Abstract
This case report demonstrates bilateral adrenal hemorrhage in a fifty-year old man with severe COVID-19 pneumonia. We discuss how adrenal hemorrhage can be one of the possible complications of COVID-19. The case also shows how adrenal hemorrhage can be diagnosed incidentally in a scan performed for a different reason given the difficulty of clinical diagnosis and the non-specific clinical presentation.
Keywords: COVID-19, Adrenal hemorrhage, Cardiopulmonary, Genitourinary, Diagnostic radiology
Introduction
Coronavirus Disease 2019 (COVID-19) is an infectious disease which was first identified in Wuhan, China, in December 2019. Since then, the virus has spread rapidly across the world. In March 2020, the rapid increase of the number of COVID-19 cases worldwide led the World Health Organization (WHO) to announce it as a global pandemic. The presentation of COVID-19 infection is variable and ranges widely from asymptomatic infection to life-threatening Acute Respiratory Distress Syndrome (ARDS). Although COVID-19 is well known as a respiratory infection, many extrapulmonary manifestations have also been reported as COVID-19 complications [1]. We report a case of bilateral adrenal hemorrhage in a previously well fifty-year old man with severe COVID-19 pneumonia.
Clinical presentation
A fifty-year old male presented to the Emergency department (ED) with a 2-week history of recurrent fevers and malaise with a 2-day history of worsening shortness of breath and cough. His past medical history includes hypertension and a right adrenal adenoma. On arrival he was hypoxic and febrile with pulse oximetry reading 85% on room air and temperature of 38.1. On examination, he had bilateral crepitations on chest auscultation. Cardiovascular and gastrointestinal clinical examinations were unremarkable (No hypotension or tachycardia).
A chest radiograph on admission demonstrated bilateral air space opacities in keeping with severe classic/probable COVID-19 as per British Society of Thoracic Imaging (BSTI) reporting guidance. The polymerase chain reaction (PCR) swab test performed on admission confirmed COVID-19 infection.
Admission blood tests showed hemoglobin (Hb) of 150 g/L, white cell count (WCC) of 6.0 × 109/L, platelet count of 154 × 109/L, serum sodium of 135 mmol/L, potassium of 3.9 mmol/L, and D-dimer of 10613. The initial working clinical diagnosis was COVID-19 infection with possible secondary pulmonary embolism, given the recent increase of shortness of breath, low oxygen saturation and highly elevated D-dimer. A Computed Tomography Pulmonary Angiogram (CTPA) was ordered, and a therapeutic dose of Enoxaparin (15,000 Units) was administered in ED. The patient was admitted to a COVID ward and started on supportive treatment and continued on a daily treatment dose of Enoxaparin.
On Day 1 of his admission, he developed vague bilateral flank discomfort. This was not severe or debilitating and his observations remained stable aside from persistent oxygen requirement.
A CTPA was performed on day 2 of his admission which showed no evidence of pulmonary embolism, but incidental adrenal lesions which were suspicious for adrenal hemorrhages (see Figs. 1 and 2 – CTPA images). A subsequent CT Abdomen and Pelvis with Contrast was performed which confirmed these findings with no evidence of active bleeding. (See Fig. 3 – CT AP images). The Enoxaparin was suspended and a specialist assessment with the Endocrinology team was requested. A random cortisol level following the CT scan was 734 nmol/L (normal range 185 – 624). He did not have any signs, symptoms or biochemical evidence of adrenal insufficiency, therefore corticosteroid medications were not started at this point. The general surgical team were also consulted who recommended starting regular IV Tranexamic Acid.
Fig. 1.
Axial CT scan image demonstrating multifocal areas of peripheral ground glass consolidation in keeping with classic/probable COVID-19 infection.
Fig. 2.
Axial CT image from CT Pulmonary Angiogram (CTPA). Hyperdense ovoid suprarenal lesions incidentally detected on the last slice of the CTPA stack with surrounding high attenuating collections and fat stranding. These features suggested the bilateral adrenal hematomas and subsequent abdominal imaging was recommended.
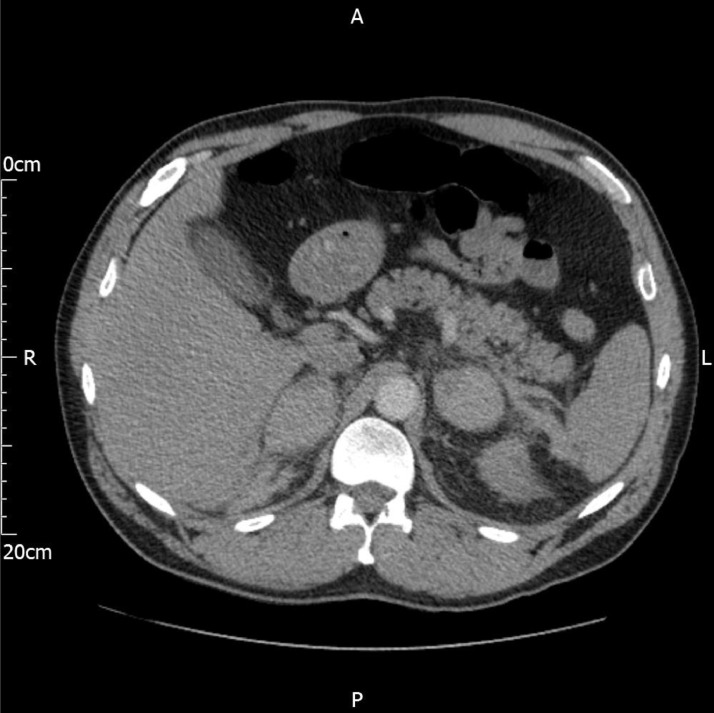
Fig. 3.
Multiphase coronal CT images from a CT Abdomen and Pelvis study. The scan phases include A) Non-contrast, B) Arterial phase, and C) Portal-Venous phase. Bilateral hyperdense ovoid suprarenal lesions with loss of the normal adrenal gland contour (small white arrow). Small left perirenal collection and fat stranding suggest associated retroperitoneal hemorrhage (thick white arrow). No enhancement of the lesions following contrast administration and no contrast extravasation on delayed phase images to suggest active bleeding. These features are classic of adrenal hematomas. Of note, contrast is also seen within the pelvicalyceal systems which is from the previous CTPA scan.
On day 3, the patient developed an episode of hypotension (blood pressure: 95/54) and tachycardia (heart rate: 122). He was subsequently started on IV Hydrocortisone 100mg Four times daily as per the advice from the endocrinology team. Repeat blood tests showed worsening inflammatory markers and thrombocytopenia with white cell count (WCC) of 13 × 109/L and platelets count of 88 × 109/L. He also developed a diffuse right leg swelling with unilateral pitting edema. An Ultrasound (US) Venous Doppler scan was requested; however, anticoagulation was not started at this point.
The US Doppler scan was performed on Day 4 which was positive for deep vein thrombosis (DVT) in the right leg and occlusion of the right common femoral vein. Of note, this was not present on the previous CT scan. Following the advice of the hematology team, the patient was started on a therapeutic dose of Enoxaparin divided into 2 doses per day (8,000 Units two times daily).
The patient suffered a cardiac arrest on Day 5 after becoming unresponsive. The initial rhythm was pulseless electrical activity (PEA) requiring 4 cycles of cardio-pulmonary resuscitation before there was restoration of spontaneous circulation. The patient was intubated and ventilated. Post resuscitation ECG confirmed the presence of a new right bundle branch block (RBBB) with a bedside Echocardiogram demonstrating a dilated right ventricle with interventricular septal bulging and severe tricuspid regurgitation suggesting acute severe right heart strain. This led to a clinical diagnosis of a massive pulmonary embolism. The patient was transferred to the Intensive Care Unit with thrombolysis started using an IV Alteplase infusion.
Unfortunately, the patient suffered another PEA cardiac arrest on the ICU. No return of circulation despite 7 cycles of CPR. At this point his pupils were noted to be fixed and dilated. It was decided that further CPR attempts would be futile and the patient was pronounced deceased. No autopsy was performed to confirm the cause of death; however, COVID-19 was the registered cause of death.
Discussion
Novel coronavirus (SARS-CoV-2) is a global pandemic, which is continuing its spread across the world. In late 2019, the first cases of COVID-19 were identified in Wuhan City, China. The clinical presentation of COVID-19 ranges widely from a mild flu-like illness to acute respiratory distress syndrome. A wide range of complications has also been associated with COVID-19 including, but not limited to, sepsis, thromboembolism, and multi-organ failure [2].
Non traumatic adrenal hemorrhage cases were first associated with cases of septicemia, also known as Waterhouse-Friderichsen syndrome [3]. Multiple organisms have been linked to this syndrome [4]. Additional cases related to severe viral infections have also been identified such as Cytomegalovirus and Parvovirus B12 viruses [5,6].
Diagnosis is established by CT or Magnetic Resonance Imaging (MRI). Acute hemorrhage is characterized by a non-enhancing mixed attenuation mass [7]. Initial investigations are typically tailored to look for any evidence of active bleeding which can manifest as contrast extravasation on a multiphase contrast enhanced CT scan [7]. MRI can be considered in cases where there is a contraindication to iodine-based contrast agents, or if the hematoma is suspected to be subacute or chronic [7,8].
Recently, a few adrenal hemorrhage cases have been reported in COVID-19 positive patients. Although the exact mechanism of adrenal hemorrhage in these cases is still unclear, it is thought that severe hyperinflammatory response and cytokine storm play an important role [9,10]. The cytokine storm is an unregulated severe inflammatory response to the viral infection. It results in high levels of acute-response cytokines (TNF and IL-1β), chemotactic cytokines (IL-8 and MCP-1). Other cytokines such as IL-6, granulocyte-colony stimulating factor (G-CSF), monocyte chemoattractant protein 1 (MCP1), macrophage inflammatory protein (MIP)1α, and tumor necrosis factor (TNF)-α have also found to be elevated. These high cytokines levels lead to endothelial dysfunction, vascular injury, and adrenal parenchymal damage and hemorrhage [11].
Furthermore, the vascular anatomy of the adrenals glands makes them more prone to turbulent flow or stasis. Although adrenal glands are supplied by a network of arterioles arising from different arteries, the venous drainage is limited to a single suprarenal vein [12]. In situations of high physiological stress such as sepsis, adrenocorticotrophic hormone (ACTH) secretion increases, which stimulates blood flow to the adrenal glands. This may exceed the limited venous drainage capacity of the adrenal gland leading to a predominant venous hemorrhage [13].
In the case described above, as well as the systemic inflammatory stress as a direct result of COVID-19 infection, the patient received therapeutic anticoagulation which likely contributed to the degree of hemorrhage. The clinical challenge in this case was managing the patient with a concurrent venous thrombosis.
In conclusion, spontaneous bilateral adrenal hemorrhage is rare, and potentially lethal. The physiological stress of severe COVID infection combined with therapeutic anticoagulation likely led to adrenal hemorrhage in this case. Understanding the need for urgent investigations and monitoring for clinical signs, symptoms and biochemical markers for adrenal insufficiency is paramount.
Raised suspicion and a multidisciplinary approach, for both resuscitation and definitive management, are essential for a successful outcome
Learning Points
-
1.
The CT findings in adrenal hemorrhage
-
2.
Adrenal hemorrhage can be diagnosed incidentally in a scan performed for another reason.
-
3.
Despite being rare, adrenal hemorrhage should be considered as a possible complication of COVID-19.
Patient consent statement
The patient unfortunately has passed away. We have tried to reach his guardians to get their consent but we could not. We have not included any personal details in the case report.
References
- 1.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. Epub 2020 Jul 10. PMID: 32651579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorbalenya Alexander E., Baker Susan C., Baric Ralph S., de Groot Raoul J, Drosten Christian, Anastasia A. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karki B.R., Sedhai Y.R., Bokhari S.R.A. Waterhouse-Friderichsen syndrome. [PubMed]
- 4.Fox B. Disseminated intravascular coagulation and the Waterhouse-Friderichsen syndrome. Arch Dis Child. 1971;46(249):680–685. doi: 10.1136/adc.46.249.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adem P.V., Montgomery C.P., Husain A.N., Koogler T.K., Arangelovich V., Humilier M. Staphylococcus aureus sepsis and the Waterhouse-Friderichsen syndrome in children. N Engl J Med. 2005;353(12):1245–1251. doi: 10.1056/NEJMoa044194. [DOI] [PubMed] [Google Scholar]
- 6.Karki B.R., Sedhai Y.R., Bokhari S.R.A. StatPearls [Internet] StatPearls Publishing; Treasure Island (FL): 2020. Waterhouse-Friderichsen syndrome. 2021 Jan–. PMID: 31855354. [PubMed] [Google Scholar]
- 7.Jordan E, 1, Poder L, Courtier J, Sai V, Jung A, Coakley FV. Imaging of nontraumatic adrenal hemorrhage. AJR Am J Roentgenol. 2012;199(1):W91–W98. doi: 10.2214/AJR.11.7973. [DOI] [PubMed] [Google Scholar]
- 8.Kawashima A., Sandler C.M., Ernst R.D., Takahashi N., Roubidoux M.A., Goldman S.M. Imaging of nontraumatic hemorrhage of the adrenal gland. Radiographics. 1999;19(4):949–963. doi: 10.1148/radiographics.19.4.g99jl13949. [DOI] [PubMed] [Google Scholar]
- 9.Bhaskar S., Sinha A., Banach M., Mittoo S., Weissert R., Kass J.S. Cytokine storm in COVID-19—immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM consortium position paper. Front Immunol. 2020;11(1648) doi: 10.3389/fimmu.2020.01648. art. no. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panigrahy D., Gilligan M.M., Huang S., Gartung A., Cortés-Puch I., Sime P.J. Inflammation resolution: a dual-pronged approach to averting cytokine storms in COVID-19? Cancer Metastasis Rev. 2020;39:337–340. doi: 10.1007/s10555-020-09889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guarner J., Paddock C.D., Bartlett J., Zaki S.R. Adrenal gland hemorrhage in patients with fatal bacterial infections. Mod Pathol. 2008;21(9):1113–1120. doi: 10.1038/modpathol.2008.98. [DOI] [PubMed] [Google Scholar]
- 12.Fox B. Venous infarction of the adrenal glands. J Pathol. 1976;119(2):65–89. doi: 10.1002/path.1711190202. [DOI] [PubMed] [Google Scholar]
- 13.Piccioli A., Chini G., Mannelli M., Serio M. Bilateral massive adrenal hemorrhage due to sepsis: report of two cases. J Endocrinol Invest. 1994;17(10):821–824. doi: 10.1007/BF03347786. [DOI] [PubMed] [Google Scholar]