Abstract
Background
We previously summarized outcomes for 46 cladribine tablets (CladT)-treated patients with multiple sclerosis (MS) and confirmed or suspected COVID-19, as reported to the Merck KGaA Global Patient Safety Database. This report updates on these findings, to 15 January 2021, for a total of 272 reported cases of COVID-19 among CladT recipients.
Methods
Case definitions: confirmed (COVID-19 diagnostic test was positive); suspected (no confirmatory test performed/reported). Cases fulfilling the criteria of hospitalized, medically significant, or fatal were designated as serious and outcomes were classified per usual pharmacovigilance practice.
Results
The evaluable cohort comprised 261 patients (confirmed COVID-19, n=160; suspected, n=101); an additional 11 patients had symptoms compatible with COVID-19 but were not evaluated further given their negative diagnostic tests. Median time to onset of COVID-19 from the most recent preceding CladT treatment course was 162 days (n=139). Outcomes were: recovered/recovering, n=133 (51%); not recovered/not resolved, n=19 (7%); died, n=1 (0.4%); and not reported/missing/pending, n=108 (41%). Of the total cohort, 40 (15%) experienced serious COVID-19.
Conclusion
Our results suggest that CladT-treated patients with MS are generally not at greater risk of serious disease and/or a severe outcome with COVID-19 compared with the general population and other patients with MS who acquired COVID-19.
Key words: Cladribine tablets, COVID-19, Multiple sclerosis
The COVID-19 pandemic continues to cause unprecedented disruption to normal social and economic life, with 113.4 million cases reported worldwide and over 2.5 million deaths (as of 28 February 2021; https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/). For patients with multiple sclerosis (MS), the pandemic has been of particular concern because of the co-morbidities of this disease as well as the disease-modifying therapies (DMTs) used in its management. Against this background, advice on COVID-19 for patients with MS has been issued by the likes of the MS International Federation (http://www.emsp.org/news-messages/coronavirus-disease-covid-19-and-multiple-sclerosis/) and others, including guidance on continued use of DMTs during the pandemic and the need to discuss specific risks with their MS healthcare provider (https://ms-coalition.org/guidance-for-the-use-of-disease-modifying-therapies-during-the-covid-19-pandemic/).
The fast-evolving nature of the pandemic has been accompanied by numerous case reports of patients with MS who acquired COVID-19 while on treatment with DMTs (Sormani et al., 2021), including cladribine tablets (Celius, 2020; Dersch et al. 2020). Indeed, we previously published a summary of real-world outcomes for 46 cladribine tablets-treated patients with MS and confirmed or suspected COVID-19 (as of 29 June 2020) (Jack et al., 2020). Here, we report an update on our original findings.
In the current analysis, also conducted on data extracted from the Merck KGaA Global Patient Safety Database, COVID-19 cases were defined as confirmed if a diagnostic test was reported as positive, while cases were described as suspected if no confirmatory test was performed or reported. Cases fulfilling the criteria of hospitalized, considered to be medically significant, or fatal were designated as serious, while outcomes were classified as recovered/recovering, not recovered, or not reported/missing/pending in accordance with usual pharmacovigilance practice.
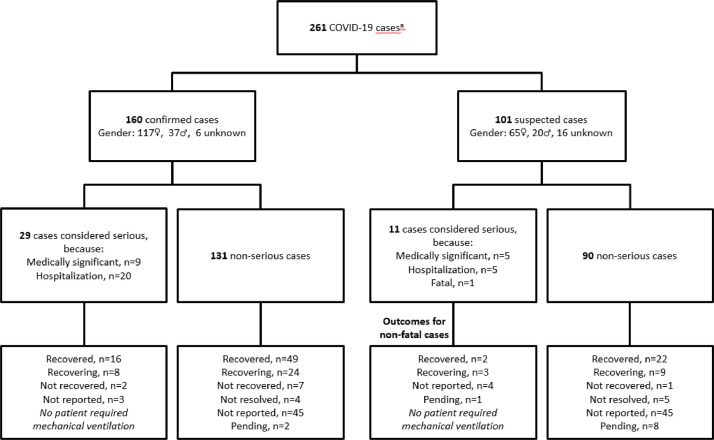
Since our initial report, the pharmacovigilance database now includes 272 patients with confirmed or suspected COVID-19 (as of 15 January 2021). Of the 272 patients, 11 had symptoms compatible with COVID-19 but were not evaluated further because they had negative PCR tests. The evaluable cohort therefore comprised 261 patients (confirmed COVID-19, n=160; suspected, n=101 [Fig. 1 ]), who were predominantly female (182/261, 70%) with a median age of 41 years. A small proportion of patients was aged ≥60 years, with a higher proportion of those with serious COVID-19 aged ≥60 years (Table 1 ). Excluding the minor proportion of patients who were asymptomatic, typically having had close contact with a COVID-19-positive individual (21/261, 8%), the majority had mild to moderate respiratory symptoms. Among 160 confirmed cases, 29 were classified as serious due to hospitalization (n=20) or because the reporting physician had noted “medically significant” (n=9). Among the 101 suspected COVID-19 cases, 11 were classified as serious (hospitalization, n=5; “medically significant”, n=5; fatal, n=1). Forty cases (15%) were therefore categorized as serious (Table 1). As per our earlier report (Jack et al., 2020), we found no indication for relevant involvement of other organ systems and no ischemic complications were reported. Several patients who experienced COVID-19 onset shortly before commencing Year 2 of treatment with cladribine tablets had treatment delayed until symptoms resolved, but were successfully treated thereafter.
Fig. 1.
Overview of COVID-19 cases and outcomes in patients treated with cladribine tablets, to 15 January 2021.
Table 1.
Demographics and patient characteristics for suspected or confirmed COVID-19 cases (to 15 January 2021).
| All patients (n=261)a | |
|---|---|
| Median age, years (range) | 41 (18–73) |
| Aged ≥60 years, n (%) | 15 (5.7) |
| Not reported, n (%) | 51 (19.5) |
| Sex, n (%) | |
| Male | 57 (21.8) |
| Female | 182 (69.7) |
| Not reported | 22 (8.4) |
| Confirmed COVID-19, n (%) | 160 (61.3) |
| Asymptomatic, n (%) | 21 (8.0) |
| Median time to COVID-19 from most recent treatment course, days (range) | 162 (0–643); n=139 |
| Most recent course of cladribine tablets treatment before COVID-19, n (%) | |
| Year 1 | 87 (33.3) |
| Year 2 | 32 (12.3) |
| Unknown or not reported | 142 (54.4) |
| Serious COVID-19 cases (n=40) | |
| Median age, years (range) | 47 (24–73) |
| Aged ≥60 years, n (%) | 7 (17.5) |
| Not reported, n (%) | 1 (2.5) |
| Sex, n (%) | |
| Male | 9 (22.5) |
| Female | 31 (77.5) |
| Confirmed COVID-19, n (%) | 29 (72.5) |
| Median time to COVID-19 from most recent treatment course, days (range) | 162 (4–643); n=31 |
| Most recent course of cladribine tablets treatment before COVID-19, n (%) | |
| Year 1 | 19 (47.5) |
| Year 2 | 3 (7.5) |
| Unknown or not reported | 18 (45.0) |
An additional 11 patients had symptoms compatible with COVID-19 but were not evaluated further given negative PCR tests.
Outcomes are summarized in Fig. 1 and Table 2 , including outcomes according to arbitrary age categories (<60 years and ≥60 years). For all cases combined, 51% of patients (133/261) recovered or were recovering from the disease at the time of the analysis; the corresponding value was 72.5% (29/40) for patients categorized as having serious COVID-19. Among the suspected COVID-19 cases one fatality was reported, a patient who died in late 2020 having experienced pneumonia and renal failure; the results of COVID-19 testing were not provided. This case fatality rate of 0.4% for the total cohort compares favorably with worldwide population findings (fatality rate of 2.2%, as of 28 February 2021; https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/). Moreover, the findings suggest that cladribine tablets-treated patients who acquire COVID-19 are generally not at greater risk of serious disease and/or a severe outcome, including mortality, compared with pharmacovigilance sources for other DMTs in patients with MS (Louapre et al., 2020; Sharifian-Dorche et al., 2021; Sormani et al., 2021).
Table 2.
Summary of outcomes (to 15 January 2021), including outcomes according to agea.
| All patients | Total(n=261) | Aged<60 years (n=195) | Aged≥60 years (n=15) |
|---|---|---|---|
| Recovered/recovering, n (%) | 133 (51.0) | 109 (55.9) | 5 (33.3) |
| Not recovered/not resolved, n (%) | 19 (7.3) | 15 (7.7) | 2 (13.3) |
| Died, n (%) | 1 (0.4) | 0 | 1 (6.7) |
| Not reported/missing/pending, n (%) | 108 (41.4) | 71 (36.4) | 7 (46.7) |
| Serious COVID-19 cases | Total(n=40) | Aged<60 years (n=32) | Aged≥60 years (n=7) |
|---|---|---|---|
| Recovered/recovering, n (%) | 29 (72.5) | 25 (78.1) | 3 (42.9) |
| Not recovered/not resolved, n (%) | 2 (5.0) | 2 (6.3) | 0 |
| Died, n (%) | 1 (2.5) | 0 | 1 (14.3) |
| Not reported/missing/pending, n (%) | 8 (20.0) | 5 (15.6) | 3 (42.9) |
Age not known for 51 patients, including 1 patient in the serious COVID-19 subgroup.
The time to onset of COVID-19 from the most recent preceding treatment with cladribine tablets is of interest because of the reduction in circulating lymphocyte counts that occurs following each annual treatment course (at the beginning of Years 1 and 2). The total lymphocyte nadir occurs in months 2–3 after the start of each annual course, with lower lymphocyte counts usually observed in Year 2 of treatment. Data on timing of COVID-19 onset with respect to start of the most recent annual treatment course with cladribine tablets were available for 139/261 patients (53%), and corresponded to a median of 162 days (i.e. approximately 5 months after the start of the preceding course; range, 0–643 days). Among those with available data (n=119), many patients had completed Year 1 of treatment only before COVID-19, while a small proportion had completed Year 2. Findings for median time to onset of COVID-19 among the cohort of serious patients were similar to the total cohort, although a slightly higher proportion were in their first year of treatment with cladribine tablets (Table 1). Very few patients had total lymphocyte counts measured at or around the time of COVID-19, and therefore it was not possible to determine if there was a link between low lymphocyte counts and disease onset. However, the median timing of onset of COVID-19 and the absence of a preponderance towards patients in Year 2 of treatment does not suggest a direct link between reduced lymphocyte counts associated with cladribine tablets and the risk of COVID-19.
We examined the potential impact of treatment with cladribine tablets on the ability of patients to mount an antibody response to the SARS-CoV-2 virus. In our database, a serology test was reported for 17 patients treated with cladribine tablets and all had a positive result. However, the details of the tests performed and their results were usually not specified. In several patients a positive serology result was found approximately 2–3 months after the infection. Similar experience has been reported by some (Preziosa et al., 2020) but not all authors (Gelibter et al., 2021) concerning a serological response after acquiring COVID-19 in patients treated with cladribine tablets. Further studies are needed to evaluate the impact of cladribine tablets on antibody response, not only in response to native infection but also COVID-19 vaccination.
There are some limitations concerning our analysis that should be highlighted. First, the safety database reflects voluntary reporting of pharmacovigilance data, and therefore data can be incomplete. Second, we are aware that two case reports from our database have been independently reported (Celius, 2020; Dersch et al. 2020). This potential for overlap – which exists for any pharmacovigilance database – should be taken into account concerning the precise number of patients with MS who have been affected by COVID-19 during treatment with cladribine tablets. These limitations aside, our analysis reached similar conclusions to those reported from international and national registries of COVID-19 among patients with MS (Louapre et al., 2020; Sormani, 2020).
In summary, our updated findings suggest that patients treated with cladribine tablets for MS are generally not at greater risk of serious disease and/or a severe outcome with COVID-19 compared with either the general population or the population of MS patients.
Declaration of Competing Interest
DJ and AN are employees of Merck KGaA, Darmstadt, Germany. DD is an employee of EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, a business of Merck KGaA, Darmstadt, Germany. AG is an employee of Merck, Aubonne, Switzerland, a division of Merck KGaA, Darmstadt, Germany.
Author Contributions
DJ and AG: Study design, data analysis and interpretation. DD: Data analysis and interpretation. AN: Study design, data curation, analysis and interpretation. All authors contributed to writing of the article and in the decision to submit for publication.
Funding and Role of Funding Source
This study was sponsored by Merck KGaA, Darmstadt, Germany. As noted under author contributions, the sponsor was involved with study design and in the collection, analysis, and interpretation of data. Medical writing assistance was provided by Steve Winter of inScience Communications, Springer Healthcare Ltd, UK, and was funded by Merck KGaA, Darmstadt, Germany.
References
- Celius E.G. Normal antibody response after COVID-19 during treatment with cladribine. Mult. Scler. Relat. Disord. 2020;46 doi: 10.1016/j.msard.2020.102476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dersch R., et al. COVID-19 pneumonia in a multiple sclerosis patient with severe lymphopenia due to recent cladribine treatment. Mult. Scler. 2020;26:1264–1266. doi: 10.1177/1352458520943783. [DOI] [PubMed] [Google Scholar]
- Gelibter S., et al. COVID-19 with no antibody response in a multiple sclerosis patient treated with cladribine: Implication for vaccination program? Mult. Scler. Relat. Disord. 2021;49 doi: 10.1016/j.msard.2021.102775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack D., et al. Favorable outcomes after COVID-19 infection in multiple sclerosis patients treated with cladribine tablets. Mult. Scler. Relat. Disord. 2020;46 doi: 10.1016/j.msard.2020.102469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C., et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.2581. online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preziosa P., et al. COVID-19 in cladribine-treated relapsing-remitting multiple sclerosis patients: a monocentric experience. J. Neurol. 2020 doi: 10.1007/s00415-020-10309-4. online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifian-Dorche M., et al. COVID-19 and disease-modifying therapies in patients with demyelinating diseases of the central nervous system: A systematic review. Mult. Scler. Relat. Disord. 2021;50 doi: 10.1016/j.msard.2021.102800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P. An Italian programme for COVID-19 infection in multiple sclerosis. Lancet Neurol. 2020;19:481–482. doi: 10.1016/S1474-4422(20)30147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann. Neurol. 2021;89(4):780–789. doi: 10.1002/ana.26028. online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]