Abstract
Coagulopathy, characterized by a high D-dimer level, is a common pathological occurrence in coronavirus disease 2019 (COVID-19) and is associated with poor prognosis. Severe cases with COVID-19 is associated with a significantly higher risk of deep vein thrombosis and acute pulmonary embolism. Pulmonary intravascular coagulopathy is the characteristic coagulopathy in COVID-19. Unlike sepsis-induced coagulopathy and disseminated intravascular coagulation, which are manifestations of systemic coagulopathy, pulmonary intravascular coagulopathy is a manifestation of a local coagulation disorder in the lung. The progression from pulmonary intravascular coagulopathy to sepsis-induced coagulopathy or disseminated intravascular coagulation in the context of COVID-19 may indicate that the patient's coagulation dysfunction has progressed from local to systemic. Exploring the associated coagulation disease will aid in the understanding of the pathophysiological mechanisms underlying severe COVID-19.
Keywords: COVID-19, Coagulopathy, Disseminated intravascular coagulation, Deep vein thrombosis, Acute pulmonary embolism
Introduction
Coronavirus disease 2019 (COVID-19) has become a global pandemic since it was first reported in Wuhan, China in December 2019. Despite its significant health and economic impacts, neither specific treatments nor effective antiviral drugs have been developed to date to combat this disease [1]. COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The binding of the SARS-CoV-2 spike protein to angiotensin-converting enzyme 2 (ACE2) receptors, which are highly expressed in type II pneumocytes [2], initiates marked inflammatory cell infiltration in lung tissue that results in a pulmonary and/or systemic inflammatory response [3]. The lung is the primary target organ of SARS-CoV-2, and acute lung injury and acute respiratory distress syndrome (ARDS) are the most common complications observed in COVID-19 patients [4]. However, SARS-CoV-2 infection has also been reported to be associated with coagulation disorders, characterized by high D-dimer levels, which are associated with poor prognosis [5], [6], [7]. Autopsy studies on COVID-19 patients have highlighted the presence of significant pulmonary microvascular disseminated microthrombosis [8,9]. In addition, emerging data suggest that severe COVID-19 is associated with a significantly higher risk of deep vein thrombosis (DVT) and acute pulmonary embolism (APE) [10,11]. However, because the characteristics of coagulopathy of COVID-19 are different from those of sepsis-induced coagulopathy (SIC) and disseminated intravascular coagulation (DIC), a new term has been presented, namely, pulmonary intravascular coagulopathy (PIC) [12].
What are the characteristics of COVID-19-related coagulopathy?
The initial coagulopathy in COVID-19 is characterized by a significant increase in D-dimer levels [5], [6], [7]. Meanwhile, abnormalities in prothrombin time (PT), activated partial thromboplastin time (APTT), and platelet count are relatively uncommon or mild Table 1 [5], [6], [7], [10], [13], [14], [15], [16], [17], [18]. In addition, a high fibrinogen level may indicate hypercoagulability, and thromboelastographic studies have revealed hypercoagulation in patients with COVID-19 [19,20]. Moreover, high incidences of DVT and APE have been reported in COVID-19 patients [10,11]. Given the characteristics of COVID-19 associated coagulopathy, several guidelines recommend routine heparin anticoagulation treatment [21,22]; however, the timing and indication of anticoagulant treatment remain controversial [23]. Moreover, there are reports of persistently high incidence rates of DVT and APE in patients with severe COVID-19 admitted to the intensive care unit (ICU) despite the administration of a therapeutic dose of heparin [24]. Nevertheless, Tang et al. [15] suggested that heparin anticoagulation may reduce mortality in patients whose D-dimer levels are increased by more than six-fold or those with SIC scores ≥4.
Table 1.
Literature on the association between coagulation abnormalities or markers of thrombosis and hemostasis in patients with COVID-19.
| Authors | Severity | N | Platelet count (× 109/L) | Prothrombin time (s) | Activated partial thromboplastin time (s) | Fibrinogen (g/L) | D-dimer (mg/L) | Antithrombin III (80–120%) |
|---|---|---|---|---|---|---|---|---|
| Wang D et al. [5] | 36 in ICU | 138 | No significant difference between the ICU and non-ICU groups (median: 142 vs. 165) | No significant difference between the ICU and non-ICU groups (median: 13.2 vs. 12.9) | No significant difference between the ICU and non-ICU groups (median: 30.4 vs. 31.7) | NA | D-dimer levels were significantly higher in the ICU group than in the non-ICU group (median: 0.41 vs. 0.16) | NA |
| Tang N et al. [6] | NA | 183 | NA | NA Significantly higher in non-survivors than in survivors (median: 15.5 vs. 13.6; normal range: 11.5-14.5) | No significant difference (median: 44.8 vs. 41.2; normal range: 29–42) | No significant difference (median: 5.16 vs. 4.51) | Non-survivors had significantly higher levels than survivors (median: 2.12 vs. 0.61) | No significant difference (median: 84 vs. 91) |
| Zhou F et al. [7] | NA | 191 | NA | Significantly higher in non-survivors than in survivors (median: 12.1 vs. 11.4), and PT was associated with mortality | NA | NA | Non-survivors has significantly higher levels than survivors (median: 5.2 vs. 0.6). D-dimer levels greater than 1 mg/L were associated with increase mortality | NA |
| Helms J et al. [10] | All in ICU | 150 | 80% normal | 108 people in the normal range | 100 people in the normal range | Most patients (>95%) had an increase (median: 6.99) | Most patients (>95%) had elevated D-dimer levels (median: 2.27) | All normal |
| Fogarty H et al. [13] | 23 in ICU | 83 | 83.1% normal, only 5 showed a decrease | No increase | No increase | Significant increase (median: 4.7). None of the patients had a decrease at any time | D-dimer levels were significantly increased. Above the normal range in 67% | NA |
| Huang C et al. [14] | 13 in ICU | 41 | 4.88% thrombocytopenia | PT was higher in ICU patients (median: 12.2 vs. 10.7) | No significant difference between ICU and non-ICU groups | NA | D-dimer levels were higher in the ICU patients (median: 2.4 vs. 0.5) | NA |
| Tang N et al. [15] | All had severe disease | 449 | Lower in non-survivors than in survivors, but all were within the normal range. The platelet count was negatively correlated with 28-day mortality | Significantly higher in non-survivors than that in survivors (median: 16.5 vs. 14.6). PT was positively correlated with 28-day mortality | NA | NA | Significantly higher in non-survivors than in survivors (median: 4.70 vs. 1.47). When D-dimer levels were 6-fold higher than the upper limit of normal, mortality was reduced by 20% in patients treated with heparin | NA |
| Chen N et al. [16] | NA | 99 | 12% thrombocytopenia | Above the normal range in 30% | Above the normal range in 16% | NA | Above the normal range in 36% | NA |
| Reun B et al. [17] | NA All in ICU | 75 | Normal (median: 270) | NA | NA | Increase (median: 7.3) | Increase (median: 48.6) | No significant change (median: 0.91) |
| Yin S et al. [18] | All had severe disease | 553 | Significantly lower in patients with other diseases than in patients with COVID-19 (median: 188 vs. 215) | No significant difference between other pathogens and SARS-CoV-2 (median: 16.2 vs. 15.2) | NA | NA | Undifferentiated between other pathogens and SARS-CoV-2 (median: 2.52 vs. 1.94) | NA |
ICU: Intensive care unit; NA: Not available; PT: Prothrombin time.
What is PIC?
The concept of PIC was recently proposed by McGonagle et al. [12] who described PIC as a macrophage activation syndrome (MAS) associated with diffuse pulmonary immunothrombosis in COVID-19 patients [12]. The increase in circulating D-dimer concentrations reflects pulmonary vascular bed thrombosis with fibrinolysis, while the increase in myocardial enzyme concentrations reflects acute ventricular stress caused by pulmonary hypertension (PH) [12]. Several studies have reported finding high levels of infiltration by macrophages and other immune cells in the lung tissue of COVID-19 non-survivors. A similar change in immune cell infiltration was noted as a histological change in severe acute respiratory syndrome (SARS) [25], [26], [27]–28], supporting that PIC may be associated with MAS. However, it remains unclear whether these deceased patients had secondary bacterial or fungal pneumonia. Additionally, other autopsy studies have also demonstrated that the pathological pulmonary changes in COVID-19 patients were mainly due to lymphocyte infiltration [9,29,30], suggesting that PIC is not associated with MAS. These observations highlight the need to further explore the mechanism underlying the inflammatory response and the association between inflammation and coagulation in COVID-19-associated acute lung injury.
There are no standardized diagnostic criteria for sepsis-associated coagulation disease, and the SIC scoring system developed by Iba et al. [31] in 2016 includes only two coagulation indicators (platelet count and PT) and excludes fibrinogen and fibrin degradation products. Despite there being only two components, subsequent studies have shown that this SIC scoring system has a significantly superior sensitivity for predicting clinical outcomes in sepsis when compared with the International Society on Thrombosis and Haemostasis and the Japanese Association for Acute Medicine scoring systems for DIC [31,32]. The results of several studies have suggested that SIC may be an early phase of DIC [33], [34]–35] and that SIC can progress to DIC if the underlying etiology of sepsis is not resolved [27,29]. Unlike SIC or DIC, the main characteristic of COVID-19-associated PIC is the large increase in D-dimer levels, an indicator of fibrinolysis, while abnormalities in other coagulation indicators are rare (Table 2).
Table 2.
Summary of the differences among PIC, SIC, and DIC.
| Item | DIC diagnostic criteria | SIC | PIC | |
|---|---|---|---|---|
| ISTH | JAAM | |||
| Underlying disease | 0 point | 0 point | 0 point | NA |
| Platelet count (×109/L) | 50–100: 1 point < 50: 2 points |
≤ 120 or > 30% reduction/24 h: 1 point < 80 or > 50% reduction/24 h: 3 points |
100–150: 1 point < 100: 2 points |
Normal or slight decrease |
| Fibrin-related marker | FDP, D-dimer, SF Moderate increase: 2 points Strong increase: 3 points |
FDP (µg/mL) ≥ 10 but < 25: 1 point ≥ 25: 3 points |
None | Increase in D-dimer levels |
| Fibrinogen (g/L) | < 1: 1 point | None | None | Normal or a slight increase |
| PT | Prolonged PT (s) 3–6: 1 point > 6: 2 points |
PT ratio ≥ 1.2: 1 point |
PT ratio 1.2–1.4: 1 point > 1.4: 2 points |
Normal or a slight increase |
| Others | NA | SIRS score ≥ 3:1 point | Four items SOFA* = 1: 1 point ≥ 2: 2 points |
NA |
| Diagnosis | ≥ 5 points | ≥ 4 points | ≥ 4 points (coagulopathy) | NA |
DIC: Disseminated intravascular coagulation; FDP: Fibrin degradation product; ISTH: International Society on Thrombosis and Haemostasis; JAAM: Japanese Association for Acute Medicine; PIC: Pulmonary intravascular coagulopathy; PT: Prothrombin time; SIC: Sepsis-induced coagulopathy; SF: Soluble fibrin; SIRS: Systemic inflammatory response syndrome; SOFA: Sequential organ failure assessment; NA: Not available.
Four-item SOFA includes respiratory SOFA, cardiovascular SOFA, hepatic SOFA, and renal SOFA.
COVID-19: DIC, SIC, or PIC?
PIC is the characteristic coagulation dysfunction in COVID-19 patients; however, it remains unclear whether the changes in coagulation progress linearly from PIC to SIC and then to DIC. A comparison between the incidence of coagulopathy in patients with COVID-19 and SARS is shown in Table 3 [6,10,13,15,36,37]. Helms et al. [10] reported that 14.7% of ICU patients with COVID-19 had SIC. Meanwhile, a study by Tang et al. [15] found that 21.6% of COVID-19 patients had a SIC score of ≥ 4 and that 71.4% of non-survivors met the criteria for DIC, which was diagnosed at an average of 4 days after admission [6]. However, other studies reported fewer patients with DIC and fewer clinical manifestations, such as bleeding (Table 3) [10,13]. Research indicates that COVID-19 patients diagnosed with DIC [6] or SIC [15] have higher mortality than patients without these complications. Although no definite diagnostic criteria for PIC are available, an increase in D-dimer levels is a feature of this coagulopathy. Additionally, a high D-dimer level is associated with the severity of COVID-19 [6,7,14].
Table 3.
Comparison of the incidence of coagulopathy in patients with COVID-19 and SARS.
| Authors | Severity | N | SIC | DIC |
|---|---|---|---|---|
| COVID-19 | ||||
| Tang et al. [6] | NA | 183 | NA | 71.4% of non-survivors met ISHT-DIC criteria; only 1 (0.6%) survivor met DIC criteria 4 days after admission |
| Helms et al. [10] | All in ICU | 150 | Only 22 patients (14.7%) had a positive SIC score | Only 6 patients (2.7%) had a positive JAAM score, 144 patients (96%) had a normal JAAM-DIC score. All patients had a normal ISTH-DIC score |
| Fogarty et al. [13] | 23 in ICU | 83 | NA | None met DIC criteria at the time of admission |
| Tang et al. [15] | All had severe COVID-19 | 449 | 21.6% had a SIC score ≥ 4. Heparin treatment was associated with lower mortality in patients with a SIC score ≥ 4, but not in those with a SIC score < 4 | NA |
| Lodigiani et al. [36] | 48 in ICU | 388 | NA | 2.1% of patients met overt DIC criteria, no bleeding complications occurred |
| SARS | ||||
| Wong et al. [37] | NA | 157 | NA | 2.5% of patients developed overt DIC |
COVID-19: Coronavirus disease 2019; DIC: Disseminated intravascular coagulation; ICU: Intensive care unit; ISHT: International Society on Thrombosis and Haemostasis; JAAM: Japanese Association for Acute Medicine; SARS: Severe acute respiratory syndrome; SIC: Sepsis-induced coagulopathy; NA: Not available.
Hypercoagulability and a high risk of thrombosis are also important features of COVID-19 coagulation complications, and may aid in better understanding the differences among PIC, SIC, and DIC. The available literature relating to the incidence of venous thrombosis-associated events in COVID-19 and other types of pneumonia is shown in Table 4 [10,11,24,36,[38], [39], [40], [41], [42], [43], [44], [45], [46], [47]. These studies showed a markedly high incidence of thrombotic complications, particularly APE, in patients with COVID-19 (Table 4) [10,38]. This highlights that COVID-19-induced coagulation disorders are concentrated in the lung; however, systemic coagulation dysfunction may not be significant or specific.
Table 4.
Literature on the incidence of venous thrombosis-related events in patients with COVID-19 and those with other types of pneumonia.
| Authors | Severity | N | VTE | DVT | APE |
|---|---|---|---|---|---|
| COVID-19 | |||||
| Helms et al. [10] | All in ICU | 150 | NA | 2.00% | 16.70% |
| Cui et al. [11] | Severe disease | 81 | 25.0% | NA | NA |
| Llitjos et al. [24] | NA | 26 | 69.0% | NA | 23.00% |
| Lodigiani et al. [36] | NA | 362 | 4.4% | 1.70% | 2.80% |
| Klok et al. [38] | NA | 184 | 27.0% | 2.89% | 24.03% |
| Demelo-Rodríguez et al. [39] | NA | 156 | NA | 14.70% | NA |
| Wichmann et al. [40] | Autopsy findings | 12 | NA | 58.00% | 33.00% |
| Influenza A | |||||
| Obi ATet al. [41] | 36 with ARDS | 36 | 44.0% | 28.00% | 28.00% |
| Avnon et al. [42] | All in MICU | 20 | 25.0% | 15.00% | NA |
| CDC [43] | All in SICU | 10 | NA | NA | 50.00% |
| Harms et al. [44] | Autopsy findings | 8 | NA | NA | 62.50% |
| Mauad et al. [45] | Autopsy findings | 21 | NA | NA | 19.00% |
| SARS | |||||
| Chong et al. [46] | Autopsy findings | 8 | NA | 37.50% | 50.00% |
| Lew et al. [47] | 46 in ICU | 199 | NA | 7.54% | 5.53% |
APE: Acute pulmonary embolism; ARDS: Acute respiratory distress syndrome; CDC: Center for Disease Control and Prevention; COVID-19: Coronavirus disease; DVT: Deep vein thrombosis; ICU: Intensive care unit; MICU: Medical intensive care unit; SARS: Severe acute respiratory syndrome; SICU: Surgical intensive care unit; VTE; Venous thromboembolism; NA: Not available.
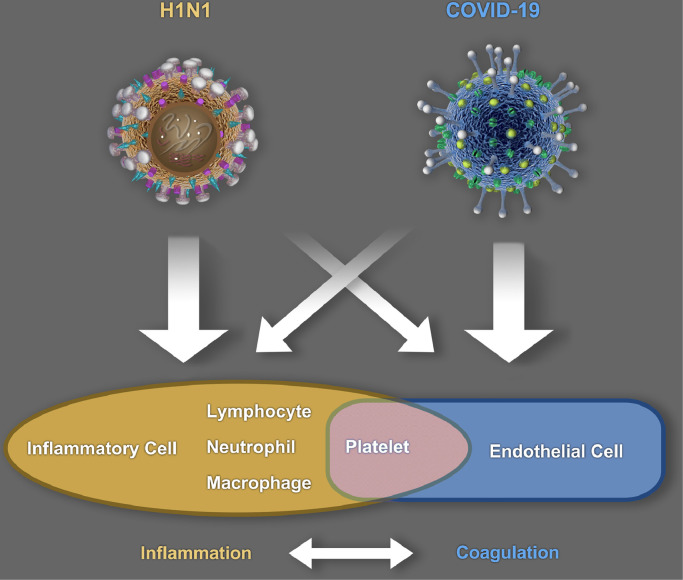
The limited evidence available indicates that the coagulopathy associated with non-COVID-19 pneumonia or ARDS is different from the PIC caused by SARS-CoV-2. The incidence of pulmonary embolism in COVID-19 patients with ARDS is six-fold higher than that in non-ARDS COVID-19 patients [10]. A recent autopsy study revealed severe endothelial cell damage and extensive microvascular thrombosis in the lungs of COVID-19 patients and reported widespread thrombosis with microangiopathy [9]. The incidence of alveolar capillary microthrombus and the amount of new vessel growth (predominantly through a mechanism of intussusceptive angiogenesis) in patients with COVID-19 are substantially higher than those in influenza A (H1N1) patients [9]. Our limited clinical experience and a review of existing studies also indicate that the incidence of massive alveolar effusion (which is generally caused by an increase in pulmonary microvascular permeability) in influenza A patients with severe ARDS is significantly higher than that seen in COVID-19 patients. This highlights the need to further explore the differences in clinical manifestations and pathophysiological mechanisms between COVID-19 and influenza A patients. Because of the central role of endothelial cell injury in coagulopathy, we propose the following hypothesis (Fig. 1): The pulmonary microvascular endothelial cell injuries in H1N1-infected patients might be mainly caused by pulmonary inflammatory responses, while the endothelial cell damage seen in COVID-19 patients might be mainly caused by the direct invasion of SARS-CoV-2. COVID-19-induced PIC may be associated with ACE2, a major component of the renin–angiotensin–aldosterone system, which is involved in the regulation of inflammatory coagulation processes and the fibrinolysis system [3,12,48]. SARS-CoV-2 is more pathogenic than SARS-CoV-1 because it has an at least 10- to 20-fold higher binding affinity for ACE2 [49]. This allows SARS-CoV-2 to more efficiently enter host cells, replicate, and damage cells, particularly endothelial cells [2,3]. Importantly, ACE2 is considered to have anti-inflammatory properties [50], which may explain the occult onset and protracted duration of COVID-19 (Fig. 1).
Fig. 1.
Differences in the pathogenesis of SARS-CoV-2 and H1N1. COVID-19: Coronavirus disease 2019; H1N1: Influenza A; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Overall, the main differences between PIC, SIC, and DIC are that PIC is the manifestation of a local coagulation disorder in the lung, while SIC and DIC are the manifestations of systemic coagulopathy. The possible mechanisms underlying PIC are as follows: (1) Thrombus formation and fibrin deposition in the lung activate the fibrinolytic system, resulting in an increase in D-dimer concentrations; or (2) although pulmonary thrombosis consumes clotting factors, these can be supplemented by the liver; thus, the changes in PT and APTT may be masked. Because fibrinogen is an acute-phase protein, the liver increases fibrinogen synthesis when systemic inflammation occurs, thereby increasing the concentration of fibrinogen in COVID-19 patients. When COVID-19 patients progress to SIC or DIC, this may indicate that their coagulation dysfunction has progressed from local to systemic. Multiple factors may be involved in the progression of PIC to SIC/DIC, including viremia, liver dysfunction, secondary infection, secondary multiple organ dysfunction, shock, severe hypoxia, and/or iatrogenic factors (e.g., continuous renal replacement therapy and frequent blood drawing).
The following questions warrant further discussion
Is PIC unique to COVID-19 or is it a characteristic of viral pneumonia-associated coagulopathy in general?
The incidence of APE in influenza A and SARS is also high, while the incidence of DIC in SARS is only 2.5% (Table 4) [38]. Currently, there are relatively few reports on the differences in coagulopathy between COVID-19 and other types of viral pneumonia [51], and further research is needed.
Which is the COVID-19 associated embolism, APE or pulmonary artery thrombosis (PAT)?
Studies have shown that the incidence of DVT is significantly lower than that of APE in patients with COVID-19 (Table 4). Consequently, some researchers believe that PAT may be the accurate term for COVID-19 associated embolism [52], [53]–54], as thrombi might form de novo in the pulmonary vascular system, rather than originating at a distant site. In addition, the high incidence of alveolar capillary microthrombus in COVID-19 patients [9] might indicate a more significant localized activation of the coagulation system and risk of thrombosis in the lung, rather than a systemic involvement.
Is pulmonary thrombosis associated with right ventricular dysfunction or PH?
Transthoracic echocardiography is a technique commonly used to evaluate right ventricular function in COVID-19 patients [55]. Dweck et al. [56] observed a 33% incidence of right heart dysfunction, a 15% incidence of right ventricular dilatation, and an 8% incidence of PH. Right heart dysfunction and PH in COVID-19 patients are caused by a variety of factors, such as ARDS, increased pulmonary vascular resistance due to hypoxia, and pulmonary thrombosis [11]. Van Dongen and colleagues reported a case of a patient with COVID-19 who developed PH diagnosed by echocardiography, without pulmonary embolism, likely as a result of residual marked pulmonary parenchymal abnormalities in combination with microvascular damage of the pulmonary arteries [57].
Is the absence of a D-dimer component for determining the SIC score reasonable?
The clinical significance of the fibrinolysis system in sepsis-related coagulation diseases merits re-examination. Studies have reported that the fibrinolytic shutdown that occurs in sepsis is characterized by increased plasminogen activator inhibitor 1 activity that leads to a low D-dimer level [58,59]. These results are in contrast to the coagulation changes observed in patients with COVID-19.
Conclusions
In conclusion, PIC is the characteristic coagulopathy in COVID-19 patients. Unlike SIC and DIC, which are manifestations of systemic coagulopathy, PIC is a manifestation of a local coagulation disorder in the lung. The progression from PIC to SIC or DIC in COVID-19 may indicate that a patient's coagulation dysfunction has progressed from local to systemic. In this context, exploring the associated coagulation disease will aid in the understanding of the pathophysiological mechanisms of severe COVID-19.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Funding
This work was supported by the Scientific Project of The Educational Department of Liaoning Province (Grant no. ZF2019010) and China Medical University's COVID-19 prevention- and control-related research projects. The sponsors did not play any role in the study design; collection, analysis, or interpretation of data; writing of the report; or deciding to submit the article for publication.
Managing Editor: Jingling Bao
References
- 1.Berlin D.A., Gulick R.M., Martinez F.J. Severe Covid-19. N Engl J Med. 2020;383(25):2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 2.Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J., et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J Pathol. 2020;251(3):228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henry B.M., Vikse J., Benoit S., Favaloro E.J., Lippi G. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: a novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin Chim Acta. 2020;507:167–173. doi: 10.1016/j.cca.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X., Ma X. Acute respiratory failure in COVID-19: is it "typical" ARDS. Crit Care. 2020;24(1):198. doi: 10.1186/s13054-020-02911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding Y., Wang H., Shen H., Li Z., Geng J., Han H., et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200(3):282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGonagle D., O'Donnell J.S., Sharif K., Emery P., Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2(7):e437–e445. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fogarty H., Townsend L., Ni Cheallaigh C., Bergin C., Martin-Loeches I., Browne P., et al. More on COVID-19 coagulopathy in Caucasian patients. Br J Haematol. 2020;189(6):1060–1061. doi: 10.1111/bjh.16791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beun R., Kusadasi N., Sikma M., Westerink J., Huisman A. Thromboembolic events and apparent heparin resistance in patients infected with SARS-CoV-2. Int J Lab Hematol. 2020;42(Suppl 1):19–20. doi: 10.1111/ijlh.13230. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2020:1–4. doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spiezia L., Boscolo A., Poletto F., Cerruti L., Tiberio I., Campello E., et al. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120(6):998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V., et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M., et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., Stergiou G.S., Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020;189(5):846–847. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Llitjos J.F., Leclerc M., Chochois C., Monsallier J.M., Ramakers M., Auvray M., et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18(7):1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oudit G.Y., Kassiri Z., Jiang C., Liu P.P., Poutanen S.M., Penninger J.M., et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39(7):618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholls J.M., Poon L.L., Lee K.C., Ng W.F., Lai S.T., Leung C.Y., et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361(9371):1773–1778. doi: 10.1016/s0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franks T.J., Chong P.Y., Chui P., Galvin J.R., Lourens R.M., Reid A.H., et al. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Hum Pathol. 2003;34(8):743–748. doi: 10.1016/s0046-8177(03)00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Q., Wang R.S., Qu G.Q., Wang Y.Y., Liu P., Zhu Y.Z., et al. Gross examination report of a COVID-19 death autopsy. Fa Yi Xue Za Zhi. 2020;36(1):21–23. doi: 10.12116/j.issn.1004-5619.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian S., Hu W., Niu L., Liu H., Xu H., SY Xiao. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients With Lung Cancer. J Thorac Oncol. 2020;15(5):700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iba T., Nisio M.D., Levy J.H., Kitamura N., Thachil J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: a retrospective analysis of a nationwide survey. BMJ Open. 2017;7(9) doi: 10.1136/bmjopen-2017-017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iba T., Arakawa M., Di Nisio M., Gando S., Anan H., Sato K., et al. Newly proposed sepsis-induced coagulopathy precedes international society on thrombosis and haemostasis overt-disseminated intravascular coagulation and predicts high mortality. J Intensive Care Med. 2020;35(7):643–649. doi: 10.1177/0885066618773679. [DOI] [PubMed] [Google Scholar]
- 33.Iba T., Levy J.H., Warkentin T.E., Thachil J., van der Poll T., Levi M. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost. 2019;17(11):1989–1994. doi: 10.1111/jth.14578. [DOI] [PubMed] [Google Scholar]
- 34.Ding R., Wang Z., Lin Y., Liu B., Zhang Z., Ma X. Comparison of a new criteria for sepsis-induced coagulopathy and international society on thrombosis and haemostasis disseminated intravascular coagulation score in critically ill patients with sepsis 3.0: a retrospective study. Blood Coagul Fibrinolysis. 2018;29(6):551–558. doi: 10.1097/MBC.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iba T., Levy J.H., Yamakawa K., Thachil J., Warkentin T.E., Levi M. Proposal of a two-step process for the diagnosis of sepsis-induced disseminated intravascular coagulation. J Thromb Haemost. 2019;17(8):1265–1268. doi: 10.1111/jth.14482. [DOI] [PubMed] [Google Scholar]
- 36.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong R.S., Wu A., To K.F., Lee N., Lam C.W., Wong C.K., et al. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ. 2003;326(7403):1358–1362. doi: 10.1136/bmj.326.7403.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klok F.A., Kruip M., van der Meer N., Arbous M.S., Gommers D., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demelo-Rodríguez P., Cervilla-Muñoz E., Ordieres-Ortega L., Parra-Virto A., Toledano-Macías M., Toledo-Samaniego N., et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res. 2020;192:23–26. doi: 10.1016/j.thromres.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Obi A.T., Tignanelli C.J., Jacobs B.N., Arya S., Park P.K., Wakefield T.W., et al. Empirical systemic anticoagulation is associated with decreased venous thromboembolism in critically ill influenza A H1N1 acute respiratory distress syndrome patients. J Vasc Surg Venous Lymphat Disord. 2019;7(3):317–324. doi: 10.1016/j.jvsv.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Avnon L.S., Munteanu D., Smoliakov A., Jotkowitz A., Barski L. Thromboembolic events in patients with severe pandemic influenza A/H1N1. Eur J Intern Med. 2015;26(8):596–598. doi: 10.1016/j.ejim.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention (CDC). Intensive-care patients with severe novel influenza A (H1N1) virus infection - Michigan, June 2009. MMWR Morb Mortal Wkly Rep. 2009;58(27):749–52. [PubMed]
- 44.Harms P.W., Schmidt L.A., Smith L.B., Newton D.W., Pletneva M.A., Walters L.L., et al. Autopsy findings in eight patients with fatal H1N1 influenza. Am J Clin Pathol. 2010;134(1):27–35. doi: 10.1309/AJCP35KOZSAVNQZW. [DOI] [PubMed] [Google Scholar]
- 45.Mauad T., Hajjar L.A., Callegari G.D., da Silva L.F., Schout D., Galas F.R., et al. Lung pathology in fatal novel human influenza A (H1N1) infection. Am J Respir Crit Care Med. 2010;181(1):72–79. doi: 10.1164/rccm.200909-1420OC. [DOI] [PubMed] [Google Scholar]
- 46.Chong P.Y., Chui P., Ling A.E., Franks T.J., Tai D.Y., Leo Y.S., et al. Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: challenges in determining a SARS diagnosis. Arch Pathol Lab Med. 2004;128(2):195–204. doi: 10.1043/1543-2165(2004)128<195:AODDTS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 47.Lew T.W., Kwek T.K., Tai D., Earnest A., Loo S., Singh K., et al. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290(3):374–380. doi: 10.1001/jama.290.3.374. [DOI] [PubMed] [Google Scholar]
- 48.Felmeden D.C., Lip G.Y. The renin-angiotensin-aldosterone system and fibrinolysis. J Renin Angiotensin Aldosterone Syst. 2000;1(3):240–244. doi: 10.3317/jraas.2000.036. [DOI] [PubMed] [Google Scholar]
- 49.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. .e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J., et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J Pathol. 2020;251(3):228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ng K.H., Wu A.K., Cheng V.C., Tang B.S., Chan C.Y., Yung C.Y., et al. Pulmonary artery thrombosis in a patient with severe acute respiratory syndrome. Postgrad Med J. 2005;81(956):e3. doi: 10.1136/pgmj.2004.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Engelke C., Riedel M., Rummeny E.J., Marten K. Pulmonary haemangiosarcoma with main pulmonary artery thrombosis imitating subacute pulmonary embolism with infarction. Br J Radiol. 2004;77(919):623–625. doi: 10.1259/bjr/52485284. [DOI] [PubMed] [Google Scholar]
- 54.Ishizaka N., Kage N., Iida H., Mutoh S., Hirata Y., Komuro I., et al. Massive pulmonary artery thrombosis, pulmonary hypertension and untreated atrial septal defect. Cardiology. 2002;97(1):53–54. doi: 10.1159/000047421. [DOI] [PubMed] [Google Scholar]
- 55.Venkata C., Aruchamy S., Kasal J. Acute right ventricular dysfunction in a critically Ill patient with COVID-19. CASE (Phila). 2020;4(6):474–6. DOI: 10.1016/j.case.2020.08.007 [DOI] [PMC free article] [PubMed]
- 56.Dweck M.R., Bularga A., Hahn R.T., Bing R., Lee K.K., Chapman A.R., et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 2020;21(9):949–958. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Dongen C.M., Janssen M.T., van der Horst R.P., van Kraaij D.J., Peeters R.H., van den Toorn L.M., et al. Unusually rapid development of pulmonary hypertension and right ventricular failure after COVID-19 pneumonia. Eur J Case Rep Intern Med. 2020;7(7) doi: 10.12890/2020_001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Semeraro F., Colucci M., Caironi P., Masson S., Ammollo C.T., Teli R., et al. Platelet drop and fibrinolytic shutdown in patients with sepsis. Crit Care Med. 2018;46(3) doi: 10.1097/CCM.0000000000002919. e221-221e228. [DOI] [PubMed] [Google Scholar]
- 59.Levy J.H., Koster A., Quinones Q.J., Milling T.J., Key N.S. Antifibrinolytic therapy and perioperative considerations. Anesthesiology. 2018;128(3):657–670. doi: 10.1097/ALN.0000000000001997. [DOI] [PMC free article] [PubMed] [Google Scholar]