Abstract
Purpose: GI-4000, a series of recombinant yeast expressing four different mutated RAS proteins, was evaluated in subjects with resected ras-mutated pancreas cancer.
Methods: Subjects (n = 176) received GI-4000 or placebo plus gemcitabine. Subjects' tumors were genotyped to identify which matched GI-4000 product to administer. Immune responses were measured by interferon-γ (IFNγ) ELISpot assay and by regulatory T cell (Treg) frequencies on treatment. Pretreatment plasma was retrospectively analyzed by matrix-assisted laser desorption/ionization-time-of-flight (MALDI-ToF) mass spectrometry for proteomic signatures predictive of GI-4000 responsiveness.
Results: GI-4000 was well tolerated, with comparable safety findings between treatment groups. The GI-4000 group showed a similar pattern of median recurrence-free and overall survival (OS) compared with placebo. For the prospectively defined and stratified R1 resection subgroup, there was a trend in 1 year OS (72% vs. 56%), an improvement in OS (523.5 vs. 443.5 days [hazard ratio (HR) = 1.06 [confidence interval (CI): 0.53–2.13], p = 0.872), and increased frequency of immune responders (40% vs. 8%; p = 0.062) for GI-4000 versus placebo and a 159-day improvement in OS for R1 GI-4000 immune responders versus placebo (p = 0.810). For R0 resection subjects, no increases in IFNγ responses in GI-4000–treated subjects were observed. A higher frequency of R0/R1 subjects with a reduction in Tregs (CD4+/CD45RA+/Foxp3low) was observed in GI-4000–treated subjects versus placebo (p = 0.033). A proteomic signature was identified that predicted response to GI-4000/gemcitabine regardless of resection status.
Conclusion: These results justify continued investigation of GI-4000 in studies stratified for likely responders or in combination with immune check-point inhibitors or other immunomodulators, which may provide optimal reactivation of antitumor immunity.
ClinicalTrials.gov Number: NCT00300950.
Keywords: K-ras, pancreas cancer, T cell response, clinical trial, immunotherapy
Introduction
The ras oncogene and its RAS protein gene product contain the most common oncogene-related mutations in human cancer, with 90% of pancreas cancers harboring mutant RAS proteins.1,2 Mutations in the ras oncogene occur in conserved locations, specifically codons 12, 13, and 61,3 and the number of mutations that can occur is limited to a few predominant amino acid substitutions. RAS oncoproteins are theoretically ideal targets for cancer immunotherapy because aberrant signaling through RAS contributes to uncontrolled cell proliferation and tumorigenesis.
Cancer immunotherapies have employed many strategies to generate immune responses4–10 including cellular immunotherapies, which are showing much promise in advanced hematological cancers11,12 and immune check-point inhibitors, which have substantial activity in a number of solid tumors including melanoma,13 nonsmall cell lung cancer (NSCLC),14 and squamous cell head and neck cancers.15,16 In the study described here, our immunotherapeutic approach is based on the use of heat-killed recombinant Saccharomyces cerevisiae yeast as vectors, which are engineered to express target protein antigens. These yeast cells can activate dendritic cells and generate T cell cytotoxicity against target cells expressing viral and cancer antigens.17–23
The GI-4000 product series consists of four different yeast-based products that target the seven most common ras mutations at codons 12 and 61, all of which result in constitutive activation of RAS. Because of the central role for RAS activation in tumor proliferation, targeted destruction of cells harboring mutant RAS proteins could result in therapeutic benefit in human cancers. A phase 1 study in patients with pancreas and colorectal cancer indicated that GI-4000 was safe, well tolerated, and immunogenic.24 A phase 2b study in NSCLC patients also indicated that GI-4000 was well tolerated, and appeared to confer an overall survival (OS) benefit as compared with historical controls.25 Here we report the results of a randomized prospective trial of adjuvant gemcitabine versus gemcitabine plus GI-4000 in patients with resected pancreas cancer. The primary end-point was improvement in recurrence-free survival. Exploratory proteomic analysis was performed retrospectively to investigate signatures that might predict responsiveness to GI-4000.
Methods
Study oversight
The study protocol was approved by institutional review boards at each trial site. All patients gave written informed consent.
Study design
This study was a randomized placebo-controlled double-blind adjuvant trial conducted at 27 investigational sites in the United States and 5 international sites in India and Bulgaria. After screening and informed consent, tumor tissue from surgical resection specimens was subjected to ras genomic sequencing. Subjects with mutations at either codon 12 or 61 positions represented in one of the GI-4000 products were eligible for study enrollment.
Objectives
The primary objective of the study was to evaluate an improvement in recurrence-free survival with GI-4000 treatment. Key secondary objectives were to evaluate OS, safety, and immunogenicity.
Variables
Demographic and baseline characteristics included age, gender, ethnic origin, time since diagnosis, tumor type, stage and grade, tumor biomarker levels, and ras gene mutations.
Interventions
The study drug consisted of four different yeast-based products targeting the four most common ras mutations at codon 12 and the three most common ras mutations at codon 61 (GI-4014: G12V, Q61L, Q61R; GI-4015: G12C, Q61L, Q61R; GI-4016: G12D, Q61L, Q61R; GI-4020: G12R, Q61L, Q61H). Each subject received only the specific product containing the mutation identified in his or her tumor. The yeast strains were engineered to express the K-ras mutation insert sequences as previously described.21
The study population consisted of patients with resected pancreas cancer who had a product-related mutation in ras and an R0 or R1 resection by pancreaticoduodenectomy or pylorus-preserving pancreaticoduodenectomy procedure. An R0 resection was defined as no microscopic residual tumor at the resection margin. An R1 resection was defined as residual microscopic but not gross evidence of tumor at the resection margin. After enrollment, subjects were randomized in a 1:1 ratio to either GI-4000 or placebo, both combined with gemcitabine. It should be noted that adjuvant gemcitabine monotherapy was used as the control because at the time the trial was designed and recruited, neither recent data from ESPAC-4 nor data comparing gemcitabine with FOLFIRINOX were available, making gemcitabine monotherapy the standard of care. Randomization was prospectively stratified based on resection status (R0/R1). Subjects were dosed subcutaneously with 40 yeast units (YU; 1 YU = 107 yeast cells) GI-4000 or with placebo (saline) for three weekly doses (0.5 mL/10 YU to each of four injection sites), starting 21 to 35 days after resection. Gemcitabine 1000 mg/m2 intravenous infusion was started on study Day 24. Monthly doses of GI-4000 or placebo were administered after initiation of gemcitabine to coincide with monthly chemotherapy holidays. Administration of gemcitabine proceeded until six monthly cycles were completed, intolerance occurred, study withdrawal, disease progression, or death. Administration of study drug proceeded until study withdrawal, disease recurrence, death, or completion of 60 months of therapy. A schematic of dosing for GI-4000 and gemcitabine is given in Table 1.
Table 1.
Dosing Schedule and Immune Sampling Schedule
| Day | 1 | 8 | 15 | 24 | 31 | 38 | 44 | 52 | 59 | 66 | 72 | 80 | 87 | 94 | 100 | 108 | 115 | 122 | 128 | 136 | 143 | 150 | 156 | 164 | 171 | 178 | 184 | 212 | 240 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GEM | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||||||
| GI-4000 | x | x | x | x | x | x | x | x | x | x | x | Monthly | ||||||||||||||||||
| Immune sample | x | x | x | x | x | x | x | x | Quarterly Thereafter |
Subjects were followed for up to 60 months after randomization and thereafter rolled into a long-term safety and outcomes protocol with an intended follow-up period of up to 15 years from treatment initiation.
Tumor tissue sequencing
Cellular genomic DNA was extracted from biopsy material and analyzed to identify ras mutations as previously described.24
Immunology analyses
Analyses were performed on samples blinded to treatment. Peripheral blood mononuclear cells (PBMCs) were collected and cryopreserved until use. Testing was performed on samples from subjects enrolled at sites in the United States only. Interferon-γ (IFNγ) ELISpot assays were performed as previously described.25 Immunophenotyping by flow cytometry evaluated the frequency of regulatory T cell (Treg) fractions,26 using PBMCs from baseline and Day 15 or Day 24 time points.
Exploratory proteomic analysis
Baseline plasma samples were retrospectively analyzed by matrix-assisted laser desorption/ionization (MALDI) time-of-flight (ToF) mass spectrometry.
Statistical methods
A Bayesian statistical approach was used to analyze efficacy on a quarterly basis using time to recurrence as the primary efficacy end-point and time to mortality as a key secondary efficacy end-point. Enrollment was expanded beyond the originally planned 100 patients based on the probability of improved efficacy for time to recurrence of <0.95 and >0.70 (this range of probabilities represents a strong trend, i.e., not yet definitive) and if an estimate of increased time to recurrence and mortality exceeded 2 months during enrollment. The efficacy analysis supported sample size expansion up to 176 patients overall, with 39 patients in the R1 subgroup and 137 patients in the R0 subgroup. Enrollment was permitted to continue until the prespecified limits were met. Once the boundaries were exceeded, the study ceased to accrue new patients.
Results
Participants
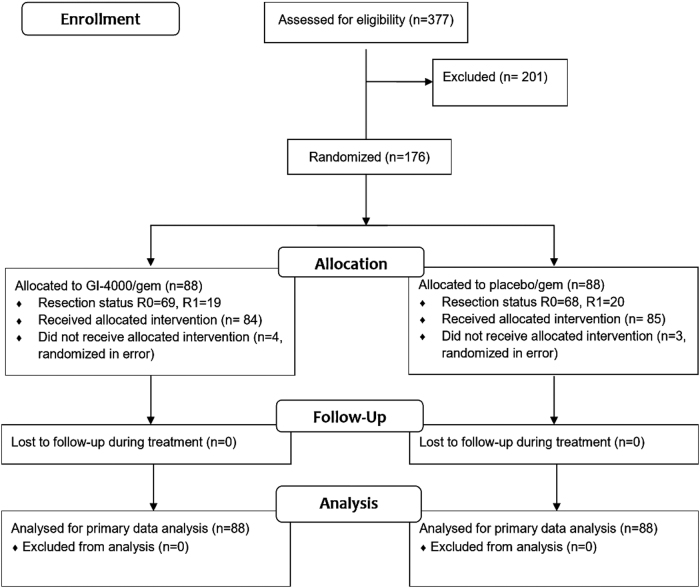
Study disposition is shown in Figure 1. A total of 377 R0/R1 subjects were screened and 176 subjects were subsequently randomized to receive GI-4000 + gemcitabine (88 subjects), or placebo + gemcitabine (88 subjects) between June 5, 2006, and April 30, 2010. These subjects comprised the intent-to-treat (ITT) population. The safety population consisted of a total of 169 subjects who received at least one dose of study drug: 84 subjects received GI-4000 and 85 subjects received placebo. The primary reasons for screened subjects failing to enroll included either the lack of a K-ras mutation in their tumor or the presence of a mutation not represented in the GI-4000 products.
FIG. 1.
Study disposition. n, number of subjects.
Unless otherwise stated, analyses are for the ITT population who underwent R0/R1 resection. The most common reason for study discontinuation in both treatment groups was death (111 subjects, 63.1% of the ITT population).
Table 2 summarizes the baseline demographic and disease characteristics. The mean age was 62.1 years and the majority of the treated subjects were white (80.7%) and men (58.5%). The ras mutations present in tumors were similar between treatment groups and most subjects with R0/R1 resection in both treatment groups had either a G12V (44.3%) or a G12D (43.2%) mutation. Most subjects in both groups had a baseline Eastern Cooperative Oncology Group (ECOG) Performance Status of either Grade 1 (59.1%) or Grade 0 (25.0%).
Table 2.
Summary of Baseline Demographic and Disease Characteristics for All Enrolled Subjects
| |
n (%) |
||
|---|---|---|---|
| Parameter | GI-4000 + gemcitabine | Placebo + gemcitabine | Total |
| Age (years) | 63.4 | 60.8 | 62.1 |
| Gender | |||
| Women | 34 (38.6%) | 39 (44.3%) | 73 (41.5%) |
| Men | 54 (61.4%) | 49 (55.7%) | 103 (58.5%) |
| Race | |||
| White | 71 (80.7%) | 71 (80.7%) | 142 (80.7%) |
| African American | 7 (8.0%) | 7 (8.0%) | 14 (8.0%) |
| Asian | 4 (4.5%) | 4 (4.5%) | 8 (4.5%) |
| Hispanic | 6 (6.8%) | 5 (5.7%) | 11 (6.3%) |
| Other | 0 | 1 (1.1%) | 1 (0.6%) |
| ECOG performance status | |||
| Grade 0 | 22 (25.0%) | 22 (25.0%) | 44 (25.0%) |
| Grade 1 | 52 (59.1%) | 52 (59.1%) | 104 (59.1%) |
| Grade 2 | 8 (9.1%) | 6 (6.8%) | 14 (8.0%) |
| Grade 3 | 0 (0.0%) | 1 (1.1%) | 1 (0.6%) |
| Not reported | 6 (6.8%) | 7 (8.0%) | 13 (7.4%) |
| ras mutation | |||
| G12Va | 41 (46.6%) | 37 (42.0%) | 78 (44.3%) |
| G12C | 3 (3.4%) | 0 (0.0%) | 3 (1.7%) |
| G12D | 35 (39.8%) | 41 (46.6%) | 76 (43.2%) |
| G12R | 7 (8.0%) | 8 (9.1%) | 15 (8.5%) |
| Q61H | 2 (2.3%) | 2 (2.3%) | 4 (2.3%) |
| Primary tumor | |||
| T1 | 7 (8.0%) | 9 (10.2%) | 16 (9.1%) |
| T2 | 8 (9.1%) | 10 (11.4%) | 18 (10.2%) |
| T3 | 70 (79.5%) | 68 (77.3%) | 138 (78.4%) |
| T4 | 3 (3.4%) | 0 (0.0%) | 3 (1.7%) |
| Not reported | 0 (0.0%) | 1 (1.1%) | 1 (0.6%) |
| Regional lymph node status | |||
| N0 | 25 (28.4%) | 20 (22.7%) | 45 (25.6%) |
| N1 | 34 (38.6%) | 36 (40.9%) | 70 (39.8%) |
| N1a | 6 (6.8%) | 9 (10.2%) | 15 (8.5%) |
| N1b | 23 (26.1%) | 22 (25.0%) | 45 (25.6%) |
| Not reported | 0 (0.0%) | 1 (1.1%) | 1 (0.6%) |
| CA 19–9 (U/mL) postoperative | |||
| Number of subjects | 84 | 84 | 168 |
| Median | 24.9 | 16.1 | 18.8 |
| Normal | 59 (67.0%) | 63 (71.6%) | 122 (69.3%) |
| Abnormalb | 25 (28.4%) | 21 (23.9%) | 46 (26.1%) |
| Not reported | 4 (4.5%) | 4 (4.5%) | 8 (4.5%) |
G, glycine; C, cysteine; D, aspartic acid; R, arginine; Q, glutamine; H, histidine; L, leucine; V, valine.
CA 19-9 values >35 U/mL were classified as abnormal.
ECOG, Eastern Cooperative Oncology Group.
Most primary tumors were stage pT3 (138 subjects, 78.4%) and there were no significant differences here between the GI-4000 and placebo cohorts (79.5% vs. 77.3%, respectively). Three subjects had T4 primary lesions and all were randomized to the GI-4000 group. The status of regional lymph node involvement was comparable between treatment groups. A higher percentage of subjects in the placebo group than in the GI-4000 group had metastasis in a single regional lymph node (10.2% vs. 6.8%, respectively), whereas metastasis in multiple regional lymph nodes occurred in a similar percentage of subjects in the GI-4000 and placebo groups (26.1% vs. 25.0%, respectively).
Efficacy
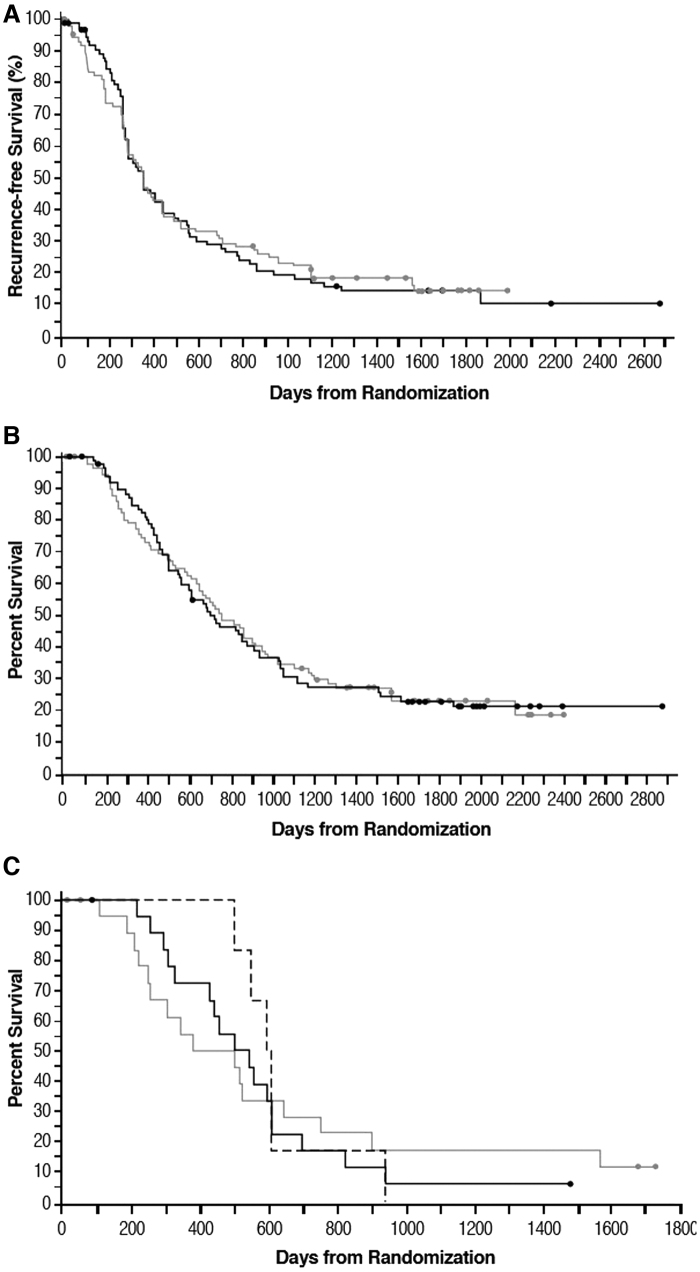
The median time from randomization to recurrence was similar for the GI-4000 and placebo groups at 354 and 357 days, respectively (hazard ratio [HR] = 1.01 [95% confidence intervals (CIs): 0.73–1.41], p = 0.936). The percentage of subjects free of recurrence in the GI-4000 group was similar to that of the placebo group (18.2% vs. 17.0%, respectively). The median time from randomization to death was also similar for the GI-4000 and placebo groups: 698 versus 751 days, respectively (HR = 1.01 [CI: 0.72–1.42], p = 0.956). Kaplan–Meier estimates of the duration of radiological recurrence-free survival and of OS from randomization show comparable patterns for both treatment groups (Fig. 2).
FIG. 2.
Recurrence-free survival (A) and OS (B, C) based on Kaplan–Meier estimates of all enrolled subjects (ITT population) (A, B) or ITT subjects with R1 resection (C) from date of randomization. Black line, GI-4000; gray line, placebo; dashed black line, GI-4000 treated R1 IFNγ ELISpot responders. Circles, censored data. A single subject in the R1 placebo group was an IFNγ ELISpot responder and had an OS of 382 days. IFNγ, interferon-γ; ITT, intent-to-treat; OS, overall survival.
For the prospectively defined and stratified R1 subgroup, there was a nonsignificant trend for improved OS with GI-4000 treatment, with an advantage in 1-year survival for GI-4000 versus placebo (72.2% vs. 55.6%, respectively), and an improvement in median OS of 80 days for GI-4000 versus placebo (523.5 vs. 443.5 days, HR = 1.06 [CI: 0.53–2.13], p = 0.872).
Safety
The side effect and safety profiles of subjects receiving GI-4000/gemcitabine were similar to those of subjects receiving placebo/gemcitabine. Table 3 summarizes treatment emergent adverse events (TEAEs) occurring in at least 5% of ITT subjects and occurring in ≥30 of all subjects. The most frequent TEAEs were fatigue (55.1%), nausea (51.7%), anemia (42.6%), diarrhea (42.6%), and neutropenia (41.5%). Overall, the frequencies of adverse events were comparable between treatment groups and consistent with events expected in the population being studied. The TEAE that occurred with a notably higher incidence in the GI-4000 group than the placebo group was injection site pain (25.0% vs. 3.4%). A notably higher incidence in the placebo group occurred with the TEAE of depression (11.4% GI-4000 vs. 23.9% placebo).
Table 3.
Summary of Treatment Emergent Adverse Events for ≥5% of all Subjects and ≥30 of All Subjects
| MedDRA System Organ Class Preferred term | GI-4000 + Gemcitabine N = 88 | Placebo + Gemcitabine N = 88 | Total N = 176 |
|---|---|---|---|
| Blood and lymphatic system disorders, n (%) | |||
| Anemia | 36 (40.9) | 39 (44.3) | 75 (42.6) |
| Neutropenia | 37 (42.0) | 36 (40.9) | 73 (41.5) |
| Thrombocytopenia | 15 (17.0) | 17 (19.3) | 32 (18.2) |
| Gastrointestinal disorders, n (%) | |||
| Abdominal pain | 33 (37.5) | 32 36.4) | 65 (36.9) |
| Constipation | 26 (29.5) | 27 (30.7) | 53 (30.1) |
| Diarrhea | 31 (35.2) | 44 (50.0) | 75 (42.6) |
| Nausea | 46 (52.3) | 45 (51.1) | 91 (51.7) |
| Vomiting | 25 (28.4) | 24 (27.3) | 49 (27.8) |
| General disorders and administration site conditions, n (%) | |||
| Fatigue | 47 (53.4) | 50 (56.8) | 97 (55.1) |
| Edema, peripheral | 29 (33.0) | 26 (29.5) | 55 (31.3) |
| Pyrexia | 25 (28.4) | 30 (34.1) | 55 (31.3) |
| Injection site reactionsa | 40 (45.5) | 8 (9.1) | 48 (27.3) |
| Metabolism and nutrition disorders, n (%) | |||
| Anorexia | 16 (18.2) | 16 (18.2) | 32 (18.2) |
| Musculoskeletal and connective tissue disorders, n (%) | |||
| Back pain | 22 (25.0) | 19 (21.6) | 41 (23.3) |
| Nervous system disorders, n (%) | |||
| Dizziness | 16 (18.2) | 17 (19.3) | 33 (18.8) |
| Headache | 20 (22.7) | 19 (21.6) | 39 (22.2) |
| Psychiatric disorders, n (%) | |||
| Depression | 10 (11.4) | 21 (23.9) | 31 (17.6) |
| Insomnia | 22 (25.0) | 15 (17.0) | 37 (21.0) |
includes injection site erythema, induration, and pain.
Immunogenicity
IFNγ ELISpot response
There was no difference in the frequency of ELISpot responders between the treatment groups with 22 of 67 (32.8%) subjects in the GI-4000 group versus 23 of 62 (37.1%) subjects in the placebo-treated group (Table 4). However, there was a nonsignificant increase in frequency of ELISpot responders in the R1 subgroup treated with GI-4000, with 6 of 15 subjects tested for GI-4000 versus 1 of 12 subjects tested for placebo (40.0% vs. 8.3%; p = 0.062). In addition, there was an improvement in median OS of 159 days for the R1 GI-4000 ELISpot immune responders versus all placebo-treated subjects (p = 0.810). For the R0 subgroup, there were comparable categorical ELISpot responses in both treatment groups with 16 of 52 (30.8%) responders in the GI-4000 group compared with 22 of 50 (44.0%) responders in the placebo group.
Table 4.
Summary of Immune Responders
| Treatment | IFNγ ELISpot response |
Naïve Treg fraction reduction |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R1 |
R0 |
R0/R1 |
R1 |
R0 |
R0/R1 |
|||||||
| Total subjects tested | Responders (%) | Total subjects tested | Responders(%) | Total subjects tested | Responders (%) | Total subjects tested | Responders (%) | Total subjects tested | Responders (%) | Total subjects tested | Responders (%) | |
| GI-4000 | 15 | 6 (40.0)a | 52 | 16 (30.8) | 67 | 22 (32.8) | 9 | 3 (33.3) | 42 | 11 (26.2) | 51 | 14 (27.4)b |
| Placebo | 12 | 1 (8.3) | 50 | 22 (44.0) | 62 | 23 (37.1) | 7 | 1 (14.3) | 34 | 3 (8.8) | 41 | 4 (9.7) |
An increase or decrease in Tregs on treatment was defined as at least a twofold change in frequency from baseline.
p = 0.062, Pearson chi-square test, for GI-4000 versus placebo groups.
p = 0.033, Pearson chi-square test, for GI-4000 versus placebo groups.
IFNγ, interferon-γ.
Treg phenotyping
There was a threefold greater frequency for a decrease in naive Tregs (CD4+CD45RA+Foxp3low) in the GI-4000 group, with 14 of 51 subjects (27.4%) showing a twofold or greater decrease compared with 4 of 41 (9.7%) placebo subjects (p = 0.033) (Table 4).
Proteomic analysis
Baseline plasma samples (44 in the GI-4000 group and 46 in the placebo group) were retrospectively analyzed by exploratory MALDI-ToF mass spectrometry using previously described methods.27 A classifier, BDX-001, was created using a strongly regularized logistic regression combination of five nearest neighbor classifiers composed of single or pairs of 100 mass spectral features (Supplementary Data). The training set for the classifier consisted of 23 samples from GI-4000–treated patients. Classifier performance was assessed on the remaining 21 samples for the GI-4000 group and all 46 placebo group samples.
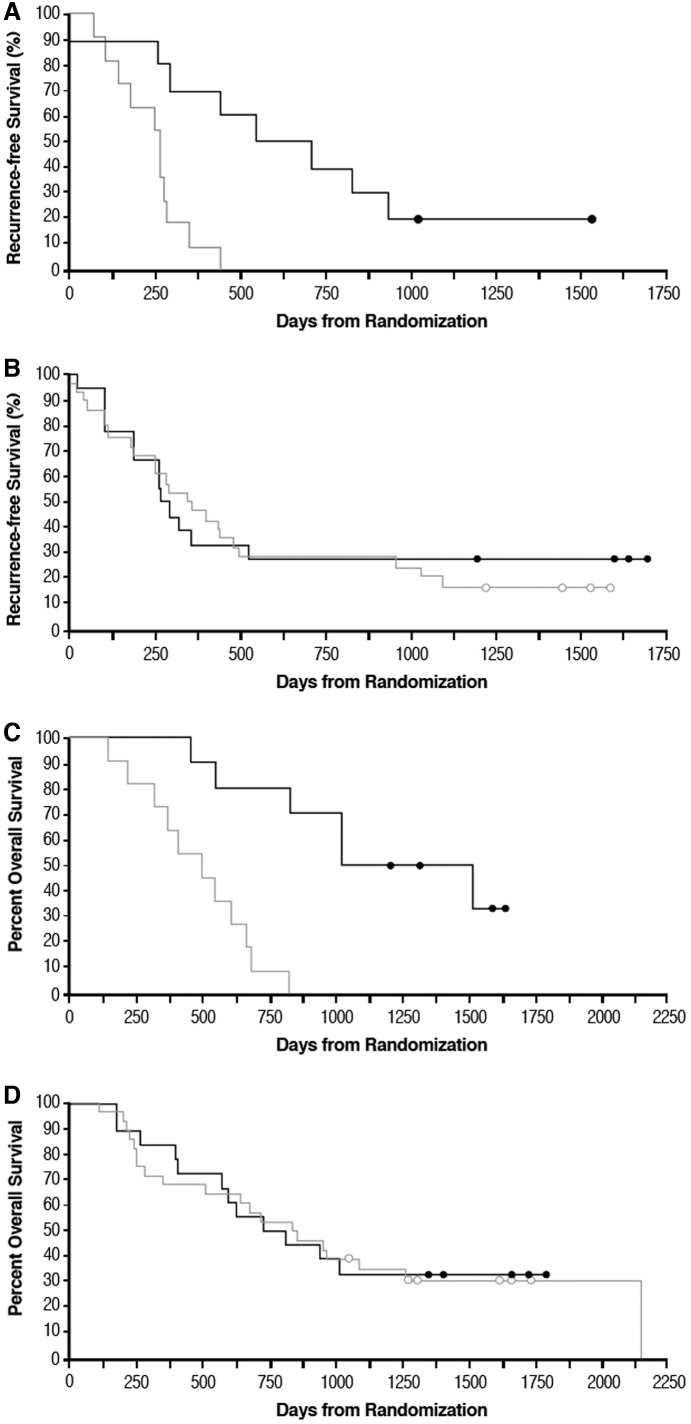
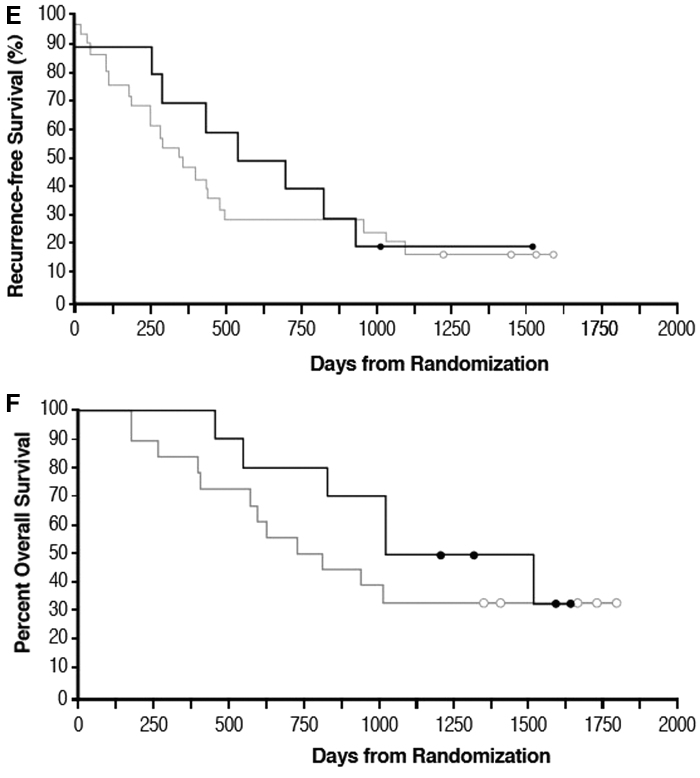
The classifier divided subjects into two classes, BDX-001+ and BDX-001−, with, respectively, better and worse outcomes when treated with GI-4000: treated subjects classified as BDX-001+ had a 12.0 month improvement in recurrence-free survival compared with GI-4000–treated subjects classified as BDX-001− (HR = 0.30 [CI: 0.07–0.49], p = 0.002, Fig. 3A). In contrast, there was no improvement in recurrence-free survival between BDX-001+ and BDX-001− placebo subjects (unfavorable 2.4 months difference, HR = 1.11 [CI: 0.57–2.18], p = 0.754, Fig. 3B). When used to evaluate OS, the proteomic classifier also predicted better and worse survival for subjects in the GI-4000 group (25.4 months improvement BDX-001+ vs. BDX-001−, HR = 0.21 [CI: 0.04–0.31], p < 0.001, Fig. 3C) but not the placebo group (HR = 1.03 [CI: 0.50–2.10], p = 0.944, Fig. 3D). BDX-001+ subjects treated with GI-4000 had improved recurrence-free survival and OS compared with BDX-001+ placebo subjects with an 11.5 months improvement in recurrence-free survival: 20.7 months versus 9.2 months (HR = 0.80 [CI: 0.34–1.91], p = 0.623) and a nonsignificant 16.4 months improvement in median OS (41.9 months for GI-4000 vs. 25.5 months for placebo, HR = 0.65 [CI: 0.26–1.67], p = 0.384) (Fig. 3E and F, respectively).
FIG. 3.
Recurrence-free survival (A, B, E) and OS (C, D, F) based on Kaplan–Meier estimates for subjects treated with GI-4000 or placebo and analyzed for proteomic signature. Baseline samples from 44 subjects in the GI-4000–treated group and 46 subjects in the placebo-treated group were available to investigate the proteomic signature. Samples from GI-4000–treated subjects were further subdivided into training (n = 23) and test (n = 21) sets and all samples not used in the classifier training set were designated BDX-001+ or BDX-001−. Circles, censored data. (A) Recurrence-free survival for GI-4000–treated subjects with (n = 10, black line)/without (n = 11, gray line) BDX-001 signature. (B) Recurrence-free survival for placebo-treated subjects with (n = 18, black line)/without (n = 28, gray line) BDX-001 signature. (C) OS for GI-4000–treated subjects with (n = 10, black line)/without (n = 11, gray line) BDX-001 signature. (D) OS for placebo-treated subjects with (n = 18, black line)/without (n = 28, gray line) BDX-001 signature. (E) Recurrence-free survival for BDX-001+ GI-4000 (n = 10, black line) and placebo-treated subjects (n = 18, gray line). (F) OS for BDX-001+ GI-4000 (n = 10, black line) and placebo-treated subjects (n = 18, gray line).
When this proteomic classifier was applied to only R0 subjects from both treatment groups, an advantage in median recurrence-free survival of 13.7 months was observed for GI-4000 compared with placebo (23.2 vs. 9.5 months, HR = 0.68 [CI: 0.27–1.73], p = 0.426) and an advantage in median OS of 25.7 months was observed (49.9 months for GI-4000 vs. 24.2 months for placebo, HR = 0.46 [CI: 0.18–1.25], p = 0.135) for BDX-001+ subjects.
Discussion
This phase 2 study was a randomized double-blind placebo-controlled multicenter trial comparing GI-4000 plus gemcitabine with placebo plus gemcitabine in subjects with resected ras-mutated pancreas cancer. Subjects were prospectively stratified based on their resection status (R0/R1). Since the majority of subjects in the trial were in the R0 subgroup (137/176; 78%), the overall findings in the study (R0 and R1 subjects) mirror those of the R0 subgroup analyses, including recurrence-free survival, OS, and mortality. To appreciate the potential differences observed in these subgroups, data have, therefore, been also analyzed separately.
The R1 subgroup showed an increase in subjects with T cell ELISpot responses after GI-4000 treatment compared with placebo treatment, and nonsignificant advantages in 1-year OS for GI-4000 versus placebo and an improvement in median OS of ∼3 months for GI-4000. Furthermore, there was a nonsignificant >5 months improvement in median OS for the R1 GI-4000 ELISpot immune responders versus placebo, indicating a potential mechanism-based improvement in survival for the R1 subgroup. In contrast, the R0 group showed comparable ELISpot responses in both treatment groups, indicating that there appears to be a greater tendency for background tumor-specific immune responses in R0 subjects than in R1 subjects.
Tregs are known to be overexpressed in pancreas cancer28 and poor prognosis is associated with the presence of Tregs in the periphery or in the tumor microenvironment.29–32 In this study, GI-4000 treatment rapidly decreased the naive Treg subpopulation. This decrease could be a potential mechanism of action of GI-4000 that contributes to effects on recurrence and survival. Since the GI-4000 vector is yeast based, it may reduce the number and function of Tregs through reciprocal activation of the Th17 T cell pathway.33–35
The improved ELISpot responses seen in the GI-4000–treated R1 subgroup, together with a trend in improved survival for all GI-4000–treated R1 subjects, suggest residual antigen may be required for optimal response. Reduction in Tregs by GI-4000 may act preferentially for R1 subjects by allowing effector T cells generated by GI-4000 to infiltrate the tumor where the presence of RAS antigen within the residual tumor margins could further drive the effector T cell response. The absence of an intact tumor in R0 subjects may, therefore, not reveal these dual benefits of GI-4000 treatment. Because of the small sample size in the R1 group, if the survival benefit in this group is real, a substantially larger trial would be required to confirm it.
Improved survival with GI-4000 treatment was retrospectively defined by a proteomic signature. The difference in time to recurrence between BDX-001+ and BDX-001− subjects treated with GI-4000 was statistically significant and did not depend on resection status. These survival trends indicate that this proteomic signature predicted late recurrence in the GI-4000–treated subjects, but not placebo subjects, and could potentially be used as an enrichment bioassay to improve observed treatment effects in future clinical trials, as demonstrated for a predictive classifier in responses of NSCLC patients to erlotinib and chemotherapy.36
GI-4000 was shown to be well tolerated, with safety findings comparable between the two groups and with no differences noted for R0 and R1 subjects. Overall, the GI-4000 group showed a similar pattern of recurrence-free survival and OS compared with the placebo group.
The K-ras mutation G12C has recently been exploited to design small molecule inhibitors that show promise for NSCLC treatment.37 However, as illustrated here, mutations in K-ras in pancreatic cancer are predominantly G12V and G12D; there was only a single subject with a G12C K-ras mutation in our study. Therefore, small molecule inhibitors for deployment in pancreatic cancer are still being sought. It may be beneficial to combine GI-4000 with cellular immunotherapies such as chimeric antigen receptor T cells or tumor-infiltrating lymphocytes38,39 in pancreatic cancer as GI-4000 may synergize to provide antigen-specific stimuli for the infused T cells. In addition, use of check-point inhibitors to block T cell death pathways may provide optimal reactivation of antitumor immunity in combination with GI-4000. Clinical trials are currently in progress or planned in a number of tumor types combining GI-4000 with other immune therapies and chemotherapies.40 As previously mentioned, it should also be noted that, given the promise of new regimens using capecitabine and FOLFIRINOX,41,42 gemcitabine can probably no longer be considered standard of care in pancreatic cancer patients and any future studies will almost certainly employ a different control arm.
Conclusion
Given the current promise of immunotherapy and interest in strategies to target cancer patients likely to respond to treatments, we believe continued investigation of GI-4000 is warranted, with further prospective studies stratified for likely responders. Combination with immune check-point inhibitors or other immunomodulators may also be beneficial as this may provide optimal reactivation of antitumor immunity.
Supplementary Material
Abbreviations Used
- CI
confidence interval
- ECOG
Eastern Cooperative Oncology Group
- FU
follow-up
- HR
hazard ratio
- IFNγ
interferon-γ
- ITT
intent-to-treat
- MALDI-ToF
matrix-assisted laser desorption/ionization-time-of-flight
- N, n
number of subjects
- NSCLC
nonsmall cell lung cancer
- OS
overall survival
- PBMCs
peripheral blood mononuclear cells
- TEAEs
treatment emergent adverse events
- Treg
regulatory T cell
- YU
yeast units
Contributor Information
Collaborators: for the GI-4000 in Pancreas Cancer Study Group
Author Disclosure Statement
At the time of study conduct, D.A., C.C., and A.M. were employees of GlobeImmune, Inc., and T.C.R. was CEO of GlobeImmune, Inc. All current and former GlobeImmune authors held or hold stock and/or stock options in the company. F.E.H. and A.C. were paid consultants to GlobeImmune, Inc. GlobeImmune, Inc., sponsored and funded the study. GlobeImmune personnel were involved in the design and conduct of the study and provided logistical support during the trial. GlobeImmune personnel prepared the article and all authors contributed to the review and final decisions on the article content. Statistical analyses were performed by QST Consultations, Ltd., Allendale, MI. The following authors declare no conflicts of interest: P.M., T. B.-S., K.M., A.R., S.B.R., D.A.R., W.E.F., P.J.F., F.E.H., and A.C. H.R. and J.R. are employees of Biodesix, Inc., and hold stock and stock options in Biodesix, Inc. H.R. and J.R are inventors on U.S. patent 2017; U.S. 9,653,272, Office USPaT(ed), assigned to Biodesix, Inc., and GlobeImmune, Inc.
Funding Information
P.M. and T.B.-S. acknowledge support from the Ohio State University Comprehensive Cancer Center core grant: P30 CA016058.
Supplementary Material
Cite this article as: Muscarella P, Bekaii-Saab T, McIntyre K, Rosemurgy A, Ross SB, Richards DA, Fisher WE, Flynn PJ, Mattson A, Coeshott C, Roder H, Roder J, Harrell FE, Jr., Cohn A, Rodell TC, Apelian D (2021) A phase 2 randomized placebo-controlled adjuvant trial of GI-4000, a recombinant yeast expressing mutated RAS proteins in patients with resected pancreas cancer, Journal of Pancreatic Cancer 7:1, 8–19, DOI: 10.1089/pancan.2020.0021.
References
- 1. Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689 [PubMed] [Google Scholar]
- 2. Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22 [DOI] [PubMed] [Google Scholar]
- 3. Knauf JA, Ouyang B, Knudsen ES, et al. Oncogenic RAS induces accelerated transition through G2/M and promotes defects in the G2 DNA damage and mitotic spindle checkpoints. J Biol Chem. 2006;281:3800–3809 [DOI] [PubMed] [Google Scholar]
- 4. Wood CG. Adjuvant approaches to renal cell carcinoma. Clin Adv Hematol Oncol. 2008;6:19–21 [PubMed] [Google Scholar]
- 5. Van Poppel H, Joniau S, Van Gool SW. Vaccine therapy in patients with renal cell carcinoma. Eur Urol. 2009;55:1333–1342 [DOI] [PubMed] [Google Scholar]
- 6. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422 [DOI] [PubMed] [Google Scholar]
- 7. Quoix E, Ramlau R, Westeel V, et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncol. 2011;12:1125–1133 [DOI] [PubMed] [Google Scholar]
- 8. Oudard S, Rixe O, Beuselinck B, et al. A phase II study of the cancer vaccine TG4010 alone and in combination with cytokines in patients with metastatic renal clear-cell carcinoma: clinical and immunological findings. Cancer Immunol Immunother. 2011;60:261–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17:3520–3526 [DOI] [PubMed] [Google Scholar]
- 10. Antonia SJ, Larkin J, Ascierto PA. Immuno-oncology combinations: a review of clinical experience and future prospects. Clin Cancer Res. 2014;20:6258–6268 [DOI] [PubMed] [Google Scholar]
- 11. Roberts ZJ, Better M, Bot A, et al. Axicabtagene ciloleucel, a first-in-class CAR T cell therapy for aggressive NHL. Leuk Lymphoma. 2018;59:1785–1796 [DOI] [PubMed] [Google Scholar]
- 12. Bach PB, Giralt SA, Saltz FB. FDA approval of tisagenlecleucel: promise and complexities of a $475,000 cancer drug. JAMA. 2017;318:1861–1862 [DOI] [PubMed] [Google Scholar]
- 13. Larkin J, Chiarion-Seleni V, Gonzalez R, et al. Five-year survival with combined Nivolumab and Ipililumab in advanced melanoma. N Engl J Med. 2019;381:1535–1546 [DOI] [PubMed] [Google Scholar]
- 14. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028 [DOI] [PubMed] [Google Scholar]
- 15. Cohen EEW, Soulieres D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open label phase 3 study. Lancet. 2019;393:156–167 [DOI] [PubMed] [Google Scholar]
- 16. Chow LQM. Head and neck cancer. New Engl J Med. 2020;382:60–72 [DOI] [PubMed] [Google Scholar]
- 17. Stubbs AC, Martin KS, Coeshott C, et al. Whole recombinant yeast vaccine activates dendritic cells and elicits protective cell-mediated immunity. Nat Med. 2001;7:625–629 [DOI] [PubMed] [Google Scholar]
- 18. Bernstein MB, Chakraborty M, Wansley EK, et al. Recombinant Saccharomyces cerevisiae (yeast-CEA) as a potent activator of murine dendritic cells. Vaccine. 2008;26:509–521 [DOI] [PubMed] [Google Scholar]
- 19. Remondo C, Cereda V, Mostböck S, et al. Human dendritic cell maturation and activation by a heat-killed recombinant yeast (Saccharomyces cerevisiae) vector encoding carcinoembryonic antigen. Vaccine. 2009;27:987–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. King TH, Kemmler CB, Guo Z, et al. A whole recombinant yeast-based therapeutic vaccine elicits HBV X, S and core specific T cells in mice and activates human T cells recognizing epitopes linked to viral clearance. PLoS One. 2014;9:e101904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu Y, Bellgrau D, Dwyer-Nield LD, et al. Mutation-selective tumor remission with Ras-targeted, whole yeast-based immunotherapy. Cancer Res. 2004;64:5084–5088 [DOI] [PubMed] [Google Scholar]
- 22. Haller AA, Lauer GM, King TH, et al. Whole recombinant yeast-based immunotherapy induces potent T cell responses targeting HCV NS3 and Core proteins. Vaccine. 2007;25:1452–1463 [DOI] [PubMed] [Google Scholar]
- 23. Tanaka A, Jensen JD, Prado R, et al. Whole recombinant yeast vaccine induces antitumor immunity and improves survival in a genetically engineered mouse model of melanoma. Gene Ther. 2011;18:827–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cohn A, Morse MA, O'Neil B, et al. Whole recombinant Saccharomyces cerevisiae yeast expressing Ras mutations as treatment for patients with solid tumors bearing Ras mutations: results from a phase 1 trial. J. Immunother. 2018;41:141–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chaft JE, Litvak A, Arcila ME, et al. Phase II study of the GI-4000 KRAS vaccine after curative therapy in patients with stage I-III adenocarcinoma harboring a KRAS G12C, G12D or G12V mutation. Clin Lung Cancer. 2014;15:405–410 [DOI] [PubMed] [Google Scholar]
- 26. Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911 [DOI] [PubMed] [Google Scholar]
- 27. Weber JS, Sznol M, Sullivan RJ, et al. A serum protein signature associated with outcome after anti–PD-1 therapy in metastatic melanoma. Cancer Immunol Res. 2018;6:79–86 [DOI] [PubMed] [Google Scholar]
- 28. Liyanage UK, Moore TT, Joo H-G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761 [DOI] [PubMed] [Google Scholar]
- 29. Bengsch F, Knoblock DM, Liu A, et al. CTLA-4/CD80 pathway regulates T cell infiltration into pancreatic cancer. Cancer Immunol Immunother. 2017;66:1609–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu C, Cheng H, Luo G, et al. Circulating regulatory T cell subsets predict overall survival of patients with unresectable pancreatic cancer. Int J Oncol. 2017;51:686–694 [DOI] [PubMed] [Google Scholar]
- 31. Wartenberg M, Cibin S, Zlobec I, et al. Integrated genomic and immunophenotypic classification of pancreatic cancer reveals three distinct subtypes with prognostic/predictive significance. Clin Cancer Res. 2018;24:4444–4454 [DOI] [PubMed] [Google Scholar]
- 32. Savage PA, Malchow S, Leventhal DS. Basic principles of tumor-associated regulatory T cell biology. Trends Immunol. 2013;34:33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marwaha AK, Crome SQ, Panagiotopoulos C, et al. Cutting edge: increased IL-17-secreting T cells in children with new-onset type 1 diabetes. J Immunol. 2010;185:3814–3818 [DOI] [PubMed] [Google Scholar]
- 34. Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646 [DOI] [PubMed] [Google Scholar]
- 35. Zhou L, Chong MMW, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655 [DOI] [PubMed] [Google Scholar]
- 36. Gregorc V, Novello S, Lazzari C, et al. Predictive value of a proteomic signature in patients with non-small-cell lung cancer treated with second-line erlotinib or chemotherapy (PROSE): a biomarker-stratified, randomised phase 3 trial. Lancet Oncol. 2014;15:713–721 [DOI] [PubMed] [Google Scholar]
- 37. Canon J, Rex K, Saiki AY, et al. The clinical KRAS (G12C) inhibitor AMG 510 drives anti- tumour immunity. Nature. 2019;575:217–223 [DOI] [PubMed] [Google Scholar]
- 38. Tran E, Robbins PF, Lu YC, et al. T cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med. 2016;375:2255–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zacharakis N, Chinnasamy H, Black M, et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med. 2018;24:724–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Clinical trials for GI-4000 and checkpoint inhibitors. Available at https://www.clinicaltrials.gov/ct2/results?cond=&term=quilt&cntry=&state=&city=&dist=Accessed December 4, 2020
- 41. Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024 [DOI] [PubMed] [Google Scholar]
- 42. Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–2406 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.