyguier C, Dunant A, et al. Added Value of Contrast-Enhanced Spectral Mammography in Postscreening Assessment. Breast J 2016;22(5):520–528. [DOI] [PubMed] [Google Scholar]
- 17.Lalji UC, Houben IP, Prevos R, et al. Contrast-enhanced spectral mammography in recalls from the Dutch breast cancer screening program: validation of results in a large multireader, multicase study. Eur Radiol 2016;26(12):4371–4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houben IPL, Van de Voorde P, Jeukens CRLPN, et al. Contrast-enhanced spectral mammography as work-up tool in patients recalled from breast cancer screening has low risks and might hold clinical benefits. Eur J Radiol 2017;94:31–37. [DOI] [PubMed] [Google Scholar]
- 19.Cheung YC, Juan YH, Lin YC, et al. Dual-Energy Contrast-Enhanced Spectral Mammography: Enhancement Analysis on BI-RADS 4 Non-Mass Microcalcifications in Screened Women. PLoS One 2016;11(9):e0162740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houben IP, Vanwetswinkel S, Kalia V, et al. Contrast-enhanced spectral mammography in the evaluation of breast suspicious calcifications: diagnostic accuracy and impact on surgical management. Acta Radiol 2019;60(9):1110–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neeter LMFH, Houben IPL, Nelemans PJ, et al. Rapid Access to Contrast-Enhanced spectral mammogRaphy in women recalled from breast cancer screening: the RACER trial study design. Trials 2019;20(1):759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tennant SL, James JJ, Cornford EJ, et al. Contrast-enhanced spectral mammography improves diagnostic accuracy in the symptomatic setting. Clin Radiol 2016;71(11):1148–1155. [DOI] [PubMed] [Google Scholar]
- 23.Lu Z, Hao C, Pan Y, Mao N, Wang X, Yin X. Contrast-Enhanced Spectral Mammography Versus Ultrasonography: Diagnostic Performance in Symptomatic Patients with Dense Breasts. Korean J Radiol 2020;21(4):442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorin V, Yagil Y, Yosepovich A, et al. Contrast-Enhanced Spectral Mammography in Women With Intermediate Breast Cancer Risk and Dense Breasts. AJR Am J Roentgenol 2018;211(5):W267–W274. [DOI] [PubMed] [Google Scholar]
- 25.Jochelson MS, Dershaw DD, Sung JS, et al. Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology 2013;266(3):743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lobbes MB, Lalji UC, Nelemans PJ, et al. The quality of tumor size assessment by contrast-enhanced spectral mammography and the benefit of additional breast MRI. J Cancer 2015;6(2):144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Travieso-Aja MDM, Naranjo-Santana P, Fernández-Ruiz C, et al. Factors affecting the precision of lesion sizing with contrast-enhanced spectral mammography. Clin Radiol 2018;73(3):296–303. [DOI] [PubMed] [Google Scholar]
- 28.Cheung YC, Juan YH, Lo YF, Lin YC, Yeh CH, Ueng SH. Preoperative assessment of contrast-enhanced spectral mammography of diagnosed breast cancers after sonographic biopsy: Correlation to contrast-enhanced magnetic resonance imaging and 5-year postoperative follow-up. Medicine (Baltimore) 2020;99(5):e19024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim EY, Youn I, Lee KH, et al. Diagnostic Value of Contrast-Enhanced Digital Mammography versus Contrast-Enhanced Magnetic Resonance Imaging for the Preoperative Evaluation of Breast Cancer. J Breast Cancer 2018;21(4):453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali-Mucheru M, Pockaj B, Patel B, et al. Contrast-Enhanced Digital Mammography in the Surgical Management of Breast Cancer. Ann Surg Oncol 2016;23(Suppl 5):649–655. [DOI] [PubMed] [Google Scholar]
- 31.Rosen EL, Blackwell KL, Baker JA, et al. Accuracy of MRI in the detection of residual breast cancer after neoadjuvant chemotherapy. AJR Am J Roentgenol 2003;181(5):1275–1282. [DOI] [PubMed] [Google Scholar]
- 32.Lobbes MB, Prevos R, Smidt M, et al. The role of magnetic resonance imaging in assessing residual disease and pathologic complete response in breast cancer patients receiving neoadjuvant chemotherapy: a systematic review. Insights Imaging 2013;4(2):163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iotti V, Ravaioli S, Vacondio R, et al. Contrast-enhanced spectral mammography in neoadjuvant chemotherapy monitoring: a comparison with breast magnetic resonance imaging. Breast Cancer Res 2017;19(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ElSaid NA, Mahmoud HGM, Salama A, Nabil M, ElDesouky ED. Role of contrast enhanced spectral mammography in predicting pathological response of locally advanced breast cancer post neo-adjuvant chemotherapy. Egypt J Radiol Nucl Med 2017;48(2):519–527. [Google Scholar]
- 35.Patel BK, Hilal T, Covington M, et al. Contrast-Enhanced Spectral Mammography is Comparable to MRI in the Assessment of Residual Breast Cancer Following Neoadjuvant Systemic Therapy. Ann Surg Oncol 2018;25(5):1350–1356. [DOI] [PubMed] [Google Scholar]
- 36.Barra FR, Sobrinho AB, Barra RR, et al. Contrast-Enhanced Mammography (CEM) for Detecting Residual Disease after Neoadjuvant Chemotherapy: A Comparison with Breast Magnetic Resonance Imaging (MRI). BioMed Res Int 2018;2018:8531916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sung JS, Stamler S, Brooks J, et al. Breast Cancers Detected at Screening MR Imaging and Mammography in Patients at High Risk: Method of Detection Reflects Tumor Histopathologic Results. Radiology 2016;280(3):716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007;57(2):75–89 [Published correction appears in CA Cancer J Clin 2007;57(3):185.]. [DOI] [PubMed] [Google Scholar]
- 39.Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst 2014;106(10):dju255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jochelson MS, Pinker K, Dershaw DD, et al. Comparison of screening CEDM and MRI for women at increased risk for breast cancer: A pilot study. Eur J Radiol 2017;97:37–43. [DOI] [PubMed] [Google Scholar]
- 41.Klang E, Krosser A, Amitai MM, et al. Utility of routine use of breast ultrasound following contrast-enhanced spectral mammography. Clin Radiol 2018;73(10):908.e11–908.e16. [DOI] [PubMed] [Google Scholar]
- 42.Sung JS, Lebron L, Keating D, et al. Performance of Dual-Energy Contrast-enhanced Digital Mammography for Screening Women at Increased Risk of Breast Cancer. Radiology 2019;293(1):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hogan MP, Amir T, Sevilimedu V, Sung J, Morris EA, Jochelson MS. Contrast-Enhanced Digital Mammography Screening for Intermediate Risk Women with a History of Lobular Neoplasia. AJR Am J Roentgenol (in press). [DOI] [PMC free article] [PubMed]
- 44.Suter MB, Pesapane F, Agazzi GM, et al. Diagnostic accuracy of contrast-enhanced spectral mammography for breast lesions: A systematic review and meta-analysis. Breast 2020;53:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hobbs MM, Taylor DB, Buzynski S, Peake RE. Contrast-enhanced spectral mammography (CESM) and contrast enhanced MRI (CEMRI): Patient preferences and tolerance. J Med Imaging Radiat Oncol 2015;59(3):300–305. [DOI] [PubMed] [Google Scholar]
- 46.Phillips J, Miller MM, Mehta TS, et al. Contrast-enhanced spectral mammography (CESM) versus MRI in the high-risk screening setting: patient preferences and attitudes. Clin Imaging 2017;42:193–197. [DOI] [PubMed] [Google Scholar]
- 47.Zanardo M, Cozzi A, Trimboli RM, et al. Technique, protocols and adverse reactions for contrast-enhanced spectral mammography (CESM): a systematic review. Insights Imaging 2019;10(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeukens CR, Lalji UC, Meijer E, et al. Radiation exposure of contrast-enhanced spectral mammography compared with full-field digital mammography. Invest Radiol 2014;49(10):659–665. [DOI] [PubMed] [Google Scholar]
- 49.James JR, Pavlicek W, Hanson JA, Boltz TF, Patel BK. Breast Radiation Dose With CESM Compared With 2D FFDM and 3D Tomosynthesis Mammography. AJR Am J Roentgenol 2017;208(2):362–372. [DOI] [PubMed] [Google Scholar]
- 50.Fallenberg EM, Dromain C, Diekmann F, et al. Contrast-enhanced spectral mammography: Does mammography provide additional clinical benefits or can some radiation exposure be avoided? Breast Cancer Res Treat 2014;146(2):371–381. [DOI] [PubMed] [Google Scholar]
- 51.Nori J, Gill MK, Vignoli C, et al. Artefacts in contrast enhanced digital mammography: how can they affect diagnostic image quality and confuse clinical diagnosis? Insights Imaging 2020;11(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gluskin J, Click M, Fleischman R, Dromain C, Morris EA, Jochelson MS. Contamination artifact that mimics in-situ carcinoma on contrast-enhanced digital mammography. Eur J Radiol 2017;95(1):147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yagil Y, Shalmon A, Rundstein A, et al. Challenges in contrast-enhanced spectral mammography interpretation: artefacts lexicon. Clin Radiol 2016;71(5):450–457. [DOI] [PubMed] [Google Scholar]
- 54.van Nijnatten TJA, Jochelson MS, Pinker K, et al. Differences in degree of lesion enhancement on CEM between ILC and IDC. BJR Open 2019;1(1):20180046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chou CP, Lewin JM, Chiang CL, et al. Clinical evaluation of contrast-enhanced digital mammography and contrast enhanced tomosynthesis--Comparison to contrast-enhanced breast MRI. Eur J Radiol 2015;84(12):2501–2508. [DOI] [PubMed] [Google Scholar]
- 56.Huang H, Scaduto DA, Liu C, et al. Comparison of contrast-enhanced digital mammography and contrast-enhanced digital breast tomosynthesis for lesion assessment. J Med Imaging (Bellingham) 2019;6(3):031407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.GE Healthcare . GE Healthcare Receives FDA Clearance of the Industry’s First Contrast-Enhanced Mammography Solution for Biopsy. https://www.ge.com/news/press-releases/ge-healthcare-receives-fda-clearance-industry%e2%80%99s-first-contrast-enhanced-mammography. Published June 9, 2020. Accessed October 8, 2020. [Google Scholar]
- 58.Patel BK, Ranjbar S, Wu T, et al. Computer-aided diagnosis of contrast-enhanced spectral mammography: A feasibility study. Eur J Radiol 2018;98:207–213. [DOI] [PubMed] [Google Scholar]
- 59.Marino MA, Pinker K, Leithner D, et al. Contrast-Enhanced Mammography and Radiomics Analysis for Noninvasive Breast Cancer Characterization: Initial Results. Mol Imaging Biol 2020;22(3):780–787. [DOI] [PMC free article] [PubMed] [Google Scholar]