Abstract
Objective
To estimate the seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies in health care personnel.
Methods
The Mayo Clinic Serology Screening Program was created to provide a voluntary, two-stage testing program for SARS-CoV-2 antibodies to health care personnel. The first stage used a dried blood spot screening test initiated on June 15, 2020. Those participants identified as reactive were advised to have confirmatory testing via a venipuncture. Venipuncture results through August 8, 2020, were considered. Consent and authorization for testing was required to participate in the screening program. This report, which was conducted under an institutional review board–approved protocol, only includes employees who have further authorized their records for use in research.
Results
A total of 81,113 health care personnel were eligible for the program, and of these 29,606 participated in the screening program. A total of 4284 (14.5%) of the dried blood spot test results were “reactive” and warranted confirmatory testing. Confirmatory testing was completed on 4094 (95.6%) of the screen reactive with an overall seroprevalence rate of 0.60% (95% CI, 0.52% to 0.69%). Significant variation in seroprevalence was observed by region of the country and age group.
Conclusion
The seroprevalence for SARS-CoV-2 antibodies through August 8, 2020, was found to be lower than previously reported in other health care organizations. There was an observation that seroprevalence may be associated with community disease burden.
Abbreviations and Acronyms: DBS, dried blood spot; HCP, health care personnel
The coronavirus disease 2019 (COVID-19) pandemic has placed unprecedented pressures on health care organizations and citizens around the world. Mayo Clinic, which spans clinical operations in Arizona, Florida, Minnesota, Iowa, and Wisconsin, like many other health care organizations, quickly adapted to the emerging demands to provide high-quality care while working to educate and protect the workforce. Personal protective equipment strategies specific to the patient, environment, and care being provided were implemented to protect the workforce. In addition, to reduce the likelihood of disease transmission across Mayo Clinic, a large percentage of non–patient facing employees were transitioned to remote work.
Starting in June 2020, with endorsement from Mayo Clinic leadership, and with funding made available through contributions to the organization, Mayo Clinic designed, initiated, and completed an enterprise-wide seroprevalence study for detection of antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among Mayo Clinic health care personnel (HCP), including employees. Participation in this study by Mayo Clinic HCP was entirely voluntary, and this brief report summarizes the findings of this point prevalence of SARS-CoV-2 seroprevalence.
Methods
Design of Testing Protocols
The overarching goal of the Mayo Clinic Serology Screening Program was to provide a single timepoint, seroprevalence estimate for the presence of antibodies against SARS-CoV-2 among our staff. The timing of the screening program was tied to the transitional period that saw the resumption of elective clinical operations and relaxation of shelter-in-place restrictions across the country. Health care personnel at Mayo Clinic were to be tested in quick succession, on a volunteer basis, across geographically diverse regions of the country, while maintaining physical distancing expectations, shelter-in-place restrictions, infection control precautions, and remote work locations. Large-scale testing using venipuncture-collected serum specimens was deemed infeasible due to the clinical practice responsibilities for emergent and routine services and the resources (eg, time, phlebotomists, and physical space) required to obtain samples from more than 80,000 potential participants. Thus, a two-stage testing approach was designed and implemented with a robust internal communications campaign to ensure awareness of the opportunity for HCP serology testing.
The first stage of serologic testing used a dried blood spot (DBS) collection method. The advantage of DBS specimens was that they could be obtained by finger stick from a large-scale population in temporary collection rooms across Mayo Clinic sites. These temporary collection rooms were identified by individual site leadership and typically consisted of conference rooms and other large, open spaces that were temporarily vacant at the time. Trained phlebotomists supervised the general collection, but individual participants comfortable with self-administration of the finger stick were allowed to self-collect the sample under supervision of a phlebotomist. This model allowed for fewer phlebotomy resources as multiple participants could be observed by a single phlebotomist. As a part of the registration process (described below), participants were required to designate a testing site and travel to the site, if needed, to complete the testing.
After collection of the DBS sample, the cards were air dried for at least 1 hour and subsequently bulk shipped overnight to the central testing laboratory in Rochester, Minnesota. Results of the DBS test were reported as either “negative” or “reactive” (described below). Whereas a result of negative concluded HCP participation in the study, individuals with a reactive result were advised to complete confirmatory testing using an alternative serologic testing platform on a venipuncture-drawn serum sample. Results from the DBS test were reported in the participant’s medical records and available on their Mayo Clinic patient portal, which also provided the means to schedule the follow-up confirmatory test if needed. Blood draws for confirmatory testing were processed on site, or if necessary, shipped to Rochester, Minnesota, for processing.
An important aspect of the testing plan was occupational follow-up based on the serology results. Although most seropositive individuals are past their communicable period, there was a need to develop a follow-up protocol to interpret results in the context of occupational health and safety. Participants who received a confirmed positive anti–SARS-CoV-2 venous antibody result were directed to complete an electronic occupational health screening evaluation through an internal website. The online evaluation was used to determine if the HCP had concern for active or recent infection by evaluation of recent symptoms and exposure. When indicated, follow-up testing with a molecular test was coordinated for the HCP.
SARS-CoV-2 Serologic Testing Details
Dried Blood Spot Collection and Extraction
The Euroimmun (Lubeck, Germany) enzyme-linked immunosorbent assay (ELISA) detects immunoglobulin G (IgG)–class antibodies to the SARS-CoV-2 spike subunit 1 (S1) and has US Food and Drug Administration Emergency Use Authorization for testing of serum. This assay was verified and implemented for testing of patient sera, without deviation from the manufacturer instructions for use, in our Clinical Laboratory Improvement Amendments–certified high-complexity laboratory. Subsequently, the Euroimmun IgG ELISA was validated for evaluation of blood dried on filter paper. The DBS sample collection was performed by finger stick using a single-use safety lancet to capture capillary blood onto a Whatman ProteinSaver 903 collection card. The collection card was then allowed to dry for a minimum of 1 hour before transfer to the laboratory at room temperature. Subsequently, a single 3-mm punch from the collection card was made and placed into a 96-well round bottom polypropylene plate. Next, 150 μL of elution buffer (phosphate-buffered saline with 2% bovine serum albumin and 0.5% Tween) was added to each well, and the microtiter plate was covered with an aluminum seal and incubated for 2 hours at 2°C to 8°C while on a rotator set to 200 rpm. Then, 150 μL of this extract was transferred to labeled polystyrene test tubes, to which 150 μL of phosphate-buffered saline was added. Next, 100 μL of the DBS extract was added, without dilution, to S1-coated wells in a 96-well plate. All subsequent processes, steps, and index value determinations were performed as described previously for serum samples.1 Based on receiver-operator curve analysis during validation of the DBS assay on the Euroimmun anti–SARS-CoV-2 IgG ELISA, sample index values of <0.71 and ≥0.71 were used to report negative or reactive results, respectively. Participants with a negative DBS result did not require additional testing, whereas reactive results prompted the order of confirmatory testing on venipuncture-drawn serum. Following the evaluation of 20,730 samples, no confirmed positive cases were found with index values between 0.71 and 0.75. Based on this finding, to reduce the number of venipuncture blood draws, the reactive result threshold was increased to ≥0.75 for the remainder of the study period.
High-Throughput Anti–SARS-CoV-2 Serologic Assays for Confirmatory Antibody Testing
Confirmatory testing of venipuncture-collected serum samples from initially DBS-reactive HCP was performed by the Roche Diagnostics Elecsys Anti–SARS-CoV-2 Total Antibody electrochemiluminescence immunoassay (ECLIA; Indianapolis, IN) at all Mayo Clinic enterprise sites, with the exception of the Mayo Clinic campus in Arizona, where confirmatory testing on serum was performed using the Ortho-Clinical Diagnostics anti–SARS-CoV-2 IgG chemiluminescence immunoassay (Rochester, NY). Both the Roche Diagnostics and Ortho-Clinical Diagnostics assays have US Food and Drug Administration Emergency Use Authorization for testing of sera and were used without deviation from the manufacturer supplied information for use as described previously.1 The Roche Diagnostics ECLIA assay detects total antibodies, without differentiation between immunoglobulin classes, to a recombinant nucleocapsid protein, whereas the Ortho-Clinical Diagnostics chemiluminescence immunoassay detects IgG-class antibodies against S1. The threshold for a positive result for both assays is a signal-to-cutoff ratio of ≥1.0.
Patient Authorization for Testing
The Mayo Clinic Serology Screening Program was designed to be voluntary at the onset for all employees and eligible HCP. The costs associated with testing were covered by benefactor support to Mayo Clinic. All costs associated with specimen collection and testing were provided at no charge to participants. To minimize administrative burden and ensure compliance with federal and state requirements, an online authorization tool was created to expedite enrollment and generation of confirmatory test orders. The Web-based registration included language indicating authorization for testing and consent for reporting of the results. Importantly, the consent process generated the clinical order for the confirmatory test without requiring a designated physician to authorize the order. The collection of DBS tests commenced on June 15, 2020.
Selection of Records for Research
This analysis was conducted by means of an electronic review of the health care record after approval of the Mayo Clinic Institutional Review Board. Only records authorized for use in research were included in the analysis to ensure compliance with the Minnesota research authorization statute. Given that Mayo Clinic maintains a single medical record across the entire practice, the research authorization was applied without discrimination to site. To ensure participants had access to testing at Mayo Clinic sites, only HCP with a home-state location in Arizona, Florida, Minnesota, Iowa, or Wisconsin were included in this report.
Records were analyzed in subgroups of HCP who participated in the Mayo Clinic Serology Screening Program or those who received testing for other indications. A specific laboratory code was used to identify the DBS test in the HCP Mayo Clinic Serology Screening Program. This allowed for the identification of all HCP who participated in the screening program.
To address the objective of establishing a point prevalence estimate for seroprevalence of SARS-CoV-2, and to study how prior test results may have impacted participation in the screening program, all test results reported in the medical record from the start of the COVID-19 pandemic through August 8, 2020, were included.
Statistical Considerations
The Mayo Clinic Serology Screening Program used a cascade of two tests. A confirmatory venipuncture test was required following a reactive DBS test to determine the final seroprevalence. For statistical reporting of the seroprevalence, the confirmatory venipuncture was required to have been completed within 30 days of the DBS test and no later than August 8, 2020. For participants who did not complete the confirmatory testing after a reactive DBS test, the serology result was considered negative for the confirmatory testing in the analysis due to the selection of a highly sensitive threshold for the DBS screen.
A second analysis was conducted using all COVID-19 test results in the medical record through August 8, 2020. In this analysis, an overall positivity calculation was performed for HCP who had either a molecular (reverse transcription polymerase chain reaction [RT-PCR]) or serologic test that resulted as positive at any time before or on August 8, 2020.
Data are summarized using standard summary statistics. Limited data including demographic characteristics from each HCP was used in the analysis. Information including primary work function, work unit, and any other data that could lead to identification of the individual was not extracted from the participant’s record. The variability in prevalence estimates are summarized using 95% CIs without finite population correction. Although the sampling fraction in some sites was such that more precise estimates could be obtained by finite population correction, summaries assuming a simple random sample were used to provide a conservative estimate of the error margin. χ2 tests were used to test if there were factors associated with engagement in the Mayo Clinic Serology Screening Program. For these tests, two-sided P values less than 0.05 were considered statistically significant. An exploratory analysis examined the relationship of community-reported COVID-19 cases by health referral regions through August 8, 2020. The Pearson correlation coefficient was used to describe the association of community cases with estimated seroprevalence. The health referral regions were determined by Federal Information Processing System county codes. The mapping of Federal Information Processing System codes to health referral regions is provided as a supplement. Community cases of COVID-19 were obtained from the New York Times public repository.2 Coronavirus disease 2019 counts were indexed by the total population of the health referral region. Census counts obtained from USA Facts.3 Statistical analysis was conducted using R version 3.6.2.
Results
A total of 81,113 HCP were eligible for this study and had previously authorized their records for research purposes. The mean (SD) age of eligible HCP was 44.2 (13.0) years, and 71.5% (n=58,024) were female. Of those eligible, 29,606 participated in the Mayo Clinic Serology Screening Program. Supplemental Figure 1 summarizes the participation rates by the state of residence, age, and sex. The percentage of females participating in the Mayo Clinic Serology Screening Program was higher than those that did not (75.7% [n=22,414 of 29,606] vs 69.1% [n=35,610 of 51,507]; P<.001) whereas the age participating HCP was lower (43.6 [12.0] years vs 44.6 [13.5] years; P<.001). The participation rate in the Mayo Clinic Serology Screening Program differed based on prior RT-PCR test results (P<.001), with an increase in the number of participants with prior RT-PCR testing engaging in the Mayo Clinic Serology Screening Program (20.2% [5994 of 29,606] vs 18.1% [9326 of 51,507]).
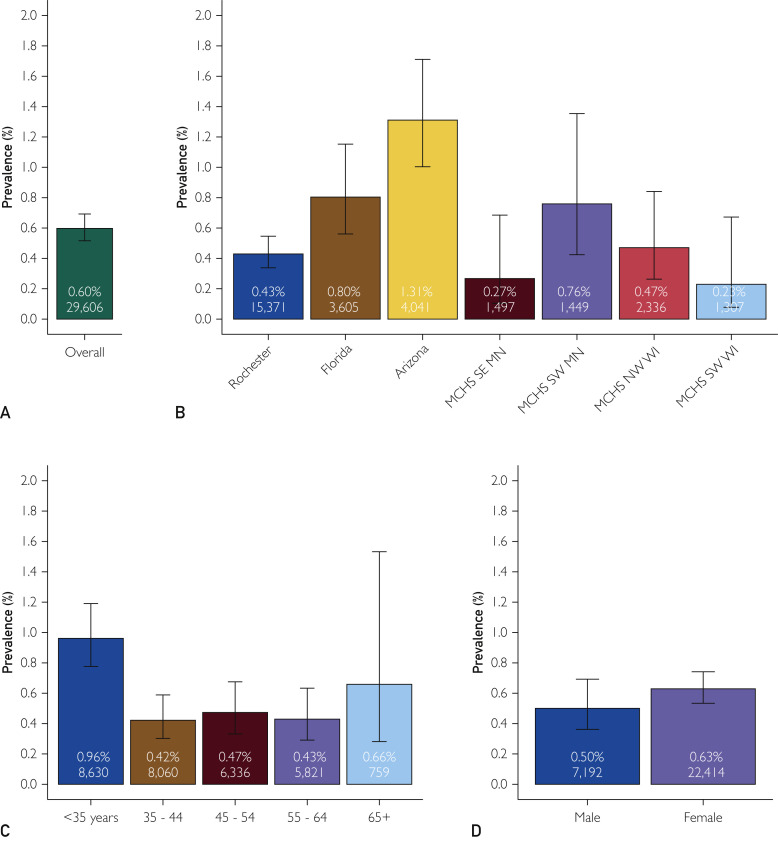
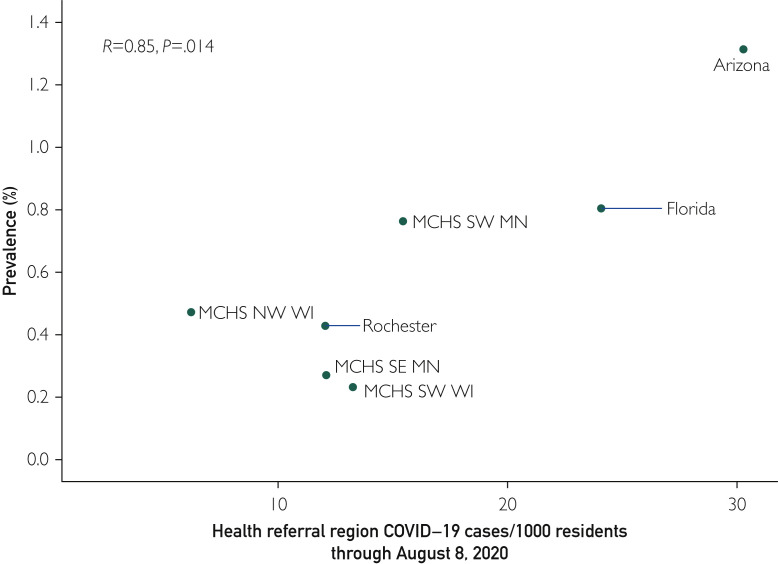
A total of 4284 (N=29606,14.5%) of the DBS test results were reactive and warranted confirmatory venous serology testing. Confirmatory testing was completed on 4094 (95.6%) of the screen reactive participants. Figure 1 presents the comprehensive confirmatory results as well as selected subgroups available for analysis. The overall seroprevalence rate was 0.60% (177 of 29,606, 95% CI, 0.52% to 0.69%). Significant variation in the seropositive prevalence was observed over the test site regions encompassing the Mayo Clinic enterprise (P<.001). The regions with higher disease activity early in the pandemic (eg, Florida and Arizona) were among the test sites with the highest seroprevalence (Figure 2 ). The seroprevalence was not found to be statistically different by sex (P=0.22), but it differed statistically by age group (P<.001) (Figure 1).
Figure 1.
Estimated seroprevalence overall and by selected entral testing laboratory in Rochester stratification factors. Estimated seroprevalence is shown as determined by the Roche Diagnostics total antibody test (except for Arizona which used the Ortho-Clinical Diagnostics immunoglobulin G [IgG] antibody test). (A) The overall prevalence of the 29,606 health care personnel (HCP) studied. (B) Results are shown by test region. The test region was the location at which the HCP submitted the dried blood spot specimen and may not reflect the primary work location. (C,D) Results are broken down by age groups and sex, respectively. Error bars are 95% CIs. Percentages reported in each bar show the estimated seroprevalence. For each category, the total sample size is also given. MCHS, Mayo Clinic Health System; MN, Minnesota; NW, northwest; SE, southeast; SW, southwest; WI, Wisconsin.
Figure 2.
Association of community coronavirus disease 2019 (COVID-19) cases and estimated seroprevalence. Shown are the cumulative numbers of positive test results by health referral region through August 8, 2020. The association of cumulative test results and seroprevalence is measured by the Pearson correlation coefficient. MCHS, Mayo Clinic Health System; NW MN, northwest; SE, southeast; SW, southwest.
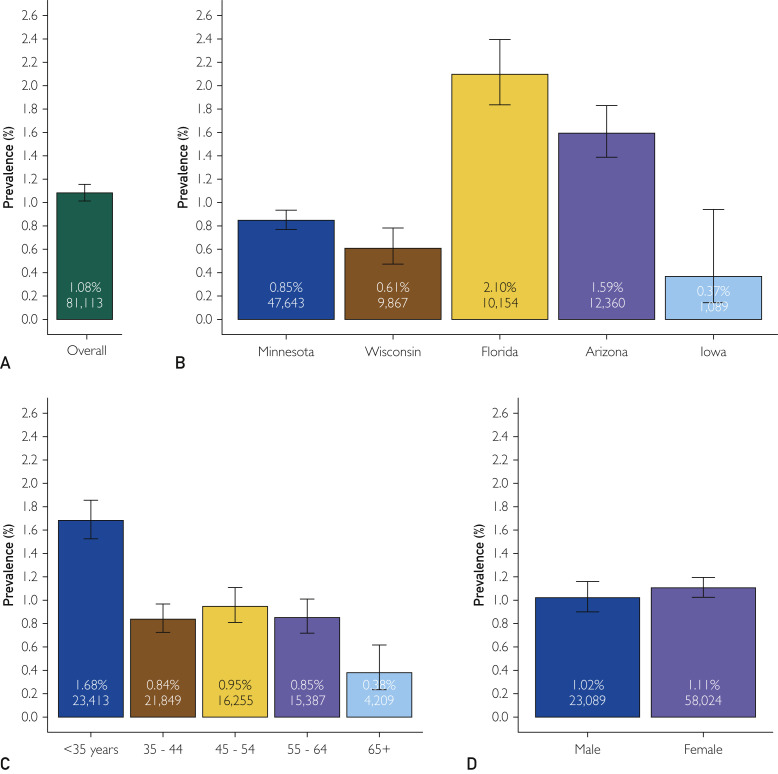
To examine the data more fully, all SARS-CoV-2 molecular and serologic test results were reviewed. An additional 9780 HCP had at least one SARS-CoV-2 test performed, with the majority (n=9326) having at least one molecular test result. In total, 48.6% (39,386 of 81,113) of HCP had at some point been tested for SARS-CoV-2 by either molecular or serology by August 8, 2020. In Figure 3 , the overall prevalence of any positive test result for SARS-CoV-2 infection is presented. The estimated prevalence for any past or current infection was 1.08% (95% CI, 1.01% to 1.16%). Consistent with the early association with community disease burden, results varied by state (P<.001) with Florida and Arizona remaining as the regions with the highest prevalence. Unlike Figure 1 where the geographic region was coded to reflect the test location for the DBS test, these results group HCP based on their home state location. Across the enterprise, prevalence was higher among younger HCP (P<.001) and did not differ by sex (P=.30).
Figure 3.
Estimated prevalence for any positive coronavirus disease 2019 (COVID-19) test. This figure reports the estimated prevalence of positive COVID-19 tests originating from either molecular or serum-based serologic tests. (A) Shown is the overall prevalence of the 81,113 health care personnel (HCP) studied. (B) The results are broken down by the participant’s home state. (C,D) Shows results broken down by age groups and sex, respectively. Error bars are 95% CIs. Percentages reported in each bar are the estimated seroprevalence. For each category, the total sample size is also given.
Discussion
The seroprevalence of SARS-CoV-2 antibodies was estimated to be 0.60% for the entire Mayo Clinic enterprise through August 8, 2020. There was significant geographic variation, with areas undergoing greater community disease transmission and burden being associated with higher seroprevalence among HCP. Notably, in the broader analysis that observed all prior SARS-CoV-2 testing, including RT-PCR and prior serologic test results, the estimated prevalence for the enterprise was higher at 1.08%.
The associated trend of seroprevalence with geographic location in the data was to be expected. The Arizona test sites were experiencing the highest COVID-19 case rates across the Mayo Clinic enterprise during the period of the Mayo Clinic Serology Screening Program, consistent with a surge in community transmission. The Mayo Clinic campus in Florida is located in a state that consistently had some of the highest number of community cases in the country during the study period. The association with age group showed younger HCP to be more likely to have prior infection. Although the available records for this report did not include location of suspected exposure (community, household, occupational), the trend in increased seroprevalence in younger HCP is likely multifactorial. Higher rates of asymptomatic infection in younger individuals may contribute to more unrecognized exposures to peers. Other possible factors include potential lower adherence rates for recommended physical distancing practices and/or personal protective equipment, as well as a higher likelihood of interactions in the community during this period.
In the primary seroprevalence results, there was notable discordance between the percentage of reactive DBS test results and those positive by the confirmatory test (ie, 14.5% vs. <1%, respectively). The DBS assay threshold for determination of reactivity was set low to provide high sensitivity (>98%), at the cost of assay specificity. One artifact of this test cascade was that reactive DBS test results may have been considered as a positive result by many HCP based on questions that were received by the operations team throughout the duration of the screening program. These questions occurred despite the operations team providing numerous handouts, signage, video recorded guidance, and more general communications developed to guide the interpretation of the results. Given this confusion, it was presumed that HCP with reactive results who did not provide a venipuncture-drawn serum sample for confirmatory testing abstained either because they had prior test results available (eg, prior positive RT-PCR test) or did not appreciate the role of confirmatory testing (eg, the HCP interpreted the DBS result as a positive test result and did not pursue further testing). The primary analysis also assumed that those who did not complete the testing cascade were antibody-negative. Based on test validation studies for the DBS assay and selection of the threshold value by which reactive or negative qualitative results were determined, it is highly improbable that the 190 HCP who did not complete the necessary confirmatory test were truly SARS-CoV-2 antibody–positive. To address this limitation, the study extracted all previously performed SARS-CoV-2 RT-PCR or serologic test results for the HCP, and associated prior testing with engagement in the Mayo Clinic Serology Screening Program. Although the overall positivity rate of prior testing was found to be higher, the estimated prevalence was still approximately 1%. In the unlikely occasion that all 190 were seropositive, the seroprevalence would only be expected to be as high as 1.24%.
Seroprevalence estimates from other large health care systems differ from what we observed in our institution. A 13.7% seroprevalence rate was reported in health care workers in New York City around the same time that the Mayo Clinic study was conducted.4 Similarly, a study of 3248 health care workers from 13 academic medical centers estimated the seroprevalence at 6%.5 Outside the health care settings, regional clusters of disease outbreaks resulted in seroprevalence estimates from 3.8% to more than 10%.6 The study by Bejema et al7 quantified the regional, age, sex, and temporal trends across the United States. This study, while cross-sectional and not designed to provide a national estimate of the seroprevalence, noted trends similar to our study with respect to region- and age-specific differences in seroprevalence. Our estimate of the prevalence of SARS-CoV-2 exposure in the Mayo Clinic Serology Screening Program was markedly lower even when all molecular test results were included in the prevalence estimate. There are a number of possible explanations for this. At the time of the study, there were limited outbreaks of SARS-CoV-2 in the Midwest and HCP from Minnesota and Wisconsin locations may have had lower overall community-exposure risk. Mayo Clinic also adopted precautions including universal masking requirements for HCP early in the pandemic which may have contributed to the reduced prevalence of past infection in all HCP, including those in Florida and Arizona where community transmission was increased during the study period.8
The presence of anti-spike IgG antibodies in a person could have important implications for vaccination policies. Early in the pandemic, there was considerable uncertainty with respect to the association of seroprevalence to longer-term immunity.6 More recently, the Oxford University Hospitals Staff Testing Group reported a marked reduction in risk for subsequent PCR–confirmed infection in the individuals that were seropositive.9
Study Limitations
There are several key limitations to this analysis. First, as noted above, the two-stage testing approach required collection of a new sample for all initially DBS reactive HCP, and 190 individuals did not complete the necessary confirmatory testing. Another key limitation is the potential bias in participation. Roughly one-third of the employed HCP across Mayo Clinic participated in this screening program. Participation was voluntary, which may have resulted in self-selection bias among the HCP. However, it is assumed that individuals with prior known exposure or infection confirmed by molecular testing would be more likely to participate in serology screening to identify presence of antibody response. Therefore, it is assumed that a systematic/random selection of HCP would result in lower seroprevalence compared with the results of this study. Further, the analysis was restricted to HCP with medical records authorized by the individuals for research purposes. Although testing outside of Mayo Clinic sites was available in several regions, few HCP have reported positive molecular test results performed elsewhere to occupational health. Thus, it would appear unlikely that unreported test results would strongly influence our findings given the broad access to testing available at Mayo Clinic. We further attempted to minimize the impact of access to testing by limiting the analysis to HCP with home states of Arizona, Florida, Minnesota, Wisconsin, and Iowa. This removed some HCP who worked remotely in non–direct patient care roles before and during the COVID-19 pandemic.
Conclusion
The overall seroprevalence rate of antibodies to SARS-CoV-2 in HCP was found to be low through early August 2020 across the Mayo Clinic enterprise. These results, which largely span the first 6 months of the pandemic, do suggest that community prevalence influences the seroprevalence of health care workers despite personal protective equipment use in the workplace. Thus, further testing in the future would be warranted to observe how the seroprevalence changes over time, particularly as the community spread of the virus intensified in during winter of 2020–2021.
Acknowledgments
Funding for the Mayo Clinic Serology Screening Program for Healthcare Personnel was made possible through the generous support of benefactors to Mayo Clinic. Drs Carter, Theel, and Breecher are co-first authors.
Footnotes
Potential Competing Interests: The authors report no potential competing interests.
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Theel E.S., Harring J., Hilgart H., Granger D. Performance characteristics of four high-throughput immunoassays for detection of IgG antibodies against SARS-CoV-2. J Clin Microbiol. 2020;58(8) doi: 10.1128/JCM.01243-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.New York Times public repository. https://github.com/nytimes/covid-19-data
- 3.USA Facts Our nation, in numbers. https://usafacts.org/
- 4.Moscola J., Sembajwe G., Jarrett M. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City Area. JAMA. 2020;324(9):893–895. doi: 10.1001/jama.2020.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Self W.H., Tenforde M.W., Stubblefield W.B. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network — 13 academic medical centers, April–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(35):1221–1226. doi: 10.15585/mmwr.mm6935e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckerle I., Meyer B. SARS-CoV-2 seroprevalence in COVID-19 hotspots. Lancet. 2020;396(10250):514–515. doi: 10.1016/S0140-6736(20)31482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bajema K.L., Wiegand R.E., Cuffe K. Estimated SARS-CoV-2 seroprevalence in the US as of September 2020. JAMA Intern Med. 2021;181(4):450–460. doi: 10.1001/jamainternmed.2020.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parkulo M.A., Brinker T.M., Bosch W., Palaj A., Deruyter M.L. Risk of SARS-CoV-2 transmission among coworkers in a surgical environment. Mayo Clin Proc. 2021;96(1):152–155. doi: 10.1016/j.mayocp.2020.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lumley S.F., O’Donnell D., Stoesser N.E. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384(6):533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.