Abstract
Introduction
The treatment of SARS CoV2 (Severe Acute Respiratory Syndrome corona virus 2) also known as COVID-19 (corona virus disease 2019) continues to remain an enigma even after six months of the pandemic. Hydroxychloroquine (HCQ) has been one of the most widely tested drugs for SARS CoV2 on account of its antiviral properties. However the results so far have been far from categorical. The meta-analyses conducted till date are also lacking in precision and appropriateness. This systematic review and meta-analysis addresses the efficacy and safety of HCQ in SARS CoV2 by overcoming the limitations of earlier meta-analysis.
Methods
A total of 5 prominent medical databases were searched and fourteen studies (n = 12455) were included in the systematic review and meta-analyses. The data on survival, alleviation of symptoms, conversion of RT PCR positivity to negativity, use and efficacy in presence of co-morbidities (Hypertension, diabetes and heart disease) and cardiac and gastrointestinal side effects were extracted. Meta-analysis was applied to calculate the pooled estimates. Fixed-effects model results were chosen since I2 was <25%.Meta-analysis was conducted using STATA version 13 (StataCorp LP, College Station, TX, USA).
Results
The pooled estimates showed that HCQ treatment did not significantly affect survival at 14 and 28 days in COVID-19 patients with respect to the control population (RR: 1.003, 95% CI: 0.983–1.022), alleviation of symptoms at day 10 (RR: 1.044, 95% CI: 0.911 1.196), success in presence of co-morbidities (RR: 1.058, 95% CI: 1.035–1.082) and conversion from RT PCR positive to RT PCR negative on day 6 (RR:1.123, 95% CI: 1.041 1.212). There was higher risk for cardiac side effects (RR: 2.012, 95% CI: 1.428 2.833) and gastrointestinal side effects (RR: 1.318, 95% CI: 0.730 2.380) in HCQ recipients.
Conclusion
There is no evidence on the safety and efficacy of HCQ either alone or in combination with other drugs in SARS CoV2 infection.
Keywords: SARS CoV2, Hydroxychloroquine, meta-Analyses, Safety, efficacy
1. Introduction
The search for a safe and effective drug for SARS CoV2 is on anvil for a considerable time. Many studies have investigated the safety and efficacy of chloroquine and hydroxychloroquine in SARS CoV2 on account of their antiviral properties [[1], [2], [3]]. Although these properties were recognized much earlier during the outbreaks of SARS CoV and MERS in 2002 and 2012 respectively, the safety and efficacy of these drugs on SARS CoV2is still contentious [4,5].This has been primarily because of the divergent reports of multiple small studies with variable designs and outcome along with the premature termination of some studies. The meta analysis performed till date have had their limitations viz. Lack of adequate data on outcome variables, lesser number of patients, outcomes unadjusted for confounding factors and lack of sensitivity analysis [6,7]. As a result, the real evidence about the safety and efficacy of HCQ in SARS CoV2has been cryptic and intriguing.
The aim of this systematic review and meta-analysis was to generate evidence on the safety and efficacy of hydroxychloroquine in the treatment of SARS CoV2 infection by overcoming the limitations of individual studies and inaccuracies of earlier meta-analysis.
2. Methods
2.1. Search strategy
This systematic review and meta-analysis was conducted and reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Statement (http://prisma-statement.org/). (Fig. 1 ) The following electronic bibliographic databases -Medline, Embase, PubMed, ClinicalTrials.gov, and the Cochrane Central Register of Controlled Trials were searched for studies from December 2019 to October 2020. Only studies published in English were chosen. No attempts were made to identify the unpublished studies. The following search terms were used for capturing the data and no filters were set in the search process- “COVID 19”, “SARS CoV2” and “hydroxychloroquine”.
Fig. 1.
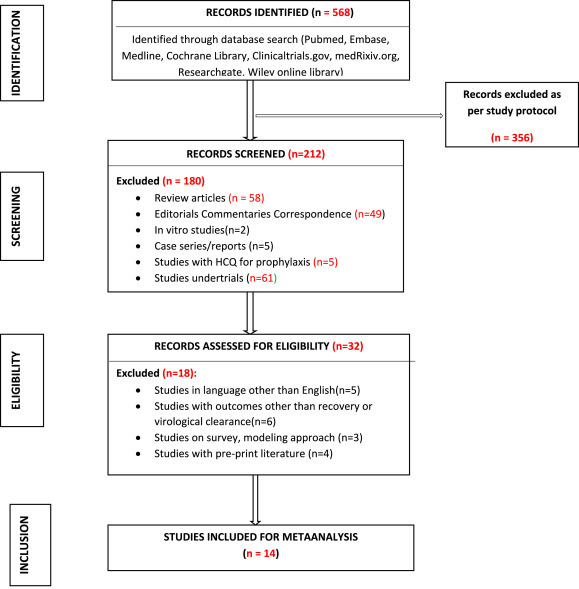
PRISMA flow-chart for selection of studies.
2.2. Inclusion and exclusion criteria
All the studies that included hydroxychloroquine either alone or in combination with other drugs for the treatment of SARS CoV2 and had a control group were eligible for inclusion. The consideration was for both clinical trials (randomized and non-randomized) and observational studies with control groups. The availability of data on the following outcomes was necessary for inclusion in the meta analysis: i) survival, ii) alleviation of clinical symptoms, iii) positive to negative conversion of nasal swab on RT-PCR iv) adverse effects of the drug on heart and gastrointestinal system and v) effect of the drug in patients with co-morbidities of hypertension, diabetes and heart disease. The studies with incomplete data were excluded.
2.3. Data extraction
Data were collected independently by two authors based on the name of the first author, year and month of publication, study design, study location, the number of patients included, and the administration of HCQ.
2.4. Outcome
The following outcomes were evaluated –alleviation of symptoms after 10 days of treatment, survival at 14 and 28 days, conversion of RT PCR positivity to negativity after 6 days, treatment effect in presence of co-morbidities -hypertension, diabetes and heart disease after 14 days and the cardiac and gastrointestinal side effects occurring during the course of HCQ use.
2.5. Analysis
Meta-analysis was applied to calculate the pooled estimates of hydroxychloroquine efficacy. We used the I 2test to estimate the approximate proportion of variability in point estimates attributed to heterogeneity other than that due to chance. Fixed-effects model results were shown sinceI 2was <25%.Meta-analysis was conducted using STATA version 13 (StataCorp LP, College Station, TX, USA).
2.6. Assessment of bias and quality
The bias tool used to evaluate RCTs and observational studies were validated under the domains of selection, performance, attrition, reporting, and others as per norms [8]. Publication bias was not assessed using a funnel plot because less than ten studies met the inclusion criteria. Two authors independently assessed within study bias using aforesaid criteria. Any discrepancies were resolved by recourse to a third author.
3. Results
A total of 568 articles were identified after database search and after evaluating their title and abstract 352 were excluded. Out of the remaining 212 articles, 180 were not relevant for meta-analysis. The full text of the final 32 articles was examined in detail and 14 were included. The 14 articles consisted of 4 randomized control trial, 1 non randomized control trial and 9 observational studies [Table 1 ]. The PRISMA chart of the studies included for meta-analysis are represented in Fig. 1.
Table 1.
Details and characteristics of studies.
| STUDY | TYPE OF STUDY | NUMBER OF PARTICIPANTS (TEST VS CONTROL) | INTERVENTION | PRIMERY OUTCOME | OUTCOME MEASURES | CONCLUSION |
|---|---|---|---|---|---|---|
| (A) Wei tang et al. BMJ, May 2020 |
Multi-center open-label Randomized controlled trail | 150 (75 vs 75) | HCQ loading dose of 1200 mg daily for three days f/b maintenance dose 800 mg daily (total treatment duration: two or three weeks for patients with mild tomoderate or severe disease, respectively) | Negative conversion by 28 days with median time for conversion | Negative conversion by 28 days, median time to negative conversion Adverse events w HCQ recipients than non-recipients |
HCQ did not resultin a significantly higher probability of negative conversion than standard of care alone Adverse events were higher with HCQ |
| (B) Joshua Geleris et al. N Engl J Med. 2020 May |
Observational study | 1376 (811 vs 545) | HCQ 600 mg twice on day 1, then 400 mg daily for a median of 5 days in Covid-19 patients with moderate-to-severe respiratory illness, | Time from study baseline to intubation or death (or time of intubation if patient died after intubation) | Time from study baseline to intubation or death (or time of intubation if patient died after intubation) | HCQ administration was not associated with either a greatly lowered or an increased risk of the composite end point of intubation ordeath. Randomized, controlled trials of HCQ in patients with Covid-19are needed |
| (C) Matthieu Mahévas et al. BMJ2020 May |
Comparative observational study | 181 (84 vs 89) | HCQ 600 mg/day within48 h of admission to hospital | Survival without ICU transferat day 21 | Survival without ICU transferat day 21, overall survival, survival without acute respiratory distress syndrome, weaning from oxygen, and discharge from hospital to home or rehabilitation (all at day 21) | No effect of HCQ in reducing ICU admissions or deaths at day 21 in patients with covid-19 pneumoniarequiring oxygen. Results do not support the use of HCQ in these patients |
| (D) Philippe Gautret et al. Travel Med Infect Dis. Mar–Apr 2020 |
Uncontrolled, non-comparative observational study | 80 | HCQ 200 mg orally three times a day for ten days with AZI (500 mg on D1 f/b 250 mg a day for the next four day |
|
|
Evidence of beneficial effect of co-administration of HCQ with AZI in COVID-19 treatment and its potential effectiveness in the early reduction of contagiousness. |
| (E) Eli S. Rosenberg et al. JAMA. May 2020 |
Retrospective multicenter cohort study | 1438 735(HCQ + AZI) vs 271 (HCQ) vs 211 (AZI) vs 221(none) |
Retrospective data collection | In-hospital mortality | In-hospital mortality. cardiac arrest and abnormal ECG findings (arrhythmia or QT prolongation). | Treatment with HCQ, AZI, or both, compared with neither treatment, was not significantly associated with differences in in-hospital mortality |
| (F) Matthieu Million et al. Travel Med Infect Dis. May 2020 |
Retrospective analysis | 1061 | HCQ (200 mg three times daily for ten days) + AZI (500 mg on day 1 followed by 250 mg daily for the next four days). | 1.Aggressive clinical course requiring oxygen, ICU transferor death after at least three days of treatment, prolongedhospitalization (≥10 days) ii) Contagiousness as assessed by PCR and culture. . |
|
HCQ + AZ combination before COVID-19complications occur is safe and associated with a very low fatality rate. |
| (G) PhilippeGautret et al. Int J Antimicrob Agents. Mar2020 |
Openlabel non-randomized clinical trial | 36 (26 vs 10) | Oral HCQ sulfate 200 mg, three times per day during ten days | Virological clearance at day-6 post-inclusion | Virological clearance at day-6 post-inclusion, clearance overtime during the study period, clinical follow-up (body temperature, respiratory rate, length of hospital stay, mortality), occurrence of side effects. | Recommend that COVID-19 patients be treated with HCQ and AZI to cure their infection and to limit the transmission of the virus to other people in order to curb the spread of COVID-19 in the world |
| (H) Yu, B et al. Sci China Life Sci, May 2020 |
Retrospective study | 550 critically ill COVID-19 patients who need mechanical ventilation (48 vs 502) | oral HCQ (200 mg twice a day for 7–10 days) |
Fatality of patients and inflammatory cytokine levels | – | HCQ on top of the basic treatments is highly effective in reducing the fatality of critically ill patients of COVID-19 through attenuation of inflammatory cytokine storm. Therefore, HCQ should be prescribed as a part of treatment for critically ill COVID-19 patients, with possible outcome of saving lives |
| (I) Joshua Barbosa et al. N Engl J Med. 2020 April |
Prospective randomized controlled trail | 63 (33 vs 30) | HCQ loading dose 400 mg BD per oral for one to two days and followed by 200 mg–400 mg once daily dose for subsequent three to four |
Effect of hydroxychloroquine usage on the need to escalate respiratory support, change in lymphocyte count, and change in neutrophil-to-lymphocyte ratio. | Hydroxychloroquine administration was associated with an increased need for escalation of respiratory support. There were no benefits of hydroxychloroquine on mortality, lymphopenia, or neutrophil-to-lymphocyte ratio improvement, recommend more judicious prescription of hydroxychloroquine for SARS-CoV-2 | |
| (J) Caleb P. Skipper et al. Ann Intern Med. 2020 |
Randomized, double-blind, placebo-controlled trial | 491 (244 vs 247) | Oral hydroxychloroquine (800 mg once, followed by 600 mg in 6–8 h, then 600 mg daily for 4 more days) or masked placebo. | Ordinal outcome by day 14 of not hospitalized, hospitalized, or intensive care unit stay or death | Symptom severity at day 5 and day 14 by 10-point visual analogue scale, nominal incidence of all hospitalizations and deaths, and incidence of study medicine withdrawal. |
Hydroxychloroquine did not substantially reduce symptom severity in outpatients with early, mild COVID-19. |
| (K) Cavalcanti A.B. et al. N Engl J Med. 2020 Sept |
multicenter, randomized, open-label, three-group, controlled trial | 667 suspected or confirmed Covid-19 (229: 221: 217) | 1:1:1 standard care: standard care plus hydroxychloroquine 400 mg twice daily: standard care plus hydroxychloroquine 400 mg twice daily plus azithromycin 500 mg once daily for 7 days |
clinical status at 15 days as assessed with the use of a seven-level ordinal scale |
|
In hospitalizes patients with mild-to-moderate Covid-19, hydroxychloroquine, alone or with azithromycin, did not improve clinical status at 15 days as compared with standard care. |
| (L) Olivier Paccoud et al. |
Observational retrospective cohort study | 84 (38 vs 46) Hospitalized mild to severe Covid-19 |
HCQ (200 mg tid for 10 days) | Time to unfavorable outcome, defined as: death, admission to an intensive care unit, or decision to withdraw or withhold life-sustaining treatments, whichever came first. |
|
no significant reduction of the risk of unfavorable outcomes was observed with hydroxychloroquine in comparison to standard of care |
| (M) Samia Arshad et al. |
Multi-center retrospective observational study | 2541 (None: HCQ: AZI: HCQ + AZI) (409: 1202: 147: 783) |
HCQ: 400 mg twice daily for 2 doses on day 1, followed by 200 mg twice daily on days 2–5. AZI: 500 mg once daily on day 1 followed by 250 mg once daily for the next 4 days. Combination severe COVID-19 patients | In-hospital mortality, assess treatment experience with HCQ vs HCQ + AZI, AZI alone, and other treatments for COVID-19. | Hydroxychloroquine alone and in combination with azithromycin was associated with reduction in COVID-19 associated mortality | |
| (N) Jean-Christophe Lagier et al. Travel Medicine and Infectious Disease, 2020 June |
Retrospective comparative study | HCQ + AZI >3days vs other Rx 3737 (3119 vs 618) |
HCQ (200 mg per oral three times daily for ten days) and AZI (500 mg on day 1 followed by 250 mg daily for the next four days) > 3 days | Poor clinical outcome: Death, transfer to the intensive care unit (ICU), ≥10 days of hospitalization and viral shedding. |
Early diagnosis, early isolation and early treatment of COVID-19 patients, with at least 3 days of HCQ-AZ lead to a significantly better clinical outcome and a faster viral load reduction than other treatments |
The results of the analysis were reported as follows:
-
1.
Survival(14 and 28 days)
-
2.Clinical recovery: in terms of
-
a.Alleviation of symptoms at day 10
-
b.Positive to Negative conversion by RT-PCR at day 6
-
c.Effect of HCQ treatment in the presence of co-morbidities viz. hypertension, diabetes and heart disease
-
a.
-
3.Occurrence of side effects with HCQ treatment
-
a.ECG changes
-
b.Gastro-intestinal side effects
-
a.
3.1. Survival
Survival rates among COVID-19 patients receiving HCQ treatment, alone or in combination with azithromycin (AZT) were compared with their controls at 14 and 28 days by various authors.
3.1.1. HCQ vs control
Mahevas et al. reported that HCQ administration did not significantly increase survival rates in COVID patients than the control groups (RR: 0.98, 95% CI: 0.89–1.08) [9].Rosenberg et al. also demonstrated that the in-hospital mortality was no different between patients on or without HCQ treatment (RR: 0.917, 95% CI: 0.85–0.99) [10]. Barbosa et al. supported no benefits of HCQ on mortality (RR:0.93, 95% CI:0.83–1.05) [11]. Cavalcanti and colleagues also suggested no significant improvement in clinical status in mild to moderate COVID-19 patients treated with HCQ (RR:1.05, 95% CI:0.56–1.96) [12]. Geleris and workers suggested a neutral effect of HCQ in aversing intubation or death (RR: 0.79, 95% CI: 0.75–0.84) [13]. Paccoud et al. suggested no significant reduction of unfavorable outcomes with HCQ (RR: 1.06, 95% CI: 0.92–1.23) [14]. However, Yu et al. however favored use of HCQ in reducing fatality in critically ill COVID-19 patients (RR:1.55, 95% CI:1.32–1.81) [15]. Work by both Arshad et al. (RR:1.18, 95% CI:1.105–1.25) and Lagier et al. (RR:1.02, 95% CI:0.99–1.05) suggested better clinical outcome and reduced mortality with HCQ [16,17]. Our analysis suggests that HCQ treatment did not significantly affect survival in COVID-19 patients with respect to the control population. (RR: 1.003, 95% CI: 0.98–1.02). (Fig. 2 ).
Fig. 2.
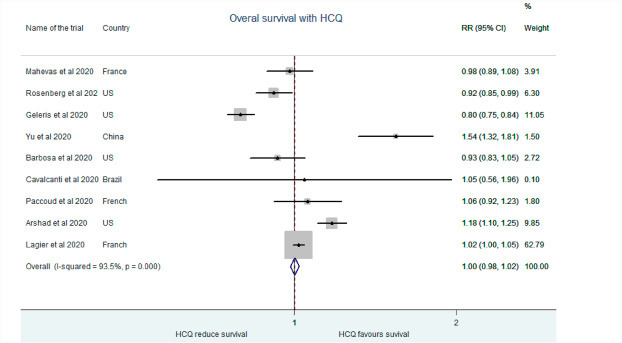
Association of HCQ administration with overall survival.
3.1.2. HCQ + AZI vs control
Comparison was also drawn between patients receiving HCQ + AZI combination and no treatment. Rosenberg et al. showed no significant difference in mortality with HCQ with or without azithromyxin than the control groups. (RR:0.85, 95% CI:0.79–0.91) [10]. Cavalcanti and workers also found no improvemnett in clinical status of mild to moderate COVID positive patients with HCQ + AZI (RR:0.79, 95% CI:0.30–1.84) [12]. However, Arshad et al. reported reducation in COVID related mortality with the combination (RR:1.09, 95% CI: 1.02–1.16) [16]. We found no significant improvement in survival between patients treated with HCQ + AZI combination than no treatment (RR:0.96, 95% CI:0.91–1.003) (Fig. 3 ).
Fig. 3.
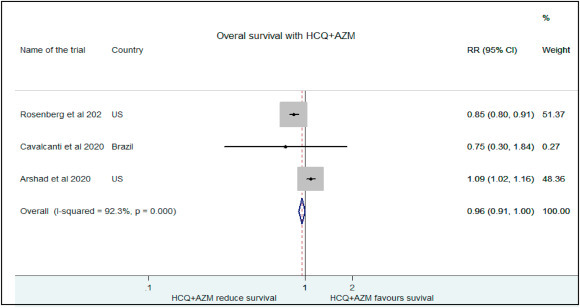
Association of HCQ+ AZI administration with overall survival.
3.2. Clinical efficacy of HCQ
Efficacy of HCQ for clinical recovery of COVID-19 patients was studied under three headings.
These were symptom alleviation at day 10, RT-PCR negative conversion at day 6 and the usein presence of co-morbidities hypertension, diabetes mellitus and heart disease.
3.2.1. Alleviation of symptoms at day 10
Tang et al. in their study demonstrated that HCQ use did not attenuate symptom relief at 28 days of treatment as compared to control group (RR:0.87, 95% CI:0.63–1.19) [18] The median time for alleviation was similar between the test and control groups (19 v 21 days; HR 1.01, 0.59 to 1.74; P = 0.97). Mahevas et al. also showed comparable proportion of patients weaned from oxygen at day 21 of treatment with or without HCQ (79% vs 74%, RR: 1.19, 95% CI: 0.81–1.75) [9]. Skipper et al. (RR:1.24, 95% CI: 0.90–1.69) and Cavalcanti et al. (RR:1.01, 95% CI:0.84–1.23)did not report much change in symptom severity between the HCQ and placebo groups [12,19]. The pooled estimate of these studies shows a cumulative risk of (1.04, 95% CI:0.91–1.19) at day 10 showing no benefit of HCQ administration on the alleviation of symptoms in treatment group as compared to control(Fig. 4 ).
Fig. 4.
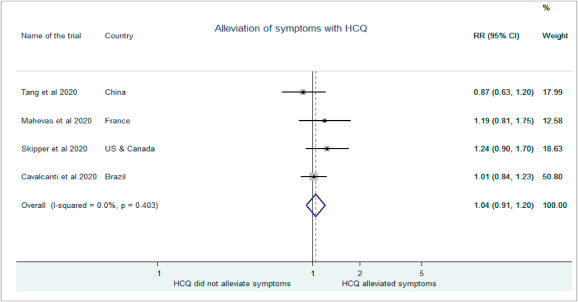
Association of HCQ administration with symptom alleviation.
3.2.2. Negative conversion of RT-PCR at day 6
Gautreta et al. in their non-comparative observational study demonstrated a strong co-relation between treatment with HCQ and negative conversion on RT-PCR in COVID-19 patients (RR: 4.57, 95% CI: 1.16–18.05) [20]. Tang et al. also showed higher rate of virological clearance in HCQ treatment group than the control population (RR: 1.05, 95% CI:0.77–1.45) [18]. Similar results were obtained by Lagier and workers (RR: 1.12, 95% CI:1.04–1.22) [17]. Our results demonstrated an increased probability of negative conversion with HCQ at day 6 with HCQ (RR: 1.12, 95% CI: 1.04–0.21).(Fig. 5 ).
Fig. 5.
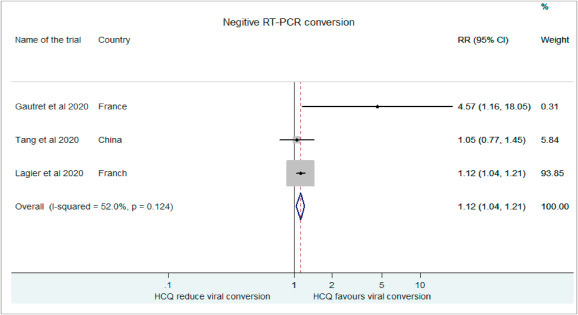
Association of HCQ administration with Negative RT-PCR conversion.
3.2.3. Successof HCQ treatment in the presence of co-morbidities
The probability of patients being treated successfully with HCQ in the presence of co-morbidities like hypertension, diabetes mellitus and heart disease was investigated. The probability of hypertensive patients being placed in the treatment group with HCQ was higher in studies by Rosenberg et al. (RR: 1.08, 95% CI: 1.003–1.13), Cavalcanti et al. (RR: 1.13, 95% CI: 0.94–1.37), Geleris et al. (RR: 2.08, 95% CI: 1.92–2.25), Yu et al. (RR: 1.09, 95% CI: 0.63–1.87), Arshad et al. (RR: 1.05, 95% CI: 0.99–1.12) and Tang et al. (RR: 1.36, 0.95%CI: 0.83–2.23 [10,12,13,15,16,18]. However, absence of hypertension favored HCQ treatment in study by Mahevas et al. (RR: 0.80, 95%CI:0.59–1.09) and Lagier et al. (RR:0.96,95% CI:0.95–1.001 [9,17]. The cumulative chance of being placed in the treatment group was higher for hypertensive COVID-19 patients as per our analysis (RR:1.06, 95% CI:1.03–1.08)(Fig. 6 ).
Fig. 6.
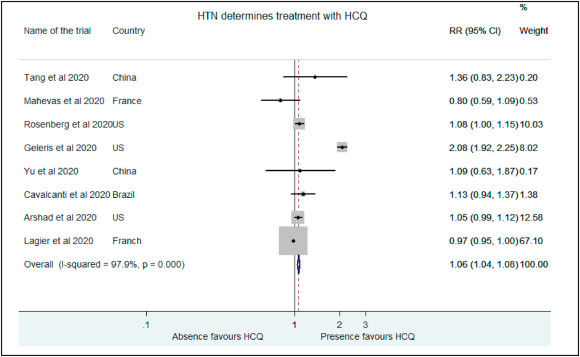
Association of HCQ administration with presence of hypertension.
Similarly, higher proportion of patients with diabetes mellitus were placed in the treatment group than the control in studies by Rosenberg et al. (RR: 1.13, 95% CI: 1.06–1.21), Cavalcanti et al. (RR: 1.16, 95% CI: 0.92–1.45), Geleris et al.(RR: 1.06, 95% CI: 0.97–1.16), Paccoud et al. (RR: 1.051, 995% CI: 0.59–1.86), Yu et al. (RR: 1.62, 95% CI: 0.88–2.99), Arshad et al. (RR: 1.09, 95% CI: 1.04–1.16), Tang et al. (RR: 1.17, 95%CI: 0.78–1.76) and Skipper et al. (RR: 1.07, 95% CI: 0.66–1.73) [10,[12], [13], [14], [15], [16],18,19]. Mahevas and colleagues (RR: 0.58, 95% CI: 0.27–1.23) and Lagier and workers (RR:0.99, 95% CI:0.97–1.03) placed non diabetics in the control arm [9,17]. Presence of diabetes favored treatment with HCQ in patients in analysis as well (RR: 1.04, 95% CI: 1.01–1.06). (Fig. 7 ).
Fig. 7.
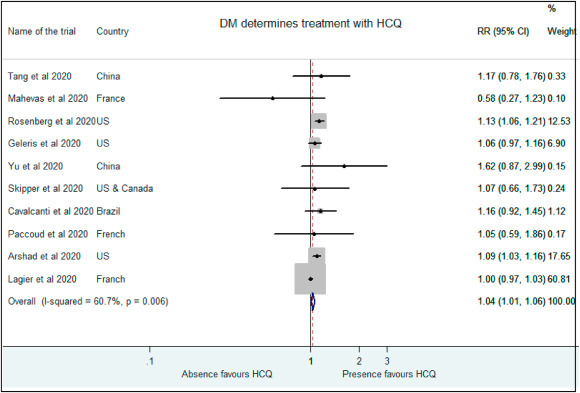
Association of HCQ administration with presence of diabetes mellitus.
Presence of heart disease also determined allocation in treatment or control groups. While Mahevas et al. (RR: 0.29, 95% CI: 0.05–1), Yu et al. (RR:0.36, 95% CI: 0.09–1.45) and Lagier et al. (RR: 0.92, 95% CI: 0.86–0.98)allocated patients with no evidence of heart disease as controls, Rosenberg and authors (RR: 1.13, 95% CI: 1.01–1.26) favored the placement of patients with heart disease in the HCQ treatment group [9,10,17] Our analysis did not find higher chance of success of HCQ treatment in patients with heart diseases (RR: 0.96, 95% CI: 0.91–1.01) (Fig. 8 ).
Fig. 8.
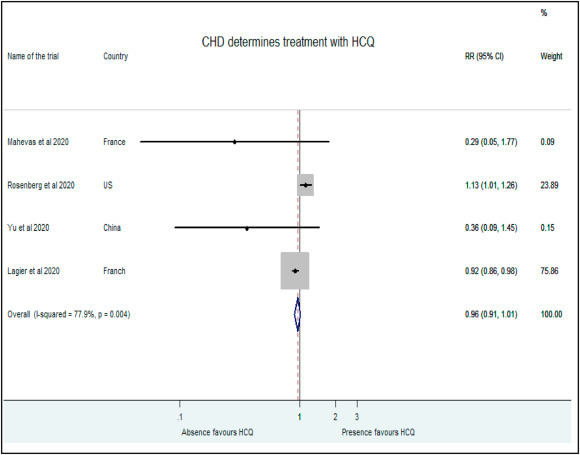
Association of HCQ administration with presence of heart disease.
Treatment with HCQ was also favored in critically ill patients by Rosenberg et al. (RR: 1.29, 95% CI: 1.21–1.38, 38.48%), Arshad et al. (RR: 1.09, 95% CI: 1.02–1.16) and Lagier et al. (RR: 1.01, 95% CI: 0.94–1.09) while Mahevas and coworkers preferred HCQ in not so severe COVID illness (RR: 0.87, 95% CI: 0.59–1.29) [9,10,17]. Our analysis suggests similar treatment effects based on point estimates for such patients in both within and outside ICU (RR: 1.14, 95% CI: 1.09–1.18). (Fig. 9 ).
Fig. 9.
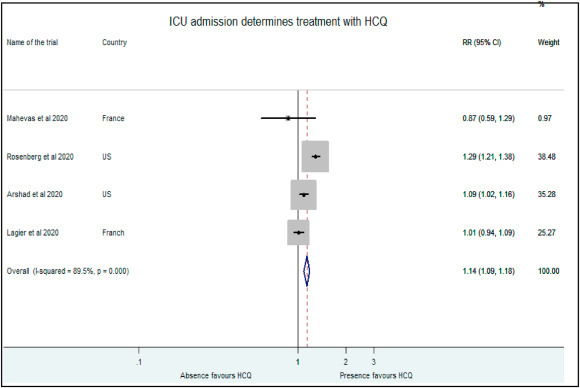
Association of HCQ administration with presence of critical illness.
3.3. Side effects with HCQ treatment
The side effects as determined after seeing the occurrence of ECG changes (arrhythmias, QTc prolongation) and gastro-intestinal manifestations (nausea, vomiting etc.) with HCQ treatment were extracted from the individual studies. Higher incidence of arrhythmias and QTc prolongation were observed in patients receiving HCQ than the control group by Mahevas et al. (RR: 8.75, 95% CI: 1.10–67.08, 2.7%) [9].Rosenberg et al. (RR: 1.93, 95% CI: 1.36–2.73, 97.23%) also demonstrated higher proportion of patients on HCQ treatment showing arrhythmias and QTc prolongation than the control group [10].Our pooled estimates found a similar association between HCQ treatment and new ECG changes (RR: 2.01, 95% CI: 1.43–2.83) (Fig. 10 ).
Fig. 10.
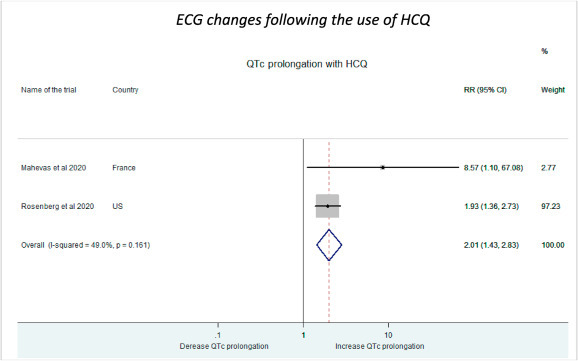
ECG changes as side effects following HCQ administration.
The incidence of gastro-intestinal adverse effects was slightly higher in patients receiving HCQ than the control group (RR: 1.32, 95% CI: 0.73–2.38).(Fig. 11 ).
Fig. 11.
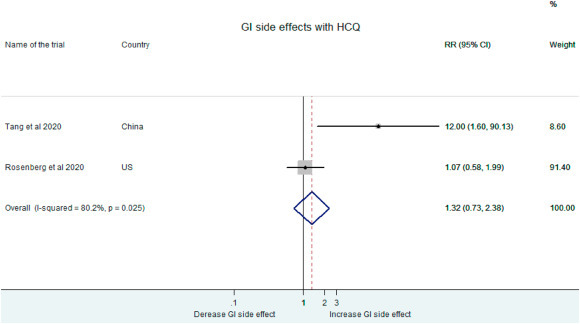
Gastro-intestinal side effects following HCQ administration.
4. Discussion
Our study is the most recent update on the safety and efficacy of HCQ in SARS CoV2 infection and in-depth analysis of its survival benefits and alleviation of symptoms. This large systematic review and meta-analysis of 12455 patients encompassing 14 studies has clearly demonstrated the lack of benefit of HCQ treatment for SARSCoV2 infection. It has additionally found higher cardiovascular side effects in the recipients of HCQ. It has assessed the benefits of using HCQ in the presence of heart disease, hypertension and diabetes which no other meta-analysis has investigated so far. It has found that the use of this drug is used more common in patients with these diseases but did not improve the outcome as compared to control. Since the objective of our study was also to overcome the limitations of earlier metaanalysis besides determining the safety and efficacy of HCQ, strict adherence to the inclusion & exclusion criteria were adopted and stringent data retrieval processes were followed. A total of 14 studies were included in the analysis and this is a significant number for making a valid observation and assessment.
Our analysis has found no survival benefit following the use of HCQ either alone or in combination with azithromycin at 28 days. Various meta-analyses conducted in the recent past also found no difference in mortality, virological clearance, and radiological improvement or their composite outcome at 28 days. We have analyzed the survival at both 14 and 28 days to clearly delineate the time period during which the individual studies reported maximum beneficial effect of the drug i.e. the period from 10 to 14 days after treatment with HCQ. However even after carefully segregating the data from these two different periods, we were unable to elicit any beneficial effect of HCQ on the survival. This strongly supports the argument that a mere reduction of viral load cannot imply an improved survival in SARS CoV2 and hyper immune response may be the key determinant. It also justifies the observed differences between in vitro and in vivo virucidal activity of HCQ published in earlier studies that demonstrated a reduced HCQ efficacy in vivo when compared to in vitro [[21], [22], [23]]. The study by Geleris et al. which contributed 1376 patients evaluated composite outcomes including survival at 28 days although other small studies investigated the outcome at both 14 days and 28 days [13].
We found no benefit with hydroxychloroquine in terms of alleviation of symptoms on day 10 and there was no difference in the RT PCR conversion from positive to negative state on day 6 in HCQ recipients. It is seen that these results are based from the studies by Rosenberg et al. Arshad et al. and Tang et al. who measured the RT PCR daily after starting the treatment till they became negative [10,16,18]. Most authors measured the same on alternate days and one author did it at the end of 14 days [9,10,13,18]. Since our analysis is based upon the alternate day data which was not measured in earlier meta-analysis it has greater reliability [24,25]. While there is no mention about the RT PCR values between days 21 to day 28 in all patients in several meta-analyses, we included the RT PCR results of day 21 days in patients who were otherwise considered as clinically recovered. Therefore our meta-analyses carry lesser risk of data duplication on the basis of their clinical profile alone. This could have magnified some the beneficial effects in earlier meta-analyses [[24], [25], [26]].
There was clearly a greater HCQ use in the patients with hypertension, diabetes and heart disease in 7 out of 10 studies although their efficacy was not unequivocal. In all patients of diabetes and heart disease, the use of the drug was similar because the studies did not reveal any dose adjustments based on their pharmacodynamic and pharmacokinetic properties. No benefits occurred in patients with co-morbidities after HCQ use even after stratification with age and concurrent use of antihypertensive medications which can elicit meaningful informations otherwise. It is thus logical to infer that co-morbid patients are unlikely to avail any benefit from HCQ use as the median age in 9 out of 12 studies was similar. It is also correct to assume that the duration of hypertension is unlikely to induce conformational change in the S-glycoprotein allowing proteolytic digestion by host cell proteases and internalization of the virion.
Safety is an important concern with the use of HCQ in patients with SARS CoV2. 12 out of 14 studies have demonstrated an increased risk of arrhythmias in HCQ recipients of SARSCoV2. However, treatment of HCQ in severe CAP was associated with an increased risk of ECG changes in our analysis and 4 studies looked into their clinical correlates. We noted that the incidences of hypotension, need for inotropes or vasopressors and the renal replacement were recorded in 11 studies but only in the control group in 7 studies [10,18,20].This may signify that some studies excluded patients who were likely to be affected by any of these side effects and reserved its use in comparatively healthier patients. However, 4 studies reported the incidence of infection although this was confounded with variations in the dose and duration of steroid use. This was the most disconcerting side effect. Corticosteroids treatment can make it possible for patients to catch influenza, which is a cause of severe CAP. Indeed some experts do recommend excluding influenza infection before beginning corticosteroids therapy in severe CAP. Therefore, it must be used cautiously for these effective drugs. However with the early usage of steroids being recommended by most guidelines the need to investigate the lowest dose to prevent any simultaneous secondary infection is of paramount importance.
Strengths of this meta-analysis were the rigorous methodology used. However, our study also has the following limitations. First, the HCQ regimens for severe SARS CoV2 requiring mechanical ventilation could not be fully elucidated; there was difference of the prescription of HCQs in these studies. Second, the definition of severe SARS CoV2 requiring mechanical ventilation was inconsistent in some studies which excluded high flow nasal cannula (HFNC) and not non invasive ventilation (NIV) from the purview of mechanical ventilation. This can confound the severity of complications in recipients of HFNC. Therefore, the studies following a consistent definition for mechanical ventilation need identification and reassessment.
Third, the usefulness of the drug has not been evaluated separately for mild, moderate and severe cases of COVID 19 and no such meta-analysis has been carried out to this effect. Certain studies have demonstrated favorable results with HCQ in mild to moderate COVID-19 patients [27].One such multinational registry analysis of 671 hospitals in 6 continents also found no benefit of HCQ. However, this study published was retracted as it is too preliminary to totally rule out the beneficial effects of HCQ in mild and moderate cases of COVID-19 [28].Even though there is a need for more concrete data with a larger sample size to substantiate this hypothesis, the possibility of beneficial effect of HCQ in mild or moderate cases cannot be entirely ruled out. This forms the basis of inclusion of HCQ in the current treatment protocol recommended for mild to moderate cases as per the Ministry of Health and Family Welfare Guidelines, Government of India [29].
Fourth, although the Infectious Diseases Society of America recommends use of hydroxychloroquine/chloroquine only in the context of a clinical trial, the position of FDA on the use of this drug has been inconsistent. The authority initially cautioned on its use in an out of hospital setting, then approving it prescription for emergency cases and finally revoking this decision [30].
5. Conclusion
Overall, HCQ therapy alone or in combination was neither effective nor safe for patients with SARS CoV2 infection and did not influence the survival. Although some efficacy was noticed in RT PCR conversion from positivity to negativity and in hypertensive patients their clinical significance requires confirmation with further high quality RCTs.
Source(s) of support
Nil.
Presentation at a meeting
None.
Declaration of competing interest
None.
References
- 1.Hashem A.M., Alghamdi B.S., Algaissi A.A., et al. Therapeutic use of chloroquine andhydroxychloroquine in COVID-19 and other viral infections- a narrative review. T ravel Med Infect Dis. 2020 May-Jun;35 doi: 10.1016/j.tmaid.2020.101735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tripathy S., Dassarma B., Roy S., Chabalala H., Matsabisa M.G. A review on possible modes of action of chloroquine/hydroxychloroquine: repurposing against SAR-CoV-2 (COVID-19) pandemic. Int. J. Antimicrob. Agents. 2020 May 22 doi: 10.1016/j.ijantimicag.2020.106028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quiros Roldan E., Biasiotto G., Magro P., Zanella I. The possible mechanisms of action of 4-aminoquinolines (chloroquine/hydroxychloroquine) against Sars-Cov-2 infection (COVID-19): a role for iron homeostasis? Pharmacol. Res. 2020 May 13;158 doi: 10.1016/j.phrs.2020.104904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent M.J., Bergeron E., Benjannet S., et al. Chloroquine is a potent inhibitor of SARS corona virus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chong Y.P., Song J.Y., Seo Y.B., Choi J.P., Shin H.S. Rapid response team. Antiviral treatment guidelines for Middle East respiratory Syndrome. Infect Chemother. 2015;47:212–222. doi: 10.3947/ic.2015.47.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodrigo C., Fernando S.D., Rajapakse S. Clinical evidence for repurposing chloroquine and hydroxychloroquine as antiviral agents: a systematic review. Clin. Microbiol. Infect. 2020 May 26;(20) doi: 10.1016/j.cmi.2020.05.016. S1198–743X. 30293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah S., Das S., Jain A., Misra D.P., Negi V.S. A systematic review of the prophylactic role of chloroquine and hydroxychloroquine in coronavirus disease-19 (COVID-19) Int J Rheum Dis. 2020;23:613–619. doi: 10.1111/1756-185X.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page M.J., Boutron I., Hansen C., Altman D.G., Hróbjartsson A. Assessing risk of bias in studies that evaluate health care interventions: recommendations in the misinformation age. J. Clin. Epidemiol. 2018;97:133–136. doi: 10.1016/j.jclinepi.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Mahévas M., Tran V.T., Roumier M., et al. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369:m1844. doi: 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg ES, Dufort EM, Udo T et al. Association of treatment with hydroxychloroquine or azithromycinwith in-hospital mortality in patients with COVID-19 in NewYork state. J. Am. Med. Assoc.. doi:10.1001/jama.2020.8630.
- 11.Barbosa J., Kaitis D., Freedman R., et al. Clinical outcomes of hydroxychloroquine in hospitalized patients with COVID-19: a quasi-randomized comparative study. N. Engl. J. Med. 2020 April [Google Scholar]
- 12.Cavalcanti Ab A.B., Zampieri F.G., Rosa R.G., et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate covid-19. N. Engl. J. Med. 2020 Sept;383:2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geleris J., Sun Y., Platt J., et al. May 14, 2020. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. at NEJM.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paccoud O, Tubach F,Baptiste A et al. Compassionate Use of Hydroxychloroquine in Clinical Practice for Patients with Mild to Severe Covid-19 in a French University Hospital. [DOI] [PMC free article] [PubMed]
- 15.Yu Bo, Li Chenze, Peng Chen, et al. Low dose of hydroxychloroquine reduces fatality of critically ill patients with COVID-19. Sci. China Life Sci. 2020 Oct;63(10):1515–1521. doi: 10.1007/s11427-020-1732-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arshad S., Kilgore P, Chaudhry Z.S., et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int. J. Infect. Dis. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagier J.C., Million M., Gautret P., et al. Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: a retrospective analysis. Trav. Med. Infect. Dis. 2020 June 36;36 doi: 10.1016/j.tmaid.2020.101791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang W., Cao Z., Han M., et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caleb P., Skipper C.P., Pastick K.A., Engen N.W., et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann. Intern. Med. 2020;173(8) doi: 10.7326/M20-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gautreta P., Lagier J.C., Parola P., et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six lay follow up: a pilot observational study. Trav. Med. Infect. Dis. 2020;34 doi: 10.1016/j.tmaid.2020.10166. 101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andreani J., Le Bideau M., Duflot I. In vitro testing of combined hydroxychloroquine and azithromycin on SARS CoV2 shows synergistic effect. Microb. Pathog. 2020 Aug;145 doi: 10.1016/j.micpath.2020.104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Touret F., de Lamballerie X. Of chloroquine and COVID-19. Antivir. Res. 2020 May;177 doi: 10.1016/j.antiviral.2020.104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann M., Kleine-Weber H., Krüger N., Müller M., Drosten C., Pöhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. BioRxiv. 2020:2020. 01.31.929042.
- 24.Rodrigo C., Fernando S.D., Rajapakse S. Clinical evidence for repurposing chloroquine and hydroxychloroquine as antiviral agents: a systematic review. Clin. Microbiol. Infect. 2020 May 26 doi: 10.1016/j.cmi.2020.05.016. S1198-743X (20)30293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das S., Bhowmick S., Tiwari S., Sen S. An updated systematic review of the therapeutic role of hydroxychloroquine in coronavirus disease-19 (COVID-19) Clin. Drug Invest. 2020 Jul;40(7):591–601. doi: 10.1007/s40261-020-00927-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chowdhury M.S., Rathod J., Gernsheimer J. A rapid systematic review of clinical trials utilizing chloroquine and hydroxychloroquine as a treatment for COVID-19. Acad. Emerg. Med. 2020 Jun;27(6):493–504. doi: 10.1111/acem.14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Z., Hu J., Zhang Z., et al. April 2020. Efficacy of Hydroxychloroquine in Patients with COVID-19: Results of a Randomized Clinical Trial. medRxiv. [Google Scholar]
- 28.Retracted, Mehra M.R., Desai S.S., Ruschitzka F., Patel A.N. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 May 22;S0140–6736(20):31180–31186. doi: 10.1016/S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.CLINICAL MANAGEMENT PROTOCOL: COVID-19. Ministry of Health and Family Welfare Directorate General of Health Services (EMR Division), Government of India. Version 5.
- 30.U.S. Food and Drug Administration Drug Safety Communication: FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-againstuse-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or Available at: Accessed on May 12, 2020.


