Abstract
Background
This laboratory study was done to evaluate the efficacy of personal protective equipment (PPE) and high-volume evacuation (HVE) against the spread of human coronavirus type 229E (HCoV-229E) during a standard dental procedure.
Methods
Patient and operator manikins were used to recreate a dental setting inside a custom-built class III cabinet–like chamber. The mouth of the patient manikin was inoculated with an HCoV-229E suspension, the viral load of which was similar to that of asymptomatic people infected with severe acute respiratory syndrome coronavirus 2. The dental procedure was performed with an air turbine handpiece and HVE for 10 seconds. The efficacy of surgical masks, N95 (filtering facepiece class 2) and filtering facepiece class 3 respirators, and face shields was tested via quantitative real-time polymerase chain reaction.
Results
The wide surface on which the inoculum was spread caused low contamination. Over the external surfaces of masks and respirators, when a face shield was not worn, viral loads ranged from 1.2 through 1.4 log10 mean gene copies per cm2. When the shield was worn, viral loads dropped below the detection limit (< 0.317 log10 gene copies/cm2) for all PPE. On the operator’s forehead, viral loads were 0.6 through 0.8 log10 gene copies/cm2. Inside the operator manikin’s mouth, viral loads were under the detection limit when using any PPE, with or without the shield. HVE did not significantly change viral loads.
Conclusions
All PPE combinations significantly reduced viral loads in the operator manikin’s mouth to below the detection limit, but HVE did not decrease viral contamination.
Practical Implications
Although caution is suggested when removing and disposing of PPE to avoid self-contamination, the combination of PPE and face shields drastically decreases the risk of transmitting human coronavirus during aerosol-generating dental procedures.
Key Words: Severe acute respiratory syndrome-coronavirus 2, aerosols, masks, respirators, face shields, personal protective equipment, high-volume evacuation, communicable disease control, dental equipment
Abbreviation Key: FFP, Filtering facepiece; FFP2, Filtering facepiece class 2; FFP3, Filtering facepiece 3; HCoV-229E, Human coronavirus type 229E; HVE, High-volume evacuation; PCR, Polymerase chain reaction; PPE, Personal protective equipment; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
As of early December 2020, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic caused more than 1,500,000 deaths worldwide.1 The second wave of COVID-19 is spreading and further threatening health, economic development, and social stability worldwide. A third wave is expected, similar to the Spanish flu diffusion in 1918 through 1920.2 , 3
SARS-CoV-2 can be found in the secretions—including saliva, which is an important reservoir4—of both symptomatic and asymptomatic infected patients.5 Because dental practitioners are potentially exposed to the saliva of asymptomatic people, especially during aerosol-generating procedures, they have been included among the professionals carrying the highest risk of developing and therefore transmitting SARS-CoV-2 infection.6, 7, 8
Several studies have found that spatter and aerosol-generating procedures in the dental operatory can spread contamination over virtually any surface, including walls and ceilings.9 , 10 Whereas spatters, having a relatively large particle size (≥ 100 μm), are too large to be inhaled, aerosol particles have a smaller diameter (< 50 μm) and can remain suspended for a long time (up to 1.5 hours) after the end of an operative procedure.11 Aerosols act as carriers of infective agents, including those reaching high saliva concentrations such as SARS-CoV-2,12 which may remain viable and infectious on surfaces for days.13 Thus, data on the efficacy of personal protective equipment (PPE) to reduce the risk of coronavirus infection via dental aerosols and spatter are urgently needed.
Data have been gathered on the efficacy of masks, respirators, and face shields against influenza viruses, and several studies have indicated the importance of devices such as face shields in blocking spatters and in preventing eye contamination.7 , 14 , 15 Many dental associations and councils worldwide and several publications recommend using nonvalved N95 (filtering facepiece [FFP] class 2 [FFP2]) standard respirators or FFP class 3 (FFP3) respirators.7 , 8 , 16 They consider the conventional, 3-layered surgical masks as providing insufficient protection during aerosol-generating procedures or when patients positive for SARS-CoV-2 are treated.17 , 18 Nevertheless, to our knowledge no study has been done to assess the efficacy of masks, respirators, and face shields against coronaviruses to date. Available data have been gathered on influenza viruses only and not in the aerosol-generating dental setting. In particular, laboratory and clinical studies showed that N95 (FFP2) respirators are significantly more effective than medical masks at reducing exposure to aerosols.19 , 20 However, the results of most studies on this topic are mixed, not allowing for reliable clinical confirmation.21
In addition to that one of the main possibilities for reducing contamination of the dental operatory via spatter and aerosol consists of using a high-volume evacuation (HVE) system.22 , 23 However, to our knowledge no study has been done to evaluate PPE efficacy against coronaviruses, and no data have been collected in the aerosol-generating dental setting.
We designed this laboratory study to evaluate the efficacy of various types of PPE and the use of HVE in reducing human coronavirus contamination of operators during conventional dental procedures. The null hypothesis was that all tested PPE and the use of HVE would significantly reduce viral concentrations.
Methods
Surgical masks were purchased from Conception et Fabrication de Produits Médicaux et Paramédicaux. Like most surgical masks, they are a class I disposable medical device compliant with the typical standard IIR IN 14683: protective masks against biological liquid projections. They show a bacterial filtration efficiency of 99%; respiratory resistance, less than 49 Pa/cm2; and splash resistance, 16 KPa or greater. KN95 (FFP2) respirators were obtained from Jinlu (filtering half-mask, Jiangsu Jinlu Group Medical Device Company). This PPE is a disposable class I medical device (category III of PPE) compliant with European Committee for Standardisation, guidelines for maintenance-free dust respirators, guideline EN 149/2001+A1/2009, and Regulation 2016/425 of the European Parliament and of the Council of March 9, 2016, on PPE and repealing Council Directive. FFP3 respirators were obtained from BLS (BLS Zer0 30 NV FFP3 R D). They are class I reusable medical devices (category III of PPE) compliant with EN 149/2001+A1/2009 and PPE (European Union) 2016/425 regulations. They show a bacterial filtration efficiency of 99.9%, respiratory resistance of 120 Pa/cm2 (inspiration) and 200 Pa/cm2 (expiration), and splash resistance. The Galaxy face shield reusable visor (Dental World) was tested. The chosen model did not include soft foam pads to prevent possible contamination sources during reuse due to incomplete disinfection. A confined environment was used that reproduced the dental operating unit and allowed a viral tracer to be used safely. The environment was equipped with manikin heads simulating the operator and the patient and was connected to vacuum pumps to maintain negative pressure and an HVE line. An air turbine operated for 10 seconds was used as the source of contamination via spatter and aerosol. The patient manikin’s mouth was inoculated with a human coronavirus type 229E (HCoV-229E) suspension, having a viral load similar to that of asymptomatic people infected with SARS-CoV-2. The efficacy of surgical masks, N95 (FFP2) and FFP3 respirators, face shields, and HVE was then assessed through quantification, via real-time polymerase chain reaction (PCR), of the viral load on PPE surfaces and on the operator’s manikin’s forehead and mouth.
Experimental dental setting
A polycarbonate pressure-tight chamber (100 × 40 × 40 centimeters) was custom-built. Two circular holes (diameter, 10 cm) and a door (35 × 25 cm) were made in the front panel. Two 50-cm long latex gloves were fitted to the circular apertures. The chamber was connected through airtight tubing to 2 laboratory vacuum pumps, a dental high-volume evacuation system (flow rate, 1,700 L/min; maximum operating head, 280 mbars) (Turbo-Smart 2V, Cattani), and handpiece tubing providing a connection for an air turbine. Two digital manometers (measuring range [standard deviation], 0-200 [0.1] mbar) and a backup analog manometer were fixed to the outer part of the front panel and used to measure the negative pressure inside the chamber (right digital manometer and analog manometer) and the differential pressure inside the mouth of the operator manikin when a mask or respirator covered its mouth and nose (left digital manometer). At the upper right corner of the chamber (Figure 1 ), 2 air leak valves were mounted to set a constant negative pressure of 15 mbar inside the chamber to avoid contamination of the surrounding area. The first valve allowed for fine-tuning the pressure, and the other allowed compensation for a higher air intake due to HVE operation. When the HVE system was not operating, the second valve was closed, and a nonreturn valve on the HVE line ensured back-contamination prevention. All holes, the panel, and tubing connections were sealed to make the chamber airtight and to bear a constant negative operating pressure of 15 mbar (Figure 1).
Figure 1.
Setup of the custom-built class III–like airtight glove box with chamber pressure control. Two access ports for gloves are shown on the front panel. The control apparatus operating the air turbine is located on the right part of the upper panel, having attached the pressurized water tank to generate the air-water cooling spray for the turbine handpiece.
A dental portable turbine unit (Zhengzhou Kongsin Trading Company) was connected to a dental air compressor and used to operate the air turbine handpiece inside the chamber (Bora, Bien-Air Dental). The latter was equipped with a cylindrical diamond bur (835KR.314.016, Komet Italia). The air pressure was set to 3.3 atms, and the speed was 320,000 rpm. The system was provided with a 1,000-mL water tank for the air-water spray (Figure 2 ). Running tap water was used to fill the tank.
Figure 2.

The air turbine and control apparatus used in the study. The pressurized water tank used to generate the air-water cooling spray for the turbine handpiece is seen at the top of the photo.
Two manikin heads (operator and patient) were fixed to custom-made holders in a vertical position inside the chamber at a conventional working distance of 25 cm (Figure 3 ). The patient manikin was equipped with resin teeth (Columbia Dentoform). The air turbine and the HVE system were oriented to simulate the position of a right-handed dentist during the preparation of the occlusal surface of the mandibular right first molar. A universal laboratory holder was used to hold the air turbine and the HVE system in the same position for all experimental runs. The air turbine was positioned 2 millimeters away from the tooth surface and oriented toward the lingual aspect of the dental arch, and the HVE tip was positioned on the opposite side of the tooth (Figure 4 ). The distance between the HVE tip and the turbine tip was 2 cm.
Figure 3.

The 2 manikins inside the chamber. On the right, the patient manikin can be seen with the air turbine and high-volume evacuation tip fixed in the same position throughout all experimental runs, as if operated by a right-handed dentist and dental assistant. On the left, the operator manikin is equipped for the first experimental run. In all runs, the chamber space between operator and patient manikins was free of materials and gloves to allow for an even aerosol spread, similar to clinical conditions.
Figure 4.
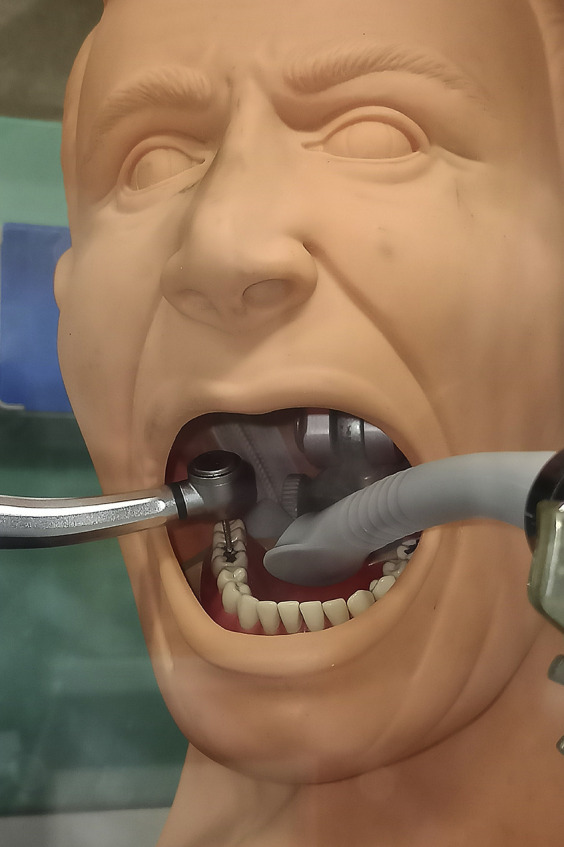
Detail of the patient manikin, showing the standardized positions of the air turbine and the high-volume evacuation system inside its mouth.
The operator manikin was sealed, making it pressure tight, save for the mouth opening. The manikin was connected to one of the manometers and a low-vacuum pump to simulate the airflow during inspiration. The operator manikin was provided with a custom-made tray (7 × 7 cm) to accommodate a 4-well plate (Nunc IVF, Merck) inside its mouth. A site was identified and marked on the manikin’s forehead to position another 4-well plate, using double-sided adhesive tape. In this way, data about viral surface contamination were gathered on 3 sites, namely the operator’s forehead, the mask surface, and the inside of the mouth of the operator manikin behind the mask (Figure 5 ). Last, a sprayer containing absolute ethanol to be used during operation procedures was inserted on the chamber’s side opposite the manikins.
Figure 5.

Detail of the operator manikin as prepared for the first experimental run, equipped with the forehead target and the surgical mask with the red mark for viral load assessment. The decontamination spray containing absolute ethanol can be seen in the background.
Preparation of viral solutions
A coronavirus (HCoV-229E, ATCC VR-740) was used as a biological tracer instead of SARS-CoV-2 for safety reasons. A suspension of HCoV-229E with a viral load (standard deviation) of 6.03 (0.04) log10 gene copies/mL, which resembles SARS-CoV-2 levels in the saliva of asymptomatic infected people,24 , 25 was prepared in an artificial saliva solution. The artificial saliva simulated the average electrolyte and mucin composition of human whole saliva and was prepared from 0.1 L of 150 mM potassium bicarbonate, 0.1 L of 100 mM sodium chloride, 0.1 L of 25 mM dipotassium hydrogen phosphate, 0.1 L of 24 mM sodium phosphate dibasic, 0.1 L of 15 mM calcium chloride, 0.1 L of 1.5 mM magnesium chloride, and 0.06 L of 25 mM citric acid. Distilled water was added to obtain 1 L of solution, a total of 2.5 g of mucin (type II, porcine gastric) was added, and the pH was adjusted to 7.0 by pipetting 4 M sodium hydroxide or 4 M hydrochloric acid solution under vigorous stirring.26 Cryovials, each containing 1 mL of stock viral suspension, were prepared and frozen at –80°C. On the day of testing, stock suspensions were thawed and stored in an ice bath. Experiments were performed in triplicate.
Operation procedures
All personnel operating the chamber wore protective equipment, including gloves, FFP3 respirators and face shields, and disposable gowns. Before starting each experimental run, 2 4-well target plates were placed in their corresponding locations (inside the operator manikin’s mouth and on its forehead) with their lids closed. A surgical mask was positioned on the operator manikin, taking care to adapt it over the nose and mouth openings and removing the 4-well lid just before positioning of the mask. A 1.9-cm2 square was marked in a central position on the external part of each mask. A sterile, leakproof plastic bag was positioned in the chamber to collect specimens after the experimental run. A micropipette with its sterile tips was also inserted into the chamber. One vial containing the viral suspension was then positioned on a stand inside the chamber.
Vacuum pumps and the HVE system were turned on to reach operating pressure conditions inside the chamber. After that, all procedures inside the chamber were performed with airtight gloves. The micropipette was used to transfer the viral solution (1 mL) to the bottom of the mandibular arch inside the patient manikin, mimicking saliva draining from the submandibular glands. The air turbine was then operated for 10 seconds to generate an aerosol containing the viral particles. After that, HVE was turned off, and 60 seconds was allowed for aerosol dispersion. The mask was then removed, and the forehead and mouth plates were covered with their lids. The mask and the 2 plates were positioned in the plastic bag and hermetically sealed. The bag was marked with the corresponding code of the experimental run. The sprayer was then used on all chamber surfaces. A total of 60 seconds was allowed for ethanol disinfection and evaporation, and then the HVE system was turned on again to remove residual ethanol completely. The chamber’s negative pressure was equalized to the atmospheric pressure; the door was opened to remove the specimen-containing bag and discard the equipment used during the run safely.
The experimental conditions tested were surgical mask PPE, no HVE; surgical mask PPE, HVE; FFP2 respirator PPE, HVE; FFP3 respirator PPE, HVE; surgical mask and face shield PPE, HVE (Figure 6 ); FFP2 respirator and face shield PPE, HVE.
Figure 6.
The setup for the fifth experimental run, testing the association of face shield and surgical mask, is visible through the transparent upper panel of the chamber. The forehead target was fixed to the upper external part of the face shield.
When the face shield was used, the forehead target was placed on the uppermost part of the shield (Figure 6).
Each experimental run was performed in triplicate.
Determination of viral loads on the target surfaces
At the end of each experimental run, the target-containing bag was immediately transferred to the virology laboratory in the next room. The wells of each target were washed with 500 μL of phosphate-buffered saline; the solution was collected in sterile Eppendorf vials and stored at –80 °C until analysis. Samples with the same surface area as the target’s wells (1.9 cm2) were cut from the external layer of the mask, inserted in Eppendorf vials containing 500 μL of phosphate-buffered saline, and stored as previously described. The presence of HCoV-229E on targets was determined via quantitative real-time PCR. RNA extraction was performed with a PureLink viral RNA/DNA kit (ThermoFisher). A total of 500 μL of viral suspension was used, and the elution was performed with 10 μL of elution buffer. RNA was retrotranscribed with a SuperScript VILO cDNA synthesis kit (ThermoFisher) and amplified with an HCoV-229E–specific real-time PCR gene assay (assay ID, Vi06439671_s1; 4331182; ThermoFisher).
Statistical analysis
All analyses were performed with SAS software (JMP 14.0, SAS Institute). Real-time PCR data were analyzed after log transformation to approach a normal distribution, which was verified by Shapiro-Wilk test. The limit of detection for quantitative real-time PCR in an error-free environment, where only sampling noise contributes to the variation, was calculated to be 3 viral copies with 95% CI.27 Considering the noise due to extraction and reverse transcription, the limit of detection was conservatively determined as 4 viral copies, according to the methodology proposed by Forootan and colleagues.28 Data were expressed as log10 viral copies per square centimeter. Homogeneity of variances was checked by Levene test, and a 2-way analysis of variance model was used considering the site (forehead, mask tissue, mouth) and experimental setting (HVE, PPE type, combination) as fixed factors. The Tukey test was used to evaluate significant differences between groups. The significance level was set to a 2-sided P < .05.
Results
When a face shield was not worn, viral loads ranged from a mean of 1.2 through 1.4 log10 gene copies/cm2 over the external surfaces of masks and respirators (Figure 7 ). When the shield was on, the viral loads dropped below the detection limit (< 0.317 log10 gene copies/cm2) for all PPE. On the operator manikin’s forehead, the viral loads ranged from 0.6 through 0.8 log10 gene copies/cm2 in all experimental runs. Inside the operator manikin’s mouth, the viral loads were less than the detection limit using any PPE, with or without a face shield, with no significant differences among surgical masks and respirators (all P > .05). The use of HVE did not significantly modify the viral loads in any experimental run (all P > .05).
Figure 7.
Results of the experimental runs (mean log10 viral copies/cm2 [standard error, shown by the whiskers]). Results are divided by the aims of the study: to compare the efficacy of masks, respirators, and face shield against human coronavirus 229E aerosolized by a conventional dental procedure (PPE) or the efficacy of high-volume evacuation (HVE) in mitigating coronavirus spread via aerosol. Blue indicates the use of a surgical mask to protect the operator, and green indicates the simultaneous use of a face shield. Experimental runs testing HVE (right) were performed with surgical masks, and are shown as light (run without HVE) or dark (run with HVE).
Discussion
Because of the SARS-CoV-2 pandemic, aerosol generation during dental procedures regained attention as a high-risk factor for airborne transmission of infectious diseases,10 , 29 , 30 and procedures for infection control and PPE use in the dental setting were updated to face this new emerging infective disease.6 , 7 , 31 Data from already known coronaviruses and other airborne-spread diseases were used,14 , 32 owing to the scarcity of information on the SARS-CoV-2 agent.6 , 7 In fact, to our knowledge this is the first study done to assess the efficacy of masks, respirators, and face shields against coronaviruses in the dental setting.
The main findings of this study have direct clinical implications. First, the highest viral loads were found on the external surfaces of the masks and respirators, and the virus was detected on the manikin’s forehead and outer part of the face shield in all runs. These results confirm a high risk of being contaminated for oral health care providers during aerosol-generating procedures. The results also outline the importance of carefully removing and disposing of masks and respirators after treating each patient, as they may concentrate any airborne pathogens on their surfaces and be a significant source of self-contamination. Viral contamination of the outer surfaces of masks was significantly higher than that of control surfaces on the manikin’s forehead. This result can be explained, considering that a vacuum pump was connected to the operator manikin to simulate inspiration via continuous air intake through the tested PPE. The airflow through the masks allowed large amounts of aerosol particles carrying the virus to settle on their external surfaces. The above findings are in agreement with those of Prospero and colleagues,33 who found that the outer surfaces of the masks were significantly more contaminated than all other surfaces. Another study was done to evaluate the contamination via respiratory viruses on the outer surfaces of surgical masks used by hospital health care workers.34 The authors found contamination on a median of 10% of the masks, concluding that respiratory pathogens on the outer surface of the masks might cause self-contamination of the operators.
Second, the use of a face shield reduced viral loads on the external surfaces of masks and respirators to less than the detection limit. This finding supports the protective effect of face shields against contamination via aerosol, confirming the importance of combined use of face shields with masks or respirators. In the dental practice, especially during the COVID-19 pandemic, face shields are highly recommended when aerosol-generating procedures are performed.7 , 14 , 15 However, limited data are available on the efficacy of face shields in blocking contamination via splashes and spatters,15 and the authors of only 1 study indirectly evaluated their efficacy in reducing aerosol diffusion; Akagi and coworkers35 performed a computational flow simulation that highlighted the relatively low efficacy of a face shield in protecting against aerosols. They found that aerosols directed toward a shield-wearing person form ring-shaped vortices that reach the shield’s top and bottom edges and form a high-velocity entrainment flow, quickly reaching the areas behind the shield.35 In contrast to these data, our study results support the protective effect of face shields against contamination via aerosol as well as via spatter.
As a third finding, in the experimental setup of our study, surgical masks and N95 (FFP2) or FFP3 respirators were equally effective in protecting the operator; even in a critical environment such as the aerosol-generating dental setting, the viral loads were below the detection limit when wearing both a surgical mask and a respirator. This finding must be interpreted with caution. Given the short duration of the test, the risk of experiencing virus transmission may still be high during long-lasting procedures, with the exclusive use of a single PPE. Nevertheless, our results are in line with those of a meta-analysis done in 202036 and a randomized controlled trial,21 which found no significant differences between masks and respirators in preventing laboratory-confirmed viral respiratory infection or clinical respiratory illness, including coronaviruses. Two randomized controlled trials conducted by another research group in 2013 and 201719 , 37 found that continuously worn N95 (FFP2) respirators provided significantly higher protection than surgical masks. In fact, N95 (FFP2) respirators are believed to provide better protection than surgical masks against viral respiratory infections, and their use whenever aerosol-generating procedures are performed is still recommended by several scientific societies.16, 17, 18 , 38 Study results produced by MacIntyre and coworkers39 showed a significantly lower efficacy of cloth masks than surgical masks. In an update to this study considering the spread of the COVID-19 pandemic, they urged the use of cloth masks only as a last resort because of their lower protection efficacy.40 This conclusion was considered when, in our study, it was decided not to test cloth masks as protective means for dental operators.
As a fourth outcome, we found no significant influences of HVE on viral contamination. Such a system has been proposed as one of the main possibilities to reduce contamination of the dental operatory via spattering and aerosols.22 , 23 The efficacy of several dry-field isolation techniques, including HVE alone, in spatter and aerosol mitigation was tested in 2020 by Ravenel and colleagues.23 In that study, the use of HVE significantly reduced spatter but not aerosol spread. Moreover, in a 2020 Cochrane review, Nagraj and colleagues22 concluded that the effects of HVE were detectable only within about 30 cm from the source of contamination (the patient’s mouth). Our results agree with these data, showing no difference in the aerosol contamination level with or without the use of HVE at a conventional working distance of 25 cm between the operator and the source of infection.
Thus, the null hypothesis must be partly rejected because all PPE combinations significantly reduced viral loads in the operator’s mouth to below the detection limit, but HVE did not influence viral contamination.
As mentioned, the main limitation of this study was the reduced duration of the aerosol-generating procedure (10 seconds). This time was selected for each experimental run to achieve dispersion of the viral inoculum through aerosol and spatters. It is clear that other dental procedures, such as dental crown preparations, last longer and produce greater amounts of aerosols. However, testing the latter procedure would have required, in turn, much larger amounts of viral inoculum that would have been difficult to produce and manage. There is a need for further evaluations with a more prolonged testing time of aerosol-spreading dental procedures to confirm whether respirators could be replaced by the more tolerated and less expensive surgical masks’ maintaining a comparable protection level.
The strengths of this study include the use of artificial saliva containing a viral concentration similar to those of asymptomatic infected patients, the high number of experimental conditions evaluated using a human coronavirus, and a methodology designed to reproduce aerosol spreading in the clinical setting as closely as possible, using a custom-built, class III–like, negative-pressure cabinet.
Conclusions
To our knowledge, this is the first quantitative analysis of human coronavirus viral loads transmitted during aerosol-generating dental procedures; it has shown that significant viral loads are deposited on the outer surfaces of masks and respirators when a face shield is not used, suggesting caution when removing and disposing of PPE to avoid self-contamination. The combination of mask or respirator and face shield reduced viral loads below the detection limit, thus decreasing the risk of the operator’s being contaminated. In the experimental setup of our study, surgical masks and N95 (FFP2) or FFP3 respirators were equally effective in protecting the operator, whereas HVE did not seem to decrease the risk of experiencing aerosol contamination.
Biographies
Dr. Ionescu is an adjunct professor and postdoctoral researcher, Oral Microbiology and Biomaterials Laboratory, Department of Biomedical, Surgical and Dental Sciences, University of Milan, Milan, Italy.
Dr. Brambilla is an associate professor and head of the Oral Microbiology and Biomaterials Laboratory, Department of Biomedical, Surgical and Dental Sciences, University of Milan, Milan, Italy.
Dr. Manzoli is a full professor and head of the Department of Medical Sciences, University of Ferrara, Italy.
Dr. Orsini is an associate professor, Department of Clinical Sciences and Stomatology, Polytechnic University of Marche, Ancona, Italy.
Dr. Gentili is a postdoctoral researcher, Department of Chemical and Pharmaceutical Sciences, University of Ferrara, Italy.
Dr. Rizzo is an associate professor, Department of Chemical and Pharmaceutical Sciences, University of Ferrara, Italy.
This article has an accompanying online continuing education activity available at: http://jada.ada.org/ce/home.
Footnotes
Disclosure. None of the authors reported any disclosures.
The present study was funded by SISOPD (Società Italiana Stomatologia Odontoiatria Protesi Dentaria: Italian Society of Stomatology, Dentistry and Dental Prosthetics) Foundation, and ANDI (Associazione Nazionale Dentisti Italiani: National Association of Italian Dentists) Progetti. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
References
- 1.Worldometer World coronavirus statistics. March 23, 2021. https://worldometer.pro/ Accessed 23 March 2021.
- 2.Barro R.J., Ursúa J.F., Weng J. The coronavirus and the great influenza pandemic: lessons from the “Spanish flu” for the coronavirus’s potential effects on mortality and economic activity. Working Paper 26866. National Bureau of Economic Research, 2020. https://www.nber.org/system/files/working_papers/w26866/w26866.pdf Accessed March 23, 2021.
- 3.Leung K., Wu J.T., Liu D., Leung G.M. First-wave COVID-19 transmissibility and severity in China outside Hubei after control measures, and second-wave scenario planning: a modelling impact assessment. Lancet. 2020;395(10233):1382–1393. doi: 10.1016/S0140-6736(20)30746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J., Li Y., Gan F., Du Y., Yao Y. Salivary glands: potential reservoirs for COVID-19 asymptomatic infection. J Dent Res. 2020;99(8):989. doi: 10.1177/0022034520918518. [DOI] [PubMed] [Google Scholar]
- 5.Pascolo L., Zupin L., Melato M., Tricarico P.M., Crovella S. TMPRSS2 and ACE2 coexpression in SARS-CoV-2 salivary glands infection. J Dent Res. 2020;99(10):1120–1121. doi: 10.1177/0022034520933589. [DOI] [PubMed] [Google Scholar]
- 6.Peng X., Xu X., Li Y., Cheng L., Zhou X., Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci. 2020;12(1):1–6. doi: 10.1038/s41368-020-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng L., Hua F., Bian Z. Coronavirus disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. J Dent Res. 2020;99(5):481–487. doi: 10.1177/0022034520914246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ge Z., Yang L., Xia J., Fu X., Zhang Y. Possible aerosol transmission of COVID-19 and special precautions in dentistry. J Zhejiang Univ Sci B. 2020;21(5):361–368. doi: 10.1631/jzus.B2010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ionescu A.C., Cagetti M.G., Ferracane J.L., Garcia-Godoy F., Brambilla E. Topographic aspects of airborne contamination caused by the use of dental handpieces in the operative environment. JADA. 2020;151(9):660–667. doi: 10.1016/j.adaj.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rautemaa R., Nordberg A., Wuolijoki-Saaristo K., Meurman J.H. Bacterial aerosols in dental practice: a potential hospital infection problem? J Hosp Infect. 2006;64(1):76–81. doi: 10.1016/j.jhin.2006.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leggat P.A., Kedjarune U. Bacterial aerosols in the dental clinic: a review. Int Dent J. 2001;51(1):39–44. doi: 10.1002/j.1875-595x.2001.tb00816.x. [DOI] [PubMed] [Google Scholar]
- 12.Santosh T.S., Parmar R., Anand H., Srikanth K., Saritha M. A review of salivary diagnostics and its potential implication in detection of Covid-19. Cureus. 2020;12(4) doi: 10.7759/cureus.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Doremalen N., Bushmaker T., Morris D.H., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gugnani N., Gugnani S. Safety protocols for dental practices in the COVID-19 era. Evid Based Dent. 2020;21(2):56–57. doi: 10.1038/s41432-020-0094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberge R.J. Face shields for infection control: a review. J Occup Environ Hyg. 2016;13(4):235–242. doi: 10.1080/15459624.2015.1095302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel J.D., Rhinehart E., Jackson M., Chiarello L., Health Care Infection Control Practices Advisory Committee 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10 suppl 2):S65–S164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herron J.B., Hay-David A.G., Gilliam A.D., Brennan P.A. Personal protective equipment and Covid 19: a risk to health-care staff? Br J Oral Maxillofac Surg. 2020;58(5):500–502. doi: 10.1016/j.bjoms.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang S., Mao Y., Jones R.M., et al. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ Int. 2020;144:106039. doi: 10.1016/j.envint.2020.106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacIntyre C.R., Chughtai A.A., Rahman B., et al. The efficacy of medical masks and respirators against respiratory infection in healthcare workers. Influenza Other Respir Viruses. 2017;11(6):511–517. doi: 10.1111/irv.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noti J.D., Lindsley W.G., Blachere F.M., et al. Detection of infectious influenza virus in cough aerosols generated in a simulated patient examination room. Clin Infect Dis. 2012;54(11):1569–1577. doi: 10.1093/cid/cis237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radonovich L.J., Simberkoff M.S., Bessesen M.T., et al. ResPECT investigators N95 respirators vs medical masks for preventing influenza among health care personnel: a randomized clinical trial. JAMA. 2019;322(9):824–833. doi: 10.1001/jama.2019.11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumbargere Nagraj S., Eachempati P., Paisi M., Nasser M., Sivaramakrishnan G., Verbeek J.H. Interventions to reduce contaminated aerosols produced during dental procedures for preventing infectious diseases. Cochrane Database Syst Rev. 2020;10:CD013686. doi: 10.1002/14651858.CD013686.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravenel T.D., Kessler R., Comisi J.C., Kelly A., Renne W.G., Teich S.T. Evaluation of the spatter-reduction effectiveness and aerosol containment of eight dry-field isolation techniques. Quintessence Int. 2020;51(8):660–670. doi: 10.3290/j.qi.a44919. [DOI] [PubMed] [Google Scholar]
- 24.Peiris J.S.M., Chu C.-M., Cheng V.C.-C., et al. HKU/UCH SARS Study Group Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wölfel R., Corman V.M., Guggemos W., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 26.Ionescu A., Brambilla E., Hahnel S. Does recharging dental restorative materials with fluoride influence biofilm formation? Dent Mater. 2019;35(10):1450–1463. doi: 10.1016/j.dental.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 27.Ståhlberg A., Kubista M. The workflow of single-cell expression profiling using quantitative real-time PCR. Expert Rev Mol Diagn. 2014;14(3):323–331. doi: 10.1586/14737159.2014.901154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forootan A., Sjöback R., Björkman J., Sjögreen B., Linz L., Kubista M. Methods to determine limit of detection and limit of quantification in quantitative real-time PCR (qPCR) Biomol Detect Quantif. 2017;12:1–6. doi: 10.1016/j.bdq.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrel S.K., Molinari J. Aerosols and spatter in dentistry: a brief review of the literature and infection control implications. JADA. 2004;135(4):429–437. doi: 10.14219/jada.archive.2004.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zemouri C., de Soet H., Crielaard W., Laheij A. A scoping review on bio-aerosols in healthcare and the dental environment. PLoS One. 2017;12(5) doi: 10.1371/journal.pone.0178007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abichandani S.J., Nadiger R. Cross-contamination in dentistry: a comprehensive overview. J Educ Ethics Dent. 2013;2(1):3–9. [Google Scholar]
- 32.Izzetti R., Nisi M., Gabriele M., Graziani F. COVID-19 transmission in dental practice: brief review of preventive measures in Italy. J Dent Res. 2020;99(9):1030–1038. doi: 10.1177/0022034520920580. [DOI] [PubMed] [Google Scholar]
- 33.Prospero E., Savini S., Annino I. Microbial aerosol contamination of dental health-care workers’ faces and other surfaces in dental practice. Infect Control Hosp Epidemiol. 2003;24(2):139–141. doi: 10.1086/502172. [DOI] [PubMed] [Google Scholar]
- 34.Chughtai A.A., Stelzer-Braid S., Rawlinson W., et al. Contamination by respiratory viruses on outer surface of medical masks used by hospital health-care workers. BMC Infect Dis. 2019;19(1):1–8. doi: 10.1186/s12879-019-4109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akagi F., Haraga I., Inage S.I., Akiyoshi K. Effect of sneezing on the flow around a face shield. Phys Fluids. 2020;32(12):127105. doi: 10.1063/5.0031150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartoszko J.J., Farooqi M.A.M., Alhazzani W., Loeb M. Medical masks vs N95 respirators for preventing COVID-19 in health-care workers: a systematic review and meta-analysis of randomized trials. Influenza Other Respir Viruses. 2020;14(4):365–373. doi: 10.1111/irv.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacIntyre C.R., Wang Q., Seale H., et al. A randomized clinical trial of three options for N95 respirators and medical masks in health workers. Am J Respir Crit Care Med. 2013;187(9):960–966. doi: 10.1164/rccm.201207-1164OC. [DOI] [PubMed] [Google Scholar]
- 38.Checchi V., Bellini P., Bencivenni D., Consolo U. COVID-19 dentistry-related aspects: a literature overview. Int Dent J. 2021;71(1):21–26. doi: 10.1111/idj.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacIntyre C.R., Seale H., Dung T.C., et al. A cluster randomised trial of cloth masks compared with medical masks in health-care workers. BMJ Open. 2015;5(4) doi: 10.1136/bmjopen-2014-006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacIntyre C.R., Tham C.D., Seale H., Chughtai A. COVID-19, shortages of masks and the use of cloth masks as a last resort [author response] BMJ Open. 2020;5(4) https://bmjopen.bmj.com/content/5/4/e006577.responses#covid-19-shortages-of-masks-and-the-use-of-cloth-masks-as-a-last-resort Accessed March 23, 2021. [Google Scholar]





