Abstract
Background
The ongoing Coronavirus Infectious Disease (COVID-19) pandemic is a global health crisis that has had a magnanimous worldwide impact on all aspects of people's lives. Several observational studies investigated the relationship between Proton Pump Inhibitors use and the risk of COVID-19 development and mortality.
Aim of the Study
The aim of this meta-analysis is to investigate the association between current PPIs use and the development of COVID-19 as well as its mortality.
Methods
Pubmed, Google Scholar, ScienceDirect and medRxiv were searched until November 21, 2020 using the following keywords: proton pump inhibitors and COVID-19 as well as their related MESH terms. The studies considered in the meta-analysis were either cohort or case-control in design and adjusted for confounding factors. The quality of the studies included in this meta-analysis was assessed using the Newcastle-Ottawa Scale. In addition, a random-effects model was used to calculate the pooled Odds Ratio (ORs) and the corresponding confidence interval (95% CI). Heterogeneity was evaluated using The Cochran's Q heterogeneity test and I2 statistic.
Results
Six observational studies with 195,230 participants were included. In this meta-analysis, current use of PPIs increased risk of COVID-19 development (OR = 1.19; 95% CI: 0.62-2.28) and mortality (OR = 1.67; 95% CI: 1.41-1.97).
Conclusions
Our meta-analysis indicates that current PPIs use significantly increased the risk of COVID-19 mortality, but it did not reach a significant threshold in regards to the risk of COVID-19 development.
Keywords: Human, Pandemic, Global Health, Gastroenterology, COVID-19, Proton Pump Inhibitors
Introduction
Since its outbreak in December of 2019 in Wuhan, China, the impact of the Coronavirus Infectious Disease 2019 (COVID-19) has been devastating on a global scale (1) with over 57,274,018 confirmed cases and 1,368,000 deaths being documented at the time of writing this article (2). Worldwide national healthcare systems have been overburdened to this current day (3).
Proton pump inhibitors (PPIs) are used in the treatment of several gastrointestinal (GI) disorders including non-ulcer dyspepsia, peptic ulcer disease, in addition to the prevention of GI bleeding in patients on antiplatelet therapy (4).
Patients diagnosed with COVID-19 can develop a variety of digestive symptoms, with a prevalence of 15%, such as nausea, vomiting, diarrhea, and loss of appetite. Furthermore, patients with GI manifestations also suffered from poorer disease outcomes (5).
Several observational studies have explored the association between PPIs use and the development/outcomes of COVID-19 (6., 7., 8., 9., 10., 11.). In a meta-analysis that has already been conducted to solely compare the outcomes of COVID-19 between PPIs users and non-users, both adjusted and non-adjusted studies were included. Consequently, this increased the risk of confounding variables present in the study and hence affected the relationship between PPIs use and COVID-19 in-hospital outcomes (12). Another meta-analysis also compared the outcomes of COVID-19 patients between PPIs users and non-users but it had cross points among the included participants (13). Thus, we have decided to conduct a meta-analysis to investigate whether current PPIs use affects not only the risk of COVID-19 development but also the mortality due to COVID-19. Moreover, we decided that the prerequisite inclusion criteria for this meta-analysis would be to only include studies which have adjusted for confounding variables.
Methods
The methods complied with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (14). The protocol which this study was built on, was not published previously. We searched Pubmed, Google Scholar, ScienceDirect, and medRxiv, a preprint repository, up to November 21, 2020 using the following keywords; proton pump inhibitors and COVID-19 and their related MESH terms without any language restrictions.
The studies were included if they were cohort or case control in design, incorporated patients with confirmed COVID-19, adjusted for confounding variables, and had available data comparing the risk of developing COVID-19 and/or mortality between current proton pump inhibitors users and non-users. The outcome was defined as COVID-19 development or severity (intensive care unit admission and need of ventilation) and mortality due to COVID-19. The retrieved articles were evaluated using the abstracts and full text independently by AT, RA, BK, and TA and any disagreement was solved by discussion. The data extraction was done by AT and it has been checked by RA, BK, and TA independently. In any case of discrepancy, consensus was achieved through discussion. There was no need to contact any corresponding authors as all the data needed was available in the included articles. The quality of observational studies was evaluated using the Newcastle-Ottawa Scale by the authors RA, BK, and TA after which it was checked by AT independently and any disagreement was solved by discussion (15).
The adjusted Odds Ratio (ORs) and the corresponding confidence interval (95% CIs) for each study were pooled through a random-effects model using Meta XL, version 5.3 (EpiGear International, Queensland, Australia). The heterogeneity was assessed using The Cochran's Q heterogeneity test and I 2 statistic. Due to the low number of studies included in this meta-analysis, the publication bias was not assessed.
Results
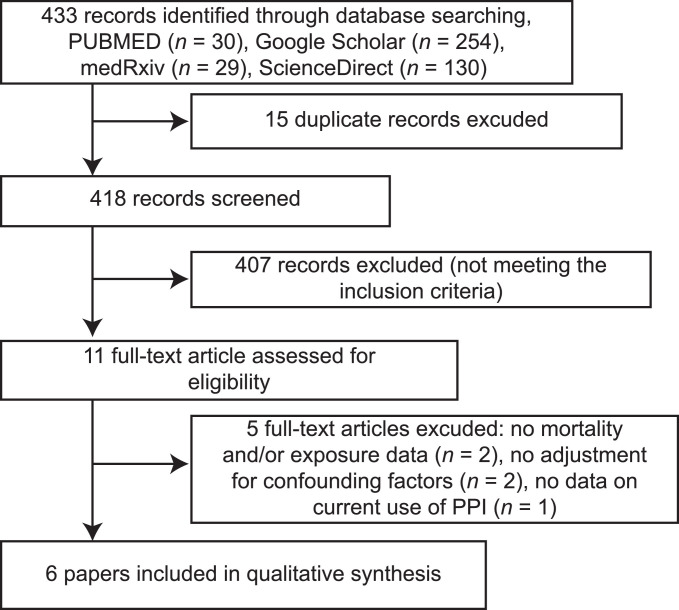
Our literature search yielded 433 potential articles. After deduplication and applying the eligibility criteria, six studies were included, four of which (6,7,9,11) reported the development of COVID-19 and four of which (7,8,10,11) reported COVID-19 mortality. The total number of participants included in the meta-analysis was 195,230 and 23.6% of them were COVID-19 positive (46,032/195,230) (Figure 1 ). Study characteristics are described (Supplementary Table 1). According to Newcastle-Ottawa Scale, all the studies included in this meta-analysis were of good quality and got a score of at least 7 out of 9 points except for one study (6) which got a 6 out of 9.
Figure 1.
PRISMA Flow Chart.
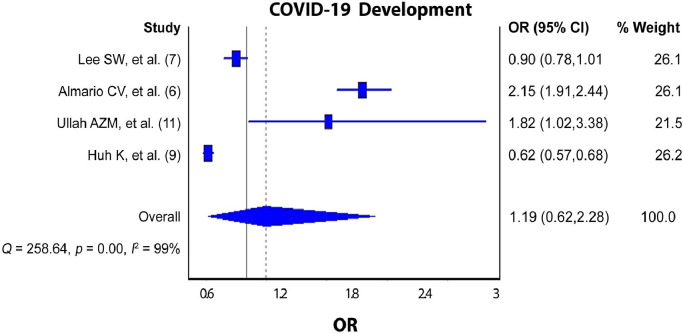
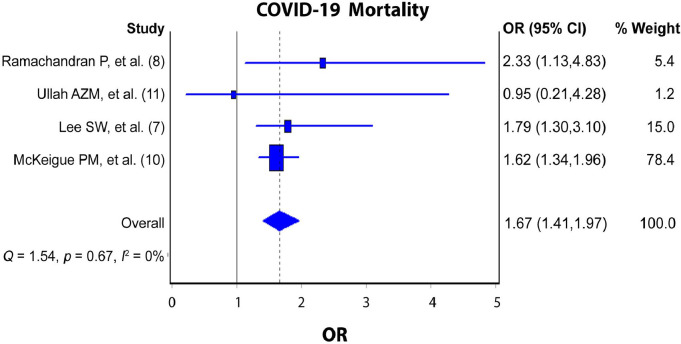
The pooled analysis revealed increased odds of COVID-19 development (Figure 2 ; pooled OR = 1.19; 95% CI: 0.62‒2.28; I2 = 99%; p-value <0.000) and mortality (Figure 3 ; pooled OR = 1.67; 95% CI: 1.41‒1.97; I2 = 0; p-value = 0.67) among current PPIs users compared to non-users.
Figure 2.
PPIs Use and the Risk COVID-19 Development.
Figure 3.
PPIs Use and COVID-19 Mortality.
Discussion
The findings can be considered reliable because our meta-analysis included a rather vast number of COVID-19 patients from 6 studies, all of which were greatly adjusted for confounding factors. Moreover, the current preliminary results revealed that the current use of PPIs significantly increased the risk of COVID-19 mortality by 62.5% (OR = 1.67; 95% CI: 1.41‒1.97). Meanwhile, the risk of developing COVID-19 among current PPIs users increased by 54.3% but did not reach significant levels (OR = 1.19; 95% CI: 0.62‒2.28).
Among the studies included in the pooled analysis of the relationship between PPIs use and COVID-19 development, two studies (7,9), found no significant increase in the risk of COVID-19 development (OR = 0.90, 0.62, respectively). Whereas the remaining studies (6,11),concluded that there was a significant increase in COVID-19 development (OR = 2.15, 1.82, respectively). On the other hand, the studies that investigated the relationship between PPIs use and mortality due to COVID-19 were more consistent in their results as three of them (7,8,10) showed that PPIs use was associated with a significant increase in the risk of mortality due to COVID-19 while one study (11) found that there was no significance.
In a pooled analysis (12) that included studies both adjusted and non-adjusted to confounding variables to investigate the relationship between the PPIs use and mortality due to COVID-19, a significant association was also found (p-value = 0.03, RR = 1.72, I 2 = 66%). However, our study showed insignificant heterogeneity (p-value = 0.67, I 2 = 0%) and only included studies that were adjusted for the presence of confounding factors. Another meta-analysis revealed that PPIs use significantly increased the risk of COVID-19 mortality (OR = 1.46; 95% CI 1.34–1.60) (13) which was similar to our findings. Nevertheless, our pooled analysis revealed a higher risk (OR = 1.67; 95% CI: 1.41‒1.97) than the previously mentioned study that included lower number of participants and cross points among them (13).
There are multiple mechanisms through which PPIs increase the incidence of enteric infections potentially caused by microbes such as Escherichia coli, Salmonella, Campylobacter jejuni, Clostridium difficile, Norovirus and others especially in elderly patients. These mechanisms include reducing gastric acidity by blocking the H+/K+-ATPase (16,17) and reducing chemotactic migration of neutrophils in response to formyl‐MLP (formyl‐methionyl‐leucyl‐phenylalanine), a potent chemo‐attractant in bacteria (15). Another mechanism that the PPIs work through is by causing severe hypochlorhydria which then modulates the microflora in the GI tract (16) leading to several changes in bacterial growth including abnormal bacterial count or overt small intestine bacterial overgrowth (SIBO) (18). Consequently, this causes dysbiosis which worsens the mortality due to viral infection (19).
Some studies have shown a strong surge in the risk of developing community acquired pneumonia (CAP) where the probability of hospitalization in patients under PPIs therapy has increased by 61.6% (20). In addition, H+/K+-ATPase are also found in the mucosal cells and ducts in the human lung (21). This suggests that PPIs may exert a pharmacological action on the human lung which rationalizes the association between COVID-19 development and PPIs use (21,22).
Multiple studies showed that COVID-19 not only affects the respiratory system, but the gastrointestinal system as well (23., 24., 25., 26.). In fact, in some patients only GI symptoms were reported (23), this can be attributed through the binding of the virus to angiotensin converting enzyme 2 (ACE 2) receptors which are abundant on the epithelial cells of the GI tract (5,25,26). The presence of SARS-CoV-2 viral RNA, using the polymerase chain reaction (PCR) (5,25,26), in stool specimens of infected patients persisted even after a negative result in the respiratory system (26) may suggest active infection, replication in the GI tract, and hence possible feco-oral transmission (5,26).
There are a few limitations of this meta-analysis that should be considered. First and foremost, the true effect of current PPIs use on the risk of COVID-19 development could be anywhere between 0.62 and 2.28 (95% CI = 0.62‒2.28). This means that the findings must be interpreted cautiously due to the line effect. Furthermore, high, and significant heterogeneity (p value = 0.00, I 2 = 99%) was observed in the studies included to assess the risk of COVID-19 development, which may be due to the differences in the proportion of COVID-19 patients and current PPIs users among the included studies. In addition, low heterogeneity (p value = 0.67, I 2 = 0%) was found in the studies included to explore the mortality due to COVID-19, because all the patients included in the analysis had COVID-19. Also, it is important to mention that the information on the patients’ adherence to PPIs use was not provided. Finally, although all the studies included were adjusted for confounding factors, they were different in extent for each study.
In conclusion, this meta-analysis provides evidence that current PPIs use significantly increases mortality due to COVID-19 but has not shown significant increase in COVID-19 development. However, there is certainly more to know regarding the current use of PPIs and its relation to COVID-19 development and related mortality, therefore we anticipate more data from prospective studies to substantiate these results.
Conflicts of Interest
The Author(s) declare(s) that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Acknowledgments
The authors thank Mr. Abdel-Rahman Mahdi Al-Husni for his linguistic assistance.
References
- 1.Paules CI, Marston HD, Fauci AS. Coronavirus Infections—More Than Just the Common Cold. JAMA. 2020;323:707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 2.WHO . World Health Organization; Geneva: 2020. WHO coronavirus disease (COVID-19) dashboard. Availabe online:Accessed November 18, 2020. [Google Scholar]
- 3.Lu H, Stratton CW, Tang Y-W. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nehra AK, Alexander JA, Loftus CG. Proton Pump Inhibitors: Review of Emerging Concerns. Mayo Clin Proc. 2018;93:240–246. doi: 10.1016/j.mayocp.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Mao R, Qiu Y, He JS. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almario CV, Chey WD, Spiegel BMR. Increased Risk of COVID-19 Among Users of Proton Pump Inhibitors. Am J Gastroenterol. 2020;115:1707–1715. doi: 10.14309/ajg.0000000000000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SW, Ha EK, Yeniova A. Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: a nationwide cohort study with propensity score matching. Gut. 2021;70:76–84. doi: 10.1136/gutjnl-2020-322248. [DOI] [PubMed] [Google Scholar]
- 8.Ramachandran P, Perisetti A, Gajendran M, et al. Prehospitalization Proton Pump Inhibitor (PPI) use and Clinical Outcomes in COVID-19. medRxiv2020. p. 2020.07.12.20151084. (Accessed November 18, 2020).
- 9.Huh K, Ji W, Kang M, et al. Association of previous medications with the risk of COVID-19: a nationwide claims-based study from South Korea. medRxiv2020. (Accessed November 18, 2020).
- 10.McKeigue PM, Kennedy S, Weir A, et al. Associations of severe COVID-19 with polypharmacy in the REACT-SCOT case-control study. medRxiv2020. p. 2020.07.23.20160747. (Accessed November 18, 2020).
- 11.Dayem Ullah AZM, Sivapalan L, Chelala C, et al. COVID-19 in patients with hepatobiliary and pancreatic diseases in East London: A single-centre cohort study. medRxiv2020. (Accessed November 18, 2020). [DOI] [PMC free article] [PubMed]
- 12.Hariyanto TI, Prasetya IB, Kurniawan A. Proton pump inhibitor use is associated with increased risk of severity and mortality from coronavirus disease 2019 (COVID-19) infection. Dig Liver Dis. 2020;52:1410–1412. doi: 10.1016/j.dld.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kow CS, Hasan SS. Use of proton pump inhibitors and risk of adverse clinical outcomes from COVID-19: a meta-analysis. J Intern Med. 2020;289:125–128. doi: 10.1111/joim.13183. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells GA, Shea B, O'Connell D. Ottawa Hospital Research Institute; 2000. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed November 18, 2020. [Google Scholar]
- 16.Bavishi C, DuPont HL. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther. 2011;34:1269–1281. doi: 10.1111/j.1365-2036.2011.04874.x. [DOI] [PubMed] [Google Scholar]
- 17.Prag C, Prag M, Fredlund H. Proton pump inhibitors as a risk factor for norovirus infection. Epidemiol Infect. 2017;145:1617–1623. doi: 10.1017/S0950268817000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis SJ, Franco S, Young G. Altered bowel function and duodenal bacterial overgrowth in patients treated with omeprazole. Aliment Pharmacol Ther. 1996;10:557–561. doi: 10.1046/j.1365-2036.1996.d01-506.x. [DOI] [PubMed] [Google Scholar]
- 19.Grayson MH, Camarda LE, Hussain SA. Intestinal Microbiota Disruption Reduces Regulatory T Cells and Increases Respiratory Viral Infection Mortality Through Increased IFNγ Production. Front Immunol. 2018;9:1587. doi: 10.3389/fimmu.2018.01587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambert AA, Lam JO, Paik JJ. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altman KW, Waltonen JD, Tarjan G. Human Lung Mucous Glands Manifest Evidence of the H+/K+-ATPase Proton Pump. Ann Otol Rhinol Laryngol. 2007;116:229–234. doi: 10.1177/000348940711600311. [DOI] [PubMed] [Google Scholar]
- 22.Aby ES, Rodin H, Debes JD. Proton Pump Inhibitors and Mortality in Individuals With COVID-19. Am J Gastroenterol. 2020;115:1918. doi: 10.14309/ajg.0000000000000992. [DOI] [PubMed] [Google Scholar]
- 23.Tian Y, Rong L, Nian W. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galanopoulos M, Gkeros F, Doukatas A. COVID-19 pandemic: Pathophysiology and manifestations from the gastrointestinal tract. World J Gastroenterol. 2020;26:4579–4588. doi: 10.3748/wjg.v26.i31.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye Q, Wang B, Zhang T. The mechanism and treatment of gastrointestinal symptoms in patients with COVID-19. Am J Physiol Gastrointest Liver Physiol. 2020;319:G245–G252. doi: 10.1152/ajpgi.00148.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su S, Shen J, Zhu L. Involvement of digestive system in COVID-19: manifestations, pathology, management and challenges. Therap Adv Gastroenterol. 2020;13 doi: 10.1177/1756284820934626. 1756284820934626. [DOI] [PMC free article] [PubMed] [Google Scholar]