Abstract
Objectives
Critical illness in COVID-19 is attributed to an exaggerated host immune response. Since neutrophils are the major component of innate immunity, we hypothesize that the quantum of activated neutrophils in the blood may predict an adverse outcome.
Design
In a retrospective study of 300 adult patients with confirmed COVID-19, we analyzed the impact of neutrophil activation (NEUT-RI), interleukin-6 (IL-6) and the established clinical risk factors of age, diabetes, obesity and hypertension on the clinical outcome.
Results
Significant predictors of the need for mechanical ventilation were NEUT-RI (Odds Ratio (OR) = 1.22, P < 0.001), diabetes (OR = 2.56, P = 0.00846) and obesity (OR = 6.55, P < 0.001). For death, the significant predictors were NEUT-RI (OR = 1.14, P = 0.00432), diabetes (OR = 4.11, P = 0.00185) and age (OR = 1.04, P = 0.00896). The optimal cut-off value for NEUT-RI to predict mechanical ventilation and death was 52 fluorescence intensity units (sensitivity 44%, specificity 88%, area under the curve 0.67 and 44%, 86%, 0.64, respectively).
Conclusion
This finding supports an aberrant neutrophil response in COVID-19, likely due to uncontained viral replication, tissue hypoxia and exacerbated inflammation, introduces a novel biomarker for rapid monitoring and opens new avenues for therapeutic strategies.
Keywords: COVID-19, Activated neutrophils, Innate immunity, Aberrant neutrophil response, Critical illness, Biomarker
Introduction
COVID-19 is a novel respiratory disease caused by SARS-CoV-2. While most patients have asymptomatic or mild self-limiting disease, approximately 17%–35% of hospitalized patients will require intensive care, primarily for acute respiratory distress syndrome (ARDS) and multiorgan failure (Wiersinga et al., 2020). For some, the turning point towards a more ominous course appears to be the onset of progressive hypoxia, exacerbated by a poorly understood host response involving a cytokine storm. Access of the virus to the pulmonary epithelia, with rapid viral expansion, alveolar wall inflammation and destruction, and compromise of the alveolar-endothelial barrier, is likely responsible for the initial impairment of alveolar gas exchange (Wiersinga et al., 2020, Huertas et al., 2020). Interstitial mononuclear inflammatory infiltrates then follow with progression to ARDS (Xu et al., 2020). With time, inflammation and edema, likely perpetuated by unchecked viral replication and the trauma of mechanical ventilation and hypercytokinemia, can lead to generalized thrombogenesis and multiorgan failure (Marini and Gattinoni, 2020).
Neutrophils are essential for their function in innate immunity and play an important role in inflammation and the repair of hypoxia-induced tissue damage (Wang, 2018, Lin and Simon, 2016). However, upon recruitment to inflamed tissues, experimental evidence indicates that neutrophils can cause collateral tissue damage, either locally or even at distant sites from reverse trans-endothelial migration (Colom et al., 2015, Woodfin et al., 2011). Focus is now being directed to their involvement in COVID-19 host immunity and whether such an aberrant response contributes to thrombosis and multiorgan damage. The role of neutrophils in COVID-19 cannot be underestimated. Rapidly replicating virus in the pulmonary epithelium and widespread clinical or subclinical tissue hypoxia are likely to attract a large number of neutrophils, and there are pointers towards collateral damage. Neutrophilia has been shown to be a predictor of poor outcome in COVID-19 (Wang et al., 2020a). Tissue infiltration by neutrophils in pulmonary capillaries and their extravasation into the alveolar space has been demonstrated in autopsy patients (Yao et al., 2020, Fox et al., 2020). There is also growing interest in neutrophil extracellular traps (NET) to contain the virus and whether this could contribute to the development of thrombosis and ARDS as typically seen in critically ill COVID-19 patients (Middleton et al., 2020, Veras et al., 2020).
Neutrophils need to be activated into functional effector cells to fulfill their role in innate immunity and tissue repair (Mayadas et al., 2014). It is understood that the neutrophil activation process begins shortly after recruitment as they roll along the endothelial walls of postcapillary venules and sample inflammatory signals (Ley, 2002). In the setting of severe COVID-19, where the extent of tissue neutrophil activity is thought to be extremely high, it is likely that activated neutrophils are constantly circulating in the peripheral blood. Their detection and quantification could assume importance in COVID-19 to determine correlations to disease outcome, improve our understanding of the disease pathophysiology and potentially monitor future targeted therapy.
There is a rapid technique to detect activated neutrophils in the peripheral blood using the Sysmex™ XN-9100 automated hematology analyzer. The Sysmex™ XN-series analyzers (Sysmex Corp., Kobe, Japan) utilize fluorescence flow-cytometry for the leukocyte differential, enabling enhanced subset differentiation based on forward and side scatter as well as RNA content. In addition to providing an accurate and reliable assessment of the complete blood count (CBC), nucleated red blood cells (nRBC) and immature granulocytes (IG), this technology also offers a set of hematological inflammation parameters that quantitatively assess the activation status of neutrophils and lymphocytes (Ustyantseva et al., 2019, Henriot et al., 2017, Yip et al., 2020, Linssen et al., 2007). This study analyzes the contribution of these novel parameters to established clinical risk factors in predicting adverse outcomes in a retrospective study of 300 patients with COVID-19 admitted to the Sultan Qaboos University Hospital, Oman (SQUH).
Methods
Between May and August 2020, over 11 weeks, a total of 331 consecutive adult patients required admission for symptomatic COVID-19 to SQUH. Testing for SARS CoV-2 was done on standard respiratory specimens for all patients by real-time polymerase chain reaction (RT-PCR). Data for all of these patients were retrospectively collected after approval by the institution’s medical research ethics committee, and a database including relevant demographic, clinical and laboratory data was created. The study aimed to look for a correlation between the novel neutrophil and lymphocyte activation markers, IG and nRBC, offered by the Sysmex™ XN-9100, along with the established clinical risk factors (age, obesity, diabetes and hypertension [HTN]) with mechanical ventilation and death.
Inclusion and exclusion criteria
All adult patients presenting to the emergency department of SQUH with symptoms consistent with COVID-19 and confirmed for SARS CoV-2 by RT-PCR from May to August 2020 were included in the study. Patients who had hemoglobinopathies, hematologic or solid malignancy on chemotherapy were excluded. Patients were also excluded if a CBC was not done at the time of admission.
Clinical parameters
Specific clinical parameters included comorbidities, the onset of symptoms, oxygen saturation by pulse oximeter at admission (normal >93%), maximum oxygen requirement (room air, nasal prongs or mask, non-invasive ventilation or mechanical ventilation), time to recovery, and death or discharge. The result of blood culture, if done at admission, was also recorded.
Hematological parameters
Central to this study is the use of the Sysmex™ XN-9100 automated cell analyzer for the detection and quantification of IG, nRBC, neutrophil reactivity intensity (NEUT-RI), neutrophil granulocyte intensity (NEUT-GI), total reactive lymphocytes (RE-Lymph) and antibody synthesizing lymphocytes (AS-Lymph).
Principle of detection of neutrophil and lymphocyte activation markers
Activated neutrophils secrete several pro-inflammatory cytokines and surface molecules for antigen presentation and activation of t-cells. In the Sysmex™ XN-DIFF channel, cells are differentiated according to their fluorescence signal intensity, size and internal structure. Activated cells have a different membrane lipid composition and a greater activity in the cytoplasm as they actively produce cytokines. Thus an increase in activity is accompanied by an increase in the number of nucleic acids that are more intensively stained with a fluorescent dye, increasing the fluorescent signal and magnitude of NEUT-RI. The parameter NEUT-RI, expressed in fluorescence intensity units (FI), reflects this neutrophil reactivity, essentially representing its metabolic activity. The 90° side scattered light of the XN-DIFF channel provides information about cell density or complexity, representing granularity. Therefore, if the complexity of neutrophils increases upon a change in functionality, e.g., by toxic granulation or vacuolization, the neutrophil cloud in the scattergram is also affected. The parameter NEUT-GI, expressed in the unit SI (scatter intensity), will change accordingly (Ustyantseva et al., 2019). Fluorescence intensity above that of normal lymphocytes with the highest fluorescence was found to be antibody-synthesizing lympho-plasmacytoid B and plasma cells. RE-Lymph (reactive lymphocytes) represents all lymphocytes with a higher fluorescence signal than the normal lymphocyte population. This parameter is given as an absolute (cells/L) and as a percentage. AS-Lymph (antibody synthesizing lymphocytes) denote the activated B lymphocytes that synthesize antibodies. They represent the lymphocyte subpopulation with the highest fluorescence signals and are part of the RE-Lymph population (Linssen et al., 2007; Sysmex, 2017).
Detection of immature granulocytes and nucleated red blood cells
The Sysmex™ XN-series auto analyzers provide accurate quantification of IG and nRBC by fluorescence technology. Absolute values (×109/L) were used in this study. The IG fraction in the CBC measured by this method comprises promyelocytes, myelocytes and metamyelocytes. Blasts and band forms are not included (Weiland et al., 2002).
IL-6 analysis
IL-6 concentration was measured on serum samples using the automated ELECSYS IL-6 immunoassay (electrochemiluminescence immunoassay) by the Cobas e-601 immunoassay analyzer (Rosche diagnostics, Switzerland) following the manufacturer’s protocol. The cut-off of IL-6 used was 7 pg/mL.
Statistical analysis
Continuous variables were described using medians with the interquartile ranges (IQR), while categorical variables were described using proportions. Pearson’s correlation was used for the correlation matrix of the laboratory variables. The impact of laboratory variables on mechanical ventilation and death was assessed using univariable logistic regression. Statistically significant factors were then entered into multivariable logistic regression. The known clinical prognostic factors for the 2 outcomes were entered into the multivariable logistic regression model to assess if the factors can independently predict these outcomes. These factors included age, diabetes, obesity and HTN. Obesity was defined as a body mass index of ≥30. An alpha threshold of 0.05 was used for assessing the statistical significance. Patients with missing weight and height values were labelled obese if treating physicians labelled them as obese before the outcome. The best cut-off value was assessed by maximizing the sensitivity and specificity. The discrimination of the cut-off values was assessed using the area under the curve (AUC). The software R program (R version 3.5.1, R Core Team, 2018) was used for all descriptive and analytical statistics.
Results
Baseline characteristics and the correlations between indices
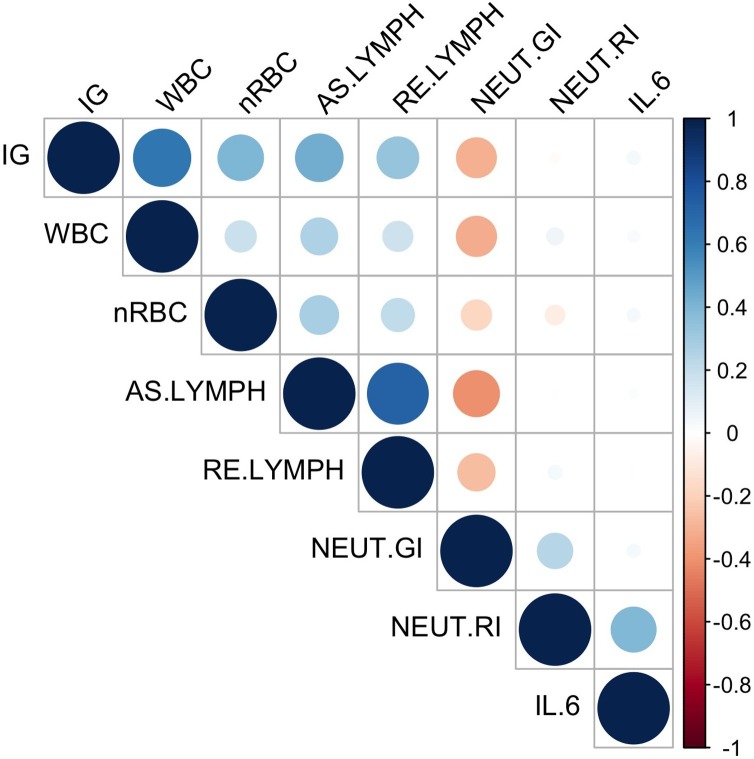
A total of 300 consecutive patients (Table 1 ) who attended the emergency department for COVID-19 were included in this study with a median age of 53 years (IQR: 41–65). The median values for nRBC, WBC, IG and NEUT-RI were 0.00 × 109/L (IQR: 0.00−0.00), 6.5 × 109/L (IQR: 4.8–9.1), 0.02 × 109/L (IQR: 0.00−0.10) and 48.8 FI (IQR: 46.3–50.9), respectively. Thirty-nine percent of the included patients had diabetes, and 10% were obese. The median IL-6 value at presentation was 37 pg/mL (IQR: 14–91). Only 7% of patients had bacteremia at presentation or during their admission. The correlation matrix between different laboratory parameters is represented in Figure 1 . Overall, the correlation between the parameters was generally in the very weak or weak category except the following: nRBC with IG (moderate, r = 0.40), IG with WBC (strong, r = 0.64), IG with AS-Lymph (moderate, r = 0.44), NEUT-GI with AS-Lymph (negative moderate, r = −0.41), and AS-Lymph with RE-Lymph (strong, r = 0.71).
Table 1.
Baseline characteristics (n = 300).
| Variable | Description |
|---|---|
| Age, median (IQR), years | 53 (41–65) |
| Gender | |
| Female | 107 |
| Male | 193 |
| nRBC, median (IQR), ×109/L | 0.00 (0.00−0.00) |
| IG, median (IQR), ×109/L | 0.02 (0.00−0.10) |
| WBC, median (IQR), (×109/L | 6.5 (4.8–9.1) |
| NEUT-GI, median (IQR), SI (scatter intensity) | 154 (150–157) |
| NEUT-RI, median (IQR), FI (fluorescence intensity) | 48.8 (46.3–50.9) |
| AS-Lymph, median (IQR), cells/L | 0.03 (0.00−0.05) |
| RE-Lymph, median (IQR), cells/L | 0.07 (0.04−0.11) |
| IL-6, median (IQR), pg/mL | 37 (14–91) |
| Comorbidities | |
| CAD | 30 |
| HTN | 118 |
| DM | 116 |
| Obesity | 31 |
| Smoking | 3 |
| Other | 103 |
| Positive blood Culture, n (%) | 9 (7%) |
IG: immature granulocytes, WBC: white blood cells, nRBC: nucleated red blood cells, AS-Lymph: antibody-synthesizing lymphocytes, RE-Lymph: reactive lymphocytes, NEUT-GI: neutrophil granularity intensity, NEUT-RI: neutrophil reactivity intensity, IL-6: interleukin-6, CAD: coronary artery disease, HTN: hypertension, DM: diabetes mellitus.
Figure 1.
Correlation Matrix.
The above figure represents the correlation matrix of the laboratory parameters for the included patients. The circle’s size represents the strength of the correlation, while the color represents the strength and direction of the correlation. IG: immature granulocytes, WBC: white blood cells, nRBC: nucleated red blood cells, AS-Lymph: antibody-synthesizing lymphocytes, RE-Lymph: reactive lymphocytes, NEUT-GI: neutrophil granularity intensity, NEUT-RI: neutrophil reactivity intensity, IL-6: interleukin-6.
Need for mechanical ventilation
During the hospital stay of the 300 patients, 66 (22%) required mechanical ventilation. In the univariable regression, IG as estimated by the analyzer (OR = 16.41, P = 0.00623), WBC (OR = 1.1, P = 0.00406) and NEUT-RI (OR = 1.17, P < 0.001) were statistically significant predictors of the need for mechanical ventilation. nRBC (P = 0.25), NEUT-GI (P = 0.52), AS-Lymph (P = 0.32), and RE Lymph (P = 0.73) did not predict the need for mechanical ventilation. In the multivariable analyses with the inclusion of age, obesity, diabetes and HTN, the statistically significant predictors of the need for mechanical ventilation were NEUT-RI (OR = 1.22, P < 0.001), diabetes (OR = 2.56, P = 0.00846) and obesity (OR 6.55, P < 0.001). Age (OR = 0.99, P = 0.799), HTN (OR = 1.40, P = 0.401), WBC (OR = 1.07, P = 0.182) and IG (OR = 7.82, P = 0.131) did not reach statistical significance. The optimal cut-off value for the NEUT-RI to predict the need for mechanical ventilation was 52 FI with a sensitivity of 44% and a specificity of 88% (AUC = 0.67).
Death
Twenty-six patients were transferred to other hospitals leading to only 274 evaluable patients for the outcome of death. Out of the 274 patients, 34 (12.4%) died during their hospital stay. In the univariable regression, NEUT-RI (OR = 1.11, P = 0.01573) was the only significant predictor of death. nRBC (P = 0.434), IG (P = 0.214), WBC (P = 0.179), NEUT GI (P = 0.28), AS-Lymph (P = 0.49) and RE-Lymph (P = 0.219) did not predict death in these patients. In the multivariable analyses with the inclusion of age, obesity, diabetes and HTN, the statistically significant predictors for death were NEUT-RI (OR = 1.14, P = 0.00432), diabetes (OR = 4.11, P = 0.00185) and age (OR = 1.04, P = 0.00896). Obesity (OR = 2.02, P = 0.271), HTN (OR = 0.63, P = 0.347) and WBC (OR = 1.06, P = 0.214) did not reach statistical significance. The optimal cut-off value for the NEUT-RI to predict death was 52 FI with a sensitivity of 44% and a specificity of 86% (AUC = 0.64).
Discussion
In our retrospective single-center study involving 300 patients admitted with COVID-19, 22% required mechanical ventilation, and the overall mortality rate was 12.4%. Multivariable analyses showed NEUT-RI (OR = 1.22, P < 0.001), diabetes (OR = 2.56, P = 0.00846) and obesity (OR = 6.55, P < 0.001) to be significant predictors of the need for mechanical ventilation while NEUT-RI (OR = 1.14, P = 0.00432), diabetes (OR = 4.11, P = 0.00185) and age (OR = 1.04, P = 0.00896) were predictors for death. Although diabetes, obesity and older age have previously been described as adverse risk factors, this is the first time that activated neutrophils, as detected by the Sysmex™ XN-9100 auto analyzer, have been shown by multivariable analysis to be an independent predictor for mechanical ventilation and death in COVID-19. This observation is significant since it implicates activated neutrophils as an important component in the pathophysiology of this disease. There are two aspects of these findings which could be relevant in COVID-19. One involves a potential overdrive of innate immunity against the virus, possibly via neutrophil traps (NETs) while the other pertains to the consequences of vast neutrophil accumulation, which could occur at sites of tissue hypoxia and inflammation.
Neutrophils home-in very early to sites of infection to clear pathogens by phagocytosis and the oxidative burst. Another less well-known mechanism is the formation of neutrophil nets (Schonrich and Raftery, 2016), which has provoked considerable interest recently for its possible role in COVID-19 (Tomar et al., 2020, Barnes et al., 2020). In a recent study, transcriptase analysis of tissue neutrophils in COVID-19 patients showed a correlation between neutrophil activation and several NET-associated genes, supporting the role of NETS in innate immunity against SARS-CoV-2. NETS include extracellular webs of DNA, histones, microbicidal proteins, and oxidative enzymes released by neutrophils to contain pathogens. In doing so, they then die in a process distinct from apoptosis or necrosis. Since excessive NETs can potentially trigger autoimmune responses and inflammation, they are normally degraded and removed from the circulation by endogenous DNase and phagocytosis (Laukova et al., 2020, Farrera and Fadeel, 2013). It is postulated that dysregulation of the normal homeostatic mechanisms to keep NETs in check could play a role in uncontrolled hyperinflammation. To support this, plasma levels of DNase I have been found to be markedly reduced in severe COVID-19, possibly due to exhaustion from overwhelming NET production. Impaired NET clearance could also be one reason why diabetes is such a significant risk factor for COVID-19 since high glucose exposure is known to interfere with phagocytosis (Pavlou et al., 2018). Recent evidence suggests that collateral damage from sustained NET formation could be involved in the thrombosis and respiratory distress often encountered in severe COVID-19 (Wang et al., 2020b).
SARS-CoV-2 involvement of the pulmonary epithelium, with influx of neutrophils and inflammation, causes impaired alveolar gas exchange and hypoxia. Although it is well-known for patients with COVID-19 to be hypoxic at presentation, it is likely that some could have subclinical hypoxemia much earlier and that the degree and extent of hypoxic tissue damage and inflammation might be significantly underestimated. Hypoxia and inflammation are intimately linked (Eltzschig and Carmeliet, 2011, Eltzschig, 2011, Xiao et al., 2019), and neutrophils and macrophages play a vital regulatory role in the resolution of inflammation and restoration of tissue integrity (Egners et al., 2016). However, there may also be untoward consequences from the large influx of neutrophils into a hypoxic environment. Hypoxia has been shown to inhibit spontaneous neutrophil apoptosis (Talla et al., 2019) and is also thought to induce degradation of azurophilic granules with the potential for exacerbation of tissue injury from spillage of granule proteins at hypoxic injury sites. While extended neutrophil survival could be beneficial for pathogen clearance, it is unclear what effects their continuing accumulation could have on an already damaged tissue environment. Additionally, it is conceivable that dysregulation of this innate immune system due to viral or host genetic factors, or both, can also contribute to the unique pathogenesis of COVID-19.
Rapid mobilization of neutrophils can occur either by demargination as in localized acute inflammation, or from the bone marrow by shortening the maturation time of post-mitotic precursors (metamyelocytes, band forms and immature neutrophils) (Orr et al., 2007). However, to compensate for this tremendous depletion and demand for mature activated neutrophils, a broader stimulation of the bone marrow compartment must occur. Hematopoietic stem cell recruitment will then increase the quantum of committed myeloid progenitors, creating a larger mitotic pool, with the potential for spill-over of IG (promyelocytes, myelocytes) into the peripheral blood. Reports from others (Mitra et al., 2020), and our observations of nRBC, IGs and even occasional blasts in the peripheral blood of patients with COVID-19, prompted us to look more closely at this phenomenon in our 300 patient cohort. We found that IGs correlated with hypoxia at admission but not with IL-6, lending support to massive NETosis and likely tissue hypoxia (rather than the cytokine storm) as causal for intense bone marrow activity to meet continued demand for mature neutrophils.
Activation of neutrophils into functional effector cells begins during adhesion and transmigration and is maximized upon interaction with specific signals within the injured or infected site (Mantovani et al., 2011). If large numbers of neutrophils are being trafficked in critically ill COVID-19 patients, the presence of activated neutrophils in the peripheral blood might reflect significant events occurring at the tissue level. Indirect evidence for this supposition has emerged in a recent article currently undergoing peer review (Meizlish et al., 2020). Using proteomic profiling, the authors report a prominent signature of neutrophil activation in the plasma of COVID-19 patients at admission that predicted increased mortality. We have shown the presence of activated neutrophils in the peripheral blood of patients with COVID-19 by directly analyzing the neutrophil population using the Sysmex™ XN-9100 automated cell analyzer. This novel technology enabled us to rapidly quantify the degree of neutrophil activation and assign a cut-off value of fluorescence intensity with predictive value for risk of mechanical ventilation and death. In a recent multi-center study done primarily to design and validate a prognostic scoring system using only hemocytometric data by the Sysmex™ technology, activated neutrophils were significantly associated with critically ill COVID-19 patients (Linssen et al., 2020). In our study, we found activated neutrophils to be an important predictor of mechanical ventilation and death. Although more studies of neutrophil activation in COVID-19 with this technology are needed to verify these results, the Sysmex™ XN-9100 cell analyzer appears to be a reliable, robust and rapid method to quantify neutrophil activation in the peripheral blood. Importantly, since the detection of activated neutrophils by this method can predict outcome, this simple and rapid automated technology could also be a valuable tool in conjunction with other NETosis markers like cell-free DNA, myeloperoxidase, elastase and citrullinated histone H3 (Perdomo et al., 2019) to monitor novel neutrophil targeted strategies. Such strategies under investigation include rapid clearance of NETs with exogenously administered DNase-1 (Park et al., 2020) and the use of the phytonutrient Resveratrol for its wide range of biological activities, especially the anti-inflammatory, antioxidant and antithrombotic properties (Giordo et al., 2021).
In summary, our study shows the presence of activated neutrophils (NEUT-RI) (as measured by the Sysmex™ XN-9100 auto-analyzer) in the peripheral blood of patients with COVID-19 at admission to be an independent predictor for mechanical ventilation and death. From a biological standpoint, these findings imply a central role for neutrophils in the disease’s pathophysiology and could potentially lead to newer therapeutic strategies for COVID-19. Importantly this non-invasive novel technology, incorporated in a multi-parameter hematology autoanalyzer to detect circulating activated neutrophils, enables rapid turn-around time for clinical and therapeutic decision making.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding source
No funding was required for this research.
Ethical approval
This study was reviewed and approved by the medical research ethics committee of the college of medicine and health sciences at the Sultan Qaboos University.
Acknowledgments
The authors would like to acknowledge Mr Selva Kumar Sundramurthy (Applications Specialist, Sysmex™ Middle East-Oman) for his expertise in the technical aspects and assistance in providing the appropriate scientific literature pertaining to the Sysmex™ hematology autoanalyzer.
References
- Barnes B.J., Androver J.M., Baxter-Stolzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med. 2020;217(6):1–7. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom B., Bodkin J.V., Beyrau M., Woodfin A., Ody C., Rourke C. Leukotriene B4-neutrophil elastase axis drives neutrophil reverse transendothelial cell migration in vivo. Immunity. 2015;42(6):1075–1086. doi: 10.1016/j.immuni.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egners A., Erdem M., Cramer T. The response of macrophages and neutrophils to hypoxia in the context of cancer and other inflammatory diseases. Mediat Inflamm. 2016;2016 doi: 10.1155/2016/2053646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig H.K. Targeting hypoxia-induced inflammation. Anesthesiology. 2011;114(2):239–242. doi: 10.1097/ALN.0b013e3182070c66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig H.K., Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364(7):656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrera C., Fadeel B. Macrophage clearance of neutrophil extracellular traps is a silent process. J Immunol. 2013;191(5):2647–2656. doi: 10.4049/jimmunol.1300436. [DOI] [PubMed] [Google Scholar]
- Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordo R., Zinellu A., Eid A.H., Pintus G. Therapeutic potential of resveratrol in COVID-19-associated hemostatic disorders. Molecules. 2021;26(4):856. doi: 10.3390/molecules26040856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriot I., Launay E., Boubaya M., Cremet L., Illiaquer M., Caillon H. New parameters on the hematology analyser XN-10 (SysmexTM) allow to distinguish childhood bacterial and viral infections. Int J lab Hematol. 2017;39(1):14–20. doi: 10.1111/ijlh.12562. [DOI] [PubMed] [Google Scholar]
- Huertas A., Montani D., Savale L., Pichon J., Tu L., Parent F. Endothelial cell dysfunction: a major player in SARS-Cov-2 infection (COVID-19)? Eur Respir J. 2020;56(1) doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukova L., Konecna B., Janovicova L., Vikova B., Celec P. Deoxyribonucleases and their applications in biomedicine. Biomolecules. 2020;10(7):1036. doi: 10.3390/biom10071036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K. Integration of inflammatory signals by rolling neutrophils. Immun Rev. 2002;186:8–18. doi: 10.1034/j.1600-065x.2002.18602.x. [DOI] [PubMed] [Google Scholar]
- Lin N., Simon M.C. Hypoxia-inducible factors: key regulators of myeloid cells during inflammation. J Clin Invest. 2016;126(10):3361–3671. doi: 10.1172/JCI84426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linssen J., Jennissen V., Hildmann J., Reisinger E., Schindler J., Malchau G. Identification and quantification of high fluorescence-stained lymphocytes as antibody synthesizing/secreting cells using the automated routine hematology analyser XE-2100. Cytometry B Clin Cytom. 2007;72(3):157–166. doi: 10.1002/cyto.b.20150. [DOI] [PubMed] [Google Scholar]
- Linssen J., Ermens A., Berrevoets M., Seghezzi M., Previtali G., van der Sar-van der Brugge S. A novel haemocytometric COVID-19 prognostic score developed and validated in an observational multicentre European hospital-based study. eLife. 2020;9(November) doi: 10.7554/eLife.63195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Cassatella M.A., Costantini C., Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11(8):519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- Marini J.J., Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323(22):2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- Mayadas T.N., Cullere X., Lowell C.A. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meizlish M.L., Pine A.B., Bishai J.D., Goshua G., Nadelmann E.R., Simonov M. A neutrophil activation signature predicts critical illness and mortality in COVID-19. medRxiv. 2020;(September) doi: 10.1101/2020.09.01.20183897. 2020.09.01.20183897. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton E.A., He X.Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A., Dwyre D.M., Schivo M., Thompson G.R., 3rd, Cohen S.H., Ku N. Leukoerythroblastic reaction in a patient with COVID-19. Am J Hematol. 2020;95(8):999–1000. doi: 10.1002/ajh.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr Y., Wilson D.P., Taylor J.M., Bannon P.G., Geczy C., Davenport M.P. A kinetic model of bone marrow neutrophil production that characterizes late phenotypic maturation. Am J Physiol Regul Integr Comp Physiol. 2007;292(4):R1707–16. doi: 10.1152/ajpregu.00627.2006. [DOI] [PubMed] [Google Scholar]
- Park H.H., Park W., Lee Y.Y., Kim H., Seo H.S., Choi D.W. Bioinspired DNase-I-coated melanin-like nanospheres for modulation of infection-associated NETosis dysregulation. Adv Sci (Weinh) 2020;7(23) doi: 10.1002/advs.202001940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlou S., Lindsay J., Ingram R., Xu H., Chen M. Sustained high glucose exposure sensitizes macrophage responses to cytokine stimuli but reduces their phagocytic activity. BMC Immunol. 2018;19(1):24. doi: 10.1186/s12865-018-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdomo J., Leung H.H.L., Ahmadi Z., Yan F., Chong J.J.H., Passam F.H. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin induced thrombocytopenia. Nat Commun. 2019;10(1):1322. doi: 10.1038/s41467-019-09160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonrich G., Raftery M. Neutrophil extracellular traps go viral. Front Immunol. 2016;7 doi: 10.3389/fimmu.2016.00366. 366: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sysmex Europe GmbH . 2017. Novel haematological parameters for rapidly monitoring the immune system response. Sysmex White Paper Infection/Inflammation; pp. 1–5. [Google Scholar]
- Talla U., Bozonet S.M., Parker H.A., Hampton M.B. Prolonged exposure to hypoxia induces an autophagy-like cell survival program in human neutrophils. J Leukoc Biol. 2019;106(6):1367–1379. doi: 10.1002/JLB.4A0319-079RR. [DOI] [PubMed] [Google Scholar]
- Tomar B., Anders H.J., Desai J., Mulay S.R. Neutrophils and neutrophil extracellular traps drive necroinflammation in COVID-19. Cells. 2020;9(6) doi: 10.3390/cells9061383. 1383 (1–8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustyantseva M., Khokhlova O.I., Agadzhanyan V.V. Innovative Technologies in the evaluation of the neutrophil functional activity in sepsis. Sysmex J Int. 2019;29(1):8–13. [Google Scholar]
- Veras F.P., Pontelli M.C., Silva C.M., Toller Kasahisa J.E., de Lima M., Nascimento D.C. SARS-CoV-2–triggered neutrophil extracellular traps mediate COVID-19 pathology. J Exp Med. 2020;217(12):1–12. doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Neutrophils in tissue injury and repair. Cell Tissue Res. 2018;371(3):531–539. doi: 10.1007/s00441-017-2785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronovirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Li Q., Yin Y., Zhang Y., Cao Y., Lin X. Excessive neutrophils and neutrophil extracellular traps in COVID-19. Front Immunol. 2020;11(article 2063):1–13. doi: 10.3389/fimmu.2020.02063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland Th, Kalkman H., Heihn H. Evaluation of the automated immature granulocyte count(IG) on Sysmex SE-2100 automated hematology analyser vs visual microscopy (NCCLS H20-A) Sysmex J Int. 2002;12(2):63–70. [Google Scholar]
- Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis and treatment of coronavirus disease 2019 (COVID-19) JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- Woodfin A., Voisin M.B., Beyrau M., Colom B., Caille D., Diapouli F.M. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol. 2011;12(8):761–769. doi: 10.1038/ni.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C. A pathological report of three COVID-19 cases by minimally invasive biopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49(5):411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. (Article in Chinese) [DOI] [PubMed] [Google Scholar]
- Yip C.Y.C., Yap E.S., De Mel S., Teo W.Z.Y., Lee C.T., Kan S. Temporal changes in immune blood cell parameters in COVID-19 infection and recovery from severe infection. Br J Haematol. 2020;190(1):33–36. doi: 10.1111/bjh.16847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Li X., Wang J., Cao J. Mechanisms of vascular endothelial cell injury in response to intermittent and/or continuous hypoxia exposure and protective effects of anti-inflammatory and anti-oxidant agents. Sleep Breath. 2019;23(2):515–522. doi: 10.1007/s11325-019-01803-9. [DOI] [PubMed] [Google Scholar]