Abstract
Aim
COVID-19 pandemic has caused extensive burden on public life and health care worldwide. This study aimed to assess circulating levels of inflammatory cytokines in adult patients who were hospitalized with COVID-19 and stratified according to age (older or younger than 65 years) aiming to explore associations between these markers of inflammation and comorbidities.
Methods
This was a cross-sectional study of 142 COVID-19 patients consecutively admitted to the University Hospital of the Federal University of São Carlos, from July to October 2020. Sociodemographic data, chronic comorbidities, and baseline NEWS2 and SOFA for clinical deterioration were obtained at hospital admission. Serum levels of inflammatory cytokines were determined by flow cytometry.
Results
Older adults with COVID-19 had higher serum levels of IL-6 and IL-10 as compared to those under 65 years of age (p < 0.001 and p = 0.003, respectively). IL-10 was independently associated with age (p = 0.04) and severity of the disease (p = 0.05), whereas serum levels of IL-6 were not directly associated with age (p = 0.5). The comorbidity index seems to be the main responsible for this, being significantly associated with IL-6 levels among those aged 65 and over (p = 0.007), in addition to the severity of the disease.
Conclusions
Higher serum levels of IL-6 and IL-10 are associated with the severity of the disease and a higher comorbidity index among adults aged 65 and over with COVID-19. This should raise awareness of the importance of comorbidity index, rather than age, during risk stratification.
Keywords: Aged, Comorbidity, COVID-19, SARS-CoV-2
1. Introduction
With more than 90 million reported cases of infection, the coronavirus 2019 disease (COVID-19) reached pandemic status in March 2020, and up to date, no specific antiviral treatment has been proven to be effective against this disease.
Currently, about 20% of those infected develop a severe inflammatory-mediated hypoxemic disease that resembles a cytokine-storm syndrome. [1] This condition seems to occur mainly as a result of a blunted IFN-I and IFN-III immune response, accompanied by high levels of pro-inflammatory cytokines such as IL-1, IL-6 and TNF-alpha. [2], [3] Most cases involve men, aging between 30 and 79 years, with one or more coexisting medical conditions such as hypertension, diabetes, chronic obstructive pulmonary disease, and cardiovascular diseases. [3], [4] Among these, poor outcomes such as acute lung injury, acute respiratory distress syndrome (ARDS), and even death have been observed. [2], [5], [6]
Such unbalanced cytokine production is also characteristic of a chronic low-grade inflammatory state shared by both aging (inflammaging) and the presence of multiple comorbidities, and increased levels of Interleukin-6 (IL-6), among other cytokines, has been described to play a central role in this process. [7], [8], [9] Moreover, in aging patients, a smaller pool of naive T and B cells associated with impaired innate immunity, also have been shown to hinder the adaptive immune response. [9], [10] Taken together, these conditions could explain why older adults with COVID-19 are more prone to develop worse outcomes.
However, there is a lack of studies investigating the profile of cytokines in adults with COVID-19, taking into account age and the role of comorbidities in this relationship. Thus, the aims of this study were to assess circulating levels of inflammatory cytokines in adult patients who were hospitalized with COVID-19 and stratified according to age (older or younger than 65 years) aiming to explore associations between these markers of inflammation and comorbidities.
2. Methods
2.1. Study design, participants and setting
This is an ongoing single-center cross-sectional study conducted at the University Hospital of the Federal University of São Carlos (HU-USFCar). In this report, 142 adults were recruited from the COVID-19 ward of the HU-UFSCar, from July to October 2020. There were no excluding criteria for sampling. The study was conducted according to the guidelines from the Declaration of Helsinki and all procedures involving human subjects were approved by the Institutional Research Ethics Committee (Number: 30184220.8.0000.5504). Written informed consent was obtained from all subjects.
2.2. Study assessments
Patients were assessed at admission for demographic data, chronic comorbidities (Charlson Comorbidity Index [CCI]), National Early Warning Score (NEWS) 2 for clinical deterioration, and Sequential Organ Failure Assessment (SOFA). Pre-hospital frailty, assessed by Clinical Frailty Scale (CFS), was defined as a score ≥5 according to this scale. Laboratory data were also reviewed, and inflammatory cytokines were assessed by commercial laboratory methods.
2.3. Systemic markers of inflammation
Within the first 12 h of admission, venous blood from COVID-19 positive patients was sampled to analyze systemic markers of inflammation. Samples were analyzed through flow cytometry using BD Accuri C6 (BD Biosciences, Franklin Lakes, NJ, USA), and serum cytokines (IL-2, IL-4, IL-6, IL-10, IFN-γ and TNF-α) were measured with cytometric bead array human inflammation kit (BD™ CBA Human Th1/Th2 Cytokine Kit, BD Biosciences, San Diego, CA, USA). The procedure was conducted following the manufacturers' instructions. Data were analyzed using FlowJo software (FlowJo LLC, Ashland, OR, USA).
2.4. Statistical analysis
Continuous data are presented as mean ± standard deviation or median [1st − 3rd quartile] according to the Shapiro-Wilk test of normality. Categorical variables are presented as counts (percentages). Comparisons between groups were performed using Wilcoxon-Mann-Whitney test for continuous variables, and Pearson’s Chi-squared test with Yates’ continuity correction for categorical variables. Multivariate linear regression models (MLRM) were used to further investigate the relationship between the circulating levels of inflammatory cytokines and age. Cytokines measurements were transformed (log10) in order for variables to meet the assumptions of statistical tests. Clinical characteristics and disease severity were selected for the multivariate analysis. All analyses were conducted using R version 4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria) in R-Studio 1.3.1093 (RStudio Inc., Boston, USA).
3. Results
Between July and October 2020, a total of 167 adults were admitted to hospital. Twenty-five patients were excluded from analysis due to lack of blood sample. (Supplementary Fig. S1) Table 1 summarizes patients characteristics. There were 142 subjects aging from 22 to 99 years (mean 60 years). About half of them were males (54.2%), with a median Charlson Comorbidity Index of 2 (ranging from a minimum 0 to a maximum 8), and a total of 36 patients (25.4%) presented high comorbidity (index ≥ 5). Most of the patients were not diabetic (76.8%) nor they had cardiovascular diseases (85.2%), nor were hypertensive (55.6%). There was a significant difference between the two age groups on almost all baseline variables, except for sex (p = 1.0), diabetes mellitus (p = 0.2), time from symptom onset to hospital admission (p = 0.7), and NEWS2 score at hospital admission (p = 0.2).
Table 1.
Baseline characteristics of the cohort.
| Characteristic | All patients (N = 142) | <65 years (n = 83) | 65+ years (n = 59) | p-value |
|---|---|---|---|---|
| Age, years | 61 [45–76] | 47 [38.5–57.5] | 79 [72.5–84.5] | <0.001 |
| Male sex | 77 (54.2) | 45 (54.2) | 32 (54.2) | 1.00 |
| Comorbidities | ||||
| Diabetes mellitus | 33 (23.2) | 16 (19.3) | 17 (28.8) | 0.2 |
| Cardiovascular diseases | 21 (14.8) | 6 (7.2) | 15 (25.4) | 0.003 |
| Hypertension | 63 (44.4) | 25 (30.1) | 38 (64.4) | <0.001 |
| Charlson Comorbidity Index | 2 [0–5] | 0 [0–2] | 5 [4 –6] | <0.001 |
| Clinical Frailty Scale | ||||
| Overall | 3 [2 –5] | 2 [1–2.5] | 6 [4.5–8] | <0.001 |
| Frailty | 47 (33.1) | 3 (3.6) | 44 (74.6) | <0.001 |
| Time from symptom onset to hospital admission, days | 7 [4–10] | 7 [4–10] | 6 [4–10.5] | 0.7 |
| NEWS2 at hospital admission | 3 [2–5] | 3 [2–5] | 3 [2–5] | 0.2 |
| SOFA at hospital admission | 2 [2–3] | 2 [2–2] | 3 [2–3] | < 0.001 |
| ICU admission | 26 (18.3) | 14 (16.9) | 12 (20.3) | 0.01 |
Continuous data are presented as mean ± standard deviation or median [1st − 3rd quartile]. Categorical variables are presented as counts (percentages).
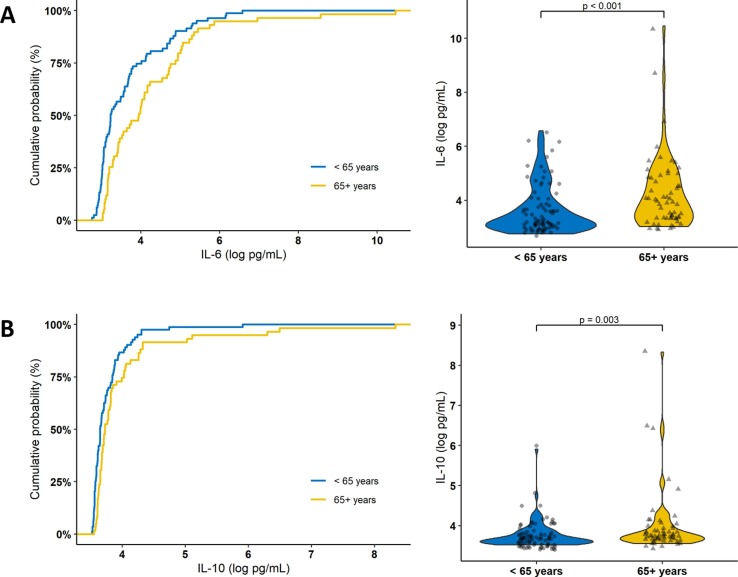
Table 2 shows the cohort cytokine profile at hospital admission by age group. IL-6 and IL-10 were significantly higher among those aged 65 and older. On the other hand, no significant difference was found in the levels of IL-2, IL-4, IFN-γ, and TNF-α between the two age groups. Fig. 1 shows the cumulative probability and distribution plots of IL-6 and IL-10 measurements in each age group. In this figure, we can observe that about 75% of the subjects aged less than 65 years had a serum IL-6 concentration below 4 log pg/mL, whereas about 50% of those aged 65 years and older had the same concentration. Similar but less pronounced results were obtained with IL-10, and accordingly, the same was observed for IL-6 to IL-10 ratio.
Table 2.
Cytokines levels according to the age-group.
| Characteristic | All patients (N = 142) | <65 years (n = 83) | 65+ years (n = 59) | p-value |
|---|---|---|---|---|
| Interleukin-2, pg/mL | 14.0 [13.1–14.7] | 13.8 [12.7–14.7] | 14.4 [13.6–14.7] | 0.1 |
| Interleukin-4, pg/mL | 19.9 [19.0–21.0] | 19.9 [18.8–20.9] | 20 [19.2–21.1] | 0.4 |
| Interleukin-6, pg/mL | 32.6 [22.1–93.4] | 25.3 [20.5–52] | 51.6 [25.5–123.0] | <0.001 |
| Interleukin-10, pg/mL | 39.7 [36.7–47.6] | 38.4 [35.8–46.6] | 41.5 [38.4–55] | 0.003 |
| Interferon-γ, pg/mL | 14.9 [13.9–17.1] | 14.8 [13.6–17] | 15.1 [14.1–17.5] | 0.3 |
| Tumor Necrosis Factor-α, pg/mL | 13.2 [12.7–14.6] | 13.1 [12.6–14.2] | 13.3 [12.9–15] | 0.2 |
| IL-6/IL-10 ratio | 0.8 [0.6–1.6] | 0.7 [0.6–1.1] | 0.9 [0.6–2.2] | 0.004 |
Continuous data are presented as median [1st − 3rd quartile].
Fig. 1.
Cumulative probability and the violin plot of the distribution of (A) IL-6 (log pg/mL) and (B) IL-10 (log pg/mL) measurements according to the age group.
Considering a possible role of comorbidity and disease severity in the observed age-related differences of IL-6 and IL-10 serum levels, we further analyzed this relationship using MLRM. Table 3 shows that aging 65 and older lost its significance after adjusting for comorbidity index. Model 3 revealed that NEWS2 score at hospital admission is the only factor independently associated with higher serum levels of IL-6. On the other hand, Table 3 (Models 4, 5 and 6) shows that higher serum levels of IL-10 (log pg/mL) remained independently associated with having 65 years and older, after adjusting for comorbidity index and disease severity.
Table 3.
Linear regression models for the association of clinical characteristics and disease severity with serum levels of IL-6 (log pg/mL) and IL-10 (log pg/mL).
| Dependent variable: IL-6 (log pg/mL) | ||||||
|---|---|---|---|---|---|---|
| Independent variables | Model 1 |
Model 2 |
Model 3 |
|||
| Standardized β Coefficient (95% CI) | p | Standardized β Coefficient (95% CI) | p | Standardized β Coefficient (95% CI) | p | |
| Age | ||||||
| <65 years | Reference | – | Reference | – | Reference | – |
| 65+ years | 0.5 (0.17–0.83) | 0.003 | 0.16 (−0.33–0.65) | 0.5 | 0.14 (−0.34 to 0.61) | 0.5 |
| Male sex | 0.16 (−0.16 to 0.49) | 0.3 | 0.17 (−0.15 to 0.49) | 0.2 | 0.11 (−0.20 to 0.43) | 0.4 |
| Charlson Comorbidity Index | – | – | 0.09 (−0.01 to 0.19) | 0.06 | 0.08 (−0.02 to 0.19) | 0.1 |
| NEWS2 at hospital admission | – | – | – | – | 0.12 (0.04–0.21) | 0.005 |
| SOFA at hospital admission | – | – | – | – | 0.02 (−0.21 to 0.24) | 0.8 |
| Dependent variable: IL-10 (log pg/mL) | ||||||
| Independent variables | Model 4 | Model 5 | Model 6 | |||
| Standardized β Coefficient (95% CI) | p | Standardized β Coefficient (95% CI) | p | Standardized β Coefficient (95% CI) | p | |
| Age | ||||||
| <65 years | Reference | – | Reference | – | Reference | – |
| 65+ years | 0.40 (0.07–0.73) | 0.02 | 0.53 (0.03–1.02) | 0.04 | 0.51 (0.02–1.01) | 0.04 |
| Male sex | 0.21 (−0.12 to 0.54) | 0.2 | 0.21 (−0.12 to 0.53) | 0.2 | 0.17 (−0.16 to 0.5) | 0.3 |
| Charlson Comorbidity Index | – | – | −0.03 (−0.14 to 0.07) | 0.5 | −0.04 (−0.15 to 0.07) | 0.4 |
| NEWS2 at hospital admission | – | – | – | – | 0.09 (0.001–0.17) | 0.05 |
| SOFA at hospital admission | – | – | – | – | −0.001 (−0.23 to 0.23) | 0.9 |
95% CI, 95% Confidence Interval.
Finally, we investigated further if there was an interaction of the age group with Charlson Comorbidity Index, adjusting it for sex and disease severity. Table 4 shows that the interaction term between age and CCI is statistically significant, that is, the effect of CCI on increasing serum levels of IL-6 (log pg/mL) varies with the age group, and it is independently and positively associated with an age of 65 years and older (p = 0.007).
Table 4.
Linear regression models for the association of the interaction term between age and CCI with serum levels of IL-6 (log pg/mL).
| Dependent variable: IL-6 (log pg/mL) | ||||
|---|---|---|---|---|
| Independent variables | Model 7 |
Model 8 |
||
| Standardized β Coefficient (95% CI) | p | Standardized β Coefficient (95% CI) | p | |
| Age | ||||
| <65 years: Comorbidity Index | 0.12 (−0.01 to 0.24) | 0.06 | 0.10 (−0.02 to 0.18) | 0.1 |
| 65+ years: Comorbidity Index | 0.12 (0.05–0.19) | <0.001 | 0.11 (0.03–0.18) | 0.007 |
| Male sex | 0.17 (−0.71 to 0.5) | 0.2 | 0.12 (−0.2 to 0.43) | 0.4 |
| NEWS2 at hospital admission | – | – | 0.12 (0.04–0.21) | 0.005 |
| SOFA at hospital admission | – | – | 0.02 (−0.21 to 0.24) | 0.8 |
95% CI, 95% Confidence Interval.
4. Discussion
Our results show that older adults with COVID-19 have higher serum levels of IL-6 and IL-10 as compared to those under 65 years of age. While IL-10 is independently associated with age and severity of the disease, serum levels of IL-6 are not directly associated with age. The comorbidity index seems to be the main responsible for this, being significantly associated with IL-6 levels among those aged 65 and over, in addition to the severity of the disease.
Similar to our results, others have demonstrated not only elevated IL-6 and IL-10 levels in some COVID-19 patients, but also that this unbalanced cytokine production predicts disease severity. [11] Accordingly, in a study that analyzed the levels of 66 soluble biomarkers in 175 Italian patients with different COVID-19 severity, Abers and colleagues [12] showed that increased levels of IL-6 and IL-10 were independently associated with mortality. Moreover, a recent meta-analysis of 44 articles (50 studies) encompassing 7865 patients has shown increased levels of IL-6 and IL-10 among those with severe disease. [13] However, most of these studies did not control for intervening factors, such as age, disease severity and comorbidities, thus, highlighting the importance of our results and pointing to the need to consider other aspects (such as disease severity and comorbidity) as adjusting factors when evaluating the risk of older people with COVID-19.
During viral diseases, including COVID-19, innate response induces infected cells to secrete several pro-inflammatory mediators, generating a potent inflammatory response. [14] IL-6 and IL-10 are produced at the sites of tissue inflammation and released into the circulation by a range of different cells, including macrophages, lymphocytes, fibroblasts, and endothelial and epithelial cells. [11] Once produced, these cytokines increase blood flow near the infection site and activate macrophages and other phagocytic cells for clearance of virus, as well as infected cells. [15]
IL-6 is a key pleiotropic mediator in several inflammatory processes including tissue damage and infection. On the other hand, IL-10 is known to have anti-inflammatory properties initiating innate and adaptive immune responses and therefore limiting pro-inflammatory responses in order to prevent tissue damage. [16] Therefore, throughout the acute course of an infection, IL-10 inhibits the activity of T cells, NK cells and macrophages that, despite necessary for viral elimination, are also key factors inducing tissue damage. Hence, while limiting collateral tissue damage, IL-10 may also prevent successful viral elimination. [17] Thus, the balance between the serum levels of IL-6 and IL-10 cytokines may be a useful tool to predict disease severity and further studies should address this feature. [18]
Yet, understanding the pathways that regulate the synthesis and production of IL-6 may be the key to understand how this cytokine participates in the severity of diseases. Although in this study the participation of TNF-α in the production of IL-6 was not verified, we suggest that other independent pathways may be involved in this regulation and that IL-10 may play a key role in this process. Accordingly, it has recently been suggested that that early induction of IL-10 upon SARS-CoV-2 infection might indeed represent a negative feedback mechanism that serves as a counter measure to inflammation caused by other proinflammatory mediators. [18] As endogenous IL-10 production increases, it might function as a proinflammatory agent that stimulates the production of other mediators of the cytokine storm. The initial evidence is limited, and further studies are warranted to confirm the role IL-10 in COVID-19.
Our study reaffirmed the importance of considering comorbidity in the context of age-related disease severity among those with COVID-19. These findings are not new; the role of several comorbidities in aggravating COVID-19 have been demonstrated in several studies. [19], [20], [21], [22] Even though, our results do shed some fresh light on the concept as a whole by showing their independent association only among those with 65 years and older.
One limitation of this study is its cross-sectional design, which does not provide the evidence of a temporal relationship between the variables and each outcome studied. However, bias owing to variations between independent and dependent variables within the same individual was reduced. Moreover, the limited sample size and use of different pre-hospital medications, which are common in this population, may represent relevant confounding factors in our study. Despite those, our results provide evidence on the relationship of inflammatory cytokines with age, highlighting the influence of covariates on it.
Taken together, our results reinforce the importance of the close monitoring of cytokine levels, with special focus on IL-6 and IL-10, which might provide valuable information on the COVID-19 severity and changes during the treatment process.
5. Conclusions
Older adults with COVID-19 have higher serum levels of IL-6 and IL-10 as compared to those under 65 years of age. This difference is associated with the severity of the disease and a higher comorbidity index among those aged 65 and over. This should raise awareness of the importance of comorbidity index, rather than age, during risk stratification.
Funding
This work was supported by FAPESP – Fundação de Amparo à Pesquisa do Estado de São Paulo (grant #2014/50867-3). ACBM had a scholarship from FAPESP #2016/23202-6.
CRediT authorship contribution statement
Rafael Luís Luporini: Investigation, Data curation, Writing - original draft. Joice M. de A. Rodolpho: Investigation, Data curation. Lauro Tatsuo Kubota: Resources. Ana Carolina Baptista Moreno Martin: Data curation. Marcia R. Cominetti: Investigation, Writing - original draft. Fernanda Freitas Anibal: Investigation, Writing - original draft. Henrique Pott-Junior: Conceptualization, Methodology, Investigation, Writing - original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cyto.2021.155507.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
References
- 1.Han H., Ma Q., Li C., Liu R., Zhao L.i., Wang W., Zhang P., Liu X., Gao G., Liu F., Jiang Y., Cheng X., Zhu C., Xia Y. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D. Blanco-Melo, B.E. Nilsson-Payant, W.C. Liu, S. Uhl, D. Hoagland, R. Moller, T.X. Jordan, K. Oishi, M. Panis, D. Sachs, T.T. Wang, R.E. Schwartz, J.K. Lim, R.A. Albrecht, B.R. tenOever, Imbalanced host response to SARS-CoV-2 drives development of COVID-19, Cell 181(5) (2020) 1036–1045 e9. [DOI] [PMC free article] [PubMed]
- 3.Garcia L.F. Immune response, inflammation, and the clinical spectrum of COVID-19. Front. Immunol. 2020;11:1441. doi: 10.3389/fimmu.2020.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonafe M., Prattichizzo F., Giuliani A., Storci G., Sabbatinelli J., Olivieri F. Inflamm-aging: why older men are the most susceptible to SARS-CoV-2 complicated outcomes. Cytokine Growth Factor Rev. 2020;53:33–37. doi: 10.1016/j.cytogfr.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quirch Miguel, Lee Jeannie, Rehman Shabnam. Hazards of the cytokine storm and cytokine-targeted therapy in patients with COVID-19: review. J. Med. Internet Res. 2020;22(8):e20193. doi: 10.2196/20193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maggio M., Guralnik J.M., Longo D.L., Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61(6):575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meftahi G.H., Jangravi Z., Sahraei H., Bahari Z. The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID-19 infection: the contribution of “inflame-aging”. Inflamm. Res. 2020;69(9):825–839. doi: 10.1007/s00011-020-01372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michaud Martin, Balardy Laurent, Moulis Guillaume, Gaudin Clement, Peyrot Caroline, Vellas Bruno, Cesari Matteo, Nourhashemi Fati. Proinflammatory cytokines, aging, and age-related diseases. J. Am. Med. Dir. Assoc. 2013;14(12):877–882. doi: 10.1016/j.jamda.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Ponnappan S., Ponnappan U. Aging and immune function: molecular mechanisms to interventions. Antioxid. Redox Signal. 2011;14(8):1551–1585. doi: 10.1089/ars.2010.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashizume M. Outlook of IL-6 signaling blockade for COVID-19 pneumonia. Inflamm. Regen. 2020;40:24. doi: 10.1186/s41232-020-00134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.M.S. Abers, O.M. Delmonte, E.E. Ricotta, J. Fintzi, D.L. Fink, A.A.A. de Jesus, K.A. Zarember, S. Alehashemi, V. Oikonomou, J.V. Desai, S.W. Canna, B. Shakoory, K. Dobbs, L. Imberti, A. Sottini, E. Quiros-Roldan, F. Castelli, C. Rossi, D. Brugnoni, A. Biondi, L.R. Bettini, M. D'Angio, P. Bonfanti, R. Castagnoli, D. Montagna, A. Licari, G.L. Marseglia, E.F. Gliniewicz, E. Shaw, D.E. Kahle, A.T. Rastegar, M. Stack, K. Myint-Hpu, S.L. Levinson, M.J. DiNubile, D.W. Chertow, P.D. Burbelo, J.I. Cohen, K.R. Calvo, J.S. Tsang, N.C.-. Consortium, H.C. Su, J.I. Gallin, D.B. Kuhns, R. Goldbach-Mansky, M.S. Lionakis, L.D. Notarangelo, An immune-based biomarker signature is associated with mortality in COVID-19 patients, JCI Insight 6(1) (2021). [DOI] [PMC free article] [PubMed]
- 13.Akbari H., Tabrizi R., Lankarani K.B., Aria H., Vakili S., Asadian F., Noroozi S., Keshavarz P., Faramarz S. The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Life Sci. 2020;258 doi: 10.1016/j.lfs.2020.118167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.A. Copaescu, O. Smibert, A. Gibson, E.J. Phillips, J.A. Trubiano, The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection, J. Allergy Clin. Immunol. 146(3) (2020) 518–534 e1. [DOI] [PMC free article] [PubMed]
- 15.Pasrija R., Naime M. The deregulated immune reaction and cytokines release storm (CRS) in COVID-19 disease. Int. Immunopharmacol. 2021;90 doi: 10.1016/j.intimp.2020.107225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmann S.R., Rösen-Wolff A., Tsokos G.C., Hedrich C.M. Biological properties and regulation of IL-10 related cytokines and their contribution to autoimmune disease and tissue injury. Clin. Immunol. 2012;143(2):116–127. doi: 10.1016/j.clim.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Rojas J.M., Avia M., Martin V., Sevilla N. IL-10: a multifunctional cytokine in viral infections. J. Immunol. Res. 2017;2017:6104054. doi: 10.1155/2017/6104054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Ligong, Zhang Hui, Dauphars Danielle J., He You-Wen. A potential role of interleukin 10 in COVID-19 pathogenesis. Trends Immunol. 2021;42(1):3–5. doi: 10.1016/j.it.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barek Md. Abdul, Aziz Md. Abdul, Islam Mohammad Safiqul. Impact of age, sex, comorbidities and clinical symptoms on the severity of COVID-19 cases: a meta-analysis with 55 studies and 10014 cases. Heliyon. 2020;6(12):e05684. doi: 10.1016/j.heliyon.2020.e05684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biswas M., Rahaman S., Biswas T.K., Haque Z., Ibrahim B. Association of sex, age, and comorbidities with mortality in COVID-19 patients: a systematic review and meta-analysis. Intervirology. 2020:1–12. doi: 10.1159/000512592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman K.E., Magder L.S., Baghdadi J.D., Pineles L., Levine A.R., Perencevich E.N., Harris A.D. Impact of sex and metabolic comorbidities on COVID-19 mortality risk across age groups: 66,646 inpatients across 613 U.S. hospitals. Clin. Infect Dis. 2020 doi: 10.1093/cid/ciaa1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mi J., Zhong W., Huang C., Zhang W., Tan L., Ding L. Gender, age and comorbidities as the main prognostic factors in patients with COVID-19 pneumonia. Am. J. Transl. Res. 2020;12(10):6537–6548. [PMC free article] [PubMed] [Google Scholar]