Abstract
Energy metabolism and appetite regulating hormones follow circadian rhythms which, when disrupted, could lead to adverse metabolic consequences. Such circadian misalignment, a mismatch between endogenous circadian rhythms and behavior, is most severely experienced by shift workers, due to nighttime wake, daytime sleep, and eating at night. However, circadian misalignment is not restricted to shift workers; milder shifts in sleep and mealtimes, termed social and eating jetlag, are highly prevalent in the general population. Social and eating jetlag result in later mealtimes, which may promote positive energy balance and weight gain. Earlier meal timing, specific to individual endogenous circadian patterns, could serve to reduce cardiometabolic disease burden and aid in weight loss and interventions should be done to test this.
Keywords: Circadian Rhythms, shift work, social jet lag, eating jet lag, energy balance, body weight
Introduction
Circadian rhythms are cyclic endogenous biological patterns following an ~24-h cycle that regulate the timing of physiology, metabolism and behavior. They initiate wake and sleep episodes at the appropriate biological time as well as signal feeding and fasting. When behaviors such as eating and sleeping fail to align with circadian cues, misalignment can occur, compromising the integrity of robust endogenous circadian rhythms [1]. Repeated disruption through mismatched timing of eating and sleeping has been shown to increase the risk of obesity, type 2 diabetes and cardiovascular disease [1,2•]. Correspondingly, shift workers, who experience chronic circadian misalignment due to complete reversal of feeding-fasting and wake-sleep behavioral cycles, provide the strongest evidence for these effects [3]. Milder desynchronizing behavioral patterns, such as variability in sleep and meal times throughout the week, are highly prevalent in modern society and have been coined social [4] and eating [5••] jetlag (differences in the midpoint of the sleep or feeding episode on free days vs. work days) (Figure 1). The timing of food intake, particularly, has come to the forefront of research efforts with studies showing that consumption of food later in the day and closer to bedtime is associated with higher weight status [6,7].
Figure 1.
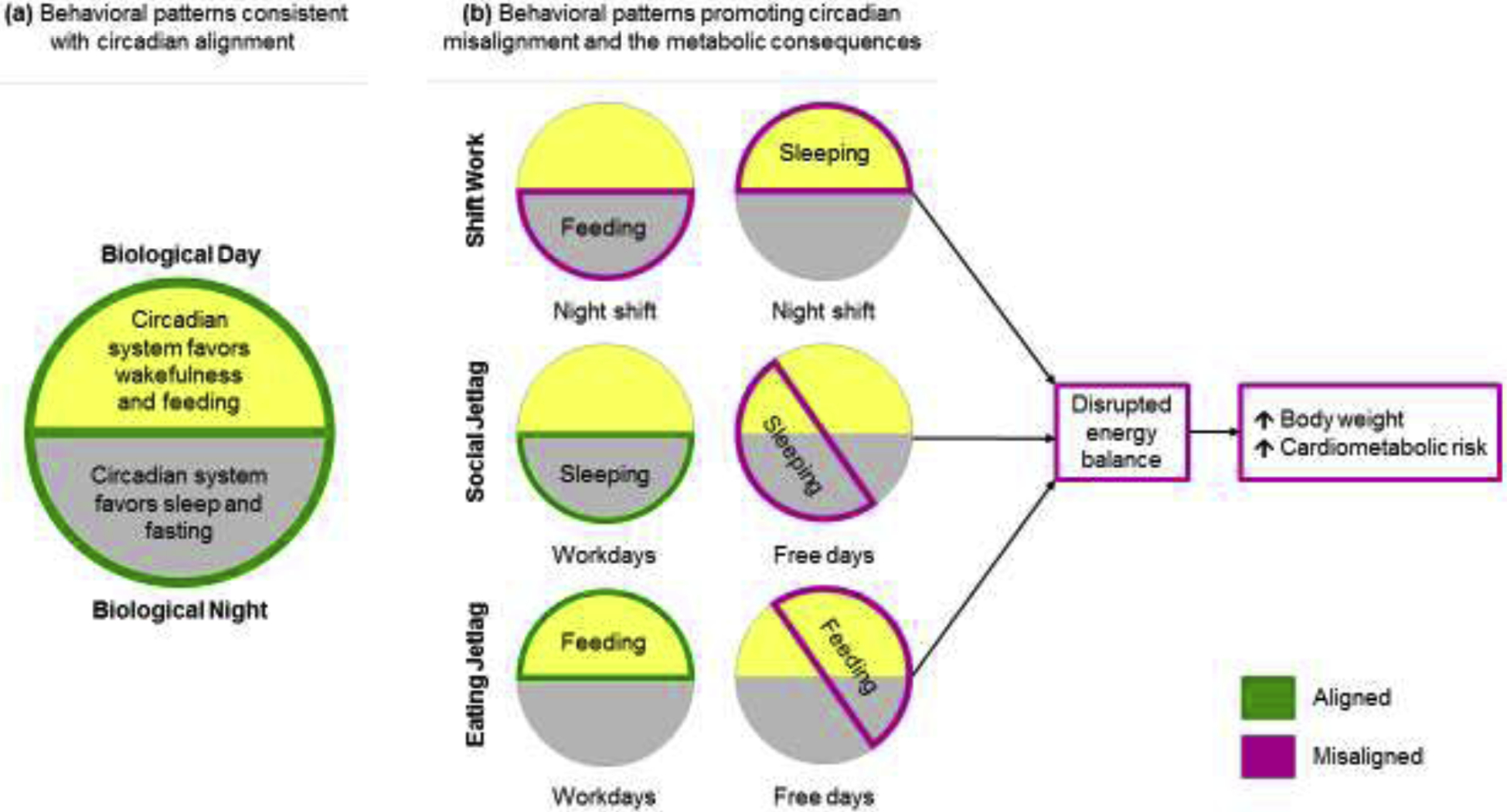
An overview of how mistimed sleeping and eating result in circadian misalignment and the metabolic consequences.
(a) The circadian system promotes wakefulness/feeding during the biological day and sleep/fasting during the biological night.
(b) Several behavioral patterns do not fit these endogenous preferences. Shift work: Awake/feeding at night and sleeping/fasting during the day, causing severe circadian misalignment. Social Jetlag: A shift in the midpoint of sleep on workdays vs. free days, causing mild circadian misalignment. Eating jetlag: A shift in the midpoint of the feeding episode on workdays vs. free days, causing mild circadian misalignment. These behavioral patterns result in a mismatch between the timing of eating/sleeping and the endogenous circadian system (indicated in magenta). Circadian misalignment is thought to disrupt energy balance, resulting in increased body weight and cardiometabolic risk.
While still in early stages, findings from this field of study have broad applicability. The demands of modern life result in many non-shift workers delaying morning meals, adopting irregular eating patterns and extending eating into the night [8,9,10]. Adjustment of meal timing in accordance with individual endogenous circadian rhythms could serve to reduce cardiometabolic disease burden. Here we present evidence on the effect of meal timing, as a disruptor of circadian rhythms, on energy balance (energy intake and energy expenditure) and body weight. Research to date has focused on leptin and ghrelin as regulators of food intake, and resting energy expenditure, all of which are known to exhibit circadian rhythmicity [11,12,13].
Entrainment of circadian clocks and circadian alignment
The endogenous circadian system is primarily controlled by an autonomous master clock in the suprachiasmatic nucleus (SCN) of the hypothalamus, which is synchronized by ambient light and entrains secondary clocks in the brain and most peripheral tissues of the body [2•]. Importantly, secondary clocks are also entrained by environmental cues and behaviors, termed “zeitgebers,” such as eating and sleeping [1,2•]. When environmental and behavioral factors are repeatedly misaligned from the SCN-driven endogenous circadian cycle, such as when food intake occurs during the night, integration of mistimed signals can disrupt the tightly controlled peripheral system, resulting in a loss of homeostasis (circadian misalignment) [2•]. Meanwhile, in conditions of circadian alignment, behavioral cues feed into peripheral circadian systems at the appropriate phase, facilitating the smooth cycling of physiological processes (Figure 1). The circadian rhythms of appetite regulating hormones, energy expenditure and substrate utilization prepare the body for specific biological responses at different times of day to maintain energy balance. For example, ghrelin levels are higher in the biological evening than the morning [12], promoting greater evening hunger. On the other hand, diet induced thermogenesis (DIT), the rise in energy expenditure after a meal, is higher after a morning meal than after an isocaloric evening meal [14,15], indicating more calories burned after a morning meal. Respiratory quotient (RQ), an index of macronutrient utilization, is highest in the biological morning, indicative of greater carbohydrate oxidation, and lowest during the biological evening, indicating greater lipid oxidation [11,16]. These rhythms have implications for health and mismatched behaviors in relation to these endogenous processes can result in adverse health effects.
Severe circadian desynchronization
Studies in mice have shown that food intake during the biological night, akin to night shift work in humans, causes a 12-h shift in peripheral clock, but not central clock, activity [17]. Such mistimed feeding results in higher body weight [18] and increased risk of metabolic syndrome and diabetes [17] relative to control mice fed during the biological day.
Studies in humans have similarly shown phase shifts in peripheral clock activity in response to inappropriate timing of sleep and food intake, while the phase of the SCN master clock remains unaffected [3,19]. Chronic shift work has been associated with metabolic disruption and positive energy balance, resulting in increased risk of obesity, type 2 diabetes, heart disease and metabolic syndrome [20]. The largest body of evidence for the impact of circadian misalignment on human health stems from clinical interventions approximating the conditions of shift work in healthy, non-shift working volunteers. Acute circadian misalignment is induced via simulated night shift or forced desynchrony protocols, in which either active and rest phases are reversed, or the day is artificially shortened/extended. This effectively shifts the behavioral patterns of sleep and eating out of phase with the endogenous rhythm of the SCN master clock, substantially altering the input received by peripheral clocks that regulate metabolism [3].
One proposed contributor to the increased risk of obesity observed in shift workers is higher energy intake due to altered levels of hunger and satiety hormones ghrelin and leptin in response to circadian misalignment. Multiple simulated shiftwork studies observed decreased leptin levels [21,22,23,24,25••] and increased ghrelin levels [12,21,25••,26] in circadian misalignment relative to circadian alignment conditions. According to a recent study [25••], these effects may be sex dependent. Indeed, night shift work (circadian misalignment) induced a 7% decrease in 24-h leptin levels and an 8% increase in wake period ghrelin levels in females whereas in males, leptin levels were increased by 11% and ghrelin levels were unchanged compared to the daytime work (circadian aligned) condition [25••]. Increased hunger and decreased satiety in response to circadian misalignment and depending on time of eating could contribute to weight gain in shift workers [20] and late eaters [27]. Meanwhile, despite higher risk of obesity and chronic disorders, studies have not reported significant differences in energy intake between shift workers by objective measure [28] or self-report [29,30,31]. However, this could reflect inherent biases in food intake measures [32].
Whereas the influence of circadian misalignment on food intake regulation are more conclusive, evidence related to energy expenditure is more equivocal. A simulated night shift intervention showed a small but significant reduction in 24-h resting energy expenditure (REE) after circadian misalignment compared to alignment [24], while similar protocols produced an increase [25••] or no effect [11,23]. The discordance in results could be explained by the finding that REE differs greatly between individuals but is very stable within a person [33]. These inter-individual differences may be in part driven by sex: Qian et al. [25••] observed distinct sex-specific differences, with REE increasing by 4.5% in females after circadian misalignment, while there was no change in males.
Circadian misalignment may also affect substrate utilization. Compared to circadian alignment, misalignment results in reduced RQ [11,16,25••], with concomitant lower carbohydrate oxidation [16,24] and higher lipid oxidation [16,24]. There is some evidence that this effect may also be sex-specific, with reduced RQ being observed in females but not in males [25••]. In general, the circadian system favors carbohydrate utilization in the biological morning and lipid utilization in the biological evening [11,16]. Circadian misalignment may cause a potentially unfavorable shift in these patterns when considered in conjunction with other circadian-controlled metabolic processes, such as glucose regulation. Indeed, multiple clinical interventions have demonstrated disrupted glucose-insulin metabolism in response to acute circadian misalignment. Postprandial glucose levels are raised in response to misaligned mealtimes and insulin sensitivity is reduced [16,22,23], which may increase risk of type 2 diabetes.
Milder circadian desynchronization
Epidemiological studies show that individuals with greater social jetlag (difference in midpoint of the sleep period between work and free days) have higher BMI, adiposity and odds of obesity, metabolic syndrome and type 2 diabetes [34,35]. In a study population with obesity-related chronic diseases, greater social jetlag was associated with consumption of more calories, saturated fat, and cholesterol at dinner, of more protein, total fat, saturated fat and cholesterol at lunch, as well as more total fat and saturated fat consumed at morning snack [36•]. Due to later waking times, social jetlag was also associated with later mealtimes for breakfast, early afternoon snack and dinner [36•]. These later consumption patterns, at odds with endogenous preference, could contribute to the observed risk of obesity in those with greater social jetlag.
Few clinical intervention studies have explored the metabolic effects of mild circadian misalignment. One observational study showed that those with later midpoint of sleep, but not necessarily social jetlag, had higher energy intakes at dinner and after 8 PM, behaviors that were associated with higher BMI, as well as higher intakes of fast food, sugar-sweetened beverages, and lower fruits and vegetables [6]. However, this study was confounded by differences in sleep duration, whereby those with later sleep times also had shorter sleep duration than those with earlier sleep times. We and others have shown that short sleep duration increases food intake [37,38]. Another study showed that sleep restriction for 2 nights followed by 2 nights of sleep recovery and another 2 nights of sleep restriction, effectively shifting midpoint of sleep by 2.5 h between sleep restriction and sleep recovery, increases food intake relative to baseline, pre-study sleep [39]. Both periods of sleep restriction increased energy intakes by ~1,000 kcal whereas recovery sleep increased intakes by ~500 kcal relative to baseline. Sex differences were observed whereby intakes were increased similarly in the two sleep restriction periods in both men and women but intakes during recovery sleep returned to baseline levels in women only. However, this study too, was confounded by differences in sleep duration throughout the sleep conditions [39].
Our lab attempted to address this research gap via a 4-phase randomized crossover pilot study with constant sleep duration [40]. Six men and women underwent four inpatient phases in which the timing, but not duration, of sleep and meals was manipulated: Normal sleep/normal meals, normal sleep/late meals, late sleep/normal meals and late sleep/late meals. Sleep and mealtimes were delayed by 3.5 h in the late conditions relative to those with normal times. Glucagon-like peptide 1 concentrations in response to a meal were higher with early sleep and mealtimes, suggesting improved satiety compared with the late meal condition [40]. On the other hand, earlier sleep and mealtimes were associated with higher ghrelin concentrations and did not influence leptin concentrations. The combination of normal sleep and mealtimes had a reducing effect on food intakes [40]. Although limited, these studies suggest a potential influence of small shifts in sleep and meal timing on regulation of energy balance. We are not aware of any study that has assessed the influence of social jetlag on REE.
Meal timing and weight status
Outside of the laboratory, several recent epidemiological studies have examined the association between timing of eating and obesity risk. Eating jetlag, the difference in midpoint of the eating period between work and free days, has been associated with higher BMI [5••]. In this epidemiological sample, eating jetlag was driven primarily by a delay in breakfast time on weekends relative to weekdays. Later meal timing in general has been associated with higher daily caloric intakes [27] and higher BMI, independent of sleep timing and duration [6]. Xiao et al. [7] associated a higher percentage of dietary intake in the morning with 50% lower odds of overweight or obesity, while a higher percentage of dietary intake in the evening was associated with 80% higher odds of overweight or obesity.
A study by Bandín et al. [41] in which mealtimes were delayed by 3.5 h while sleep was kept constant, showed decreased pre-meal REE, decreased fasting RQ and decreased carbohydrate oxidation compared to the control condition. Other studies have shown that diet-induced thermogenesis (DIT), the rise in energy expenditure in response to food intake, is consistently lower in response to an evening meal compared with a morning meal [14,15,24]. In fact, one study reported 44% lower DIT following an evening meal compared to a morning meal [15]. These findings suggest that energy homeostasis is favored when greater caloric intakes occur in the morning/early afternoon vs. the evening/night due to higher energy costs of processing foods consumed at an earlier time.
Importantly, meal timing patterns may not follow a ‘one-size-fits all’ approach. Recent findings suggest that timing of meals relative to individual circadian clock timing, marked by evening melatonin onset, is a better predictor of body composition and weight status than clock time [42,43••]. Indeed, individuals consuming a greater proportion of their daily energy intakes closer to melatonin onset (circadian clock) had higher BMI and percentage body fat than those who consumed food earlier in their biological day [42]. The same study showed that individuals with higher percentage body fat ate 8% more of their total daily calories in the biological evening, and 13% more carbohydrates in the biological afternoon, irrespective of clock hour, than individuals with lower percentage body fat [43••]. Given the circadian rhythmicity of DIT and substrate oxidation highlighted above, food consumption later in the day could result in fewer calories burned and greater carbohydrates remaining in the circulation, increasing the risk of weight gain and type 2 diabetes in susceptible individuals.
Meal timing and weight loss
Given the associations between timing of food intake and circadian alignment, it is plausible that shifting mealtimes could influence weight management. Indeed, behavioral weight loss programs have shown that greater weight loss occurs in those who consume their main daily meal earlier in the day compared to those who consume that meal later in the day [44] and in those who consume the greatest percentage of daily calories during a morning meal [45,46,47,48]. A 6-year follow-up study of bariatric surgery similarly associated earlier consumption of the main meal with greater weight loss success compared to a later main meal, an observation that could not be explained by differences in energy intake, diet composition or sleep duration [49]. These results indicate that the timing of meals and distribution of caloric intake throughout the day may be important considerations for weight management, along with traditional dietary characteristics such as energy intake and diet composition.
Conclusion
Circadian misalignment is increasingly recognized as a risk factor for obesity and cardiometabolic disease. While shift workers are most affected, there is a growing understanding that milder shifts in eating and sleeping patterns, such as social jetlag and eating jetlag, can also have adverse health consequences. Both social and eating jetlags result in later meal consumption patterns, which may result in eating at biologically unfavorable times for energy and macronutrient metabolism. Clinical intervention studies assessing the effects of these subtle shifts in sleep and meal timing are needed to uncover the mechanism by which mild forms of circadian misalignment lead to higher body weight and cardiometabolic risk. Finally, due to inter-individual differences in circadian timing, it may be important to personalize meal timing recommendations. Meal timing in relation to chronotype, a measure of innate individual preference for morning or evening shown to modulate the risk associated with late eating [50], could be considered to alleviate burden in those at high risk. An understanding of circadian rhythms and differential metabolic responses to food intake at different circadian phases can inform recommendations for temporally healthier eating patterns and should be the subject of clinical investigations.
Highlights:
Energy expenditure, substrate oxidation, and appetite follow circadian rhythms
Mistimed eating and sleeping disrupt circadian rhythms, increasing risk of obesity
Shift workers experience severe chronic circadian misalignment
Social and eating jetlag, variable sleep/eating, cause milder circadian disruption
Personalization of mealtimes could reduce circadian misalignment and improve health
Acknowledgements
This was funded in part by the National Institutes of Health [grant numbers R01 HL142648, R01 HL128226] and the American Heart Association [grant number 16SFRN27950012] (St-Onge).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Buijs FN, León-Mercado L, Guzmán-Ruiz M, Guerrero-Vargas NN, Romo-Nava F, Buijs RM: The Circadian System: A Regulatory Feedback Network of Periphery and Brain. Physiology 2016, 31:170–181. [DOI] [PubMed] [Google Scholar]
- 2.•.Challet E: The circadian regulation of food intake. Nature Reviews Endocrinology 2019, 15:393–405. [DOI] [PubMed] [Google Scholar]; A comprehensive review detailing the entrainment of circadian rhythms, circadian control of food intake and circadian desynchrozing factors.
- 3.Pickel L, Sung H-K: Feeding Rhythms and the Circadian Regulation of Metabolism. Frontiers in Nutrition 2020, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wittmann M, Dinich J, Merrow M, Roenneberg T: Social Jetlag: Misalignment of Biological and Social Time. Chronobiology International 2006, 23:497–509. [DOI] [PubMed] [Google Scholar]
- 5.••.Zerón-Rugerio M, Hernáez Á, Porras-Loaiza A, Cambras T, Izquierdo-Pulido M: Eating Jet Lag: A Marker of the Variability in Meal Timing and Its Association with Body Mass Index. Nutrients 2019, 11:2980. [DOI] [PMC free article] [PubMed] [Google Scholar]; Coined the term “eating jetlag” to describe the difference in the midpoint of the eating period between work and free days, a mild form of circadian misalignment.
- 6.Baron KG, Reid KJ, Kern AS, Zee PC: Role of Sleep Timing in Caloric Intake and BMI. Obesity 2011, 19:1374–1381. [DOI] [PubMed] [Google Scholar]
- 7.Xiao Q, Garaulet M, Scheer FAJL : Meal timing and obesity: interactions with macronutrient intake and chronotype. International Journal of Obesity 2019, 43:1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill S, Panda S: A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metabolism 2015, 22:789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.St-Onge M-P, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton P, Varady K: Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement From the American Heart Association. Circulation 2017, 135:e96–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eicher-Miller HA, Khanna N, Boushey CJ, Gelfand SB, Delp EJ: Temporal Dietary Patterns Derived among the Adult Participants of the National Health and Nutrition Examination Survey 1999–2004 Are Associated with Diet Quality. Journal of the Academy of Nutrition and Dietetics 2016, 116:283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zitting K-M, Vujovic N, Yuan RK, Isherwood CM, Medina JE, Wang W, Buxton OM, Williams JS, Czeisler CA, Duffy JF: Human Resting Energy Expenditure Varies with Circadian Phase. Current Biology 2018, 28:3685–3690.e3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian J, Morris CJ, Caputo R, Garaulet M, Scheer FAJL: Ghrelin is impacted by the endogenous circadian system and by circadian misalignment in humans. International Journal of Obesity 2019, 43:1644–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS: Independent Circadian and Sleep/Wake Regulation of Adipokines and Glucose in Humans. The Journal of Clinical Endocrinology & Metabolism 2005, 90:2537–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bo S, Fadda M, Castiglione A, Ciccone G, De Francesco A, Fedele D, Guggino A, Parasiliti Caprino M, Ferrara S, Vezio Boggio M, et al. : Is the timing of caloric intake associated with variation in diet-induced thermogenesis and in the metabolic pattern? A randomized cross-over study. International Journal of Obesity 2015, 39:1689–1695. [DOI] [PubMed] [Google Scholar]
- 15.Morris CJ, Garcia JI, Myers S, Yang JN, Trienekens N, Scheer FAJL: The Human Circadian System Has a Dominating Role in Causing the Morning/Evening Difference in Diet-Induced Thermogenesis. Obesity 2015, 23:2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, Buxton OM, Shea SA, Scheer FAJL: Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proceedings of the National Academy of Sciences 2015, 112:E2225–E2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherji A, Kobiita A, Damara M, Misra N, Meziane H, Champy M-F, Chambon P: Shifting eating to the circadian rest phase misaligns the peripheral clocks with the master SCN clock and leads to a metabolic syndrome. Proceedings of the National Academy of Sciences 2015, 112:E6691–E6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW: Circadian Timing of Food Intake Contributes to Weight Gain. Obesity 2009, 17:2100–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wehrens SMT, Christou S, Isherwood C, Middleton B, Gibbs MA, Archer SN, Skene DJ, Johnston JD: Meal Timing Regulates the Human Circadian System. Current Biology 2017, 27:1768–1775.e1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kervezee L, Kosmadopoulos A, Boivin DB: Metabolic and cardiovascular consequences of shift work: The role of circadian disruption and sleep disturbances. European Journal of Neuroscience 2020, 51:396–412. [DOI] [PubMed] [Google Scholar]
- 21.Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W, Czeisler CA, Shea SA: Adverse Metabolic Consequences in Humans of Prolonged Sleep Restriction Combined with Circadian Disruption. Science Translational Medicine 2012, 4:129ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheer FAJL Hilton MF, Mantzoros CS, Shea SA: Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences 2009, 106:4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonnissen HK, Rutters F, Mazuy C, Martens EA, Adam TC, Westerterp-Plantenga MS: Effect of a phase advance and phase delay of the 24-h cycle on energy metabolism, appetite, and related hormones. The American Journal of Clinical Nutrition 2012, 96:689–697. [DOI] [PubMed] [Google Scholar]
- 24.McHill AW, Melanson EL, Higgins J, Connick E, Moehlman TM, Stothard ER, Wright KP: Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proceedings of the National Academy of Sciences 2014, 111:17302–17307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.••.Qian J, Morris CJ, Caputo R, Wang W, Garaulet M, Scheer FAJL: Sex differences in the circadian misalignment effects on energy regulation. Proceedings of the National Academy of Sciences 2019, 116:23806–23812. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first study to identify distinct sex-specific differences in circadian misalignment’s effects on REE, RQ and appetite regulating hormones leptin and ghrelin. Suggests that circadian misalignment may affect energy balance in men and women through separate mechanisms.
- 26.Schiavo-Cardozo D, Lima MMO, Pareja JC, Geloneze B: Appetite-regulating hormones from the upper gut: disrupted control of xenin and ghrelin in night workers. Clinical Endocrinology 2013, 79:807–811. [DOI] [PubMed] [Google Scholar]
- 27.Reid KJ, Baron KG, Zee PC: Meal timing influences daily caloric intake in healthy adults. Nutrition Research 2014, 34:930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Lauren S, Chang BP, Shechter A: Objective Food Intake in Night and Day Shift Workers: A Laboratory Study. Clocks & Sleep 2018, 1:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cayanan EA, Eyre NAB, Lao V, Comas M, Hoyos CM, Marshall NS, Phillips CL, Shiao JSC, Guo Y-LL, Gordon CJ: Is 24-hour energy intake greater during night shift compared to non-night shift patterns? A systematic review. Chronobiology International 2019, 36:1599–1612. [DOI] [PubMed] [Google Scholar]
- 30.Shaw E, Dorrian J, Coates AM, Leung GKW, Davis R, Rosbotham E, Warnock R, Huggins CE, Bonham MP: Temporal pattern of eating in night shift workers. Chronobiology International 2019, 36:1613–1625. [DOI] [PubMed] [Google Scholar]
- 31.Lauren S, Chen Y, Friel C, Chang B, Shechter A: Free-Living Sleep, Food Intake, and Physical Activity in Night and Morning Shift Workers. Journal of the American College of Nutrition 2020, 39: 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subar AF, Freedman LS, Tooze JA, Kirkpatrick SI, Boushey C, Neuhouser ML, Thompson FE, Potischman N, Guenther PM, Tarasuk V, et al. : Addressing Current Criticism Regarding the Value of Self-Report Dietary Data. The Journal of Nutrition 2015, 145:2639–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melanson EL, Ritchie HK, Dear TB, Catenacci V, Shea K, Connick E, Moehlman TM, Stothard ER, Higgins J, McHill AW, et al. : Daytime bright light exposure, metabolism, and individual differences in wake and sleep energy expenditure during circadian entrainment and misalignment. Neurobiology of Sleep and Circadian Rhythms 2018, 4:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parsons MJ, Moffitt TE, Gregory AM, Goldman-Mellor S, Nolan PM, Poulton R, Caspi A: Social jetlag, obesity and metabolic disorder: investigation in a cohort study. International Journal of Obesity 2015, 39:842–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koopman ADM, Rauh SP, Van ‘T Riet E, Groeneveld L, Van Der Heijden AA, Elders PJ, Dekker JM, Nijpels G, Beulens JW, Rutters F: The Association between Social Jetlag, the Metabolic Syndrome, and Type 2 Diabetes Mellitus in the General Population: The New Hoorn Study. Journal of Biological Rhythms 2017, 32:359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.•.Mota MC, Silva CM, Balieiro LCT, Gonçalves BF, Fahmy WM, Crispim CA: Association between social jetlag food consumption and meal times in patients with obesity-related chronic diseases. PLOS ONE 2019, 14:e0212126. [DOI] [PMC free article] [PubMed] [Google Scholar]; An epidemiological study associating social jetlag with later meal timing throughout the day, higher caloric intakes, and a poorer diet (higher total fat, saturated fat and cholesterol intakes) compared to individuals with no social jetlag.
- 37.St-Onge M-P, Roberts AL, Chen J, Kelleman M, O’Keeffe M, Roychoudhury A, Jones PJ: Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. The American Journal of Clinical Nutrition 2011, 94:410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al Khatib HK, Harding SV, Darzi J, Pot GK: The effects of partial sleep deprivation on energy balance: a systematic review and meta-analysis. European Journal of Clinical Nutrition 2017, 71:614–624. [DOI] [PubMed] [Google Scholar]
- 39.Depner CM, Melanson EL, Eckel RH, Snell-Bergeon JK, Perreault L, Bergman BC, Higgins JA, Guerin MK, Stothard ER, Morton SJ, et al. : Ad libitum Weekend Recovery Sleep Fails to Prevent Metabolic Dysregulation during a Repeating Pattern of Insufficient Sleep and Weekend Recovery Sleep. Current Biology 2019, 29:957–967.e954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.St-Onge MP, Pizinger T, Kovtun K, RoyChoudhury A: Sleep and meal timing influence food intake and its hormonal regulation in healthy adults with overweight/obesity. European Journal of Clinical Nutrition 2019, 72:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bandín C, Scheer FAJL, Luque AJ, Ávila-Gandía V, Zamora S, Madrid JA, Gómez-Abellán P, Garaulet M: Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. International Journal of Obesity 2015, 39:828–833. [DOI] [PubMed] [Google Scholar]
- 42.McHill AW, Phillips AJ, Czeisler CA, Keating L, Yee K, Barger LK, Garaulet M, Scheer FA, Klerman EB: Later circadian timing of food intake is associated with increased body fat. The American Journal of Clinical Nutrition 2017, 106:1213–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.••.McHill A, Czeisler C, Phillips A, Keating L, Barger L, Garaulet M, Scheer F, Klerman E: Caloric and Macronutrient Intake Differ with Circadian Phase and between Lean and Overweight Young Adults. Nutrients 2019, 11:587. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study found that individual circadian phase better predicted the metabolic effects of meal timing than clock time, due to differences in timing of circadian rhythms between individuals. This suggests that recommendations for dietary patterns might be most effective if personalized to an individual’s endogenous circadian cycle.
- 44.Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee YC, Ordovás JM, Scheer FAJL: Timing of food intake predicts weight loss effectiveness. International Journal of Obesity 2013, 37:604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jakubowicz D, Barnea M, Wainstein J, Froy O: High Caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 2013, 21:2504–2512. [DOI] [PubMed] [Google Scholar]
- 46.Raynor HA, Li F, Cardoso C: Daily pattern of energy distribution and weight loss. Physiology & Behavior 2018, 192:167–172. [DOI] [PubMed] [Google Scholar]
- 47.Kahleova H, Lloren JI, Mashchak A, Hill M, Fraser GE: Meal Frequency and Timing Are Associated with Changes in Body Mass Index in Adventist Health Study 2. The Journal of Nutrition 2017, 147:1722–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lombardo M, Bellia A, Padua E, Annino G, Guglielmi V, D’Adamo M, Iellamo F, Sbraccia P: Morning Meal More Efficient for Fat Loss in a 3-Month Lifestyle Intervention. Journal of the American College of Nutrition 2014, 33:198–205. [DOI] [PubMed] [Google Scholar]
- 49.Ruiz-Lozano T, Vidal J, De Hollanda A, Scheer FAJL, Garaulet M, Izquierdo-Pulido M: Timing of food intake is associated with weight loss evolution in severe obese patients after bariatric surgery. Clinical Nutrition 2016, 35:1308–1314. [DOI] [PubMed] [Google Scholar]
- 50.Muñoz JSG, Cañavate R, Hernández CM, Cara-Salmerón V, Morante JJH: The association among chronotype, timing of food intake and food preferences depends on body mass status. European Journal of Clinical Nutrition 2017, 71:736–742. [DOI] [PubMed] [Google Scholar]


