Abstract
Treatment of ventilated patients with gram-negative pneumonia (GNP) is often unsuccessful. We aimed to assess the efficacy and safety of nebulized amikacin (NA) as adjunctive therapy to systemic antibiotics in this patient population. PubMed, Embase, China national knowledge infrastructure, Wanfang, and the Cochrane database were searched for randomized controlled trials (RCTs) investigating the effect of NA as adjunctive therapy in ventilated adult patients with GNP. Heterogeneity was explored using subgroup analysis and sensitivity analysis. The Grading of recommendations assessment, development, and evaluation approach was used to assess the certainty of the evidence. Thirteen RCTs with 1733 adults were included. The pooled results showed NA had better microbiologic eradication (RR = 1.51, 95% CI 1.35 to 1.69, P < 0.0001) and improved clinical response (RR = 1.23; 95% CI 1.13 to 1.34; P < 0.0001) when compared with control. Meanwhile, overall mortality, pneumonia associated mortality, duration of mechanical ventilation, length of stay in ICU and change of clinical pneumonia infection scores were similar between NA and control groups. Additionally, NA did not add significant nephrotoxicity while could cause more bronchospasm. The use of NA adjunctive to systemic antibiotics therapy showed better benefits in ventilated patients with GNP. More well-designed RCTs are still needed to confirm our results.
Subject terms: Infectious diseases, Drug therapy
Introduction
Gram‑negative pneumonia (GNP) is a common and serious infection in critically ventilated patients, which accounts for around 65% of pneumonia cases in the intensive care unit (ICU)1. It is associated with significant mortality, duration of mechanical ventilation (MV), length of ICU stay, as well as health care costs2–4. To date, despite diagnostic and antibiotics improvements, treatment failure for ventilated GNP is not infrequent5. Moreover, the presence of GNP caused by drug-resistant pathogens has significantly grown and shown difficult to be eradicated due to the poor lung penetration of intravenous antibiotics, which further complicates the treatment6,7. Therefore, therapies that increase local concentration antibiotics in the lung by adding aerosolized antibiotics (i.e., amikacin) have attracted increasing attention8.
Theoretically, nebulized amikacin (NA) can be used as an adjunctive therapeutic option in treating ventilated patients with GNP. The advantage to NA in this scenario including achieving high intra-pulmonary concentration that may be effective even for resistant pathogens, thwarting selective pressure and drug-resistant development, and extremely low concentration in the blood due to local administration, thus avoiding dose-dependent systemic toxicity9–13.
Although several clinical studies reported the merits of NA in ventilated GNP12–15, high-quality evidence to support its use remains limited. Even so, the use of NA in ventilated patients is not unusual. In 2016, a survey of 193 ICUs worldwide showed that NA was prescribed by 27% of the ICUs in clinical practice16. Interestingly, the latest American2 and European17 guidelines for the management of HAP/VAP provided opposed recommendations on the use of aerosolized antibiotics in ventilated GNP. Of note, these weak recommendations are mainly based on observational studies, with very few RCTs focusing on amikacin have been included. Additionally, a recently published meta-analysis in Chinese suggests NA improves clinical response but not mortality rate and other clinical outcomes18. However, this meta-analysis mainly included literatures in Chinese. Therefore, the efficacy and safety of NA in such a patient population remain unclear.
Recently, several studies on this topic have been published and some of them have a modest sample size, while the conclusions are inconsistent14,19,20. Thus, with the help of the strengthened power of meta-analytic techniques, the present meta-analysis aimed to review the available published RCTs to investigate the efficacy and safety of NA as adjunctive therapy in the treatment of critically ill ventilated patients with GNP.
Materials and methods
This systematic review and meta-analysis were conducted following the PRISMA guidance (http://www.prisma-statement.org) (Appendix 1). The protocol for this systematic review and meta-analysis was registered on the International Platform of Registered Systematic Review and Meta-analysis Protocols database (INPLASY202070045) and is available in full on the inplasy.com (https://doi.org/10.37766/inplasy2020.7.0045).
Search strategy
Two authors (H-BH and J-PQ) independently searched the Cochrane Library, PubMed, China national knowledge infrostructure, Wanfang and Embase database for potentially relevant studies from inception to Jun 20, 2021, which is the last search. The details in the literature search terms were summarized in Appendix 2. Our research was limited to RCTs with Chinese and English. Reference lists of relevant studies were also evaluated to ensure that all possible publications were included.
Study selection
Studies were considered eligible if they met the following criteria: (1) design: RCTs; (2) population: adult (≥ 18 years old) critically ill patients with MV (tracheal intubation or tracheostomy) and diagnosed of GNP (caused by susceptible or resistant pathogens); (3) intervention: patients were randomized to either NA group or control group (aerosolized placebo or no drug), both of which were given alongside intravenous antibiotics during the treatment period (decided by the attending physician based on available culture results or clinical guidelines provided); and (4) predefined outcomes: clinical response, mortality, microbiologic eradication, clinical pulmonary infection score (CPIS), duration of MV and length of stay in ICU. We excluded studies as following: (1) the main focus was children or pregnant women, (2) with any different therapy other than NA between two groups, (3) use of NA as monotherapy, (4) studies focused on in vitro or cystic fibrosis or just pharmacokinetic/pharmacodynamic, (5) available only in abstract form or meeting reports, and 6) studies without reporting predefined treatment outcomes.
Data extraction and outcomes
Data extraction was undertaken by H-BH and JPQindependently for included studies on study design, patient inclusion criteria, NA and control group regimens, microbiological and clinical cure criteria, as well as predefined outcomes. Authors were contacted where data were unclear or unavailable. The primary outcome was the clinical response (defined as a complete or partial resolution of clinical signs and symptoms of infection, according to the criteria by each study author). Secondary outcomes included overall mortality (defined as ICU or hospital or 28-day mortality, the longest follow-up reported was preferred), pneumonia associated mortality, microbiologic eradication (defined as no growth of the causative pathogen from any samples taken [e.g., sputum, throat swab or bronchoalveolar lavage fluid] after treatment, regardless of the clinical outcome), change of CPIS from baseline after treatment (∆CPIS), length of stay in ICU, duration of MV and adverse events of bronchospasm and nephrotoxicity. Discrepancies were identified and resolved through discussion.
Quality assessment
The two investigators also independently assessed the quality of RCTs using the risk of bias tool recommended by the Cochrane Handbook for Systematic Reviews of Interventions21. We also used Jadad score to assess the quality of included trials22. The quality of evidence resulting from the present meta-analysis was evaluated using the Grading of recommendations assessment, development, and evaluation (GRADE) approach23. Publication bias was evaluated by visually inspecting funnel plots and modified Galbraith tests.
Statistical analysis
The results from all relevant studies were combined to estimate the pooled risk ratio (RR) and associated 95% confidence intervals (CI) for dichotomous outcomes. As to the continuous outcomes, mean differences (MD) and 95% CI were estimated as effective results. Some studies reported median as the measure of treatment effect, with accompanying interquartile range (IQR). Before data analysis, we estimated mean from median and standard deviations (SD) from IQR using the methods described in previous studies24. Heterogeneity was tested by using the I2 statistic. An I2 < 50% was considered to indicate insignificant heterogeneity and a fixed-effect model was used, whereas a random-effect model was used in cases of significant heterogeneity (I2 > 50%) using the Mantel–Haenszel method25. Testing the robustness of our outcomes and exploring the potential influence factors, we performed sensitivity analyses by omitting one study in each turn to investigate the influence of a single study on the overall pooled estimate of each predefined outcome. Also, subgroup analyses were performed concerning the primary outcome by pooling studies with the following: (1) types of nebulizers (Jet or ultrasonic or vibrating nebulizer); (2) dose of NA (≥ 800 mg/day or < 800 mg/day); (3) proportion of patients with drug-resistant bacteria (including multidrug-resistant (MDR), extensively drug-resistant (XDR) or pan drug-resistant (PDR) bacteria) (100% or < 100%); (4) study design (blinded or un-blinded), and estimated models (fixed-effect or random effect models). All analyses were performed using Review Manager, Version 5.3. The quality assessment of the evidence was evaluated by GRADE profiler software version 3.6 (GRADE Working Group, 2004–2007).
Results
Searching results
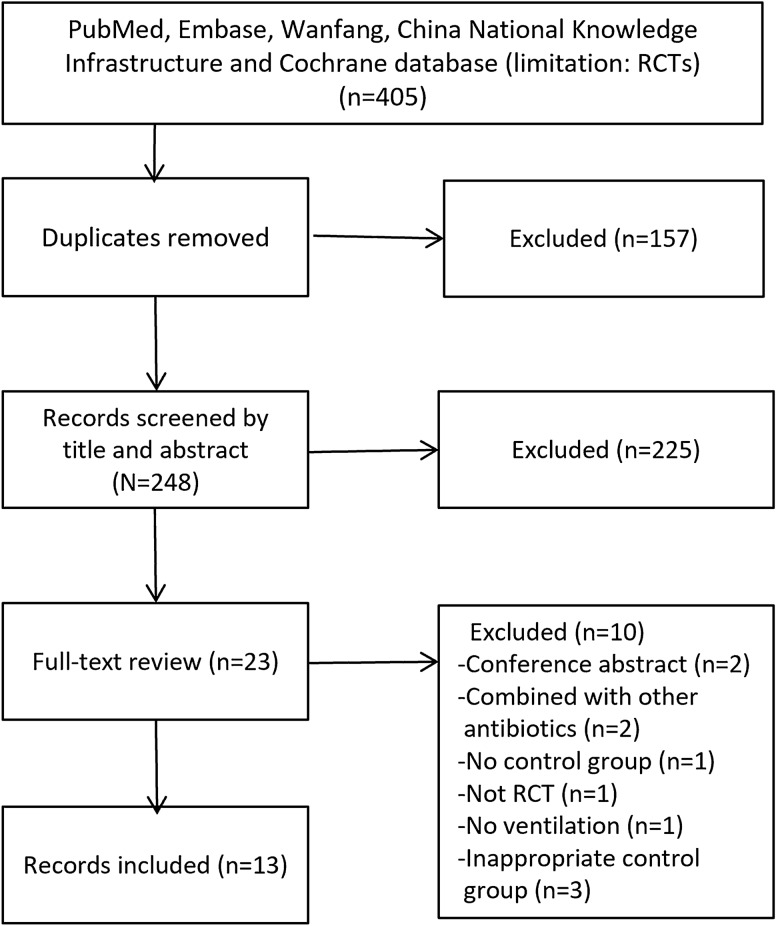
The literature search yielded 325 records through database searching, of which 24 full-text were considered for text-trials review. Finally, 13 RCTs with a total of 1733 patients met the inclusion criteria and were included in our study11,14,18,19,26–34 (Fig. 1). The details in the search strategy were shown in Appendix 2.
Figure 1.
Selection process for RCTs included in the meta-analysis.
Studies characteristics and quality assessment
The main characteristics of included RCTs and predefined outcomes are shown in Table 1 and Appendix 3, while the definitions of patient inclusion, microbiological cure criteria, and clinical response criteria are summarized in Appendix 4. All the included studies were conducted in medical-surgical ICUs. Ten20,24,25,27–34 out of the 13 RCTs were multicenter studies. A total of 1733 patients were included in intention-to-treat analysis while 1,450 patients were included in clinically evaluable. As to the type of nebulizer devices employed in the NA group, vibrating-mesh nebulizer (3 studies)11,19,26, ultrasonic nebulizer (2 study)20,34, and jet nebulizer (8 study)14,24,25,27–33 were used. During the treatment period, patients received concomitant intravenous antibiotics variable among the included studies, decided by the clinician, or based on pathogen-specific treatment criteria. Seven RCTs11,14,19,20,26,28,33 described in detail the nebulization technique, including nebulizer position, ventilator settings, humidifier, respiratory mode, and sedation during the nebulization period (Appendix 5).
Table 1.
Characteristics of the studies included in current systemic review and meta-analysis.
| Study | Study design | Type of pneumonia | Device for drug delivery | Patient characteristics (NA/Control) | NA regimen | Primary outcome | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients ITTa | No. of patients clinically evaluable | Age, mean, (year) | APACHE II mean | Patients with resistant GNPb (%) | MV, IVAB or ICU/hospital stay before NA | ||||||
| Niederman et al.19 , 2020 | PR, DB, MC | HAP, HCAP, VAP, CAP | Vibrating mesh nebulizer | 362/363 | 255/253 | 64/64 | 20/20 | 50/55 | NR | 400 mg every 12 h for 10 d | Survival at days 28–32 |
| Ammar et al.20, 2018 | PR, NB, SC | VAP | Ultrasound nebulizer | 65/32 | 30/30 | 56/55 | 20/18 | 100/100 | NR | 20 mg/kg every 8 h | Clinical response |
| Chen27 2018 | PR, NB, SC | VAP | Jet nebulizer | 55/55 | 55/55 | 73/73 | 13/13 | NR | NR | 400 mg every 12 h for 14 d | Clinical response and Bacteriological eradication |
| Liu et al.14, 2017 | PR, DB, SC | VAP | Jet nebulizer | 30/30 | 27/25 | 68/65 | 22/19 | 100/100 | MV:17 vs. 18 d; ICU stay: 16 vs. 14 d | 400 mg every 8 h for 7 d | Bacteriological eradication and new drug resistant to amikacin |
| Kollef et al.11, 2017 | PR, DB, MC | VAP | Vibrating plate electronic nebulizer | 71/72 | 71/71 | 58/62 | 19/19 | 45/29 | IVAB: 7 vs. 5 d | 300 mg twice daily for 10 d | Change from baseline in CPIS |
| Li et al.28, 2016 | PR, NB, SC | VAP | Jet nebulizer | 38/38 | 38/38 | 64/61 | 13/16 | 8/18 |
MV: 5 vs. 6 d; IVAB: 6 vs. 6 d |
400 mg every 24 h for 7 d | Clinical response |
| Ji29 2016 | PR, NB, SC | VAP | Jet nebulizer | 21/21 | 21/21 | 60/60 | NR | 100/100 | NR | 7.5 mg/kg every 12 h | Clinical response |
| Tong30 2016 | PR, NB, SC | VAP | Jet nebulizer | 45/45 | 45/45 | 45/47 | NR | NR | NR | 600 mg every 24 h, for 7–14 d | Clinical response |
| Yue31 2016 | PR, NB, SC | VAP | Jet nebulizer | 39/39 | 39/39 | 50/50 | NR | NR | NR | 600 mg every 24 h, for 14 d | Clinical response |
| Zhu32 et al., 2015 | PR, NB, SC | VAP | Jet nebulizer | 34/34 | 34/34 | 42/42 | NR | NR | NR | 7.5 mg/kg every 24 h for 8 d | Clinical response |
| Li et al.33, 2015 | PR, NB, SC | VAP | Jet nebulizer | 60/60 | 60/60 | 54/58 | 13/13 | NR |
MV: 5 vs. 6 d; IVAB: 31 vs. 22 d |
400 mg every 12 h for 7 d | Clinical response |
| Niederman et al.26,2012 | PR, DB, MC | HAP, VAP, CAP |
Vibrating mesh nebulizer |
47/22 | 47/22 | 59 /62 | 16/16 | NR |
ICU stay > 5 d: 94% vs. 82%; IVAB within two weeks: 85% vs. 86% |
400 mg every 12 h or 24 h, for 7–14 d | Patients with Cmax ≥ 6,400 ug/mL and AUC0–24 h/256 ≥ 100 |
| Meng34 2011 | PR, NB, SC | VAP | Ultrasound nebulizer | 30/30 | 29/27 | 50/49 | NR | 100/100 | NR | 600 mg every 24 h for 10–14 d | Clinical response |
APACHE II = acute physiology and chronic health evaluation II, AUC0–24 h = area under the concentration–time curve from 0 to 24 h, CAP = community acquired pneumonia, CPIS = clinical pulmonary infection score, Cmax = maximum concentration, DB = double blind, GNB = gram‑negative pneumonia, HAP = hospital-acquired pneumonia, h = hours, HCAP = healthcare-associated pneumonia, ICU = intensive care unit, IVAB = intravenous antibiotics, MC = multi-centers, Mix-ICU = medical-surgical intensive care unit, NA = nebulized amikacin, NR = not reported, PR = prospective randomized, SD = standard deviation, SC = single-center, VAP = ventilator-associated pneumonia.
aITT = intention-to-treat analysis, bdefined as multidrug‑resistant or extensively drug-resistant or pandrug-resistant gram‑negative pneumonia.
The Cochrane risk of bias score for each study is summarized in Appendix 6, Fig. S1A and S1b. Four studies11,14,19,26 were assessed to be at low risk of bias overall and nine studies20,27–34 were at high risk of bias overall. The median Jadad score of the included studies was 2.6 (range from 1 to 5, see Appendix 7). Using GRADE methodology, we evaluated the evidence for pooled data for clinical response rate, overall mortality, pneumonia associated mortality, microbiologic eradication, ∆CPIS, duration of MV, length of stay in ICU, nephrotoxicity, and bronchospasm to be moderate, moderate, moderate, low, low, very low, low, respectively (Table 2). Assessment of publication bias using visually inspecting funnel plots and modified Galbraith tests showed no potential publication bias among the included RCTs (Appendix 8, Fig. S2a and S2b) (Appendix 8, Fig. S2).
Table 2.
Grading of recommendations assessment, development, and evaluation evidence profile for the role of adjunctive aerosolized amikacin in outcomes of the meta-analysis.
| Outcome | No. of study | No. of patients | Relative effect (95% CI) | Estimated Absolute Effects | Heterogeneity I2 , (P) | Quality of the evidence (GRADE)* |
|---|---|---|---|---|---|---|
| Clinical response rate | 13 | 1450 | RR, 1.29 (1.14–1.47) | 38 more per 1000 (from 10 fewer to 89 more) | 49%, (0.02) |
⊕⊕⊕○ Moderate because of risk of bias |
| Overall mortality | 7 | 1058 | RR, 1.17 (0.91, 1.50) | 30 more per 1000 (from 16 fewer to 88 more) | 0%, (0.77) | ⊕⊕⊕○Moderate because of risk of bias |
| Pneumonia associated mortality | 7 | 1066 | RR, 1.12 (0.82, 1.52) | 15 more per 1000 (from 23 fewer to 66 more) | 0%, (0.87) |
⊕⊕⊕○ Moderate because of risk of bias |
| Microbiologic eradication | 11 | 921 | RR, 1.51 (1.35, 1.69) | 466 more per 1000 (from 163 fewer to 322 more) | 6%, (0.38) |
⊕⊕○○ Low because of risk of bias and inconsistency |
| Length of stay in ICU | 4 | 785 | – | Mean duration was 0.31 day lower (2.08 lower to 1.45 higher) | 67%, (0.03) |
⊕⊕○○ Low because of risk of bias and imprecision |
| ∆Clinical pulmonary infection score | 8 | 596 | – | Mean difference was 1.08 lower (0.11 lower to 2.27 higher) | 96%, (0.000) |
⊕⊕○○ Low because of risk of bias and imprecision |
| Duration of mechanical ventilation | 4 | 774 | – | Mean duration was 0.45 day lower (2.69 lower to 1.78 higher) | 84%, (0.0003) |
⊕⊕⊕○ Very low because of risk of bias, inconsistency and imprecision |
| Nephrotoxicity | 7 | 1026 | RR, 0.82 (0.60, 1.12) | 26 more per 1000 (from 57 fewer to 17 more) | 2%, (0.41) |
⊕⊕⊕○ Moderate because of risk of bias |
| Bronchospasm | 8 | 1097 | RR, 2.55 (1.40, 4.66) | 38 more per 1000 (from 10 fewer to 89 more) | 49%, (0.02) |
⊕⊕⊕○ Moderate because of risk of bias |
⊕⊕⊕○ moderate, ⊕⊕○○ low, ⊕○○○ very low, CI = confidence intervals, ICU = intensive care unit. RR = relative risk.
*GRADE Working Group grades of evidence.
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Primary outcome
Clinical response rate was reported in all 13 RCTs11,14,19,20,24–34. The pooled analysis showed that, compared with control, NA improved clinical response (n = 1,450; RR = 1.24; 95% CI 1.13 to 1.35; P < 0.00001), with moderate heterogeneity (I2 = 47%) among the studies (Fig. 2). In the sensitivity analysis, exclusion of any single trial did not significantly alter the overall combined RR (P value ranging from 1.22 to 1.37, with I2 from 31 to 53%), whereas most subgroup analyses based on types of nebulizers, NA dose, sample size, study quality or study design confirmed similar improved clinical response among groups. However, the use of NA did not affect clinical response rate when pooling dada limited to studies using vibrating mesh nebulizer (P = 0.90), being high quality (P = 0.84), with large sample size (P = 0.15), or with blinding design (P = 0.84) (Table 3).
Figure 2.
Forest plots of the effects of aerosolized amikacin on clinical response.
Table 3.
Subgroup analysis on primary outcome of clinical response.
| Studies number | Patient number | Event in NA group | Event in control group | Risk ratio (95% CI) | I2 | P | ||
|---|---|---|---|---|---|---|---|---|
| Types of nebulizers | Vibrating mesh nebulizer | 3 | 698 | 191 of 358 | 177 of 340 | 0.99 [0.87, 1.13] | 0% | 0.90 |
| Ultrasonic nebulizer | 2 | 114 | 23 of 59 | 14 of 57 | 1.64 [1.07, 2.53] | 0% | 0.003 | |
| Jet nebulizer | 8 | 778 | 255 of 390 | 185 of 388 | 1.37 [1.22, 1.55] | 0% | < 0.0001 | |
| Patients with resistant GNP | 100% | 4 | 210 | 73 of 107 | 45 of 103 | 1.58 [1.24, 2.00] | 0% | 0.0002 |
| < 100% | 9 | 1,240 | 401 of 629 | 324 of 611 | 1.24 [1.08, 1.42] | 54% | 0.003 | |
| Dose of NA | ≥ 800 mg/day | 7 | 652 | 301 of 462 | 254 of 460 | 1.25 [1.04, 1.51] | 59% | 0.02 |
| < 800 mg/day | 7 | 542 | 173 of 272 | 129 of 270 | 1.33 [1.16, 1.53] | 35% | < 0.0001 | |
| Sample size | < 100 | 9 | 570 | 223 of 295 | 145 of 275 | 1.28 [0.78, 2.12] | 40% | < 0.0001 |
| > 100 | 4 | 880 | 251 of 441 | 224 of 439 | 1.19 [0.94, 1.50] | 63% | 0.15 | |
| Study quality | Low | 9 | 700 | 273 of 351 | 186 of 349 | 1.46 [1.31, 1.63] | 0% | < 0.0001 |
| High | 4 | 750 | 201 of 385 | 183 of 365 | 1.01 [0.89, 1.16] | 0% | 0.84 | |
| Study design | Blinded | 4 | 750 | 201 of 385 | 183 of 365 | 1.01 [0.89, 1.16] | 0% | 0.84 |
| Unblinded | 9 | 700 | 23 of 30 | 14 of 30 | 1.46 [1.31, 1.63] | 0% | < 0.0001 |
NA = nebulized amikacin; CI = confidence interval; GNP = gram‑negative pneumonia.
Secondary outcome
There was no statistically significant differences between the NA and control groups in overall mortality (7 trials, n = 1,058; RR = 1.17; 95% CI 0.98 to 1.50; I2 = 0%; P = 0.21)11,19,20,26,28,30,33 (Appendix 9, Fig. S3) or pneumonia associated mortality (7 trials, n = 1,066; RR = 1.12; 95% CI 0.82 to 1.52; I2 = 0%; P = 0.48) [11,14,19,26,28,30,33 ,(Appendix 9, Fig. S4). The length of stay in ICU (4 trials, n = 785, MD = − 0.31 days; 95% CI − 2.08 to 1.45, I2 = 67%; P = 0.73)11,20,26,28 (Appendix 9, Fig. S5), duration of MV (4 studies, n = 774, MD = − 0.45 days; 95% CI − 2.69 to 1.78, I2 = 84%; P = 0.69)11,19,20,28 (Appendix 9, Fig. S6) and ∆CPIS (8 studies, n = 596, MD = 1.08; 95% CI − 0.11 to 2.27, I2 = 96%; P = 0.08)11,14,20,29–32,34 (Appendix 9, Fig. S7) were also similar. Eleven RCTs reported specific data on outcome of microbiologic eradication, with better microbiologic eradication using NA compared with control (11 studies, n = 921, RR = 1.32; 95% CI 1.09 to 1.59, I2 = 6%; P < 0.00001)11,14,20,24–28,30–34 (Appendix 9, Fig. S8). Further sensitivity analyses showed that exclusion of any single trial did not significantly alter the overall combined RR in all the secondary outcomes.
Eight studies presented data on bronchospasm during treatment, with 6.4% (36/562) and 2.4% (13/535) in NA and control groups11,14,19,24. Pooled the data showed significantly higher bronchospasm in the NA group (RR = 2.55; 95% CI 1.40–4.66; I2 = 0%; P = 0.002) (Appendix 9; Fig. S9). Nephrotoxicity was reported in eight studies11,14,19,26,28–30,33. In the study by Liu et al., the authors reported no significant difference in serum creatinine concentration between NA and placebo group at the time of randomization (P = 0.857) and day 7 (P = 0.614)14. The other seven studies reported renal failure rate, and pooled data showed no differences between the two groups (n = 1,026; RR = 0.82; 95% CI 0.60–1.12; I2 = 2%; P = 0.20)11,19,26,28–30,33 (Appendix 9, Fig. S10).
Discussion
The present meta-analysis assessed the role of NA as adjunctive therapy in ventilated patients with GNP. We found NA has a better microbiologic eradication and improve the clinical response. Meanwhile, NA did not affect mortality, ∆CPIS, and duration of MV or ICU stay. Additionally, NA did not add significant nephrotoxicity, while it could cause more bronchospasm.
To date, several recent meta-analyses and guidelines have suggested favorable clinical response of aerosolized antibiotics in ventilated pneumonia3–5. However, pooled results of different study designs (RCTs and observational studies), various antibiotics (aminoglycosides, colistin, and vancomycin), and different therapy strategies (adjunctive and substitution) might contribute to the significant heterogeneity among the included studies. Meanwhile, observational studies have the risk of overrated pooled estimates. To address these limitations, we focused specifically on NA used as adjunctive therapy in ventilated GNP, expanded the sample size by including recent published RCTs, and conducted robust data analyses and quality evaluation. We found NA is effective as such a therapeutic strategy for GNP. Therefore, our findings support and expand the suggestions in previous meta-analyses and guidelines.
To facilitate comparison with the previous meta-analyses4,5, we chose clinical response as the primary outcome. Indeed, from a research and clinical standpoint, the clinical response may be a more reliable parameter compared with other important clinical outcomes (e.g., CPIS, microbiologic eradication or mortality, duration of MV, and ICU stay). For instance, the CPIS was originally designed for VAP diagnosis, rather than assessing the response to treatment35, whereas mortality is an outcome not only related to GNP, but it is also influenced by many other prognostic factors (e.g., underlying diseases, the severity of illness or immunity of the host). Furthermore, clinical response was the most reported outcome and might provide more evidence to aid in the clinical decision.
Our results showed NA exhibited better clinical response. However, we should interpret this finding with caution. First, we found moderate heterogeneity among the pooled trials in this outcome. This heterogeneity could be caused by different pathogenic bacteria and the definition of clinical response between the pooled trials. Subgroup-analysis of studies with large sample size and double blinding also could not confirm this benefit of NA. Second, we could not demonstrate a significant reduction in mortality, ICU LOS, and ventilated duration. Additionally, although NA resulted in better microbiologic eradication, the eradication data varied widely among the pooled studies (ranging from 29 to 71%)11,14,18,24, which means these data can be susceptible to some clinical factors, such as microbiological detection technique, the proportion of drug-resistant GNP, systemic antibiotics therapy, or airway secretions or antibiotics contained in bronchoalveolar lavage fluid. Of note, the positive detection of microbial culture may be affected by colonization with bacteria, and the correlation has been demonstrated to be poor between the positive cultures alone and histologically confirmed pneumonia36. Thus, microbiologic eradication based on microbial culture does not necessarily mean the eradication of deep parenchymal pneumonia.
Several included studies with high quality, though reporting the negative results, provided information concerning the specific treatments in NA. This might help to explain the opposite results among the included studies. On the one hand, the severity and extension of pulmonary infection might affect the lung deposition of NA. In ventilated animal models with pneumonia, lung tissue concentrations of NA were markedly lower in pulmonary segments with confluent pneumonia and lung abscess compared to that in the early stages of lung infection. However, most patients of included RCTs received NA only after their time-consuming VAP/GNP diagnosis procedures. This, to some extent, delays the administration of NA in the early stages of GNP. Furthermore, most of these patients also received a prolonged course of MV and/or intravenous amikacin before receiving NA. This might contribute to an increase in airway biofilms and bacterial resistance, thus making lung infection treatment more difficult and ineffective.
On the other hand, several critical factors, such as aerosol particle size, type of nebulizer, physical characteristics of the carrying gas, and respiratory settings during the implementation of NA can also influence lung deposition of NA. By and large, to increase the efficiency of aerosol delivery, ultrasonic or vibrating mesh nebulizers producing low flow turbulence, volume-control mode with the constant inspiratory flow and appropriate end-inspiratory pause (representing about 20% of the duty cycle) are preferred; whereas heating and humidification that increase the diameter of the aerosol particles (> 5 μm), decelerating flows, spontaneous modes or ventilator-patient asynchrony during NA period should be avoided. In one RCT focusing on nebulized antibiotics in VAP, the authors chose vibrating mesh nebulizers and filled out the well-designed checklist before NA to standardize and optimize the nebulization procedure. However, the total extrapulmonary (nebulizer chamber, the inspiratory limb of the respiratory circuit, and the expiratory filter) depositions of amikacin were as high as 40%. Therefore, it can be conceivable that in clinical practice, as shown in the included RCTs in the current study (Appendix 4), the efficiency of actual aerosol delivery may be lower. However, this may also mean that there is still ample space for improvement in nebulized techniques in the future.
This study has several limitations. First, most of included studies14,18,24 had a sample size of fewer than 100 patients, which might be subject to overestimation of effect size. Second, definitions and timing assessment of microbiologic eradication, the dose of amikacin used, as well as disease severity varied among the included RCTs. This might lead to observed heterogeneity, thus impairing the robustness of our findings. Third, the duration of MV before NA, time to start NA, and pathogens varied across included RCTs. The original plan of subgroup analysis to further explore trials based on the above diversities was hampered by insufficient data. Finally, the results of some subgroup analyses should be interpreted with caution due to insufficient studies, i.e., type of nebulizers or study design.
Conclusion
In summary, based on the current evidence, the use of NA adjunctive to systemic antibiotics therapy showed better benefits in ventilated patients with GNP. However, the overall quality of included studies is poor and more well-designed RCTs are still needed to confirmed our results.
Supplementary Information
Abbreviations
- CPIS
Clinical pulmonary infection score
- CI
Confidence interval
- GNP
Gram-negative pneumonia
- GRADE
Grading of recommendations assessment, development, and evaluation
- ICU
Intensive care unit
- MD
Mean difference
- MV
Mechanical ventilation
- NA
Nebulized amikacin
- RR
Risk ratio
- RCTs
Randomized controlled trials
- SD
Standard deviations
Author contributions
Dr. J.-P.Q. contributed data collection, analysis, and drafting of the article. Dr. Y.Z. and Dr. H.Z. contributed to data collection and analysis. Dr. Y.X. and Dr. B.D. contributed to the design and revisions of this manuscript. Dr. H.-B.H. was responsible for the conception of the study and the integrity of the work as a whole, from inception to publication of the article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-86342-8.
References
- 1.Gaynes R, Edwards JR. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 2005;41(6):848–854. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 2.Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American Thoracic Sosciety. Clin. Infect. Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu F, He LL, Che LQ, et al. Aerosolized antibiotics for ventilator-associated pneumonia: A pairwise and Bayesian network meta-analysis. Crit. Care (Lond. Engl.) 2018;22(1):301. doi: 10.1186/s13054-018-2106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zampieri FG, Nassar AP, Jr, Gusmao-Flores D, Taniguchi LU, Torres A, Ranzani OT. Nebulized antibiotics for ventilator-associated pneumonia: A systematic review and meta-analysis. Crit. Care (Lond. Engl.) 2015;19:150. doi: 10.1186/s13054-015-0868-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sweeney DA, Kalil AC. Why don't we have more inhaled antibiotics to treat ventilator-associated pneumonia? Clin. Microbiol. Infect. 2019;25(10):1195–1199. doi: 10.1016/j.cmi.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Panidis D, Markantonis SL, Boutzouka E, Karatzas S, Baltopoulos G. Penetration of gentamicin into the alveolar lining fluid of critically ill patients with ventilator-associated pneumonia. Chest. 2005;128(2):545–552. doi: 10.1378/chest.128.2.545. [DOI] [PubMed] [Google Scholar]
- 7.Sieger B, Berman SJ, Geckler RW, Farkas SA. Empiric treatment of hospital-acquired lower respiratory tract infections with meropenem or ceftazidime with tobramycin: A randomized study Meropenem Lower Respiratory Infection Group. Crit. Care Med. 1997;25(10):1663–1670. doi: 10.1097/00003246-199710000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Wood GC, Swanson JM. An update on aerosolized antibiotics for treating hospital-acquired and ventilator-associated pneumonia in adults. Ann. Pharmacother. 2017;51(12):1112–1121. doi: 10.1177/1060028017723934. [DOI] [PubMed] [Google Scholar]
- 9.Roberts MC. Antibiotic toxicity, interactions and resistance development. Periodontol. 2000;2002(28):280–297. doi: 10.1034/j.1600-0757.2002.280112.x. [DOI] [PubMed] [Google Scholar]
- 10.Palmer LB, Smaldone GC. Reduction of bacterial resistance with inhaled antibiotics in the intensive care unit. Am. J. Respir. Crit. Care Med. 2014;189(10):1225–1233. doi: 10.1164/rccm.201312-2161OC. [DOI] [PubMed] [Google Scholar]
- 11.Kollef MH, Ricard JD, Roux D, et al. A randomized trial of the amikacin fosfomycin inhalation system for the adjunctive therapy of gram-negative ventilator-associated pneumonia: IASIS Trial. Chest. 2017;151(6):1239–1246. doi: 10.1016/j.chest.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 12.Lu Q, Yang J, Liu Z, Gutierrez C, Aymard G, Rouby JJ. Nebulized ceftazidime and amikacin in ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Am. J. Respir. Crit. Care Med. 2011;184(1):106–115. doi: 10.1164/rccm.201011-1894OC. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein I, Wallet F, Nicolas-Robin A, Ferrari F, Marquette CH, Rouby JJ. Lung deposition and efficiency of nebulized amikacin during Escherichia coli pneumonia in ventilated piglets. Am. J. Respir. Crit. Care Med. 2002;166(10):1375–1381. doi: 10.1164/rccm.200204-363OC. [DOI] [PubMed] [Google Scholar]
- 14.Liu C, Zhang YT, Peng ZY, et al. Aerosolized amikacin as adjunctive therapy of ventilator-associated pneumonia caused by multidrug-resistant gram-negative bacteria: A single-center randomized controlled trial. Chin. Med. J. 2017;130(10):1196–1201. doi: 10.4103/0366-6999.205846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassan NA, Awdallah FF, Abbassi MM, Sabry NA. Nebulized versus IV amikacin as adjunctive antibiotic for hospital and ventilator-acquired pneumonia postcardiac surgeries: A randomized controlled trial. Crit. Care Med. 2018;46(1):45–52. doi: 10.1097/CCM.0000000000002695. [DOI] [PubMed] [Google Scholar]
- 16.Sole-Lleonart C, Roberts JA, Chastre J, et al. Global survey on nebulization of antimicrobial agents in mechanically ventilated patients: A call for international guidelines. Clin. Microbiol. Infect. 2016;22(4):359–364. doi: 10.1016/j.cmi.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Rello J, Sole-Lleonart C, Rouby JJ, et al. Use of nebulized antimicrobials for the treatment of respiratory infections in invasively mechanically ventilated adults: A position paper from the European Society of Clinical Microbiology and Infectious Diseases. Clin. Microbiol. Infect. 2017;23(9):629–639. doi: 10.1016/j.cmi.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Huang CX, Zhan XN, Su JH, et al. Efficacy and safety of intravenous antibiotics combined with inhalation of amikacin versus intravenous antibiotics for treatment of ventilator-associated pneumonia: A systematic review and meta-analysis. Int. J. Respir. 2020;40(7):521–530. [Google Scholar]
- 19.Ammar MA, Abdalla W. Effect of extended infusion of meropenem and nebulized amikacin on gram-negative multidrug-resistant ventilator associated pneumonia. Saudi J. Anesth. 2018;12(1):89–94. doi: 10.4103/sja.SJA_148_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niederman MS, Alder J, Bassetti M, et al. Inhaled amikacin adjunctive to intravenous standard-of-care antibiotics in mechanically ventilated patients with gram-negative pneumonia (INHALE): A double-blind, randomized, placebo-controlled, phase 3, superiority trial. Lancet. Infect. Dis. 2020;20(3):330–340. doi: 10.1016/S1473-3099(19)30574-2. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomized trials. BMJ (Clin. Res. ed) 2011;343:5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 23.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin. Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 24.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clin. Res. ed.) 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niederman MS, Chastre J, Corkery K, Fink JB, Luyt CE, Garcia MS. BAY41-6551 achieves bactericidal tracheal aspirate amikacin concentrations in mechanically ventilated patients with gram-negative pneumonia. Intensive Care Med. 2012;38(2):263–271. doi: 10.1007/s00134-011-2420-0. [DOI] [PubMed] [Google Scholar]
- 27.Chen S. Clinical efficacy of aerosol inhalation of amikacin in the treatment of elderly patients with ventilator-associated pneumonia and its effects on blood gas indexes and inflammatory factors. Anhui Med. Pharm. J. 2018;22(5):965–967. [Google Scholar]
- 28.Li H, Yang CH, Xue YY, et al. Clinical efficacy of nebulized amikacin in the treatment of ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Nei Ke Ji Wei Zhong Zheng Za Zhi. 2016;22(5):335–337. [Google Scholar]
- 29.Ji HH. Patients with ventilation-associated pneumonia caused by MDR-pseudomonas aeruginosa. China Prac Med. 2016;11(14):138–139. [Google Scholar]
- 30.Tong K. Efficacy of nebulized amikacin in treatment of ventilator-associated pneumonia. Lin Chuang Fei Ke Za Zhi. 2016;21(10):1862–1864. [Google Scholar]
- 31.Yue XZ. Curative effect and feasibility of aerosol inhalation of amikacin in the treatment of ventilator associated pneumonia. Chin Clin. Ration. Drug Use. 2016;9(5):38–39. [Google Scholar]
- 32.Zhu JD, Jiang HT, Luo JG, et al. Clinical value of amikacin in treatment of Acinetobacter baumannii ventilator associated pneumonia. Chin. J. Microecol. 2015;27(11):1319–1321. [Google Scholar]
- 33.Li H, Yang CH, Xue YY, et al. Efficacy of nebulized antibiotics in treatment of ventilator-associated pneumonia. Chinese Journal of Respir. Crit. Care Med. 2015;14(3):273–277. [Google Scholar]
- 34.Meng FL. Evaluation of efficacy of aerolized amikacin in the treatment of ventilator associated pneumonia (D). Bang Bu, Bang bu medical university. 2013.
- 35.Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and non bronchoscopic "blind" bronchoalveolar lavage fluid. Am. Rev. Respir. Dis. 1991;143(5 Pt 1):1121–1129. doi: 10.1164/ajrccm/143.5_Pt_1.1121. [DOI] [PubMed] [Google Scholar]
- 36.Tejerina E, Esteban A, Fernandez-Segoviano P, et al. Accuracy of clinical definitions of ventilator-associated pneumonia: Comparison with autopsy findings. J. Crit. Care. 2010;25(1):62–68. doi: 10.1016/j.jcrc.2009.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.