Abstract
Purpose
TO assess perceptions and implications of COVID-19 infection across the spectrum of individuals with visually impairment (VI) and those with normal sight.
Design
Prospective cross-sectional comparative study.
Methods
Setting: institutional. Patients: 232 patients and their caregivers. Four groups were created based on better eye characteristics: blind (best-corrected distance visual acuity [BCDVA] <3/60 or visual field <10 central degrees); severe VI (BCDVA ≤3/60 to <6/60; vertical cup-to-disc ratio ≥0.85 or neuroretinal rim width ≤0.1); moderate VI (BCDVA ≤6/60 to <6/18); or no or mild VI (controls: BCDVA ≥6/18) based on International Classification of Diseases-10 criteria and Foster and Quigley's consensus definition of glaucoma. Procedure: telephone questionnaires. Main outcome measures: differences in perceptions and implications of COVID-19 infection across various levels of VI. Caregiver perceptions were a secondary outcome measure.
Results
Surveys were completed by 232 participants, with 58 participants in each VI group. Mean age was 58.9 ± 13.2 years old. Greater degrees of VI were associated with older age (P = .008) and lower education level (P = .046). Blind participants more commonly perceived vision as a risk factor for contracting COVID-19 (P = .045), were concerned about access to health care (P <.001), obtained news through word of mouth (P <.001), and less commonly wore masks (P = .003). Controls more commonly performed frequent handwashing (P = .001), were aware of telemedicine (P = .029), and had fewer concerns about social interactions (P = .020) than groups with substantial VI. All caregivers reported more frequent patient care since the COVID-19 pandemic began.
Conclusions
The pandemic might have had a disproportionate impact on the visually impaired, and evidence-based assessments of COVID-19 health outcomes in this population are warranted.
CCORONAVIRUS disease 2019 (COVID-19) was declared a pandemic by the World Health Organization on March 11, 2020. The disease and its consequent shutdown brought various challenges to patients with visual impairment (VI) who were seeking eye care either electively or on an emergent basis. Analysis revealed that ophthalmology lost more patient volume than any other specialty during the acute phase of COVID-19 infection in the United States1 and possibly worldwide. In India, as in most countries across the world, travel restrictions were in place, private eye clinics were closed, and multispecialty hospitals focused all efforts on the overwhelming numbers of COVID-19 patients.2 Although all nonurgent cases were cancelled, a few specialized eye hospitals remained open to serve patients with urgent problems, including the Aravind Eye Care System (AECS), Pondicherry, a multispecialty tertiary eye care center serving 550,000 outpatients per year at the base hospital and 12 primary eye care centers (Vision Centers).
Studies evaluating an increased risk of contracting COVID-19 infection or worse outcomes in the setting of VI have not been performed. However, the presence of comorbidities such as hypertension, diabetes, chronic cardiac, pulmonary, or renal diseases, autoimmune disease, cancer, and tuberculosis have been associated with poor COVID-19 prognoses.3, 4, 5 Additionally, a decline in emergency department visits for conditions other than COVID-19 disease has been associated with higher morbidity and mortality rates due to other causes.6 , 7 Individuals with VI face certain disadvantages that may make them vulnerable to worse health outcomes.8, 9, 10 An unpublished survey of more than 1,900 individuals from the United States with VI revealed concerns regarding safety of public transit and taxis and access to health care, groceries, and other key essentials.11 Additionally, concomitant disabilities were reported in 40% of individuals with VI, and 60% of individuals with VI stated that they felt particularly vulnerable if exposed to COVID-19 infection.12
Currently, there is no evidence regarding differences in the perceptions of COVID-19 between VI individuals and those with normal vision. The present study aimed to assess the perceptions and implications of the COVID-19 pandemic in patients and their caregivers across the spectrum of VI. These perceptions included knowledge of COVID-19 infection, experience with COVID-19 infection, preventive measures undertaken, perceptions of VI affecting COVID-19 outcomes, delivery of eye care and associated obstacles, general physical and mental health, and caregiver perceptions.
METHODS
This prospective cross-sectional comparative study was performed using telephone surveys from July 20, 2020, to August 15, 2020. Institutional review board approval was obtained at AECS-Pondicherry, and research adhered to the tenets of the Declaration of Helsinki. The medical records database was used to obtain demographic data, ophthalmic findings, diagnoses, best-corrected distance visual acuity (BCDVA), and the telephone numbers of patients who presented to AECS-Pondicherry between July 2019 and February 2020. During their in-person visit to AECS prior to the onset of the pandemic, all participants underwent a complete ophthalmic examination including BCDVA, slit lamp biomicroscopy, gonioscopy, a fundus examination, and if able, visual field examination and optical coherence tomography imaging. Patients were consecutively recruited from the database based on their registration date (starting July, 2019), until the planned sample size was achieved.
Inclusion criteria included status as an established patient >18 years old, active telephone number, fluency in the regional language (Tamil), and willingness to participate. Exclusion criteria included incisional surgery or laser procedures performed elsewhere after their most recent visit to AECS, presence of neurocognitive disorders, and status as a health care worker, as this group might have been more familiar with COVID-19 infection. We also interviewed caregivers whom the patients identified as being responsible for their basic needs. Patients were divided into 4 groups based on the degree of their VI in the better eye, as shown in Table 1 .13 , 14 Those with moderate VI, severe VI, or blindness were considered case groups, whereas those with mild or no VI were controls. Furthermore, VI was classified as either reversible or irreversible based on diagnosis. For example, cataract, pterygium, and keratitis were classified as reversible, whereas proliferative diabetic retinopathy, glaucoma, and retinitis pigmentosa were classified as irreversible. In patients with multiple diagnoses, the diagnosis with the more advanced disease state (presumably responsible for greater VI) was considered primary.
Table 1.
Categorization Based on Visual Impairment
| Visual Impairment Category | Classification Type | Definition |
|---|---|---|
| Blind | International Classification of Diseases-1013 | BCDVA worse than 3/60 or a visual field < 10 degree in radius around central fixation in the better eye |
| Severe Visual Impairment (fulfilled either of the following criteria) | International Classification of Diseases-1013 | BCDVA worse than 6/60 but better than or equal to 3/60 in the better eye |
| Foster and Quigley Criteria - Advanced Disc Damage14 | Vertical CDR or CDR asymmetry greater than or equal to 99.5th percentile (CDR 0.85) in normal population; neuroretinal rim width reduced to ≤ 0.1 of CDR | |
| Moderate Visual Impairment | International Classification of Diseases-1013 | BCDVA worse than 6/18 but better than or equal to 6/60 in the better eye |
| No / Mild Visual Impairment (controls) | International Classification of Diseases-1013 | BCDVA better than or equal to 6/18 in the better eye |
BCDVA: Best corrected distance visual acuity
CDR: Cup-to-disc ratio
A pilot study was performed in July 2020 in 40 participants (10 in each category) to validate survey questions, assess feasibility, and calculate sample size. During the phone call, survey details were discussed, and participants were asked to provide oral consent. Caregivers, if available, were also free to accept or decline the survey. The validated patient survey questionnaire and the caregiver survey questionnaire were administered in Tamil by 1 of 4 experienced study coordinators.
Statistical Analysis
We performed statistical analyses using SPSS version 27.0 software (IBM Analytics, Chicago, Illinois, USA). Continuous variables were presented as mean ± SD. Proportions (%) were used to describe categorical variables. Between-group comparisons were performed using 1-way analysis of variance (ANOVA) for continuous variables (age) and χ2 tests for categorical variables (and all other variables). Post-hoc Tukey honest significant difference tests were performed using significant P values for multiple comparisons and to uncover specific differences between groups. P values <.05 were considered significant. Estimation of sample size was performed by considering outcomes from the pilot study, which included the same surveys included in the main study. To allow for 80% power and an alpha of .05, an estimated 232 participants (58 in each group) were required to identify differences among varying levels of VI in regard to obtaining information about COVID-19 and perceptions regarding vision as a risk factor for disease contraction.
RESULTS
Characteristics of the Study Group
A total of 470 participants were eligible for the study, and contact was made with 248 households. Of this group, 10 individuals declined participation, and 6 individuals had died (cause of death was not asked). The questionnaire was completed by 232 participants, with 58 participants in each group. Baseline characteristics of survey respondents and nonrespondents are represented in Table 2 and Supplemental Table 1, respectively. Caregivers of 132 participants (56.9% of total) responded to the survey. Participation by caregivers varied across groups, and was more common among caregivers of blind participants (72.4% vs. 34.5%-60.3%, respectively; P <.001).
Table 2.
Characteristics of Patient Survey Respondents
| Controls N = 58 | Moderate VI N = 58 | Severe VI N = 58 | Blindness N = 58 | Total N = 232 | P-value * | ||
|---|---|---|---|---|---|---|---|
| Age | 54.6 ± 11.7a | 57.7 ± 13.2a | 61.1 ± 12.4b | 62.2 ± 14.3b | 58.9 ± 13.2 | 0.008 | |
| Females (%) | 43.1 % | 46.6% | 32.8% | 37.9% | 40.1% | 0.451 | |
| Education Level | No schooling | 6 (10.3)a | 18 (31.1)a | 12 (20.7)a | 10 (17.2)a | 46 (19.8) | 0.046 |
| Elementary school | 18 (31)a | 21 (36.2)a | 27 (46.6)a | 24 (41.4)a | 90 (38.8) | ||
| Secondary or high school | 22 (37.9)a | 15 (25.9)a | 14 (24.1)a | 19 (32.8)a | 70(30.2) | ||
| Graduate or postgraduate | 12 (20.7)a | 4 (6.9)b | 5 (8.6)b | 5 (8.6)b | 26 (11.2) | ||
| Residential Setting | Urban or semi-urban | 26 (44.8) | 24 (41.4) | 20 (34.5) | 19 (32.8) | 89 (38.4) | 0.496 |
| Rural | 32 (55.2) | 34 (58.6) | 38 (65.5) | 39 (67.2) | 143 (61.6) | ||
| Diagnosis | Early cataract | 42 (72.4)a | 17 (29.3)b | 0c | 0c | 59 (25.4) | < 0.001 |
| Advanced cataract | 0a | 2 (3.4)b | 15 (25.9)c | 10(17.2)b | 27 (11.6) | ||
| Refractive error | 7 (12.1)a | 0b | 1 (1.7)b | 0b | 8 (3.4) | ||
| Conjunctival disorder | 5 (8.6)a | 0b | 0b | 0b | 5 (2.2) | ||
| Corneal disorder | 2 (3.4)a | 2 (3.4)a | 1 (1.7)a | 1 (1.70)a | 6 (2.6) | ||
| Non-proliferative diabetic retinopathy | 2 (3.4)a | 0a | 1 (1.7)a | 0a | 3 (1.3) | ||
| Proliferative diabetic retinopathy | 0a | 9 (15.5)a | 7 (12.1)a | 2 (3.4)a | 18 (7.8) | ||
| Other retinal disorder∞ | 0a | 21 (36.2)b | 10 (17.2)c | 4 (6.9)c | 35 (15.1) | ||
| Optic neuropathy | 0a | 5 (8.6)b | 1 (1.7)a | 0a | 6 (2.6) | ||
| Glaucoma | 0a | 2 (3.4)a | 22 (37.9)b | 41 (70.7)c | 65 (28.2) | ||
| Primary Diagnosis Associated WithIrreversible Vision Loss | 0a | 37 (63.8)b | 40 (69.0)b | 47 (81.0)c | 124 (53.4) | < 0.001 | |
*Post-hoc Tukey's Honest Significant Difference tests were performed for P-values less than 0.05 to uncover specific differences between groups. Groups that share a common superscript within a row are not significantly different, while those do not share a common superscript are significantly different.
∞Other retinal disorders included age-related macular degeneration, retinitis pigmentosa, myopic degeneration, and other forms of retinal disease.
VI= Visual impairment
Older patients were commonly more visually impaired (P = .008), controls were more likely to have a graduate degree (P = .046). The frequency of irreversible blinding primary diagnoses varied among groups and were most common among blind participants (81.0%) and least common among controls (0%) (P <.001). Questions and answers for the patient survey are reported in Table 3 and for the caregiver survey are reported in Table 4 .
Table 3.
Patient Questionnaire
| Controls N = 58 | Moderate VI N = 58 | Severe VI N = 58 | Blindness N = 58 | Total N = 232 | P-value * | ||
|---|---|---|---|---|---|---|---|
| Have you heard about the COVID-19 pandemic? | Yes | 57 (98.3) | 58 (100) | 58 (100) | 57 (89.3) | 230 (99.1) | 0.569 |
| How did you find out about the COVID-19 pandemic? | Word of mouth | 13 (22.4)a | 23 (39.7)b | 26 (44.8)b | 41 (70.7)c | 103 (44.4) | < 0.001 |
| TV (audio and visual) | 54 (93.1)a | 55 (94.8)a | 54 (93.1)a | 40 (69.0)b | 203 (87.5) | < 0.001 | |
| Newspaper (in print or online) | 19 (32.8) | 19 (32.8) | 10 (17.2) | 10 (17.2) | 58 (25.0) | 0.059 | |
| Social media | 9 (15.5) | 5 (8.6) | 3 (5.2) | 2 (3.4) | 19 (8.2) | 0.086 | |
| TV audio only | 0a | 0a | 1 (1.7)a | 4 (6.9)b | 5 (2.2) | 0.032 | |
| Have you been sick with COVID-19? | Yes | 1 (1.7) | 0 | 0 | 0 | 1 (0.4) | 0.390 |
| No | 57 (98.3) | 58 (100) | 58 (100) | 58 (100) | 231 (99.6) | ||
| I am not sure | 0 | 0 | 0 | 0 | 0 | ||
| Do you know anyone who has been sick with COVID-19? | Yes | 8 (13.8) | 4 (6.9) | 2 (3.4) | 3 (5.2) | 17 (7.3) | 0.290 |
| No | 49 (84.5) | 54 (93.1) | 55 (94.8) | 55 (94.8) | 213 (91.8) | ||
| I am not sure | 1 (1.7) | 0 | 1 (1.7) | 0 | 2 (0.9) | ||
| Have you taken any preventive measures? | Avoid leaving the home | 27 (46.6)a | 52 (89.7)b | 33 (56.9)b | 45 (77.6)c | 157 (67.7) | < 0.001 |
| Wearing a mask | 56 (96.6)a | 48 (82.8)a | 55 (94.8)a | 45 (77.6)b | 204 (87.9) | 0.003 | |
| Frequent hand washing | 49 (84.5) a | 33 (56.9)b | 42 (72.4)b | 30 (51.7)b | 154 (66.4) | 0.001 | |
| Social distancing from others | 21 (36.2)a | 3 (5.2)b | 8 (13.8)c | 8 (13.8)c | 40 (17.2) | < 0.001 | |
| Other | 13 (22.4) | 5 (8.6) | 14 (24.1) | 16 (27.6) | 48 (20.7) | 0.061 | |
| None | 0 | 0 | 0 | 0 | 0 | ||
| If not, why not? | I do not feel the virus will affect me | 1 (1.7) | 0 | 0 | 1 (1.7) | 2 (0.9) | 0.569 |
| I lack the resources | 0 | 0 | 0 | 0 | 0 | ||
| Do you feel your vision may put you at greater risk of contracting COVID-19? | Yes | 2 (3.4)a | 7 (12.1)a | 2 (3.4)a | 8 (13.8)b | 19 (8.2) | 0.045 |
| No | 54 (93.1)a | 51 (87.9)a | 56 (96.6)a | 50 (86.2)a | 211 (90.9) | ||
| Maybe | 0a | 0a | 0a | 0a | 0 | ||
| I am not sure | 2 (3.4)a | 0a | 0a | 0a | 2 (0.9) | ||
| Do you feel your vision may put you at greater risk of poor outcomes if you contract COVID-19? | Yes | 2 (3.4) | 8 (13.8) | 2 (3.4) | 10 (17.2) | 22 (9.5) | 0.053 |
| No | 51 (87.9) | 48 (82.8) | 54 (93.1) | 48 (82.8) | 201 (86.6) | ||
| Maybe | 1 (1.7) | 0 | 1 (1.7) | 0 | 2 (0.9) | ||
| I am not sure | 4 (6.9) | 2 (3.4) | 1 (1.7) | 0 | 7 (3.0) | ||
| Do you feel that your eye treatment may put you at greater risk of contracting COVID-19 or suffering worse disease? | Yes | 6 (10.3) | 8 (13.8) | 8 (13.8) | 12 (20.7) | 34 (14.7) | 0.310 |
| No | 49 (84.5) | 50 (86.2) | 48 (82.8) | 45 (77.6) | 192 (82.8) | ||
| Maybe | 0 | 0 | 1 (1.7) | 1 (1.7) | 2 (0.9) | ||
| I am not sure | 3 (5.2) | 0 | 1 (1.7) | 0 | 4 (1.7) | ||
| Do you feel your vision has worsened during the pandemic? | Yes | 9 (15.5) | 8 (13.8) | 14 (24.1) | 16 (27.6) | 47 (20.3) | 0.356 |
| No | 49 (84.5) | 49 (84.5) | 43 (74.1) | 41 (70.7) | 182 (78.4) | ||
| Maybe | 0 | 0 | 1 (1.7) | 1 (1.7) | 2 (0.9) | ||
| I am not sure | 0 | 1 (1.7) | 0 | 0 | 1 (0.4) | ||
| Did you face any additional difficulties because of the lockdown? | Obtaining eye medications | 1 (1.7) | 7 (12.1) | 3 (5.2) | 3 (5.2) | 14 (6.0) | 0.123 |
| Seeing the eye doctor | 6 (10.3)a | 48 (82.8)b | 25 (43.1)c | 22 (37.9)c | 101 (43.5) | < 0.001 | |
| Undergoing an eye procedure | 4 (6.9)a | 1 (1.7)b | 12 (20.7)a | 12 (20.7)a | 29 (12.5) | 0.002 | |
| I have not faced any above difficulties | 47 (81.0)a | 8 (13.8)b | 24 (41.4)c | 24 (41.4)c | 103 (44.4) | < 0.001 | |
| If you had difficulty obtaining eye medications or seeing the eye doctor, what were some reasons for this? | Unable to travel to pharmacy or hospital | 1 (1.7)a | 10 (17.2)b | 6 (10.3)a | 1 (1.7)a | 18 (7.8) | 0.003 |
| Lack of transport due to the lockdown | 10 (17.2)a | 50 (86.2)b | 35 (60.3)c | 35 (60.3)c | 130 (56.0) | < 0.001 | |
| I did not feel it was safe to travel | 2 (3.4) | 3 (5.2) | 4 (6.9) | 8 (13.8) | 17 (7.3) | 0.153 | |
| I was too sick to travel | 0 | 1 (1.7) | 0 | 1 (1.7) | 2 (0.9) | 0.569 | |
| Financial burden of lockdown | 0a | 0a | 3 (5.2)b | 0a | 3 (1.3) | 0.028 | |
| Medications were not available | 0 | 0 | 0 | 0 | 0 | ||
| Would you prefer going to a vision center rather than a base hospital? | Yes | 9 (15.5)a | 34 (58.6)b | 6 (10.3)a | 3 (5.2)c | 52 (22.4) | < 0.001 |
| Would you undergo a laser, injection, or surgical intervention during this period if advised by your eye physician? | Yes | 32 (55.2)a | 42 (72.4)b | 46 (79.3)b | 43 (74.1)b | 163 (70.3) | 0.002 |
| No | 11 (19.0)a | 15 (25.9)a | 8 (13.8)a | 6 (10.3)a | 40 (17.2) | ||
| It depends on the intervention | 10 (17.2)a | 1 (1.7)a | 2 (3.4)a | 8 (13.8)a | 21 (9.1) | ||
| I am not sure | 5 (8.6)a | 0a | 2 (3.4)a | 1 (1.7)a | 8 (3.4) | ||
| Would you appear for regular follow up after the procedure as per medical advice? | Yes | 38 (65.5)a | 43 (74.1)a | 49 (84.5)a | 48 (82.8)a | 178 (76.7) | 0.013 |
| No | 10 (17.2)a | 14 (24.1)a | 7 (12.1)a | 6 (10.3)a | 37 (15.9) | ||
| I prefer delaying my appointments | 3 (5.2)a | 0a | 0a | 3 (5.2)a | 6 (2.6) | ||
| I am not sure | 7 (12.1)a | 1 (1.7)b | 2 (3.4)b | 1 (1.7)b | 11 (4.7) | ||
| If advised for frequent examinations based on your condition, how often would you be able to come? | Weekly | 5 (8.6)a | 1 (1.7)a | 3 (5.2)a | 1 (1.7)a | 10 (4.3) | < 0.001 |
| Monthly | 19 (32.8)a | 9 (15.5)b | 29 (50.0)a | 27 (46.6)a | 84 (36.2) | ||
| Exam once in 3 months | 5 (8.6)a | 20 (34.5)b | 3 (5.2)a | 3 (5.2)a | 31 (13.4) | ||
| Exam once in 6 months | 2 (3.4)a | 12 (20.7)b | 3 (5.2)a | 2 (3.4)a | 19 (8.2) | ||
| Depends on lockdown relaxation | 19 (32.8)a | 3 (5.2)b | 12 (20.7)a | 19 (32.8)a | 53 (22.8) | ||
| I am not sure | 8 (13.8)a | 13 (22.4)a | 8 (13.8)a | 6 (10.3)a | 35 (15.1) | ||
| Do you have difficulty in the following? | Seeing even when wearing glasses | 0a | 41 (70.7)b | 8 (13.8)c | 8 (13.8)c | 57 (24.6) | < 0.001 |
| Hearing | 1 (1.7)a | 4 (6.9)a | 8 (13.8)a | 7 (12.1)a | 20 (8.6) | 0.087 | |
| Concentrating, remembering, or making decisions | 1 (1.7)a | 10 (17.2)b | 2 (3.4)a | 3 (5.2)a | 16 (6.9) | 0.004 | |
| Walking or climbing stairs | 1 (1.7)a | 13 (22.4)b | 7 (12.1)b | 10 (17.2)b | 31 (13.4) | 0.008 | |
| Dressing or bathing | 0a | 0a | 0a | 6 (10.3)b | 6 (2.6) | < 0.001 | |
| Doing errands alone such as doctor visits, shopping | 0 | 2 (3.4) | 0 | 2 (3.4) | 4 (1.7) | 0.254 | |
| Do not wish to answer | 2 (3.4) | 0 | 1 (1.7) | 0 | 3 (1.3) | 0.294 | |
| I don't have any difficulty | 53 (91.4)a | 11 (19.0)b | 37 (63.8)c | 36 (62.1)c | 137 (59.1) | < 0.001 | |
| Over the past 12 months, has a doctor or health provider told you that you have any of the following? | Diabetes | 15 (25.9) | 25 (43.1) | 25 (43.1) | 20 (34.5) | 85 (36.6) | 0.164 |
| Heart disease | 0 | 3 (5.2) | 2 (3.4) | 4 (6.9) | 10 (4.3) | 0.554 | |
| Pulmonary disorder (asthma, COPD, etc.) | 0 | 0 | 4 (6.9) | 3 (5.2) | 7 (3.0) | 0.057 | |
| Hearing impairment | 0a | 0a | 5 (8.6)a | 8 (13.8)b | 13 (5.6) | 0.002 | |
| Epilepsy or neurologic disease | 0 | 0 | 0 | 0 | 0 | - | |
| Arthritis | 1 (1.7) | 1 (1.7) | 5 (8.6) | 6 (10.3) | 13 (5.6) | 0.080 | |
| Are overweight or underweight | 0 | 0 | 0 | 0 | 0 | - | |
| Psychiatric disorder | 0 | 0 | 0 | 0 | 0 | - | |
| Significant physical disability | 0 | 0 | 0 | 1 (1.7) | 1 (0.4) | 0.390 | |
| Developmental or intellectual disability | 0 | 0 | 0 | 0 | 0 | - | |
| Other | 10 (17.2) | 17 (29.3) | 12 (20.7) | 17 (29.3) | 56 (24.1) | 0.311 | |
| None of the above | 36 (62.1)a | 24 (41.4)b | 23 (39.7)b | 22 (37.9)b | 105 (45.3) | 0.030 | |
| If you have an underlying health condition, do you feel your condition makes you more likely to suffer from complications if you contract COVID-19? | Yes | 7 (31.8) | 5 (14.7) | 3 (8.6) | 3 (8.3) | 18 (14.2) | 0.111 |
| No | 15 (68.2) | 28 (82.4) | 32 (91.4) | 33 (91.7) | 108 (85.0) | ||
| I am not sure | 0 | 1 (2.9) | 0 | 0 | 1 (0.8) | ||
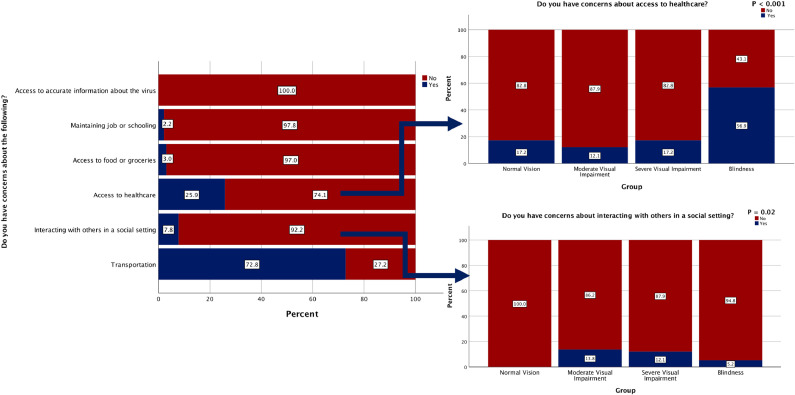
| Do you have concerns about the following? | Transportation | 34 (58.6)a | 50 (86.2)b | 42 (72.4)c | 43 (74.1)c | 169 (72.8) | 0.011 |
| Interacting with others in a social setting | 0a | 8 (13.8)b | 7 (12.1)b | 3 (5.2)b | 18 (7.8) | 0.020 | |
| Access to healthcare | 10 (17.2)a | 7 (12.1)b | 10 (17.2)a | 33 (56.9)c | 60 (25.9) | < 0.001 | |
| Access to food and groceries | 6 (10.3)a | 0b | 1 (1.7)b | 0b | 7 (3.0) | 0.002 | |
| Maintaining your job or schooling | 1 (1.7)a | 0a | 4 (6.9)b | 0a | 5 (2.2) | 0.032 | |
| Access to accurate information about the virus | 0 | 0 | 0 | 0 | 0 | - | |
| I am not sure | 0 | 1 (1.7) | 0 | 0 | 1 (0.4) | 0.390 | |
| I have no concerns | 21 (36.2)a | 6 (10.3)b | 15 (25.9)a | 13 (22.4)a | 55 (23.7) | 0.012 | |
| Since COVID-19 began, have you had more anxiety, fear, concern about your health? | Yes | 8 (13.8)a | 14 (24.1)b | 4 (6.9)a | 5 (8.6)a | 31 (13.4) | 0.029 |
| If you developed COVID-19 symptoms (cough, sore throat, fever), would you seek medical care? | Yes | 58 (100) | 58 (100) | 57 (98.3) | 57 (98.3) | 230 (99.1) | 0.421 |
| No | 0 | 0 | 1 (1.7) | 0 | 1 (0.4) | ||
| I am not sure | 0 | 0 | 0 | 1 (1.7) | 1 (0.4) | ||
| Have you heard of telehealth or virtual care? | Yes | 11 (19.0)a | 5 (8.6)b | 2 (3.4)b | 4 (6.9)b | 22 (9.5) | 0.029 |
*Post-hoc Tukey's Honest Significant Difference tests were performed for P-values less than 0.05 to uncover specific differences between groups. Groups that share a common superscript within a row are not significantly different, while those that do not share a common superscript are significantly different.
COVID-19 = Coronavirus disease
VI = Visual impairment
Table 4.
Caregiver Questionnaire
| Controls | Moderate VI | Severe VI | Blindness | Total | P-value * | ||
|---|---|---|---|---|---|---|---|
| Number of caregivers available for the survey | 20 (34.5)a | 35 (60.3)b | 35 (60.3)b | 42 (72.4)c | 132 (56.9) | < 0.001 | |
| If this section cannot be completed, choose a reason | Caregiver was present, but was unable/did not want to answer | 3 (7.9) | 1 (4.3) | 2 (8.7) | 3 (18.8) | 9 (9.0) | 0.470 |
| Caregiver was not present | 32 (84.2) | 22 (95.7) | 20 (87.0) | 13 (81.3) | 87 (87.0) | ||
| The patient does not have a caregiver who live with him/her | 3 (7.9) | 0 | 1 (4.3) | 0 | 4 (4.0) | ||
| Has the frequency of your care for Mr./Mrs.___ changed since COVID-19 began? | Yes, more frequent visits/care | 20 (100) | 35 (100) | 35 (100) | 42 (100) | 132 (100) | - |
| Yes, less frequent visits/care | 0 | 0 | 0 | 0 | 0 | ||
| No | 0 | 0 | 0 | 0 | 0 | ||
| If there has been a change, select possible reasons for this. | Fear of contracting the virus or transmitting to others | 17 (29.3)a | 32 (55.2)b | 30 (51.7)b | 41 (70.7)c | 120 (51.7) | < 0.001 |
| Transportation issues due to the lockdown | 3 (5.2)a | 17 (29.3)b | 5 (8.6)a | 1 (1.7)c | 26 (11.2) | < 0.001 | |
| Increased need to care | 0a | 1 (1.7)a | 5 (8.6)a | 8 (13.8)b | 14 (6.0) | 0.006 | |
| I have been sick with COVID-19 | 0 | 0 | 0 | 0 | 0 | - | |
| Other | 1 (1.7) | 0 | 2 (3.4) | 2 (3.4) | 5 (2.2) | 0.522 | |
| Have you been able to take adequate precautions (wearing a mask, frequent handwashing, etc.) to prevent infection while caring for Mr./Ms.___? | Yes | 19 (95) | 35 (100) | 35 (100) | 42 (100) | 131 (99.2) | 0.130 |
| Since COVID-19 began, have you had more anxiety, fear, concern about your health? | Yes | 1 (5.0) | 1 (2.9) | 0 | 0 | 2 (1.5) | 0.357 |
*Post-hoc Tukey's Honest Significant Difference tests were performed for P-values less than 0.05 to uncover specific differences between groups. Groups that share a common superscript within a row are not significantly different, while those that do not share a common superscript are significantly different.
COVID-19 = Coronavirus disease
VI = Visual impairment
Knowledge of Covid-19 Infection
Awareness of the pandemic was similar among all groups, but modes of information transfer varied. Reliance on TV (audio and visual) to obtain news was less common among blind participants (69.0% vs. 93.1%-94.8%, respectively; P <.001), who were more likely to depend only on TV audio (6.9% vs. 0%-1.7%, respectively; P = .032) or word of mouth (70.7% vs. 22.4%-44.8%, respectively; P <.001).
Experience with COVID-19 and Preventive Measures
Only 1 participant reported being positive for COVID-19, and few individuals overall knew of others who had been sick with COVID-19 infection (3.4%-14.8%, respectively; P = .290). Preventive measures varied among groups (Figure 1 ). Mask-wearing varied among groups, and was least common among blind participants (77.6% vs. 82.8%-96.6%, respectively; P = .003). Likewise, frequent handwashing varied among groups and was more common among controls (84.5% vs. 51.7%-72.4%, respectively; P = .001).
Figure 1.
Participant responses regarding COVID-19 preventive measures. (Left) All participants’ responses. (Right) Each group's response regarding mask-wearing and frequent handwashing.
VI and Perceptions of COVID-19 Infection
The feeling that vision status puts the patient at greater risk of contracting COVID-19 disease varied across groups, with the highest percentage found in blind participants (13.8% vs. 3.4%-12.1%, respectively; P = .045) (Figure 2 ). Likewise, concerns about access to health care differed among groups, with the highest percentage reporting these concerns among blind participants (56.9% vs. 12.1%-17.2%, respectively; P <.001) (Figure 3 ). Furthermore, the feeling that vision status would lead to poor COVID-19 outcomes differed among groups, with the highest proportion among blind participants (17.2% vs. 3.4-13.8%, respectively; trending toward significance: P = .053). However, no differences were detected among groups regarding eye treatment leading to poor COVID-19 outcomes (P = .310). The majority of participants (72.8%) noted concerns about transportation, more commonly among those with moderate VI (86.2% vs. 58.6%-74.1%, respectively; P = .011). Concerns about social interaction varied among groups, with lowest concerns among controls (0% vs. 5.2%-13.8%, respectively; P = .020) (Figure 3). Controls were also more likely to have concerns about accessing food and groceries (10.3% vs. 0%-1.7%, respectively; P = .002) than other VI groups.
Figure 2.
Participants’ responses regarding COVID-19 contraction risk or poor outcomes in the setting of vision status or eye treatment. (Left) All participants’ responses. (Right) Each group's response regarding vision as a risk factor for COVID-19 contraction.
Figure 3.
Participants’ responses regarding concerns during COVID-19. (Left) All participants’ responses. (Right) Each group's response regarding concerns about health care access and social interactions.
Delivery of Eye Care and Associated Obstacles
Difficulty seeing the eye doctor was reported with varying frequency, most commonly by those with moderate VI (82.8% vs. 10.3%-43.1%, respectively; P <.001). Difficulty accessing eye medications was reported infrequently (1.7%-12.1%; P = .123), and no participant reported a lack of medication availability, potentially because the medication supply chain was intact. Telehealth care was recognized with varying frequency across groups, more commonly by controls (19% vs. 3.4%-8.6%, respectively; P = .029). Additionally, willingness to undergo a medically advised intervention and appearing for regular follow-up after procedures varied across groups, with the lowest proportion among controls ([55.2% vs. 72.4%-79.3%, respectively; P = .002] and [65.5% vs. 74.4%-84.5%, respectively; P = .013], respectively).
General Physical and Mental Health
Difficulty in walking or climbing stairs was reported at differing levels across groups, with the lowest proportion among controls (1.7% vs. 12.1%-22.4%, respectively; P = .008). Blind participants were the only group to report difficulty with dressing or bathing (10.3% vs. 0%, respectively; P <.001) and more commonly reported a diagnosed hearing impairment than other groups (13.8% vs. 0-8.6%, respectively; P = .002). Of those who reported an underlying health condition, 14.2% felt that their condition would make them more likely to suffer from worse complications due to COVID-19 infection, with no differences between groups (P = .111). In regard to mental health, increased fear, anxiety, or concern about health was present across groups, with a greater rate among those with moderate VI (24.1% vs. 6.9%-13.8%, respectively; P = .029).
Caregiver Perceptions
Caregivers in all groups reported more frequent visits to survey participants’ homes since the COVID-19 pandemic began. Reasons for increased visits varied across groups, and caregivers of blind participants were more likely to report patients’ fears of contracting the virus or transmitting the virus to others (70.7% vs. 29.3%-55.2%, respectively; P <.001) or increased need for care (13.8% vs. 0%-8.6%, respectively; P = .006) as reasons for more frequent visits. The majority of caregivers reported that they had been able to take adequate precautions to prevent infection while caring for the surveyed participants (95%-100%; P = .130).
DISCUSSION
The survey demonstrated that viewpoints on the implications of COVID-19 infection on eye care, general and mental health, and essential activities varied among VI groups, and serious concerns were more commonly reported among individuals with substantial VI. The perspectives of physicians, trainees, and industry during the COVID-19 pandemic have been extensively published,15, 16, 17, 18, 19 but the literature for the perspectives of visually impaired patients has been limited.11 , 20
VI is a major cause of disability worldwide21 and has been associated with increased mortality,22 multiple chronic comorbidities,23 , 24 anxiety and depression,10 and decreased quality of life.25 , 26 People with disabilities have been reported to be at higher risk of COVID-19 infection and worse outcomes,27 but disease prevalence and outcomes among the visually impaired have not been adequately examined. The present study was not intended to detect differences among groups in regard to rate of infection, as only 1 participant overall reported being positive for COVID-19 infection. However, most participants suffered from problems related to the COVID-19 shutdown. The degree of VI had an impact on the types and magnitudes of these challenges. Blind participants were most likely to perceive vision as a risk factor for contracting COVID-19 illness (P = .045) and worse disease outcomes (trending toward significance: P = .053) (Figure 2). Overall, groups with greater proportions of irreversible blinding disease, including those with moderate and severe VI, as well as blindness, reported greater challenges associated with COVID-19 and its shutdown. These challenges included difficulty in seeing the eye doctor (P <.001), lack of transport (P <.001), and financial burden (P = .028).
Additionally, preventive measures varied among VI groups, as mask-wearing was least commonly reported by blind participants (P = .003), and frequent handwashing was most common among controls (P = .001) (Figure 1). These visually demanding preventive measures may be challenging for individuals with substantial VI, whose daily activities and mobility are limited at baseline.28, 29, 30, 31 Furthermore, given their greater concerns about the risk of contracting COVID-19 infection, individuals with worse vision might have been staying isolated, lessening the need for preventive measures such as mask wearing and hand washing. In agreement with prior studies,28, 29, 30, 31 the present participants described impediments to mobility, including limitations in dressing and bathing among the blind (P <.001), whereas controls denied difficulty walking or climbing stairs (P = .008). However, these limitations might have predated the COVID-19 pandemic, and associated changes before and during the pandemic were not specifically assessed. These findings highlight the limitations that individuals with disabilities, especially VI, face in following strict social distancing and hygiene protocols to prevent COVID-19 transmission.32 , 33
Moreover, insufficient knowledge of preventive measures might have contributed to lower adherence to safe practices by those with substantial VI.32 Although the majority of participants (99.1%) were aware of the pandemic, blind participants more commonly relied on word of mouth to obtain information (P <.001). Not being able to access print or video reports might have made this group vulnerable to incomplete information about modes of transmission and techniques for prevention. Furthermore, 13.8% of those with blindness and 8.6% of those with severe VI had a diagnosed concomitant hearing impairment, as compared to 0% in the other groups (P = .002); this might have been associated with their older age34 and worse functioning than VI alone.35 , 36
Even without COVID-19, visually impaired individuals experience increased challenges to access to health care, from the identification of a potential problem to the ongoing management of diagnosed illnesses.37 In the present sample, blind participants were the most concerned about health care access (P <.001) (Figure 3). These obstacles were augmented by the forced closure of many health systems and increased dependence on telehealth.38 , 39 The present survey sought to learn various VI groups’ understanding of alternative health care access options and found that, although awareness of telehealth was overall low (9.5%), controls were more commonly familiar with this modality of care (P = .029). Additionally, the majority of survey participants (72.8%) reported travel concerns, including limitations to seeing an ophthalmologist or to going to the pharmacy. Seeing an eye doctor was a challenge for 43.5% of all participants and was highest among those with moderate VI (P <.001). Additionally, this group most commonly reported anxiety and fear in the COVID-19 era (P = .029), difficulty seeing with glasses (P <.001), and difficulty with transportation (P = .011). This group's substantial proportions of treatable disorders such cataracts (29.3%) and retinal disorders (36.2%) and possible delayed intervention might have led to increased anxiety, whereas other groups might have had no need for interventions (controls) or untreatable advanced irreversible disease (severe VI, blindness). Furthermore, controls might have had greater concerns regarding access to food and groceries because they might have been more likely to perform those tasks for the entire household, whereas those with substantial VI might have relied on a caregiver to perform those tasks.
This survey demonstrated that concerns associated with instrumental activities of daily living and social interactions varied by level of VI. Although previous studies have revealed similar findings,28 , 29 , 40 the present study is the first to report this in the setting of the COVID-19 shutdown. Those with any level of VI reported concerns regarding social interaction (5.2%-13.8%) compared to 0% in participants without VI (P = .02) (Figure 3). VI has been associated with stigma and discrimination,32 potentially leading to limited social interactions; this might have been aggravated during the COVID-19 shutdown. Additionally, those with severe VI had concerns about maintaining employment (P = .032). Unemployment became a global problem during the pandemic,41 and remote work may be especially challenging for groups with VI, as collaboration by electronic and video conferencing is dependent on visual input and feedback.42
Worsening of vision during the shutdown was reported by similar proportions of all groups (13.8%-27.6%; P = .356). This self-reported deterioration might have been due to the lack of standard vision care and the delay of all nonemergent eye procedures such as intravitreal injections and cataract surgery. Additionally, increased near work in working from home might have led to greater eye strain and dry eye disease, which have been shown to lead to functional VI.43 Moreover, the overall low rate of self-reported worsening of vision may be due to the limited duration of the shutdown at the time of this survey (4 months). Glaucoma, cataract, and some retinal diseases can progress slowly, and we expect that worsening of vision may become more prevalent as the shutdown is prolonged.
This study had several limitations. Although the survey had a high response rate (93.5%), the study might have selected patients who were well enough to participate by telephone. Second, although the study relied on multiple classifications (International Classification of Diseases-10 criteria13 and Foster and Quigley's consensus definition of glaucoma14) to define the patient groups, all existing criteria were not included in order to prevent overlapping classifications. Importantly, the American Academy of Ophthalmology supports the conclusion that people experience functional limitations when BCDVA in the better-seeing eye is less than 20/40,44 and groups were not compared based on this criterion. Additionally, multiple aspects of VI (BCDVA, contrast sensitivity, stereoacuity, and so forth) have been found to contribute to limitations to mobility,9 and only BCVDA was assessed at the last visit, which might have changed over time. Moreover, VI categories grouped together wide ranges of visual acuity (eg, 6/18-6/60 for moderate VI), which might have missed differences between VI within categories. Additionally, similar to prior studies, greater VI was associated with older age,9 , 45 possibly affecting our study's outcomes.45 Furthermore, because responses to these survey questions were not obtained before the COVID-19 pandemic, it is difficult to say what differences among groups existed at baseline versus changed after the onset of the pandemic. Furthermore, this study was performed at a time when strict travel restrictions were in place, possibly limiting the rate of COVID-19 infection. The dynamic state of the pandemic might have led to changes in perceptions about the disease over time; these were not captured by the present study.
Finally, our study was performed in a sample consisting of South Indian participants at a tertiary care center, potentially limiting its applicability elsewhere. However, inclusion of standardized multiple-choice responses allowed for consistency in the survey, which could be administered elsewhere. Of note, health care offerings in most parts of India are available to all regardless of financial resources; patients can pay to be seen at private hospitals or can receive free care at government-run institutions. In this study, all patients were cared for at the AECS, which offers services on a sliding scale payment basis, including free care for those who are unable to pay.
Assessing patient perceptions of COVID-19 and its regulations is especially important among those with moderate or worse VI, as limitations and concerns significantly differed in these groups. This survey demonstrated that those with blindness had greater concerns about their visual disability, putting them at greater risk of virus contraction, had more difficulty in adhering to standard preventive measures, and were at greater risk of receiving incomplete news, as they relied on word of mouth. Visual limitations might have been augmented by concomitant hearing impairment and older age. The pandemic response must include individuals with VI and other disabilities, as evidence-based assessments inclusive of all populations are necessary to demonstrate a potential disproportionate impact of COVID-19.
Acknowledgments
ALL AUTHORS HAVE COMPLETED AND SUBMITTED THE ICMJE FORM FOR DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST and none were reported.
Funding/Support: Publication of this article was supported by the US National Institutes of health (to Pradeep Y. Ramulu), the American Glaucoma Society (to Alan L. Robin), and an American Glaucoma Society Mentoring for Advancement of Physician Scientists grant (to Aakriti Garg Shukla). Financial Disclosures: Alan L. Robin is an employee of the American Glaucoma Society. Pradeep Y. Ramulu has financial arrangements with Thea Pharmaceuticals, Ivantis, WL Gore, and Perfuse Therapeutics. Alan L. Robin has financial arrangements with Versant Health and Perfuse Therapeutics. Aakriti Garg Shukla has financial arrangements with Allergan. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Supplemental Material available at AJO.com.
Appendix. Supplementary materials
References
- 1.StrataSphere . 2020. Strata Decision Technology. The national patient and procedure volume tracker: six-month analysis of patient and procedure volume at 275 hospitals shows an industry in slow recovery.https://www.stratadecision.com/article/55-percent-fewer-americans-sought-hospital-care-in-march-april-due-to-covid-19-drivinga-clinical-and-financial-crisis-in-u-s-healthcare/ Available at: Accessed May 11, 2020. [Google Scholar]
- 2.TIME Magazine . 2020. Indian prime minister Narendra Modi announces total lockdown of 1.3 billion people for 21 days.https://time.com/5808348/india-coronavirus-lockdown Available at: Accessed September 10, 2020. [Google Scholar]
- 3.Boulle A, Davies MA, Hussey H, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1198. ciaa1198. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Almeida-Pititto B, Dualib PM, Zajdenverg L, et al. Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: a meta-analysis. Diabetol Metab Syndr. 2020;12:75. doi: 10.1186/s13098-020-00586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Lusignan S, Joy M, Oke J, et al. Disparities in the excess risk of mortality in the first wave of COVID-19: cross sectional study of the English sentinel network. J Infect. 2020;81(5):785–792. doi: 10.1016/j.jinf.2020.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masroor S. Collateral damage of COVID-19 pandemic: delayed medical care. J Card Surg. 2020;35(6):1345–1347. doi: 10.1111/jocs.14638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pikoulis E, Solomos Z, Riza E, et al. Gathering evidence on the decreased emergency room visits during the coronavirus disease 19 pandemic. Public Health. 2020;185:42–43. doi: 10.1016/j.puhe.2020.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swenor BK, Lee MJ, Tian J, Varadaraj V, Bandeen-Roche K. Visual impairment and frailty: examining an understudied relationship. J Gerontol A Biol Sci Med Sci. 2020;75(3):596–602. doi: 10.1093/gerona/glz182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swenor BK, Simonsick EM, Ferrucci L, et al. Visual impairment and incident mobility limitations: the health, aging and body composition study. J Am Geriatr Soc. 2015;63(1):46–54. doi: 10.1111/jgs.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng DD, Bokman CL, Lam BL, et al. Longitudinal relationships between visual acuity and severe depressive symptoms in older adults: the Salisbury Eye Evaluation study. Aging Ment Health. 2016;20(3):295–302. doi: 10.1080/13607863.2015.1008985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flatten Inaccessibility . 2020. Flatten the Inaccessibility Curve: COVID-19 and the Impacts on People who are Blind or have Low Vision.https://flatteninaccessibility.com Available at: . Accessed June 21, 2020. [Google Scholar]
- 12.Rosenblum LP. Unprecedented times call for unprecedented collaboration: how two COVID-19 surveys were created with input from across the field of visual impairment to analyze the needs of adults, students, teachers, and orientation and mobility practitioners. J Vis Impair Blind. 2020;114(3):237–239. [Google Scholar]
- 13.World Health Organization . 2020. International statistical classification of diseases and related health problems 10th revision (ICD-10)-WHO version. Diseases of the eye and adnexa. Available at: https://icd.who.int/browse10/2016/en#/H53-H54. Accessed June 16, 2020. [Google Scholar]
- 14.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86(2):238–242. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen RWS, Abazari A, Dhar S, et al. Living with COVID-19: a perspective from New York area ophthalmology residency program directors at the epicenter of the pandemic. Ophthalmology. 2020;127(8):e47–e48. doi: 10.1016/j.ophtha.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Husain R, Zhang X, Aung T. Challenges and lessons for managing glaucoma during COVID-19 pandemic: perspectives from Asia. Ophthalmology. 2020;127(9):e63–e64. doi: 10.1016/j.ophtha.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanjay S, Garg A, Shetty N, Shetty R, Pindipapanahalli N, Shetty KB. Changes in the interaction of ophthalmologists and ophthalmic industry during and after COVID-19 lockdown: a perspective. Indian J Ophthalmol. 2020;68(7):1487–1488. doi: 10.4103/ijo.IJO_1239_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duong AT, Van Tassel SH, Alzaga Fernandez AG, et al. Medical education and path to residency in ophthalmology in the COVID-19 era: a perspective from medical student educators. Ophthalmology. 2020;127(11):e95–e98. doi: 10.1016/j.ophtha.2020.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan MA, Sivalingam A, Haller JA. Perceptions of occupational risk and changes in clinical practice of United States vitreoretinal surgery fellows during the COVID-19 pandemic. Ophthalmol Retina. 2020;4(12):1181–1187. doi: 10.1016/j.oret.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kothari M, Rathod V, Sugathan S, Kothari MM. A pilot study on the perspectives of pediatric ophthalmologists and their patients towards online consultation during COVID-19 lockdown in India. Indian J Ophthalmol. 2020;68(7):1494–1495. doi: 10.4103/ijo.IJO_1306_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuper H, Mathenge W, Macleod D, et al. Mortality during 6 years of follow-up in relation to visual impairment and eye disease: results from a population-based cohort study of people aged 50 years and above in Nakuru, Kenya. BMJ Open. 2019;9(6) doi: 10.1136/bmjopen-2019-029700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garin N, Olaya B, Lara E, et al. Visual impairment and multimorbidity in a representative sample of the Spanish population. BMC Public Health. 2014;14:815. doi: 10.1186/1471-2458-14-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Court H, McLean G, Guthrie B, Mercer SW, Smith DJ. Visual impairment is associated with physical and mental comorbidities in older adults: a cross-sectional study. BMC Med. 2014;12:181. doi: 10.1186/s12916-014-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Assi L, Rosman L, Chamseddine F, et al. Eye health and quality of life: an umbrella review protocol. BMJ Open. 2020;10(8) doi: 10.1136/bmjopen-2020-037648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broman AT, Munoz B, Rodriguez J, et al. The impact of visual impairment and eye disease on vision-related quality of life in a Mexican-American population: proyecto VER. Invest Ophthalmol Vis Sci. 2002;43(11):3393–3398. [PubMed] [Google Scholar]
- 27.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721–727. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 29.Lam BL, Christ SL, Zheng DD, et al. Longitudinal relationships among visual acuity and tasks of everyday life: the Salisbury Eye Evaluation study. Invest Ophthalmol Vis Sci. 2013;54(1):193–200. doi: 10.1167/iovs.12-10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swenor BK, Muñoz B, West SK. A longitudinal study of the association between visual impairment and mobility performance in older adults: the Salisbury Eye Evaluation study. Am J Epidemiol. 2014;179(3):313–322. doi: 10.1093/aje/kwt257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swenor BK, Simonsick EM, Ferrucci L, et al. Visual impairment and incident mobility limitations: the health, aging and body composition study. J Am Geriatr Soc. 2015;63(1):46–54. doi: 10.1111/jgs.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Senjam SS. Impact of COVID-19 pandemic on people living with visual disability. Indian J Ophthalmol. 2020;68(7):1367–1370. doi: 10.4103/ijo.IJO_1513_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.HUB Journal . Johns Hopkins University.; 2020. COVID-19 poses unique challenges for people with disabilities.https://hub.jhu.edu/2020/04/23/how-covid-19-affects-people-with-disabilities Available at: Accessed June 19, 2020. [Google Scholar]
- 34.Swenor BK, Ramulu PY, Willis JR, Friedman D, Lin FR. The prevalence of concurrent hearing and vision impairment in the United States. JAMA Intern Med. 2013;173(4):312–313. doi: 10.1001/jamainternmed.2013.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneck ME, Lott LA, Haegerstrom-Portnoy G, Brabyn JA. Association between hearing and vision impairments in older adults. Ophthalmic Physiol Opt. 2012;32(1):45–52. doi: 10.1111/j.1475-1313.2011.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mudie LI, Varadaraj V, Gajwani P, et al. Dual sensory impairment: The association between glaucomatous vision loss and hearing impairment and function. PLoS One. 2018;13(7) doi: 10.1371/journal.pone.0199889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cupples ME, Hart PM, Johnston A, Jackson AJ. Improving health care access for people with visual impairment and blindness. BMJ. 2012;344:e542. doi: 10.1136/bmj.e542. [DOI] [PubMed] [Google Scholar]
- 38.Cheng CS. Consultant-delivered care in telehealth and phone consultations during the COVID-19 shutdown period. ANZ J Surg. 2020;90(9):1825. doi: 10.1111/ans.16172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atherly A, Van Den Broek-Altenburg E, Hart V, Gleason K, Carney J. Consumer Reported Care Deferrals Due to the COVID-19 Pandemic, and the Role and Potential of Telemedicine: Cross-Sectional Analysis. JMIR Public Health Surveill. 2020;6(3):e21607. doi: 10.2196/21607. PMID: 32833661; PMCID: PMC7498465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desrosiers J, Wanet-Defalque MC, Témisjian K, et al. Participation in daily activities and social roles of older adults with visual impairment. Disabil Rehabil. 2009;31(15):1227–1234. doi: 10.1080/09638280802532456. [DOI] [PubMed] [Google Scholar]
- 41.Crain's Detroit Business. April 30, 2020 07:21 AM. Coronavirus impact could extend to 57 million U.S. jobs, McKinsey says. April 30, 2020. Available at: https://www.crainsdetroit.com/economy/coronavirus-impact-could-extend-57-million-us-jobs-mckinsey-says. Accessed April 6, 2021.
- 42.Ginley B. Working remotely if you are visually impaired. British Journal of Visual Impairment. 2020 doi: 10.1177/0264619620925702. [DOI] [Google Scholar]
- 43.Mathews PM, Ramulu PY, Swenor BS, et al. Functional impairment of reading in patients with dry eye. Br J Ophthalmol. 2017;101(4):481–486. doi: 10.1136/bjophthalmol-2015-308237. [DOI] [PubMed] [Google Scholar]
- 44.Fontenot JL, Bona MD, Kaleem MA, et al. Vision rehabilitation preferred practice pattern. Ophthalmology. 2018;125(1):P228–P278. doi: 10.1016/j.ophtha.2017.09.030. [DOI] [PubMed] [Google Scholar]
- 45.Swenor BK, Lee MJ, Varadaraj V, Whitson HE, Ramulu PY. Aging with vision loss: a framework for assessing the impact of visual impairment on older adults. Gerontologist. 2020;60(6):989–995. doi: 10.1093/geront/gnz117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.