Abstract
Fluoroquinolines, the widely used antibacterial antibiotics, have been shown to interact with human DNA topoisomerases supporting their use as repurposed cancer drugs in humans. In this communication molecular docking of eleven Fluoroquinolines against predicted structure of Candida albicans DNA Topoisomerase II is reported for the first time. C. albicans topoisomerase II structure prediction was done by using homology modeling tool. Ligand preparation and molecular docking with C. albicans topoisomerase II were done by using Autodock tool. These antibiotics formed hydrogen bond with good binding affinity at ARG 841, GLN803, ALA840 amino acid residues in the active site of C. albicans Topoisomerase II. We hypothesize that DNA toposiomerases may be the targets of Fluroquinoline group of antibiotics in C. albicans causing inhibition of growth.
Keywords: Fluoroquinolines, Repurposing, Molecular docking, DNA Topoisomerase II, Candida albicans
Introduction
DNA topological states in humans are manipulated by DNA Topoisomerase I and Topoisomerase II enzymes. Replication, transcription, translation and DNA repair basically depend upon Topoisomerase activity (Bertozzi et al. 2011, 2014). Topoisomerase II is the main target for anticancer drugs. In bacteria DNA gyrase Topo IV is the main target for Fluoroquinolines (Skok et al. 2020; Nitiss, 2009). Fluoroquinolines are also responsible for induction of apoptosis (Pommier, 2013). Molecular docking of Fluoroquinolines with Topoisomerase II in Mycobacterium tuberculosis is reported by Anand et al. (2011). Fluroqunionline group of antibiotics can interact with human DNA topoisomerases supporting the repurposing of these molecules as anticancer agents (Jadhav et al. 2017). Topoisomerase II inhibitors like, Aclarubicin, Doxorubicin and Mitoxantrone show antifungal activity against C. glabrata and Cryptococcus neoformans. Current study suggests that selected DNA Topoisomerase II inhibitors may be a promising class of compounds for the development of novel antifungal agents (Steverding et al. 2012).
Fluoroquinolines are broad spectrum fourth generation antibacterial antibiotics (Aldred et al. 2014). Gatifloxacin and Moxifloxacin are reported to have anti Candida activity alone and in combination with antifungal drugs like Amphotericin B, Voriconazole, Fluconazole and Caspofungin (Stergiopoulou et al. 2009; Deren et al. 2010; Yalcin et al. 2010). In vivo studies have shown that Levofloxacin, Moxifloxacin and Prulifloxacin can reduce fungal infection by decreasing gut colonization in a murine model (Maraki et al. 2011). In rabbit model, synergistic effect of Moxifloxacin and Amphotericin B against C. albicans endophthalmitis infection have been reported (Deren et al. 2010). Moxifloxacin and Gatifloxacin are known to have anti-candida activity in vitro, inhibit yeast to hyphal form morphogenesis and biofilm formation (Ozdek et al. 2006; Jadhav and Karuppayil, 2017). Moxifloxacin is reported to inhibit the cell cycle and biofilm formation in C. albicans (Jadhav et al. 2017). We hypothesize that DNA Topoisomerase II may be one of the targets of fluroqunolines in C. albicans. In the current study the potential of binding of fluroquniolines to DNA Topoisomerase II in C. albicans is explored.
Homology model of C. albicans DNA Topoisomerase II
The nucleotide sequence of C. albicans Topoisomerase II have 74% identity with Human DNA Topoisomerase II alpha gene and 70.53% identity with Human DNA Topoisomerase II beta gene. Amino acid sequence of C. albicans Topoisomerase II is 48.43% similar to DNA Topoisomerase II alpha protein. Primary sequence of C. albicans Topoisomerase II was retrieved in FASTA format from Uniprot public domain protein database (Uniprot accession no. P87078). Retrieved sequence was submitted to Phyre 2 homology modeling program for modeling of three dimensional structure of protein (Kelley et al. 2015). Tertiary structure was predicted and validation of tertiary structure was done by Procheck (Laskowski et al. 2001). Tertiary structure of Topoisomerase II was used for docking studies (Kumar and Bora 2014).
In the present study a combined Ligand and structure based virtual screening for fluoroquinolines which binds with the predicted model of C. albicans Topoisomerase II was done. Fluoroquinolines were found to bind with predicted structures of C. albicans Topoisomerase II at active site. This indicates that fluoroquinolines are active against C. albicans Topoisomerase II activity and may cause inhibition of growth and morphogenesis (Jadhav et al. 2017). Fluoroquinolines tested in this study showed binding affinity at active site and formed Hydrogen bond with ARG 841, GLN803, ALA840 amino acid residues (Table 1).
Table 1.
Molecular docking results of Fluoroquinolines with Candida albicans Topoisomerase II
| Sr. no. | Ligand | Run no | Interacting residue | Interacting atoms (amino acid…ligand) | H bond formed | Binding energy (kcal/mol) | Electrostatic energy |
|---|---|---|---|---|---|---|---|
| 1 | Ciprofloxacin | 30 | ARG841 | HE…O1 | 1 | − 6.51 | − 0.31 |
| 2 | Enoxacin | 27 | ARG841 | HE…O1 | 1 | − 6.93 | − 0.31 |
| 3 | Finafloxacin | 14 | ALA840 | HN…O1 | 2 | − 7.03 | − 0.6 |
| ARG841 | HN…O2 | ||||||
| 4 | Flumexacin | 51 | ARG841 | HN…O2 | 2 | − 6.19 | − 0.6 |
| ARG841 | HE…O2 | ||||||
| 5 | Gatifloxacin | 22 | GLN803 | OE1…N3 | 1 | − 6.89 | − 0.57 |
| 6 | Levofloxacin | 55 | ALA840 | HN…O2 | 1 | − 7.7 | − 0.61 |
| 7 | Lomefloxacin | 57 | ARG841 | HN…O1 | 1 | − 6.95 | − 0.65 |
| 8 | Norfloxacin | 56 | ARG841 | HE…O1 | 1 | − 7.34 | − 0.67 |
| 9 | Ofloxacin | 51 | ALA840 | HN…O2 | 1 | − 7.7 | − 0.6 |
| 10 | Sarafloxacin | 54 | ARG841 | HE…O1,O3 | 1 | − 6.7 | − 0.68 |
| 11 | Sparafloxacin | 42 | ARG841 | HN…O2 | 1 | − 8.26 | − 0.76 |
Potential inhibitors of C. albicans DNA Topoisomerase II
The combination of structure- and ligand-based computational methods has been successfully applied in many drug design projects (Drwal et al. 2013). Therefore, the exploration of differences in structure and function of Topoisomerase II is important, and the development of selective Topoisomerase II inhibitors may be a beneficial strategy in the search for new antifungal drugs with improved clinical safety. Etoposide, teniposide, doxorubicin, daunorubicin, epirubicin, idarubicin and mitoxantrone are well known topoisomerase II inhibitors (Hande 2008). Some of these antibiotics are reported to possess anticancer and antifungal properties. These studies are supported by in silico studies where these molecules have been shown to interact with human topoisomerase (Jadhav and Karuppayil 2017; Jadhav et al. 2017). DNA topoisomerase inhibitors possess antifungal activity against clinical isolates of C. albicans. Aclarubicin exhibits fungistatic activity while daunorubicin, doxorubicin, idarubicin and β lapachone, influences C. albicans morphology (Kwok et al. 2010).
Moxifloxacin and Levofloxacin are active against the bacterial pathogens, Staphylococcus aureus and Sterptococcus pneumoniae which are responsible for upper and lower respiratory tract infections, meningitis and acute otitis (Keating and Scott. 2004). Moxifloxacin is also active against Mycobacterium tuberculosis and C. albicans (Mdluli and Ma 2007; Jadhav et al. 2017). Moxifloxacin protected cyclophosphamide-injected mice from C. albicans induced lung infection and significantly reduced pneumonia, weight loss, and mortality despite the lack of direct antifungal activity. This is most likely due to an immunomodulating activity conferred by Moxifloxacin (Shalit et al. 2002). Along with antifungal activity Moxifloxacin exhibit anti-tuberculosis activity. Docking studies and in vitro studies has shown the potential activity of methoxy Fluoroquinolones against MDR-TB (Anand et al. 2011). DNA topoisomerase inhibitors effect the growth and morphology of C. albicans suggesting a possible role as antifungal agents in the treatment of C. albicans infections (Kwok et al. 2010).
Molecular docking of repurposed drugs
Molecular docking of fluoroquinolines against C. albicans DNA Topoisomerase II were done by using AutoDock® 1.5.6rc2 (Kumar and Bora 2014). The structures Fluroqunionlines were retrieved from Pubchem tool, chemical structure followed by 2D structure cleaning, 3D optimization and viewing by using marvine view. The Autodock Tools package version 1.5.6rc2 was used to generate the docking input files (Molecular Graphics laboratory, The Scripps Research Institute, San Diego, California). For Docking, a grid spacing of 0.375 Å and 60 × 60 × 60 number of points was used. Before docking addition of non-polar hydrogen was done by using Autodock 1.5.6rc2. Three dimensional structure of C. albicans Topoisomerase II and each ligand structure were converted to PDBQT format (Kumar and Bora 2014; Morris et al. 1998).
The default optimization parameters were used for docking with the Lamarckian Genetic Algorithm was used with a population size of 150 dockings. Autodock® tools generated 60 possible binding conformations of ligand with protein i.e. 60 runs for each docking by using Genetic Algorithm (GALS) searches. The grid box used for specifying the search space was set at 60 × 60 × 60 centered on of Protein with a default grid point spacing of 0.375 Å. Autogrid was used to obtain pre calculated grid maps. − 44.36133, 21.27667 and 14.656 these are x, y and z co-ordinates were used during Grid preparation. Docking of FQs with predicted structure on C. albicans Topoisomerase II was done. After completion of docking most suitable conformation was chosen based on lowest docked energy. Selected conformations were analyzed by Autodock® tool and Discovery studio® (Kumar and Bora, 2014; Morris et al. 1998) (Fig. 1).
Fig. 1.

Predicted structure of C. albicans Topoisomerase II
After docking of fluoroquinolines with C. albicans Topo II, 60 best runs having lowest binding energy was chosen for building complex of Ligand and Topoisomerase II protein. Out of the eleven antibiotics, Sparafloxacin has shown higher binding affinity with Topoisomerase II (binding energy is -8.26 kcal/mol) and forms hydrogen bond at ARG 841 amino acid residue. Ciprofloxacin and Enoxacin formed hydrogen bond with ARG 841 with binding energies − 6.51 and 6.93 kcal/mol. Finafloxacin was found to form two H bond with ALA 840 and ARG 841 binding energies − 7.03 kcal/mol. Flumequine was observed to bind with ARG 841 by two H bonds with binding energy − 6.19 kcal/mol (Fig. 2).
Fig. 2.
PROCHECK analysis of predicted structure of C. albicans Topoisomerase II
Gatifloxacin has been found to form one hydrogen bond at GLN803 amino acid residue with binding energy − 6.89 kcal/mol. Levofloxacin and Ofloxacin formed H bond with ALA 840 having same binding energy − 7.7 kcal/mol. Lomefloxacin and Norfloxacin has shown binding with amino acid residue ARG 481 with binding energy − 6.95 and − 7.3 kcal/mol. Sarafloaxcin formed H-bond with ARG 841 having binding energy − 6.7 kcal/mol. Since all fluoroquinolines showed binding affinity with C. albicans Topoisomerase II, FQs can be considered for in vitro and in vivo studies for anti-Candida activity in future (Figs. 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13).
Fig. 3.

Docking of ciprofloxacin with Candida albicans Topoisomerase II
Fig. 4.

Docking of enoxacin with Candida albicans Topoisomerase II
Fig. 5.

Docking of finafloxacin with Candida albicans Topoisomerase II
Fig. 6.

Docking of flumefloxacin with Candida albicans Topoisomerase II
Fig. 7.

Docking of gatifloxacin with Candida albicans Topoisomerase II
Fig. 8.
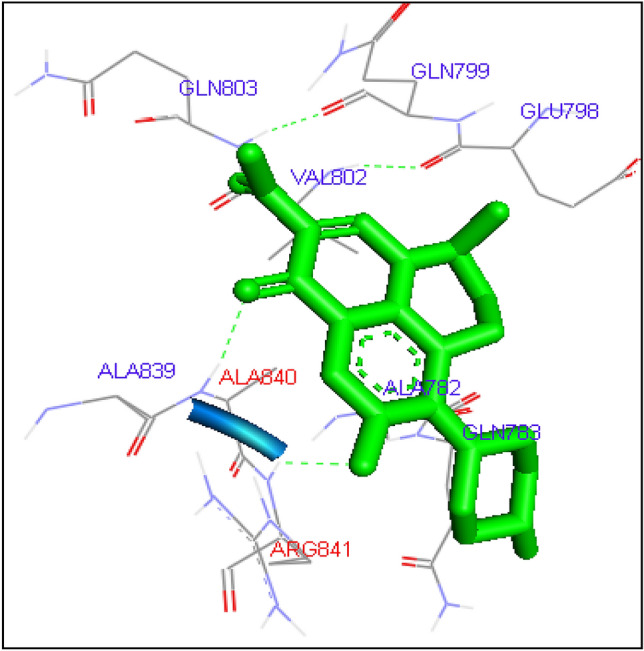
Docking of levofloxacin with Candida albicans Topoisomerase II
Fig. 9.

Docking of lomefloxacin with Candida albicans Topoisomerase II
Fig. 10.

Docking of norfloxacin with Candida albicans Topoisomerase II
Fig. 11.

Docking of ofloxacin with Candida albicans Topoisomerase II
Fig. 12.
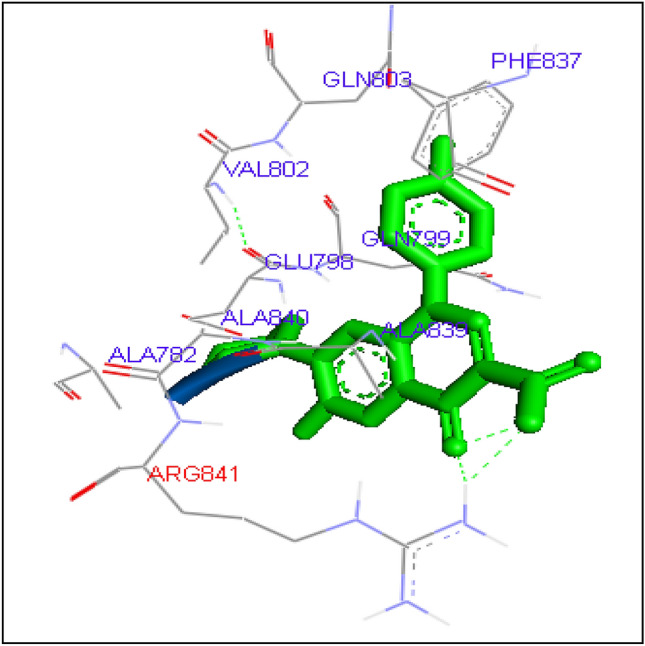
Docking of sarafloxacin with Candida albicans Topoisomerase II
Fig. 13.

Docking of sparafloxacin with Candida albicans Topoisomerase II
In this communication molecular docking of fluoroquinolines were performed against the predicted structure of C. albicans Topoisomerase II. Molecular docking shows that fluoroquinolines bind within the active site of C. albicans Topoisomerase II and interact with amino acids ARG 841, GLN803, ALA 840, suggesting potential inhibition of C. albicans. Fluoroquinolines thus may be useful candidates in the treatment of Candida infections and could be used in conjunction with standard antifungal drugs to reduce side effects and multi-drug resistance. Further, in vitro and in vivo studies are essential to confirm the Topoisomerase inhibitory activity of fluoroquinolines and their use in combination with other drugs.
Acknowledgements
AKJ and SMK is thankful to DY Patil Education Society (Deemed to be University), Kolhapur, 416006, Maharashtra, India for providing funding support for Research.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aldred J, Kerns J, Osherof N. Mechanism of quinolone action and resistance. Biochemistry. 2014;53:1565–1574. doi: 10.1021/bi5000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand S, Somasundaram S, Doble M, Paramasivan C. Docking studies on novel analogues of 8 methoxyfluoroquinolones against GyrA mutants of Mycobacterium tuberculosis. BMC StructBiol. 2011;11(1):1–13. doi: 10.1186/1471-2210-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertozzi D, Iurlaro R, Sordet O, Marinello J, Zaffaroni N, Capranico G. Characterization of novel antisense HIF-1α transcripts in human cancers. Cell Cycle. 2011;10(18):3189–3197. doi: 10.4161/cc.10.18.17183. [DOI] [PubMed] [Google Scholar]
- Bertozzi D, Marinello J, Manzo S, Fornari F, Gramantieri L, Capranico G. The natural inhibitor of DNA topoisomerase I, camptothecin, modulates HIF-1α activity by changing miR expression patterns in human cancer cells. Mol Cancer Ther. 2014;13(1):239–248. doi: 10.1158/1535-7163.MCT-13-0729. [DOI] [PubMed] [Google Scholar]
- Deren Y, Özdek S, Kalkanci A, Akyürek N, Hasanreisoglu B. Comparison of antifungal efficacies of moxifloxacin, liposomal amphotericin B, and combination treatment in experimental Candida albicansendophthalmitis in rabbits. Can J Microbiol. 2010;56(1):1–7. doi: 10.1139/W09-112. [DOI] [PubMed] [Google Scholar]
- Drwal MN, Agama K, Pommier Y, Griffith R. Development of purely structure-based pharmacophores for the Topoisomerase I-DNA-ligand binding pocket. J Comput Aided Mol Des. 2013;27(12):1037–1049. doi: 10.1007/s10822-013-9695-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hande K. Topoisomerase II inhibitors. Update Cancer Ther. 2008;3(1):13–26. doi: 10.1016/j.uct.2008.02.001. [DOI] [Google Scholar]
- Jadhav A, Karuppayil M. Molecular docking studies on thirteen fluoroquinolines with human topoisomerase II a and b. In Silico Pharmacol. 2017;5(1):1–12. doi: 10.1007/s40203-017-0024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav A, Bansode B, Phule D, Amruta S, Rajendra P, Gade W, Kharat K, Karuppayil M. The antibacterial agent, moxifloxacin inhibits virulence factors of Candida albicans through multitargeting. World J MicrobiolBiotechnol. 2017;33(5):1–9. doi: 10.1007/s11274-017-2264-z. [DOI] [PubMed] [Google Scholar]
- Keating G, Scott L. Moxifloxacin. Drugs. 2004;64(20):2347–2377. doi: 10.2165/00003495-200464200-00006. [DOI] [PubMed] [Google Scholar]
- Kelley L, Mezulis S, Yates C, Wass M, Sternberg M. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10(6):845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Bora U. Molecular docking studies of curcumin natural derivatives with DNA topoisomerase I and II-DNA complexes. InterdiscipSciComput Life Sci. 2014;6(4):285–291. doi: 10.1007/s12539-012-0048-6. [DOI] [PubMed] [Google Scholar]
- Kwok S, Schelenz S, Wang X, Steverding D. In vitro effect of DNA topoisomerase inhibitors on Candida albicans. Med Mycol. 2010;48(1):155–160. doi: 10.3109/13693780903114934. [DOI] [PubMed] [Google Scholar]
- Laskowski R, MacArthur M, Thornton JM (2001) PROCHECK: validation of protein structure coordinates. In: International tables of crystallography, vol F. Crystallography of biological macromolecules. Kluwer Academic Publishers, The Netherlands, pp 722–725
- Maraki S, Lionakis S, Ntaoukakis M, Barbounakis E, Ntasis E, Kofteridis D, Samonis G. Effects of levofloxacin, moxifloxacin and prulifloxacin on murine gut colonization by Candida albicans. Med Mycol. 2011;49(4):419–423. doi: 10.3109/13693786.2010.538443. [DOI] [PubMed] [Google Scholar]
- Mdluli K, Ma Z. Mycobacterium tuberculosis DNA gyrase as a target for drug discovery. Infect Disord Drug Targets. 2007;7(2):159–168. doi: 10.2174/187152607781001763. [DOI] [PubMed] [Google Scholar]
- Morris G, Goodsell D, Halliday R, Huey R, Hart W, Belew R, Olson A. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J ComputChem. 1998;19(14):1639–1662. [Google Scholar]
- Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer. 2009;9:338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdek S, Miller D, Flynn P, Flynn H. In vitro antifungal activity of the fourth generation fluoroquinolones against Candida isolates from human ocular infections. Ocular ImmunolInflamm. 2006;14(6):347–351. doi: 10.1080/09273940600976953. [DOI] [PubMed] [Google Scholar]
- Pommier Y. Drugging topoisomerases: lessons and challenges. ACS ChemBiol. 2013;8:82–95. doi: 10.1021/cb300648v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalit I, Horev-Azaria L, Fabian I, Blau H, Kariv N, Shechtman I, Kletter Y. Immunomodulatory and protective effects of moxifloxacin against Candida albicans-induced bronchopneumonia in mice injected with cyclophosphamide. Antimicrob Agent Chemother. 2002;46(8):2442–2449. doi: 10.1128/AAC.46.8.2442-2449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skok Z, Zidar N, Kikelj D, Ilaš J, Dual inhibitors of human DNA topoisomerase II and other cancer-related targets. J Med Chem. 2020;63:884–890. doi: 10.1021/acs.jmedchem.9b00726. [DOI] [PubMed] [Google Scholar]
- Stergiopoulou T, Meletiadis J, Sein T, Papaioannidou P, Tsiouris I, Roilides E, Walsh T. Comparative pharmacodynamic interaction analysis between ciprofloxacin, moxifloxacin and levofloxacin and antifungal agents against Candida albicans and Aspergillus fumigatus. J AntimicrobChemother. 2009;63(2):343–348. doi: 10.1093/jac/dkn473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steverding D, Evans P, Msika L, Riley B, Wallington J, Schelenz S. In vitro antifungal activity of DNA topoisomerase inhibitors. Med Mycol. 2012;50(3):333–336. doi: 10.3109/13693786.2011.609186. [DOI] [PubMed] [Google Scholar]
- Wang S, Miller W, Milton J, Vicker N, Stewart A, Charlton P, Denny WA. Structure–activity relationships for analogues of the phenazine-based dual topoisomerase I/II inhibitor XR11576. Bioorg Med Chem Lett. 2002;12(3):415–418. doi: 10.1016/S0960-894X(01)00770-3. [DOI] [PubMed] [Google Scholar]
- Yalcin B, Kalkanci A, Gürelik F, Fidan I, Kustimur S, Ozdek S. In vitro synergistic effect of moxifloxacin and amphotericin B combination against Candida strains. MikrobBult. 2010;44(1):65–70. [PubMed] [Google Scholar]



