To the Editor:
Immune checkpoint inhibitors (ICIs) offer potential life-saving benefits to patients with cancer but can cause multisystem immune-related adverse events (irAEs) that, when severe, may lead to significant morbidity and mortality.1,2 Data regarding the safety and efficacy of ICIs in patients with cancer receiving maintenance dialysis are extremely limited because these patients were excluded from all pivotal clinical trials of ICIs. Addressing this knowledge gap is particularly important because cancer rates are higher in patients receiving dialysis than in the US general population.3 Currently, there are few published cases of ICI use in patients receiving dialysis.4–7 We aimed to characterize cancer response rates and the incidence of overall and severe (grade 3/4) irAEs in nonselected patients undergoing dialysis receiving ICIs at Partners Healthcare.
ICI treatment start dates were determined by oncology infusion records. Patients with at least 1 end-stage renal disease diagnosis code before the ICI start date were reviewed to confirm that they were receiving maintenance hemodialysis or peritoneal dialysis at the time of ICI initiation. Clinical details such as demographics, comorbid conditions, medications, cancer type, irAEs, disease status, and patient survival were ascertained through chart review. Severity of irAEs was determined by Common Terminology Criteria for Adverse Events, reviewed by an oncologist, and confirmed by a subspecialist.8 The Institutional Review Board approved this study and waived the need for informed consent.
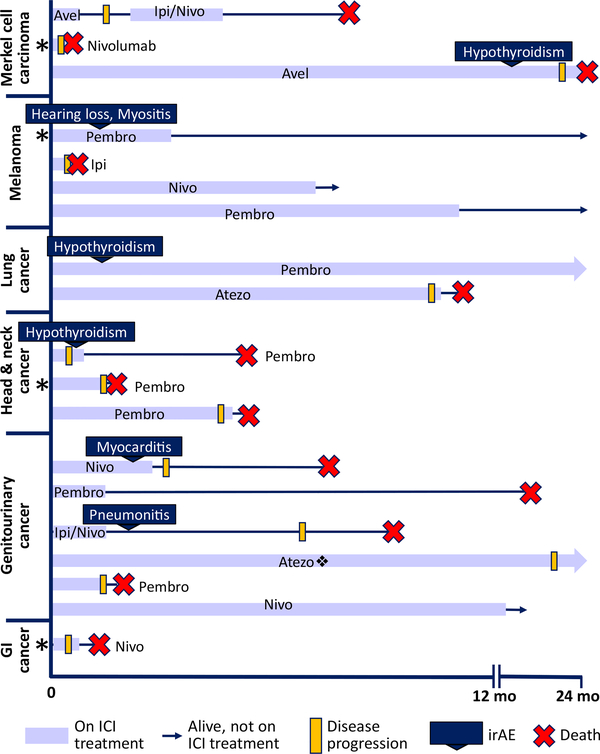
We identified 19 dialysis patients who received ICI therapy between 2013 and 2019. Table 1 shows baseline characteristics and malignancy type. Thirteen patients (68%) received PD-1 inhibitors; 4 (21%), PD-L1 inhibitors; 1 (5%), a CTLA-4 inhibitor; and 1 (5%), combination PD-1 and CTLA4 inhibitor therapy. All patients received standard ICI dosing regimens without dose reduction (Table S1). Overall, 6 patients (32%) experienced irAEs and 2 (11%) experienced an irAE of grade 3/4 toxicity (pneumonitis and myocarditis). Neither of the high-grade irAEs was confirmed by tissue biopsy. Three patients received systemic corticosteroids (≥60 mg/d) and permanently discontinued ICI therapy due to irAEs; 2 experienced full recovery, while 1 patient with suspected ICI-associated myocarditis had a persistently low left ventricular ejection fraction and died 5 months later of an unclear cause. Three (16%) patients had new-onset hypothyroidism requiring levothyroxine. Regarding cancer outcomes, 8 (42%) survived more than 12 months or are still in remission, including 3 of 4 patients with melanoma (Fig 1).
Table 1.
Patient Characteristics
| Characteristic | Value |
|---|---|
| Age, y | 71 ± 11 |
| Female sex | 8 (42%) |
| White, non-Hispanic | 1 7 (90%) |
| Cancer group | |
| Genitourinary | 6 (32%) |
| Melanoma | 4 (21%) |
| Merkel cell | 3 (16%) |
| Head and neck | 3 (16%) |
| Lung | 2 (11 %) |
| Gastrointestinal | 1 (5%) |
| ICI class | |
| PD-1 | 13 (68%) |
| PD-L1 | 4 (21%) |
| CTLA-4 | 1 (5%) |
| Combination CTLA-4/PD-1 | 1 (5%) |
| Medical comorbid conditions | |
| Diabetes mellitus | 6 (32%) |
| Hypertension | 1 7 (90%) |
| Coronary artery disease | 5 (26%) |
| Prior kidney transplant | 4 (21%) |
| Dialysis modality | |
| Peritoneal dialysis | 4 (21%) |
| Hemodialysis | 15 (79%) |
| Dialysis vintage, y | 1.7 [1.0–2.5] |
| Length of ICI treatment, d | 98 [42–327] |
| Reason for discontinuing immunotherapy | |
| Progression | 9 (47%) |
| Ongoing or completed treatment | 4 (21%) |
| irAE | 3 (16%) |
| Non-irAE side effectsa | 2 (11 %) |
| Death | 1 (5%) |
| irAEs | 6 (32%) |
| Grade 3–4 irAEs | 2 (11 %) |
Note: Values given as mean ± standard deviation, count (percent), or median [interquartile range]. The irAEs prompting discontinuation were myocarditis, pneumonitis, adrenalitis, and myositis.
In both cases, patients discontinued due to fatigue.
Figure 1.
Clinical course of the 19 patients treated with ICIs for advanced malignancies while receiving dialysis, grouped by cancer type. Patients with arrows survived beyond 2 years or are still alive at last follow-up. Eight patients (42%) survived longer than 12 months or are still in remission.*Patient who had a failed kidney transplant. ❖Patient who received 2 additional cycles of Pembro after 24 months of Atezo. Abbreviations: Atezo, atezolizumab; Avel, avelumab; Ipi, ipilimumab; Nivo, nivolumab; Pembro, pembrolizumab.
Notably, 4 patients in this case series had prior failed kidney transplants. Three of the 4 had progressive malignancy and died within 2 months of starting ICI therapy; 2 of these patients were still receiving low-dose transplant immunosuppression (prednisone, 5 mg, daily and prednisone, 10 mg, daily). Patient 4 was weaned off all immunosuppression therapy before starting ICI treatment for melanoma and has since been in remission for 2 years (Fig 1). None had abdominal pain or other clinical signs or symptoms of rejection of the failed allograft.
Our dual-center study on the characteristics and clinical courses of patients receiving ICI therapy while on dialysis substantially increases the total number of reported cases of this patient population. A recent literature review reported that 3 of the 13 (23%) patients undergoing dialysis receiving ICIs experienced grade 3/4 irAEs, including pneumonitis, encephalitis, and pemphigoid rash.4 A report of 8 patients with renal cell carcinoma receiving ICIs while receiving dialysis reported grade 3 adverse events in 2 cases.6 Due to the limited number of patients included in these studies, it is impossible to make definite conclusions regarding the safety and efficacy of ICIs in dialysis patients; however, we suspect that the high rate of severe irAEs previously reported in dialysis patients may be due to the reporting bias that exists in case reports. In the general population, 40% to 60% of patients receiving ICIs experience irAEs at some point during therapy.9 Because ICIs are nonrenally eliminated, no dosage adjustment is recommended for patients with decreased glomerular filtration rates, and given the high molecular weight of ICIs, clearance with dialysis is likely to be minimal. However, pharmacokinetic studies examining dialytic clearance do not exist.10 Our study is limited by the possible biases of a small retrospective cohort study, as well as the lack of definitive histologic phenotyping of irAEs because none was diagnosed through tissue biopsy. It is important to note that common comorbid conditions, laboratory abnormalities, and symptoms affecting dialysis patients may complicate the diagnosis of irAEs. The optimal management of patients with a prior kidney transplant who develop irAEs requires future study. The reporting of real-world outcomes of treatment with these novel anticancer therapies is of particular value for the dialysis population, which has a 5-year incidence of cancer of nearly 10% and has limited access to safety information due to exclusion from clinical trials.3,11 Additional larger studies, ideally involving multicenter registries, are needed to more precisely define the incidence and outcomes of irAEs in patients receiving dialysis receiving ICIs.
Supplementary Material
Table S1: ICI doses, frequencies, and number of doses administered for patients in the cohort.
Acknowledgments
Support: MES was supported by NIH K23 DK117014. TGN was supported in part through a gift from A. Curtis Greer and Pamela Kohlberg. Funders did not have any role in study design; data collection, analysis, or reporting; or the decision to submit for publication.
Financial Disclosure: MES has received grant support from Gilead Sciences, Abbvie, and Merck & Co. and has participated in scientific advisory board meetings for Abbvie, Merck & Co, and Gilead unrelated to the current research. TGN has acted as a consultant for Parexel, Bristol-Myers Squibb, Aprea Therapeutics, H3 Biomedicine, and Intrinsic Imaging unrelated to the current research. The remaining authors declare that they have no relevant financial interests.
Contributor Information
Ian A. Strohbehn, Department of Medicine, Liver Center, Massachusetts General Hospital.
Meghan Lee, Department of Medicine, Liver Center, Massachusetts General Hospital.
Harish Seethapathy, Division of Nephrology, Department of Medicine, Massachusetts General Hospital.
Donald Chute, Division of Nephrology, Department of Medicine, Massachusetts General Hospital.
Osama Rahma, Department of Medical Oncology, Dana Farber Cancer Institute, Department of Medicine, Massachusetts General Hospital, Boston, MA.
Amanda Guidon, Division of Neuromuscular Medicine, Department of Neurology, Department of Medicine, Massachusetts General Hospital, Boston, MA.
Tomas G. Neilan, Divisions of Cardiology, Department of Medicine, Massachusetts General Hospital, Boston, MA.
Daniel A. Zlotoff, Divisions of Cardiology, Department of Medicine, Massachusetts General Hospital, Boston, MA.
Daniel Okin, Pulmonary and Critical Care Medicine, Department of Medicine, Massachusetts General Hospital, Boston, MA.
Michelle Rengarajan, Endocrinology, Department of Medicine, Massachusetts General Hospital, Boston, MA.
Kerry Reynolds, Hematology and Oncology, Department of Medicine, Massachusetts General Hospital, Boston, MA.
Meghan E. Sise, Division of Nephrology, Department of Medicine, Massachusetts General Hospital.
References
- 1.Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shingarev R, Glezerman IG. Kidney complications of immune checkpoint inhibitors: a review. Am J Kidney Dis. 2019;74(4): 529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler AM, Olshan AF, Kshirsagar AV, et al. Cancer incidence among US Medicare ESRD patients receiving hemodialysis, 1996–2009. Am J Kidney Dis. 2015;65(5):763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheun H, Kim M, Lee H, Oh KH, Keam B. Safety and efficacy of immune checkpoint inhibitors for end-stage renal disease patients undergoing dialysis: a retrospective case series and literature review. Invest New Drugs. 2019;37(3):579–583. [DOI] [PubMed] [Google Scholar]
- 5.Ansari J, Ali M, Farrag A, Ali AM, Alhamad A. Efficacy of nivolumab in a patient with metastatic renal cell carcinoma and end-stage renal disease on dialysis: case report and literature review. Case Rep Immunol. 2018;2018:1623957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vitale MG, Baldessari C, Milella M, et al. Immunotherapy in dialysis-dependent cancer patients: our experience in patients with metastatic renal cell carcinoma and a review of the literature. Clin Genitourin Cancer. 2019;17(5):e903–e908. [DOI] [PubMed] [Google Scholar]
- 7.Osman-Garcia I, Congregado-Ruiz CB, Lendinez-Cano G, Baena-Villamarin C, Conde-Sanchez JM, Medina-Lopez RA. Outcomes and safety of biweekly and monthly nivolumab in patients with metastatic renal cell carcinoma and dialysis: three case reports and literature review. Urol Int. 2020;104(3–4):323–326. [DOI] [PubMed] [Google Scholar]
- 8.Common Terminology Criteria for Adverse Events (CTCAE) Version 5. In: Services USDoHaH, editor. 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5×7.pdf. Accessed May 22, 2020.
- 9.Som A, Mandaliya R, Alsaadi D, et al. Immune checkpoint inhibitor-induced colitis: a comprehensive review. World J Clin Cases. 2019;7(4):405–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centanni M, Moes D, Troconiz IF, Ciccolini J, van Hasselt JGC. Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin Pharmacokinet. 2019;58(7):835–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitchlu A, Shapiro J, Amir E, et al. Representation of patients with chronic kidney disease in trials of cancer therapy. JAMA. 2018;319(23):2437–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: ICI doses, frequencies, and number of doses administered for patients in the cohort.