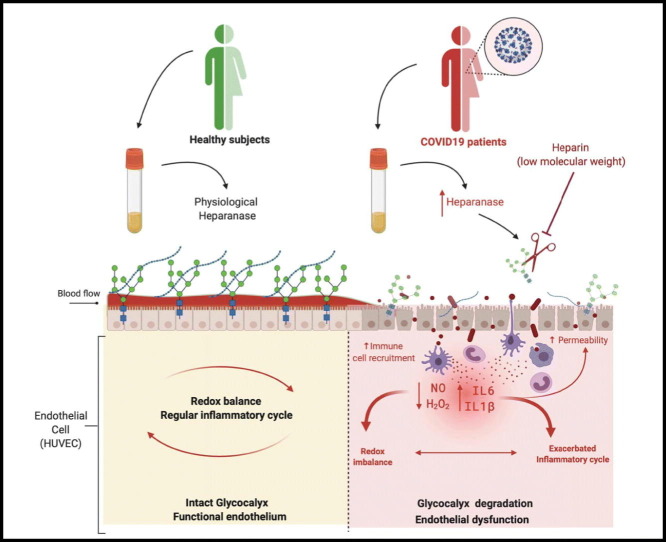
Abstract
The severe forms and worsened outcomes of COVID-19 (coronavirus disease 19) are closely associated with hypertension and cardiovascular disease. Endothelial cells express Angiotensin-Converting Enzyme 2 (ACE2), which is the entrance door for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The hallmarks of severe illness caused by SARS-CoV-2 infection are increased levels of IL-6, C-reactive protein, D-dimer, ferritin, neutrophilia and lymphopenia, pulmonary intravascular coagulopathy and microthrombi of alveolar capillaries. The endothelial glycocalyx, a proteoglycan- and glycoprotein-rich layer covering the luminal side of endothelial cells, contributes to vascular homeostasis. It regulates vascular tonus and permeability, prevents thrombosis, and modulates leukocyte adhesion and inflammatory response. We hypothesized that cytokine production and reactive oxygen species (ROS) generation associated with COVID-19 leads to glycocalyx degradation. A cohort of 20 hospitalized patients with a confirmed COVID-19 diagnosis and healthy subjects were enrolled in this study. Mechanisms associated with glycocalyx degradation in COVID-19 were investigated. Increased plasma concentrations of IL-6 and IL1-β, as well as increased lipid peroxidation and glycocalyx components were detected in plasma from COVID-19 patients compared to plasma from healthy subjects. Plasma from COVID-19 patients induced glycocalyx shedding in cultured human umbilical vein endothelial cells (HUVECs) and disrupted redox balance. Treatment of HUVECs with low molecular weight heparin inhibited the glycocalyx perturbation. In conclusion, plasma from COVID-19 patients promotes glycocalyx shedding and redox imbalance in endothelial cells, and heparin treatment potentially inhibits glycocalyx disruption.
Keywords: COVID-19, Glycocalyx, Heparan sulfate proteoglycans, Low molecular weight heparin, Vascular dysfunction, Endothelial cells
Graphical abstract
1. Introduction
The correlation between viral infection and cardiovascular disease gained strength in the last year with the emergence of the new β-coronavirus disease 2019 (COVID-19) [1]. The severe forms and worsened outcomes of COVID-19 are closely associated with hypertension and cardiovascular disease [2] and up to February 2021 the COVID-19 pandemic has affected more than one hundred and eleven million people worldwide [3].
Caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), COVID-19 may trigger intense and diffuse lung injury, that may progress to acute respiratory distress syndrome (ARDS), leading to respiratory failure and death [4,5]. SARS-CoV-2 [6] activates the immune system and increases cytokines production such as IL-1β, IL-6 and TNF-α [7], stimulates reactive oxygen species (ROS) generation [8] and coagulation cascade, increasing the risk for thrombosis in the macro and microvasculature [9]. Of importance, as reviewed by Libby and Lüscher [10], “COVID-19 is, in the end, an endothelial disease” [10].
Endothelial cells provide a crucial interface in host defenses, producing inflammatory cytokines, ROS and reactive nitrogen species (RNS), which contribute to immune responses against viral infections [11,12]. However, when inappropriately or excessively produced, ROS and RNS disrupt endothelial cell and vascular function [13,14]. The endothelial glycocalyx is one of the most redox-sensitive components that covers endothelial cells [15] and is composed of membrane-attached proteoglycans, glycosaminoglycan chains and glycoproteins [16]. The heparan sulfate proteoglycans, as syndecan and glypican, are the predominant constituent of the glycocalyx, ranging from 50 to 90% [17].
The glycocalyx structure contributes to vascular homeostasis. It modulates vascular tonus, acts as a selective permeable barrier, controlling vascular permeability, prevents microvascular thrombosis, and regulates endothelial cells interactions with immune cells [15], modulating leukocyte adhesion and inflammatory responses (see review [18]). In addition, the vascular endothelial glycocalyx responds to shear stress and is responsible for mechano-transduction signaling, being pivotal for adequate nitric oxide (NO) production [[19], [20], [21], [22], [23]], and redox control [24].
The degradation of glycocalyx is thought to contribute to vascular dysfunction in viral infections as in dengue [25,26], diseases caused by hantavirus [27], ebola virus [28] and influenza virus [29]. The vascular endothelial glycocalyx is more easily damaged in older adults than younger adults, and in common comorbidities such as chronic kidney disease [30], stroke [31,32], diabetes [33], and heart failure [34].
We hypothesized that the cytokine storm and redox imbalance present in plasma from COVID-19 patients leads to glycocalyx degradation, increasing cardiovascular risk. Therefore, this study determined the effects of plasma from SARS-CoV-2-infected patients on the glycocalyx structure of human umbilical vein endothelial cells (HUVECs).
2. Materials and methods
2.1. Human samples
The Brazilian National Committee for Ethics in Research (CONEP) approved all procedures performed in the study (CONEP CAAE: 30248420.9.0000.5440 and 30816620.0.0000.5440). In addition, written informed consent was obtained from all recruited patients.
Twenty hospitalized patients in the Hospital das Clínicas de Ribeirão Preto (Ribeirao Preto Medical School) with a RT-PCR of nasopharyngeal samples [35] proved SARS-CoV-2 infection as well as the detection of specific antibodies IgM and IgG against SARS-CoV-2 were confirmed in plasma samples. Blood from COVID-19 patients was collected between days 1 to 5 after admission to the emergency room in the Hospital das Clínicas de Ribeirão Preto. In addition, a control group with seven sex-matched healthy subjects was included in this study. Blood samples from all volunteers were collected in tubes containing ethylenediaminetetraacetic acid (EDTA), to obtain the plasma and in tubes without anticoagulant, to obtain the serum. Samples were stored at −80 °C for later analysis. Table 1 summarizes clinical information, biochemical parameters and therapeutic treatments for COVID-19 patients.
Table 1.
Baseline characteristics of COVID-19 patients.
| Characteristics | COVID-19 patients (n = 20) | Critical COVID-19 (n = 10) | Mild-to-severe COVID-19 (n = 10) | p value |
|---|---|---|---|---|
| Demographic | ||||
| Age (years), median IQR | 57 (50–70) | 57 (50–70) | 59 (47–71) | 0.94 |
| Male gender (%) | 48% | 70% | 30% | 0.08 |
| Female gender (%) | 52% | 30% | 70% | 0.08 |
| Comorbidities | ||||
| Hypertension (%) | 65% | 70% | 60% | 0.68 |
| Heart disease | 50% | 40% | 30% | 0.68 |
| Diabetes (%) | 45% | 30% | 70% | 0.20 |
| Obesity (%) | 50% | 30% | 70% | 0.09 |
| BMI, median, IQR | 28 (25–35) | 27.7 (25–31) | 31.3 (24–42) | 0.72 |
| Clinical characteristics | ||||
| Days of symptoms (mean, ±sd) | 13 ± 5 | 13 ± 4 | 13 ± 6 | 0.82 |
| Hospitalization (days), mean, ±sd | 18 ± 11 | 26 ± 11 | 11 ± 4 | 0.0004⁎ |
| Mechanical ventilation (%) | 50% | 100% | 0 | 0.0001⁎ |
| Acute kidney injury (%) | 35% | 50% | 20% | 0.185 |
| Mortality rate (%) | 10% | 20% | 0 | 0.0001⁎ |
| Laboratory tests | ||||
| Creatinine, mg/dl, mean (IQR) | 1.1 ± 0.9 | 1.23 ± 1.34 | 0.94 ± 0.47 | 0.85 |
| Lymphocyte; mm3, median (IQR) | 1100 (600–1650) | 650 (475–1550) | 1295 (975–2450) | 0.09 |
| Platelets; ×103/l (IQR) | 246 (217–338) | 251 (218–369) | 243 (186–313) | 0.85 |
| RCP; mg/dl, median (IQR) | 13 (9–20) | 10.8 (6.9–14.1) | 7.1 (1.9–13.3) | 0.23 |
| D dimer; mg/dl, median (IQR) | 1.19 (0.93–1.98) | 1.3 (1–3.5) | 1 (0.8–1.7) | 0.43 |
| Lactate; mg/dl, median (IQR) | 2.2 (1.7–2.6) | 2.5 (2–2.6) | 1.7 (1.5–2.5) | 0.09 |
| Lactate dehydrogenase, U/l(IQR) | 361 (302–434) | 340 (292–438) | 383 (278–498) | 0.89 |
| Ferritin, ng/ml (IQR) | 608 (345–1076) | 608 (246–635) | 600 (345–1409) | 0.51 |
| Fibrinogen (mg/dl) | 686 (619–815) | 796 (619–833) | 683 (617–799) | 0.55 |
| Severity | ||||
| PaO2/FiO2 ratio, median (IQR) | 205 (139–315) | 158 (122–194) | 272 (211–358) | 0.01⁎ |
| SOFA score | 2 (1–3) | 3 (2–4.5) | 2 (1–2) | 0.005⁎ |
| SAPS-3 | 47 (38–50) | 47 (42–51) | 42 (34–47) | 0.11 |
BMI: body mass index; IQR: interquartile range; sd: standard deviation; RCP: reactive C protein; PaO2/FiO2 ratio: the ratio of arterial oxygen partial pressure (PaO2 in mm Hg) to fractional inspired oxygen; SOFA: sequential organ failure assessment; SAPS-3: simplified acute physiology score III.
p < 0.05, comparison between mild-to-severe and critical patients with COVID-19.
2.2. IgM-IgG combined antibody test
Analysis of IgG and IgM anti-SARS-CoV-2 antibodies was performed in serum from healthy subjects (control) and COVID-19 patients using the rapid test Asan Easy Test® COVID-19 IgG/IgM from Asan Pharmaceutical (Gyeonggi-do, Korea). Healthy subjects were not previously exposed to SARS-CoV-2 until to the moment of blood collection (Supplementary Fig. 1). The text is based in an immunochromatographic assay for the rapid qualitative detection of IgG and IgM antibodies through combination of particles coated with SARS-CoV-2 antigen. The Agência Nacional de Vigilância Sanitária (National Agency of Sanitary Vigilance, ANVISA, Brazil) licensed the Asan Easy Test COVID-19 IgG/IgM in May of 2020 (https://consultas.anvisa.gov.br/#/saude/q/?numeroRegistro=80198110005).
2.3. ELISA assay
Plasma samples were used to evaluate circulating cytokine levels by enzyme-linked immunosorbent assay (ELISA) using Human DuoSet ELISA (R&D Systems, Minneapolis, MN, USA). The assay was performed according to the manufacturer's instructions and the detection limits were as follows: TNF-α, 15.62–2000 pg/ml; IL-6, 9.37–1200 pg/ml; and IL-1β, 3.90–500 pg/ml.
Glycocalyx components, as hyaluronan (DuoSet ELISA) and heparan sulfate proteoglycans (ELISA, Cloud-Clone Corporation, Katy, TX, USA), were determined in plasma samples and in the supernatants of HUVECs (exposed to plasma for 12 h) by ELISA assay, according to the manufacturer's protocol. The detection limit for hyaluronan was 0.37–90 ng/ml and for heparan sulfate proteoglycans was 15.6–1000 pg/ml.
Heparanase activity was measured in plasma samples using Simple Step ELISA kit (Abcam, Cambridge, United Kingdom) and the detection limit established by manufacturer's guidelines was 125–8000 pg/ml.
2.4. TBARS assay
Thiobarbituric acid reactive substance (TBARS) assay was performed to measure malondialdehyde (MDA), which is an end product of lipid peroxidation and indicative of oxidative stress [36], using plasma samples according to the manufacturer's datasheet (Cayman Chemical, Ann Arbor, MI, USA).
2.5. HUVECs cells
Human umbilical vein endothelial cells (HUVECs) were purchased from ATCC cell lines (American Type Culture Collection, Manassas, VA – USA), cultured in Dulbecco's modified eagle's medium (DMEM) supplemented with sodium bicarbonate, HEPES, penicillin, streptomycin, amphotericin B, fetal bovine serum (FBS, 10%). Before any experiment, HUVECs were submitted to serum starvation (FBS 3%) [37,38]. Cells were cultured for at least 3 days before the experiments and used at passages 4–6.
2.6. Cytotoxicity assay
Supernatants of naive/non-treated HUVECs (Control), HUVECs treated with plasma from healthy subjects and HUVECs treated with plasma from COVID-19 patients (12 h of treatment) were used to measure lactate dehydrogenase (LDH), a stable cytosolic enzyme that is released upon cell lysis or damage, using the CytoTox 96® Non-Radioactive Cytotoxicity colorimetric assay according to the manufacturer's instructions (G1780, Promega Corporation, Madison, WI, USA).
2.7. Measurement of ROS and RNS in HUVECs
HUVECs were cultured at 4 × 104 cells/well in a 96-well assay plate in DMEM with fetal bovine serum (-FBS) 10% for 24 h (h). After 24 h, cells were serum deprived (3 h), washed twice with PBS (phosphate buffered saline) and incubated with 100 μL of DAF-2DA (5 μM + L-arginine 1 mM, for 30 min), or carboxy-H2DCFA (10 μM, for 60 min), or 7-CBA (20 μM, for 30 min), or DHE (2.5 μM, for 30 min). After this period, HUVECs were treated with plasma (10%) from healthy subjects and COVID-19 patients for 30 min.
The fluorescence produced by DAF-2DA (488 nm/530 nm; excitation/emission), 7-CBA (332 nm/475 nm), carboxy-H2DCFA (370 nm/420 nm; Excitation/Emission), DHE (379 nm/420 nm) was measured at a microplate reader (FlexStation-3, Molecular Devices, San Jose, CA, USA).
2.8. Western blot
HUVECs were cultured in DMEM and after serum deprivation for 3 h [37,38], the confluent HUVECs received no treatment (Control) or were treated with a “pool” of plasma (10%) - 2 plasma samples per pool were randomly chosen - from healthy subjects or COVID-19 patients for 30 min, 1 h, 12 h and 24 h. In a subsequent set of experiments, the time of 12 h to treat cells with plasma from COVID-19 patients in presence of low molecular weight heparin (LMWH, 10 μg/ml) was established. After treatments, cells were lysed in RIPA buffer supplemented with a protease inhibitor cocktail (PIC, P8340, Sigma-Aldrich, St Louis, MO, USA) and phosphatase inhibitors [NaF (1 mmol/l), Na3VO4 (1 mmol/l) and PMSF (10 mmol/l)]. Protein (30 μg) obtained from each sample was submitted to electrophoresis on polyacrylamide gel (8 to 15%) and transferred to a nitrocellulose membrane. Next, membranes were incubated with primary antibodies (overnight) against syndecan-1 (#sc-12765), syndecan-4 (#sc-12766) and glypican-1 (#sc-101827). The bands were detected by a chemiluminescent system (ImageQuant LAS 400, GE Life Science, Chicago, IL, USA). β-Actin was used to normalize the results. The bands were quantified with the ImageJ Software (NIH Image).
2.9. Immunofluorescence
HUVECs were plated in glass coverslips pretreated with 0.4% gelatin. When cells reached confluence, they were serum deprived for 3 h and treated for 12 h with plasma (10%) from control subjects, COVID-19 patients or COVID-19 patients in the presence of LMWH (10 μg/ml). Cells were fixed with 4% paraformaldehyde and permeabilized with triton 0.1% in HBSS. Cells were incubated with glypican-1 (#sc-101827) and CD34 (#IS63230-2) antibodies overnight at 4 °C. Then, cells were washed and incubated with Alexa Fluor ™ 647 and 488 antibodies for 2 h at room temperature followed up by incubation with DAPI. Slides were mounted and images obtained on a LSM 780 System on the Axio Observer microscope using Zen software. Images were quantified with ImageJ (NIH). Data are presented as relative fluorescent units (RFU, fluorescence on 647 channel normalized by the number of cells in the field assessed by DAPI staining).
2.10. Statistical analysis
The results are expressed as the mean ± standard error of the mean (SEM) and interquartile interval (IQR), depending on distribution tested by Shapiro Wilk test. In the experiments where HUVECs were used, n indicates the number of independent experiments. The statistical significance was determined by unpaired Student's t-test and one-way ANOVA with Tukey post-hoc test. The statistical analysis was performed with the Prism GraphPad 5.0 software and differences statistically significant were considered when p < 0.05.
3. Results
A total of 27 subjects were included in the present study, 20 patients that tested positive to SARS-CoV-2 and 7 healthy individuals in the control group. Similar numbers of men and women with COVID-19 were included (48% men and 52% women). The demographic, clinical, and biochemical characteristics for COVID-19 patients are shown in Table 1, and information from healthy subjects are shown in Supplementary Fig. 1.
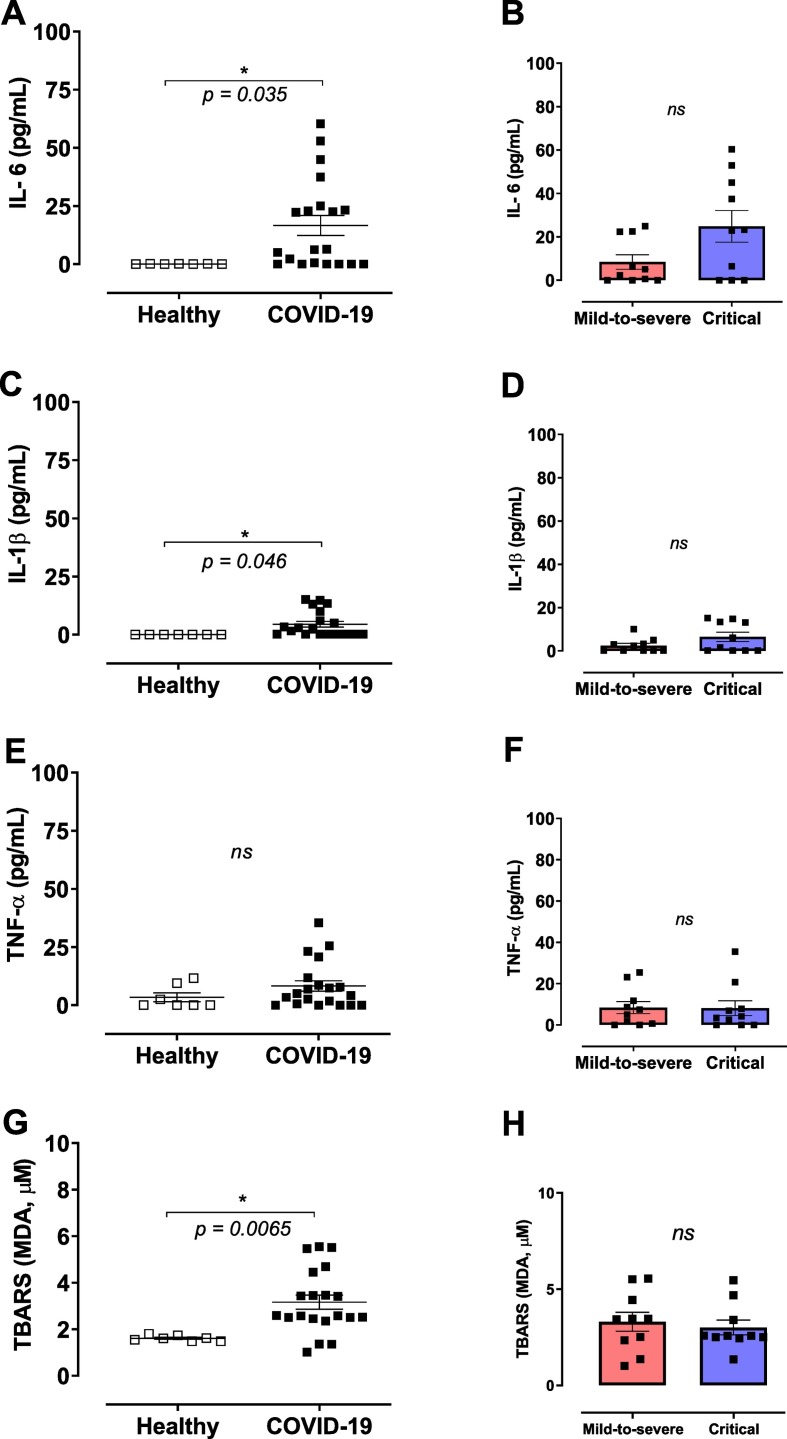
In all analyses, healthy subjects versus COVID-19 patients as well as mild-to-severe versus critical individuals with COVID-19 were compared. PaO2/FiO2 ratio was used to identify mild-to-severe and critical patients. 70% of critical patients were men, reinforcing sex differences in the severity of disease. Patients infected with SARS-CoV-2 showed significant higher plasma levels of IL-6 (Fig. 1A) and IL-1β (Fig. 1C) compared to healthy subjects. TNF-α levels did not differ between the groups (Fig. 1E). In addition, splitting data frames in moderate-to-severe and critical cases of COVID-19, showed no differences for IL-6, IL-1β or TNF-α levels, as shown in Fig. 1B, D, and F, respectively. Moreover, MDA levels were increased in patients with SARS-CoV-2 infection, in comparison to healthy subjects (Fig. 1G). MDA levels did not differ between moderate-to-severe and critical patients diagnosed with COVID-19 (Fig. 1H).
Fig. 1.
COVID-19 patients exhibit high concentrations of cytokines and augmented lipid peroxidation. Plasma was obtained from healthy controls and COVID-19 patients; cytokines were measured by ELISA and lipid peroxidation was determined by malondialdehyde (MDA) levels, which is an end product of lipid peroxidation and indicative of oxidative stress. (A) IL-6, (B) IL1-β, (C) TNF-α and (D) MDA in plasma from healthy controls (n = 7) and COVID-19 patients (n = 20). Data are shown as mean ± SEM. Student's t-test. *p < 0.05; ns: non-significant.
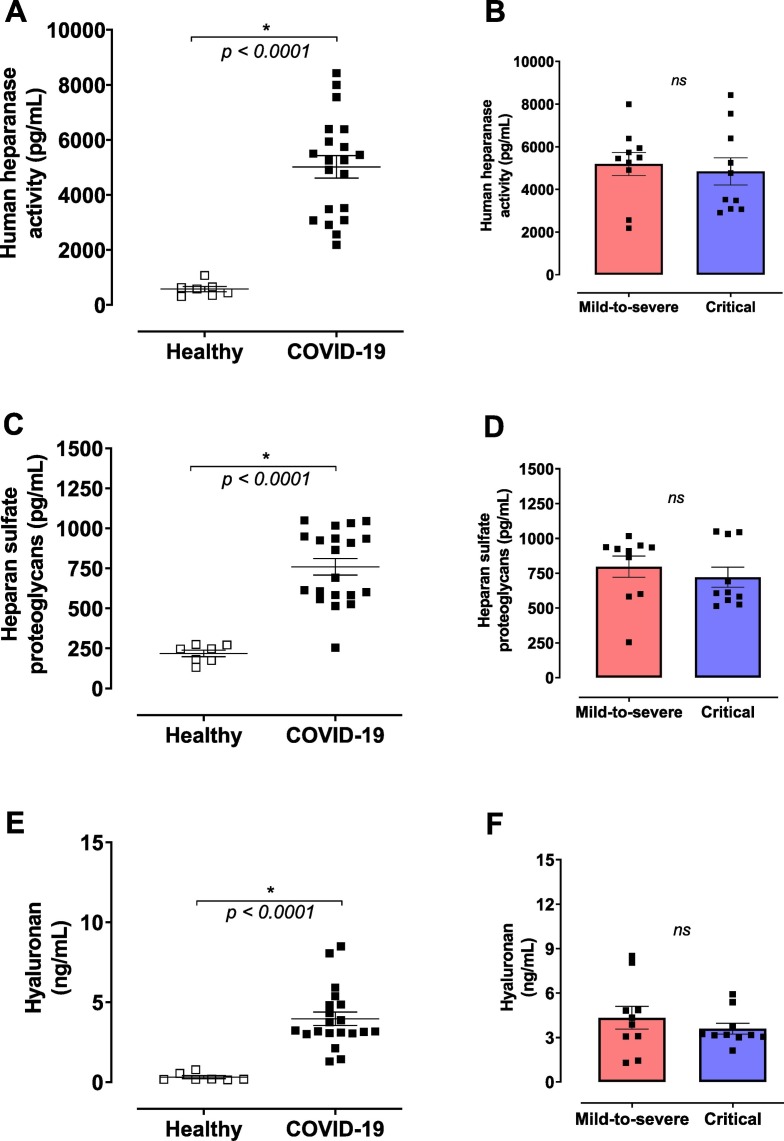
Glycocalyx integrity was evaluated by heparanase activity, by heparan sulfate and hyaluronan levels. Heparanase activity was significantly higher in the plasma from COVID-19 patients compared to healthy controls (Fig. 2A). In addition, circulating levels of heparan sulfate were also increased during SARS-CoV-2 infection (Fig. 2C), possibly due to the increased heparanase activity observed in patients with COVID-19. Hyaluronan plasma levels were also increased in COVID-19 patients compared to the control group (Fig. 2E). The analyses showed no differences between mild-to-severe and critical patients (Fig. 2B, D and F).
Fig. 2.
COVID-19 patients exhibit high concentrations of degradation glycocalyx components. Plasma was obtained from healthy controls and COVID-19 patients and glycocalyx components measured by ELISA. (A) Human heparanase activity, (B) heparan sulfate proteoglycans, and (C) hyaluronan in plasma from healthy controls (n = 7) or COVID-19 patients (n = 20). Data are shown as mean ± SEM. Student's t-test. *p < 0.05; ns: non-significant.
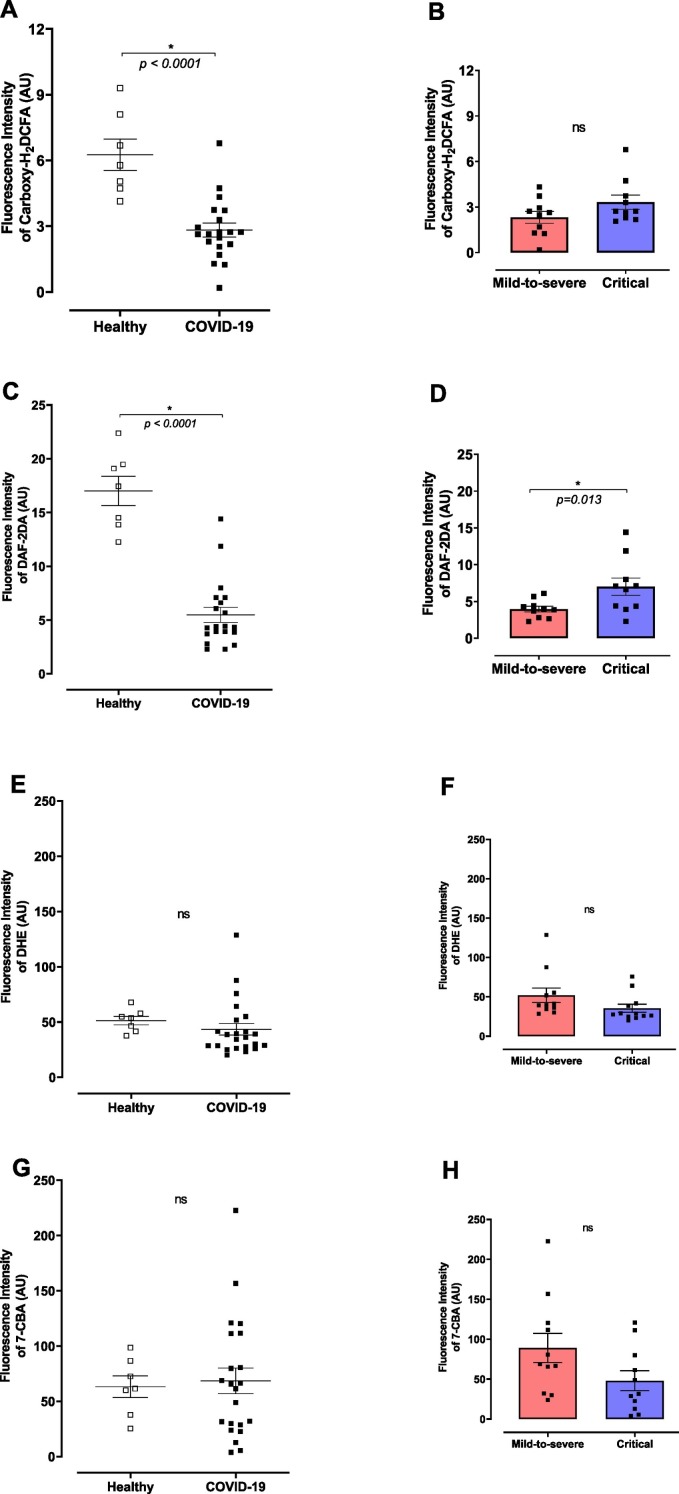
Considering that plasma samples from COVID-19 patients displayed higher levels of cytokines, MDA and heparanase activity, the effects of plasma from COVID-19 patients on the glycocalyx of healthy endothelial cells were determined. HUVECs stimulated for 30 min with plasma from COVID-19 patients showed lower concentrations of H2O2 (Fig. 3A) and NO (Fig. 3C), but not changes were observed in superoxide anion or peroxynitrite levels (Fig. 3E and G, respectively). No differences were observed in H2O2, superoxide anion or peroxynitrite levels between HUVECs stimulated with plasma from mild-to-severe and critical patients with COVID-19 (Fig. 3B, F and H, respectively). However, NO levels were higher in HUVECs treated with plasma from severe COVID-19 patients (Fig. 3D).
Fig. 3.
Plasma from COVID-19 patients reduces H2O2 and NO in healthy endothelial cells. (A) Hydrogen peroxide (H2O2), (B) nitric oxide (NO), (C) superoxide anion (O2•−), and peroxynitrite (ONOO−) levels in HUVECs treated with plasma from healthy subjects or COVID-19 patients. H2O2 was measured by Carboxy-H2DCFA (10 μM), NO by DAF-2DA (5 μM), peroxynitrite by 7-CBA (5 μM), and superoxide anion by DHE (2.5 μM) in 4 × 104 HUVECs cells. The values are expressed by as Fluorescence Intensity. Data are shown as mean ± SEM. Student's t-test. *p < 0.05; ns: non-significant.
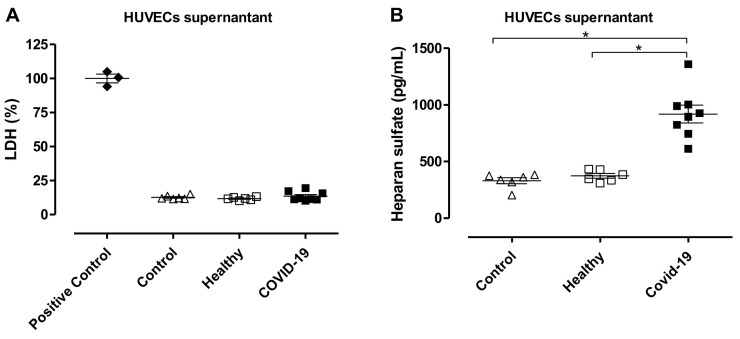
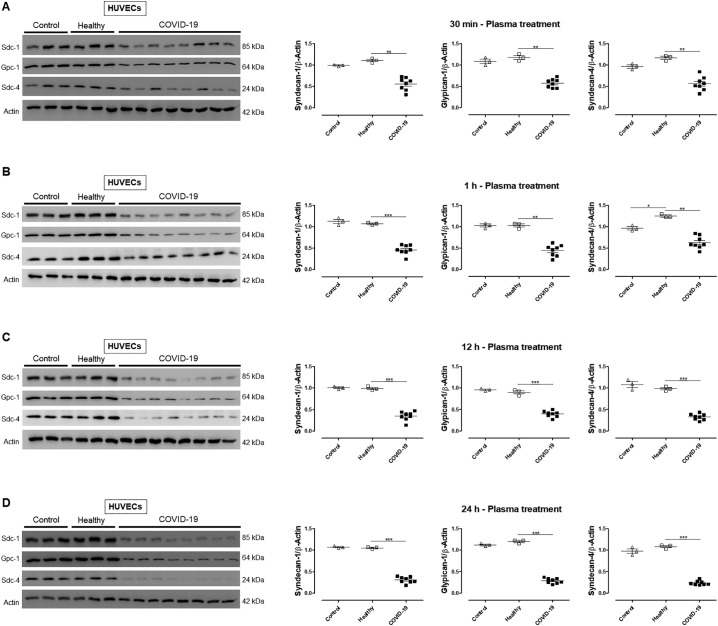
Since ROS imbalance may lead to glycocalyx degradation, we questioned whether HUVECs treated with plasma from COVID-19 patients exhibit glycocalyx shedding. HUVECs treated with plasma from COVID-19 patients or treated with plasma from healthy subjects for 12 h showed similar levels of LDH compared to Control HUVECs, indicating that plasma treatment did not damage or decrease viability of the cells (Fig. 4A). In addition, heparan sulfate levels were increased in HUVECs treated with plasma from COVID-19 patients for 12 h, demonstrating glycocalyx shedding (Fig. 4B). To confirm glycocalyx disruption, HUVECs were exposed to plasma for various time points (30 min, 1 h, 12 h, and 24 h). Treatment of HUVECs with plasma from COVID-19 patients decreased protein levels of important glycocalyx components such as syndecan-1, glypican-1, and syndecan-4 (Fig. 5 ). As treatment for 12 h did not decrease cells viability, but significantly decreased the expression of the analyzed proteins, this time point was used to evaluate the effects of heparin/LMWH.
Fig. 4.
Treatment of HUVECs with plasma from COVID-19 patients stimulates glycocalyx shedding but does not decrease cell viability. (A) Lactate dehydrogenase (LDH) levels and (B) heparan sulfate proteoglycans measured in supernatant from HUVECs without any treatment (Control, n = 6), HUVECs treated with plasma from healthy subjects (n = 6) or plasma from COVID-19 patients (n = 8). Data are shown as mean ± SEM. Student's t-test. *p < 0.05.
Fig. 5.
Plasma from COVID-19 patients reduces the expression of glycocalyx components. Immunoblot representative images (A, B, C, and D) and densitometric analysis (right) of protein levels of syndecan-1, glypican-1, and syndecan-4 in HUVECs non-treated (Control), treated with plasma from healthy subjects and COVID-19 patients for 30 min (A), 1 h (B), 12 h (C) and 24 h (D). The values are normalized by β-actin and expressed by absolute values. Data are representative of at least 3 experiments and are shown as mean ± SEM. Student's t-test. *p < 0.05. We processed images of blots changing brightness and contrast and we applied equally over the entire image.
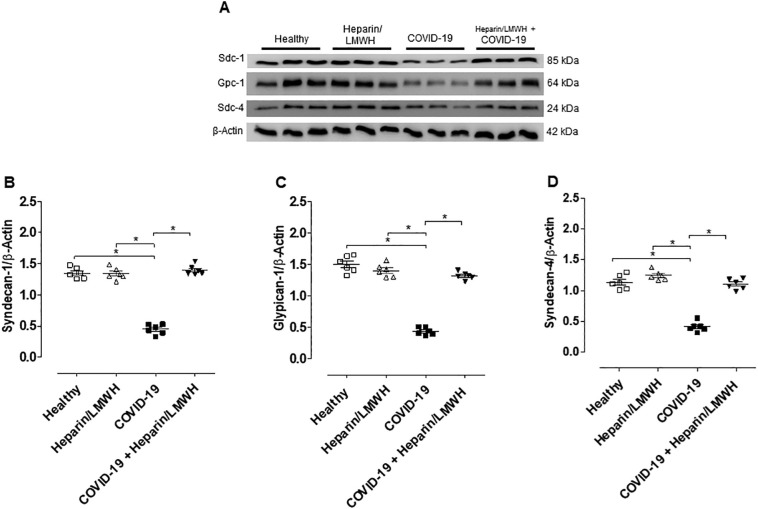
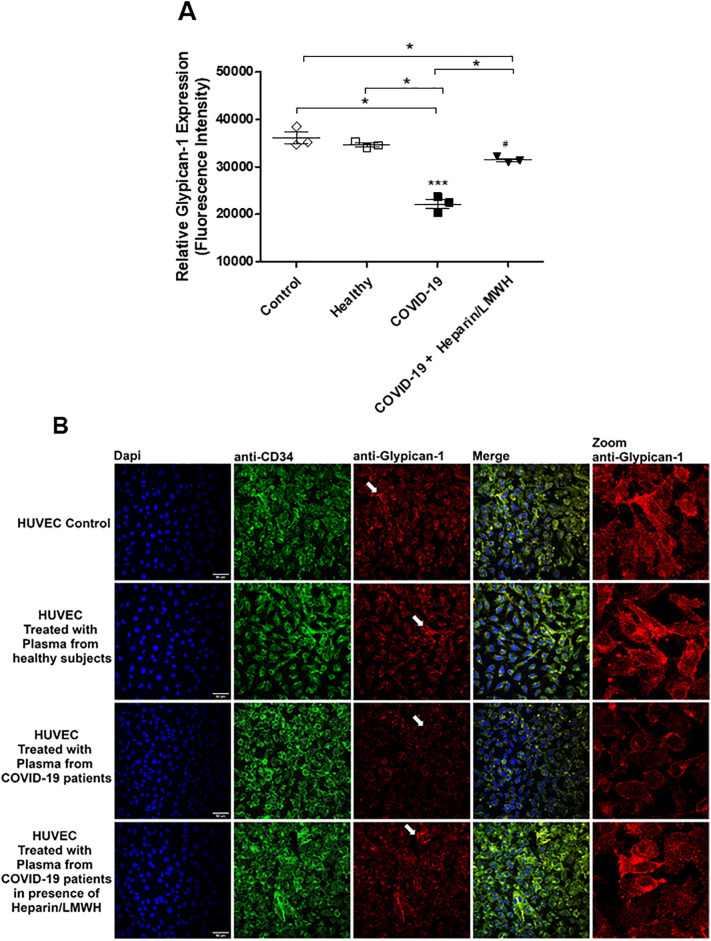
To determine the role of heparanase on glycocalyx degradation, HUVECs were treated with plasma from COVID-19 patients in the presence of heparin/LMWH. Heparin/LMWH prevented the decreased expression of syndecan-1, glypican-1 and syndecan-4 (Fig. 6 ). These results were confirmed by fluorescence images. HUVECs exhibited decreased fluorescence intensity for glypican-1 in the presence of plasma from SARS-CoV-2-positive patients compared to plasma from healthy subjects. Furthermore, heparin/LMWH abrogated the decreased fluorescence intensity of glypican-1 observed in cells exposed to plasma from COVID-19 patients (Fig. 7 ).
Fig. 6.
Heparin/LMWH abrogates the reduced levels of glycocalyx components induced by plasma of COVID-19 patients. Immunoblot representative images (up) and densitometry analysis (A, B, and C) of protein levels of syndecan-1, glypican-1, and syndecan-4 in HUVECs treated with plasma from healthy subjects and COVID-19 patients for 12 h in presence of Heparin/LMWH (10 μg/ml, 12 h). The values are normalized by β-actin and expressed as absolute values. Data are representative of at least 6 experiments and are shown as mean ± SEM. One-way ANOVA followed by Tukey post-test. *p < 0.05. We processed images of blots changing brightness and contrast and we applied equally over the entire image.
Fig. 7.
Heparin/LMWH abrogates the reduced levels of glycocalyx components induced by plasma from COVID-19 patients. Immunofluorescence (40× magnification) of glypican-1 in HUVECs treated with plasma from healthy subjects and COVID-19 patients in the presence of heparin/LMWH or vehicle. Data are representative of at least 3 experiments and are shown as mean ± SEM. One-way ANOVA followed by Tukey post-test. *p < 0.05.
4. Discussion
This study shows that plasma from COVID-19 patients promotes endothelial glycocalyx shedding. Glycocalyx disruption occurs in healthy endothelial cells (HUVECs) and is associated with high levels of cytokines and redox imbalance. The impairment of endothelial barrier function, represented by glycocalyx perturbation, contributes to endothelial dysfunction, procoagulant, and thrombotic events. In addition, in vitro treatment with heparin/LMWH prevents endothelial glycocalyx disruption in HUVECs exposed to plasma from COVID-19 patients.
Reports from China showed that about 10–15% of mild COVID-19 cases may progress to severe illness, and 15–20% of severe cases may become critical, with critical cases requiring treatment in intensive care units (ICU) [5]. It is important to mention that the criteria to define severely ill patients vary among studies. Individuals with cardiovascular and metabolic comorbidities such as hypertension, obesity, and type 2 diabetes are more likely to develop severe symptoms of COVID-19 and to be classified into as mild-to-severe and critical cases of COVID-19 [39]. In addition, mild-to-severe versus critical patients may show differences during the development of COVID-19, and critical cases can rapidly progress to death.
Our study used a cohort of elderly patients with comorbidities, one of the main risk groups. Although no significant differences were observed in the rate of hypertension and diabetes between mild-to-severe and critical cases of COVID-19 (Table 1), many, but not all, studies show differences in the occurrence of comorbidities between mild-to-severe versus critical patients. In a public hospital in New York City during the first month of the COVID-19 pandemic, similar rates of cardiovascular comorbidities and type 2 diabetes were observed between ICU versus non-ICU patients [40]. On the other hand, hypertension was prevalent in a critical group compared to a mild-to-severe group (p < 0.0078) in a cohort of forty-one patients positive to COVID-19 hospitalized at the Fifth Medical Center of PLA General Hospital in Beijing (China) [41]. Of note, a similar percentage of patients with obesity was present in these groups of patients [41].
In addition, differences in platelet activation and platelet-monocyte aggregate formation were reported between critical and mild-to-severe patients. However, the mild-to-severe patients were younger than the critical group [42]. Differences in endothelial glycocalyx were also reported in critically ill patients with COVID-19, but basically only men (95%) were enrolled in the study [43]. In the present study, however, samples from men and women were similar, with 48% of male patients, reinforcing that the cohort is representative for both aging and comorbidities.
Viral infections demand an immediate immune response of host metabolism to create an effective antiviral response [44]. SARS-CoV-2 triggers a cascade of acute biological events, such as increased synthesis of ROS in immune and non-immune cells, promoting redox imbalance. ROS are by-products of a wide range of physiological reactions that play essential roles in living organisms. However, excessive ROS induced by infections may disrupt redox balance and lead to vascular pathology.
COVID-19 patient's exhibit increased extracellular traps (NETs), which are indicative of neutrophil activation [45,46]. Activated polymorphonuclear neutrophils migrate to target tissues of inflammation and enhance ROS generation [47]. In addition, many viruses inhibits nuclear factor erythroid 2-related factor 2 (Nrf2) activation, which is responsible for antioxidant defense [48]. Moreover, decreased expression of the antioxidant enzyme superoxide dismutase 3 (ec-SOD3) in the lungs of elderly patients infected with SARS-CoV-2 [49] has been reported. These studies reinforce that viral infection disrupts the antioxidant defense system [50], a potential mechanism associated with increased ROS generation.
Human coronaviruses activate NF-κB signaling, the major contributor to inflammation and oxidative damage during SARS-CoV infection [51]. Furthermore, the viral infection induces mitochondrial ROS production to promote efficient viral replications [52]. All these events contribute to overproduction of ROS and lead to oxidative stress and cellular damage, including lipid peroxidation and DNA oxidation [53]. MDA levels were increased in plasma from COVID-19 patients as compared to healthy individuals, which indicates a state of oxidative stress. High levels of ROS were also detected in fresh sputum of COVID-19 patients [8].
Despite the damage caused by ROS excess, ROS are essential signaling molecules for the progression of inflammatory processes and production of cytokines and chemokines [47]. Costela-Ruiz et al. [7] reviewed several studies that show hyperproduction of cytokines and interleukins in plasma and blood from COVID-19 patients. During cellular infection, RNA virus promotes NLRP3 (Nod-like receptor pyrin domain-containing 3) inflammasome activation [54,55]. NLRP3 converts inactive pro-caspase-1 to active caspase-1 [56], and then caspase-1 cleaves pro-IL-1β forming active IL-1β [57]. SARS-CoV-2 infection is associated with the activation and maturation of IL-1β, a key cytokine in the cytokine storm produced by coronavirus. IL-1β activates other proinflammatory cytokines, such as IL-6 and TNF-α [58]. In the present study, plasma from COVID-19 patients exhibited increased levels of IL-1β and IL-6, but no differences in the levels of TNF-α.
Proinflammatory cytokines, as IL-1β and IL-6, and ROS activate enzymes named sheddases, such as heparanase, metalloproteinases (MMPs) and hyaluronidase, which induce glycocalyx degradation [28,59]. Regarding the activation of MMPs, ROS decrease levels of tissue inhibitors of MMPs (TIMPs), thus increasing the activity of MMPs [60], which cleave the protein core of the syndecan proteoglycan, promoting shedding of the syndecan's family [61]. Heparanase is an endoglycosidase that cleaves the side chains of heparan sulfate present in the structure of syndecan and glypican families and, therefore, disrupts the glycocalyx [62]. Hyaluronidase degrades hyaluronan into fragments via hydrolysis of the disaccharides at hexosaminidic β (1 to 4) linkages [63]. A recent study demonstrated that heparanase activity was increased in both non-ICU (intensive care unit) and ICU patients with COVID-19 compared to healthy individuals [64], reinforcing data from our study. The increased heparanase activity was linked to increased heparan sulfate fragments present in the plasma from COVID-19 patients. In addition, hyaluronan fragments were also increased in COVID-19 patients, confirming that SARS-CoV-2 infection is associated with glycocalyx degradation. Although, Stahl et al. [43] did not find differences in heparanase-1 activity between COVID-19 patients and healthy subjects, using sublingual sidestream darkfield (SDF) image, they showed in vivo that endothelial glycocalyx thickness is decreased on the perfused boundary region in patients with COVID-19.
We then addressed whether plasma from patients with COVID-19 alters vascular homeostasis, by inducing endothelial glycocalyx degradation. HUVECs treated with plasma from COVID-19 patients showed reduced H2O2 and NO levels compared to endothelial cells treated with plasma from healthy individuals. Several studies have shown that the endothelial glycocalyx is the main sensor that activates mechanotransduction in vascular cells, creating immediate response to shear stress and inducing NO production [21,65]. Pharmacological tools that remove or degrade heparan sulfate and other glycocalyx constituents block NO production in endothelial cells [[20], [21], [22], [23]]. These studies reinforce our results that glycocalyx is disrupted in HUVECs exposed to plasma from patients with COVID-19, since NO production was decreased. It is important to mention that plasma from critically ill patients exhibited increased NO, an event that may be linked to NO derived from iNOS, whose expression is 2–3 fold higher following infection [66]. H2O2 is an intracellular messenger that modulates several endothelial cell functions. Its rigidly regulated concentration modulates endothelial cell growth and proliferation, endothelium-dependent vasorelaxation and vascular remodeling, and also inflammatory responses [67]. Low levels of H2O2 decrease survival and proliferation of vascular smooth muscle cells [68], and the decreased H2O2 levels in HUVECs exposed to plasma from infected patients may impair vascular homeostasis.
HUVECs exposed to plasma from patients with COVID-19 exhibited decreased expression of specific components of the glycocalyx, including glypican-1, syndecan-1 and syndecan-4. Expression of these heparan sulfate proteoglycans were reduced after 30 min and 1 h of exposure to plasma from COVID-19 patients. Longer exposure times, 12 h and 24 h, promoted a drastic decrease in the expression of glypican-1, syndecan-1 and syndecan-4, confirming the degradation of glycocalyx. Syndecan-1 was increased in blood from COVID-19 patients, indicated shedding of glycocalyx and reinforce our data [43]. Intact heparan sulfate chains of the glycocalyx serve as binding sites for antithrombin III, the main anticoagulant molecule that inhibits coagulant factors [69,70]. Likewise, tissue factor pathway inhibitor (TFPI) can also bind to heparan sulfates and inhibits the early stages of procoagulant process [71]. Therefore, the endothelial glycocalyx has a role as antithrombotic and anticoagulant, and its degradation may be directly associated with thrombotic events and pro-coagulant effects in SARS-CoV2 infection. A review with 1026 patients with SARS-CoV-2 infection showed that at least 40% were considered at high risk of venous thromboembolism [72]. In addition, coagulation disorders were reported in patients infected by the new coronavirus, and more than 70% of non-survivors patients developed disseminated intravascular coagulation (DIC) [73]. Moreover, bleeding was a significant cause of death in positive cases of COVID-19, which was associated with increased plasma D-dimer levels during the admission of patients, making D-dimer an excellent predictor of coagulation disorders, bleeding complications, and thrombotic events [74].
Heparanase is a unique mammalian enzyme that degrades heparan sulfate chains [75], and its inhibition may promote beneficial effects on COVID-19 patients, such as preventing coagulation disorders and avoiding thrombotic events by blocking glycocalyx shedding and disruption. In the present study, the treatment of HUVECs exposed to plasma from COVID-19 patients with heparin/LMWH was effective in preventing glycocalyx degradation. Accordingly, heparin/LMWH has been shown to inhibit heparanase [[76], [77], [78], [79], [80]]. Corroborating our data, non-ICU patients positive for SARS-CoV-2 that received prophylactic doses of dalteparin/LMWH (5000 IU, daily) exhibited reduced heparanase activity [64]. Several clinical studies demonstrated beneficial effects of heparin/LMWH during lung injury; and decreased the mortality of patients with acute respiratory distress syndrome, according review by Li et al. [81]. Moreover, heparin inhibited the infection caused by SARS-CoV strain HSR1 in Vero cells by 50% [82] and blocked of the SARS-CoV pseudovirus spike protein at the HEK293 cells, thus preventing the entry of the virus on host cells [83]. Furthermore, heparin has recently shown to induce a conformational change on the SARS-CoV-2 spike protein domain, preventing the virus attachment to host cells [84] and effectively inhibiting SARS-CoV-2 infection in vitro [85].
Prevention of endothelial glycocalyx degradation may effectively preserve endothelial barrier function, avoiding endothelial dysfunction as well as coagulant disorders and thrombotic events observed in critical and severe patients with COVID-19. In addition, our study reinforces the potential use of heparin and its derivatives in SARS-CoV-2 infection. More clinical trials are necessary to find the appropriate, effective dose of heparin/LMWH to improve outcomes in COVID-19 patients [86]. Therefore, knowledge on the mechanisms associated of SARS-CoV-2 infection and vascular dysfunction may certainly contribute to the development of new therapeutic strategies aiming to treat COVID-19 patients and to avoid cardiovascular complications.
Although we have used classical methods to show that plasma of patients with COVID-19 increases the enzyme responsible for the degradation of heparan sulfate proteoglycans and for the leakage of the glycocalyx components, more robust techniques, such as electron microscopy, are important to provide accurate information on the thickness of the glycocalyx. Nevertheless, our data clearly suggest that plasma from COVID-19 patients disturbs the glycocalyx of HUVEC, decreasing the content of specific components of the glycocalyx and promoting shedding of heparan sulfate, which indicates that COVID-19 induces glycocalyx degradation.
5. Conclusion
Our study shows that plasma from hospitalized COVID-19 patients contains increased levels of glycocalyx components and increased heparanase activity, indicating glycocalyx disruption. Moreover, plasma from COVID-19 patients also induces glycocalyx shedding and disturbs redox balance in healthy HUVECs. LMWH/heparin inhibits glycocalyx perturbation induced by plasma from COVID-19 patients.
The following is the supplementary data related to this article.
A) Antibodies testing in healthy subjects. B) Image representative of antibodies testing. C) Baseline characteristics of healthy subjects.
Funding
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grants 2020/05270-0 and 2013/08216-2 – Research Center in Inflammatory Diseases to VLDB and RCT, respectively), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Simone R. Potje and Tiago J. Costa are FAPESP post-doctoral fellows (grants 2016/21239-0 and 2017/25116-2, respectively).
CRediT authorship contribution statement
SRP, TJC and RT designed the research. MNB and MAM supervised the clinical study. SRP, TJC, TFCFS, RBM and KSGS performed experiments. SRP, TJC, and RT analyzed and interpreted data. SRP, TJC, MNB, CELA, VLDB, EA, PLJ, RDRO, DSZ, CB, MAM and RT discussed data. SRP and TJC wrote the manuscript. All authors approved the manuscript.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
The authors thank Muriel C. R. O. Berti, Basílica B. Muniz, and Carla P. Manzato for excellent technical assistance. Graphical abstract was created at BioRender.com license.
References
- 1.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang S., Wang J., Liu F., Liu J., Cao G., Yang C., et al. COVID-19 patients with hypertension have more severe disease: a multicenter retrospective observational study. Hypertens. Res. 2020;43:824–831. doi: 10.1038/s41440-020-0485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(CSSE-JHU) CfSSaEaJHU COVID-19 Dashboard. https://coronavirus.jhu.edu/map.html
- 4.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;13:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miripour Z.S., Sarrami-Forooshani R., Sanati H., Makarem J., Taheri M.S., Shojaeian F., et al. Real-time diagnosis of reactive oxygen species (ROS) in fresh sputum by electrochemical tracing; correlation between COVID-19 and viral-induced ROS in lung/respiratory epithelium during this pandemic. Biosens. Bioelectron. 2020;165 doi: 10.1016/j.bios.2020.112435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patell R., Bogue T., Koshy A., Bindal P., Merrill M., Aird W.C., et al. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136:1342–1346. doi: 10.1182/blood.2020007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libby P., Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Meo S., Reed T.T., Venditti P., Victor V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxidative Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kvietys P.R., Granger D.N. Role of reactive oxygen and nitrogen species in the vascular responses to inflammation. Free Radic. Biol. Med. 2012;52:556–592. doi: 10.1016/j.freeradbiomed.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa T.J., Barros P.R., Arce C., Santos J.D., da Silva-Neto J., Egea G., Dantas A.P., Tostes R.C., Jiménez-Altayó F. The homeostatic role of hydrogen peroxide, superoxide anion and nitric oxide in the vasculature. Free Radic. Biol. Med. 2021;162:615–635. doi: 10.1016/j.freeradbiomed.2020.11.021. [DOI] [PubMed] [Google Scholar]
- 14.Incalza M.A., D’Oria R., Natalicchio A., Perrini S., Laviola L., Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018;100:1–19. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Möckl L. The emerging role of the mammalian glycocalyx in functional membrane organization and immune system regulation. Front. Cell. Dev. Biol. 2020;8:253. doi: 10.3389/fcell.2020.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinbaum S., Tarbell J.M., Damiano E.R. The structure and function of the endothelial glycocalyx layer. Annu. Rev. Biomed. Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 17.Reitsma S., Slaaf D.W., Vink H., van Zandvoort M.A., oude Egbrink M.G. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alphonsus C.S., Rodseth R.N. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia. 2014;69:777–784. doi: 10.1111/anae.12661. [DOI] [PubMed] [Google Scholar]
- 19.Bartosch A.M.W., Mathews R., Tarbell J.M. Endothelial glycocalyx-mediated nitric oxide production in response to selective AFM pulling. Biophys. J. 2017;113:101–108. doi: 10.1016/j.bpj.2017.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dragovich M.A., Chester D., Fu B.M., Wu C., Xu Y., Goligorsky M.S., et al. Mechanotransduction of the endothelial glycocalyx mediates nitric oxide production through activation of TRP channels. Am. J. Physiol. Cell. Physiol. 2016;311:C846–C853. doi: 10.1152/ajpcell.00288.2015. [DOI] [PubMed] [Google Scholar]
- 21.Florian J.A., Kosky J.R., Ainslie K., Pang Z., Dull R.O., Tarbell J.M. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ. Res. 2003;93:e136–e142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- 22.Pahakis M.Y., Kosky J.R., Dull R.O., Tarbell J.M. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem. Biophys. Res. Commun. 2007;355:228–233. doi: 10.1016/j.bbrc.2007.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yen W., Cai B., Yang J., Zhang L., Zeng M., Tarbell J.M., et al. Endothelial surface glycocalyx can regulate flow-induced nitric oxide production in microvessels in vivo. PLoS One. 2015;10 doi: 10.1371/journal.pone.0117133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubio-Gayosso I., Platts S.H., Duling B.R. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2247–H2256. doi: 10.1152/ajpheart.00796.2005. [DOI] [PubMed] [Google Scholar]
- 25.Suwarto S., Sasmono R.T., Sinto R., Ibrahim E., Suryamin M. Association of endothelial glycocalyx and tight and adherens junctions with severity of plasma leakage in dengue infection. J. Infect. Dis. 2017;215:992–999. doi: 10.1093/infdis/jix041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang T.H., Alonso S., Ng L.F., Thein T.L., Pang V.J., Leo Y.S., et al. Increased serum hyaluronic acid and heparan sulfate in dengue fever: association with plasma leakage and disease severity. Sci. Rep. 2017;7 doi: 10.1038/srep46191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connolly-Andersen A.M., Thunberg T., Ahlm C. Endothelial activation and repair during hantavirus infection: association with disease outcome. Open Forum Infect. Dis. 2014;1 doi: 10.1093/ofid/ofu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becker B.F., Jacob M., Leipert S., Salmon A.H., Chappell D. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. Br. J. Clin. Pharmacol. 2015;80:389–402. doi: 10.1111/bcp.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benatti M.N., Fabro A.T., Miranda C.H. Endothelial glycocalyx shedding in the acute respiratory distress syndrome after flu syndrome. J. Intensive Care. 2020;8 doi: 10.1186/s40560-020-00488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Padberg J.S., Wiesinger A., di Marco G.S., Reuter S., Grabner A., Kentrup D., et al. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis. 2014;234:335–343. doi: 10.1016/j.atherosclerosis.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 31.DellaValle B., Hasseldam H., Johansen F.F., Iversen H.K., Rungby J., Hempel C. Multiple soluble components of the glycocalyx are increased in patient plasma after ischemic stroke. Stroke. 2019;50:2948–2951. doi: 10.1161/STROKEAHA.119.025953. [DOI] [PubMed] [Google Scholar]
- 32.Martens R.J., Vink H., van Oostenbrugge R.J., Staals J. Sublingual microvascular glycocalyx dimensions in lacunar stroke patients. Cerebrovasc. Dis. 2013;35:451–454. doi: 10.1159/000348854. [DOI] [PubMed] [Google Scholar]
- 33.Broekhuizen L.N., Lemkes B.A., Mooij H.L., Meuwese M.C., Verberne H., Holleman F., et al. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia. 2010;53:2646–2655. doi: 10.1007/s00125-010-1910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wadowski P.P., Hülsmann M., Schörgenhofer C., Lang I.M., Wurm R., Gremmel T., et al. Sublingual functional capillary rarefaction in chronic heart failure. Eur. J. Clin. Investig. 2018;48 doi: 10.1111/eci.12869. [DOI] [PubMed] [Google Scholar]
- 35.Nalla A.K., Casto A.M., Huang M.W., Perchetti G.A., Sampoleo R., Shrestha L., et al. Comparative performance of SARS-CoV-2 detection assays using seven different primer-probe sets and one assay kit. J. Clin. Microbiol. 2020:58. doi: 10.1128/JCM.00557-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armstrong D., Browne R. The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory. Adv. Exp. Med. Biol. 1994;366:43–58. doi: 10.1007/978-1-4615-1833-4_4. [DOI] [PubMed] [Google Scholar]
- 37.Chen Z., Oliveira S.D.S., Zimnicka A.M., Jiang Y., Sharma T., Chen S., et al. Reciprocal regulation of eNOS and caveolin-1 functions in endothelial cells. Mol. Biol. Cell. 2018;29(10):1190–1202. doi: 10.1091/mbc.E17-01-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pirkmajer S., Chibalin A.V. Serum starvation: caveat emptor. Am. J. Physiol. Cell. Physiol. 2011;301(2):C272–C279. doi: 10.1152/ajpcell.00091.2011. [DOI] [PubMed] [Google Scholar]
- 39.Almeida-Pititto B., Dualib P.M., Zajdenverg L., Dantas J.R., de Souza F.D., Rodacki M., Bertoluci M.C., Brazilian Diabetes Society Study Group (SBD) Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: a meta-analysis. Diabetol. Metab. Syndr. 2020;12:75. doi: 10.1186/s13098-020-00586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Filardo T.D., Khan M.R., Krawczyk N., Galitzer H., Karmen-Tuohy S., Coffee M., Schaye V.E., Eckhardt B.J., Cohen G.M. Comorbidity and clinical factors associated with COVID-19 critical illness and mortality at a large public hospital in New York City in the early phase of the pandemic (March–April 2020) PLoS One. 2020;15(11) doi: 10.1371/journal.pone.0242760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song J.W., Zhang C., Fan X., Meng F.P., Xu Z., Xia P., Cao W.J., et al. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-17240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hottz E.D., Azevedo-Quintanilha I.G., Palhinha L., Teixeira L., Barreto E.A., Pão C.R.R., Righy C., Franco S., Souza T.M.L., Kurtz P., Bozza F.A., Bozza P.T. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136(11):1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stahl K., Gronski P.A., Kiyan Y., Seeliger B., Bertram A., Pape T., et al. Injury to the endothelial glycocalyx in critically ill patients with COVID-19. Am. J. Respir. Crit. Care Med. 2020;202:1178–1181. doi: 10.1164/rccm.202007-2676LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanchez E.L., Lagunoff M. Viral activation of cellular metabolism. Virology. 2015;479-480:609–618. doi: 10.1016/j.virol.2015.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veras F.P., Pontelli M.C., Silva C.M., Toller-Kawahisa J.E., de Lima M., Nascimento D.C., et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020:217. doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A., et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020:5. doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramezani A., Nahad M.P., Faghihloo E. The role of Nrf2 transcription factor in viral infection. J. Cell. Biochem. 2018;119:6366–6382. doi: 10.1002/jcb.26897. [DOI] [PubMed] [Google Scholar]
- 49.Abouhashem A.S., Singh K., Azzazy H.M.E., Sen C.K. Is low alveolar type II cell. Antioxid. Redox Signal. 2020;33:59–65. doi: 10.1089/ars.2020.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Treitinger A., Spada C., Verdi J.C., Miranda A.F., Oliveira O.V., Silveira M.V., et al. Decreased antioxidant defence in individuals infected by the human immunodeficiency virus. Eur. J. Clin. Investig. 2000;30:454–459. doi: 10.1046/j.1365-2362.2000.00642.x. [DOI] [PubMed] [Google Scholar]
- 51.DeDiego M.L., Nieto-Torres J.L., Regla-Nava J.A., Jimenez-Guardeño J.M., Fernandez-Delgado R., Fett C., et al. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J. Virol. 2014;88:913–924. doi: 10.1128/JVI.02576-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng M.L., Weng S.F., Kuo C.H., Ho H.Y. Enterovirus 71 induces mitochondrial reactive oxygen species generation that is required for efficient replication. PLoS One. 2014;e113234:9. doi: 10.1371/journal.pone.0113234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sena C.M., Leandro A., Azul L., Seiça R., Perry G. Vascular oxidative stress: impact and therapeutic approaches. Front. Physiol. 2018;9:1668. doi: 10.3389/fphys.2018.01668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allen I.C., Scull M.A., Moore C.B., Holl E.K., McElvania-TeKippe E., Taxman D.J., et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.da Costa L.S., Outlioua A., Anginot A., Akarid K., Arnoult D. RNA viruses promote activation of the NLRP3 inflammasome through cytopathogenic effect-induced potassium efflux. Cell Death Dis. 2019;10 doi: 10.1038/s41419-019-1579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 57.Zhou R., Tardivel A., Thorens B., Choi I., Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 58.Conti P., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Frydas I., et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34:327–331. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 59.Uchimido R., Schmidt E.P., Shapiro N.I. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit. Care. 2019;23 doi: 10.1186/s13054-018-2292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siwik D.A., Colucci W.S. Regulation of matrix metalloproteinases by cytokines and reactive oxygen/nitrogen species in the myocardium. Heart Fail. Rev. 2004;9:43–51. doi: 10.1023/B:HREV.0000011393.40674.13. [DOI] [PubMed] [Google Scholar]
- 61.Ramnath R., Foster R.R., Qiu Y., Cope G., Butler M.J., Salmon A.H., et al. Matrix metalloproteinase 9-mediated shedding of syndecan 4 in response to tumor necrosis factor α: a contributor to endothelial cell glycocalyx dysfunction. FASEB J. 2014;28:4686–4699. doi: 10.1096/fj.14-252221. [DOI] [PubMed] [Google Scholar]
- 62.Garsen M., Rops A.L., Rabelink T.J., Berden J.H., van der Vlag J. The role of heparanase and the endothelial glycocalyx in the development of proteinuria. Nephrol. Dial. Transplant. 2014;29:49–55. doi: 10.1093/ndt/gft410. [DOI] [PubMed] [Google Scholar]
- 63.Wang T., Chen R., Liu C., Liang W., Guan W., Tang R., et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7:e362–e363. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buijsers B., Yanginlar C., de Nooijer A., Grondman I., Maciej-Hulme M.L., Jonkman I., et al. Increased plasma heparanase activity in COVID-19 patients. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.575047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ando J., Yamamoto K. Flow detection and calcium signalling in vascular endothelial cells. Cardiovasc. Res. 2013;99:260–268. doi: 10.1093/cvr/cvt084. [DOI] [PubMed] [Google Scholar]
- 66.Fang W., Jiang J., Su L., Shu T., Liu H., Lai S., Ghiladi R.A., Wang J. The role of NO in COVID-19 and potential therapeutic strategies. Free Radic. Biol. Med. 2021;163:153–162. doi: 10.1016/j.freeradbiomed.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc. Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 68.Brown M.R., Miller F.J., Li W.G., Ellingson A.N., Mozena J.D., Chatterjee P., et al. Overexpression of human catalase inhibits proliferation and promotes apoptosis in vascular smooth muscle cells. Circ. Res. 1999;85:524–533. doi: 10.1161/01.res.85.6.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quinsey N.S., Greedy A.L., Bottomley S.P., Whisstock J.C., Pike R.N. Antithrombin: in control of coagulation. Int. J. Biochem. Cell Biol. 2004;36:386–389. doi: 10.1016/s1357-2725(03)00244-9. [DOI] [PubMed] [Google Scholar]
- 70.Shimada K., Kobayashi M., Kimura S., Nishinaga M., Takeuchi K., Ozawa T. Anticoagulant heparin-like glycosaminoglycans on endothelial cell surface. Jpn. Circ. J. 1991;55:1016–1021. doi: 10.1253/jcj.55.1016. [DOI] [PubMed] [Google Scholar]
- 71.Kato H. Regulation of functions of vascular wall cells by tissue factor pathway inhibitor: basic and clinical aspects. Arterioscler. Thromb. Vasc. Biol. 2002;22:539–548. doi: 10.1161/01.atv.0000013904.40673.cc. [DOI] [PubMed] [Google Scholar]
- 72.Wang G., Kostidis S., Tiemeier G.L., Sol W.M.P.J., de Vries M.R., Giera M., et al. Shear stress regulation of endothelial glycocalyx structure is determined by glucobiosynthesis. Arterioscler. Thromb. Vasc. Biol. 2020;40:350–364. doi: 10.1161/ATVBAHA.119.313399. [DOI] [PubMed] [Google Scholar]
- 73.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Al-Samkari H., Karp Leaf R.S., Dzik W.H., Carlson J.C.T., Fogerty A.E., Waheed A., et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shu J., Santulli G. Heparanase in health and disease: the neglected housekeeper of the cell? Atherosclerosis. 2019;283:124–126. doi: 10.1016/j.atherosclerosis.2019.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bar-Ner M., Eldor A., Wasserman L., Matzner Y., Cohen I.R., Fuks Z., et al. Inhibition of heparanase-mediated degradation of extracellular matrix heparan sulfate by non-anticoagulant heparin species. Blood. 1987;70:551–557. [PubMed] [Google Scholar]
- 77.Bitan M., Mohsen M., Levi E., Wygoda M.R., Miao H.Q., Lider O., et al. Structural requirements for inhibition of melanoma lung colonization by heparanase inhibiting species of heparin. Isr. J. Med. Sci. 1995;31:106–118. [PubMed] [Google Scholar]
- 78.Irimura T., Nakajima M., Nicolson G.L. Chemically modified heparins as inhibitors of heparan sulfate specific endo-beta-glucuronidase (heparanase) of metastatic melanoma cells. Biochemistry. 1986;25:5322–5328. doi: 10.1021/bi00366a050. [DOI] [PubMed] [Google Scholar]
- 79.Naggi A., Casu B., Perez M., Torri G., Cassinelli G., Penco S., et al. Modulation of the heparanase-inhibiting activity of heparin through selective desulfation, graded N-acetylation, and glycol splitting. J. Biol. Chem. 2005;280(13) doi: 10.1074/jbc.M414217200. [DOI] [PubMed] [Google Scholar]
- 80.Pala D., Rivara S., Mor M., Milazzo F.M., Roscilli G., Pavoni E., et al. Kinetic analysis and molecular modeling of the inhibition mechanism of roneparstat (SST0001) on human heparanase. Glycobiology. 2016;26:640–654. doi: 10.1093/glycob/cww003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li J., Li Y., Yang B., Wang H., Li L. Low-molecular-weight heparin treatment for acute lung injury/acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. Int J Clin Exp Med. 2018;2:414–422. [Google Scholar]
- 82.Vicenzi E., Canducci F., Pinna D., Mancini N., Carletti S., Lazzarin A., et al. Coronaviridae and SARS-associated coronavirus strain HSR1. Emerg. Infect. Dis. 2004;10:413–418. doi: 10.3201/eid1003.030683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lang J., Yang N., Deng J., Liu K., Yang P., Zhang G., et al. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLoS One. 2011;6 doi: 10.1371/journal.pone.0023710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mycroft-West C.J., Su D., Pagani I., Rudd T.R., Elli S., Gandhi N.S., et al. Heparin inhibits cellular invasion by SARS-CoV-2: structural dependence of the interaction of the Spike S1 receptor-binding domain with heparin. Thromb. Haemost. 2020;12:1700–1715. doi: 10.1055/s-0040-1721319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kwon P.S., Oh H., Kwon S.J., Jin W., Zhang F., Fraser K., et al. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell. Discov. 2020;6 doi: 10.1038/s41421-020-00192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gozzo L., Viale P., Longo L., Vitale D.C., Drago F. The potential role of heparin in patients with COVID-19: beyond the anticoagulant effect. A review. Front. Pharmacol. 2020;11:1307. doi: 10.3389/fphar.2020.01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Antibodies testing in healthy subjects. B) Image representative of antibodies testing. C) Baseline characteristics of healthy subjects.