Abstract
Introduction
For decisions on glioblastoma surgery, the risk of complications and decline in performance is decisive. In this study, we determine the rate of complications and performance decline after resections and biopsies in a national quality registry, their risk factors and the risk-standardized variation between institutions.
Methods
Data from all 3288 adults with first-time glioblastoma surgery at 13 hospitals were obtained from a prospective population-based Quality Registry Neuro Surgery in the Netherlands between 2013 and 2017. Patients were stratified by biopsies and resections. Complications were categorized as Clavien-Dindo grades II and higher. Performance decline was considered a deterioration of more than 10 Karnofsky points at 6 weeks. Risk factors were evaluated in multivariable logistic regression analysis. Patient-specific expected and observed complications and performance declines were summarized for institutions and analyzed in funnel plots.
Results
For 2271 resections, the overall complication rate was 20 % and 16 % declined in performance. For 1017 biopsies, the overall complication rate was 11 % and 30 % declined in performance. Patient-related characteristics were significant risk factors for complications and performance decline, i.e. higher age, lower baseline Karnofsky, higher ASA classification, and the surgical procedure. Hospital characteristics, i.e. case volume, university affiliation and biopsy percentage, were not. In three institutes the observed complication rate was significantly less than expected. In one institute significantly more performance declines were observed than expected, and in one institute significantly less.
Conclusions
Patient characteristics, but not case volume, were risk factors for complications and performance decline after glioblastoma surgery. After risk-standardization, hospitals varied in complications and performance declines.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11060-021-03697-8.
Keywords: Glioblastoma, Neurosurgical procedures, Postoperative complications, Karnofsky performance status, Quality of health care, Patient outcome assessment
Introduction
For patients with glioblastoma, neurosurgeons aim to maximize tumor removal, while preserving functional integrity, to prolong patient survival with acceptable quality of life [1]. Based on various factors, including age, symptoms, general condition, comorbidity, tumor location and extent, perceived balance between procedural risks and anticipated benefit and patient preference, the decision is sometimes made to biopsy rather than to resect, and to limit the extent of resection. An important argument to make neurosurgical decisions and to counsel patients is the risk of complications and a decline in performance.
Standards are lacking for the indication to biopsy and for the extent of tumor removal. Consequently a large range of options is available for neurosurgical teams which can be considered an opportunity for highly patient-tailored decisions, but at the same time could result in considerable practice variation and therefore outcome variation. We have previously reported on variation in 30-day mortality and 2-year survival outcome among institutes from the same registry [2].
The literature contains surgical reports with varying rates of adverse events after glioblastoma surgery. Instead of selectively citing the rate that suits the present decision best, it is probably better to use real-world data as source for arguments in these neurosurgical decisions, preferably using one’s own outcome data. To this end the Dutch Society for Neurosurgery has initiated a quality registry, which contains outcome data from all patients who had glioblastoma surgery in all institutes.
In this study, we determine the rate and severity of complications and Karnofsky performance decline after resections and biopsies in a nation-wide quality registry, their risk factors and the risk-standardized variation between institutions.
Materials and methods
Dutch quality registry neuro surgery
The Dutch Society for Neurosurgery (http://www.nvvn.org) established the Quality Registry for Neuro Surgery in 2011 (http://www.qrns.nl). This registry aims to provide feedback to all institutions with neurosurgical units on patient outcomes and treatment variation for self-assessment and quality-monitoring. Neurosurgeons, nurse specialists in neuro-oncology and trained physician assistants prospectively enter patient data in the registry. Participation in the registry is mandatory for all intitutions providing glioblastoma surgery. Each institution is represented in the collaborative for glioblastoma surgery with several meetings per year for the methods design and interpretation of results. Outcomes are reported to the Dutch Society for Neurosurgery annually.
Patients
We studied all 3288 patients who had first-time surgery for glioblastoma at all 13 hospitals in the Netherlands. Patients had their surgery between 1/1/2013, when the complication severity was included, and 12/31/2017. We collected data for patients 18 years or older at surgery and a histopathological diagnosis of glioblastoma according to the WHO 2007 criteria until 2015 and the WHO 2016 criteria thereafter [3].
Data collection
Demographic and clinical information consisted of age at diagnosis, gender, Karnofsky performance status before surgery, type of surgery (biopsy or resection), and dates of treatment, last follow-up and death. A surgical procedure was considered a biopsy, when tissue was taken for diagnosis only, either by needle biopsy or open biopsy.
Treatment decisions for patients were made in multidisciplinary tumor board meetings in all hospitals. Image-guided navigation was available in all hospitals, fluorescence-guidance in ten hospitals, intraoperative stimulation mapping in nine hospitals, ultrasound in three hospitals, and intraoperative MRI in none of the hospitals.
Because this data was collected for evaluation of quality of care in accordance with the Dutch Quality Act for Healthcare (http://wetten.overheid.nl/BWBR0007850/2015-01-01) and the New Healthcare Quality, Complaints and Disputes Act (https://wetten.overheid.nl/BWBR0037173/2019-05-01), individual written informed consent was not needed. The study was not subject to the Medical Research Involving Human Subjects Act (WMO, https://wetten.overheid.nl/BWBR0009408/2018-08-01), therefore ethical approval was waived, and de-identified data had been collected of patients not alive by a trusted third party (http://www.sivz.nl/en).
Outcome measures and risk predictors
The main outcome measures to evaluate variation were specified in the consensus item set: the risk-standardized complications and performance alterations at 6 weeks postoperative. The severity of complications was graded by the revised Clavien-Dindo classification. This classification ranks complications based on the therapy used to treat the complication and has been reported to be an objective, simple, reliable, and reproducible way of reporting adverse events after surgery [4], and consists of five grades, i.e. I: any deviation from normal, not requiring treatment, II: requiring medication, III: requiring an intervention, IV: requiring intensive care management, and V: death. This grading was added to the registry in 2013. For institutional comparison, we analyzed complication severity as complications of grade II and higher. Performance alterations were calculated by subtracting the baseline Karnofsky performance score prior to surgery from the Karnofsky performance score at 6 weeks after surgery. For institutional comparison, negative performance change of more than ten points was considered a performance decline [5].
To account for risk differences between institutions, we explored these patient-related characteristics as predictors for outcomes: age at surgery, gender, American Society of Anesthesiologists physical status (ASA) classification, baseline Karnofsky performance, and year of treatment; and the institution-related characteristics: case volume, university hospital, and biopsy percentage.
Statistical analysis
For each patient a risk prediction was calculated based on the patient characteristics that were identified in multivariable logistic regression models. The model with the lowest Akaiki’s information criterium was selected as a trade-off between goodness of fit and model simplicity. Patient-specific risk prediction allowed for risk-standardized comparison between expected and observed complication grades and performance decline for institutes. To compare outcomes between institutes, the expected number of events based on an institute’s patient population was plotted against the ratio of number of observed and expected events in funnel plots. Institutes with achievements outside the 95 % confidence intervals were considered significantly deviant from the expectation. Institutes, providing less than 85 % of outcome measures, were considered to contribute insufficient data and their results were therefore uninformative.
Results
Of the 3288 patients, 2271 (69 %) had a resection, the others a biopsy only. The complication severity was missing for 417 (13 %). A baseline or follow-up performance score to calculate the performance change was missing for 440 (13 %). This was mainly due to 3 of 13 institutes, f, h, and i, with more than 15 % of outcome measures missing. Patient characteristics per institute and institutional characteristics are listed in Table 1.
Table 1.
Characteristics of patients and hospitals with complication grading and performance decline per institute and overall
| Institute | a | b | c | d | e | f | g | h | i | j | k | l | m | Overall |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 80 | 112 | 119 | 120 | 181 | 197 | 236 | 237 | 335 | 387 | 413 | 422 | 449 | 3288 |
| Number of complete cases for complication grading | 79 | 112 | 104 | 115 | 157 | 7 | 229 | 144 | 256 | 384 | 413 | 422 | 449 | 2871 |
| Percentage of complete cases for complication grading | 99 % | 100 % | 87 % | 96 % | 87 % | 4 % | 97 % | 61 % | 76 % | 99 % | 100 % | 100 % | 100 % | 87 % |
| Number of complete cases for performance changes | 80 | 112 | 119 | 112 | 173 | 52 | 235 | 126 | 234 | 383 | 357 | 421 | 444 | 2848 |
| Percentage of complete cases for performance changes | 100 % | 100 % | 100 % | 93 % | 96 % | 26 % | 100 % | 53 % | 70 % | 99 % | 86 % | 100 % | 99 % | 87 % |
| Patient-related characteristics | ||||||||||||||
| Number of females | 28 | 52 | 49 | 39 | 65 | 79 | 89 | 96 | 124 | 127 | 149 | 163 | 162 | 1222 |
| Percentage of females | 35 % | 46 % | 41 % | 33 % | 36 % | 40 % | 38 % | 41 % | 37 % | 33 % | 36 % | 39 % | 36 % | 37 % |
| Mean age | 61.1 | 61.3 | 62.8 | 62.2 | 61.4 | 59.5 | 60.6 | 60.7 | 60.1 | 57.7 | 62.0 | 62.9 | 66.1 | 61.6 |
| s.d. of age | 10.9 | 11.6 | 11.0 | 12.8 | 11.1 | 15.2 | 12.8 | 14.0 | 11.8 | 14.2 | 11.3 | 13.3 | 90.2 | 35.8 |
| ASA classification | ||||||||||||||
| ASA I | 46 | 24 | 28 | 12 | 33 | 40 | 42 | 46 | 123 | 141 | 150 | 51 | 77 | 813 |
| ASA II | 28 | 61 | 64 | 83 | 105 | 88 | 154 | 133 | 108 | 191 | 215 | 305 | 199 | 1734 |
| ASA III | 5 | 23 | 5 | 18 | 25 | 15 | 38 | 25 | 75 | 51 | 43 | 57 | 52 | 432 |
| ASA IV | 1 | 1 | 0 | 1 | 1 | 0 | 2 | 1 | 2 | 0 | 3 | 6 | 6 | 24 |
| ASA V | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 6 |
| ASA missing | 0 | 3 | 21 | 6 | 16 | 53 | 0 | 32 | 27 | 3 | 2 | 1 | 115 | 279 |
| Preoperative Karnofsky performance score | ||||||||||||||
| KPS 100 | 8 | 2 | 19 | 16 | 11 | 34 | 14 | 8 | 33 | 83 | 53 | 24 | 23 | 328 |
| KPS 90 | 36 | 32 | 48 | 38 | 61 | 68 | 60 | 79 | 96 | 126 | 193 | 154 | 124 | 1115 |
| KPS 80 | 12 | 24 | 29 | 13 | 45 | 29 | 65 | 62 | 90 | 72 | 87 | 136 | 107 | 771 |
| KPS 70 | 4 | 24 | 11 | 18 | 29 | 29 | 28 | 25 | 59 | 53 | 20 | 52 | 97 | 449 |
| KPS 60 | 6 | 18 | 5 | 18 | 19 | 6 | 32 | 18 | 32 | 32 | 9 | 35 | 58 | 288 |
| KPS 50 | 8 | 11 | 4 | 8 | 14 | 2 | 29 | 13 | 11 | 17 | 7 | 16 | 23 | 163 |
| KPS 40 | 3 | 1 | 0 | 3 | 1 | 1 | 2 | 4 | 4 | 1 | 1 | 2 | 5 | 28 |
| KPS 30 | 1 | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 1 | 2 | 1 | 1 | 3 | 13 |
| KPS 20 | 2 | 0 | 2 | 0 | 1 | 1 | 3 | 7 | 2 | 1 | 0 | 1 | 3 | 23 |
| KPS 10 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 5 |
| KPS missing | 0 | 0 | 0 | 6 | 0 | 26 | 0 | 21 | 5 | 0 | 42 | 0 | 5 | 105 |
| Year of treatment | ||||||||||||||
| 2013 | 22 | 26 | 27 | 27 | 23 | 37 | 48 | 37 | 54 | 59 | 81 | 76 | 87 | 604 |
| 2014 | 16 | 25 | 15 | 20 | 47 | 47 | 46 | 49 | 74 | 79 | 88 | 77 | 75 | 658 |
| 2015 | 12 | 22 | 18 | 25 | 35 | 44 | 45 | 56 | 88 | 67 | 80 | 81 | 100 | 673 |
| 2016 | 22 | 22 | 28 | 27 | 33 | 31 | 50 | 36 | 89 | 76 | 99 | 87 | 83 | 683 |
| 2017 | 8 | 17 | 31 | 21 | 43 | 38 | 47 | 59 | 30 | 106 | 65 | 101 | 104 | 670 |
| Hospital-related characteristics | ||||||||||||||
| Academic setting | No | No | No | No | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | 7 |
| Number of patients with biopsy | 20 | 33 | 49 | 36 | 61 | 130 | 57 | 45 | 72 | 99 | 164 | 106 | 145 | 1017 |
| Percentage of patients with biopsy | 25 % | 29 % | 41 % | 30 % | 34 % | 66 % | 24 % | 19 % | 21 % | 26 % | 40 % | 25 % | 32 % | 31 % |
| Number of patients with complication grade II–V | 2 | 15 | 3 | 13 | 13 | 1 | 39 | 19 | 28 | 22 | 39 | 45 | 58 | 297 |
| Percentage of patients with complication grade II–V | 3 % | 13 % | 3 % | 11 % | 7 % | 1 % | 17 % | 8 % | 8 % | 6 % | 9 % | 11 % | 13 % | 9 % |
| Number of patients with performance decline | 15 | 8 | 38 | 24 | 51 | 4 | 47 | 19 | 63 | 72 | 74 | 129 | 120 | 664 |
| Percentage of patients with performance decline | 19 % | 7 % | 32 % | 20 % | 28 % | 2 % | 20 % | 8 % | 19 % | 19 % | 18 % | 31 % | 27 % | 20 % |
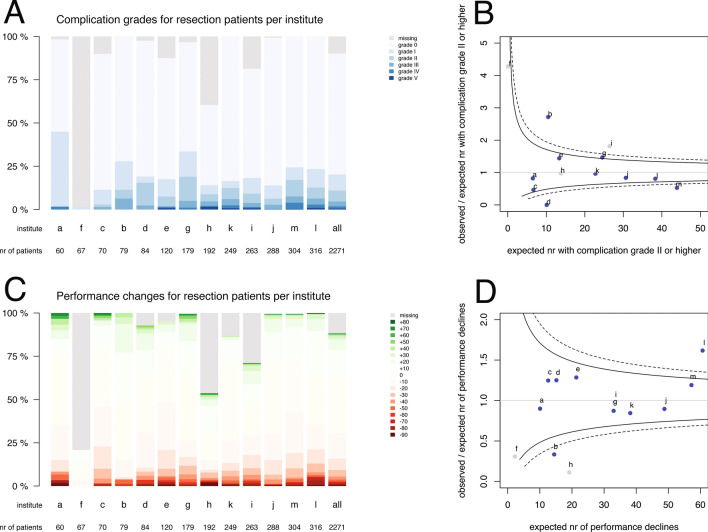
The observed complication severity and performance changes at 6 weeks postoperative are plotted in Fig. 1 for resections and in Fig. 2 for biopsies. For resections over all institutes, complications of any severity were observed in 459 (20 %) patients, grade II or higher in 250 (11 %), grade III or higher in 105 (5 %) and grade IV or higher in 41 (2 %) (Fig. 1a). Complications of grade II or higher for resections ranged between 0 % (institute f) and 19 % (institute g). And a performance decline was observed in 359 (16 %) resection patients, a stable performance in 1427 (63 %), and a performance improvement in 217 (10 %) (Fig. 1c). Performance decline for resections varied from 0 % (institute f) to 23 % (institute c). For biopsies over all institutes, complications of any grade were observed in 112 (11 %) patients, grade II or higher in 47 (5 %), grade III or higher in 22 (2 %) and grade IV or higher in 12 (1 %) (Fig. 2a). Complications of grade II or higher for biopsies varied from 0 % (institute d) to 18 % (institute b). And a performance decline was observed in 305 (30 %) biopsy patients, a stable performance in 518 (51 %), and a performance improvement in 22 (2 %) (Fig. 2c). Performance decline for biopsies ranged between 2 % (institute h) and 58 % (institute l).
Fig. 1.
Distributions of a Clavien-Dindo complication severity and c Karnofsky performance changes for resection patients per institute, sorted by volume of resection cases over 5 years and funnel plots for observed and expected b complication grade II or higher and d performance decline. The color codes are provided in the legends. Each dot represents an institute indicated by a letter corresponding to Table 1. Blue dots indicate institutes with less than 15 % outcome measures missing, grey dots institutes with more than 15 % missing. The solid funnels are 95 % control limits, the dotted funnels 99 % control limits
Fig. 2.
Distributions of a Clavien-Dindo complication severity and c Karnofsky performance changes for biopsy patients per institute, sorted by volume of biopsy cases over five years and funnel plots for observed and expected b complication grade II and higher and d performance decline. The color codes are provided in the legends. Each dot represents an institute indicated by a letter corresponding to Table 1. Blue dots indicate institutes with less than 15 % outcome measures missing, grey dots institutes with more than 15 % missing. The solid funnels are 95 % control limits, the dotted funnels 99 % control limits
To determine the risk factors for complication severity and performance decline, we first plotted the patient and the institute characteristics to these outcomes (Supplementary Fig. 1). Next, the association between characteristics and a complication grade II or higher, or a performance decline was evaluated in multivariable logistic regression models. A higher risk of a complication grade II or higher was associated with lower baseline Karnofsky (odds ratio, 95 % confidence interval: 0.97, 0.97–0.98), a higher ASA classification (ASA-II to ASA-I: 1.6, 1.1–2.2; ASA-III to ASA-I: 1.8, 1.2–2.8; ASA-IV to ASA-I: 3.0, 1.0-7.9; ASA-V to ASA-I: 1.2, 0.1–11), and a resection (compared to a biopsy: 2.7, 1.9–3.8). This model has an AIC of 1774 and the interaction terms were not significantly associated. Year of treatment, patient age and gender were not associated with a complication grade II or higher. A higher risk of performance decline of more than 10 points was associated with a higher age (1.02, 1.01–1.03), higher ASA classification (ASA-II to ASA-I: 1.2, 0.97–1.6; ASA-III to ASA-I: 1.6, 1.1–2.2; ASA-IV to ASA-I: 2.6, 1.0-6.3; ASA-V to ASA-I: 5.8, 0.93-45) and a biopsy (compared to a resection: 2.3, 1.9–2.7). This model has an AIC of 2922 and a significant interaction term between age and ASA classification. Year of treatment and gender were not associated with performance decline. Of note, the institution characteristics overall case volume, university hospital and biopsy percentage were not associated with complication severity nor with performance decline (Supplementary Figs. 1 and 2).
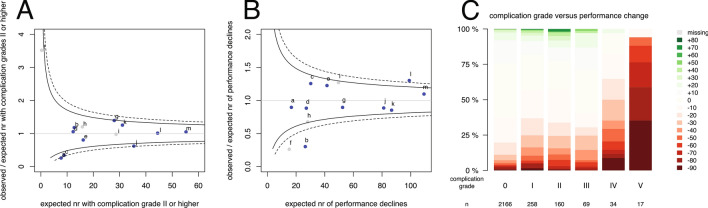
The between-institution variation in complication severity and performance decline is displayed as funnel plot for all patients in Fig. 3a, b. Ratios higher than 1.0 indicate more adverse events than expected based on risk standardization. In three institutes, a, c, and j, the number of observed patients with complications was significantly less than expected (Fig. 3a). In institute l significantly more performance declines were observed than expected, and in b significantly less (Fig. 3b). Other institutions had ratios within the control limits, i.e. observed events were according to expectations. As the type of intervention was a strong risk factor for outcomes, funnel plots were generated separately for the subgroup with a resection (Fig. 1b, d) and for the subgroup with a biopsy (Fig. 2b, d).
Fig. 3.
Funnel plot for all patients per institute of risk-standardized expected number of events and the ratio of observed and expected number of events for a a complication grade II and higher, and b for a performance decline of more than ten points. Each dot represents an institute indicated by a letter corresponding to Table 1. Blue dots indicate institutes with less than 15 % outcome measures missing, grey dots institutes with more than 15 % missing. The solid funnels are 95 % control limits, the dotted funnels 99 % control limits. Correlation between complication grades and performance changes (c)
The correlation between Clavien-Dindo classification and the change in Karnofsky performance was low (Kendall’s tau correlation: − 0.14, Fig. 3c). No complication was observed in 198 (55 %) of 359 resection patients with a performance decline and in 228 (75 %) of 305 biopsy patients with a performance decline. Conversely, a performance improvement was observed in 109 (45 %) of 250 resection patients with a complication grade II or higher and in 7 (13 %) of 47 biopsy patients with a complication grade II or higher.
Discussion
The main findings of this study are: (1) any complication is observed in 20 % after resection and in 11 % after biopsy; a performance decline was observed in 16 % after resection and in 30 % after biopsy, (2) risk factors for a complication were lower baseline Karnofsky, higher ASA classification, and a resection; risk factors for a performance decline were higher age, higher ASA classification and a biopsy; institutional case volume, biopsy percentage and university hospital were not associated with complications nor performance decline, (3) patient outcomes among institutes vary more in complications than in performance decline.
Variation between institutions in complication outcomes and performance changes has not been published for glioblastoma surgery. Compared to the extensive literature on benefits of glioblastoma surgery, the literature on adverse outcome is limited. In these reports, the definitions of surgical complications, the classifications of patient condition and the timing of assessment are far from uniform. Complications after glioblastoma resection varied between 15 % [6], 19 % [7], 23 % [8], 24 % [9], and 68 % [10]. Complications of biopsies varied between 3 % [11], 6 % [12], 7 % [13], 8 % [14], 9 % [15] ,12 % [16], and 13 % [17]. We now demonstrate the outcome variation in adverse events after glioblastoma surgery among teams using identical definitions and risk-standardization.
Some reports have used the Karnofsky performance score. The median Karnofsky performance before and after surgery were reported as similar [18–21]. The performance change is more informative for risk assessment in individual patients. For instance, a performance decline was observed in 5 % [22], 10 % [23], and 39 % [7].
Other measures of adverse outcome of glioblastoma surgery have been documented. Several reports narrow down patient condition to neurologic outcome [24–26]. Yet, others have used readmission rate as surrogate marker [6]. Even more scarce are reports on health-related quality of life after surgery [27, 28].
Apart from divergent complication definitions as a source of variation in the literature, the timing of assessment also varied: 21 days [9], 30 days [8, 23], 6 weeks [7], 3 months [26], and 6 months after surgery [24]. Too early assessment would inadvertently include transient neurologic dysfunctions and exclude late complications from surgery. Too late assessment would include decline from tumor progression or adverse events from other treatments. Therefore, we selected 6 weeks after surgery, typically immediately before the start of radiotherapy.
To compare complication outcomes between neurosurgical reports, a consensus definition of complications, their severity and their timing of assessment is essential. Few classifications have been proposed [29–32]. Ambiguity arises in classifying adverse events when judgment of deviation from the expected course is required. We have chosen to use the revised Clavien-Dindo classification, because it avoids this ambiguity from expectations and allows for direct comparison with other surgical procedures. In general surgery this has been proven to be an objective, simple, reliable, and reproducible way of reporting adverse postoperative events [4, 33]. This classification is based on the type of therapy required to treat the complication and was devised to eliminate subjective interpretation of serious adverse events, because it is based on events that are usually well-documented and easily verified. To put the observed complication risk of glioblastoma surgery into perspective, any complication was observed in 10 % after radical prostatectomy [34], 29 % after hepatocellular carcinoma resections [35], 29 % after noncardiac thoracic operations [36], 47 % after pancreatic adenocarcinoma resections [37], and 61 % after pancreaticoduodenectomies [38].
The Clavien-Dindo classification and the change in Karnofsky performance can both detect adverse surgical events, although these measure different aspects of patient outcome and their correlation was low. Others have observed more performance decline in patients with higher grade complications in a general neurosurgical population [30, 39]. An explanation for the discordance between the two measures in our data may be that post-operative neurological deficit is not scored in the Clavien-Dindo classification, when it does not require additional treatment. Another explanation may be that not every performance decline is due to surgical complications, but can also be due to early glioblastoma progression. We have previously identified early progression as frequent cause of death within 30 days [2]. Early progression may also explain the discordance between 30 % performance decline after biopsy and 16 % decline after resection.
The implications for clinical practice from this work are that the risk factors for complications, i.e. lower baseline Karnofsky and higher ASA classification, can be used for patient counseling. For example, consider two patients indicated for a resection: a 75-year-old patient with a KPS of 70, and an ASA classification of III, and a 25-year-old patient with a KPS of 90, and an ASA classification of I. The first patient has a risk of 18 % for a relevant complication and 24 % for performance decline, whereas the second patient 7 % and 14 %, respectively (see also https://nvvn-qrns-gbm.shinyapps.io/patient_risk_prediction/). Ideally, patient counseling should be based on institute-specific data, which is now available for these hospitals. Others have identified higher age and tumor location as risk factors for complications [10, 23]. The systematic collection of imaging characteristics has been scheduled for our registry from 2020 to be able to evaluate tumor volume and location as determinant of patient outcome in future analyses.
The discussions based on these results in the registry collaborative and in the institutions have been constructive and should contribute to outcome improvement programs for all institutes. Nevertheless this improvement has yet to be determined. A recent systematic review on the effectiveness of quality improvement collaboratives showed a statistically significant improvement of at least 50 % of the primary outcome in 73 % of studies [40]. The elements for success from collaboratives have not been identified [41, 42]. The aim for our collaborative on glioblastoma surgery is to further reduce complications and performance decline, rather than facilitate regression to a common mean outcome with no outliers. We will expand the registry with more detailed neurological outcome and complication diagnosis to be able to address potential strategies for improvement.
Strengths of this study include a comprehensive population-based nation-wide prospective registry with standardized definitions of severity and timing of complications and performance decline. As limitations, we did not specify the neurological outcomes, the complication diagnosis and potential causes and we did not measure the health-related quality of life. Imaging data including tumor volume and location was so far not systematically collected and consequently risk-standardization could be improved. Some treatment-related characteristics that may be of interest as predictors were not systematically collected in the registry, such as corticosteroid use, surgical technique and extent of resection. Data verification by audits could support the data quality.
Conclusions
Any complication in glioblastoma surgery is observed in 11 % after biopsy and in 20 % after resection, and a performance decline was observed in 30 % after biopsy and in 16 % after resection. The risk factors for a complication were lower baseline Karnofsky, higher ASA classification, and a resection. The risk factors for a performance decline were higher age, higher ASA classification and a biopsy. Institution case volume, academic status and biopsy percentage were not associated with complications nor performance decline. Complications and performance declines vary between hospitals.
Supplementary Informatiom
Below is the link to the electronic supplementary material.
Supplemental Figure 1 Boxplots of patient and hospital characteristics versus complication grades and performance changes. Supplementary Material 1 (TIF 7373 kb)
Supplemental Figure 2 Scatterplots of hospital characteristics versus complication grades and performance changes. Each dot represents an institution with corresponding identification. Blue dots indicate institutes with less than 15 % outcome measures missing, grey dots institutes with more than 15 % missing. The solid dots indicate university hospitals, the open dots indicate general hospitals. The solid black lines plot the estimated regression coefficients, and the dotted black lines represent the 95 % confidence intervals of the regression coefficients. The solid grey line represents the overall population percentage of complications grade 2 and higher, and percentage of performance declines over 20 points. Supplementary Material 2 (TIF 1658 kb)
Compliance with ethical standards
Conflict of interest
Author declares that they have no conflict of interest to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sanai N, Berger MS. Surgical oncology for gliomas: the state of the art. Nat Rev Clin Oncol. 2018;15:112–125. doi: 10.1038/nrclinonc.2017.171. [DOI] [PubMed] [Google Scholar]
- 2.De Witt Hamer PC, Ho VKY, Zwinderman AH, et al. Between-hospital variation in mortality and survival after glioblastoma surgery in the Dutch Quality Registry for Neuro Surgery. J Neurooncol. 2019;144:313–323. doi: 10.1007/s11060-019-03229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 4.Clavien PA, Barkun J, De Oliveira ML, et al. The clavien-dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 5.Chaichana KL, Halthore AN, Parker SL, et al. Factors involved in maintaining prolonged functional independence following supratentorial glioblastoma resection. J Neurosurg. 2010;114:604–612. doi: 10.3171/2010.4.jns091340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nuño M, Ly D, Ortega A, et al. Does 30-day readmission affect long-term outcome among glioblastoma patients? Neurosurgery. 2014;74:196–204. doi: 10.1227/NEU.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 7.Gulati S, Jakola AS, Nerland US, et al. The risk of getting worse: surgically acquired deficits, perioperative complications, and functional outcomes after primary resection of glioblastoma. World Neurosurg. 2011;76:572–579. doi: 10.1016/j.wneu.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Li YM, Suki D, Hess K, Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J Neurosurg. 2016;124:977–988. doi: 10.3171/2015.5.JNS142087. [DOI] [PubMed] [Google Scholar]
- 9.Chang SM, Parney IF, McDermott M, et al. Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project. J Neurosurg. 2003;98:1175–1181. doi: 10.3171/jns.2003.98.6.1175. [DOI] [PubMed] [Google Scholar]
- 10.Ening G, Osterheld F, Capper D, et al. Risk factors for glioblastoma therapy associated complications. Clin Neurol Neurosurg. 2015;134:55–59. doi: 10.1016/j.clineuro.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Tilgner J, Herr M, Ostertag C, Volk B. Validation of intraoperative diagnoses using smear preparations from stereotactic brain biopsies: Intraoperative versus final diagnosis - Influence of clinical factors. Neurosurgery. 2005;56:257–263. doi: 10.1227/01.NEU.0000148899.39020.87. [DOI] [PubMed] [Google Scholar]
- 12.Malone H, Yang J, Hershman DL, et al. Complications following stereotactic needle biopsy of intracranial tumors. World Neurosurg. 2015;84:1084–1089. doi: 10.1016/j.wneu.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 13.Kongkham PN, Knifed E, Tomber MS, Bernstein M. Complications in 622 cases of frame-based stereotactic biopsy, a decreasing procedure. Can J Neurol Sci. 2008;35:79–84. doi: 10.1017/S0317167100007605. [DOI] [PubMed] [Google Scholar]
- 14.Field M, Witham TF, Flickinger JC, et al. Comprehensive assessment of hemorrhage risks and outcomes after stereotactic brain biopsy. J Neurosurg. 2001;94:545–551. doi: 10.3171/jns.2001.94.4.0545. [DOI] [PubMed] [Google Scholar]
- 15.Sciortino T, Fernandes B, Conti Nibali M, et al. Frameless stereotactic biopsy for precision neurosurgery: diagnostic value, safety, and accuracy. Acta Neurochir (Wien) 2019;161:967–974. doi: 10.1007/s00701-019-03873-w. [DOI] [PubMed] [Google Scholar]
- 16.Dammers R, Haitsma IK, Schouten JW, et al. Safety and efficacy of frameless and frame-based intracranial biopsy techniques. Acta Neurochir (Wien) 2008;150:23–29. doi: 10.1007/s00701-007-1473-x. [DOI] [PubMed] [Google Scholar]
- 17.McGirt MJ, Woodworth GF, Coon AL, et al. Independent predictors of morbidity after image-guided stereotactic brain biopsy: a risk assessment of 270 cases. J Neurosurg. 2005;102:897–901. doi: 10.3171/jns.2005.102.5.0897. [DOI] [PubMed] [Google Scholar]
- 18.Ringel F, Pape H, Sabel M, et al. Clinical benefit from resection of recurrent glioblastomas: results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro Oncol. 2016;18:96–104. doi: 10.1093/neuonc/nov145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sang S, Wanggou S, Wang Z, et al. Clinical long-term follow-up evaluation of functional neuronavigation in adult cerebral gliomas. World Neurosurg. 2018;119:e262–e271. doi: 10.1016/j.wneu.2018.07.127. [DOI] [PubMed] [Google Scholar]
- 20.Heiland DH, Haaker G, Watzlawick R, et al. One decade of glioblastoma multiforme surgery in 342 elderly patients: what have we learned? J Neurooncol. 2018;140:385–391. doi: 10.1007/s11060-018-2964-8. [DOI] [PubMed] [Google Scholar]
- 21.Roux A, Peeters S, Zanello M, et al. Extent of resection and Carmustine wafer implantation safely improve survival in patients with a newly diagnosed glioblastoma: a single center experience of the current practice. J Neurooncol. 2017;135:83–92. doi: 10.1007/s11060-017-2551-4. [DOI] [PubMed] [Google Scholar]
- 22.Chaichana KL, Cabrera-Aldana EE, Jusue-Torres I, et al. When gross total resection of a glioblastoma is possible, how much resection should be achieved? World Neurosurg. 2014;82:e257–e265. doi: 10.1016/j.wneu.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 23.Sawaya R, Hammoud M, Schoppa D, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044–1055. doi: 10.1097/00006123-199805000-00054. [DOI] [PubMed] [Google Scholar]
- 24.McGirt MJ, Mukherjee D, Chaichana KL, et al. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65:463–469. doi: 10.1227/01.NEU.0000349763.42238.E9. [DOI] [PubMed] [Google Scholar]
- 25.Rahman M, Abbatematteo J, De Leo EK, et al. The effects of new or worsened postoperative neurological deficits on survival of patients with glioblastoma. J Neurosurg. 2017;127:123–131. doi: 10.3171/2016.7.JNS16396. [DOI] [PubMed] [Google Scholar]
- 26.De Witt Hamer PCPC, Robles SGSG, Zwinderman AHAH, et al. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. 2012;30:2559–2565. doi: 10.1200/JCO.2011.38.4818. [DOI] [PubMed] [Google Scholar]
- 27.Jakola AS, Gulati S, Weber C, et al. Postoperative deterioration in health related quality of life as predictor for survival in patients with glioblastoma: a prospective study. PLoS ONE. 2011 doi: 10.1371/journal.pone.0028592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sagberg LM, Solheim O, Jakola AS. Quality of survival the 1st year with glioblastoma: a longitudinal study of patient-reported quality of life. J Neurosurg. 2016;124:989–997. doi: 10.3171/2015.4.JNS15194. [DOI] [PubMed] [Google Scholar]
- 29.Landriel Ibañez FA, Hem S, Ajler P, et al. A new classification of complications in neurosurgery. World Neurosurg. 2011;75:709–715. doi: 10.1016/j.wneu.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Gozal YM, Aktüre E, Ravindra VM, et al. Defining a new neurosurgical complication classification: lessons learned from a monthly Morbidity and Mortality conference. J Neurosurg. 2020;132:272–276. doi: 10.3171/2018.9.JNS181004. [DOI] [PubMed] [Google Scholar]
- 31.Wong JM, Panchmatia JR, Ziewacz JE, et al. Patterns in neurosurgical adverse events: intracranial neoplasm surgery. Neurosurg Focus. 2012;33:1–3. doi: 10.3171/2012.7.FOCUS12183. [DOI] [PubMed] [Google Scholar]
- 32.Rolston JD, Han SJ, Lau CY, et al. Frequency and predictors of complications in neurological surgery: national trends from 2006 to 2011: clinical article. J Neurosurg. 2014;120:736–745. doi: 10.3171/2013.10.JNS122419. [DOI] [PubMed] [Google Scholar]
- 33.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agarwal PK, Sammon J, Bhandari A, et al. Safety profile of robot-assisted radical prostatectomy: a standardized report of complications in 3317 patients. Eur Urol. 2011;59:684–698. doi: 10.1016/j.eururo.2011.01.045. [DOI] [PubMed] [Google Scholar]
- 35.Fan ST, Mau Lo C, Poon RTP, et al. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg. 2011;253:745–758. doi: 10.1097/SLA.0b013e3182111195. [DOI] [PubMed] [Google Scholar]
- 36.Seely AJE, Ivanovic J, Threader J, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg. 2010;90:936–942. doi: 10.1016/j.athoracsur.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 37.Peng P, Hyder O, Firoozmand A, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:1478–1486. doi: 10.1007/s11605-012-1923-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Topal B, Fieuws S, Aerts R, et al. Pancreaticojejunostomy versus pancreaticogastrostomy reconstruction after pancreaticoduodenectomy for pancreatic or periampullary tumours: a multicentre randomised trial. Lancet Oncol. 2013;14:655–662. doi: 10.1016/S1470-2045(13)70126-8. [DOI] [PubMed] [Google Scholar]
- 39.Schiavolin S, Broggi M, Acerbi F, et al. The impact of neurosurgical complications on patients’ health status: a comparison between different grades of complications. World Neurosurg. 2015;84:36–40. doi: 10.1016/j.wneu.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Wells S, Tamir O, Gray J, et al. Are quality improvement collaboratives effective? A systematic review. BMJ Qual Saf. 2018;27:226–240. doi: 10.1136/bmjqs-2017-006926. [DOI] [PubMed] [Google Scholar]
- 41.Nadeem E, Olin SS, Hill LC, et al. Understanding the components of quality improvement collaboratives: a systematic literature review. Milbank Q. 2013;91:354–394. doi: 10.1111/milq.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schouten LMT, Hulscher MEJL, Van Everdingen JJE, et al. Evidence for the impact of quality improvement collaboratives: systematic review. Bmj. 2008;336:1491–1494. doi: 10.1136/bmj.39570.749884.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]