Abstract
There is an urgent need to develop novel compounds that prevent the deleterious effects of opioids such as fentanyl on minute ventilation while, if possible, preserving the analgesic actions of the opioids. We report that L-glutathione ethyl ester (GSHee) may be such a novel compound. In this study, we measured tail flick latency (TFL), arterial blood gas (ABG) chemistry, Alveolar-arterial gradient, and ventilatory parameters by whole body plethysmography to determine the responses elicited by bolus injections of fentanyl (75 μg/kg, IV) in male adult Sprague–Dawley rats that had received a bolus injection of GSHee (100 μmol/kg, IV) 15 min previously. GSHee given alone had minimal effects on TFL, ABG chemistry and A-a gradient whereas it elicited changes in some ventilatory parameters such as an increase in breathing frequency. In vehicle-treated rats, fentanyl elicited (1) an increase in TFL, (2) decreases in pH, pO2 and sO2 and increases in pCO2 (all indicative of ventilatory depression), (3) an increase in Alveolar-arterial gradient (indicative of a mismatch in ventilation-perfusion in the lungs), and (4) changes in ventilatory parameters such as a reduction in tidal volume, that were indicative of pronounced ventilatory depression. In GSHee-pretreated rats, fentanyl elicited a more prolonged analgesia, relatively minor changes in ABG chemistry and Alveolar-arterial gradient, and a substantially milder depression of ventilation. GSHee may represent an effective member of a novel class of thiolester drugs that are able to prevent the ventilatory depressant effects elicited by powerful opioids such as fentanyl and their deleterious effects on gas-exchange in the lungs without compromising opioid analgesia.
Subject terms: Drug discovery, Systems biology
Introduction
Fentanyl is a high-potency opioid receptor (OR) agonist prescribed to treat pain1,2. The misuse-abuse of fentanyl causes adverse effects including opioid-induced respiratory depression (OIRD)1. Fentanyl has high affinity for μ-ORs3–6 and also activates δ- and κ-ORs7–10. Mechanisms underlying the effects of fentanyl have been studied11–14. Henderson et al.15 found that fentanyl-induced analgesia, decreased tidal volume (Vt) and increased Alveolar-arterial (A-a) gradient (ventilation-perfusion mismatch)16, was reduced by a peripherally-restricted μ-OR antagonist, naloxone methiodide. This agrees with reports that the analgesia and OIRD elicited by morphine and heroin are attenuated by naloxone methiodide17–19. As such, fentanyl may act on peripheral structures, brain regions without a blood–brain barrier, and brain structures within the blood brain barrier15,20.
Intravenous L-cysteine ethyl ester (L-CYSee) reverses the effects of morphine on arterial blood-gas (ABG) chemistry in tracheotomized rats21. L-CYSee is membrane-permeable22,23, readily enters peripheral tissues and brain24, and increases intracellular pools of cysteine25,26 via a membrane-associated carboxylesterase27. Increased availability of cysteine alters redox status of cells28,29 and enhances production of glutathione (GSH)30,31, which exerts redox effects and S-glutathiolation of proteins32, and production of hydrogen sulfide30,31, which increases minute ventilation (Ve) via actions in carotid bodies33. Enhanced biovailability of L-cysteine and L-GSH promotes formation of S-nitrosothiols such as S-nitrosocysteine and S-nitrosylation status of functional proteins34–37. S-nitrosothiols exert diverse effects by the S-nitrosylation of functional proteins38,39 and S-nitrosothiols in brainstem40 and peripheral structures41–43 exert positive effects on ventilatory function. In contrast, there is evidence that morphine alters redox status of cells to an oxidative state44 and reduces cellular GSH levels45.
Mendoza et al.21 reasoned that L-CYSee or its free radical cation46 reversed the effects of morphine via its reductive capacity, which down-regulates ORs47 or affects OR function by effects on membrane-associated proteins48. The efficacy of L-CYSee raises the question as to whether L-GSH ethyl ester (GSHee) can modulate OIRD. GSHee is hydrolyzed to GSH by esterases in plasma and cells49–51. Peripheral administration of GSHee increases the concentrations of GSHee, GSH and cysteine in blood cells, peripheral organs49–51 and cerebrospinal fluid52, but not brain tissue53. Nonetheless, central administration or direct application of GSHee to isolated neurons elicits robust increases in GSH levels53. Elevation of GSH levels by GSHee protects neurons from oxidative or metabolic stress, excitotoxicity, ischemia and ischemia–reperfusion injury53–64. GSHee protects endothelial cells65 and hepatic mitochondria66,67 from endotoxin-induced injury, and protects hepatic mitochondria68 and cardiac cells from ischemia–reperfusion injury69 and improves their functional recovery. GSHee enhances neuron survival, and stabilizes spinal cord blood flow after injury70,71. GSHee also enhances insulin sensitivity72, and decreases allergen-induced airway hyper-responsiveness73,74.
A unique intracellular enzymatic cascade is involved in the bidirectional conversion of L-cysteine to γ-glutamyl-L-cysteine to γ-L-glutamyl-L-cysteinylglycine (GSH)75–77. These compounds play vital roles in maintaining redox homeostasis, protecting cells from oxidative damage and the toxicity of xenobiotic electrophiles75–77. Our laboratory is exploring the biological activities and particularly the ventilatory actions of the ethyl and methyl ester derivatives of these thiols against OIRD and as mentioned, we have determined that L-CYSee can elicit immediate reversal of the negative effects of morphine on ABG in anesthetized, tracheotomized rats but it is important to note that L-CYSee was not effective in rats without a tracheotomy, obviously suggesting that L-CYSee had negative effects in the upper airway and that better thiolester therapeutics than L-CYSee need to be considered. Despite the wealth of information about the biological activities of GSH and GSHee, the possibility that GSHee has the ability to modulate any of the pharmacological effects of opioids has not been explored to date. On the basis of the evidence presented above, the major objectives of the present study were (1) to determine the effects of GSHee on ventilatory performance in freely-moving adult rats, and (2) to determine whether the prior administration of GSHee is able to ameliorate the negative effects of fentanyl on breathing and gas-exchange in freely-moving adult rats. The results demonstrate that GSHee (100 μmol/kg, IV) has pronounced positive effects on breathing in freely-moving adult male Sprague–Dawley rats and that it markedly attenuates the deleterious effects elicited by subsequent injection of fentanyl (75 μg/kg, IV) on minute ventilation, A-a gradient and ABG chemistry while extending the analgesic actions of the OR agonist. In contrast, the injection of GSH itself (100 μmol/kg, IV), while having positive effects on breathing did not attenuate the negative effects of this dose of fentanyl on breathing parameters.
Methods
Rats and surgical procedures
All studies were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23) revised in 1996. In addition, all studies were carried out in compliance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines (http://www.nc3rs.org.uk/page.asp?id=1357). The protocols were approved by the Animal Care and Use Committees of the University of Virginia and Case Western Reserve University. Adult male Sprague–Dawley rats (Harlan, Madison, WI, USA) were implanted with jugular vein catheters under 2% isoflurane anesthesia and some rats received femoral arterial catheters15,78,79. The rats were allowed at least four days to recover from surgery before use. All arterial catheters were flushed daily with heparin solution (50 units heparin in phosphate-buffered saline at 0.1 M, pH 7.4). All catheters were flushed with phosphate-buffered saline (0.1 M, pH 7.4) approximately four hours before commencement of the experiments. All studies were performed in a quiet laboratory with relative humidity of 50 ± 2% and room temperature of 21.3 ± 0.2 °C.
Antinociception protocols
Antinociception was determined by a radiant heat tail-flick (TF) assay15,78,79. The intensity of the light was adjusted so that baseline TF latencies were approximately 3 s. A cutoff time of 12 s was set to minimize damage to the tail. Baseline TF latencies were established and after 15 min, the rats were injected with vehicle (saline, 1 ml/kg, IV; n = 9 rats; 316 ± 2 g) or GSHee (100 μmol/kg, IV; n = 9; 317 ± 2 g) and TF latencies were then recorded after 5 and 15 min. At that point, all of the rats received an injection of fentanyl (75 μg/kg, IV) and TF latencies were recorded at 15, 30, 60, 120, 180 and 240 min thereafter. The data are shown as actual TF latencies (sec) and as “maximum possible effect” (%MPE) using the formula, %MPE = [(post-injection TF latency − baseline TF latency)/(12 − baseline TF latency)] × 100.
Protocols for blood gas measurements and determination of Arterial-alveolar gradient
Arterial blood samples (100 μL) were taken 15 min before, 1, 7.5 and 15 min after injection of vehicle (saline, IV; n = 9 rats; 322 ± 2 g) or GSHee (100 μmol/kg, IV; n = 9 rats; 320 ± 2 g) and 1, 5, 10, 15 and 20 min after injection of fentanyl (75 μg/kg, IV). The pH, pCO2, pO2 and sO2 of the samples were measured using a Radiometer blood-gas analyzer (ABL800 FLEX). A-a gradient measures the difference between alveolar and arterial blood O2 concentrations15,16,80,81. A decrease in PaO2, without a change in A-a gradient is caused by hypo-ventilation whereas a decrease in PaO2 with an increase in A-a gradient indicates ventilation–perfusion mismatch15,16,82. A-a gradient = PAO2 − PaO2, where PAO2 is the partial pressure of alveolar O2 and PaO2 is pO2 in arterial blood. PAO2 = [(FiO2 × (Patm − PH2O) − (PaCO2/respiratory quotient)], where FiO2 is the fraction of O2 in inspired air; Patm is atmospheric pressure; PH2O is the partial pressure of H2O in inspired air; PaCO2 is pCO2 in arterial blood; and respiratory quotient (RQ) is the ratio of CO2 eliminated/O2 consumed. We took FiO2 of room-air to be 21% = 0.21, Patm to be 760 mmHg, and PH2O to be 47 mmHg16,80,81. We took the RQ value of our adult male rats to be 0.983,84.
Body temperature (BT) protocols
Changes in BT impact the size of recorded flow-related variables in plethysmography chambers85. Our chambers do not monitor BT, but it is imperative to record BT to understand its influence on GSHee on fentanyl-induced changes in ventilation. Adult male Sprague–Dawley rats were placed in separate open plastic boxes and allowed 60–90 min to acclimatize. BT was recorded as described previously86. A thermistor probe, inserted 5–6 cm into the rectum to allow regular recording of BT, was connected to a telethermometer (Yellow Springs Instruments) was taped to the tail. BT was recorded every 5 min during acclimatization to establish baseline values. One group of rats received vehicle (saline, 1 ml/kg, IV; n = 9 rats; 321 ± 2 g) and another received GSHee (100 μmol/kg, IV; n = 9 rats; 318 ± 2 g) and BT was recorded at 5 and 15 min post-injection. Both groups then received an injection of fentanyl (75 μg/kg, IV) and BT was recorded 5, 10, 15, 20, 25 and 30 min later.
Whole-body plethysmography measurement of ventilatory parameters
Ventilatory parameters were recorded in freely-moving rats by whole body plethysmography (PLY3223; Data Sciences International, St. Paul, MN) as described previously15,87–97. The rats were allowed 60 min to acclimatize to the chambers and to allow true resting ventilatory parameters to be established. One group of rats received vehicle (saline, 1 ml/kg, IV; n = 9 rats; 326 ± 2 g) and another received GSHee (100 μmol/kg, IV; n = 9 rats; 329 ± 2 g). After 15 min, all rats received an injection of fentanyl (75 μg/kg, IV). The effects of bolus injections of GSH (100 μmol/kg, IV; 324 ± 3 g; n = 9 rats) or vehicle (saline; 326 ± 2 g; n = 9 rats) on fr, Vt and Ve were determined in freely-moving male Sprague–Dawley rats and the subsequent effects of fentanyl (75 μg/kg, IV) given 15 min were also determined in both groups of rats. Due to the closeness of the body weights of the two groups of rats, ventilatory data are shown without body weight corrections. Parameters were breathing frequency (fr), tidal volume (Vt), minute ventilation (Ve), inspiratory time (Ti), expiratory time (Te), peak inspiratory (PIF) and peak expiratory (PIF) flows. The provided software (Fine Pointe, BUXCO) constantly corrected digitized values for changes in chamber temperature and humidity. Pressure changes associated with the respiratory waveforms were then converted to volumes (i.e., Vt, PIF and PEF) using the algorithm of Epstein and colleagues98,99. Specifically, factoring in chamber temperature and humidity, the cycle analyzers filtered the acquired signals, and BUXCO algorithms (Fine Pointe) generated an array of box flow data that identified a waveform segment as an acceptable breath. From that data vector, the minimum and maximum values were determined. Flows at this point were considered to be “box flow” signals. From this array, the minimum and maximum box flow values were determined and multiplied by a compensation factor provided by the selected algorithm98,99, thus producing Vt, PIF and PEF values that were used to determine accepted and rejected waveforms reported as Rejection Index (non-eupneic breathing)97. All directly recorded parameters including Rejection Index (see below) were extracted from the raw waveforms using Data Sciences International (St. Paul, MN, USA) proprietary Biosystem XA software (version 2.9.0.2) and proprietary FinePointe software (version v2.8.0), as described previously87–97 and as detailed in the Data Sciences International/Buxco website reference to the list of parameters provided by FinePointe Software using whole body plethysmography (https://www.datasci.com/products/buxco-respiratory-products/finepointe-whole-body-plethysmography). The BioSystem XA software extracts the waveforms that are analyzed by the FinePointe software that uses National Instruments Measurement Studio to perform these analyses (http://zone.ni.com/reference/en-XX/help/372636F-01/mstudiowebhelp/html/5d5b3031/).
Righting reflex
Separate groups of adult male Sprague–Dawley rats were used to evaluate the effects of GSHee (100 μmol/kg, IV) on the duration of fentanyl (75 μg/kg, IV)-induced impairment of the righting reflex (inability to stand on all four legs). Each rat was placed in an open plastic chamber to allow the duration of the loss of righting reflex to be accurately recorded. The time at which the rat spontaneously stood on all four paws was taken as the point of recovery100–102. In this study, one group of rats (320 ± 2 g, n = 12) received an injection of vehicle (saline) and after 15 min, an injection of fentanyl. A second group of rats (323 ± 2 g, n = 12) received an injection of GSHee and after 15 min an injection of fentanyl. The duration of effect of fentanyl was defined as the time interval from the time of injection of fentanyl administration to the recovery of righting reflex.
Statistics
The recorded data (1 min bins) and derived parameters, Vt/Ti and Response Area (cumulative percent changes from pre-values) were taken for statistical analyses. The pre-drug 1 min bins excluded occasional marked deviations from resting due to movements or scratching by the rats. These exclusions ensured accurate determinations of baseline parameters. The data are presented as mean ± SEM. All data were analyzed by one-way or two-way analysis of variance followed by Student's modified t test with Bonferroni corrections for multiple comparisons between means using the modified error mean square term (EMS) from the ANOVA103. The modified t-statistic is t = (mean group 1—mean group 2)/[s x (1/n1 + 1/n2)1/2] where s2 = the mean square within groups term from the ANOVA (the square root of this value is taken for the modified t-statistic formula) and n1 and n2 are the number of rats in each group. Based on an elementary inequality called Bonferroni’s inequality, a conservative critical value for the modified t-statistics is obtained from tables of t-distribution using a significance level of P/m, where m is the number of comparisons between groups to be performed. The degrees of freedom are those for the mean square for within group variation from the ANOVA table. In most cases, the critical Bonferroni value cannot be obtained from conventional tables of the t- distribution but may be approximated from widely available tables of the normal curve by t* = z + (z + z3)/4n, where n is the degrees of freedom and z is the critical normal curve value for P/m104. As demonstrated by Wallenstein et al.103 the Bonferroni procedure is recommended for general use since it is easiest to apply, has the widest range of applications, and gives critical values that will be lower than those of other procedures if the investigator is able to limit the number of comparisons, and that will be only slightly larger than those of other procedures if many comparisons are made. A value of P < 0.05 was taken as the initial level of statistical significance103,104.
Results
Tail-flick latencies
As summarized in Fig. 1, a bolus injection of GSHee (100 μmol/kg, IV) or vehicle (VEH, IV) did not alter TFL as measured 5 and 15 min after administration. The subsequent injection of fentanyl (75 μg/kg, IV) elicited robust antinociception for at least 180 min in vehicle-treated rats. As also seen in Fig. 1, the duration of the fentanyl-induced antinociception was greater in GSHee-pretreated rats than in the vehicle-pretreated rats.
Figure 1.
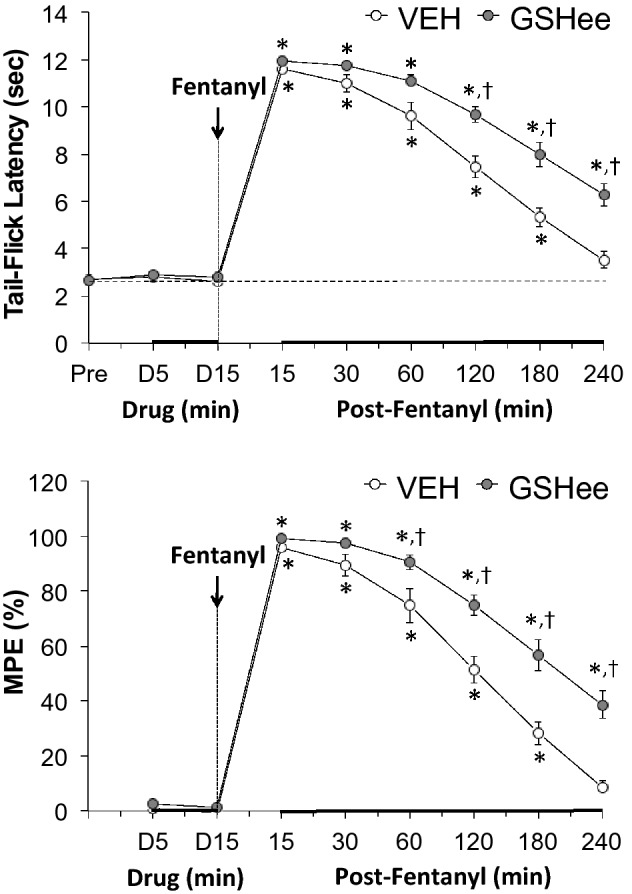
Upper panel: Effects of fentanyl (75 μg/kg, IV) on tail-flick latencies in rats pretreated with vehicle (VEH; 1 ml/kg, IV) or GSHee (100 μmol/kg, IV). Lower panel: Data in the upper panel expressed as maximal possible effect (MPE, %). All data are presented as mean ± SEM. There were 9 rats in each group. The data were analyzed by repeated measures ANOVA followed by multiple comparison testing as detailed in the Methods section. *P < 0.05/6 comparisons per group, significant change from post-drug (i.e., 15, 30, 60, 120, 180 or 240 min post-fentanyl versus D15 value). †P < 0.05/6 between group comparisons, GSHee versus vehicle.
Arterial blood-gas chemistry and A-a gradient
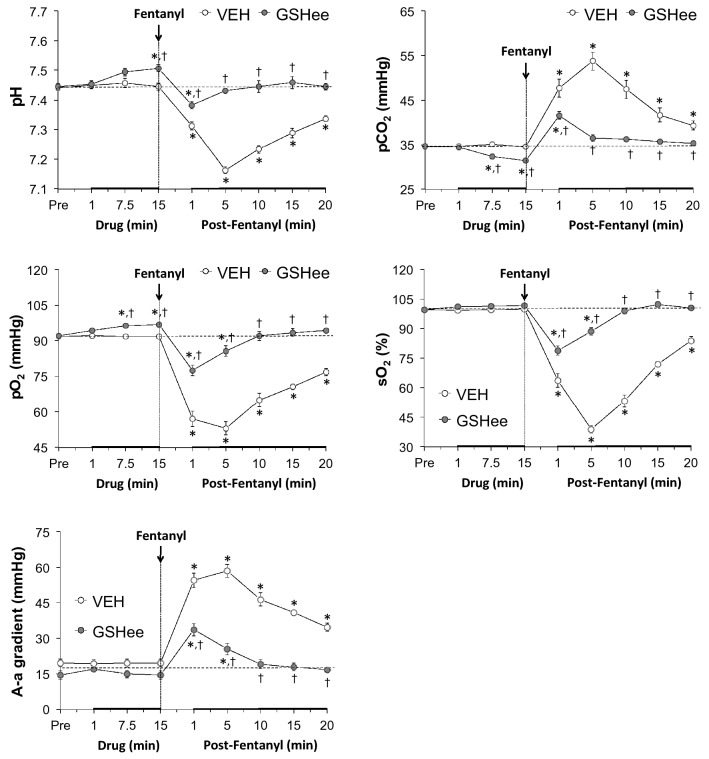
As summarized in Fig. 2, the injection of GSHee (100 μmol/kg, IV) elicited minor but significant increases in pH and pO2 that were accompanied by a minor but significant decrease in pCO2. These responses were still evident 20 min after injection of fentanyl. GSHee did not affect sO2 or A-a gradient. The injection of fentanyl (75 μg/kg, IV) in vehicle-treated rats elicited dramatic decreases in arterial blood pH, pO2 and sO2 that were accompanied by substantial increases in pCO2 and A-a gradient (Fig. 2). The changes in ABG chemistry and A-a gradient were markedly smaller in magnitude and of substantially shorter duration in the GSHee-pretreated rats.
Figure 2.
Effects of fentanyl (75 μg/kg, IV) on arterial blood pH, pO2, pCO2 and sO2 values and Alveolar-arterial (A-a) gradients in rats pretreated with vehicle (VEH; 1 ml/kg, IV) or GSHee (100 μmol/kg, IV). The data are presented as mean ± SEM. The data were analyzed by repeated measures ANOVA followed by multiple comparison testing as detailed in the Methods section. There were 9 rats in each group. *P < 0.05/5 comparisons per group, significant change from post-drug. †P < 0.05/5 between group comparisons, GSHee versus vehicle.
Body temperature
The changes in BT elicited by injection of fentanyl (75 μg/kg, IV) in vehicle-treated or GSHee (100 μmol/kg, IV)-treated rats are summarized in Supplemental Table 1. Prior to the injections, both groups had similar resting BT values. Neither vehicle nor GSHee affected BT as recorded at 5 and 15 min post-injection. The injection of fentanyl in vehicle-treated rats elicited a small but significant hyperthermia. Fentanyl elicited a similar minor hyperthermia in the GSHee-treated rats.
Ventilatory parameters
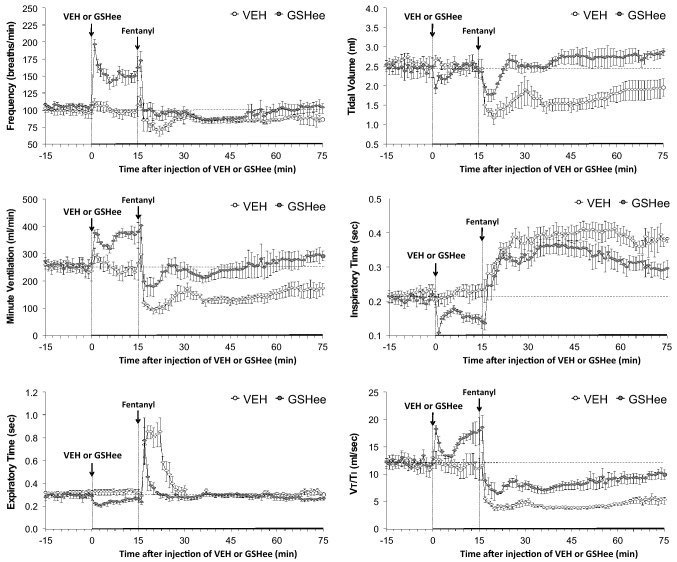
As summarized in Fig. 3, the injection of GSHee elicited a prompt and sustained increase in breathing frequency (fr) that was accompanied by expected decreases in inspiratory time (Ti) and expiratory time (Te). GSHee did not affect tidal volume (Vt) and so the sustained increase in minute ventilation (Ve) was due entirely to the increase in fr. The changes in Vt and Ti resulted in a biphasic increase in inspiratory drive (Vt/Ti). The injection of vehicle did not alter any of the above ventilatory parameters. Subsequent injection of fentanyl in vehicle-treated rats elicited (a) a decrease in fr associated with a pronounced and sustained increase in Ti and a pronounced but shorter in duration increase in Te, and (b) sustained decreases in Vt, Ve and inspiratory drive (Vt/Ti). Subsequent injection of fentanyl in GSHee-treated rats elicited (i) a substantially smaller decrease in fr that was accompanied by a substantially smaller increase in Te but a smaller reduction of the increase in Ti, and (ii) smaller decreases in Vt, Ve and inspiratory drive.
Figure 3.
Effects of fentanyl (75 μg/kg, IV) on frequency of breathing (top left panel), tidal volume (top right panel), inspiratory time (middle left panel), minute ventilation (middle right panel), expiratory time (bottom left panel) and tidal volume/inspiratory time (Vt/Ti) in rats pretreated with vehicle (VEH; 1 ml/kg, IV) or GSHee (100 μmol/kg, IV). The data are presented as mean ± SEM. There were 9 rats in each group. The stippled horizontal line denotes average resting values before injection of GSHee or vehicle.
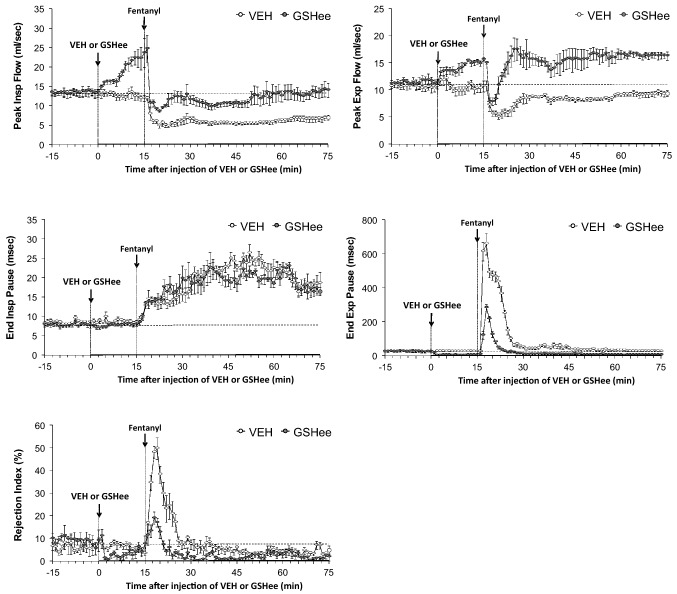
As shown in the top panels of Fig. 4, GSHee elicited robust increases in peak inspiratory flow (PIF) and peak expiratory flow (PEF) that were still present at the time fentanyl was injected 15 min later. Fentanyl elicited pronounced and sustained decreases in PIF and PEF in the vehicle-treated rats. The injection of fentanyl elicited a robust and sustained decrease in PIF in the GSHee-treated rats but the levels of PIF did not fall markedly below baseline (pre-GSHee levels). In contrast, fentanyl elicited a transient reduction in PEF with the levels quickly returning and remaining at the elevated levels elicited by GSHee. As shown in the middle panels of Fig. 4, GSHee elicited robust decreases in end expiratory pause (EEP), but only minor changes in end inspiratory pause (EIP) (more readily seen in Supplemental Fig. 1). The subsequent injection of fentanyl elicited pronounced and sustained increases in EIP that were similar in the vehicle- and GSHee-treated rats. In contrast, fentanyl elicited a marked increase in EEP for approximately 25 min in the vehicle-treated rats, but a markedly smaller response of lesser duration in the GSHee-treated rats. Finally, the bottom panel of Fig. 4 shows that GSHee elicited a fall in Rejection Index (Rinx, decrease in non-eupneic breathing) that had transpired by the time fentanyl was given. The subsequent injection of fentanyl elicited a marked increase in Rejection Index for approximately 15 min in vehicle-treated rats, but a markedly smaller increase of lesser duration in GSHee-treated rats. Rejection Index tended to drop in both groups of fentanyl-injected rats, most noticeably about 30 min after injection of fentanyl (45 min after injection of vehicle or GSHee).
Figure 4.
Effects of fentanyl (75 μg/kg, IV) on peak inspiratory flow (top left panel), peak expiratory flow (top right panel), end inspiratory pause (middle left panel), end expiratory pause (middle right panel), and Rejection Index (bottom left panel) in rats pretreated with vehicle (VEH; 1 ml/kg, IV) or GSHee (100 μmol/kg, IV). The data are presented as mean ± SEM. There were 9 rats in each group. The stippled horizontal line denotes average resting values before injection of GSHee or vehicle.
Supplemental Fig. 1 summarizes the cumulative responses elicited by injections of vehicle (VEH) or GSHee (top panel) recorded over the 15 min-period prior to injection of fentanyl and those elicited by subsequent injection of fentanyl recorded for 60 min (bottom panel). The injection of vehicle did not elicit cumulative changes in any ventilatory parameter. GSHee elicited cumulative increases in Ve, which was due solely to increases in fr not Vt. GSHee elicited cumulative decreases in Ti and Te; cumulative increases in inspiratory drive (Vt/Ti), PIF, and PEF; no cumulative change in EIP, but cumulative decreases in EEP and Rejection Index. The subsequent injection of fentanyl in the vehicle-treated rats (bottom panel) elicited cumulative decreases in fr, Vt and Ve; cumulative increases in Ti and to a lesser extent Te; cumulative decreases in PIF and PEF; and cumulative increases in EIP, EEP and Rejection Index. Pretreatment with GSHee prevented/attenuated/reversed the fentanyl-induced cumulative decreases in Vt, Ve, Vt/Ti, PIF, PEF, and fentanyl-induced cumulative increases in EIP, EEP and Rejection Index. We also investigated whether pre-treatment with GSH (100 μmol/kg, IV) itself would modulate the ventilatory depressant effects of fentanyl (75 μg/kg, IV). The baseline (Pre) fr, Vt and Ve values in the two groups of rats used in this study are presented in Supplemental Table 3. There were no-between group differences in any parameter. The changes in fr, Vt and Ve elicited by the injection of vehicle (VEH, saline) or GSH and the subsequent responses elicited by fentanyl are summarized in Supplemental Fig. 2. As can be seen, the injection of GSH elicited a prompt but short-lived increase in Freq with minor effects on TV that resulted in short-lived increases in MV. The responses elicited by the subsequent injection of fentanyl were similar in the vehicle- or GSH-treated rats.
Behaviors
Despite the relatively pronounced increase in Freq in the rats that received GSHee, the rats that did not display any obvious behavioral signs (e.g., movement, squealing, scratching or sniffing) and the rats that were lying quietly at the time of injection, remained so immediately during and following the injection. In addition, the duration of the pronounced sedation elicited by fentanyl was very similar in vehicle- or GSHee-treated rats. More specifically, we determined that in other groups of adult male Sprague–Dawley rats that injection of fentanyl (75 μg/kg, IV) caused immediate sedation (the rats quickly become immobile and lay on their side with eyes most often closed).The full return of the righting-reflex in vehicle-treated rats (43 ± 6 min, n = 12) and GSHee (100 μmol/kg, IV)-treated rats (54 ± 9 min, n = 12) were similar to one another (P > 0.05, GSHee versus vehicle).
Discussion
The intravenous injection of 100 μmol/kg (33.54 mg/kg) of GSHee elicited an array of responses in freely-moving adult male Sprague–Dawley rats and had dramatic effects on the responses elicited by subsequent injection of fentanyl (100 μmol/kg). It is likely that GSHee exerts its effects in naïve rats by increasing levels of GSH and metabolites (γ-glutamylcysteinyl and cysteine) in cells (e.g., neurons) in which GSH53–74 and the metabolites82,105–108 affect a number of biological processes. It is unlikely that GSHee directly inhibits OR function (loss of affinity or down-regulation of membrane accessible receptors) since GSHee augmented the analgesic effects of fentanyl, which are known to be OR-mediated15. It is well known that morphine can markedly lower GSH and cysteine levels in brain and peripheral tissues109–117. To our knowledge, there is no literature describing the effects of fentanyl on tissue GSH levels. However, one study reported that fentanyl causes an increase in production of reactive oxygen species in freshly isolated peripheral blood lymphocytes118, which arguably may involve a reduction in cellular reduced thiol levels. It must be noted that it is far from clear how a proposed fentanyl-induced decrease in intracellular GSH and cysteine levels would contribute to fentanyl-induced depression of ventilation. Moreover, it is not clear whether the ability of GSHee to blunt the ventilatory depressant effects of fentanyl is due to enhancement of GSH and cysteine levels in relevant tissues such as the brainstem and carotid bodies. It should also be noted that the ability of fentanyl to enhance the production of reactive oxygen species in lymphocytes is far from definitive with respect to how fentanyl depresses ventilation or how GSHee modulates the effects of fentanyl. The finding that the injection of GSH itself did not blunt the ventilatory depressant effects of fentanyl raises the tentative suggestion that the bioactivity of GSHee may be due to the enhanced ability of the thiolester to enter cells that control breathing and that this intracellular entry is related to the sustained efficacy of GSHee against some of the actions of fentanyl.
With respect to analgesia, GSHee did not elicit observable changes in analgesia status of freely moving rats per se whereas the duration of fentanyl analgesia was increased in rats pretreated with the thiolester. The mechanisms by which GSHee augments fentanyl-induced analgesia are probably multi-factorial and likely to involve modulation of analgesia-signaling pathways within the periphery, spinal cord and brain15. Indeed, it may be possible that GSHee modulates opioid receptor signaling processes that result in fentanyl becoming a “biased” ligand so that the opioid receptor signaling pathways now favor analgesia rather than respiratory depression119,120. There is no literature on whether GSH or GSHee can alter the bias of fentanyl-induced signaling but molecular studies that look at the effects of GSH and GSHee on the interaction between ligand-occupied opioid receptors and coupling of Gproteins and b-arrestins for example, would seem to be feasible119,120. Whatever the mechanism, opioid-induced analgesia augmentation is a very important attribute for an OIRD drug candidate. The dose of fentanyl we chose for this study (75 μg/kg, IV) elicited a robust decrease in minute ventilation (approximately 50% over a period of at least 60 min) and so represents a substantial level of ventilatory impairment by which to examine the effects of the 100 μmol/kg dose of GSHee. However, it is clear that the effects of lower and higher doses of GSHee need to be evaluated against higher doses of fentanyl that are known to cause a more dangerous depression of breathing121. This is to fully characterize the full efficacy of GSHee but also to take into account recent evidence from elegant in situ brainstem studies showing that morphine affects key areas of the respiratory network in a dose-dependent manner122.
The ability of GSHee to affect ventilatory parameters in naïve rats was as complex as it was impressive. First, GSHee elicited a sustained increase in Ve via the sustained increase in fr that was accompanied by the expected decreases in both Ti and Te, whereas it had minimal effects on Vt. It would seem likely that GSHee drives fr by actions within the carotid bodies by mechanisms including direct effects on neurotransmitter release from primary glomus cells. However, Gonzalez et al.123 provided evidence that GSH did not activate chemoreceptor cells in normoxia or modify hypoxic activation of these cells even though incubation of carotid body glomus cells with reduced GSH increased GSH-reducing potential. It remains possible that nitrosyl derivatives of GSHee such as S-nitrosoglutathione and S-nitrosocysteinylglycine or S-nitrosocysteine drive an increase in fr by activating carotid body glomus cells, chemoafferent terminals in the carotid bodies, or by actions in brain sites such as the nucleus tractus solitarius40,124, a key nucleus of ventilatory processing. Despite minimal effects on Vt (actual volume of air taken in each breath), GSHee did elicit a sustained increase in both PIF and PEF, which suggests the thiolester either directly affected the force of contraction of skeletal muscle in the extrinsic (inspiratory) and intrinsic (expiratory) intercostals and/or diaphragm. This would be consistent with considerable in vitro evidence that GSH enhances both Ca2+-dependent and Ca2+-independent skeletal muscle contractility125–127.
As we reported previously15, the injection of fentanyl elicits much longer increases in Te than Ti in vehicle-treated rats. As such, it would appear that the effects of fentanyl on the processes driving active inspiration in intact rats are of much longer duration than those that occur during passive expiration. Saunders and Levitt128 provided elegant demonstration that the fentanyl-induced changes in Te and Ti occurred in parallel in the in situ arterially-perfused rat brainstem preparation. We therefore presume that sensory inputs or other mechanisms not available in the in situ presentation are responsible for the exaggerated effects on Ti as opposed to Te in vivo. The mechanisms by which fentanyl exerts its longer-lasting effects on Ti remain to be determined. The effects on GSHee on the fentanyl-induced changes in Te (GSHee dramatically curtailed the effects of fentanyl on Te) were perhaps more remarkable than they were on Ti (GSHee somewhat diminished the magnitude rather than the duration of the longer lasting elevation in Ti). We cannot speculate on how GSHee elicits these effects but it is clear that it is available to interfere with the processes by which fentanyl suppresses active and passive phases of breathing. Fentanyl would be expected to cause non-eupneic breathing with more variation in phase length as the respiratory rate drops122,129. Indeed, in the present study we found that the relative increases in Ti and Te were substantially different during the peak decreases in fr elicited by fentanyl (approximately + 5 min post-injection, see Fig. 3). As can be seen in Supplementary Table 4, Te lengthened considerably more than Ti at the + 5 min time-point such that the Ti/Te ratio fell dramatically (-37 ± 5%). Moreover, at the 30 min post-fentanyl time-point when fr was close to return to pre-values (see Fig. 3), Ti was still substantially longer than pre-fentanyl whereas Te had returned to pre-values so that the Ti/Te ratio rose dramatically (+ 81 ± 9%). As also seen in Fig. 3, Ti values were substantially lower at the + 5 and + 30 min time-points post-fentanyl in the GSHee-treated rats whereas Te values were equivalent to pre-values at both times, reinforcing the general observation that GSHee differentially effects the ventilatory effects of fentanyl.
Under physiological circumstances, the ratio of Vt/Ti is an accepted index of inspiratory drive although it could be argued that changes in PIF could be taken as a similar index of changes in inspiratory drive14. However, the changes in Vt/Ti and PIF elicited by GSHee were not exactly the same (GSHee caused an initial transient spike in Vt/Ti not seen in PIF) although Vt/Ti and PIF both rose steadily between 5 to 15 min following injection. The injection of fentanyl elicited robust and long-lasting decreases in both Vt/Ti and PIF in vehicle-treated rats. However, it is clear that the depressant effects of fentanyl on PIF in the GSHee-treated rats were substantially less than the effects on Vt/Ti. As such, one possible interpretation of these findings is that GSHee does not entirely blunt the processes by which fentanyl suppresses inspiratory drive (Vt/Ti) it does have more pronounced effects on the processes by which fentanyl suppresses PIF.
Another compelling finding was that GSHee markedly reduced the occurrence of non-eupneic breaths (usually expressed as apneas, type 1 and 2 sighs) despite actually increasing fr, which usually is a stimulus to enhance non-eupneic breathing87. This would tentatively suggest that GSHee exerts positive effects in brain regions such as the Kölliker-Fuse and pre-Bötzinger complex, which generate/control rhythmic breathing121–132, although to our knowledge there is no reports as to the direct effects of GSH in these neuronal complexes. With respect to the rejection index, it must be noted that the summation of sighs and apneas under non-eupneic breathing under the rejection index is obviously simplistic and does not do justice to the different mechanisms that elicit these individual respiratory patterns. Sighs are reported to result from activation of a neuronal circuit in the preBötzinger complex/parafacial group133 while apnea results from direct inhibition of the preBötzinger complex or inhibition of drive to phase-switching neurons in the preBötzinger complex134. An increase in respiratory drive elicited by GSGee may decrease the number of sighs and decrease the number of apneas, but that would not be defined definitively by a change in the rejection index since this index also includes sniffs and changes in respiratory waveforms due to behavioral movements87, although it must be noted that the fentanyl-treated rats (those that received vehicle or GSHee) were basically immobile throughout the ventilatory study. The ventilatory effects of GSHee resulted in predictable (minor) changes in ABG chemistry including an increase in pH, a decrease in pCO2 and an increase in pO2. There was minimal change in A-a gradient suggesting that GSHee did not directly affect gas-exchange processes in the lungs.
The injection of fentanyl to vehicle (saline)-pretreated rats elicited a sustained reduction in fr that was associated with pronounced long-lasting increases in Ti and EIP (at least 60 min) and pronounced but shorter-lived increases in Te and EEP (about 15 min in duration). Fentanyl also elicited a marked and sustained (at least 60 min) decrease in Vt (coupled to the decrease in fr resulting in a marked decrease in Ve), inspiratory drive (Vt/Ti), PIF and PEF. Finally, fentanyl caused a marked increase in Rejection Index (elevated disordered breathing) for 10–15 min. The potential sites and mechanisms of action underlying these effects of fentanyl have been addressed in detail elsewhere11–15. Consistent with our previous study15, the injection of fentanyl was associated with minor increases in BT that would have minimal direct effects on breathing.
Our major findings were that the ventilatory depressant effects and negative changes in ABG chemistry and A-a gradient elicited by fentanyl were markedly attenuated in rats pretreated with GSHee. With respect to fr, it would be easy to conclude that fentanyl still exerts dramatic effects since fentanyl elicited a rather precipitous fall in fr even though the values did not fall below baseline values. GSHee had little effect on the increase in Ti elicited by fentanyl. However, GSHee had a dramatic impact on the duration of fentanyl-induced increase in Te. Indeed, the substantially smaller fentanyl-induced increase in Te in the presence of GSHee suggests that GSHee enhances the central processes driving the end of expiration (inspiratory on-switch) and thus decrease expiratory duration. In addition, the thiolester may directly combat the ability of fentanyl to suppress the central processes driving expiration under fentanyl-induced hypoxic/hypercapnic conditions and/or the direct inhibitory effects of fentanyl on expiratory muscle activity11–15, although it should be noted that there is evidence that opioids can actually increase expiratory muscle activity under certain circumstances135. The effects of GSHee on the ability of fentanyl to lower PIF and PEF may also provide some discrimination as to potential sites and mechanisms of action of the thiolester. Remembering that GSHee elicited sustained increases in PIF and PEF, it was evident that fentanyl was able to elicit strong reductions in these parameters. The difference was that PIF fell to levels approximately equivalent to baseline values (before any drug was given) whereas the fentanyl-induced reduction in PEF was short-lived and PEF rapidly returned to the elevated levels seen after injection of GSHee. As such, it is possible that GSH-dependent processes may be more influential on central and peripheral mechanisms driving expiratory chest-muscles than inspiratory chest-muscles.
Opioid analgesics (e.g., fentanyl, morphine, etorphine, buprenorphine, methadone, butorphanol, oxymorphone) have been demonstrated to increase A-a gradient in a variety of species including humans136–138, goats139, rabbits140, dogs141, impala142, and rats15,94,95. As discussed by Meyer et al.139, and Henderson et al.15, opioids increase A-a gradients by impairing ventilation-perfusion ratios in the lungs (ventilation-perfusion mismatch). For example, opioids decrease pulmonary perfusion via hypoxia-induced pulmonary vasoconstriction143, and by direct pulmonary vasoconstriction144,145 by mechanisms including centrally-mediated activation of sympathetic nerve activity to the lungs146, and induction of histamine release in the lungs144,147. As such, the ability of GSHee to overcome the fentanyl-induced increase in A-a gradient is most likely due to the direct GSHee-induced increase in Ve and potentially by interfering with any of the other mechanisms described immediately above.
The possibility that GSHee directly blunted the mechanisms by which fentanyl depressed breathing is supported by the evidence that GSHee did not affect baseline Vt values but markedly blunted the magnitude and duration of fentanyl-induced reduction fentanyl-induced reduction in Vt. This effect on Vt suggests that GSHee or its functional metabolites/nitrosylated species block central and peripheral signaling pathways by which fentanyl suppresses Vt15. This key effect of GSHee is connected to the findings that GSHee reduced the ability of fentanyl to lower pH, pO2 and sO2 while elevating pCO2 (all consistent with a fall in Ve) and blunted the fentanyl-induced increase A-a-gradient (i.e., impaired gas-exchange in the lungs). The ability GSHee to suppress fentanyl-induced disordered (non-eupneic) breathing raises the possibility that GSH-dependent mechanisms in key brainstem sites responsible for the control of breathing may be recruited to prevent the negative effects of fentanyl on eupneic breathing. An important consideration with respect to the ability of GSHee to prevent the deleterious effects of fentanyl on breathing was that the opioid appeared to elicit its full sedative effects in the presence of the GSHee (e.g., righting-reflex times and general observations of behavior could not discriminate between the effects of fentanyl in vehicle-treated or GSHee-treated rats). It could be expected that abrupt arousal may have led to enhanced breathing but as this did not appear to happen it is likely that GSHee interacted directly with the neural pathways/cellular mechanisms responsible for fentanyl-induced OIRD.
In summary, GSHee and L-CYSee21 may represent the first examples of a novel class of thiolester compounds that are able to effectively prevent the ventilatory depressant effects of powerful opioids such as fentanyl (and perhaps other high potency opioids such as sufentanil and alfentanil) and their deleterious effects on breathing stability and gas-exchange within the lungs without compromising the analgesic actions of the opioids. The obvious caveat is that we have only tested GSHee against a 75 mg/kg dose of fentanyl, that while eliciting a substantial degree of ventilatory depression, it does not approach the lethality of higher doses of this opioid. The ability of GSHee to prevent the negative effects of fentanyl on Vt is a key factor in the therapeutic potential of this thiolester in the treatment of OIRD. However, the vital importance of the ability of GSHee to overcome the negative effects of fentanyl on gas-exchange in the lungs cannot be over-emphasized. The administration protocol used in this study highlights the ability of GSHee to affect ventilatory parameters and to blunt the negative effects of fentanyl on breathing. It would certainly be preferable that GSHee be delivered in a dose form that would not elicit profound responses prior to administration of an opioid and we are establishing whether lower doses or a constant infusion can achieve blockade of fentanyl-induced OIRD without eliciting dramatic effects on ventilation prior to administration of the opioid. For reasons that are obvious in terms of clinical use and the current opioid crisis, we are also establishing whether GSHee can elicit a prompt and sustained reversal of the negative effects of opioids without eliciting unwanted side-effects such as hyperventilation.
Pretreatment with GSHee blunted the effects of the subsequent injection of fentanyl in a manner that suggest that the actions are heavily dependent on the actions of fentanyl itself on some parameters but not others. More specifically, pretreatment with GSHee elicited a sustained attenuation of the long-lasting negative effects of fentanyl on Vt, Vt/Ti and PIF whereas it had relatively minimal effects on the long-lasting effects of fentanyl on Ti and EIP. In addition, pretreatment with GSHee elicited a pronounced attenuation of the relatively short-lived effects of fentanyl on fr and Te, EEP and Rejection Index. As such, it is evident the GSHee reaches the systems/circuitry that are involved in some of the actions of fentanyl but not others. A search of the literature has not yielded any information as to whether GSH/GSHee may be a ventilatory stimulant in humans and/or whether they blunt the ventilatory depressant effects of opioids. As such, the key question remains as to whether the efficacy of GSHee in rats will be translated into humans.
GSHee could be an addition to the important strategy to mitigate OIRD via co-treatment with non-OR ventilatory stimulants that do not affect opioid-induced analgesia. Several classes of non-OR ventilatory stimulants are currently being investigated with many acting in the brainstem respiratory network including D1-dopamine receptor agonists, 5-hydroxytryptamine receptor modulators, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor agonists (ampakines), thyrotropin-releasing hormone, the endogenous peptide glycyl-glutamine, and phospodiesterase-4 inhibitors. Others include doxapram and GAL021 that act on K+-channels on O2-sensing cells of the carotid bodies. In their definitive review, Dahan et al.148 critically appraised the efficacy of these ventilatory stimulants and concluded that “none of the experimental drugs are adequate for therapeutic use in OIRD and all need further study of efficacy and toxicity. Nonetheless, Dahan et al.148 did point out that all of these drugs “highlight potential mechanisms of action and possible templates for further study and development”. We believe that GSHee could be added to this exciting list.
Supplementary Information
Acknowledgements
The authors wish to thank the staff at the Animal Care Facilities at Galleon Pharmaceuticals, Inc. and the University of Virginia. The authors also wish to acknowledge Ms. Chelsea Csuhran and Ms. Yvonnda West for their important contribution to data collection and data analyses.
Author contributions
M.W.J., F.K., J.N.B. and S.J.L. designed and conceived all of the experiments. S.M.B., J.M.S., W.J.M. and A.P.Y. performed the studies. F.K., F.C. and S.J.L. collated and analyzed the data. M.W.J., J.N.B., J.M.S. and S.J.L. wrote the manuscript. All authors have approved the final version of the manuscript and agree to account for all aspects of the work. All persons designated as authors qualify for authorship, and all of those qualify for authorship are listed.
Funding
This work was supported by grants to S.J.L from Galleon Pharmaceuticals, Inc.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-86458-x.
References
- 1.Nelson L, Schwaner R. Transdermal fentanyl: pharmacology and toxicology. J. Med. Toxicol. 2009;5:230–241. doi: 10.1007/BF03178274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston KD. The potential for mu-opioid receptor agonists to be anti-emetic in humans: a review of clinical data. Acta Anaesthesiol. Scand. 2010;54:132–140. doi: 10.1111/j.1399-6576.2009.02115.x. [DOI] [PubMed] [Google Scholar]
- 3.Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician. 2008;11(2 Suppl):S133–S153. [PubMed] [Google Scholar]
- 4.Hajiha M, DuBord MA, Liu H, Horner RL. Opioid receptor mechanisms at the hypoglossal motor pool and effects on tongue muscle activity in vivo. J. Physiol. 2009;587:2677–2692. doi: 10.1113/jphysiol.2009.171678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol. Pharmacol. 1994;45:330–334. [PubMed] [Google Scholar]
- 6.Huang P, Kehner GB, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist. J. Pharmacol. Exp. Ther. 2001;297:688–695. [PubMed] [Google Scholar]
- 7.Yeadon M, Kitchen I. Multiple opioid receptors mediate the respiratory depressant effects of fentanyl-like drugs in the rat. Gen. Pharmacol. 1990;21:655–664. doi: 10.1016/0306-3623(90)91013-H. [DOI] [PubMed] [Google Scholar]
- 8.Zhu J, Xue JC, Law PY, Claude PA, Luo LY, Yin J, Chen C, Liu-Chen LY. The region in the mu opioid receptor conferring selectivity for sufentanil over the delta receptor is different from that over the kappa receptor. FEBS Lett. 1996;384:198–202. doi: 10.1016/0014-5793(96)00312-2. [DOI] [PubMed] [Google Scholar]
- 9.Butelman ER, Ball JW, Kreek MJ. Comparison of the discriminative and neuroendocrine effects of centrally penetrating kappa-opioid agonists in rhesus monkeys. Psychopharmacology. 2002;164:115–120. doi: 10.1007/s00213-002-1195-y. [DOI] [PubMed] [Google Scholar]
- 10.Gharagozlou P, Hashemi E, DeLorey TM, Clark JD, Lameh J. Pharmacological profiles of opioid ligands at kappa opioid receptors. BMC Pharmacol. 2006;6:3. doi: 10.1186/1471-2210-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahan A, Sarton E, Teppema L, Olievier C. Sex-related differences in the influence of morphine on ventilatory control in humans. Anesthesiology. 1988;88:903–913. doi: 10.1097/00000542-199804000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Dahan A, Aarts L, Smith TW. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology. 2010;112:226–238. doi: 10.1097/ALN.0b013e3181c38c25. [DOI] [PubMed] [Google Scholar]
- 13.Sarton E, Teppema L, Dahan A. Sex differences in morphine-induced ventilatory depression reside within the peripheral chemoreflex loop. Anesthesiology. 1999;90:1329–1338. doi: 10.1097/00000542-199905000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Stein C, Clark JD, Oh U, Vasko MR, Wilcox GL, Overland AC, Vanderah TW, Spencer RH. Peripheral mechanisms of pain and analgesia. Brain Res. Rev. 2009;60:90–113. doi: 10.1016/j.brainresrev.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson F, May WJ, Gruber RB, Discala JF, Puskovic V, Young AP, Baby SM, Lewis SJ. Role of central and peripheral opiate receptors in the effects of fentanyl on analgesia, ventilation and arterial blood-gas chemistry in conscious rats. Respir. Physiol. Neurobiol. 2014;191:95–105. doi: 10.1016/j.resp.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein PD, Goldhaber SZ, Henry JW. Alveolar-arterial oxygen gradient in the assessment of acute pulmonary embolism. Chest. 1995;107:139–143. doi: 10.1378/chest.107.1.139. [DOI] [PubMed] [Google Scholar]
- 17.Lewanowitsch T, Irvine RJ. Naloxone methiodide reverses opioid-induced respiratory depression and analgesia without withdrawal. Eur. J. Pharmacol. 2002;445:61–67. doi: 10.1016/S0014-2999(02)01715-6. [DOI] [PubMed] [Google Scholar]
- 18.Lewanowitsch T, Irvine RJ. Naloxone and its quaternary derivative, naloxone methiodide, have differing affinities for mu, delta, and kappa opioid receptors in mouse brain homogenates. Brain Res. 2003;964:302–305. doi: 10.1016/S0006-8993(02)04117-3. [DOI] [PubMed] [Google Scholar]
- 19.Lewanowitsch T, Miller JH, Irvine RJ. Reversal of morphine, methadone and heroin induced effects in mice by naloxone methiodide. Life Sci. 2006;78:682–688. doi: 10.1016/j.lfs.2005.05.062. [DOI] [PubMed] [Google Scholar]
- 20.Campbell C, Weinger MB, Quinn M. Alterations in diaphragm EMG activity during opiate-induced respiratory depression. Respir. Physiol. 1995;100:107–117. doi: 10.1016/0034-5687(94)00119-K. [DOI] [PubMed] [Google Scholar]
- 21.Mendoza J, Passafaro R, Baby S, Young AP, Bates JN, Gaston B, Lewis SJ. L-Cysteine ethyl ester reverses the deleterious effects of morphine on, arterial blood-gas chemistry in tracheotomized rats. Respir. Physiol. Neurobiol. 2013;189:136–143. doi: 10.1016/j.resp.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukui K, Kaneda M, Takahashi E, Washio M, Doi K. Protective effects of sulfhydryl compounds on HOCl-induced intracellular Ca2+ increase in single rat ventricular myocytes. J. Mol. Cell Cardiol. 1994;26:455–461. doi: 10.1006/jmcc.1994.1056. [DOI] [PubMed] [Google Scholar]
- 23.Clancy R, Cederbaum AI, Stoyanovsky DA. Preparation and properties of S-nitroso-L-cysteine ethyl ester, an intracellular nitrosating agent. J. Med. Chem. 2001;44:2035–2038. doi: 10.1021/jm000463f. [DOI] [PubMed] [Google Scholar]
- 24.Servin AL, Goulinet S, Renault H. Pharmacokinetics of cysteine ethyl ester in rat. Xenobiotica. 1988;18:839–847. doi: 10.3109/00498258809041722. [DOI] [PubMed] [Google Scholar]
- 25.Hobbs MJ, Butterworth M, Cohen GM, Upshall DG. Structure-activity relationships of cysteine esters and their effects on thiol levels in rat lung in vitro. Biochem. Pharmacol. 1993;45:1605–1612. doi: 10.1016/0006-2952(93)90301-C. [DOI] [PubMed] [Google Scholar]
- 26.Deneke SM. Thiol-based antioxidants. Curr. Top. Cell Regul. 2000;36:151–180. doi: 10.1016/S0070-2137(01)80007-8. [DOI] [PubMed] [Google Scholar]
- 27.Butterworth M, Upshall DG, Cohen GM. A novel role for carboxylesterase in the elevation of cellular cysteine by esters of cysteine. Biochem. Pharmacol. 1993;46:1131–1137. doi: 10.1016/0006-2952(93)90460-E. [DOI] [PubMed] [Google Scholar]
- 28.Métayer S, Seiliez I, Collin A, Duchêne S, Mercier Y, Geraert PA, Tesseraud S. Mechanisms through which sulfur amino acids control protein metabolism and oxidative status. J. Nutr. Biochem. 2008;19:207–215. doi: 10.1016/j.jnutbio.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 31.Kimura H. Hydrogen sulfide: from brain to gut. Antioxid. Redox Signal. 2010;12:1111–1123. doi: 10.1089/ars.2009.2919. [DOI] [PubMed] [Google Scholar]
- 32.Hill BG, Bhatnagar A. Role of glutathiolation in preservation, restoration and regulation of protein function. IUBMB Life. 2007;59:21–26. doi: 10.1080/15216540701196944. [DOI] [PubMed] [Google Scholar]
- 33.Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. H2S mediates O2 sensing in the carotid body. Proc. Natl. Acad. Sci. USA. 2010;107:10719–10724. doi: 10.1073/pnas.1005866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gow AJ, Buerk DG, Ischiropoulos H. A novel reaction mechanism for the formation of S-nitrosothiol in vivo. J. Biol. Chem. 1991;272:2841–2845. doi: 10.1074/jbc.272.5.2841. [DOI] [PubMed] [Google Scholar]
- 35.Kharitonov VG, Sundquist AR, Sharma VS. Kinetics of nitrosation of thiols by nitric oxide in the presence of oxygen. J. Biol. Chem. 1995;270:28158–28164. doi: 10.1074/jbc.270.47.28158. [DOI] [PubMed] [Google Scholar]
- 36.Keszler A, Zhang Y, Hogg N. Reaction between nitric oxide, glutathione, and oxygen in the presence and absence of protein: how are S-nitrosothiols formed? Free Radic. Biol. Med. 2010;48:55–64. doi: 10.1016/j.freeradbiomed.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu TM, Ho SC. Similarity and dissimilarity of thiols as anti-nitrosative agents in the nitric oxide-superoxide system. Biochem. Biophys. Res. Commun. 2011;404:785–789. doi: 10.1016/j.bbrc.2010.12.059. [DOI] [PubMed] [Google Scholar]
- 38.Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 39.Foster MW, McMahon TJ, Stamler JS. S-nitrosylation in health and disease. Trends Mol. Med. 2003;9:160–168. doi: 10.1016/S1471-4914(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 40.Lipton AJ, Johnson MA, Macdonald T, Lieberman MW, Gozal D, Gaston B. S-nitrosothiols signal the ventilatory response to hypoxia. Nature. 2001;413:171–174. doi: 10.1038/35093117. [DOI] [PubMed] [Google Scholar]
- 41.Gaston B, Drazen JM, Loscalzo J, Stamler JS. The biology of nitrogen oxides in the airways. Am. J. Respir. Crit. Care Med. 1994;149:538–551. doi: 10.1164/ajrccm.149.2.7508323. [DOI] [PubMed] [Google Scholar]
- 42.Gaston B, Singel D, Doctor A, Stamler JS. S-nitrosothiol signaling in respiratory biology. Am. J. Respir. Crit. Care Med. 2006;173:1186–1193. doi: 10.1164/rccm.200510-1584PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoyanovsky D, Murphy T, Anno PR, Kim YM, Salama G. Nitric oxide activates skeletal and cardiac ryanodine receptors. Cell Calcium. 1997;21:19–29. doi: 10.1016/S0143-4160(97)90093-2. [DOI] [PubMed] [Google Scholar]
- 44.Polanco MJ, Alguacil LF, Albella B, Segovia JC, González-Martín C. Yohimbine prevents the effect of morphine on the redox status of neuroblastomaxglioma NG108-15 cells. Toxicol. Lett. 2009;189:115–120. doi: 10.1016/j.toxlet.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 45.Macchia I, Palamara AT, Bué C, Savini P, Ciriolo M, Gaziano R, di Francesco P. Increased replication of Sendai virus in morphine-treated epithelial cells: evidence for the involvement of the intracellular levels of glutathione. Int. J. Immunopharmacol. 1999;21:185–193. doi: 10.1016/S0192-0561(98)00080-0. [DOI] [PubMed] [Google Scholar]
- 46.Osburn S, Steill JD, Oomens J, O'Hair RA, van Stipdonk M, Ryzhov V. Structure and reactivity of the cysteine methyl ester radical cation. Chemistry. 2011;17:873–879. doi: 10.1002/chem.201002042. [DOI] [PubMed] [Google Scholar]
- 47.Cox BM, Leslie FM, Dunlap CE. The use of ascorbate as a probe of opioid receptor structure: evidence for two independent mechanisms of receptor destruction by ascorbate. J. Recept. Res. 1980;1:329–354. doi: 10.3109/10799898009044104. [DOI] [PubMed] [Google Scholar]
- 48.Laragione T, Gianazza E, Tonelli R, Bigini P, Mennini T, Casoni F, Massignan T, Bonetto V, Ghezzi P. Regulation of redox-sensitive exofacial protein thiols in CHO cells. Biol. Chem. 2006;387:1371–1376. doi: 10.1515/BC.2006.172. [DOI] [PubMed] [Google Scholar]
- 49.Anderson ME, Powrie F, Puri RN, Meister A. Glutathione monoethyl ester: preparation, uptake by tissues, and conversion to glutathione. Arch. Biochem. Biophys. 1985;239:538–548. doi: 10.1016/0003-9861(85)90723-4. [DOI] [PubMed] [Google Scholar]
- 50.Grattagliano I, Wieland P, Schranz C, Lauterburg BH. Effect of oral glutathione monoethyl ester and glutathione on circulating and hepatic sulfhydrils in the rat. Pharmacol. Toxicol. 1994;75:343–347. doi: 10.1111/j.1600-0773.1994.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 51.Grattagliano I, Wieland P, Schranz C, Lauterburg BH. Disposition of glutathione monoethyl ester in the rat: glutathione ester is a slow release form of extracellular glutathione. J. Pharmacol. Exp. Ther. 1995;272:484–488. [PubMed] [Google Scholar]
- 52.Anderson ME, Underwood M, Bridges RJ, Meister A. Glutathione metabolism at the blood-cerebrospinal fluid barrier. FASEB J. 1989;3:2527–2531. doi: 10.1096/fasebj.3.13.2572501. [DOI] [PubMed] [Google Scholar]
- 53.Zeevalk GD, Manzino L, Sonsalla PK, Bernard LP. Characterization of intracellular elevation of glutathione (GSH) with glutathione monoethyl ester and GSH in brain and neuronal cultures: relevance to Parkinson's disease. Exp. Neurol. 2007;203:512–520. doi: 10.1016/j.expneurol.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robinson MK, Ahn MS, Rounds JD, Cook JA, Jacobs DO, Wilmore DW. Parenteral glutathione monoester enhances tissue antioxidant stores. J. Parenter. Enteral. Nutr. 1992;16:413–418. doi: 10.1177/0148607192016005413. [DOI] [PubMed] [Google Scholar]
- 55.Leeuwenburgh C, Ji LL. Glutathone and glutathione ethyl ester supplementation of mice alter glutathione homeostasis during exercise. J. Nutr. 1998;128:2420–2426. doi: 10.1093/jn/128.12.2420. [DOI] [PubMed] [Google Scholar]
- 56.Neuschwander-Tetri BA, Ferrell LD, Sukhabote RJ, Grendell JH. Glutathione monoethyl ester ameliorates caerulein-induced pancreatitis in the mouse. J. Clin. Invest. 1993;89:109–116. doi: 10.1172/JCI115550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arai T, Takeyama N, Tanaka T. Glutathione monoethyl ester and inhibition of the oxyhemoglobin-induced increase in cytosolic calcium in cultured smooth-muscle cells. J. Neurosurg. 1999;90:527–532. doi: 10.3171/jns.1999.90.3.0527. [DOI] [PubMed] [Google Scholar]
- 58.Anderson MF, Nilsson M, Sims NR. Glutathione monoethylester prevents mitochondrial glutathione depletion during focal cerebral ischemia. Neurochem. Int. 2004;44:153–159. doi: 10.1016/S0197-0186(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 59.Anderson M, Nilssona M, Erikssona P, Sims N. Glutathione monoethyl ester provides neuroprotection in a rat model of stroke. Neurosci. Lett. 2004;354:163–165. doi: 10.1016/j.neulet.2003.09.067. [DOI] [PubMed] [Google Scholar]
- 60.Ceccon M, Giusti P, Facci L, Borin G, Imbesi M, Floreani M, Skaper SD. Intracellular glutathione levels determine cerebellar granule neuron sensitivity to excitotoxic injury by kainic acid. Brain Res. 2000;862:83–89. doi: 10.1016/S0006-8993(00)02074-6. [DOI] [PubMed] [Google Scholar]
- 61.Singhal RK, Jain A. Glutathione ethyl ester supplementation prevents mortality in newborn rats exposed to hyperoxia. Biol. Neonate. 2000;77:261–266. doi: 10.1159/000014225. [DOI] [PubMed] [Google Scholar]
- 62.Rush T, Liu X, Nowakowski AB, Petering DH, Lobner D. Glutathione-mediated neuroprotection against methylmercury neurotoxicity in cortical culture is dependent on MRP1. Neurotoxicology. 2012;33:476–481. doi: 10.1016/j.neuro.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Netzahualcoyotzi C, Tapia R. Degeneration of spinal motor neurons by chronic AMPA-induced excitotoxicity in vivo and protection by energy substrates. Acta Neuropathol. Commun. 2015;3:27. doi: 10.1186/s40478-015-0205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kahl A, Stepanova A, Konrad C, Anderson C, Manfredi G, Zhou P, Iadecola C, Galkin A. Critical role of flavin and glutathione in complex I-mediated bioenergetic failure in brain ischemia/reperfusion injury. Stroke. 2018;49:1223–1231. doi: 10.1161/STROKEAHA.117.019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morris PE, Wheeler AP, Meyrick BO, Bernard GR. Escherichia coli endotoxin-mediated endothelial injury is modulated by glutathione ethyl ester. J. Infect. Dis. 1995;172:1119–1122. doi: 10.1093/infdis/172.4.1119. [DOI] [PubMed] [Google Scholar]
- 66.Uedono Y, Takeyama N, Yamagami K, Tanaka T. Lipopolysaccharide-mediated hepatic glutathione depletion and progressive mitochondrial damage in mice: protective effect of glutathione monoethyl ester. J. Surg. Res. 1997;70:49–54. doi: 10.1006/jsre.1997.5068. [DOI] [PubMed] [Google Scholar]
- 67.Aggarwal S, Dimitropoulou C, Lu Q, Black SM, Sharma S. Glutathione supplementation attenuates lipopolysaccharide-induced mitochondrial dysfunction and apoptosis in a mouse model of acute lung injury. Front. Physiol. 2012;3:161. doi: 10.3389/fphys.2012.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grattagliano I, Vendemiale G, Lauterburg BH. Reperfusion injury of the liver: role of mitochondria and protection by glutathione ester. J. Surg. Res. 1999;86:2–8. doi: 10.1006/jsre.1999.5620. [DOI] [PubMed] [Google Scholar]
- 69.Guarnieri C, Turinetto B, Colì G, Muscari C, Cattabriga I, Vaona I, Finelli C, Pigini F, Caldarera CM. Effect of glutathione monoethyl ester on glutathione level and cardiac energetics in reperfused pig heart. Res. Commun. Chem. Pathol. Pharmacol. 1993;81:33–44. [PubMed] [Google Scholar]
- 70.Guízar-Sahagún G, Ibarra A, Espitia A, Martínez A, Madrazo I, Franco-Bourland RE. Glutathione monoethyl ester improves functional recovery, enhances neuron survival, and stabilizes spinal cord blood flow after spinal cord injury in rats. Neuroscience. 2005;130:639–649. doi: 10.1016/j.neuroscience.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 71.del RayoGarrido M, Silva-García R, García E, Martiñón S, Morales M, Mestre H, Flores-Domínguez C, Flores A, Ibarra A. Therapeutic window for combination therapy of A91 peptide and glutathione allows delayed treatment after spinal cord injury. Basic Clin. Pharmacol. Toxicol. 2013;112:314–318. doi: 10.1111/bcpt.12023. [DOI] [PubMed] [Google Scholar]
- 72.Guarino MP, Macedo MP. Co-administration of glutathione and nitric oxide enhances insulin sensitivity in Wistar rats. Br. J. Pharmacol. 2006;147:959–965. doi: 10.1038/sj.bjp.0706691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kloek J, van Ark I, de Clerck F, Bloksma N, Nijkamp FP, Folkerts G. Modulation of airway hyperresponsiveness by thiols in a murine in vivo model of allergic asthma. Inflam. Res. 2003;52:126–131. doi: 10.1007/s000110300025. [DOI] [PubMed] [Google Scholar]
- 74.Fitzpatrick AM, Jones DP, Brown LA. Glutathione redox control of asthma: from molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012;17:375–408. doi: 10.1089/ars.2011.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oestreicher J, Morgan B. Glutathione: subcellular distribution and membrane transport1. Biochem. Cell Biol. 2019;97:270–289. doi: 10.1139/bcb-2018-0189. [DOI] [PubMed] [Google Scholar]
- 77.Sies H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999;27:916–921. doi: 10.1016/S0891-5849(99)00177-X. [DOI] [PubMed] [Google Scholar]
- 78.Lewis SJ, Meller ST, Brody MJ, Gebhart GF. Reduced nociceptive effects of i.v. 5-HT in the SHR. Clin. Exp. Hypertens. 1991;A13:849–857. doi: 10.3109/10641969109042089. [DOI] [PubMed] [Google Scholar]
- 79.Meller ST, Lewis SJ, Brody MJ, Gebhart GF. The peripheral nociceptive actions of intravenously administered 5-HT in the rat requires dual activation of both 5-HT2 and 5-HT3 receptor subtypes. Brain Res. 1991;561:61–68. doi: 10.1016/0006-8993(91)90749-L. [DOI] [PubMed] [Google Scholar]
- 80.Torda TA. Alveolar-arterial oxygen tension difference: a critical look. Anaesth. Intensive Care. 1981;9:326–330. doi: 10.1177/0310057X8100900403. [DOI] [PubMed] [Google Scholar]
- 81.Story DA. Alveolar oxygen partial pressure, alveolar carbon dioxide partial pressure, and the alveolar gas equation. Anesthesiology. 1996;84:1011. doi: 10.1097/00000542-199604000-00036. [DOI] [PubMed] [Google Scholar]
- 82.Joshi G, Hardas S, Sultana R, St Clair DK, Vore M, Butterfield DA. Glutathione elevation by γ-glutamyl cysteine ethyl ester as a potential therapeutic strategy for preventing oxidative stress in brain mediated by in vivo administration of adriamycin: implication for chemobrain. J. Neurosci. Res. 2007;85:497–503. doi: 10.1002/jnr.21158. [DOI] [PubMed] [Google Scholar]
- 83.Stengel A, Coskun T, Goebel M, Wang L, Craft L, Alsina-Fernandez J, Rivier J, Taché Y. Central injection of the stable somatostatin analog ODT8-SST induces a somatostatin2 receptor-mediated orexigenic effect: role of neuropeptide Y and opioid signaling pathways in rats. Endocrinology. 2010;151:4224–4235. doi: 10.1210/en.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chapman CD, Dono LM, French MC, Weinberg ZY, Schuette LM, Currie PJ. Paraventricular nucleus anandamide signaling alters eating and substrate oxidation. NeuroReport. 2012;23:425–429. doi: 10.1097/WNR.0b013e32835271d1. [DOI] [PubMed] [Google Scholar]
- 85.Mortola JP, Frappell PB. On the barometric method for measurements of ventilation, and its use in small animals. Can. J. Physiol. Pharmacol. 1998;76:937–944. doi: 10.1139/y99-001. [DOI] [PubMed] [Google Scholar]
- 86.Kregel KC, Kenney MJ, Massett MP, Morgan DA, Lewis SJ. Role of nitrosyl factors in the hemodynamic adjustments to heat stress in the rat. Am. J. Physiol. 1997;273:H1537–H1543. doi: 10.1152/ajpheart.1997.273.3.H1537. [DOI] [PubMed] [Google Scholar]
- 87.Getsy PM, Davis J, Coffee GA, May WJ, Palmer LA, Strohl KP, Lewis SJ. Enhanced non-eupneic breathing following hypoxic, hypercapnic or hypoxic-hypercapnic gas challenges in conscious mice. Respir. Physiol. Neurobiol. 2014;204:147–159. doi: 10.1016/j.resp.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kanbar R, Stornetta RL, Cash DR, Lewis SJ, Guyenet PG. Photostimulation of Phox2b medullary neurons activates cardiorespiratory function in conscious rats. Am. J. Respir. Crit. Care Med. 2010;182:1184–1194. doi: 10.1164/rccm.201001-0047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Palmer LA, May WJ, deRonde K, Brown-Steinke K, Lewis SJ. Hypoxia-induced ventilatory responses in conscious mice: gender differences in ventilatory roll-off and facilitation. Resp. Physiol. Neurobiol. 2013;185:497–505. doi: 10.1016/j.resp.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Palmer LA, May WJ, deRonde K, Brown-Steinke K, Gaston B, Bates JN, Lewis SJ. Ventilatory responses during and following exposure to a hypoxic challenge in conscious mice deficient or null in S-nitrosoglutathione reductase. Respir. Physiol. Neurobiol. 2013;185:571–581. doi: 10.1016/j.resp.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Palmer LA, deRonde K, Brown-Steinke K, Gunter S, Jyothikumar V, Forbes M, Lewis SJ. Hypoxia-induced changes in protein S-nitrosylation in female mouse brainstem. Am. J. Respir. Cell. Mol. Biol. 2015;52:37–45. doi: 10.1165/rcmb.2013-0359OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Young AP, Gruber RB, Discala JF, May WJ, Palmer LA, Lewis SJ. Co-activation of μ- and δ-opioid receptors elicits tolerance to morphine-induced ventilatory depression via generation of peroxynitrite. Respir. Physiol. Neurobiol. 2013;186:255–264. doi: 10.1016/j.resp.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Henderson F, May WJ, Gruber RB, Young AP, Palmer LA, Gaston B, Lewis SJ. Low-dose morphine elicits ventilatory excitant and depressant responses in conscious rats: Role of peripheral μ-opioid receptors. Open J. Mol. Integr. Physiol. 2013;3:111–124. doi: 10.4236/ojmip.2013.33017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.May WJ, Henderson F, Gruber RB, Discala JF, Young AP, Bates JN, Palmer LA, Lewis SJ. Morphine has latent deleterious effects on the ventilatory responses to a hypoxic-hypercapnic challenge. Open J. Mol. Integr. Physiol. 2013;3:134–145. doi: 10.4236/ojmip.2013.33019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.May WJ, Gruber RB, Discala JF, Puskovic V, Henderson F, Palmer LA, Lewis SJ. Morphine has latent deleterious effects on the ventilatory responses to a hypoxic challenge. Open J. Mol. Integr. Physiol. 2013;3:166–180. doi: 10.4236/ojmip.2013.34022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gaston B, May WJ, Sullivan S, Yemen S, Marozkina NV, Palmer LA, Bates JN, Lewis SJ. Essential role of hemoglobin beta-93-cysteine in post-hypoxia facilitation of breathing in conscious mice. J. Appl. Physiol. 2014;116:1290–1299. doi: 10.1152/japplphysiol.01050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baby SM, Gruber RB, Young AP, MacFarlane PM, Teppema LJ, Lewis SJ. Bilateral carotid sinus nerve transection exacerbates morphine-induced respiratory depression. Eur. J. Pharmacol. 2018;834:17–29. doi: 10.1016/j.ejphar.2018.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Epstein MA, Epstein RA. A theoretical analysis of the barometric method for measurement of tidal volume. Respir. Physiol. 1978;32:105–210. doi: 10.1016/0034-5687(78)90103-2. [DOI] [PubMed] [Google Scholar]
- 99.Epstein RA, Epstein MA, Haddad GG, Mellins RB. Practical implementation of the barometric method for measurement of tidal volume. J. Appl. Physiol. 1980;49:1107–1115. doi: 10.1152/jappl.1980.49.6.1107. [DOI] [PubMed] [Google Scholar]
- 100.Yu C, Yuan M, Yang H, Zhuang X, Li H. P-glycoprotein on blood-brain barrier plays a vital role in fentanyl brain exposure and respiratory toxicity in rats. Toxicol. Sci. 2018;164:353–362. doi: 10.1093/toxsci/kfy093. [DOI] [PubMed] [Google Scholar]
- 101.Ren J, Ding X, Greer JJ. Countering opioid-induced respiratory depression in male rats with nicotinic acetylcholine receptor partial agonists varenicline and ABT 594. Anesthesiology. 2020;132:1197–1211. doi: 10.1097/ALN.0000000000003128. [DOI] [PubMed] [Google Scholar]
- 102.Ren J, Ding X, Greer JJ. 5-HT1A receptor agonist Befiradol reduces fentanyl-induced respiratory depression, analgesia, and sedation in rats. Anesthesiology. 2015;122:424–434. doi: 10.1097/ALN.0000000000000490. [DOI] [PubMed] [Google Scholar]
- 103.Wallenstein S, Zucker CL, Fleiss JL. Some statistical methods useful in circulation research. Circ. Res. 1980;47:1–9. doi: 10.1161/01.RES.47.1.1. [DOI] [PubMed] [Google Scholar]
- 104.Winer BJ. Statistical principles of experimental design. McGraw-Hill Book Co; 1971. pp. 752–809. [Google Scholar]
- 105.Chinta SJ, Rajagopalan S, Butterfield DA, Andersen JK. In vitro and in vivo neuroprotection by γ-glutamylcysteine ethyl ester against MPTP: relevance to the role of glutathione in Parkinson's disease. Neurosci. Lett. 2006;402:137–141. doi: 10.1016/j.neulet.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 106.Reed TT, Owen J, Pierce WM, Sebastian A, Sullivan PG, Butterfield DA. Proteomic identification of nitrated brain proteins in traumatic brain-injured rats treated postinjury with γ-glutamylcysteine ethyl ester: insights into the role of elevation of glutathione as a potential therapeutic strategy for traumatic brain injury. J. Neurosci. Res. 2009;87:408–417. doi: 10.1002/jnr.21872. [DOI] [PubMed] [Google Scholar]
- 107.Yalcin A, Armagan G, Turunc E, Konyalioglu S, Kanit L. Potential neuroprotective effect of γ-glutamylcysteine ethyl ester on rat brain against kainic acid-induced excitotoxicity. Free Radic. Res. 2010;44:513–521. doi: 10.3109/10715761003645964. [DOI] [PubMed] [Google Scholar]
- 108.Henderson M, Rice B, Sebastian A, Sullivan PG, King C, Robinson RA, Reed TT. Neuroproteomic study of nitrated proteins in moderate traumatic brain injured rats treated with gamma glutamyl cysteine ethyl ester administration post injury: insight into the role of glutathione elevation in nitrosative stress. Proteomics Clin. Appl. 2016;10:1218–1224. doi: 10.1002/prca.201600004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roberts SM, Skoulis NP, James RC. A centrally-mediated effect of morphine to diminish hepatocellular glutathione. Biochem. Pharmacol. 1987;36:3001–3005. doi: 10.1016/0006-2952(87)90215-2. [DOI] [PubMed] [Google Scholar]
- 110.Skoulis NP, James RC, Harbison RD, Roberts SM. Perturbation of glutathione by a central action of morphine. Toxicology. 1989;57:287–302. doi: 10.1016/0300-483X(89)90117-0. [DOI] [PubMed] [Google Scholar]
- 111.Skoulis NP, James RC, Harbison RD, Roberts SM. Depression of hepatic glutathione by opioid analgesic drugs in mice. Toxicol. Appl. Pharmacol. 1989;99:139–147. doi: 10.1016/0041-008X(89)90119-1. [DOI] [PubMed] [Google Scholar]
- 112.Goudas LC, Carr DB, Maszczynska I, Marchand JE, Wurm WH, Greenblatt DJ, Kream RM. Differential effect of central versus parenteral administration of morphine sulfate on regional concentrations of reduced glutathione in rat brain. Pharmacology. 1997;54:92–97. doi: 10.1159/000139474. [DOI] [PubMed] [Google Scholar]
- 113.Lee J, Kim MS, Park C, Jung EB, Choi DH, Kim TY, Moon SK, Park R. Morphine prevents glutamate-induced death of primary rat neonatal astrocytes through modulation of intracellular redox. Immunopharmacol. Immunotoxicol. 2004;26:17–28. doi: 10.1081/IPH-120029941. [DOI] [PubMed] [Google Scholar]
- 114.Guzmán DC, Vázquez IE, Brizuela NO, Alvarez RG, Mejía GB, García EH, Santamaría D, de Apreza ML, Olguín HJ. Assessment of oxidative damage induced by acute doses of morphine sulfate in postnatal and adult rat brain. Neurochem. Res. 2006;31:549–554. doi: 10.1007/s11064-006-9053-7. [DOI] [PubMed] [Google Scholar]
- 115.Guzmán DC, Brizuela NO, Alvarez RG, García EH, Mejía GB, Olguín HJ. Cerebrolysin and morphine decrease glutathione and 5-hydroxyindole acetic acid levels in fasted rat brain. Biomed. Pharmacother. 2009;63:517–521. doi: 10.1016/j.biopha.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 116.Calderón-Guzmán D, Osnaya-Brizuela N, García-Alvarez R, Hernández-García E, Juárez-Olguín H. Oxidative stress induced by morphine in brain of rats fed with a protein deficient diet. Hum. Exp. Toxicol. 2009;28:577–582. doi: 10.1177/0960327109102798. [DOI] [PubMed] [Google Scholar]
- 117.Trivedi M, Shah J, Hodgson N, Byun HM, Deth R. Morphine induces redox-based changes in global DNA methylation and retrotransposon transcription by inhibition of excitatory amino acid transporter type 3-mediated cysteine uptake. Mol. Pharmacol. 2014;85:747–757. doi: 10.1124/mol.114.091728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Delogu G, Moretti S, Antonucci A, Marandola M, Tellan G, Sale P, Carnevali R, Famularo G. Apoptogenic effect of fentanyl on freshly isolated peripheral blood lymphocytes. J. Trauma. 2004;57:75–81. doi: 10.1097/01.TA.0000075349.66640.3E. [DOI] [PubMed] [Google Scholar]
- 119.Grim TW, Acevedo-Canabal A, Bohn LM. Toward directing opioid receptor signaling to refine opioid therapeutics. Biol. Psychiatry. 2020;87:15–21. doi: 10.1016/j.biopsych.2019.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grim TW, Schmid CL, Stahl EL, Pantouli F, Ho JH, Acevedo-Canabal A, Kennedy NM, Cameron MD, Bannister TD, Bohn LM. A G protein signaling-biased agonist at the μ-opioid receptor reverses morphine tolerance while preventing morphine withdrawal. Neuropsychopharmacology. 2020;45:416–425. doi: 10.1038/s41386-019-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ren J, Ding X, Funk GD, Greer JJ. Ampakine CX717 protects against fentanyl-induced respiratory depression and lethal apnea in rats. Anesthesiology. 2009;110:1364–1370. doi: 10.1097/ALN.0b013e31819faa2a. [DOI] [PubMed] [Google Scholar]
- 122.Varga AG, Reid BT, Kieffer BL, Levitt ES. Differential impact of two critical respiratory centres in opioid-induced respiratory depression in awake mice. J. Physiol. 2020;598:189–205. doi: 10.1113/JP278612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gonzalez C, Sanz-Alyayate G, Agapito MT, Obeso A. Effects of reducing agents on glutathione metabolism and the function of carotid body chemoreceptor cells. Biol. Chem. 2004;385:265–274. doi: 10.1515/BC.2004.021. [DOI] [PubMed] [Google Scholar]
- 124.Ohta H, Bates JN, Lewis SJ, Talman WT. Actions of S-nitrosocysteine in the nucleus tractus solitarii are unrelated to release of nitric oxide. Brain Res. 1997;746:98–104. doi: 10.1016/S0006-8993(96)01188-2. [DOI] [PubMed] [Google Scholar]
- 125.Lamb GD, Posterino GS. Effects of oxidation and reduction on contractile function in skeletal muscle fibres of the rat. J. Physiol. 2003;546:149–163. doi: 10.1113/jphysiol.2002.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Murphy RM, Dutka TL, Lamb GD. Hydroxyl radical and glutathione interactions alter calcium sensitivity and maximum force of the contractile apparatus in rat skeletal muscle fibres. J. Physiol. 2008;586:2203–2216. doi: 10.1113/jphysiol.2007.150516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mollica JP, Dutka TL, Merry TL, Lamboley CR, McConell GK, McKenna MJ, Murphy RM, Lamb GD. S-glutathionylation of troponin I (fast) increases contractile apparatus Ca2+ sensitivity in fast-twitch muscle fibres of rats and humans. J. Physiol. 2012;590:1443–1463. doi: 10.1113/jphysiol.2011.224535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Saunders SE, Levitt ES. Kolliker-Fuse/Parabrachial complex mu opioid receptors contribute to fentanyl-induced apnea and respiratory rate depression. Respir. Physiol. Neurobiol. 2020;275:103388. doi: 10.1016/j.resp.2020.103388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bachmutsky I, Wei XP, Kish E, Yackle K. Opioids depress breathing through two small brainstem sites. Elife. 2020;9:e52694. doi: 10.7554/eLife.52694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Smith JC, Abdala AP, Borgmann A, Rybak IA, Paton JF. Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci. 2013;36:152–162. doi: 10.1016/j.tins.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dhingra RR, Dutschmann M, Galán RF, Dick TE. Kölliker-Fuse nuclei regulate respiratory rhythm variability via a gain-control mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017;312:R172–R188. doi: 10.1152/ajpregu.00238.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang CF, Feldman JL. Efferent projections of excitatory and inhibitory preBötzinger complex neurons. J. Comp. Neurol. 2018;526:1389–1402. doi: 10.1002/cne.24415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Del Negro CA, Funk GD, Feldman JL. Breathing matters. Nat. Rev. Neurosci. 2018;19:351–367. doi: 10.1038/s41583-018-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Palkovic B, Marchenko V, Zuperku EJ, Stuth EAE, Stucke AG. Multi-level regulation of opioid-induced respiratory depression. Physiology (Bethesda) 2020;35:391–404. doi: 10.1152/physiol.00015.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Haouzi P, Mellen N, McCann M, Sternick M, Guck D, Tubbs N. Evidence for the emergence of an opioid-resistant respiratory rhythm following fentanyl overdose. Respir. Physiol. Neurobiol. 2020;277:103428. doi: 10.1016/j.resp.2020.103428. [DOI] [PubMed] [Google Scholar]
- 136.Teichtahl H, Wang D, Cunnington D, Kronborg I, Goodman C, Prodromidis A, Drummer O. Cardiorespiratory function in stable methadone maintenance treatment (MMT) patients. Addict. Biol. 2004;9:247–253. doi: 10.1080/13556210412331292578. [DOI] [PubMed] [Google Scholar]
- 137.Wang D, Teichtahl H, Drummer O, Goodman C, Cherry G, Cunnington D, Kronborg I. Central sleep apnea in stable methadone maintenance treatment patients. Chest. 2005;128:1348–1356. doi: 10.1378/chest.128.3.1348. [DOI] [PubMed] [Google Scholar]
- 138.Goetz AM, Rogers PL, Schlichtig R, Muder RR, Diven WF, Prior RB. Adult respiratory distress syndrome associated with epidural fentanyl infusion. Crit. Care Med. 1994;22:1579–1583. doi: 10.1097/00003246-199410000-00012. [DOI] [PubMed] [Google Scholar]
- 139.Meyer LC, Fuller A, Mitchell D. Zacopride and 8-OH-DPAT reverse opioid-induced respiratory depression and hypoxia but not catatonic immobilization in goats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R405–R413. doi: 10.1152/ajpregu.00440.2005. [DOI] [PubMed] [Google Scholar]
- 140.Shafford HL, Schadt JC. Respiratory and cardiovascular effects of buprenorphine in conscious rabbits. Vet. Anaesth. Analg. 2008;35:326–332. doi: 10.1111/j.1467-2995.2007.00383.x. [DOI] [PubMed] [Google Scholar]
- 141.Jacobson JD, McGrath CJ, Smith EP. Cardiorespiratory effects of induction and maintenance of anesthesia with ketamine-midazolam combination, with and without prior administration of butorphanol or oxymorphone. Am. J. Vet. Res. 1994;55:543–550. [PubMed] [Google Scholar]
- 142.Meyer LC, Hetem RS, Fick LG, Mitchell D, Fuller A. Effects of serotonin agonists and doxapram on respiratory depression and hypoxemia in etorphine-immobilized impala (Aepyceros melampus) J. Wildl. Dis. 2010;46:514–524. doi: 10.7589/0090-3558-46.2.514. [DOI] [PubMed] [Google Scholar]
- 143.Nunn JF. Nunn’s applied respiratory physiology. Butterworth Leinemann; 1993. [Google Scholar]
- 144.Hakim TS, Grunstein MM, Michel RP. Opiate action in the pulmonary circulation. Pulm. Pharmacol. 1992;5:159–165. doi: 10.1016/0952-0600(92)90036-G. [DOI] [PubMed] [Google Scholar]
- 145.Santiago TV, Edelman NH. Opioids and breathing. J. Appl. Physiol. 1985;59:1675–1685. doi: 10.1152/jappl.1985.59.6.1675. [DOI] [PubMed] [Google Scholar]
- 146.Roquebert J, Delgoulet C. Cardiovascular effects of etorphine in rats. J. Auton. Pharmacol. 1988;8:39–43. doi: 10.1111/j.1474-8673.1988.tb00167.x. [DOI] [PubMed] [Google Scholar]
- 147.Mather L. Opioid analgesic drugs. In: Nimmo W, Rowbotham D, Smith G, editors. Anaesthesia. 2. Blackwell Scientific; 1994. [Google Scholar]
- 148.Dahan A, van der Schrier R, Smith T, Aarts L, van Velzen M, Niesters M. Averting opioid-induced respiratory depression without affecting analgesia. Anesthesiology. 2018;128:1027–1037. doi: 10.1097/ALN.0000000000002184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.