Abstract
Sars-Cov-2 infection is still a healthcare emergency and acute respiratory distress failure with Diffuse Alveolar Damage (DAD) features is the main causes of patients’ death. Pathogenic mechanisms of the disease are not clear yet, but new insights are necessary to improve therapeutic management, to prevent fatal irreversible multi-organ damage and to adequately follow up those patients who survive. Here we investigated, by histochemistry and immunohistochemistry, a wide number of mapped lung specimens taken from whole body autopsies of 7 patients dead of COVID-19 disease. Our data confirm morphological data of other authors, and enlarge recent reports of the literature suggesting that Endothelial–Mesenchymal Transition might be central to COVID-19 lung fibrosing lesions. Furthermore, based upon recent acquisition of new roles in immunity and vascular pathology of the CD31 molecule, we hypothesize that this molecule might be important in the development and treatment of COVID-19 pulmonary lesions. These preliminary findings need further investigations to shed light on the complexity of Sars-Cov-2 disease.
Keywords: COVID-19, Diffuse alveolar damage, Fibrosis, Endothelial-Mesenchymal Transition, CD31
1. Introduction
In-depth analyses on pathomechanisms leading to Sars-Cov-2 patients’ death are critical to appropriate therapeutic management of symptomatic subjects and to follow up those who survive. Aspects of Diffuse Alveolar Damage (DAD), often with thromboembolism and superimposed infections, were described in the lung tissue of COVID-19 deceased patients [1,2]. Recently the Endothelial-Mesenchymal Transition (End-MT) process was suggested to have an important role in COVID-19 pulmonary fibrosis by Eapen et al. [3]. Here we investigated with histochemistry and immunohistochemistry lung specimens from whole body COVID-19+ autopsies to deepen aspects of the disease pathogenesis possibly useful to rationalize therapies.
2. Methods
Whole body autopsies were performed in 7 consecutive COVID-19+ patients died of the disease in the Sub-Intensive and Intensive Care Unit of San Paolo Hospital, University of Milan, Italy (patients’ clinical data are summarized in Table 1 ). Autopsies were performed in safety within 1−2 hours from death to avoid post-mortem artefacts.
Table 1.
Patients’ clinical data.
| Patient | Age | Sex | SARS-CoV-2 swab at admission | Respiratory symptoms before hospitalization (days) | Sub-intensive care (days) | Intensive care (days) | Type of ventilation | Associated diseases | Overlapping infections during hospitalization |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 55 | M | + | 0 | 16 | 0 | no | diabetes mellitus, obesity, chronic kidney failure, peripheral vasculopathy, OSAS | none |
| 2 | 58 | M | + | 0 | 7 | 9 | NIV/CPAP | diabetes mellitus, obesity, hypertension | none |
| 3 | 54 | M | + | 1 | 10 | 19 | CPAP/MV | MRSA e A.Baumanii | |
| 4 | 56 | M | + | 2 | 9 | 18 | NIV/MV | ulcerative colitis, hypertension | Pseudmonas Aeruginosa |
| 5 | 68 | M | + | 10 | 17 | 21 | NIV/MV | none | |
| 6 | 54 | M | + | 2 | 4 | 14 | NIV/CPAP/MV | none | |
| 7 | 64 | F | + | 2 | 0 | 7 | NIV/CPAP/MV | hypertension, hypothyroidism, BPCO | none |
NIV = Non-Invasive Ventilation.
CPAP = Continuous Positive Airway Pressure.
MV = Mechanical Ventilation.
OSAS = Obstructive Sleep Apnea Syndrome.
COPD = Chronic Obstructive Pulmonary Disease.
MRSA = Methicillin-Resistant Staphylococcus Aureus.
Consecutive 3-μm sections from extensively mapped, routinely paraffin-embedded, lung tissue samples were explored by immunohistochemistry with the automatic immunostainer DAKO OMNIS (DAKO, Glostrup, Denmark).
All lung samples were tested for Sars-Cov-V2 (2019-nCoV) Nucleoprotein (NP) antigen (Sino Biological) to confirm the virus presence [4].
In order to assess tissue changes in epithelial and vascular lung architecture, we used Cytokeratin 7, TTF1, Napsin A, Vimentin, Calponin, α-Actin, Desmin, CD34, CD31 and ERG antibodies. Inflammatory background was characterized with antibodies against CD20, CD3, CD4, CD8, CD56, MUM1, CD15 and CD163.
Fibrin and collagen were highlighted with the Masson’s Trichrome and PTAH stainings.
Parasites other than Sars-Cov-V2 virus were evaluated with Gram and PAS stains.
For each patient, three areas with different stages of disease evolution (early, proliferative and fibrotic) were selected and 3 high power fields (X40) were evaluated in each area.
For each marker, a semi-quantitative score (none = 0, mild = 1, moderate = 2, severe = 3) was applied as previously described [5], reporting the mean score value of each area (early, proliferative and fibrotic).
Slides were simultaneously evaluated by all the pathologists of the study.
Lung sections from 7 non COVID-19+ post-bacterial pneumonia with fibroblastic proliferations were used as controls.
3. Results
In all cases Sars-Cov-V-2 infection was documented by immunoreactivity for NP viral antigen in respiratory epithelium, pneumocytes, endothelium and inflammatory cells. GRAM stain was positive in two patients with overlapping infection.
All patients, including that from Sub-Intensive Care Unit, had similar pulmonary microscopic aspects, with prevalent early/exudative and intermediate/proliferative phases of DAD and minimal collagen deposition.
No differences in score values were observed among immune cell groups in DAD phases. Since early lung injury the inflammatory infiltrate was predominantly constituted by macrophages, diffusely detected in damaged parenchyma.
Macrophages with unusual morphology (bi-nucleation and eosinophilic large nucleoli) and increased strong CD31 and CD163 immunoreactivity were mixed with hyperplastic atypical and sometimes metaplastic pneumocytes engulfing alveolar spaces. This aspect was present also in DAD proliferative phase, where their number still increased and a fibroblastic foci entrapment was evident. Only in the fibrosing phase macrophages began slightly to decrease, crushed by the fibrosis. Faint fibrotic modification in vessels are appreciable since early phases: weak blue collagen deposition around medium-sized vessels was accompanied with discontinuity in CD34 and, to a lesser extent, in CD31 immunostaining of capillaries.
In areas with proliferative DAD aspects, fibroblastic foci, both as small plugs and elongated interlacing bundles of spindle cells, showed intermediate/high expression of α-Actin and CD31 in early lesions; in more advanced lesions, α-Actin displayed no modification while CD31 immunoreactivity decreased to loss when a concomitant cytoplasmic accumulation of slightly blue collagen material was appreciated.
All cases showed damaged vascular network: in early lesions swollen endothelium in capillaries of alveolar septa showed discontinuous and reduced CD34, ERG and CD31 immunostaining. In fields with early proliferative DAD features small and medium-sized vessels exhibited homogenous eosinophilic thickened walls and narrowed lumen in close continuity with oval or spindle cells of fibroblastic foci; these cells were strongly positive to CD31 and showed intermediate immunoreactivity to α-Actin and Vimentin, with only a weak staining for Calponin. Fibroblastic spindle cells in proliferative DAD fields reduced CD31 staining, acquired strong immunoreactivity for Vimentin, Calponin and α-Actin, and were negative to ERG and CD34; their cytoplasm turned from red to blue with Masson’s Trichrome, due to accumulation of collagenous material. In the most damaged lung fields (late proliferative/early fibrosing lesions), vessels were unrecognizable and spindle cells, with bluish cytoplasmic collagen with Masson staining, were negative to CD34, ERG and CD31. In control lungs fibrotic areas, endothelial markers (CD34, ERG and CD31) showed disruption but not reduction in the immunohistochemical expression.
Results are depicted in Fig. 1, Fig. 2 , and detailed in Table 2 .
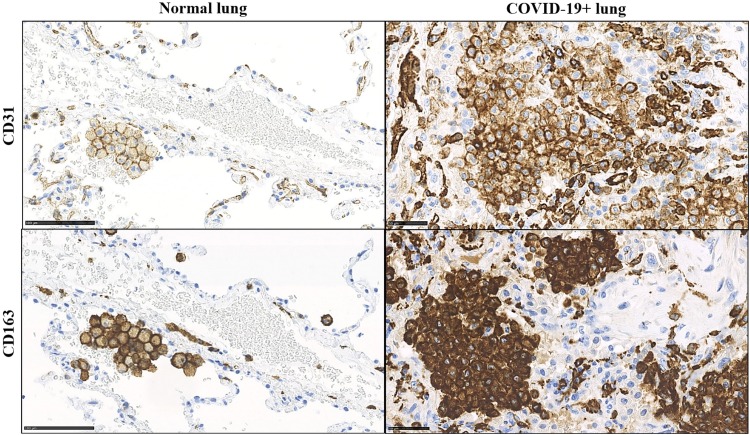
Fig. 1.
Aberrant CD31 and CD163 expression in alveolar macrophage accumulated in COVID-19+ patients. In normal lung parenchyma, small groups of macrophages are detectable in the alveolar spaces; membrane cytoplasmic immunostaining for CD31 and CD163 is appreciable in these cells. In COVID-19+ patient macrophages are increased in number and show CD31 and CD163 overexpression at both membrane and cytoplasmic levels.
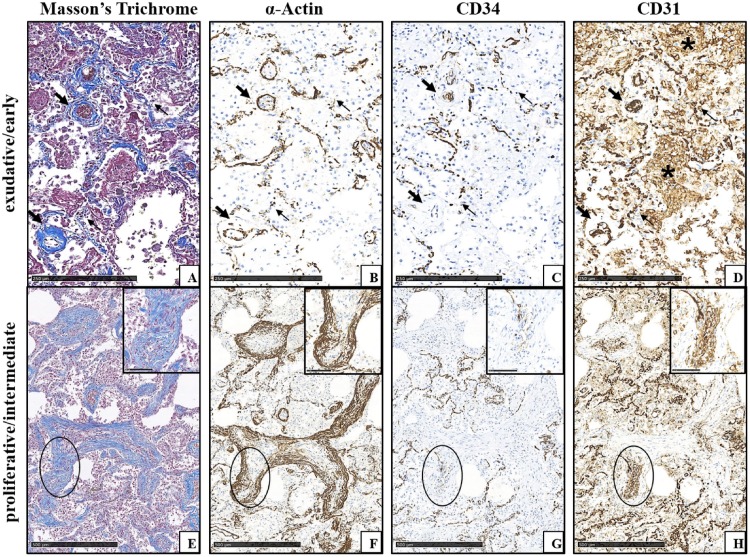
Fig. 2.
COVID-19+ lung lesion features in different phases (same patient, consecutive tissue sections from paraffin-embedded specimens blocks). A-D) Early lung damage with exudative DAD features in the alveolar septa (thin arrows) and around medium-sized vessels (thick arrows). A) Initial blue collagen deposition in the alveolar septa and around vessels highlighted by Masson’s Trichrome staining. B) α-Actin immunoreactivity is easily appreciated in the walls of vessels, and is fragmented in the capillaries of the alveolar septa. C) CD34 immunoreactivity is decreased in vessels and in septal capillaries, where it is also fragmented. D) CD31 immunostaining in vascular compartment at all levels is strong, but appears fragmented in alveolar capillaries; CD31+ macrophages accumulated in alveolar spaces are appreciable (asterisks). E-H) Advanced lung damage with myofibroblastic foci with proliferative DAD features (circles). E) Myofibroblastic foci are here organized as bundles of spindle cells; blue collagen production is detectable both in the cytoplasm of myofibroblastic cells and, to a lesser extent, in the extracellular matrix (inset of the circled area). Masson’s Trichrome staining. F) α-Actin immunoreactivity is easily appreciable in the outer myofibroblastic cells of fibroblastic foci (inset of the circled area). G) CD34 immunostaining is lost in the outer areas of organizing fibrosis (inset of the circled area). H) In myofibroblastic foci with disappeared/minimal CD34 immunoreactivity, CD31 staining is still present in myofibroblastic cells and in filamentous elongated structures in the centre of the proliferation (inset of the circled area).
Table 2.
Markers distribution of immune cell infiltrate, epithelial respiratory cells, and of vessels and fibroblastic foci in COVID-19+ lung DAD lesions. Markers are scored as specified in the material and methods section.
| Phase of disease evolution |
|||
|---|---|---|---|
| Marker | Exudative/Early | Proliferative/ Intermediate | Fibrosing/ Late |
| CD20 | 1 | 1 | 1 |
| CD3 | 2 | 2 | 1 |
| CD4 | 1 | 1 | 1 |
| CD8 | 2 | 2 | 2 |
| CD56 | 1 | 1 | 0 |
| MUM1 | 1 | 1 | 0 |
| CD15 | 3 | 3 | 1 |
| CD 163 | 3 | 3 | 2 |
| CK7 | 2 | 2 | 2 |
| TTF-1 | 2 | 1 | 1 |
| Napsin | 3 | 3 | 2 |
| Desmin | 1 | 2 | 2 |
| α-Actin | 2 | 3 | 3 |
| Vimentin | 2 | 3 | 3 |
| Calponin | 1 | 2 | 3 |
| Masson | 0 | 2 | 3 |
| CD34 | 2 | 0 | 0 |
| CD31 | 2 | 3* | 3* |
| ERG | 2 | 0 | 0 |
For Each marker, the mean values of scores attributed to 3 different areas representative of each evolutionary phase in patients’ slides are here reported. No important differences were found among inflammatory cells but macrophages, which accumulated with disease progression. CD31 was progressively lost (as ERG) by vessels near fibroblastic foci, but its expression increased in macrophages in evolving lesions (*) (see text and figures).
4. Discussion
Our findings are in line with data in the literature about DAD morphological features and inflammatory infiltrates in patients died of COVID-19 respiratory distress, suggesting a complex dysfunction in immune response necessary to fight infection and to limit exaggerated reparative response to lung damage [6]. Parenchymal injury, independently of patients’ age, clinical history, disease length and permanence in the Sub-Intensive or Intensive Care Unit, is a fibrosing process in different stages of evolution, whose features, characterized in this work, further support recent literature data focusing on End-MT as the process involved in Sars-Cov-2-induced damage [3]. End-MT is characterized by endothelial cell disaggregation, vascular progressive loss of polarization and immunophenotype, and acquisition of motility and extracellular matrix antigens [6]. In this study morphological aspects of vascular alterations, and histo- and immunohisto- chemical modifications of in spindle cells in their close proximity during disease evolution, support the hypothesis of an endothelial cell transformation in pro-fibrotic myofibroblast, similar to the fibroblast-like cell at intermediate state of End-MT described by Hashimoto [7]. In fact, from early to intermediate lesions, cells in close relationship with damaged vessel walls acquire spindle cell morphology, motility (α-Actin expression) and mesenchymal markers while still maintaining vascular stigmata (CD31 expression); in late proliferative or at the beginning of fibrosing phase, these “mesenchymal” cells progressively acquire collagen-producing histochemical features while contextually losing, until negativity, all vascular antigens, lastly CD31. This event might possibly represent a crucial “point of no return” for a rescue of lung architecture and for the acquisition of fibrosing characteristics; further investigations might probably add important information to CD31 role in the process. This is particularly true considering that immune cell infiltrate in COVID-19+ lung parenchyma is peculiarly rich in alveolar macrophages with CD31 overexpression. Though this molecule is a well-known endothelial marker, it is also expressed by macrophages and other inflammatory cells [8,9] with important roles in mediation of pro- and anti-inflammatory signalling, and immuno-tolerance [6,9], in leucocyte-leucocyte engagement and interaction [9], and in immune cell proliferation, apoptosis and migration [8]. In macrophages, CD31 was reported to be involved in the promotion of reparative polarized functions [10] and, interestingly, in this work, a co-overexpression of this antigen with CD163, a M2 macrophage molecule associated to tissue remodelling and repair, was found in these cells. As recent evidences hypothesize a possible macrophage control on endothelial plasticity involved in vasculopathy and fibrosis under pathological conditions [11], our data might suggest COVID-19+ macrophages involvement in dysregulation of lung tissue repair, with exuberant remodelling features, or altered endothelial plasticity during the End-MT process, or defective mechanisms triggering the apoptosis-mediated clearance of myofibroblasts at the end of the virus-induced alveolar wound repair. Lung damage would be the result of a vicious positive feed-back loop of persistent damage, failed controlled regeneration, and of myofibroblast–fibroblast persistence, activation, and resistance to apoptosis [11].
Furthermore, CD31 macrophage overexpression might suggest a dysfunction in their antigen presenting cell properties [12] and immune cell recruitment and coordination at the site of infection; absence of significant differences among immune cell groups in the progression COVID-19+ lung might support this hypothesis and suggest a macrophage functional defect as an early event in lung damage.
In conclusion, our results support current literature data suggesting End-MT as a process heavily involved in lung fibrosis of COVID-19+ patients [3]. Moreover, on the basis of research information about CD31 role in immune cell and tissue repair regulation, our results suggest that further studies on this molecule and lung macrophages might add important information in the understanding of COVID-19 pathogenesis and treatment.
Authors statement
All Authors have seen and approved the final version of the manuscript being submitted.
They warrant that the article is the authors' original work, hasn't received prior publication and isn't under consideration for publication elsewhere.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
We would like to thank all the staff members of the Pathology lab for the diligence, technical skills and willingness spent in this research.
References
- 1.Bussani R., Schneider E., Zentilin L., et al. Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. EBioMedicine. 2020;61 doi: 10.1016/j.ebiom.2020.103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calabrese F., Pezzuto F., Fortarezza F., et al. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European Pulmonary Pathologists. Virchows Archiv. 2020;477:359–372. doi: 10.1007/s00428-020-02886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eapen M.S., Lu W., Gaikwad A.V., et al. Endothelial to mesenchymal transition: a precursor to post-COVID-19 interstitial pulmonary fibrosis and vascular obliteration? Eur. Respir. J. 2020;56(4) doi: 10.1183/13993003.03167-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bulfamante G.P., Perrucci G.L., Falleni M., et al. Evidence of SARS-CoV-2 transcriptional activity in Cardiomyocytes of COVID-19 patients without clinical signs of cardiac involvement. Biomedicines. 2020;8:626–639. doi: 10.3390/biomedicines8120626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorward D.A., Russell C.D., Um I.H., et al. Tissue-specific immunopathology in fatal COVID-19. Am. J. Respir. Crit. Care Med. 2021;203:192–201. doi: 10.1164/rccm.202008-3265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manetti M., Romano E., Rosa I., et al. Endothelial-to-mesenchymal transition contributes to endothelial dysfunction and dermal fibrosis in systemic sclerosis. Ann. Rheum. Dis. 2017;76(5):924–934. doi: 10.1136/annrheumdis-2016-210229. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto N., Phan S.H., Imaizumi K., et al. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2010;43(2):161–172. doi: 10.1165/rcmb.2009-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y.Y., Kong L.Q., Zhu X.D., et al. CD31 regulates metastasis by inducing epithelial-mesenchymal transition in hepatocellular carcinoma via the ITGB1-FAK-Akt signaling pathway. Cancer Lett. 2018;429:29–40. doi: 10.1016/j.canlet.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Marelli-Berg F.M., Clement M., Mauro C., Caligiuri G. An immunologist’s guide to CD31 function in T-cells. J. Cell. Sci. 2013;126:2343–2352. doi: 10.1242/jcs.124099. [DOI] [PubMed] [Google Scholar]
- 10.Andreata F., Syvannarath V., Clement M., et al. Macrophage CD31 signaling in dissecting aortic aneurysm. J. Am. Coll. Cardiol. 2018;72(1):45–57. doi: 10.1016/j.jacc.2018.04.047. [DOI] [PubMed] [Google Scholar]
- 11.Horowitz J.C., Thannickal V.J. Mechanisms for the resolution of organ fibrosis. Physiol. Bethesda (Bethesda) 2019;34(1):43–55. doi: 10.1152/physiol.00033.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H., Liu L., Zhang D., et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020 doi: 10.1016/S0140-6736(20)30920-X. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]