Abstract
The growth and development of watermelon and melon are affected by abiotic stresses such as cold, salinity and drought. Plant superoxide dismutase (SOD) proteins exerted great effects on plant growth, development and response to abiotic stresses. However, little is known about the characteristics of watermelon and melon SOD gene families and their expression patterns under abiotic stresses. In this study, the genome-wide identification of SOD genes and their expression patterns under abiotic stresses has been done in watermelon and melon. Seven SODs were identified in watermelon and melon, respectively. Chromosome location indicated that the SODs were dispersedly distributed on 4–6 chromosomes. Almost all the SOD proteins contained 300 amino acids or less and the intron numbers of SODs ranged from 5 to 7. On the basis of phylogenetic analysis, the SODs were classified into six sub-groups which was also verified by similar motif composition, gene structure and sub-cellular location. Gene ontology analysis displayed that many SOD proteins participated in binding, catalytic, antioxidant activity and stimulus–response. Cis-regulatory elements related to stresses and hormones were found in the promoters of the SODs. Based on the quantitative real-time PCR, most of CmSOD and ClSOD genes showed obvious up-regulation under low-temperature, NaCl and PEG6000 treatments. The abiotic stress-responsive SOD genes were identified to improve watermelon and melon tolerance against abiotic stresses. This was a preliminary study to describe the genome-wide analysis of SOD gene family in watermelon and melon, and the results would facilitate further study of gene cloning and functional verification of SOD genes response to abiotic stresses in watermelon and melon.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-02726-7.
Keywords: Genome-wide, Superoxide dismutase, Expression pattern, Watermelon, Melon
Introduction
It is widely known that there are lots of reactive oxygen species (ROS) produced in plant tissues and cells under low-temperature, heat, high-salt and drought stresses. Excessive ROS such as superoxide ion (O2−), hydrogen peroxide (H2O2) and hydroxyl radicals (OH−) can lead to plant cell death and the reduction of its yield and quality. Recently, more and more researches probed into superoxide dismutases (SODs) due to their important antioxidant function, which can eliminate ROS produced in plant cells exposed to adverse conditions (Alscher et al. 2002). As a group of metalloenzyme, plant SODs are mainly categorized as Cu/ZnSODs, FeSODs, and MnSODs according to their metal cofactors (Abreu and Cabelli 2010). At present, SOD gene families have been reported in many plants, such as Arabidopsis thaliana (Kliebenstein et al. 1998), Populus (Molina-Rueda et al. 2013), Oryza sativa (Nath et al. 2014), Solanum lycopersicum (Feng et al. 2016), Brassica juncea and B. rapa (Verma et al. 2019) and so on. Moreover, studies revealed that plant SODs could form a first defense line against abiotic stresses including cold, drought, salinity and heat. For example, over-expression of FeSOD increased superoxide-scavenging capacity and thereby improved the winter survival of transgenic alfalfa plants (McKersie et al. 2000). Over-expression of the yeast SOD2 in A. thaliana improved seed germination and seedling salt tolerance (Gao et al. 2003). Over-expression of a cytosolic Cu/ZnSOD, cloned from Potentilla atrosanguinea, in potato resulted in enhancing net photosynthetic rates and stomatal conductance compared to that in wild-type plants under drought stress (Pal et al. 2012). Over-expression of Tamarix albiflonum MnSOD enhanced higher activity of antioxidant enzymes and increased more proline, soluble sugar and biomass of transgenic cotton plants than wild-type plants under water stress (Zhang et al. 2014). When compared to the WT during cold stress, a Cu/ZnSOD which cloned from P. atrosanguinea was over-expressed in A. thaliana, and then displayed higher levels of total antioxidant enzyme activities, chlorophyll content, total soluble sugars, proline content and lower levels of ROS, ion leakage (Shafi et al. 2014). Under drought stress, white clover FeSOD, Cu/ZnSOD and MnSOD genes exhibited more abundant transcripts, while cold and cadmium stress mainly up-regulated Cu/ZnSOD genes (Zhang et al. 2015). Most of banana SOD genes were increased at the transcriptional levels under heat stress but down-regulated in response to drought stress (Feng et al. 2015). Over-expression of a Cu/ZnSOD gene from Arachis hypogaea enhanced tolerance to salinity and dehydration stress in transgenic tobacco plants (Negi et al. 2015). The grain quality of rice with high expression of MSD1 grown at high-temperature condition was significantly better than that of wild type, and MSD1-knock-down rice was susceptible to heat stress (Shiraya et al. 2015). Most of tomato SOD genes were altered under salt or drought stress conditions (Feng et al. 2016), similar report was found in B. juncea and B. rapa SOD genes (Verma et al. 2019). Above researches indicated that although these SOD genes could display the different expression patterns or roles under various abiotic stresses or in different plant species, SOD genes played a critical and positive role in different plants response to various abiotic stresses.
Watermelon (Citrullus lanatus L. 2n = 2x = 22) and Melon (Cucumis melo L. 2n = 2x = 24) belong to the important Cucurbitaceae family, which grow throughout the world and play important roles in nutritional and economic affairs of human being. In recent years, there were few researches about the characteristics of watermelon and melon SOD gene families and their expression patterns under low-temperature, salt and osmotic stresses. To comprehensively understand the putative roles of SOD genes in watermelon and melon, thus, the author tried to analyze the genome-wide identification and expression profiles, obtaining the basic information of watermelon and melon SOD genes, which included genome distribution, evolutionary divergence, gene structure, conserved motif, promoter analysis and putative expression patterns under low-temperature, salt and osmotic stresses, to facilitate gene cloning and functional verification of SOD genes to improve watermelon and melon growth and development under low-temperature, salt and osmotic stresses.
Materials and methods
Sequence retrieval and identification
In this study, two methods were used to search potential SODs of watermelon and melon. First, the amino acid sequences of published A. thaliana SOD proteins (Kliebenstein et al. 1998) were retrieved from The A. thaliana Information Resource (TAIR) (https://www.arabidopsis.org/), and used as a query file to search the candidate SOD proteins of watermelon and melon in Cucurbit Genomics Database (CGD) (http://cucurbitgenomics.org/) by BlastP, the advanced options included that number descriptions and number alignments were set at 5 and e-value was set at 1e-2. Second, the proteomes of watermelon and melon were downloaded from CGD to establish local watermelon and melon protein databases using BLAST 2.2.28 for Stand Alone Blast; then, the Hidden Markov Model (HMM) profiles of Cu/ZnSOD (PF00080), Fe-MnSOD (PF00081, PF02777) were downloaded from the Pfam Database (Finn et al. 2014; El-Gebali et al. 2019), and used to search SODs from watermelon and melon local protein databases using HMMER3.0 (the cutoff of e-value was 1e-10) (Finn et al. 2011).
To confirm the presence of the Sod_Cu, Sod_Fe_N or Sod_Fe_C domains in watermelon and melon SOD protein sequences, the web tools of the Interpro program (http://www.ebi.ac.uk/interpro/) and the SMART program (http://smart.embl.de/) were applied to test these SOD proteins. If protein sequence contains a Sod_Cu domain (PF00080 or IPR001424), it was considered to be Cu/ZnSOD. If protein sequence contains Sod_Fe_N (PF00081 or IPR019831) and Sod_Fe_C (PF02777 or IPR019832) domains, it was considered to be Fe-MnSOD. The coding sequences (CDS) and protein sequences of SOD members in watermelon and melon were listed in Table S1. The protein length, molecular weight (Mw), and theoretical isoelectric point (pI) were computed by the ProtParam tool (https://web.expasy.org/protparam/); sub-cellular locations were predicted by the Euk-mPLoc 2.0 tool (Chou and Shen 2010) and ProtComp version 9.0 server (http://www.softberry.com).
Chromosomal location, phylogenetic and syntenic analysis
The mappings of physical locations of the SOD genes on watermelon and melon chromosomes were used by Mapchart 2.2 (Plant Research International, Wageningen, The Netherlands). To identify the phylogenetic relationship of SODs among Arabidopsis, watermelon and melon, their protein sequences were aligned by MUSCLE with Neighbor-Joining cluster method, and then, phylogenetic tree was constructed using MEGA X (Kumar et al. 2018) through Maximum Likelihood method with 1000 bootstrap replicates and WAG + G model (Felsenstein 1985; Whelan and Goldman 2001). The analysis of sequence similarities was conducted using the EBI-EMBOSS pairwise sequence alignment server (Chojnacki et al. 2017). Syntenic relationships and duplication events among SOD genes were analyzed by MCScanX software (Wang et al. 2012) and visualized the results via Circos (Krzywinski et al. 2009). The homologous genes among Arabidopsis, watermelon and melon SOD gene families were analyzed using Orthofinder 2.3.11 (Emms and Kelly 2019).
Gene structure, conserved motifs and sequence alignment
Intron/exon structure analysis was carried out using the Gene Structure Display Server (Hu et al. 2015). The analysis of motif distribution and types was taken through the MEME 5.3.0 suite that followed these parameters: optimum motif width ≥ 20 and ≤ 150 and the maximum number of motifs set at 10 (Bailey et al. 2006). The motif distribution and logos were visualized by Evolview version 2 (He et al. 2016) and WebLogo 3.7.4 (Schneider and Stephens 1990; Crooks et al. 2004), respectively, and the motif types were classified by NCBI conserved domain database (CDD) (Marchler-Bauer et al. 2015). Protein sequence alignment was constructed by ClustalX 2.0 (Larkin et al. 2007), and then, the identity and similarity boxes and conserved lines were marked by Boxshade 3.21 (https://embnet.vital-it.ch/software/BOX_form.html).
Promoter sequence analysis and gene ontology annotation
To identify the cis-regulatory elements in AtSOD, ClSOD and CmSOD genes, 2000 bp upstream sequences of initiation codons of the genes were downloaded from TAIR and CGD, respectively, and listed in Table S1. Then, cis-regulatory elements were predicted by the PlantCARE server (Lescot et al. 2002). Functional annotation of SOD sequences and data mining on the resulting annotations were performed by Blast2GO (Conesa and Götz 2008) and verified using AmiGO database (http://amigo.geneontology.org/amigo).
Plant materials, growth condition and stress treatments
To facilitate gene cloning, tissue culture and transgenic function verification in later-stage research, watermelon inbreeding line “J-2” and melon inbreeding line “Huanghemi” were selected to use for gene expression analysis under abiotic stresses. The seeds were germinated in the glass Petri dish with two layers water-saturated filter papers in a controlled chamber (28 °C day/night, 24-h dark cycle). Before germination, the seeds were sterilized by a 10% hypochlorous acid solution for 10 min first, then washed three times with distilled water, and soaked in distilled water for 8 h. After germination for 3 days, the seeds were sowed into pots (one seedling in one pot) containing vermiculite and peat (3:1). The seedlings were grown in the light growth incubator at 28 ± 2 °C and with 16-h light/8-h dark photoperiod. 21-day-old seedlings were used for abiotic treatments in triplicates. For salinity and osmotic treatments, plant samples were irrigated by 250 mM NaCl and 20% (w/v) polyethylene glycol (PEG6000), respectively, until the two kinds of liquid flowed out from the bottom of the pots, and the control were irrigated by water. For low-temperature treatment, plant samples were placed in the growth cabinet at 8 °C with a photoperiod of 16-h light and 8-h dark, taking plants at 28 ± 2 °C with 16-h light/8-h dark as the control. Then, after treatments for 3 and 12 h, leaf samples were gathered, respectively, quick-frozen in liquid nitrogen, and stored at − 80 °C until RNA isolation.
RNA isolation and qRT-PCR
Total RNA was extracted from each sample using a Trizol reagent (Tiangen, Beijing, China). cDNA synthesis was performed using a PrimeScriptTM RT reagent kit with a gDNA Eraser (Takara, Dalian, China). Quantitative real-time PCR (qRT-PCR) was set up by a SYBR Green Master mix (Takara, Dalian, China) on Roche LightCycler 96 Real-Time PCR System (Roche, Basel, Switzerland). The qRT-PCR primers of CmSOD, ClSOD and actin genes were listed in Table S2. The qRT-PCR reactions were carried out in a 20-µL volume including 10 µL of 2 × SYBR Green Master mix, 0.6 µL of each primer (10 µM), 6.8 µL of double-distilled water and 2 µL of cDNA template. The PCR cycling conditions were as follows: 95 °C for 30 s, followed by 50 cycles of 95 °C for 5 s and 60 °C for 34 s. The specificity of the product was confirmed by dissociation curve analysis after each PCR run. The expression level of ‘Ck-3 h’ was used as a calibrator. The relative expression levels were calculated by the comparative 2−ΔΔCt method first (Livak and Schmittgen 2001), and then, analyzed by the two-way ANOVA of R software to test whether the results were statistically different or not. After that, the results were presented by the “pheatmap” and “ggplot2” functions of R software. The Ct values of CmSOD and ClSOD genes were listed in Tables S8 and S9, respectively.
Results
Identification and properties analysis
Under the premise of published genome (Garcia-Mas et al. 2012; Guo et al. 2013), the searching tasks for SOD genes in C. lanatus and C. melo were completed. The putative SOD members were searched by BlastP with the reported SOD protein sequences and then examined for the presence of SOD domains with the Interpro and the SMART tools. Finally, seven SOD members were obtained in watermelon and melon, respectively. For convenience, these SOD genes were renamed according to their A. thaliana orthologs based on the ortholog analysis of the Gramene database (Liang et al. 2008) and Orthofinder (Emms and Kelly 2019). If two or more SOD genes had the same A. thaliana homologous, one additional number was added after their first number to differentiate them. As detailed in Table 1, a total of 14 SOD proteins in watermelon and melon were classified into two major groups (Cu/ZnSODs and Fe-MnSODs). The Cu/ZnSOD groups were consisted of eight SOD proteins containing copper/zinc SOD domain (four ClCSDs and four CmCSDs), and the Fe-MnSODs groups had six SOD proteins with iron/manganese SOD, alpha-hairpin domain and iron/manganese SOD, C-terminal domain (three in watermelon and melon, respectively). In addition, the coding sequences (CDS) of these SODs ranged from 459 to 894 bp and the predicted protein lengths varied in size from 152 to 297 amino acids. Furthermore, the molecular weight of the SOD proteins ranged from 15.12 to 33.81 kDa and the theoretical isoelectric point (pI) from 5.28 to 9.00. Subcellular locations displayed that ClMSD1 and CmMSD1 were located on the mitochondrion, with the rest members on the chloroplast or cytoplasm.
Table 1.
Properties of SOD genes and proteins in watermelon and melon
| Name | Gene locus | Chromosome | CDS length(bp) | Protein | Subcellular location by Em | Subcellular location by PC | Predicted Pfam domain | ||
|---|---|---|---|---|---|---|---|---|---|
| Length (aa) | MW (KDa) | pI | |||||||
| ClCSD1-1 | Cla011299 | Chr3 | 459 | 152 | 15.12 | 5.59 | Ch | Cy | CZ |
| ClCSD1-2 | Cla019840 | Chr2 | 459 | 152 | 15.44 | 5.31 | Ch | Cy | CZ |
| ClCSD2 | Cla008698 | Chr2 | 654 | 217 | 22.10 | 5.82 | Ch | Cy | CZ |
| ClCSD3 | Cla012125 | Chr4 | 450 | 149 | 14.97 | 6.74 | Ch | Ch | CZ |
| ClMSD1 | Cla008101 | Chr3 | 708 | 235 | 26.13 | 7.95 | Mi | Mi | IMA, IMC |
| ClFSD2 | Cla010691 | Chr7 | 888 | 295 | 33.49 | 6.52 | Ch | Ch | IMA, IMC |
| ClFSD3 | Cla011317 | Chr3 | 804 | 267 | 30.92 | 7.75 | Ch | Ch | IMA, IMC |
| CmCSD1-1 | MELO3C015374 | Chr2 | 459 | 152 | 15.22 | 5.28 | Ch | Cy | CZ |
| CmCSD1-2 | MELO3C004342 | Chr5 | 459 | 152 | 15.45 | 5.31 | Ch | Cy | CZ |
| CmCSD2 | MELO3C026955 | Chr11 | 654 | 217 | 22.01 | 5.87 | Ch | Cy | CZ |
| CmCSD3 | MELO3C008809 | Chr8 | 474 | 157 | 15.91 | 6.53 | Ch/Cy | Ch | CZ |
| CmMSD1 | MELO3C020487 | Chr12 | 630 | 209 | 23.17 | 9.00 | Mi | Mi | IMA, IMC |
| CmFSD2 | MELO3C017624 | Chr7 | 894 | 297 | 33.81 | 6.28 | Ch | Ch | IMA, IMC |
| CmFSD3 | MELO3C015351 | Chr2 | 804 | 267 | 30.82 | 8.24 | Ch | Ch | IMA, IMC |
MW molecular weight; pI isoelectric points; Ch chloroplast; Cy cytoplasm; Mi mitochondrion; CZ copper/zinc superoxide dismutase; IMA iron/manganese superoxide dismutases, alpha-hairpin domain; IMC iron/manganese superoxide dismutases, C-terminal domain
Phylogenetic analysis
The SOD proteins of A. thaliana, C. lanatus and C. melo were utilized to construct phylogenetic tree with Maximum Likelihood method. Moreover, to provide further evidence for identification of A. thaliana, C. lanatus and C. melo SOD orthologs, sequence similarities analysis were calculated using the EBI-EMBOSS pairwise sequence alignment server (see Table S3). As listed in Fig. 1, 21 SOD proteins were divided into three groups (11 Cu/ZnSODs, three MnSODs and seven FeSODs) according to higher bootstrap, with 11 Cu/ZnSODs in three sub-groups (CSD1, CSD2 and CSD3), three MnSODs in one sub-group (MSD1) and seven FeSODs in two sub-groups (FSD2 and FSD3) according to their correlative A. thaliana orthologs. CSD1 sub-group included four SOD proteins (ClCSD1-2, CmCSD1-2, CmCSD1-1 and ClCSD1-1), with sequence similarities ranging from 83.6 to 90.8% compared to putative AtCSD1 ortholog. In CSD2 sub-group, ClCSD2 and CmCSD2 shared 76.1 and 77.5% sequence similarities with AtCSD2 ortholog, respectively. In CSD3 sub-group, ClCSD3 and CmCSD3 had more than 75% homology with AtCSD3. The MnSODs group is the smallest in three groups, including one ClMSD and one CmMSD, with 83.3 and 71.4% homology compared to AtMSD1, respectively. ClFSD2 and CmFSD2 in FSD2 sub-group shared about 69% similarities with AtFSD2, and ClFSD3 and CmFSD3 in FSD3 sub-group about 80% similarities with AtFSD3. Phylogenetic analysis also identified seven ortholog pairs (bootstrap ≥ 72) among C. lanatus and C. melo SOD homologs (see also Fig. 1). Most of the pairs showed more than 90% sequence similarity, including one pair (ClCSD1-2 and CmCSD1-2) with the highest sequence similarity (96.7%) (Table S3).
Fig. 1.
Maximum Likelihood phylogenetic tree and motif distribution of SOD protein sequences. The tree was obtained using WAG + G model with 1000 bootstrap replicates. Three groups included copper/zinc SODs (circles), manganese SODs (triangles) and iron SODs (stars). A. thaliana, C. lanatus and C. melo SOD proteins were marked by black, green and yellow symbols, respectively. The distribution of SOD conserved motifs was analyzed by the online tool MEME 5.3.0, then the result of tree and motif distribution were revealed via Evolview version 2
Conserved motif and intron/exon structure analysis
As shown in Fig. 1, through the MEME analysis, the motif distributions were as follows: motif 1 and 5 were only shared in Cu/ZnSODs; motif 3 and 4 were only shared in Fe-MnSODs; motif 2 and 7 were only presented in FeSODs, motif 6 was only in MnSODs, motif 8 was only in AtFSD3, ClFSD3 and CmFSD3, motif 9 was in two CSD2 (ClCSD2, CmCSD2), two FSD2 (ClFSD2, CmFSD2) and two FSD3 (ClFSD3, CmFSD3), and motif 10 was only in ClFSD2 and CmFSD2. In addition, CDD analysis showed that motif 1 was related to copper/zinc SOD domain, motif 2 and 6 were related to iron/manganese SOD dismutase, alpha-hairpin domain and iron/manganese SOD dismutase, C-terminal domain, motif 3 was related to iron/manganese SOD dismutase, alpha-hairpin domain (Fig.S1 and Table S4).
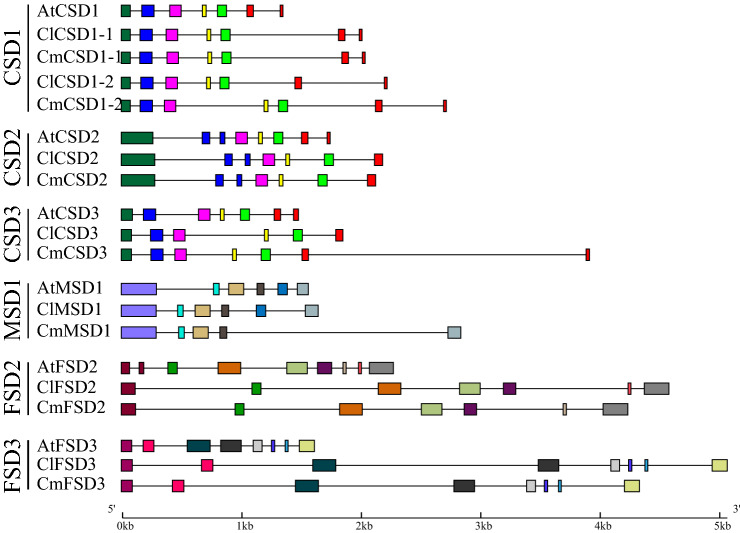
Intron and exon structure of AtSOD, ClSOD and CmSOD genes were marked by black lines and various similar color squares based on similarities in their coding amino acid sequences (Molina-Rueda et al. 2013; Hu et al. 2015). As indicated in Fig. 2, the intron numbers of AtSOD, ClSOD and CmSOD genes ranged from 4 to 8, with most SOD genes containing six introns; in addition, intron/exon structures were largely conserved in each sub-group of A. thaliana, C. lanatus and C. melo, especially CSD1 and FSD3 sub-group, but with a few differences. For example, in CSD2 sub-group, exons 7 and 8 were fused in ClCSD2 and CmCSD2. Similar phenomenon was detected in CSD3 sub-group that exons 6 and 7 were fused in ClCSD3. In addition, there were some differences in 3′ region among AtMSD1 and CmMSD1 genes, and the intron/exon structure differences were observed in FSD2 sub-group, with exons 1 and 2 fusing in ClFSD2 and CmFSD2, and exon 7 lacking in ClFSD2 and exon 8 lacking in CmFSD2. In addition, most of the introns were longer in ClSODs and CmSODs than in AtSODs. All the phenomena of intron/exon loss/gain in SOD genes were similar to the results in previous report (Molina-Rueda et al. 2013).
Fig. 2.
Intron and exon structure of SOD genes in Arabidopsis, watermelon and melon. Intron and exon were represented by lines and squares. Similar exons had same color according to similarities in their coding amino acid sequences within each sub-group (CSD1, CSD2, CSD3, MSD1, FSD2 and FSD3). These SOD genes were classified according to Fig. 1
Sequence alignment analysis
The high conserved patterns were found in SOD protein sequences based on multiple sequence alignment results that were generated by ClustalX 2.0 and marked by Boxshade 3.21 (Molina-Rueda et al. 2013; Larkin et al. 2007). As detailed in Fig.S2, black and grey boxes showed identical and similar amino acid sequences, respectively. Many metal-binding sites were found in CSDs, MSDs and FSDs, with the sites of CSDs in motif 1, the sites of MSDs in motif 3, 4 and 6, and the sites of FSDs in motif 2, 3 and 4. For example, in CSD proteins, the copper atom binding sites were His-118, 120 and 192, and zinc atom binding sites were His-143, 152 and Asp-155. Moreover, a bridging histidine residue (His-135) and conserved cysteine residues (Cys-129 and 218) involved in disulphide bond formation (Fig.S2A). In MSD proteins, the metal-binding sites were His-55, 103, 196 and Asp-192 (Fig.S2B), but there were no His-196 and Asp-192 residues in CmMSD1 protein. In FSD proteins, the metal-binding conserved residues were His-82, 135, 238 and Asp-234, but there was no Asp-234 residue in ClFSD2 and His-238 residue in CmFSD2, respectively (Fig.S2C). In addition, Tyr-63 and Tyr-90 residues were essential for catalytic activity in MSD and FSD proteins, respectively.
Chromosome mapping, syntenic analysis and gene duplication
Based on the physical location of ClSODs and CmSODs on their chromosomes, the chromosomal distributions were mapped by Mapchart2.2 tool. As shown in Fig. 3, four out of the 11 watermelon chromosomes and six out of the 12 melon chromosomes possessed of SOD genes, respectively, other chromosomes without SOD genes were not displayed. Watermelon chromosome 3 and 2 had three and two ClSOD genes, and chromosome 4 and 7 had only one ClSOD gene, respectively (Fig. 3a). Except for two CmSOD genes locating in chromosome 2, all of the rest five chromosomes (5, 7, 8, 11 and 12) had only one CmSOD gene, respectively (Fig. 3b).
Fig. 3.
Chromosomes distribution and synteny analysis of SOD genes. A, ClSOD genes on watermelon chromosomes; B, CmSOD genes on melon chromosomes; chromosomal distance were given in Mbp. The numbers represented the physical location of SOD genes. C, Synteny analysis of SOD genes on Arabidopsis, watermelon and melon chromosomes. The chromosome colors of Arabidopsis, watermelon and melon were marked as red, green and blue. Different color curves represented the syntenic regions among AtSOD, ClSOD and CmSOD genes
Evolutionary relationships among AtSODs, ClSODs and CmSODs were analyzed by MCScanX software and visualized via Circos. As detailed in Fig. 3c and Table S5-1, there were 20 collinear gene pairs such as AtCSD1/ClCSD1-1/CmCSD1-1, AtCSD2/ClCSD2/CmCSD2, AtCSD3/ClCSD3/CmCSD3, AtFSD2/ClFSD2/CmFSD2, ClCSD1-1/CmCSD1-1/CmCSD1-2, ClCSD1-2/CmCSD1-1/CmCSD1-2, ClMSD1/CmMSD1 and ClFSD3/CmFSD3. Among them, 3 gene pairs (AtFSD1/AtFSD2, ClCSD1-1/ClCSD1-2 and CmCSD1-2/CmCSD1-1) were identified as segmental duplication. In addition, these collinear gene pairs were also identified as six orthogroups (OG1–OG6) using Orthofinder software (Emms and Kelly 2019) (Table S5-2).
Promoter region analysis
Cis-regulatory elements play important roles in plant growth and response to abiotic and biotic stresses, which determine expression patterns of the genes such as tissue-specific, stress-responsive and hormone-responsive (Fujita et al. 2006; Walther et al. 2007). As shown in Fig. 4 and Table S6, two major classes were identified: stress-responsive and hormone-responsive. First, stress-responsive elements consisted of ARE, LTR, MBS and TC-rich element, which were responsive to anaerobic induction, low-temperature stress, drought inducible, defense and stress, respectively; second, hormone-responsive elements included ABRE, AuxRR-core, TGA-element, CGTCA-motif, GARE-motif, P-box, TATC-box and TCA-element, which were related to abscisic acid (ABA), auxin (IAA), Methyl jasmonate (MeJA), gibberellins (GA) and salicylic acid (SA). In addition, the promoters of 14 SOD genes contained one or two W-box elements (TTGACC, WRKY-binding sites), which implied the genes might be regulated by WRKY proteins. All these results indicated that SOD genes might enhance plant resistance to abiotic stresses via being induced by exogenous or endogenous hormone, or being regulated by transcriptional factors.
Fig. 4.
Cis-regulatory elements in the 2000-bp upstream regions of SOD genes in Arabidopsis, watermelon and melon. Numbering was from translation start codons. The same Cis-regulatory elements were marked by same color
Gene ontology annotation
The annotations of ClSOD and CmSOD proteins were performed using Blast2GO (Table S7). In biological process, all 14 SOD proteins were predicted to be related to “superoxide metabolic process” (GO:0006801). Among them, seven ClSODs also possessed “removal of superoxide radicals” (GO:0019430) and “oxidation–reduction process” (GO:0055114), and seven CmSODs also possessed “response to stress” (GO:0006950). In addition, ClCSD3 was found to be involved in responding to abiotic stresses such as ozone (GO:0071457), salt stress (GO:0071472), high light intensity (GO:0071486) and UV-B (GO:0071493); some SODs (ClCSD1-2, ClCSD2, CmCSD1-2 and CmCSD2) maybe participate in regulating plant growth, such as “seed trichome elongation” (GO:0090378), reproduction (GO:0000003) and “cell differentiation” (GO:0030154). Molecular function prediction showed all the SOD genes had “metal ion binding” (GO:0046872) and catalytic activity including “superoxide dismutase activity” (GO:0004784) or “oxidoreductase activity” (GO:0016491). Cellular component prediction indicated majority of Cu/ZnSODs were found in cytosol, plastid, peroxisome, cytoplasm, and MnSODs in mitochondrion, which coincided with the sub-cellular prediction results for SOD proteins.
Expression analysis of CmSODs and ClSODs under low temperature, NaCl and PEG treatments
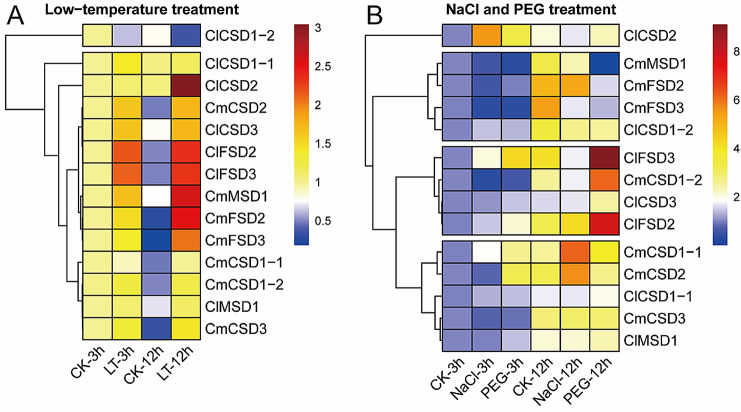
To further understand the possible roles of CmSODs and ClSODs in response to abiotic stresses, the expression levels were carried out under low-temperature (8 °C), osmotic (PEG6000) and salt (NaCl) stresses, respectively. As shown in the Fig. 5 and Fig.S3, most CmSODs and ClSODs displayed significantly up-regulated expression patterns compared to untreated control, with a few down-regulated. For example, under low-temperature treatment, seven CmSOD and six ClSOD genes were up-regulated from 0.5- to 10.7-fold-change, however, ClCSD1-2 was down-regulated 0.5-fold change when treated for 3 or 12 h. Under NaCl treatment, up-regulated expression levels from 0.7- to 4.5-fold-change were observed in three SOD genes (CmCSD1-1 and CmCSD2 at 12 h, ClCSD2 at 3 h), however, the relative expression levels of CmFSD3 and ClFSD3 showed down-regulated expression patterns from 1.3- to 2.3-fold-change when treated for 12 h. Under PEG6000 treatment, five CSDs (CmCSD1-1, CmCSD1-2, CmCSD2, ClCSD2 and ClCSD3) were up-regulated form 0.5- to 2.0-fold-change when treated for 3 or 12 h, however, ClCSD1-2 was down-regulated 0.4-fold-change when treated for 12 h. In addition, decreased expression levels of more than 2.3-fold change were found in three CmSOD genes (CmFSD2, CmFSD3 and CmMSD1), on the contrary, ClFSD2 and ClFSD3 showed increased expression levels of more than 1.0-fold change when treated for 3 and 12 h.
Fig. 5.
21-day-old plants were treated with low temperature (8 °C), 20% (w/v) PEG 6000 and 250 mM NaCl for 3 and 12 h before the mature leaves were harvested. Expression of the CmSOD and ClSOD genes were normalized to those of CmActin and ClActin, and shown relative to the expression of CK at 3 h, respectively
Discussion
Watermelon and melon are the major vegetable crops of the world, but their yield and quality are affected by various abiotic stresses. Previous studies showed that SOD genes exerted great impact upon plant growth and resistance to stresses in that the genes could build a defense line against reactive oxygen species (ROS) produced by various stresses. SOD genes appeared in various plant species, and due to the huge differences of their genome sizes and gene duplications, the number of SOD genes varied from one plant to another, such as six in Larix kaempferi (Han et al. 2019), seven in A. thaliana (Kliebenstein et al. 1998), eight in Setaria italica (Wang et al. 2018), nine in S. lycopersicum (Feng et al. 2016), 12 in Populus (Molina-Rueda et al. 2013), 18 in B. rapa (Verma et al. 2019) and 25 in M. acuminata (Feng et al. 2015). In this study, the numbers of SOD genes in C. lanatus and C. melo were seven (four Cu/ZnSODs, one MnSODs and two FeSODs) and seven (four Cu/ZnSODs, one MnSODs and two FeSODs), respectively. Although the genome sizes of C. lanatus (425 Mb) and C. melo (450 Mb) were twice more than that of A. thaliana (135 Mb) and half less than that of S. lycopersicum (960 Mbp), respectively, there was not significantly increase or decrease in the numbers of SOD genes, which suggested relative conservation in numbers. Nonetheless, a few differences were detected in the numbers of CSD1 and FSD genes. The discrepancy may be attributed to gene duplications, i.e., segmental and tandem duplications, which have the significant importance in expanding the members of gene family (Zhang 2003; Barker et al. 2012). For instance, segmental duplication played a key role in the expansion of cotton SOD genes (Zhang et al. 2016), segmental and tandem duplication in tomato SOD genes (Feng et al. 2016), tandem duplication in cucumber SOD genes (Zhou et al. 2017). In this study, through sequence similarity and syntenic analysis, two adjacent gene pairs (ClCSD1-1 and ClFSD3 in Chr3, CmCSD1-1 and CmFSD3 in Chr2) were not identified as a tandem duplicated event because of large differences in protein sequence similarity, however, segmental duplications were identified between two pairs of paralogs (ClCSD1-1 in chr3 and ClCSD1-2 in chr2, CmCSD1-1 in chr2 and CmCSD1-2 in chr5), which shared 84.2 and 88.8% protein sequence similarity, respectively. This result indicated that segmental duplication most likely exerted an important influence on the expansion of ClSODs and CmSODs, respectively.
The amino acid length of most SOD proteins was less than 300 aa in plants such as Populus (Molina-Rueda et al. 2013) and M. acuminata (Feng et al. 2015). In addition, plant CSD proteins had been reported to be found in cytoplasm or chloroplast, FSD proteins in chloroplast and MSD proteins in mitochondrion (Feng et al. 2016; Verma et al. 2019). The result of amino acid length and sub-cellular location of ClSODs and CmSODs was similar to the previously reported studies. Phylogenetic analysis revealed that ClSODs and CmSODs were closely clustered into three groups (Cu/ZnSODs, MnSODs and FeSODs), including seven pairs of orthologs, which their bootstrap value ranged from 86 to 100%. The similar results were also found in GRF and MLO protein families in Cucurbitaceae (Baloglu 2014; Iovieno et al. 2015). Phylogenetic analysis also revealed that MnSODs and FeSODs were separated by 100% bootstrap value, based on the previous reports that plants MnSODs and FeSODs shared a common ancestor but a significant difference was existed in their separate evolutionary process (Feng et al. 2016; Miller 2012). In addition, sequence similarity and motif analysis were also carried out to increase reliability of the phylogenic tree, and found that seven pairs of orthologs had higher similar protein sequences (about 90%) and same motifs, respectively. The result showed that these ortholog pairs may be have similar biological functions, which need to be verified by gene cloning and biological functional analysis of ClSOD and CmSOD genes in the later researches.
Gene structure analysis revealed that the intron numbers of ClSODs and CmSODs ranged from 4 to 7. Among them, there were relatively conservative in cytoplasm/chloroplast CSD1 genes (six introns), cytoplasm/chloroplast CSD2 genes (six introns), chloroplast FSD2 genes (six introns) and chloroplast FSD3 genes (seven introns), which suggested high conservation in their evolutionary process. However, the differences were also observed in chloroplast CSD3 genes (five in ClCSD3 and six in CmCSD3) and mitochondrion MSD1 genes (five in ClMSD1 and four in CmMSD1). Thus, this result did not support the earlier report that the intron patterns of plant SODs were highly conserved, with seven introns in cytosolic genes and eight introns in chloroplastic genes (Fink and Scandalios 2002). In addition, this phenomenon was reported in S. lycopersicum (Feng et al. 2016) and B. juncea (Verma et al. 2019). The variation in exon/intron number were ascribed to the mechanisms of exon/intron gain/loss, exonization/pseudoexonization and insertion/deletion, which also resulted in gene structural divergence and functional differentiation (Xu et al. 2012; Baloglu 2014; Feng et al. 2016; Verma et al. 2019). Multiple sequence alignment results presented that majority of ClSOD and CmSOD proteins possessed conserved metal-binding sites, however, three SOD proteins (CmMSD1, ClFSD2 and CmFSD2) lacked of partial metal-binding sites because of their partial exons loss in 3′ regions, which will also be the focus in the future research whether these loss sites would affect the biological function of SOD proteins.
Abiotic factors such as drought, low temperature, and salinity have serious influence on the yield and quality of watermelon and melon. Previous reports revealed that the cis-regulatory elements in the promoters of other plants SOD genes were related to these stresses (Wang et al. 2018; Han et al. 2019). To better understand the roles of ClSODs and CmSODs under various environmental stresses, cis-regulatory elements in the promoter sequence were analyzed. Several stress-responsive elements such as ARE, LTR, MBS, TC-rich elements and W-box were identified in the promoters of ClSODs and CmSODs, which implied that the transcription factors would interact with these cis-regulatory elements to enhance the stress tolerance of watermelon and melon. In addition, other researches on S. lycopersicum, B. rapa, and M. acuminate indicated that various SOD genes displayed different expression patterns induced or inhibited by these stresses (Feng et al. 2015, 2016; Verma et al. 2019), and over-expression of SODs in transgenic plants could increase their resistance to adverse conditions (Gao et al. 2003; Zhang et al. 2014). To understand the roles of CmSODs and ClSODs in response to low-temperature, salt and osmotic stresses, their expression levels were also discussed. For low-temperature treatment, more SOD genes were up-regulated from 0.5- to 10.7-fold change, respectively, possibly because of LTR and TC-rich elements in their promoters. In addition, although MBS- and TC-rich elements related to abiotic stresses were also found in the promoters of some CmSOD and ClSOD genes, different expression patterns of these genes were observed under salt and PEG treatment. For example, CmCSD2 at 12 h and ClCSD2 at 3 h were up-regulated 1.0-fold change, however, CmFSD3 and ClFSD3 were down-regulated at 12 h under salt treatment. The expression of CmFSD2 and CmFSD3 decreased more than two-fold change, while increased expression levels were found in ClFSD2 and ClFSD3 genes under PEG treatment. These results suggested that different SOD genes in watermelon and melon could play different roles in response to various abiotic stresses. Similar results have also been found in other plant species (Feng et al. 2016; Verma et al. 2019).
Conclusions
In this study, a total of seven ClSODs and seven CmSODs were identified in watermelon and melon and these genes were anchored in several chromosomes, respectively. Our study also provided detailed information about ClSODs and CmSODs, including sequence characteristics, gene structure, motif distribution, phylogenetic relationship, syntenic analysis, cis-element distribution, gene ontology annotation and expression patterns. Especially, several SOD genes displayed different expression patterns under low-temperature, salt and PEG treatments, suggesting that they might play different roles in response to these stresses. This study enabled us to further our research and to identify stress-related SOD genes to offer some insight into the response of watermelon and melon to abiotic stresses under field conditions.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The author thanks Mr. Jiannong Zhang in Gansu Agricultural University for his providing melon and watermelon inbreeding lines. The author also thanks Ms. Lilan Chen in Gansu Agricultural University for her help with manuscript revision.
Author contribution
GYZ, QD and BQW designed, conceived and performed the experiments. GYZ and QD carried out the bioinformatics, edited the data, figures and tables, draft the manuscript. All the authors read and approved the final manuscript.
Funding
This work was supported by Scientific research start-up funds for openly-recruited doctors (2017RCZX-30) of Science and Technology Innovation Funds of Gansu Agricultural University, China.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abreu IA, Cabelli DE. Superoxide dismutases-a review of the metal-associated mechanistic variations. Biochem Biophys Acta. 2010;1804(2):263–274. doi: 10.1016/j.bbapap.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Alscher RG, Erturk N, Heath LS. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot. 2002;53(372):1331–1341. doi: 10.1093/jxb/53.372.1331. [DOI] [PubMed] [Google Scholar]
- Bailey TL, Williams N, Misleh C, et al. MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006;34(Web Server issue):W369–W373. doi: 10.1093/nar/gkl198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloglu MC. Genome-wide in silico identification and comparison of Growth Regulating Factor (GRF) genes in Cucurbitaceae family. Plant Omics J. 2014;7(4):260–270. doi: 10.1097/01.tp.0000332556.64365.e7. [DOI] [Google Scholar]
- Barker MS, Baute GJ, Liu SL. Duplications and turnover in plant genomes. Plant Genome Divers. 2012;1:155–169. doi: 10.1007/978-3-7091-1130-7_11. [DOI] [Google Scholar]
- Chojnacki S, Cowley A, Lee J, et al. Programmatic access to bioinformatics tools from EMBL-EBI update: 2017. Nucleic Acids Res. 2017;45(Web Server issue):W550–W553. doi: 10.1093/nar/gkx273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou KC, Shen HB. A new method for predicting the subcellular localization of eukaryotic proteins with both single and multiple sites: Euk-mPLoc 2.0. PLoS ONE. 2010;5(4):e9931. doi: 10.1371/journal.pone.0009931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conesa A, Götz S. Blast2GO: a comprehensive suite for functional analysis in plant genomics. Int J Plant Genomics. 2008;2008:619832. doi: 10.1155/2008/619832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, et al. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gebali S, Mistry J, Bateman A, et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019;47(Database issue):D427–D432. doi: 10.1093/nar/gky995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emms D, Kelly S. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 2019;20(1):238–252. doi: 10.1186/s13059-019-1832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Feng X, Lai Z, Lin Y, et al. Genome-wide identification and characterization of the superoxide dismutase gene family in Musa acuminata cv. Tianbaojiao (AAA group) BMC Genomics. 2015;16:823. doi: 10.1186/s12864-015-2046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng K, Yu J, Cheng Y, et al. The SOD gene family in tomato: identification, phylogenetic relationships, and expression patterns. Front Plant Sci. 2016;7:1279. doi: 10.3389/fpls.2016.01279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink RC, Scandalios JG. Molecular evolution and structure–function relationships of the superoxide dismutase gene families in angiosperms and their relationship to other eukaryotic and prokaryotic superoxide dismutases. Arch Biochem Biophys. 2002;399(1):19–36. doi: 10.1006/abbi.2001.2739. [DOI] [PubMed] [Google Scholar]
- Finn RD, Clements J, Eddy SR. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 2011;39(Web Server issue):W29–W37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Bateman A, Clements J, et al. Pfam: the protein families database. Nucleic Acids Res. 2014;42(Database issue):D222–D230. doi: 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Fujita Y, Noutoshi Y, et al. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol. 2006;9(4):436–442. doi: 10.1016/j.pbi.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Gao X, Ren Z, Zhao Y, et al. Overexpression of SOD2 increases salt tolerance of Arabidopsis. Plant Physiol. 2003;133(4):1873–1881. doi: 10.1104/pp.103.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mas J, Benjak A, Sanseverino W, et al. The genome of melon (Cucumis melo L.) Proc Nat Acad Sci U S A. 2012;109(29):11872–11877. doi: 10.1073/pnas.1205415109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Zhang J, Sun H, et al. The draft genome of watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions. Nat Genet. 2013;45(1):51–58. doi: 10.1038/ng.2470. [DOI] [PubMed] [Google Scholar]
- Han XM, Chen QX, Yang Q, et al. Genome-wide analysis of superoxide dismutase genes in Larix kaempferi. Gene. 2019;686:29–36. doi: 10.1016/j.gene.2018.10.089. [DOI] [PubMed] [Google Scholar]
- He Z, Zhang H, Gao S, et al. Evolview v2: an online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Res. 2016;44(Web Server issue):W236–W241. doi: 10.1093/nar/gkw370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Jin J, Guo AY, et al. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2015;31(8):1296–1297. doi: 10.1093/bioinformatics/btu817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovieno P, Andolfo G, Schiavulli A, et al. Structure, evolution and functional inference on the Mildew Locus O (MLO) gene family in three cultivated Cucurbitaceae spp. BMC Genomics. 2015;16:1112. doi: 10.1186/s12864-015-2325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein DJ, Monde RA, Last RL. Superoxide dismutase in Arabidopsis: an eclectic enzyme family with disparate regulation and protein localization. Plant Physiol. 1998;118(2):637–650. doi: 10.1104/pp.118.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywinski M, Schein J, Birol I, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19(9):1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, et al. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lescot M, Déhais P, Thijs G, et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Jaiswal P, Hebbard C, et al. Gramene: a growing plant comparative genomics resource. Nucleic Acids Res. 2008;36(Database issue):D947–D953. doi: 10.1093/nar/gkm968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A, Derbyshire MK, Gonzales NR, et al. CDD: NCBI's conserved domain database. Nucleic Acids Res. 2015;43(Database issue):D222–D226. doi: 10.1093/nar/gku1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKersie BD, Murnaghan J, Jones KS, et al. Iron-superoxide dismutase expression in transgenic alfalfa increases winter survival without a detectable increase in photosynthetic oxidative stress tolerance. Plant Physiol. 2000;122(4):1427–1437. doi: 10.1104/pp.122.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AF. Superoxide dismutases: ancient enzymes and new insights. FEBS Lett. 2012;586(5):585–595. doi: 10.1016/j.febslet.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Rueda JJ, Tsai CJ, Kirby EG. The Populus superoxide dismutase gene family and its responses to drought stress in transgenic poplar overexpressing a pine cytosolic glutamine synthetase (GS1a) PLoS ONE. 2013;8(2):e56421. doi: 10.1371/journal.pone.0056421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath K, Kumar S, Poudyal RS, et al. Developmental stage-dependent differential gene expression of superoxide dismutase isoenzymes and their localization and physical interaction network in rice (Oryza sativa L.) Genes Genomics. 2014;36(1):45–55. doi: 10.1007/s13258-013-0138-9. [DOI] [Google Scholar]
- Negi NP, Shrivastava DC, Sharma V, et al. Overexpression of CuZnSOD from Arachis hypogaea alleviates salinity and drought stress in tobacco. Plant Cell Rep. 2015;34(7):1109–1126. doi: 10.1007/s00299-015-1770-4. [DOI] [PubMed] [Google Scholar]
- Pal AK, Acharya K, Vats SK, et al. Over-expression of PaSOD in transgenic potato enhances photosynthetic performance under drought. Biol Plant. 2012;57(2):359–364. doi: 10.1007/s10535-012-0277-x. [DOI] [Google Scholar]
- Schneider TD, Stephens RM. Sequence Logos: a new way to display consensus sequences. Nucleic Acids Res. 1990;18:6097–6100. doi: 10.1093/nar/18.20.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafi A, Dogra V, Gill T, et al. Simultaneous over-expression of PaSOD and RaAPX in transgenic Arabidopsis thaliana confers cold stress tolerance through increase in vascular lignifications. PLoS ONE. 2014;9(10):e110302. doi: 10.1371/journal.pone.0110302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraya T, Mori T, Maruyama T, et al. Golgi/plastid-type manganese superoxide dismutase involved in heat-stress tolerance during grain filling of rice. Plant Biotechnol J. 2015;13(9):1251–1263. doi: 10.1111/pbi.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D, Lakhanpal N, Singh K. Genome-wide identification and characterization of abiotic-stress responsive SOD (superoxide dismutase) gene family in Brassica juncea and B. rapa. BMC Genomics. 2019;20(1):227. doi: 10.1186/s12864-019-5593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther D, Brunnemann R, Selbig J. The regulatory code for transcriptional response diversity and its relation to genome structural properties in A. thaliana. PLoS Genet. 2007;3(2):e11. doi: 10.1371/journal.pgen.0030011.eor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Tang H, Debarry JD, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40(7):e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Song H, Zhang B, et al. Genome-wide identification, characterization, and expression analysis of superoxide dismutase (SOD) genes in foxtail millet (Setaria italica L.) 3 Biotech. 2018;8(12):486. doi: 10.1007/s13205-018-1502-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan S, Goldman N. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol Biol Evol. 2001;18:691–699. doi: 10.1093/oxfordjournals.molbev.a003851. [DOI] [PubMed] [Google Scholar]
- Xu G, Guo C, Shan H, et al. Divergence of duplicate genes in exon-intron structure. Proc Natl Acad Sci USA. 2012;109(4):1187–1192. doi: 10.1073/pnas.1109047109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JZ. Evolution by gene duplication: an update. Trends Ecol Evol. 2003;18:292–298. doi: 10.1016/S0169-5347(03)00033-8. [DOI] [Google Scholar]
- Zhang DY, Yang HL, Li XS, et al. Overexpression of Tamarix albiflonum TaMnSOD increases drought tolerance in transgenic cotton. Mol Breeding. 2014;34:1–11. doi: 10.1007/s11032-014-0015-5. [DOI] [Google Scholar]
- Zhang Y, Li Z, Peng Y, et al. Clones of FeSOD, MDHAR, DHAR genes from white clover and gene expression analysis of ROS-scavenging enzymes during abiotic stress and hormone treatments. Molecules. 2015;20(11):20939–20954. doi: 10.3390/molecules201119741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li B, Yang Y, et al. Genome-wide characterization and expression profiles of the superoxide dismutase gene family in Gossypium. Int J Genomics. 2016;2016:8740901. doi: 10.1155/2016/8740901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Hu L, Wu H, et al. Genome-wide identification and transcriptional expression analysis of cucumber superoxide dismutase (SOD) family in response to various abiotic stresses. Int J Genomics. 2017;2017:7243973. doi: 10.1155/2017/7243973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.