Abstract
Background
Various cardiovascular risk factors are thought to modify atherosclerosis in a similar fashion (ie, by increasing the magnitude of coronary artery disease [CAD]). However, coronary CT angiography allows precision phenotyping of plaque characteristics through use of radiomics.
Purpose
To assess whether different cardiovascular risk factors have distinctive contributions to the changes in plaque morphologic features over time.
Materials and Methods
Individuals with or without HIV infection and cocaine use and without cardiovascular symptoms underwent coronary CT angiography between May 2004 and August 2015. In the current HIPAA-compliant study, the effects of cocaine use, HIV infection, and atherosclerotic cardiovascular disease (ASCVD) risk on the temporal changes (mean ± standard deviation, 4.0 years ± 2.3 between CT angiographic examinations) in CAD structure were analyzed by using radiomic analysis. The changes in radiomic features were analyzed by using linear mixed models, with correction for factors that may change plaque structure: high‐sensitivity C‐reactive protein level, statin use, positive family history of CAD, and total plaque volume to account for any potential intrinsic correlation between volume and morphologic features. Clusters among significant radiomic features were identified by using hierarchical clustering. Bonferroni-corrected P values less than .00004 (.05 divided by 1276) were considered to indicate significant differences.
Results
Of 1429 participants, 300 with CAD confirmed at coronary CT angiography were randomly selected (mean age, 48 years ± 7; 210 men, 226 people infected with HIV, 174 people who use cocaine) and 1276 radiomic features were quantified for each plaque. Cocaine use was significantly associated with 23.7% (303 of 1276) of the radiomic features, HIV infection was significantly associated with 1.3% (17 of 1276), and elevated ASCVD risk was significantly associated with 8.2% (104 of 1276) (P < .00004 for all). Parameters associated with elevated ASCVD risk or cocaine use and HIV infection did not overlap. There were 13 clusters among the 409 parameters, eight of which were affected only by cocaine use and three of which were affected only by ASCVD risk.
Conclusion
Radiomics-based precision phenotyping indicated that conventional risk factors, cocaine use, and HIV infection each had different effects on CT angiographic morphologic changes in coronary atherosclerosis over 4 years.
© RSNA, 2021
Online supplemental material is available for this article.
See also the editorial by Schoepf and Emrich in this issue.
Summary
Radiomics-based precision phenotyping indicates that conventional risk factors, cocaine use, and HIV infection each have different effects on CT angiographic morphologic changes in coronary atherosclerosis over 4 years.
Key Results
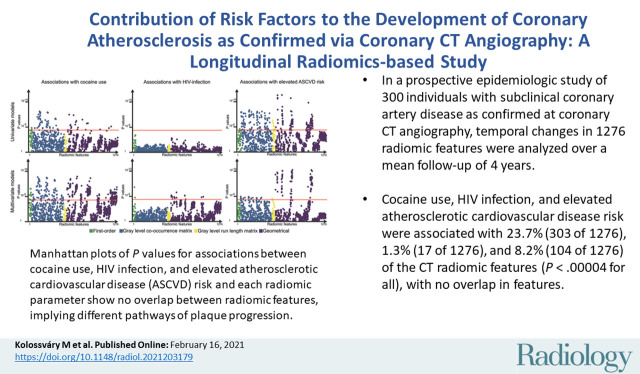
■ In a prospective epidemiologic study of 300 individuals with subclinical coronary artery disease as confirmed at coronary CT angiography, temporal changes in 1276 radiomic features were analyzed over a mean follow-up of 4 years.
■ Cocaine use, HIV infection, and elevated atherosclerotic cardiovascular disease risk were associated with 23.7% (303 of 1276), 1.3% (17 of 1276), and 8.2% (104 of 1276) of the CT radiomic features (Bonferroni-corrected P < .00004 for all), with no overlap in features.
Introduction
Although the associations between cardiovascular risk factors and adverse clinical outcomes are well established, considerable interindividual variation in outcomes cannot be explained (1–4). A better understanding of the magnitude and manner in which cardiovascular risk factors can affect subclinical coronary artery disease (CAD) may help improve understanding of the therapeutic effects of drug therapies to maximize beneficial effects.
Coronary CT angiography permits in vivo noninvasive assessment of CAD with unprecedented detail, even at subclinical stages (5). Both conventional and nonconventional risk factors (such as substance use or HIV infection) affect different pathways of CAD progression at a molecular level (6–8). However, current in vivo evidence suggests that these pathways are interconnected, acting synergistically to increase the magnitude of coronary plaque (7,9). However, conventional imaging markers that characterize only the amount (magnitude) of coronary plaque hold limited information regarding the structural composition of CAD.
We hypothesized that the magnitude of coronary plaque alone might be insufficient to identify differences in CAD phenotypes. Volumetric analysis may misleadingly imply that different factors result in similar CAD morphologic features and therefore promote CAD through similar pathomechanisms. In this pilot study, we assessed whether radiomic analysis (10–12) of coronary plaque phenotypes by using CT angiography could help differentiate between the effects of risk factors known to promote CAD through different molecular mechanisms. These primary mechanisms are through inflammation (HIV infection and cocaine use) or lipid accumulation (atherosclerotic cardiovascular disease [ASCVD] risk).
Materials and Methods
Study Design and Participants
The institutional review board approved the study protocol, and all study participants provided written informed consent. All procedures used in this study were in accordance with the Health Insurance Portability and Accountability Act, local and federal regulations, and the Declaration of Helsinki.
Between May 2004 and August 2015, 1429 individuals with no cardiovascular symptoms were prospectively enrolled in epidemiologic observational studies investigating the effects of HIV, antiretroviral therapy, cocaine use, inflammation, and other associated factors on CAD (13,14). In previous publications involving this cohort, the associations between HIV infection and clinical characteristics of CAD (presence of CAD and presence of different plaque types) were investigated based on cross-sectional data (13,14). In this study, we evaluated the longitudinal change of coronary plaque by using radiomic analysis to assess the relationship between traditional and nontraditional cardiovascular risk factors.
Study participants who underwent two CT angiographic examinations at least 1 year apart were identified. Inclusion criteria included suitable image quality for quantitative analysis and greater than 10% coronary stenosis on the most recent coronary CT angiogram (15). Robust sample size estimates for our hypothesis were not available because there are no data available for such analyses. Therefore, on the basis of sample sizes in prior radiomic investigations in the field of cardiovascular imaging (3,16,17) and the high computational and time requirements for segmentation and analysis, we chose to randomly include 300 study participants who met inclusion criteria.
Immediately before each CT examination, study participants underwent a detailed interview to obtain information about medical history and risk factors, including alcohol consumption, cocaine use, and cigarette smoking. A physical examination was performed, and anthropometric characteristics were recorded. Routine clinical laboratory blood chemistry tests were conducted. ASCVD risk scores were calculated as a measure of overall cardiovascular risk based on the American College of Cardiology and American Heart Association guidelines at the time of CT angiography (18). Elevated ASCVD risk was defined as at least 7.5%, referring to low to borderline risk as per American College of Cardiology and American Heart Association guidelines. Cocaine use has been associated with CAD and was defined as use by any route for at least 6 months administered at least four times a month (13,14). All participants were tested for HIV with an enzyme-linked immunosorbent assay; if the result was positive, the diagnosis was confirmed with a Western blot test. Medical chart review was used to confirm the information on medical history and medications provided by the study participants.
CAD Assessment and Segmentation
Coronary plaque was defined as any discernible coronary artery wall thickening that was identified in at least two perpendicular imaging planes and caused at least a 10% reduction in lumen caliber (15,19). Coronary CT angiograms were loaded into dedicated plaque segmentation software (QAngioCT, version 3.1.3.13; Medis Medical Imaging Systems), which has been validated for use in the assessment of coronary plaque relative to intravascular US (20). The software incorporates adaptive lumen attenuation corrected on an individual scan basis by using gradient filters in combination with intensity values of the arteries. Software contouring of the vessel and luminal wall was corrected manually as needed, as reported previously (21). Voxels between the vessel and luminal borders were considered plaque and were extracted in the original image spaces as Digital Imaging and Communications in Medicine images by using dedicated software for volumetric and radiomic analyses (QAngioCT 3D workbench, version 1.4.0.2; Medis Medical Imaging Systems).
Radiomic Calculations
Exported Digital Imaging and Communications in Medicine data sets were loaded into the open-source Radiomics Image Analysis software package (version 1.4.2; https://CRAN.R-project.org/package=RIA) in the R environment (22). First-order, gray-level co-occurrence matrix, gray-level run length matrix, and geometry-based parameters were derived, resulting in a total of 1276 features. Detailed methods are available in Appendix E1 (online) and previous publications (16).
Statistical Analysis
We used linear mixed models to analyze our longitudinal data and to account for the inherently intrapatient clustering of our data (23). Univariable models were calculated by adding each predictor (cocaine use, HIV infection, and elevated ASCVD risk) and time as fixed effects to the model, where each radiomic feature (continuous variable) was the outcome. For multivariable models, all predictors and time were included as fixed effects, with additional adjustment for high‐sensitivity C‐reactive protein, plaque volume, statin use, and positive family history. To account for the familywise error rate inflation, we used the Bonferroni correction; therefore, two-sided P values less than .00004 (0.05 divided by 1276) were considered to indicate statistically significant differences.
To assess how many distinctive information clusters (latent structural components) are represented by the significant radiomic features, we conducted hierarchical clustering analysis, using the 1-R2 value from pairwise linear regression analysis between significant parameters as a distance metric. We calculated the within-clusters sum of squares for cutting the cluster dendrogram into two to 50 different clusters to find the best trade-off between within-cluster sum of squares and the number of clusters.
For additional sensitivity analysis, we also stratified the population according to sex and age, where 51 years was the median age of the population (young refers to participants younger than 51 years at both timepoints; old refers to participants older than 51 years at the second visit). We also stratified the individuals by cocaine use and ASCVD risk to assess potential interactions between these parameters of interest. To compare the cluster dendrogram of each subgroup, we calculated the cophenetic correlation between them. Furthermore, we created Bk plots, where we plotted the Fowlkes-Mallows values for cutting the dendrogram trees to k different clusters, where k was a number between two and 50 (24). The analysis plan is summarized in Appendix E1 (online). All calculations were performed in the R environment (version 3.6.1; https://www.r-project.org) by one author (M.K., 8 years of experience in statistics) and was supervised by another author (S.L., 30 years) (25).
Results
Characteristics of Study Participants
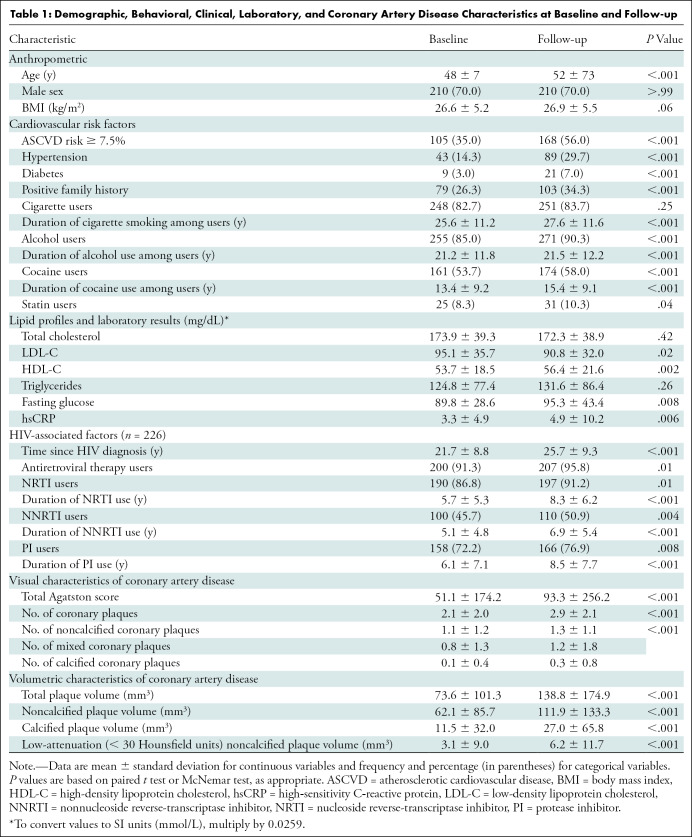
Of 1429 participants with asymptomatic CAD, 300 individuals with subclinical CAD were randomly included, resulting in an analysis of 600 coronary CT angiograms. The 300 individuals (mean age ± standard deviation, 48 years ± 7; 210 men) underwent follow-up CT angiography a mean of 4.0 years ± 2.3 later. Of the 300 individuals, 161 (53.7%) reported cocaine use at baseline; this number increased to 174 (58.0%) at follow-up (P < .001). Among participants, 105 (35.0%) had increased risk for ASCVD scores of 7.5% or greater at baseline, and 168 (56.0%) had increased risk at follow-up (P < .001). Overall, 226 (75.3%) had HIV infection at the time of the first CT examination. Detailed demographic, behavioral, clinical, and laboratory characteristics of participants and clinical characteristics of CAD are presented in Table 1.
Table 1:
Demographic, Behavioral, Clinical, Laboratory, and Coronary Artery Disease Characteristics at Baseline and Follow-up
Associations between Risk Factors and Coronary Plaque Morphology
In univariable analysis, 24.0% (306 of 1276) of all radiomic parameters were significantly associated with at least one of the risk factors. Cocaine use was significantly associated with 5.6% (72 of 1276) of the parameters (P < .00004), whereas HIV infection was not associated with any feature (0 of 1276). Elevated ASCVD risk was significantly associated with 18.3% (234 of 1276) of the parameters. None of the radiomic features that were significantly associated with cocaine use overlapped with those associated with elevated ASCVD risk.
Among the correction factors, high-sensitivity C-reactive protein level and statin use were not associated with any of the radiomic features in univariable analysis, whereas positive family history was significantly associated with two features (0.2%), and plaque volume was significantly associated with the change of 1076 radiomic metrics (84.3%) (P =.00004) (.05 divided by 1276).
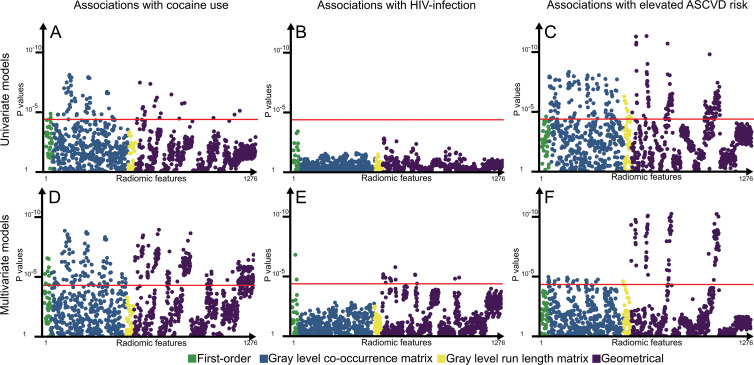
In the multivariable analysis, 32.0% (409 of 1276) of the radiomic features showed association with cocaine use, HIV infection, or elevated ASCVD risk. Cocaine use and HIV infection were associated with 23.7% (303 of 1276) and 1.3% (17 of 1276) of the radiomic features. Elevated ASCVD risk score was associated with 8.2% (104 of 1276) of the parameters. Similarly, there was no overlap among radiomic features associated with elevated ASCVD risk or cocaine use and HIV infection. However, 88.2% (15 of 17) of HIV infection–associated parameters were also significantly associated with cocaine use. Among the correction factors, only total plaque volume showed associations (83.8% of the parameters [1069 of 1276]). Manhattan plots of P values are presented in Figure 1, and results stratified by radiomic classes are presented in Table E1 (online). Manhattan plots of P values for the correction factors are presented in Figure E1 (online).
Figure 1:
Manhattan plots of P values for associations between cocaine use, HIV infection, and elevated atherosclerotic cardiovascular disease (ASCVD) risk and each radiomic parameter. A–C, P values for univariate associations between each radiomic feature and cocaine use, HIV infection, and elevated ASCVD risk in univariate models. D–F, P values for associations between each radiomic feature and cocaine use, HIV infection, and elevated ASCVD risk in multivariate models corrected for high-sensitivity C-reactive protein level as the most common marker of inflammation, positive family history of coronary artery disease (CAD) as an indicator of potential genetic predisposition for CAD progression, statin use because it is known to modify the composition and development of coronary plaques, and the plaque volume itself because we wished to correct for any potential intrinsic correlation between volume and morphologic characteristic. Radiomic parameters are situated on the x-axis in the same order of each subplot, and the corresponding P values are located on the y-saxis. Points above the red line (P = .00004) indicate radiomic features in which the given predictor showed a significant association. There was no overlap between radiomic features associated with cocaine use or elevated ASCVD risk, potentially implying different pathways of plaque progression.
Cluster Analysis of Significant Radiomic Features
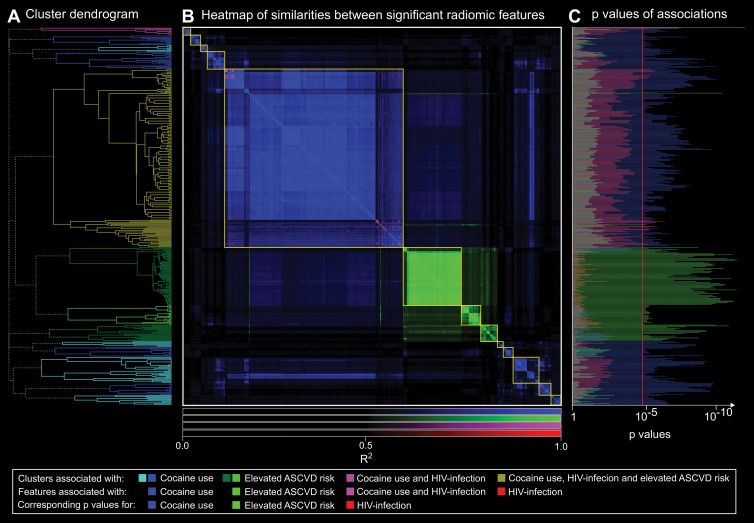
Among the 409 significant radiomic features, we found 13 separate significant clusters. Eight contained only parameters associated with cocaine use, and three were affected only by elevated ASCVD risk. One cluster contained eight parameters associated with long-term cocaine use; two of these eight were also affected by HIV infection. The largest cluster contained 193 radiomic features, of which two were specific to HIV infection, two were specific to increased ASCVD risk, 15 were associated with chronic cocaine use and HIV infection, and the remaining 164 parameters were associated only with long-term cocaine use. A heatmap showing the similarity matrix and cluster dendrogram is presented in Figure 2.
Figure 2:
A, Hierarchical cluster dendrogram of radiomic features significantly associated with cocaine use, HIV infection, and/or elevated atherosclerotic cardiovascular disease (ASCVD) risk. Clusters are color-coded depending on risk factor with which features were associated. B, Heatmap of R2 values for linear regressions between each pair of significant radiomic features (n = 409). Elements of the heatmap are color coded depending on risk factor with which features were associated. Clusters are outlined in yellow. C, Corresponding P values for cocaine use, HIV infection, and increased ASCVD for each radiomic feature. Features are reordered based on hierarchical clustering to correspond to the dendrogram. Bars extending farther than the red line (P = .00004) indicate significant associations. Results from hierarchical clustering indicate that there are distinct morphologic feature sets that are associated with only specific risk factors. Furthermore, P values for cocaine use among clusters associated with cocaine use were magnitudes lower than for HIV infection and especially elevated ASCVD risk. In addition, P values for elevated ASCVD risk for the three clusters containing only radiomic features associated with elevated ASCVD risk were magnitudes lower than for cocaine use in HIV infection. These results potentially imply distinct pathways of coronary atherosclerosis progression because modifying effects of cocaine use and conventional cardiovascular risk factors are clearly separable.
Cluster analysis indicated that not only are the effects of cocaine use and ASCVD independent of each other in multivariable analysis, there is also a clear separation in terms of which structural components are affected by which risk factors. Furthermore, clusters affected by cocaine use are split into two distinct areas on the left and right of the clusters associated with ASCVD risk, indicating that cocaine use may have two different effects on CAD morphologic changes. Even though HIV infection was associated with some radiomics features, they were part of clusters also containing parameters associated with cocaine use, elevated ASCVD risk, or both. Detailed results broken down into radiomic classes can be found in Table E2 (online).
Analysis of P Values among the Clusters
On the basis of our results, for clusters associated with cocaine use, the P values for the association between the radiomic features and cocaine use were significantly (P < .05 for all) smaller than for HIV infection or elevated ASCVD risk (Table 2).
Table 2:
Distribution of P Values Associated with Cocaine Use, HIV Infection, and Increased Risk for Atherosclerotic Cardiovascular Disease among Clusters
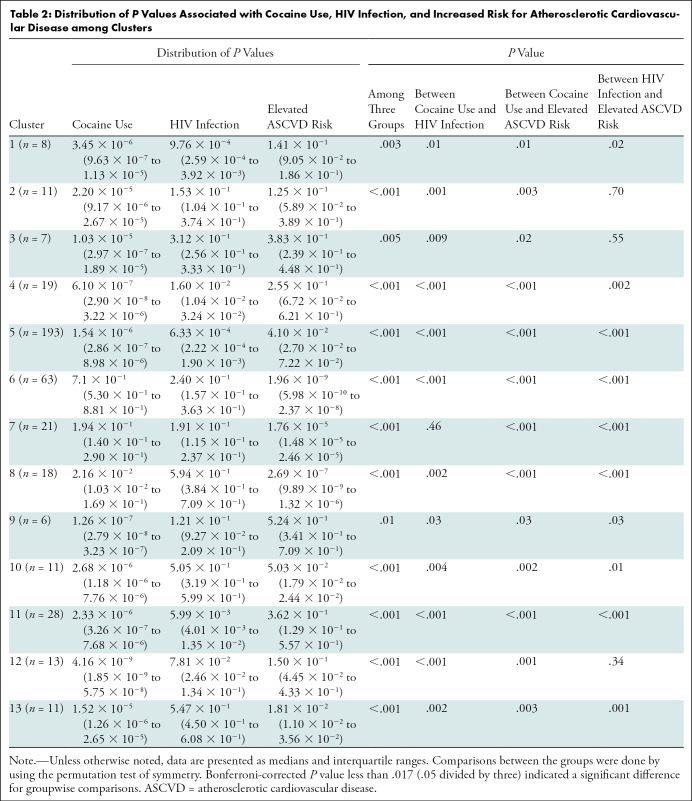
Stratification Analysis Based on Sex and Age
Demographic characteristics stratified by sex and age are shown in Tables E3 and E4 (online).
Briefly, stratification based on sex showed that a clear separation between clusters remained in both populations, with differences in the strength of the associations between sexes. For the first four clusters, HIV infection showed a stronger association with radiomic features in women than in men. In addition, in the three clusters that were influenced only by ASCVD, the associations were stronger in men. Furthermore, for the last five clusters, cocaine use had a more prominent effect in women.
In regard to our age-based stratification analyses, the effect of HIV infection overwhelmed the effects of the other risk factors among younger (below the median age of 51 years) participants. Even in clusters six through eight, which were associated only with ASCVD risk in the total population, among young individuals, HIV infection had a stronger association with radiomic features than ASCVD risk or cocaine use. For older participants, the associations showed similar patterns than in the whole population. Detailed results can be found in Appendix E1 (online), and a summary of the results is given in Figure 3.
Figure 3:
Corresponding P values for associations between cocaine use, HIV infection, elevated atherosclerotic cardiovascular disease (ASCVD) risk and the significant radiomic features stratified by sex and age. A, B, Corresponding P values for associations between cocaine use, HIV infection, and increased ASCVD for each radiomic feature stratified by sex. C, D, Corresponding P values for associations between risk factors and each significant radiomic feature stratified by age based on the median age of 51 years. The features are reordered according to hierarchical clustering. Bars extending farther than the red line (P = .00004) indicate significant associations. The sex-based results indicate sex-specific contributions of the different risk factors on coronary atherosclerosis morphologic features. Furthermore, age stratification indicates that different risk factors may have different contributions to atherosclerosis depending on the individual’s age.
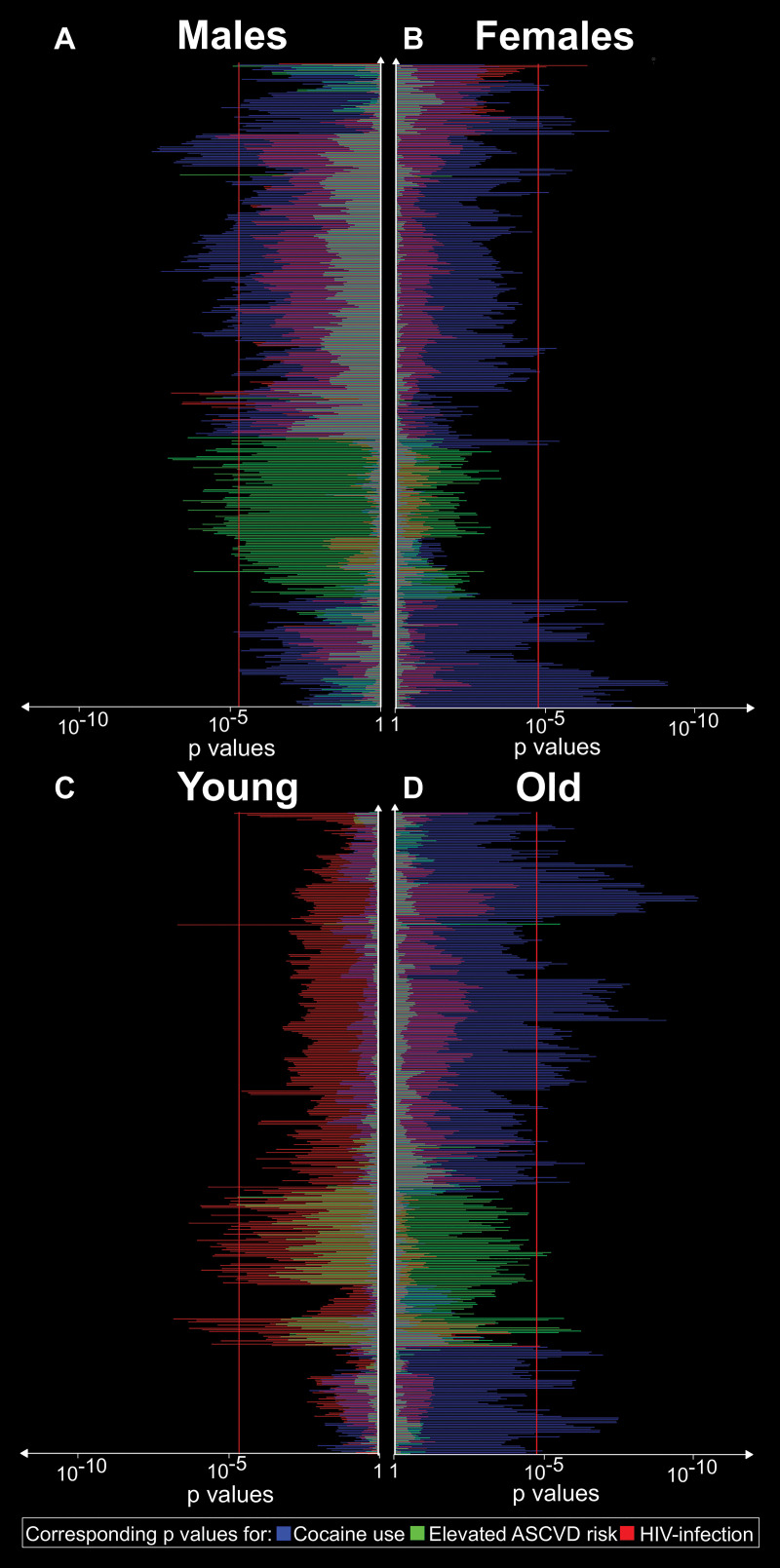
Stratification Analysis Based on Cocaine Use and ASCVD Risk
Among participants who used cocaine, 20 radiomic features were associated with elevated ASCVD, all of which were also associated in the total population. Among participants who did not use cocaine, 86 features were associated with increased ASCVD risk (P < .00004), of which 89.5% (77 of 86) were also significant among the total population. The remaining nine features had P values of .000046–.003582 in the general population.
Among individuals with elevated ASCVD risk, 78 features showed associations with cocaine use, of which 97.4% (76 of 78) were also associated in the general population. The P values of the remaining two parameters were .000072 and .00076 in the general population. Among individuals with low ASCVD risk, none of the parameters showed any association with cocaine use. Detailed results are presented in Appendix E1 (online), and a summary of the results is given in Figure 4.
Figure 4:
Bar chart of P values for radiomic features affected by cocaine use or elevated atherosclerotic cardiovascular disease (ASCVD) risk stratified by disease subgroups. A, Corresponding P values for cocaine use among the total population, elevated ASCVD risk, and low ASCVD risk subgroups, shown in different shades of blue. B, Corresponding P values for ASCVD risk among the total population and among cocaine user and nonuser subgroups, shown in different shades of green. Bars above the red line (P = .00004) indicate significant associations. Results indicate that modifying effects of cocaine use may require a susceptible environment (increased ASCVD risk) to occur. However, once it is present, it modifies the morphologic features of atherosclerosis differently than ASCVD risk. In addition, ASCVD risk may have a more profound effect among cocaine nonusers, which may imply that the effects of cocaine use on morphologic changes overwhelm the effects of ASCVD.
Robustness of Clusters among Different Risk Groups
The robustness of clusters among different risk groups is presented in Appendix E1 (online). Cophenetic correlation (c) showed high values among the total population; between men and women, young and old individuals, and participants who used and did not use cocaine; and among individuals with elevated ASCVD risk (c > 0.90 for all). These results indicate the relative stability of our features regardless of the disease class, implying the robustness of our results.
Discussion
Because the overall number of life-years lost attributable to coronary artery disease (CAD) is increasing (26) and because CAD is still the main cause of avoidable death among older adults (27), we need to better understand the disease course of CAD. Our pilot radiomics-based precision phenotyping study on the temporal changes in coronary plaque composition showed the following: (a) Different risk factors may have distinct contributions to the temporal changes in plaque morphologic features because we found no overlap in radiomic features associated with conventional cardiovascular risk factors or cocaine use and HIV infection. (b) Sex may not only affect the magnitude of CAD but also may modulate the effects of risk factors on CAD, resulting in different phenotypes potentially explaining sex differences in adverse cardiovascular outcomes. (c) The effect of risk factors may differ throughout life (eg, HIV infection had a more profound effect on structural changes of CAD in younger participants than in older individuals). (d) Complex interactions may be present between risk factors, potentially increasing or decreasing the effects of each other (eg, the effects of cocaine use were present only in individuals with elevated atherosclerotic cardiovascular disease risk).
Clinically, atherosclerosis is believed to be a lipid-driven chronic inflammatory disease of the intimal layers of the arteries, wherein the effects of inflammation and lipoproteins are interconnected and synergistically amplify the effects of each other (6,28). However, basic science research shows that different factors have specific effects on the intimal layer of the arteries. Cocaine use impairs nitric oxide release and increases the levels of cell and leukocyte adhesion molecules, causing migration of white blood cells into the intimal layers (29,30). HIV infection causes the chronic activation of the innate immune system, which results in increased levels of inflammatory cytokines (such as interleukin-6, CD14, and CD163) and activation of white blood cells results in chronic vasculopathy (31–33). However, ASCVD risk score is based on decades of cardiovascular research focused on the lipid theory of atherosclerosis development (34). Although the effects of these factors can be differentiated at a molecular level and may result in specific alteration at a histologic level, current imaging solutions did not enable differentiation between these factors in vivo (35,36). However, radiologic imaging provides a unique opportunity to assess the temporal changes in disease processes, which may help improve understanding of how different risk factors affect the course of the disease. To our knowledge, our results are the first in vivo findings that suggest effects of different exposures on CAD may be distinguishable from each other. This implies that rather than having a complex interconnected network of factors contributing to the development of atherosclerosis, the effects of different risk factors may correspond to specific known or unknown pathways of disease progression.
Our study had limitations. First, all study participants were Black. Although racial homogeneity could be considered a strength, the findings derived from this study population might not be generalizable to other racial or ethnic groups without caution. Second, information on cocaine use was obtained from self-reported questionnaires and may not represent actual information on substance use. However, there is no better feasible way to obtain such information from so many participants. Third, all participants were scanned using the same manufacturers’ equipment, and segmentations were conducted by the same expert. Similarities in imaging and quantification may allow identification of these differences.
In conclusion, our results suggest that different risk factors may each determine the course of atherosclerosis. Our results also suggest that interpatient variability of coronary artery disease on coronary CT angiography, regardless of conventional cardiovascular risk, is caused by environmental factors—not just genetic factors. These include nontraditional risk factors, such as HIV infection and cocaine use. Although our analyses do not permit identification of molecular pathways of coronary artery disease (CAD) directly, they may inspire basic sciences to investigate potential mechanisms of atherosclerosis development in a controlled bench environment. Further clinical studies and extensive validation are warranted to investigate the associations between markers, proteomics, metabolomics, genetics, and radiomic features and to assess the contribution and interactions of specific disease pathways to the development of specific CAD phenotypes. Furthermore, analyses should also focus on the effect of specific phenotypic clusters on later cardiovascular outcomes.
APPENDIX
SUPPLEMENTAL FIGURES
Acknowledgments
Acknowledgement
The authors thank the study participants. Without their participation and commitment, this study would not have been possible.
Supported by the National Institute on Drug Abuse (grants R01DA12777, R01DA15020, R01DA25524, R21DA048780, and U01DA040325).
Disclosures of Conflicts of Interest: M.K. Activities related to the present article: is the creator and developer of Radiomics Image Analysis, which was used for the generation of the radiomic features. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. G.G. disclosed no relevant relationships. D.A.B. disclosed no relevant relationships. E.K.F. disclosed no relevant relationships. R.N.M. disclosed no relevant relationships. T.S.K. disclosed no relevant relationships. S.C. disclosed no relevant relationships. S.B. disclosed no relevant relationships. S.L. disclosed no relevant relationships. H.L. disclosed no relevant relationships.
Abbreviations:
- ASCVD
- atherosclerotic cardiovascular disease
- CAD
- coronary artery disease
References
- 1.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140(11):e563–e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sytkowski PA, Kannel WB, D’Agostino RB. Changes in risk factors and the decline in mortality from cardiovascular disease. The Framingham Heart Study. N Engl J Med 1990;322(23):1635–1641. [DOI] [PubMed] [Google Scholar]
- 3.Oikonomou EK, Williams MC, Kotanidis CP, et al. A novel machine learning-derived radiotranscriptomic signature of perivascular fat improves cardiac risk prediction using coronary CT angiography. Eur Heart J 2019;40(43):3529–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oikonomou EK, Marwan M, Desai MY, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet 2018;392(10151):929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maurovich-Horvat P, Ferencik M, Voros S, Merkely B, Hoffmann U. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol 2014;11(7):390–402. [DOI] [PubMed] [Google Scholar]
- 6.Bäck M, Yurdagul A Jr, Tabas I, Öörni K, Kovanen PT. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol 2019;16(7):389–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsue PY, Waters DD. HIV infection and coronary heart disease: mechanisms and management. Nat Rev Cardiol 2019;16(12):745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim ST, Park T. Acute and Chronic Effects of cocaine on cardiovascular health. Int J Mol Sci 2019;20(3):E584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmadi A, Argulian E, Leipsic J, Newby DE, Narula J. From subclinical atherosclerosis to plaque progression and acute coronary events: JACC state-of-the-art review. J Am Coll Cardiol 2019;74(12):1608–1617. [DOI] [PubMed] [Google Scholar]
- 10.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology 2016;278(2):563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolossváry M, Kellermayer M, Merkely B, Maurovich-Horvat P. Cardiac computed tomography radiomics: a comprehensive review on radiomic techniques. J Thorac Imaging 2018;33(1):26–34. [DOI] [PubMed] [Google Scholar]
- 12.Aerts HJ. The potential of radiomic-based phenotyping in precision medicine: a review. JAMA Oncol 2016;2(12):1636–1642. [DOI] [PubMed] [Google Scholar]
- 13.Lai S, Fishman EK, Lai H, et al. Long-term cocaine use and antiretroviral therapy are associated with silent coronary artery disease in African Americans with HIV infection who have no cardiovascular symptoms. Clin Infect Dis 2008;46(4):600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai H, Moore R, Celentano DD, et al. HIV Infection itself may not be associated with subclinical coronary artery disease among African Americans without cardiovascular symptoms. J Am Heart Assoc 2016;5(3):e002529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SCOT-HEART Investigators ; Newby DE, Adamson PD, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med 2018;379(10):924–933. [DOI] [PubMed] [Google Scholar]
- 16.Kolossváry M, Karády J, Szilveszter B, et al. Radiomic features are superior to conventional quantitative computed tomographic metrics to identify coronary plaques with napkin-ring sign. Circ Cardiovasc Imaging 2017;10(12):e006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolossváry M, Park J, Bang JI, et al. Identification of invasive and radionuclide imaging markers of coronary plaque vulnerability using radiomic analysis of coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging 2019;20(11):1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;74(10):e177–e232 [Published corrections appear in J Am Coll Cardiol 2019;74(10):1429–1430 and J Am Coll Cardiol 2020;75(7):840.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Achenbach S, Moselewski F, Ropers D, et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation 2004;109(1):14–17. [DOI] [PubMed] [Google Scholar]
- 20.Boogers MJ, Broersen A, van Velzen JE, et al. Automated quantification of coronary plaque with computed tomography: comparison with intravascular ultrasound using a dedicated registration algorithm for fusion-based quantification. Eur Heart J 2012;33(8):1007–1016. [DOI] [PubMed] [Google Scholar]
- 21.Symons R, Morris JZ, Wu CO, et al. Coronary CT Angiography: variability of CT scanners and readers in measurement of plaque volume. Radiology 2016;281(3):737–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolossvary M. RIA: Radiomics image analysis toolbox for grayscale images. https://cran.r-project.org/web/packages/RIA/index.html. Published April 9, 2019. Accessed February 15, 2020.
- 23.Morrell CH, Brant LJ, Ferrucci L. Model choice can obscure results in longitudinal studies. J Gerontol A Biol Sci Med Sci 2009;64(2):215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer M, Ronald J, Vernuccio F, et al. Reproducibility of CT radiomic features within the same patient: influence of radiation dose and CT reconstruction settings. Radiology 2019;293(3):583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2018. [Google Scholar]
- 26.GBD 2017 Causes of Death Collaborators . Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392(10159):1736–1788 [Published corrections appear in Lancet 2019;393(10190):e44 and Lancet 2018;392(10160):2170.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathers CD, Stevens GA, Boerma T, White RA, Tobias MI. Causes of international increases in older age life expectancy. Lancet 2015;385(9967):540–548. [DOI] [PubMed] [Google Scholar]
- 28.Libby P. Inflammation in atherosclerosis. Nature 2002;420(6917):868–874. [DOI] [PubMed] [Google Scholar]
- 29.Havranek EP, Nademanee K, Grayburn PA, Eichhorn EJ. Endothelium-dependent vasorelaxation is impaired in cocaine arteriopathy. J Am Coll Cardiol 1996;28(5):1168–1174. [DOI] [PubMed] [Google Scholar]
- 30.Gan X, Zhang L, Berger O, et al. Cocaine enhances brain endothelial adhesion molecules and leukocyte migration. Clin Immunol 1999;91(1):68–76. [DOI] [PubMed] [Google Scholar]
- 31.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013;382(9903):1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011;204(8):1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kearns A, Gordon J, Burdo TH, Qin X. HIV-1-associated atherosclerosis: unraveling the missing link. J Am Coll Cardiol 2017;69(25):3084–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Summerhill VI, Grechko AV, Yet SF, Sobenin IA, Orekhov AN. The atherogenic role of circulating modified lipids in atherosclerosis. Int J Mol Sci 2019;20(14):E3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Micheletti RG, Fishbein GA, Fishbein MC, et al. Coronary atherosclerotic lesions in human immunodeficiency virus-infected patients: a histopathologic study. Cardiovasc Pathol 2009;18(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Micheletti RG, Fishbein GA, Currier JS, Singer EJ, Fishbein MC. Calcification of the internal elastic lamina of coronary arteries. Mod Pathol 2008;21(8):1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.