Abstract
Although the previous success of bladder tissue engineering demonstrated the feasibility of this technology, most polyester based scaffolds used in previous studies possess inadequate mechanical properties for organs that exhibit large deformation. The present study explored the use of various biodegradable elastomers as scaffolds for bladder tissue engineering and poly (carbonate-urethane) urea (PCUU) scaffolds mimicked urinary bladder mechanics more closely than polyglycerol sebacate-polycaprolactone (PGS-PCL) and poly (ether-urethane) urea (PEUU). The PCUU scaffolds also showed cyto-compatibility as well as increased porosity with increasing stretch indicating its ability to aid in infiltration of smooth muscle cells. Moreover, a bladder outlet obstruction (BOO) rat model was used to test the safety and efficacy of the PCUU scaffolds in treating a voiding dysfunction. Bladder augmentation with PCUU scaffolds led to enhanced survival of the rats and an increase in the bladder capacity and voiding volume over a 3 week period, indicating that the high-pressure bladder symptom common to BOO was alleviated. The histological analysis of the explanted scaffold demonstrated smooth muscle cell and connective tissue infiltration. The knowledge gained in the present study should contribute towards future improvement of bladder tissue engineering technology to ultimately aide in the treatment of bladder disorders.
Keywords: Bladder outlet obstruction, Biaxial mechanical testing, Bladder smooth muscle cell, Cyto-compatibility, Voiding volume
INTRODUCTION
Various conditions including congenital malformations and cancers of the lower urinary tract affect approximately 400 million people worldwide.1 Current surgical treatments for these disorders rely on the use of autologous intestinal segments and xenografts such as small intestinal submucosa, which suffer from various complications including mechanical mismatch and graft shrinkage.26 To address this problem, tissue engineering approaches have been explored where the urinary bladder has been successfully engineered in vitro and implanted in both dogs and humans.3,20 The neo-bladders fabricated with the autologous cell-seeded scaffolds made of polyglycolic acid (PGA) and poly-dl-lactide-co-glycolide 50:50 (PLGA) exhibited capacities and compliance greater than the pre-cystectomy level of the patients’ bladders eleven months post-surgery and the retrieved tissues displayed a normal cellular organization.3 However, more recent clinical trials of the same approach demonstrated multiple adverse events such as bowel obstruction and bladder rupture without improving capacity or compliance, which resulted in discontinuation of the study.12 Previous studies have shown that Collagen-PGA composite scaffolds possessed twice the stiffness (Young’s modulus ~ 0.001 MPa) compared to native bladder tissue (Young’s modulus ~ 0.002 MPa).6 Hence, biomaterials issues such as scaffold mechanical property mismatch may need to be reevaluated. In addition, a comprehensive review of twenty eight bladder tissue engineering studies concluded that though pre-clinical studies using healthy animals showed promising outcomes, subsequent clinical trials could not replicate the results revealing dissimilar outcomes in different clinical/disease backgrounds.28 Thus, to study the effect of tissue engineering for bladder augmentation in a relevant clinical scenario, animal models with dysfunctional bladders have to be evaluated. Therefore, the objective of this study was to characterize a viable bladder tissue scaffold (patch) which mimics the mechanical properties of the bladder; maintains the viability and morphology of the BSMC seeded in it and finally, tested in vivo in a dysfunctional bladder model to evaluate its true efficacy.
In pursuit of this goal, the present study explored the use of biodegradable elastomers, namely, polyglycerol sebacate –polycaprolactone (PGS-PCL), poly (ether-urethane) urea (PEUU), and poly (carbonate-urethane) urea (PCUU) as scaffold materials for urinary bladder tissue engineering since these materials have previously been shown to exhibit high extensibility and biocompatibility.9,24 For example, Sant and colleagues demonstrated that, compared to electrospun PCL-only (mesh) scaffolds, electrospun (ES) fibrous PGS-PCL scaffolds supported greater cell attachment and proliferation of human umbilical vein endothelial cells (HUVECs) and mitral valve interstitial cells (MVICs).23,24 In separate studies, Courtney et al. and Stankus et al. demonstrated that electrospun PEUU scaffolds simulated the anisotropic mechanical response of vascular tissues4,29 and promoted smooth muscle neo-tissue formation in rats with sub-acute myocardial infraction.7 Studies on the PCUU scaffold showed that though it degraded slower than PEUU (when implanted subcutaneously in rats at the end of 8 weeks), it also promoted native smooth muscle infiltration and growth similar to PEUU.9 In addition to cytocompatibility, PGS-PCL, PEUU and PCUU exhibited high tensile strength (10–35 MPa)9 and elongation (400–800%)9,23 which are ideal for applications toward the bladder that demonstrate a peak stress (~ 1.6 MPa) and elongation of 150–350% when subjected to uniaxial tension.15
The present study focused on characterization of the mechanical behavior and the in vitro cytocompatibility of these biodegradable elastomers for urinary bladder tissue engineering applications. In addition, we conducted a pilot bladder augmentation study using a rat model of bladder outlet obstruction (BOO) to determine the efficacy of the biomaterial. BOO is a bladder dysfunction induced by benign prostatic hyperplasia or urethral structure14 and is one of the more common problems in both males and females. The BOO bladders tend to exhibit overactive bladder symptoms and, thus, the BOO model is a popular model for bladder dysfunction.14 In the present study, during the 21-day implantation period, voiding volume and bladder capacity were calculated at every 7 day time-period to assess the impact of bladder augmentation with a PCUU patch. Furthermore, histological analyses were performed to characterize host cell-biomaterial interactions.
MATERIALS AND METHODS
Materials
All elastomeric scaffolds were prepared using conventional electrospinning methods with conditions described in previous report.24,29 Briefly, for PGS-PCL scaffolds the PGS and PCL polymers (33 wt%) were dissolved in a mixture of ethanol and anhydrous chloroform so that the PGS: PCL weight ratio was kept constant at 2:1. Electrospinning was carried out at 12.5 kV using a 21G blunt needle and at a flow rate of 2 mL/h for 30 min.24 PEUU and PCUU scaffolds were fabricated by first dissolving the polymer in HFIP under mechanical stirring at either 5 or 12 wt%. The electrospinning was performed at 12 kV (PCUU or PEUU solution) and at 9 kV (cell culture media), which were fed at ~ 0.98–1.2 mL/h (dependent on ambient relative humidity) using two different syringe pumps (Harvard Apparatus) located at 17 cm and 4.5cm respectively from the target (mandrel at - 7 kV ~ 200 rpm). PCUU scaffolds were prepared in Normal (A) and Compliant (B) versions by varying the amount of culture media added during electrospinning. After fabrication, scaffolds were stored in DMEM supplemented with 10% FBS, 10% horse serum and 5% penicillin/streptomycin at 4 °C and sterilized under UV light overnight before use.
Uniaxial Mechanical Testing of Elastomeric Scaffolds
PGS-PCL, PEUU and PCUU test specimens (~ 30 × 3 mm) were prepared according to the ASTM standards D3039/D3039D. Using MTS Synergie 100, the specimens were subjected to uniaxial tensile loads under hydrated conditions (PBS pH 7.4 at 37 °C) at a rate of 18 mm/min until rupture.5 Since the thickness of the scaffolds (< 0.1 mm) was small compared to its length and width, tension (N/mm) was quantified instead of stress for all the elastomeric scaffolds. The mechanical behaviors of these materials were analyzed by plotting the calculated values of tension (N/mm) against stretch ratio λ (ratio of deformed to reference lengths) and by comparing the maximum tensions and stretch ratios at failure. As a reference, the physiological peak tension of the human bladder (MT) was estimated by MT = PR/2 where P is the internal pressure and R is the radius. Corresponding stress values were then estimated by T = MT/thickness. The values used in these equations for the internal radius of the normal bladder (6 mm),maximum thickness (0.4 mm), and peak pressure (75 cm H2O or 7.34 kPa) were based on reports in the literature.13,26
Biaxial Mechanical Testing of Elastomeric Scaffolds
PCUU specimens approximately 10 mm × 10 mm in size were subjected to biaxial mechanical testing using a custom tester.22 After the thickness was measured using a caliper, the PCUU specimen was mounted into the biaxial testing device using stainless hooks and sutures on each edge with an initial grip separation of 8-8.5 mm between hooks on both axes. All samples underwent a minimum of ten loading/unloading cycles with equibiaxial stress up to 300 kPa, immersed in saline at 37 °C, with a time cycle of 50 s. The stretch ratios (λx and λy) were determined by tracking markers attached onto the specimen surface and by computing the deformation gradient.8 The force measurements from the 2 N load cells were used to calculate first Piola-Kirchoff (PK) stress. The areal strain (= λXλY – 1) under 100 kPa were also computed to determine the change in area between the reference and deformed states under planar biaxial loading. Peak bladder stress in humans does not cross 100 kPa even in extreme conditions and further stress leads to rupture of the bladder under biological conditions and hence, areal strain was measured at 100 kPa.
SEM Imaging of PCUU Scaffolds
Rectangular PCUU specimens were created according to the ASTM standards D3039/D3039D with the dimensions 10 × 1 mm. These scaffolds were affixed to thin aluminum sheets (gauge – 0.0084, Amerimax, USA) under stretched states at λ = 1.0, 1.25, and 1.75. These samples were then imaged using SU 6600 scanning electron microscope (Hitachi). The percent porosity was determined semi-quantitatively from the SEM images using NIH ImageJ.
BSMC Culture on PCUU Scaffolds
Rat bladder smooth muscle cells (BSMC) were harvested and cultured as described previously.27 The PCUU scaffolds (5 mm × 5 mm) were sterilized by soaking in 70% ethanol for at least 1 h before cell seeding and then washed thoroughly with sterile phosphate buffered saline (PBS) (24–48 h). The scaffolds are then soaked overnight in sterile BSMC culture media composed of RPMI supplemented with 10% fetal bovine serum and 1% antibiotic/antimycotic (Life Technologies, NY, USA). The BSMC were suspended in 20–25 μL of BSMC culture media at a final concentration of ~ 1 × 105 cells/scaffold and seeded evenly on each scaffold. The cells were allowed to attach to scaffolds for up to 1.5 h before flooding the culture plate with additional medium. After incubation under standard cell culture conditions (humidified, 37 °C, 5% CO2/95% air) overnight, the cell-seeded scaffolds were transferred to new well plates with fresh media to separate them from the cells attached to the bottom of wells. BSMC with six passages or less were used in all experiments.
Characterization of BSMC Viability and Morphology
After 7 days in culture, BSMC on PCUU scaffolds were rinsed in sterile PBS following removal of media and stained with LIVE/DEAD assay kit according to the manufacturer’s instructions (Molecular Probes, NY, USA). The cells were visualized using a laser confocal microscopy (C1Si Confocal; Nikon Ti Eclipse) and their viability was determined semi-quantitatively by counting green (live) and red (dead) cells in images using the ImageJ software.
To examine cell morphology, BSMC cultured on PCUU scaffolds were stained with rhodamine-phalloidin. Briefly, cells were fixed in paraformaldehyde (1:50 dilution in PBS, P6148, Sigma) at room temperature for 15 min, and the excess aldehyde was quenched with 0.1 M glycine (T9284, Sigma) for 5 min. The cells were permeabilized with 0.1% triton-X 100 (T9284, Sigma) for 1 min and incubated with rhodamine-phalloidin (1:100 dilution in PBS, R415, Molecular Probes/invitrogen) for 15 min. The cell nuclei were stained with DAPI (1:100 dilution, D-1306, Molecular probes, Eugene, OR). The specimens were then subjected to triplicate PBS washes for 5 min, and imaged using laser confocal microscopy (C1Si Confocal; Nikon Ti Eclipse).
Bladder Outlet Obstruction and Augmentation
Bladder outlet obstruction (BOO) model creation followed by bladder augmentation with PCUU scaffolds on female Sprague–Dawley rats (3–6 month old, 175–199 g) were performed according to a protocol approved by Medical University of South Carolina IACUC. Briefly, under anesthesia, a sterile, lubricated catheter (P50 tubing; O.D. 1 mm) was inserted transurethrally and secured to the inguinal region using 5-0 PGA suture. The abdominal cavity was opened longitudinally and a 5-0 silk suture was tied securely around the urethra to produce outlet obstruction or tied loosely for sham animals. The inguinal stich and the catheter were removed and animals were allowed to recover. After 10 days, under anesthesia a 1 cm-long incision was made at the dome of the bladder using surgical scissors to create a defect and a square piece (1 cm × 1 cm) of PCUU scaffold was sutured to augment the bladder.
Monitoring Voiding Behavior
At the end of prescribed time periods (1,7,14, and 21 days) after PCUU implantation, the voiding volume and bladder capacity were quantified by placing each rat in a metabolic cage with a wire mesh bottom above a scale. To ensure that the rat would void recordable volumes of urine during the experiment, each animal was gavaged with 500 ¼L of drinking water prior to being placed into the cage. The scale was continuously monitored by a computer with special software (Catamount Research and Development, St. Albans, VT) which registered the time and weight (converted to volume assuming 1 g/mL density) of urine voided. After at least eight voids were recorded on each day of the experiment, the rat was returned to the animal facility. The voiding volume and bladder capacity of sham rats, normal rats, and PCUU rats at day 0 (BOO rats before scaffold implantation) were also measured as control.
Histological Analysis of Explanted Tissues
Three weeks after implantation, the rats were sacrificed and the whole bladder was harvested. The dome section of the bladder that contained the scaffold was fixed in neutral buffer saline (NBF) and specimens were prepared for histological and immunohisto-chemical analysis. The fixed samples were embedded in Richard Allen Scientific™ NEG 50™ (Thermo Scientific, USA) and frozen using Freeze Spray (Chemtronics, GA, USA). Serial sections were generated at thicknesses of 8 μm on a Microm HM505 N cryostat for conventional histology on glass slides. Bladder sections were stained with Hematoxylin and Eosin (H& E), Masson’s Trichrome and alizarain red based on standard protocols (Kits and protocols were obtained from http://www.ihcworld.com). The presence of smooth muscle in the explanted bladder samples was examined by immuno-staining with primary antibodies for a-actin (1:100 dilution, Sigma-Aldrich, USA) and smoothelin (1:100 dilution, Thermo scientific, USA).
RESULTS
Uniaxial Mechanical Testing of Elastomeric Scaffolds
The results of uniaxial tensile testing revealed that the maximum stretch and the maximum tension at failure of PGS-PCL scaffolds were on average 2.15 ± 0.28 and 0.072 ± 0.005 N/mm, respectively (Fig. 1). Under the same testing conditions, PEUU scaffolds exhibited the maximum stretch ratio and tension at failure of 2.47 ± 0.52 and 1.75 ± 0.68 N/mm, respectively (Figs. 1–3c). In contrast, PCUU scaffolds reached the maximum stretch, 2.43 ± 0.26, under a significantly (P < 0.05) lower maximum tension at failure, 0.43 ± 0.029 N/mm (Fig. 1).
FIGURE 1.

Uniaxial tensile testing of biodegradable elastomer scaffolds. Specimens were subjected to uniaxial tension at the rate of 18 mm/min until failure and average peak tension (a), peak stretch (b) were calculated from the tension-stretch relationship (c) of viscoelastic elastomer scaffolds. The parallel lines across (c) represent the corresponding values for peak physiological tension to which the human bladder is subjected in physiological conditions varying on thickness (0.2–0.4 mm). Values are mean ± SEM; analyzed by ANOVA, *p < 0.01, **p < 0.05, n = 4.
FIGURE 3.

SEM images of PCUU. Specimens were imaged under stretched state at λ = 1.0 (a), 1.25 (b), and 1.75 (c) at × 1000 magnification. The PCUU fiber alignment appears to align in the direction of the stretch (indicated by arrow) and the % porosity increased nearly 70% from λ = 1.0 to λ = 1.25.
Biaxial Testing of PCUU Scaffolds
PCUU scaffolds were further characterized by performing planar biaxial testing and the results indicated that they exhibited anisotropic mechanical behavior under equibiaxial loading (Fig. 2). At 100 kPa, the maximum stretch values for normal PCUU (A version) were 1.06 ± 0.01 and 1.14 ± 0.01 in the x- and y-axis, respectively, whereas for compliant PCUU (B version), the stretch values were 1.1 ± 0.03 and 1.2 ± 0.03 (Fig. 2a). The areal strains calculated under these loads were 0.21 ± 0.02 and 0.32 ± 0.05, for A and B versions, respectively (Fig. 2b).
FIGURE 2.

Biaxial mechanical testing of PCUU. Specimens were subjected to equi-biaxial stress up to 100 kPa, immersed in saline at 37 °C, with a loading–unloading cycle of 50 s. Mean stress (a) and areal strain (b) were calculated for normal and compliant PCUU. The compliant version of PCUU was nearly twice as complaint as the normal version of PCUU. Values are mean ± SEM; analyzed by ANOVA, *p < 0.01, n = 3.
SEM Imaging of PCUU Scaffolds
The results of the SEM imaging provided evidence that the PCUU scaffolds changes in porosity and fiber orientation dependent on the intensity of stretch. The increase in stretch from 0 to 25% led to a significant (p < 0.01) increase in porosity from 37.6 ± 3.9% to 54.9 ± 1.7% (Fig. 3). The porosity at 75% stretch was 57.1 ± 2.8% and similar to that under 25% stretch (Fig. 3).
Examination of BSMC Viability and Morphology on PCUU Scaffolds In Vitro
The fluorescence images showed high viability (90.11%) (Fig. 4a), and elongated cell morphology of BSMC which were distributed throughout the PCUU surface (Fig. 4b). The PCUU scaffolds have a small degree of auto-fluorescence while visualization leading to a high background signal and hence, a high threshold level was required to observe the staining.
FIGURE 4.
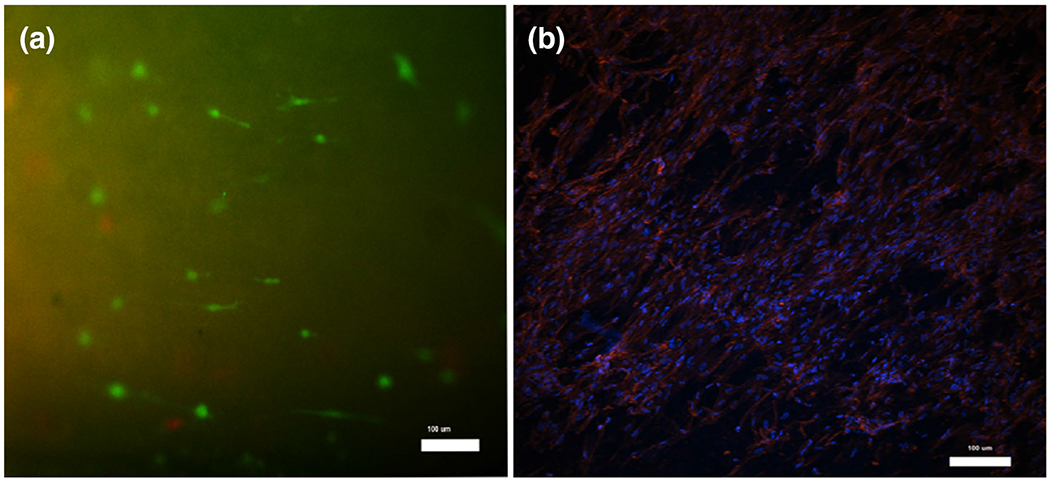
BSMC culture on electrospun PCUU scaffolds in vitro. Cells were stained using Live/Dead staining kit (a) and Rhodamine –Phalloidin (b) and imaged at 3 100 magnification after a 7-day culture period. High viability of BSMC was confirmed by the presence of 90.11% live (green) cells among the total cells in the scaffold (a) using imageJ. The Rhodamine-Phalloidin and DAPI results demonstrate F-actin fiber orientation in the well distributed and elongated, spindle-like bladder smooth muscle cells (b).
Morphology and Histological Analysis of Explanted PCUU Scaffolds
The control (Figs. 5b and Figs. 5e) and the sham (Figs. 5c and 5f) bladders both exhibited normal tissue organization displaying all three layers i.e. the urothelium, muscle and connective tissue layers. The sham rat bladder showed chronic inflammation (Fig. 5i) and disorganized collagen formation wheras the control bladder (Fig. 5h) indicated normal tissue organization. The control and sham rat bladders exhibited the presence of smooth muscle cells throughout the sections when stained for a-actin (Figs. 5k and 5l) and smoothelin (Figs. 5n and 5o), respectively.
FIGURE 5.

Histological sections of PCUU scaffold (a, d, g, j, m), control bladder (b, e, h, k, n) and sham BOO bladder (c, f, i, l, o) stained with H&E (a–c), Alizarin red (d–f), Masson’s Trichrome staining (g–i) α-actin antibody and DAPI (j–l), Smoothelin antibody and DAPI (m–o), PCUU scaffold with no primary antibody (p) at × 40 magnification. The orange colored arrows (a, d, g, j, and m) are pointing to the PCUU scaffold attached to the rat bladder in a frozen section of the experimental bladders.
By gross inspection, the intact PCUU scaffold was visible on the exterior and caliculi were present on the inner lumen of the augmented bladder upon harvest after 21 days of implantation. H&E stained sections of the explanted PCUU scaffold revealed infiltration of muscle cells and fibroblasts (Fig. 5a). The intense red color of Alizarin Red staining indicated that the bladder wall augmented with PCUU was infiltrated with calcium deposits (Fig. 5d), which was absent in the control and sham specimens (Figs. 5e and 5f). The masson’s trichrome staining revealed an acute inflammation in the PCUU explant indicated by the presence of nuclei (black) representing macrophages in the scaffold (Fig. 5g). The staining for α-actin and DAPI indicate presence of cells throughout the scaffold (Fig. 5j). However, infiltration of smooth muscle cells was limited to the surface of the scaffold in contact with bladder tissue as demonstrated by immuno-staining for smoothelin and DAPI (Fig. 5m).
Rat Voiding Behavior and Bladder Capacity Following Augmentation with PCUU
The mean voiding volumes of the control rats and the sham rats were 871.88 ± 166.14 μL (n = 3) and 526.9 ± 318.15 μL (n = 4), respectively. The mean voiding volume of PCUU augmented bladders remained similar on day 0 (639.43 ± 239.48 μL), day 1 (635.21 ± 390.55 μL) and day 7 (638.64 ± 413.33 μL) which then exhibited a increase on day 14 (815.65 ± 210.45 μL) and day 21 (968.75 ± 592.83 μL) (Table 1). The voiding frequency was 6 voids per hour on day 1, followed by a gradual decrease over the following 21 days. While the control rats did not void more than once every hour after gavaging, the sham rats voided approximately five times every hour. The time span for 8 voids was 8308 ± 5359 s on day 1, followed by a decrease on day 7 (7612 ± 700 s), leading to a gradual increase on day 14 (9973 ± 1453 s) and day 21 (10,130 ± 10,533 s) surpassing the time span of day 1. The control rats did not void more than 4 times 3 h after gavaging and the sham rat took 3963.625 ± 755.72 s for 8 voids (n = 2), with the remaining two sham rats not voiding for more than 6 times even after 2.5 h. Based, on the voiding time (time to void eight times) we could not come to any conclusions as the data was inconsistent. The maximum voiding volumes of control and sham rats were 1234 μL and 1414 μL respectively (Table 1). The maximum void measured (rat bladder capacity) for PCUU augmented bladders were similar on day 1 (1622 μL) and day 7 (1576 μL) followed by a decrease on day 14 (maximum: 1167 μL) and a subsequent increase (maximum: 1997 μL) on day 21 (Table 1).
TABLE 1.
Measurement of micturition for various samples tested.
| Sample | Number of specimens | Maximum Capacity (μL) | Average voiding volume (μL) |
|---|---|---|---|
| Control | 3 | 1234 | 871.88 ± 166.14 |
| Sham BOO | 4 | 1414 | 526.9 ± 318.15 |
| PCUU/Sham day 0 | 3 | 1371 | 639.43 ± 239.28 |
| PCUU day 1 | 3 | 1622 | 635.21 ± 390.55 |
| PCUU day 7 | 3 | 1576 | 638.4 ± 413.33 |
| PCUU day 14 | 2 | 1167 | 815.65 ± 210.45 |
| PCUU day 21 | 2 | 1997 | 968.75 ± 592.83 |
| Sham day 21 | 0 | N/A | N/A |
There are no statistical differences among voiding volumes.
DISCUSSION
The objectives of the present study were to characterize various elastomeric biomaterials for bladder tissue engineering applications in vitro and to examine the feasibility of an animal model of voiding dysfunction for evaluation of tissue engineering constructs. Since previous studies have demonstrated that both PGS-PCL and PEUU scaffolds supported cell proliferation and ECM production in vitro9,24and exhibited high extensibility9,23 we hypothesized that these scaffolds may provide a suitable biomaterial for bladder tissue engineering. However, the results of mechanical testing in the present study revealed that the maximum tension at failure for PGS-PCL was on average 0.079 N/mm (Fig. 1), an order of magnitude lower than that for the normal human bladder (0.2–0.4 N/mm).5 In addition, under similar tensile testing conditions, PEUU scaffolds displayed high stiffness under low forces (Fig. 1), unlike the bladder tissue, which exhibits a considerable toe region (large deformation under low stress) in its stress-strain curve.13 Thus, it was determined that electrospun PGS-PCL and PEUU were unsuitable for bladder tissue engineering purposes due to mechanical mismatch. In contrast, PCUU scaffolds exhibited a mechanical behavior similar to that of bladder tissue13; peak tension (0.43 ± 0.029 N/mm) comparable to native bladder tissue (0.2–0.4 N/mm); displaying relatively high compliance at low forces (Fig. 1) and high strength to withstand physiological bladder pressures19 corresponding to 100 kPa.
Although uniaxial tensile testing can provide insightful data for comparison between materials, it is not representative of physiological loading conditions in the urinary bladder, where loading occurs in all three dimensions.8 Since the urinary bladder wall can be considered an incompressible thin membrane material due to negligible thickness during loading, biaxial testing would more efficiently mimic the physiological loading.8 In the present study, the stretch ratios (deformed length divided by original length) and the areal strain of compliant PCUU scaffolds under equi-biaxial stress of 100 kPa (Fig. 2) were estimated to be in the same range as the biaxial stretch values of native bladder (~ 0.28).8 These values are in the same range as the biaxial stretch values of native bladder, which were reported to be 1.09 (X-axis) and 1.10 (Y-axis) at 100 kPa equi-biaxial stress.8 In addition, the results from these tests demonstrated that the anisotropic nature of the PCUU scaffolds was also similar to that of the urinary bladder under physiological conditions.19 Based on these findings, compliant PCUU scaffolds (B version) were selected for use in the remainder of this study for further characterization toward bladder tissue engineering applications.
The results of the present study provide evidence that PCUU scaffolds possess high porosity (Fig. 3) and cyto-compatibility (Fig. 4) indicating its ability to support growth of bladder cells to form a viable tissue. The SEM imaging results showed that the pore diameter in the PCUU scaffolds is between 5 and 20 μm across the scaffold and the fibers alignment allows for an increase in porosity under stretch (Fig. 3). Although this pore size is conducive for slender BSMC (2–5 μm diameter31) to infiltrate the scaffold under physiological loading conditions it is lower than the pore size (30–40 μm) typically necessary to promote angiogenesis.17 Thus, the current design of PCUU scaffolds may be unsuited for encouraging vascularization in vivo and will need to be modified. One method to increase pore size would be to increase the amount of culture media electro-sprayed onto the scaffold during the electrospinning processes to loosen up the fibers. In addition, an increase in mandrel translation speed leads to a decrease in fiber intersection density and the formation of a more open structure in PEUU scaffolds with greater pore sizes and % porosity.2 Thus, with some modifications to PCUU material synthesis we could eventually increase the porosity of the PCUU scaffolds to promote angiogenesis.
The in vitro cytocompatibility of PCUU scaffolds for BSMC were demonstrated by not only high viability (over 90%) (Fig. 4a), but also by the spindle-like cell morphology (Fig. 4b). Hence, our in vitro work achieved two major goals of a bladder tissue engineering scaffold: (1) we characterized the mechanical behavior of the PCUU material and demonstrated it was suitable for the bladder’s main functions to maintain low pressure during storage (indicated by its high compliance at low forces) (Fig. 1) and to withstand high pressures during voiding (indicated by its ability to withstand peak physiological bladder pressures) (Fig. 2) and (2) the PCUU scaffolds could support viability and morphology of the SMC to aid in tissue infiltration in vivo (Fig. 4).
Despite early promises of bladder tissue engineering,3 a recent report of unsuccessful clinical trials 12 suggests that the technology needs further improvement and evaluation through animal models of bladder dysfunction rather than in healthy animals.28 Thus, the present study made an attempt at creating a clinically informative study by implanting PCUU scaffolds onto bladders of BOO model rats. Animals with BOO exhibit symptoms that are typically characterized by high voiding pressure and low urine flow rate usually accompanied by small voided volume of urine.10 In the present study, both voiding volume and bladder capacity improved with time by implanting the PCUU and were comparable to those of control and sham rats (Table 1), indicating that some of the symptoms associated with BOO were alleviated. In addition, the results to date suggest that PCUU implantation lead to an increase in the survival of BOO rats (66.6% at 31 days, n = 3) compared to untreated BOO rats (0% survival at 31 days, n = 4). However, further studies to increase replicates are needed to confirm the enhancement of survival and improvement in bladder capacity after PCUU implantation.
Calculi formation is one of the common problems in animal studies when foreign objects such as tissue engineering constructs are implanted in the bladder.11,21,25 In the present study, calculi were present on the inner lumen of the augmented rat bladders upon harvest after 21 days of implantation (Fig. 5d), which were nearly absent in the control and sham specimens (Figs. 5e and 5f). The cause of stone formation on scaffolds in the bladder is only partially understood at this point. Previously, Seth and colleagues reported that the frequency and extent of bladder calculi formation on silk-based scaffolds decreased in relation to the increase in compliance of the scaffold.25 However, the most compliant silk scaffold in their study (maximum elongation of 197 ± 4%25) was not as extensible as our PCUU scaffold (maximum elongation 243 ± 26%), indicating that the mechanical properties alone may not be the determinant for calculi formation. It can be speculated that formation of calcium stones is triggered by the initial surgical insult to the bladder tissue and/or simply the presence of a foreign material, which cannot be avoided in bladder augmentation with engineered constructs. Thus, it may be necessary to take additional measures to inhibit calculi formation. For example, it has been shown that controlled release of disodiumethylenediaminetetraacetic acid (EDTA) from PLGA nanoparticle pellets placed periadventitially can lead to regression of calcification in the aorta.16 Similar anti-calcification approaches may be applicable to the bladder tissue engineering on calculi reduction.
Another important criterion for regeneration of the bladder is a proper repopulation of the implanted scaffolds with functional smooth muscle cells. The results of the present study provided evidence that, after 3 weeks of implantation, smoothelin-positive cell infiltration was only limited to near the edge of the PCUU construct (Figs. 5j and 5m). These findings are similar to those in previous studies.11,18 Jack et al. reported that after 4 weeks, acellular PLGA composite scaffolds that had been implanted in rat bladders exhibited scant SMC infiltration in the edges and no smooth muscle organization in the center.11 Similarly, Mauney et al. reported that after 3 weeks, a-actin and SM22α—positive cells were present only at the periphery of the silk-based scaffolds implanted in immunocompetent mice (CD1).18 A potential downside of the acellular scaffolds is the tendency to induce relatively higher inflammation and fibroblast ingrowth compared to cell seeded scaffolds, which could potentially reduce the time required for integration into the host bladder.30 Cell-seeded SIS grafts implanted subcutaneously in nude mice for 12 weeks exhibited layered urothelium and smooth muscle bundle formation,32 which were absent in acellular SIS grafts (control).30 Hence, future studies can build on the results obtained with acellular scaffolds and should focus the efforts on seeding scaffolds with pertinent cells prior to implantation in bladder dysfunction model animals.
The effect of SMCs on the mechanical properties of the PCUU scaffold was not analyzed in this study. However, since there was infiltration of SMCs only in the surface of PCUU scaffolds at the end of 3 weeks of implantation, we think that any significant change in mechanical and degradation properties would take longer time periods.
In summary, the present study characterized electrospun elastomeric scaffolds for bladder tissue engineering applications. Based on the results of in vitro mechanical characterization and cell culture studies, we concluded that the PCUU scaffolds were the most relevant scaffold for bladder tissue engineering among the ones we examined. For the first time, we used an established rat model of a bladder dysfunction (BOO) to evaluate the effect of augmentation with PCUU scaffolds on bladder voiding. Although the urinary stone formation problem needs to be addressed using various anti-calcification treatments, the knowledge gained in the present study should help improve the bladder tissue engineering technology to ultimately aide in treatment of bladder disorders.
ACKNOWLEDGMENTS
The authors thank Dr. Ali Khademhosseini and Dr. Shilpa Sant, Harvard University, for supplying us with the PGS-PCL scaffolds, Dr. Yi Hong, University of Pittsburgh, for supplying us with PEUU and PCUU scaffolds, Dr. Thuy Pham and Dr. Wei Sun, Georgia Institute of Technology for assistance with biaxial testing, Dr. George Wetzel, Clemson University for assistance with the Scanning electron microscope (SEM), and Mr. Chad McMahan, Clemson University, for assistance with histology. The funding for this research was provided by NIH 8P20GM103444.
REFERENCES
- 1.AdelÖw CAM, and Frey P. Synthetic hydrogel matrices for guided bladder tissue regeneration. Methods Mol. Med. 140:125–140, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Amoroso NJ, D’Amore A, Hong Y, Rivera CP, Sacks MS, and Wagner WR. Microstructural manipulation of electrospun scaffolds for specific bending stiffness for heart valve tissue engineering. Acta Biomater. 8(12):4268–4277, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atala A, Bauer SB, Soker S, Yoo JJ, and Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. The Lancet 367(9518):1241–1246, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Courtney T, Sacks MS, Stankus J, Guan J, and Wagner WR. Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials 27(19):3631–3638, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Dahms SE, Piechota HJ, Dahiya R, Lue TF, and Tanagho EA. Composition and biomechanical properties of the bladder acellular matrix graft: comparative analysis in rat, pig and human. Br. J. Urol. 82(3):411–419, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Eberli D, Filho LF, Atala A, and Yoo JJ. Composite scaffolds for the engineering of hollow organs and tissues. Methods 47(2):109–115, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Fujimoto KL, Tobita K, Merryman WD, Guan J, Momoi N, Stolz DB, et al. An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction. J. Am. Coll. Cardiol. 49(23):2292–2300, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gloeckner DC, Sacks MS, Fraser MO, Somogyi GT, de Groat WC, and Chancellor MB. Passive biaxial mechanical properties of the rat bladder wall after spinal cord injury. J. Urol. 167(5):2247–2252, 2002. [PubMed] [Google Scholar]
- 9.Hong Y, Guan J, Fujimoto KL, Hashizume R, Pelinescu AL, and Wagner WR. Tailoring the degradation kinetics of poly (ester carbonate urethane) urea thermoplastic elastomers for tissue engineering scaffolds. Biomaterials 31(15):4249–4258, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes FM, Hill HM, Wood CM, Edmondson AT, Dumas A, Foo WC, Oelsen JM, Rac G, and Purves JT. The NLRP3 inflammasome mediates inflammation produced by bladder outlet obstruction. J. Urol. 195:1598–1605, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jack GS, Zhang R, Lee M, Xu Y, Wu BM, and Rodriguez LV. Urinary bladder smooth muscle engineered from adipose stem cells and a three dimensional synthetic composite. Biomaterials 30(19):3259–3270, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joseph DB, Borer JG, De Filippo RE, Hodges SJ, and McLorie GA. Autologous cell seeded biodegradable scaffold for augmentation cystoplasty: phase II study in children and adolescents with spina bifida. J. Urol. 191(5):1389–1395, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Khurana I Excretory system. In: Essentials of Medical Physiology, edited by Pathak R, and Nasim S. Noida: Elsevier, 2008, pp. 339–340. [Google Scholar]
- 14.Kim JH, Lee HJ, and Song YS. Treatment of bladder dysfunction using stem cell or tissue engineering technique. Korean J. Urol. 55(4):228–238, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korossis S, Bolland F, Ingham E, Fisher J, Kearney J, and Southgate J. Tissue engineering of the urinary bladder: considering structure-function relationships and the role of mechanotransduction. Tissue Eng. 12(4):635–644, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Lei Y, Grover A, Sinha A, and Vyavahare N. Efficacy of reversal of aortic calcification by chelating agents. Calcif. Tissue Int. 93(5):426–435, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maddena LR, Mortisen DJ, Sussmana EM, Duprasc SK, Fugatec JA, Cuya JL, Haucha KD, Laflammea MA, Murry CE, and Ratner BD. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. PNAS 107(34):15211–15216, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mauney JR, Cannon GM, Lovett ML, Gong EM, Vizio DD, Gomez P III, Kaplan DL, Adama RM, and Estrada CR Jr. Evaluation of gel spun silk-based biomaterials in a murine model of bladder augmentation. Biomaterials 32:808–818, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagatomi J, Gloeckner DC, Chancellor M, de-Groat W, and Sacks M. Changes in the biaxial viscoelastic response of the urinary bladder following spinal cord injury. Ann. Biomed. Eng. 32(10):1409–1419, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Oberpenning F, Meng J, Yoo JJ, and Atala A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat. Biotechnol. 17(2):149– 155, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Piechota HJ, Gleason CA, Dahms SE, Dahiya R, Nunes LS, Lue TF, and Tanagho EA. Bladder acellular matrix graft: in vivo functional properties of the regenerated rat bladder. Urol. Res. 27:206–213, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sacks MS A method for planar biaxial mechanical testing that includes in-plane shear. ASME J. Biomech. Eng. 121(5):551–555, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Sant S, Hwang CM, Lee S, and Khademhosseini A. Hybrid PGS-PCL microfibrous scaffolds with improved mechanical and biological properties. J. Tissue Eng. Regen. Med. 5(4):283–291, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sant S, Iyer D, Gaharwar AK, Patel A, and Khademhosseini A. Effect of biodegradation and de novo matrix synthesis on the mechanical properties of valvular interstitial cell-seeded polyglycerol sebacate-polycaprolactone scaffolds. Acta Biomater. 9(4):5963–5973, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seth A, Chung YG, Gil ES, Tu D, Franck D, and Di Vizio D. The performance of silk scaffolds in a rat model of augmentation cystoplasty. Biomaterials 34(20):4758–4765, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sivaraman S, and Nagatomi J. Polymer-based scaffolds for urinary bladder tissue engineering. In: Polymers for Vascular and Urogenital Applications, edited by Shalaby SW, Burg KJ, and Shalaby W. Florida: CRC Press, 2012, pp. 175–200. [Google Scholar]
- 27.Sivaraman S, Ostendorff R, Fleishman B, and Nagatomi J. Tetronic®-based composite hydrogel scaffolds seeded with rat bladder smooth muscle cells for urinary bladder tissue engineering applications. J. Biomater. Sci. Polym. Ed. 26(3):196–210, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sloff M, Simaioforidis V, de Vries R, Oosterwijk E, and Feitz W. Tissue engineering of the bladder—reality or myth? A systematic review. J. Urol. 192(4):1035–1042, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Stankus JJ, Soletti L, Fujimoto K, Hong Y, Vorp DA, and Wagner WR. Fabrication of cell microintegrated blood vessel constructs through electrohydrodynamic atomization. Biomaterials 28(17):2738–2746, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoo JJ, Meng J, Oberpenning F, and Atala A. Bladder augmentation using allogenic bladder submucosa seeded with cells. Urology 51(2):221–225, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Zderic SA, Chacko S, Disanto ME, and Wein AJ. Voiding function: relevant anatomy, physiology, pharmocology and molecular aspects. In: Adult and Pediatric Urology, edited by Gillenwater JY, Grayhack JT, Howards SS, and Mitchell ME. Philadelphia: Lippincott Williams and Wilkins, 2007, pp. 1067–1068. [Google Scholar]
- 32.Zhang Y, Kropp BP, Ling HK, Cowan R, and Cheng EY. Bladder regeneration with cell-seeded small intestinal submucosa. Tissue Eng. 10:181–187, 2004. [DOI] [PubMed] [Google Scholar]


