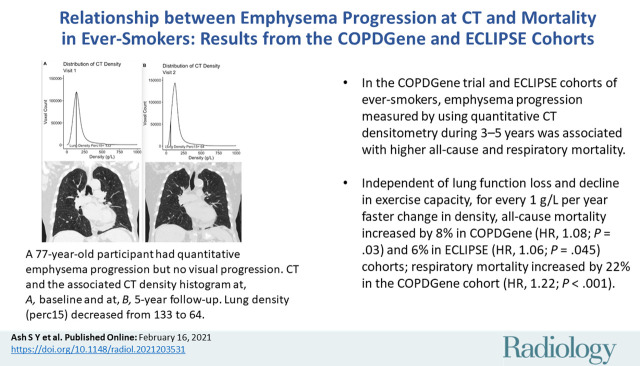
Abstract
Background
The relationship between emphysema progression and long-term outcomes is unclear.
Purpose
To determine the relationship between emphysema progression at CT and mortality among participants with emphysema.
Materials and Methods
In a secondary analysis of two prospective observational studies, COPDGene (clinicaltrials.gov, NCT00608764) and Evaluation of Chronic Obstructive Pulmonary Disease Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE; clinicaltrials.gov, NCT00292552), emphysema was measured at CT at two points by using the volume-adjusted lung density at the 15th percentile of the lung density histogram (hereafter, lung density perc15) method. The association between emphysema progression rate and all-cause mortality was analyzed by using Cox regression adjusted for ethnicity, sex, baseline age, pack-years, and lung density, baseline and change in smoking status, forced expiratory volume in 1 second, and 6-minute walk distance. In COPDGene, respiratory mortality was analyzed by using the Fine and Gray method.
Results
A total of 5143 participants (2613 men [51%]; mean age, 60 years ± 9 [standard deviation]) in COPDGene and 1549 participants (973 men [63%]; mean age, 62 years ± 8) in ECLIPSE were evaluated, of which 2097 (40.8%) and 1179 (76.1%) had emphysema, respectively. Baseline imaging was performed between January 2008 and December 2010 for COPDGene and January 2006 and August 2007 for ECLIPSE. Follow-up imaging was performed after 5.5 years ± 0.6 in COPDGene and 3.0 years ± 0.2 in ECLIPSE, and mortality was assessed over the ensuing 5 years in both. For every 1 g/L per year faster rate of decline in lung density perc15, all-cause mortality increased by 8% in COPDGene (hazard ratio [HR], 1.08; 95% CI: 1.01, 1.16; P = .03) and 6% in ECLIPSE (HR, 1.06; 95% CI: 1.00, 1.13; P = .045). In COPDGene, respiratory mortality increased by 22% (HR, 1.22; 95% CI: 1.13, 1.31; P < .001) for the same increase in the rate of change in lung density perc15.
Conclusion
In ever-smokers with emphysema, emphysema progression at CT was associated with increased all-cause and respiratory mortality.
© RSNA, 2021
Online supplemental material is available for this article.
See also the editorial by Lee and Park in this issue.
Summary
In participants with emphysema, quantitatively measured emphysema progression at CT was associated with increased mortality.
Key Results
■ In the COPDGene trial and Evaluation of Chronic Obstructive Pulmonary Disease Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) cohorts of ever-smokers, emphysema progression measured by using quantitative CT densitometry during 3–5 years was associated with higher all-cause and respiratory mortality.
■ Independent of lung function loss and decline in exercise capacity, for every 1 g/L per year faster change in density, all-cause mortality increased by 8% in COPDGene (hazard ratio [HR], 1.08; P = .03) and 6% in ECLIPSE (HR, 1.06; P = .045) cohorts; respiratory mortality increased by 22% in the COPDGene cohort (HR, 1.22; P < .001).
Introduction
Emphysema is characterized by airspace dilation and parenchymal destruction. These changes are readily apparent not only at histopathologic analysis but also visually and quantitatively at chest CT (1–3). Despite this, longitudinal assessment of the effectiveness of interventions in the treatment of emphysema and chronic obstructive pulmonary disease (COPD) has largely been focused on surrogate physiologic biomarkers and end points such as lung function and exercise capacity, rather than on emphysema itself (4). This is in part because in the past these alternative measures of disease severity were more commonly obtained than performance of CT imaging. However, the growing use of CT in lung cancer screening and other indications, and the shared risk factors between emphysema and lung cancer mean that many patients with emphysema are now undergoing serial CT examinations (5). These CT examinations represent an opportunity for monitoring the progression of diseases such as emphysema over time.
Previous work in the COPDGene and Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) cohorts has demonstrated that emphysema progression can be measured quantitatively and that it is associated with a decline in lung function (6,7). In addition, cross-sectional studies demonstrated the association between emphysema and adverse outcomes such as mortality, but there is limited evidence regarding the clinical relevance of emphysema progression or its additive importance in predicting outcomes to other measures of disease severity (8–10). We hypothesized that in smokers with emphysema, emphysema progression would be associated with both all-cause mortality and respiratory mortality. We also aimed to determine the relationship between emphysema progression at CT and mortality among participants with emphysema.
Materials and Methods
Our study was a secondary analysis of prospectively collected data from two large observational cohorts, COPDGene (clinicaltrials.gov, NCT00608764; http://www.copdgene.org/) and ECLIPSE (clinicaltrials.gov, NCT00292552; http://www.eclipse-copd.com/), both of which were approved by the individual site institutional review boards at every participating site (Appendix E1–E3 [online]) (11,12). Written informed consent was obtained from all participants in accordance with institutional review board regulations, and all data were obtained and stored in accordance with the Health Insurance Portability and Accountability Act. No informed consent was required for our study because it was a secondary analysis of previously acquired data. Data generated by the authors or analyzed during the study are available online (https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000179.v1.p1 and https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs001252.v1.p1).
Cohort Descriptions
Details regarding the COPDGene and ECLIPSE cohorts and lists of investigators and previous publications describing other analyses of these cohorts are available in the supplemental material (Appendix E1, E4–E8 [online]) (11,12). Briefly, the COPDGene Study is a multicenter longitudinal observational investigation of smokers that is focused on the epidemiologic and genetic factors associated with COPD (12). Our study included the participants who completed the baseline and 5-year follow-up visits. Imaging was performed between November 2007 and July 2017 for COPDGene. The COPDGene-specific data sets used for this study include the phase I and phase II data set from March 2019 and the mortality data set from October 2018. The ECLIPSE study was a 3-year multicenter longitudinal study largely composed of participants with COPD. Longitudinal follow-up included assessment of all-cause mortality for 8 years (5 years after the 3-year follow-up visit) (11). Imaging and clinical assessments were performed between January 2006 and March 2010, and the specific data set used for this study was generated in April 2015. Inclusion criteria for this study included complete longitudinal clinical, imaging, and lung function data and mortality follow-up data. Never-smokers were excluded except for developing the distribution-based minimum clinically important difference.
Image Analysis
Details regarding CT image acquisition and analysis for both cohorts were described previously and are available in Appendix E1 (online) (7,13). Analysis of the COPDGene CT scans was performed by using the Chest Imaging Platform (https://chestimagingplatform.org/), an extension of the Slicer open-source image analysis program (SlicerACIL, v4–10–2), and image analysis of the ECLIPSE CT scans was performed by using Pulmonary Workstation 2.0 (VIDA Diagnostics) (14,15).
Details regarding the rationale for and calculation of the baseline and longitudinal emphysema measures are available in Appendix E1 (online). Briefly, we classified participants as having emphysema at baseline if they had more than the upper limit of normal amount of tissue with a density of less than −950 HU as defined by work from the Multi-Ethnic Study of Atherosclerosis cohort (16). For longitudinal assessment on the basis of work by the Quantitative Imaging Biomarkers Alliance, we quantified emphysema by using the volume-adjusted lung density measured at the 15th percentile of the CT lung density histogram (hereafter, referred to as lung density perc15) (Fig 1) (17). To calculate lung density perc15, the lung attenuation for each CT voxel measured in Hounsfield units was first translated into a more general measure of density, grams per liter. The frequency of lung density measures for the entire lung (ie, the lung density histogram) is then used to identify the 15th percentile of lung density (lung density perc15; Fig 1) and this number is then adjusted for lung volume by using the sponge model (16–18). Increases in emphysema are expressed as decreases in density (ie, a lower lung density perc15 indicates more emphysema) (Fig 1).
Figure 1a:
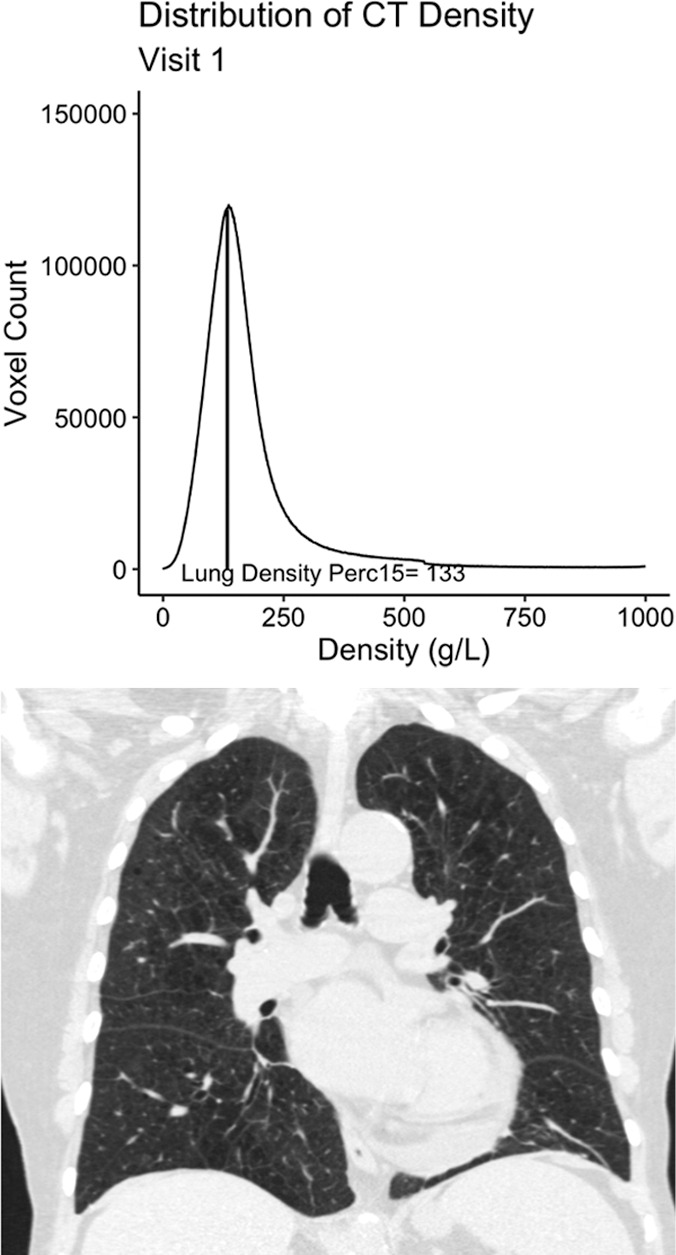
Example 77-year-old female participant from the chronic pulmonary obstructive disease (COPD) gene (COPDGene) cohort with evidence of quantitative emphysema progression but no clear visual progression of emphysema over 5 years who ultimately died during long term follow-up. (a) A representative coronal noncontrast CT image and the associated CT density histogram for the participant at baseline. (b) A representative noncontrast-enhanced coronal CT image and the associated CT density histogram for the participant at the 5-year follow-up visit. Lung density perc15 = volume-adjusted lung density measured at the 15th percentile of the CT lung density histogram.
Figure 1b:
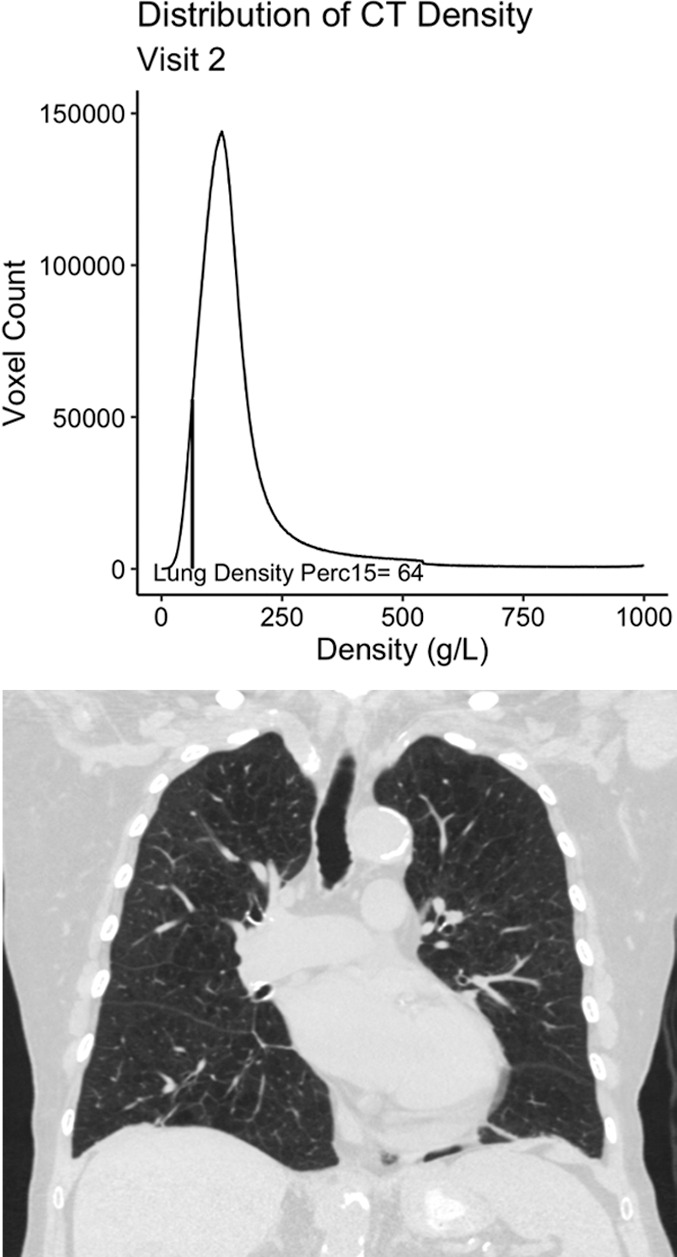
Example 77-year-old female participant from the chronic pulmonary obstructive disease (COPD) gene (COPDGene) cohort with evidence of quantitative emphysema progression but no clear visual progression of emphysema over 5 years who ultimately died during long term follow-up. (a) A representative coronal noncontrast CT image and the associated CT density histogram for the participant at baseline. (b) A representative noncontrast-enhanced coronal CT image and the associated CT density histogram for the participant at the 5-year follow-up visit. Lung density perc15 = volume-adjusted lung density measured at the 15th percentile of the CT lung density histogram.
Statistical Analysis
Details regarding the statistical analyses are available in Appendix E1 (online). Briefly, the primary analyses were performed in ever-smokers who had emphysema at baseline. Secondary analyses were performed in all smokers regardless of the baseline presence of emphysema.
Emphysema progression was analyzed by using two general approaches. For the primary analysis, the annualized rate of change in lung density perc15 was analyzed as a continuous predictor. Thus, the effects shown are for the association between a 1 g/L per year faster rate of decline in density (increase in emphysema). Secondary dichotomized analyses were performed by comparing participants who showed progression (hereafter, progressors) with those who did not (hereafter, nonprogressors). For these analyses, progression was defined in one of two ways: on the basis of the repeatability coefficient of lung density perc15 and the rate of emphysema progression in never-smoking healthy individuals. Additional details regarding the definition of progressors and nonprogressors is available in Appendix E1 (online).
Differences in the annualized rate of decline in lung density perc15 were analyzed by using t tests. The association between emphysema progression and mortality was analyzed by using multivariable Cox regression with adjustments made for both baseline and longitudinal variables including ethnicity, sex, baseline age, baseline smoking status, baseline pack-years, baseline forced expiratory volume in 1 second, baseline 6-minute walk distance, baseline lung density perc15, change in smoking status, annualized rate of change in forced expiratory volume in 1 second, and annualized rate of change in 6-minute walk distance (16,17,19–22). Additionally, in COPDGene, respiratory specific mortality was analyzed by using the Fine and Gray method. To determine whether the addition of longitudinal imaging data improved the prediction of subsequent mortality, we compared the model fit of multivariable Cox models containing all of the demographic and exposure covariates from the primary analyses and combinations of the following: baseline lung density perc15 and/or forced expiratory volume in 1 second and longitudinal lung density perc15 and/or forced expiratory volume in 1 second by using Akaike information criteria (AIC), partial likelihood ratio tests (for nonnested models), and likelihood ratio tests (for nested models) (23–25).
All statistical tests were two sided and P values less than .05 indicated statistical significance. All analyses were performed in R (version 4.0.3; R Foundation for Statistical Computing) by using RStudio (version 1.3.1093) and the tidyverse, survival, survminer, cmprsk, and nonnestcox packages.
Results
Participant Characteristics
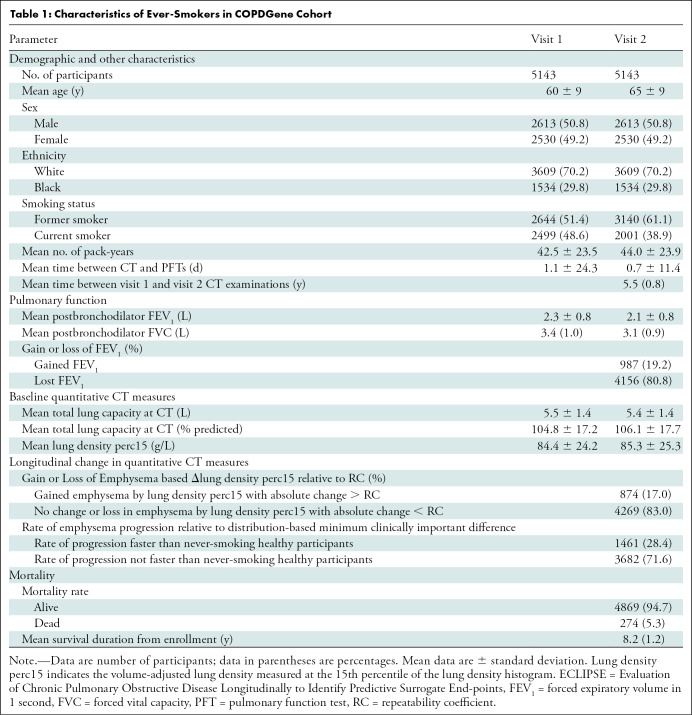
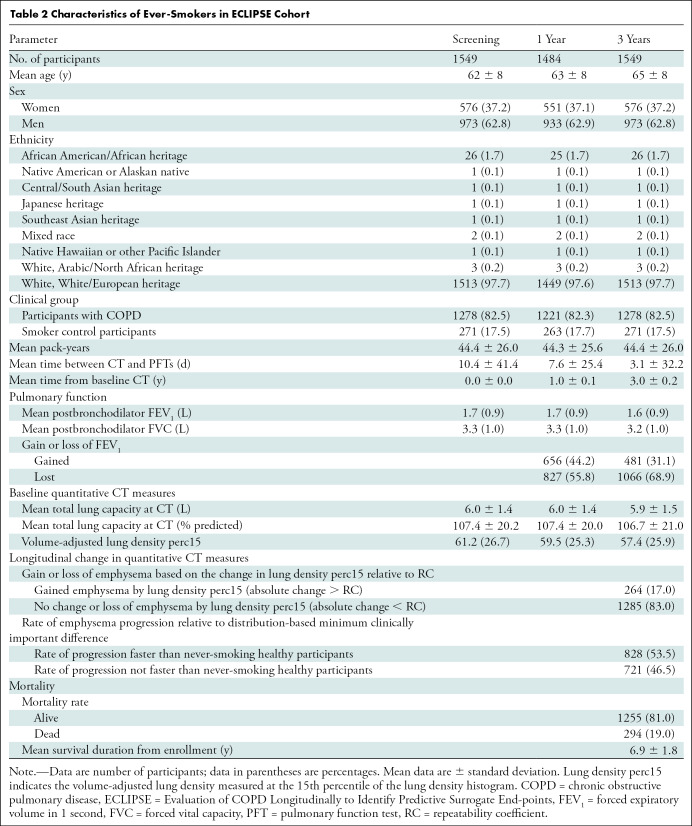
A total of 5143 of 10 198 (50.4%) participants in the COPDGene cohort (mean age at baseline, 60 years ± 9 [standard deviation]; 2613 men [51%]) and 1549 participants in the ECLIPSE cohort (mean age at baseline, 62 years ± 8; 973 men [63%]) met inclusion criteria (Tables 1, 2; Fig E1 [online]). Of those, 2097 of 5143 (40.8%) and 1179 of 1549 (76.1%) had emphysema at baseline and were included in the primary analyses, respectively (Table E1, Fig E1 [online]).
Table 1:
Characteristics of Ever-Smokers in COPDGene Cohort.
Table 2:
Characteristics of Ever-Smokers in ECLIPSE Cohort
Among participants with emphysema in the COPDGene cohort, 387 of 2097 (18.5%) had evidence of emphysema progression defined by an absolute decrease in lung density perc15 of more than the reproducibility coefficient and 617 of 2097 (29.4%) had emphysema progression defined by a rate of lung density perc15 decline faster than the distribution-based minimum clinically important difference. In the ECLIPSE cohort, 173 of 1179 (14.7%) had evidence of emphysema progression defined by an absolute decrease in lung density perc15 of more than the reproducibility coefficient and 606 of 1179 (51.4%) had emphysema progression defined by a rate of lung density perc15 decline faster the distribution-based minimum clinically important difference (Tables 1, 2). In both cohorts, the rate of emphysema progression, measured by annualized rate of change in lung density perc15, was faster in those who died than in those who survived (Fig 2). Specifically, in the COPDGene cohort, the mean rate of change in lung density perc15 in participants who lived versus those who died was 0.41 g/L per year ± 2.72 versus −0.33 g/L per year ± 2.13 (P < .001), respectively. In the ECLIPSE cohort, the mean rate of change in lung density perc15 in participants who lived versus those who died was −1.10 g/L per year ± 2.95 versus −1.50 g/L per year ± 2.72, respectively (P = .04).
Figure 2a:
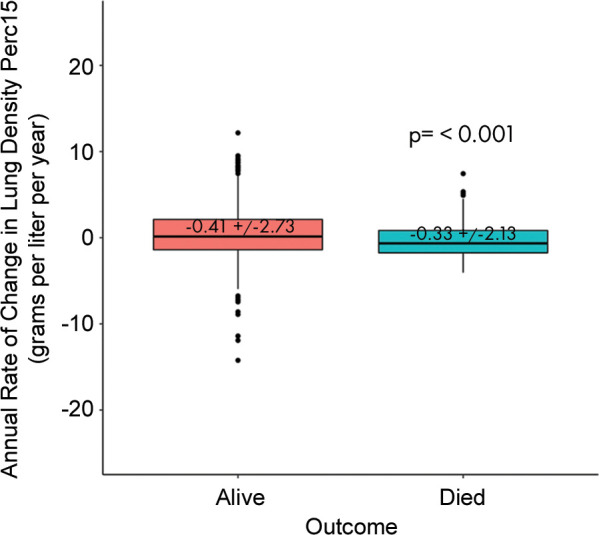
Differences in progression rate by outcome. Annualized rate of change in volume-adjusted lung density measured at the 15th percentile (referred to as lung density perc15) of the CT lung density histogram by mortality in the (a) COPDGene cohort (those with emphysema at baseline) and in the (b) Evaluation of Chronic Obstructive Pulmonary Diseaase Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) cohort (those with emphysema at baseline). Differences between rates by outcome assessed by using t tests.
Figure 2b:
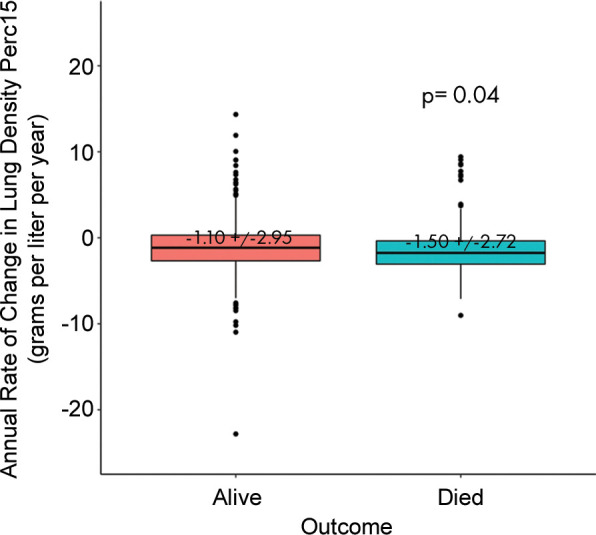
Differences in progression rate by outcome. Annualized rate of change in volume-adjusted lung density measured at the 15th percentile (referred to as lung density perc15) of the CT lung density histogram by mortality in the (a) COPDGene cohort (those with emphysema at baseline) and in the (b) Evaluation of Chronic Obstructive Pulmonary Diseaase Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) cohort (those with emphysema at baseline). Differences between rates by outcome assessed by using t tests.
All-Cause Mortality and Respiratory Mortality
Regarding the primary outcome, in ever-smokers in the COPDGene cohort with emphysema at baseline, for every 1 g/L per year faster rate of decline in lung density perc15, all-cause mortality increased by 8% (hazard ratio [HR], 1.08; 95% CI: 1.01, 1.16; P = .03) (Table 3) and respiratory mortality increased by 22% (HR, 1.22; 95% CI: 1.13, 1.31; P < .001). Similarly, in ever-smokers in the ECLIPSE cohort with emphysema at baseline, for every 1 g/L per year faster rate of decline in perc15, all-cause mortality increased by 6% (HR, 1.06; 95% CI: 1.00, 1.13; P = .045) (Table 3). In the dichotomized analyses, in the COPDGene cohort ever-smokers with emphysema at baseline who had a loss of density more than the repeatability coefficient had 65% higher mortality (HR, 1.65; 95% CI: 1.10, 2.47; P = .02) than those who did not, and those who had a rate of loss of density faster than the minimum clinically important difference had 76% higher mortality (HR, 1.76; 95% CI: 1.25, 2.48; P = .001) than those who did not. In the ECLIPSE cohort, ever-smokers with emphysema at baseline who had a rate of loss of density faster than the minimum clinically important difference had 76% higher mortality (HR, 1.63; 95% CI: 1.18, 2.24; P = .003) than those who did not. However, this association was not statistically significant in the ECLIPSE cohort when progression was defined on the basis of the repeatability coefficient (HR, 1.39; 95% CI: 0.93, 2.07; P = .19) (Table 4, Fig 3).
Table 3:
Association between Rate of Change in Emphysema Progression and Mortality
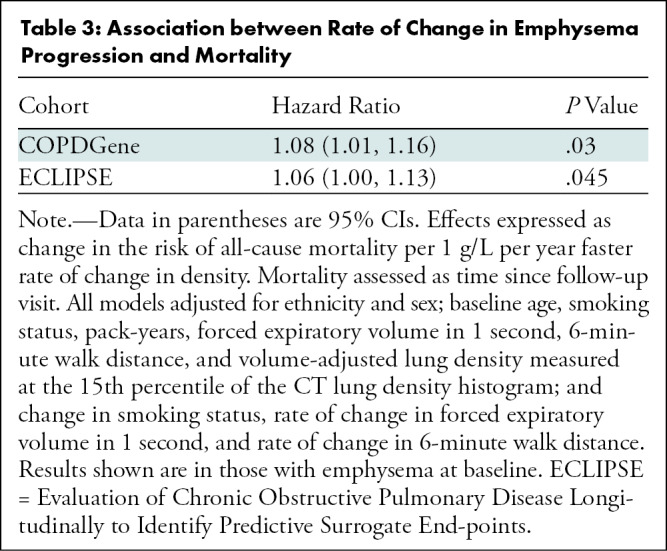
Table 4:
Association between Emphysema Progression and Mortality Responder Analysis
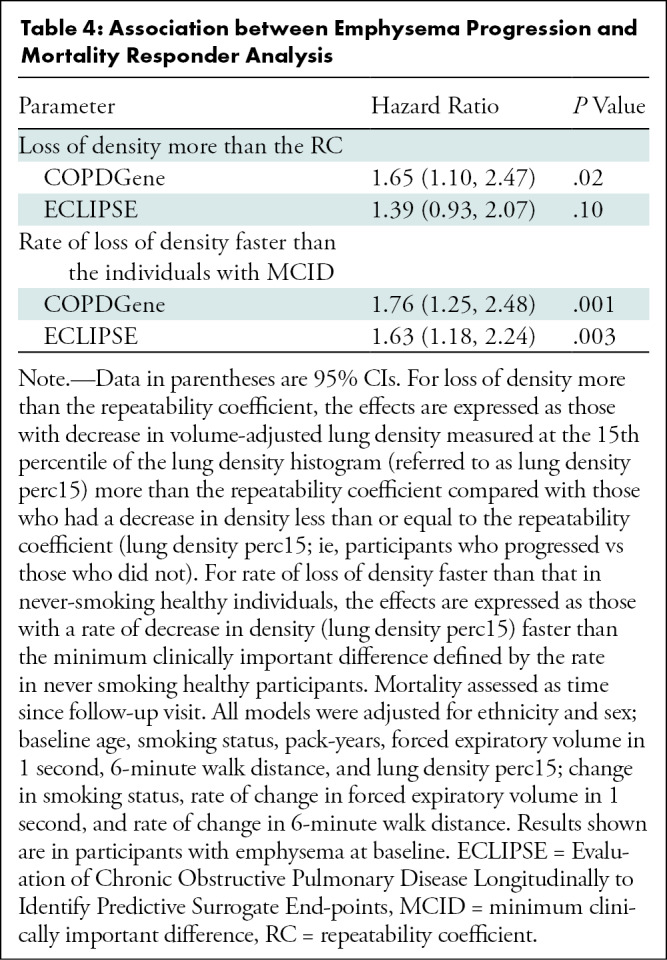
Figure 3a:
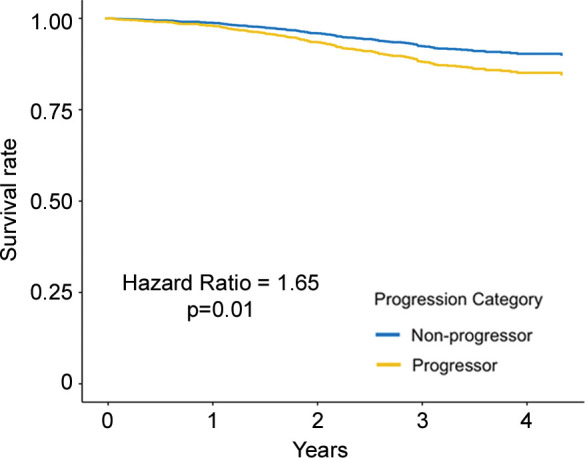
Adjusted survival curves created by using the corrected group prognosis method and by using multivariable Cox models adjusted for ethnicity; sex; baseline age; baseline pack-years of cigarette use; baseline percent predicted expiratory volume in 1 second; baseline volume-adjusted lung density measured at the 15th percentile of the CT lung density histogram (referred to as lung density perc15); and change in scanner model, body mass index, CT-measured lung volume, and smoking status. Survival curves of (a) COPDGene cohort (participants with emphysema at baseline) and (b) Evaluation of Chronic Pulmonary Obstructive Disease Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) cohort (participants with emphysema at baseline) show progressors who had a decrease of lung density perc15 more than the repeatability coefficient and nonprogressors who did not. Survival curves of (c) COPDGene cohort and (d) ECLIPSE cohort progressors in whom the rate of lung density perc15 decline was faster than the distribution-based minimum clinically important difference on the basis of the rate of change in never-smoking healthy participants.
Figure 3b:
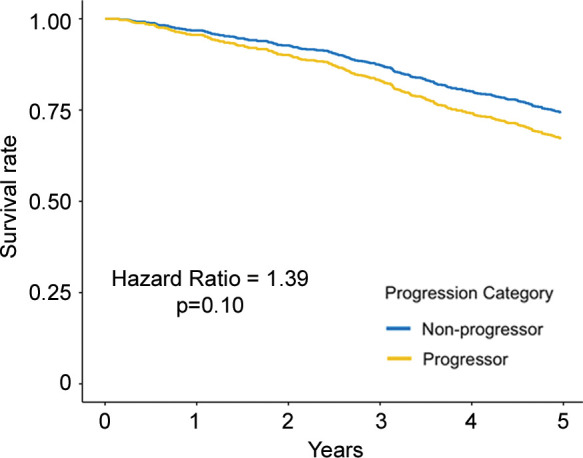
Adjusted survival curves created by using the corrected group prognosis method and by using multivariable Cox models adjusted for ethnicity; sex; baseline age; baseline pack-years of cigarette use; baseline percent predicted expiratory volume in 1 second; baseline volume-adjusted lung density measured at the 15th percentile of the CT lung density histogram (referred to as lung density perc15); and change in scanner model, body mass index, CT-measured lung volume, and smoking status. Survival curves of (a) COPDGene cohort (participants with emphysema at baseline) and (b) Evaluation of Chronic Pulmonary Obstructive Disease Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) cohort (participants with emphysema at baseline) show progressors who had a decrease of lung density perc15 more than the repeatability coefficient and nonprogressors who did not. Survival curves of (c) COPDGene cohort and (d) ECLIPSE cohort progressors in whom the rate of lung density perc15 decline was faster than the distribution-based minimum clinically important difference on the basis of the rate of change in never-smoking healthy participants.
Figure 3c:
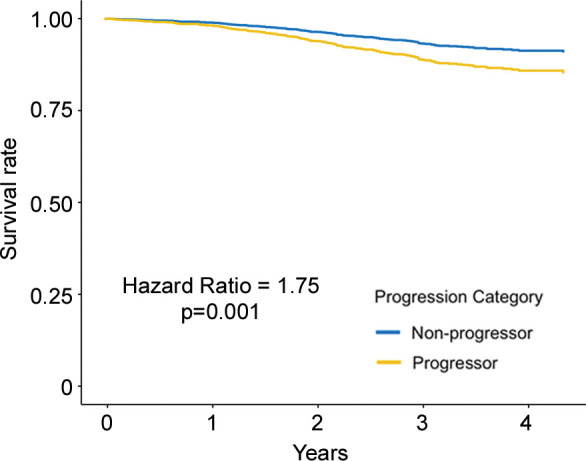
Adjusted survival curves created by using the corrected group prognosis method and by using multivariable Cox models adjusted for ethnicity; sex; baseline age; baseline pack-years of cigarette use; baseline percent predicted expiratory volume in 1 second; baseline volume-adjusted lung density measured at the 15th percentile of the CT lung density histogram (referred to as lung density perc15); and change in scanner model, body mass index, CT-measured lung volume, and smoking status. Survival curves of (a) COPDGene cohort (participants with emphysema at baseline) and (b) Evaluation of Chronic Pulmonary Obstructive Disease Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) cohort (participants with emphysema at baseline) show progressors who had a decrease of lung density perc15 more than the repeatability coefficient and nonprogressors who did not. Survival curves of (c) COPDGene cohort and (d) ECLIPSE cohort progressors in whom the rate of lung density perc15 decline was faster than the distribution-based minimum clinically important difference on the basis of the rate of change in never-smoking healthy participants.
Figure 3d:
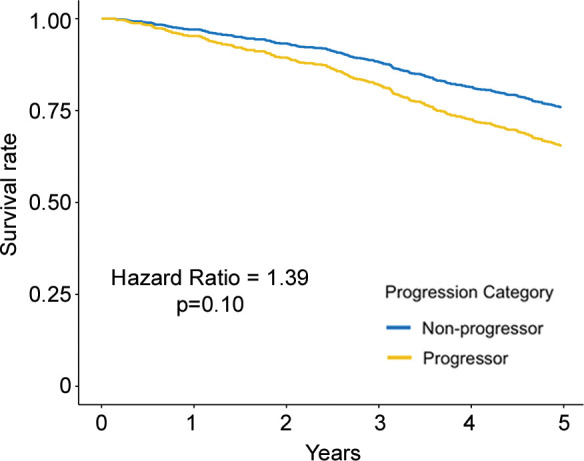
Adjusted survival curves created by using the corrected group prognosis method and by using multivariable Cox models adjusted for ethnicity; sex; baseline age; baseline pack-years of cigarette use; baseline percent predicted expiratory volume in 1 second; baseline volume-adjusted lung density measured at the 15th percentile of the CT lung density histogram (referred to as lung density perc15); and change in scanner model, body mass index, CT-measured lung volume, and smoking status. Survival curves of (a) COPDGene cohort (participants with emphysema at baseline) and (b) Evaluation of Chronic Pulmonary Obstructive Disease Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) cohort (participants with emphysema at baseline) show progressors who had a decrease of lung density perc15 more than the repeatability coefficient and nonprogressors who did not. Survival curves of (c) COPDGene cohort and (d) ECLIPSE cohort progressors in whom the rate of lung density perc15 decline was faster than the distribution-based minimum clinically important difference on the basis of the rate of change in never-smoking healthy participants.
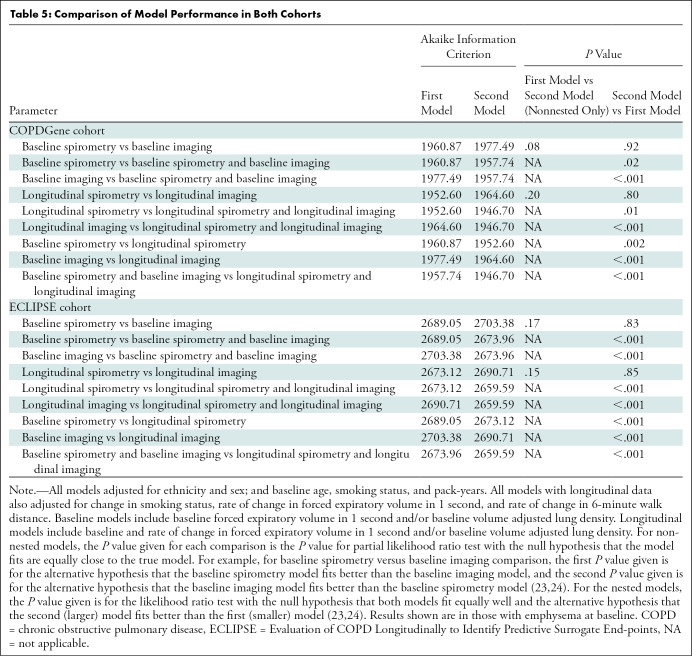
Comparing Model Performance
Regarding model fit, models with either spirometry (forced expiratory volume in 1 second) or imaging (lung density perc15) data were acquired similarly, whereas the inclusion of additional information either in the form of longitudinal information from the same modality or information from a different modality improved model fit (Table 5). For example, the addition of longitudinal imaging to longitudinal spirometry significantly improved model fit in the COPDGene cohort (longitudinal spirometry–only AIC, 1952.60; both longitudinal spirometry and longitudinal imaging AIC, 1946.70; P = .01) and ECLIPSE cohort (longitudinal spirometry–only AIC, 2673.12; both longitudinal spirometry and longitudinal imaging AIC, 2659.59; P < .001). Similarly, the addition of longitudinal imaging to baseline imaging also improved the model fit in COPDGene (baseline imaging–only AIC, 1977.49; both baseline and longitudinal imaging AIC, 1964.60; P < .001) and ECLIPSE (baseline imaging–only AIC, 2703.38; both baseline and longitudinal imaging AIC, 2690.71; P < .001). Additional comparisons are shown in Table 5.
Table 5:
Comparison of Model Performance in Both Cohorts
Secondary Analyses
Similar relationships between emphysema progression and mortality and model performance were found when all ever-smokers (ie, those with and without emphysema at baseline) were included (Tables E2–E4; Figs E2, E3 [online]). Of note, among all ever-smokers in the COPDGene cohort, models that incorporated forced expiratory volume in 1 second alone did appear to perform better than those that used lung density perc15 alone. But the addition of longitudinal imaging data to spirometry data and vice versa improved overall model fit as in the primary analyses.
Discussion
The clinical longitudinal assessment of emphysema has largely focused on surrogate functional measures of disease severity rather than the direct measurement of emphysema itself (4). This is in part because the clinical relevance of emphysema progression is unclear. Therefore, we aimed to determine whether emphysema progression was associated with mortality. We found that among ever-smokers with emphysema at baseline in two large observational cohorts, COPDGene and Evaluation of Chronic Obstructive Pulmonary Disease Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE), a faster rate of emphysema progression measured by using CT densitometry over 3–5 years was associated with higher all-cause and respiratory mortality. Specifically, for every 1 g/L per year faster decline in lung density, all-cause mortality increased by 8% in the COPDGene cohort (hazard ratio [HR], 1.08; 95% CI: 1.01, 1.16; P = .03) and 6% in the ECLIPSE cohort (HR, 1.06; 95% CI: 1.00, 1.13; P = .045), whereas respiratory increased by 22% in the COPDGene cohort (HR, 1.22; 95% CI: 1.13, 1.31; P < .001). In addition, the inclusion of multiple modalities of disease severity quantification resulted in better performance of mortality prediction models than the reliance on one modality alone. For example, the addition of longitudinal imaging to longitudinal spirometry significantly improved the fit of the model for the prediction of mortality in both the COPDGene cohort (longitudinal spirometry–only Akaike information criterion [AIC], 1952.60; both longitudinal spirometry and longitudinal imaging AIC, 1946.70; P = .01) and ECLIPSE cohort (longitudinal spirometry–only AIC, 2673.12; both longitudinal spirometry and longitudinal imaging AIC, 2659.59; P < .001). In conjunction with previous work regarding emphysema progression and cross-sectional studies about emphysema and mortality, our findings suggest that emphysema cannot only be measured over time but also that quantitative emphysema progression is clinically important (6–9,26).
These findings have several implications. For example, it is estimated that more than 6 million people in the United States are eligible for annual CT screening for lung cancer. Because of shared risk factors between lung cancer and emphysema, many of the individuals undergoing lung cancer screening may be found to have emphysema (28,29). Although the uptake of CT screening remains relatively low, this represents an opportunity to improve the early diagnosis of lung cancer and to diagnose and longitudinally measure other thoracic diseases as the rate of these studies increases.
From a practical standpoint, multiple companies already provide densitometric measurements routinely to clinical investigators, and several open-source methods for image processing and emphysema quantification are freely available (14,15,29). Based in part on this current study, the COPD Biomarkers Qualification Consortium, a group organized by the COPD Foundation (Appendix E1, E8 [online]), is working to qualify quantitative emphysema, specifically lung density perc15, with the U.S. Food and Drug Administration as a biomarker for the measurement of disease severity and progression (30). Therefore, although clear logistic hurdles remain, measurements like this could be incorporated quickly into chest CT scan reading and interpretation in the near future. Importantly, if quantitative emphysema is approved as an imaging biomarker by the U.S. Food and Drug Administration, it could also be used in clinical trials aimed at identifying disease-modifying therapies by targeting the underlying pathologic disease of emphysema rather than the physiologic result.
That is not to say that CT densitometry and our study are without limitations. CT-measured lung density is affected by a number of factors beyond the extent of emphysema (7,17,18,21,31–33). Although we attempted to account for these, their effect cannot be discounted. For example, although we used a volume-adjusted approach in our study, spirometric gating was not used and this was a limitation (34,35). Similarly, we used multivariable modeling to attempt to account for confounders such as change in smoking status and changes in lung function and exercise capacity, but the relationship between emphysema and mortality may have been affected by other potentially unmeasured confounders, especially because emphysema and COPD are systemic diseases associated with a multitude of physiologic process and comorbid illnesses (36,37).
Other limitations to this study included the use of objective quantitative measures for both the definition of emphysema and the measurement of progression. We specifically selected these quantitative measures instead of visual assessment of emphysema because although visual emphysema does correlate with quantitative measures, it is subjective and varies from reader to reader, making the assessment of progression difficult (15,38). Even objectively, it is difficult to define who is a so-called progressor or nonprogressor, as is evident from the disparate numbers of participants defined as having disease progression, depending on whether the repeatability coefficient or distribution-based minimum clinically important difference definition of progression was used. To some extent, this is true of categorizing any continuous variable, and until additional work is performed in other cohorts, we suggest that quantitative emphysema progression may be best measured as a continuous rate rather than as a dichotomous “yes” or “no.” In addition, as is evident on Figure 1, quantitative measurement may demonstrate progression in the absence of clear visual progression. A hybrid approach in which the radiologist identifies emphysema and then a quantitative method is used to measure severity and/or progression may be the best strategy to leverage the strengths of both subjective and objective image interpretation, including guarding against spurious results because of confounders. This type of workflow would also allow the radiologist to apply the appropriate biomarker in the appropriate setting (eg, densitometric measures of emphysema in patients with emphysema-predominant disease and measures of airway wall thickness in airway-predominant smoking-related lung disease). Additional work is needed to evaluate such an approach, but it could be viewed as analogous to the use of cancer antigen 125 in ovarian cancer and prostate-specific antigen in prostate cancer, where each is useful in the appropriate clinical context (39,40).
In conclusion, emphysema progression was measured in ever-smokers with emphysema from two observational cohorts: COPDGene and Evaluation of Chronic Obstructive Pulmonary Disease Longitudinally to Identify Predictive Surrogate End-points, or ECLIPSE, during 5 and 3 years, respectively. Emphysema progression measured by using CT densitometry was associated with increased mortality, and the addition of quantitative emphysema measures to models by incorporating measures of lung function improved the prediction of mortality. These findings, combined with previous cross-sectional work, suggested that objectively measured emphysema at CT is a clinically meaningful imaging biomarker that can be used for measuring disease progression. Our work is part of the support for an ongoing U.S. Food and Drug Administration review of quantitative emphysema as an imaging biomarker. Our work will enable clinicians and researchers to directly measure emphysema progression in hopes of identifying new disease-modifying therapies and improving clinical outcomes.
APPENDIX
SUPPLEMENTAL FIGURES
Acknowledgments
Acknowledgments
The authors appreciate the contributions of the COPD Foundation and the COPD Biomarker Qualification Consortium to this work, as well as the work done by the Quantitative Imaging Biomarkers Alliance and the Radiological Society of North America.
S.Y.A. and R.S.J.E. contributed equally to this work.
Study supported by National Institutes of Health (K08-HL145118 [S.Y.A.], K23-HL141651 [C.L.P.], K23-HL136905 [F.N.R.], T32-HL007633 [S.E.M.], K24-HL138188 [M.K.H.], R01-HL107246 [G.R.W., R.S.J.E.], R01-HL116931 [G.R.W., R.S.J.E.], R01-HL116933 [G.R.W., R.S.J.E.], R21-HL140422 [G.R.W., R.S.J.E.], P01-HL114501 [G.R.W.]). COPDGene study is supported by the (R01 HL089897, R01 HL089856) and by the COPD Foundation through contributions made to an industry advisory board composed of AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Siemens, and Sunovion. The ECLIPSE study is supported by GlaxoSmithKline. G.R.W. supported by Boehringer-Ingelheim Pharmaceuticals. S.Y.A. supported by the Pulmonary Fibrosis Foundation.
Disclosures of Conflicts of Interest: S.Y.A. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed ownership/dividends from Quantitative Imaging Solutions. Other relationships: disclosed no relevant relationships. R.S.J.E. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money to author for consultancy from LeukoLabs; disclosed money to author’s institution for grant from Boehringer Ingelheim; disclosed payment for lectures from Chiesi; disclosed stockholdings from Quantitative Imaging Solutions. Other relationships: disclosed no relevant relationships. S.B.F. Activities related to the present article: disclosed grant from GE Healthcare. Activities not related to the present article: disclosed money to author for consultancy from Plarean; disclosed payment for lectures from Sanofi/Regeneron; disclosed membership on scientific advisory board for Xemed; disclosed that author is co-chair for the CT Lung Density Biomarker Committee of QIBA, sponsored by RSNA and NIBIB. Other relationships: disclosed no relevant relationships. R.T.S. Activities related to the present article: disclosed employment and consortium member of GlaxoSmithKline. Activities not related to the present article: disclosed money to author for consulting fees from Immunomet, ENA Respiratory, Vocalis Health; employment until retirement in October 2019 from GlaxoSmithKline; shareholder of GlaxoSmithKline stock. Other relationships: disclosed no relevant relationships. R.A.S. Activities related to the present article: disclosed money to author for fees for participation in review activities from Kamada. Activities not related to the present article: disclosed money to author for board membership from CSL Behring; money to author for consultancies from BVertex, Mereo Biopharma; grants/grants pending from Mereo Biopharma; payment for lectures from Grifols. Other relationships: disclosed no relevant relationships. L.H.N. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed employment by AstraZeneca. Other relationships: disclosed no relevant relationships. S.R. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed unpaid board membership at Dizalk Pharma; money to author for consultancies from GlaxoSmithKline, Bergenbio, Verona, Novoventures; employment at AstraZeneca; grants/grants pending from NIH, PCORI, patent for linking gait and respiration in COPD; royalties for several chapters in books totalling less than $20 per year; money to author for stock in AstraZeneca. Other relationships: disclosed no relevant relationships. M.K.H. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money to author for consultancies from GlaxoSmithKline, Bi, AZ, Verona, Teva, Merck, Mylan; disclosed research support from Novartis, Sunovion. Other relationships: disclosed no relevant relationships. D.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed consultancy for the COPD Foundation. Other relationships: disclosed no relevant relationships. S.M.H. disclosed no relevant relationships. A.A.D. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed grants/grants pending from the National Heart, Lung, and Blood Institute. Other relationships: disclosed no relevant relationships. S.E.M. disclosed no relevant relationships. F.N.R. disclosed no relevant relationships. C.L.P. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed grants/grants pending from Alpha1 Foundation, Boehringer Ingelheim; personal fees from the Lancet Respiratory Medicine, Horizon CME. Other relationships: disclosed no relevant relationships. F.C.S. disclosed no relevant relationships. G.V.S.F. disclosed no relevant relationships. D.A.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money to author for consultancies from Parexel, Boehringer Ingelheim, Veracyte. Other relationships: disclosed no relevant relationships. G.R.W. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money to author for consultancy from Vertex; grants/grants pending from NHLBI; money to author for consultancy, advisory board, and grant from Boehringer Ingelheim; money to author from Quantitative Imaging Solutions, Janssen Pharmaceuticals, and CSL Behring. Other relationships: disclosed no relevant relationships.
Abbreviations:
- AIC
- Akaike information criterion
- COPD
- chronic obstructive pulmonary disease
- ECLIPSE
- Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points
- HR
- hazard ratio
References
- 1.Auerbach O, Hammond EC, Kirman D, Garfinkel L. Emphysema produced in dogs by cigarette smoking. JAMA 1967;199(4):241–246. [PubMed] [Google Scholar]
- 2.Goddard PR, Nicholson EM, Laszlo G, Watt I. Computed tomography in pulmonary emphysema. Clin Radiol 1982;33(4):379–387. [DOI] [PubMed] [Google Scholar]
- 3.Müller NL, Staples CA, Miller RR, Abboud RT. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest 1988;94(4):782–787. [DOI] [PubMed] [Google Scholar]
- 4.Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med 2014;189(3):250–255. [DOI] [PubMed] [Google Scholar]
- 5.National Lung Screening Trial Research Team ; Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368(21):1980–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pompe E, Strand M, van Rikxoort EM, et al. Five-year Progression of Emphysema and Air Trapping at CT in Smokers with and Those without Chronic Obstructive Pulmonary Disease: Results from the COPDGene Study. Radiology 2020;295(1):218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coxson HO, Dirksen A, Edwards LD, et al. The presence and progression of emphysema in COPD as determined by CT scanning and biomarker expression: a prospective analysis from the ECLIPSE study. Lancet Respir Med 2013;1(2):129–136. [DOI] [PubMed] [Google Scholar]
- 8.Haruna A, Muro S, Nakano Y, et al. CT scan findings of emphysema predict mortality in COPD. Chest 2010;138(3):635–640. [DOI] [PubMed] [Google Scholar]
- 9.Lynch DA, Moore CM, Wilson C, et al. CT-based Visual Classification of Emphysema: Association with Mortality in the COPDGene Study. Radiology 2018;288(3):859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green CE, Parr DG, Edgar RG, Stockley RA, Turner AM. Lung density associates with survival in alpha 1 antitrypsin deficient patients. Respir Med 2016;112:81–87. [DOI] [PubMed] [Google Scholar]
- 11.Vestbo J, Anderson W, Coxson HO, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE). Eur Respir J 2008;31(4):869–873. [DOI] [PubMed] [Google Scholar]
- 12.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD 2010;7(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González G, Ash SY, Vegas-Sánchez-Ferrero G, et al. Disease Staging and Prognosis in Smokers Using Deep Learning in Chest Computed Tomography. Am J Respir Crit Care Med 2018;197(2):193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estepar RS, Ross JC, Harmouche R, Onieva J, Diaz AA, Washko GR. Chest Imaging Platform: An Open-Source Library And Workstation For Quantitative Chest Imaging. Am J Respir Crit Care Med 2015;191:A4975. https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2015.191.1_MeetingAbstracts.A4975. [Google Scholar]
- 15.Gietema HA, Müller NL, Fauerbach PV, et al. Quantifying the extent of emphysema: factors associated with radiologists’ estimations and quantitative indices of emphysema severity using the ECLIPSE cohort. Acad Radiol 2011;18(6):661–671. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman EA, Ahmed FS, Baumhauer H, et al. Variation in the percent of emphysema-like lung in a healthy, nonsmoking multiethnic sample. The MESA lung study. Ann Am Thorac Soc 2014;11(6):898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quantitative Imaging Biomarkers Alliance. QIBA Profile: Computed Tomography: Lung Densitometry. Radiological Society of North America. http://qibawiki.rsna.org/images/e/e4/QIBA_CT_Lung_Density_Profile_090319-clean.pdf. Accessed January 25, 2021. [Google Scholar]
- 18.Dirksen A. Monitoring the progress of emphysema by repeat computed tomography scans with focus on noise reduction. Proc Am Thorac Soc 2008;5(9):925–928. [DOI] [PubMed] [Google Scholar]
- 19.Bakker ME, Stolk J, Putter H, et al. Variability in densitometric assessment of pulmonary emphysema with computed tomography. Invest Radiol 2005;40(12):777–783. [DOI] [PubMed] [Google Scholar]
- 20.Gierada DS, Yusen RD, Pilgram TK, et al. Repeatability of quantitative CT indexes of emphysema in patients evaluated for lung volume reduction surgery. Radiology 2001;220(2):448–454. [DOI] [PubMed] [Google Scholar]
- 21.Ashraf H, Lo P, Shaker SB, et al. Short-term effect of changes in smoking behaviour on emphysema quantification by CT. Thorax 2011;66(1):55–60. [DOI] [PubMed] [Google Scholar]
- 22.Celli BR. Predictors of mortality in COPD. Respir Med 2010;104(6):773–779. [DOI] [PubMed] [Google Scholar]
- 23.Fine JP. Comparing Nonnested Cox Models. Biometrika 2002;89(3):635–648. [Google Scholar]
- 24.Vuong QH. Likelihood Ratio Tests for Model Selection and Non-Nested Hypotheses. Econometrica 1989;57(2):307–333. [Google Scholar]
- 25.Sugiura N. Further Analysis of the Data by Akaike’s Information Criteria and the Finite Corrections. Commun Stat Theory Methods 1978;7(1):13–26. [Google Scholar]
- 26.Han MK, Tayob N, Murray S, et al. Association between Emphysema and Chronic Obstructive Pulmonary Disease Outcomes in the COPDGene and SPIROMICS Cohorts: A Post Hoc Analysis of Two Clinical Trials. Am J Respir Crit Care Med 2018;198(2):265–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jemal A, Fedewa SA. Lung Cancer Screening With Low-Dose Computed Tomography in the United States-2010 to 2015. JAMA Oncol 2017;3(9):1278–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moyer VA; U.S. Preventive Services Task Force . Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160(5):330–338. [DOI] [PubMed] [Google Scholar]
- 29.Thirona. Chest CT. https://thirona.eu/solutions/chestct/. Accessed December 27, 2019.
- 30.COPD Biomarker Qualification Consortium . https://www.copdfoundation.org/Research/COPD-Biomarker-Qualification-Consortium/Learn-More.aspx. Accessed December 27, 2019.
- 31.Parr DG, Dirksen A, Piitulainen E, Deng C, Wencker M, Stockley RA. Exploring the optimum approach to the use of CT densitometry in a randomised placebo-controlled study of augmentation therapy in alpha 1-antitrypsin deficiency. Respir Res 2009;10(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaker SB, Dirksen A, Laursen LC, Skovgaard LT, Holstein-Rathlou NH. Volume adjustment of lung density by computed tomography scans in patients with emphysema. Acta Radiol 2004;45(4):417–423. [DOI] [PubMed] [Google Scholar]
- 33.Stoel BC, Putter H, Bakker ME, et al. Volume correction in computed tomography densitometry for follow-up studies on pulmonary emphysema. Proc Am Thorac Soc 2008;5(9):919–924. [DOI] [PubMed] [Google Scholar]
- 34.Kalender WA, Rienmüller R, Seissler W, Behr J, Welke M, Fichte H. Measurement of pulmonary parenchymal attenuation: use of spirometric gating with quantitative CT. Radiology 1990;175(1):265–268. [DOI] [PubMed] [Google Scholar]
- 35.Moroni C, Mascalchi M, Camiciottoli G, et al. Comparison of spirometric-gated and -ungated HRCT in COPD. J Comput Assist Tomogr 2003;27(3):375–379. [DOI] [PubMed] [Google Scholar]
- 36.Divo MJ, Casanova C, Marin JM, et al. COPD comorbidities network. Eur Respir J 2015;46(3):640–650. [DOI] [PubMed] [Google Scholar]
- 37.Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;186(2):155–161. [DOI] [PubMed] [Google Scholar]
- 38.Wille MM, Thomsen LH, Dirksen A, Petersen J, Pedersen JH, Shaker SB. Emphysema progression is visually detectable in low-dose CT in continuous but not in former smokers. Eur Radiol 2014;24(11):2692–2699. [DOI] [PubMed] [Google Scholar]
- 39.Abu Hassaan SO. Monitoring ovarian cancer patients during chemotherapy and follow-up with the serum tumor marker CA125. Dan Med J 2018;65(4):B5463. [PubMed] [Google Scholar]
- 40.Dahabreh IJ, Chung M, Balk EM, et al. Active surveillance in men with localized prostate cancer: a systematic review. Ann Intern Med 2012;156(8):582–590. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.