Abstract
Objective
To explore the survival benefit of tofacitinib in addition to dexamethasone in hospitalized patients treated for coronavirus disease 2019 (COVID-19)–related pneumonia.
Patients and Methods
This is a single-center retrospective observational study. All patients who were hospitalized at Delta Regional Medical Center (a regional hospital in the Mississippi Delta) with a COVID-19 diagnosis and discharged between March 1 and September 30, 2020, are included. The primary outcome was in-hospital mortality in relation to receipt of tofacitinib alone or in addition to dexamethasone (designated as the tofacitinib group), versus dexamethasone alone (designated as the dexamethasone group).
Results
Of 269 eligible patients, 138 (51.3%) received tofacitinib uniformly and 131 (48.7%) patients received dexamethasone without tofacitinib. A total of 44 patients expired: 14 (31.8%) in the tofacitinib group and 30 (68.2%) in the dexamethasone group. The proportions of death among the tofacitinib and dexamethasone groups were, respectively, 10.1% and 22.9%. This represents a 70% reduction in odds of dying among the tofacitinib group compared to the dexamethasone group after adjusting for age and clinical parameters captured at hospitalization (adjusted odds ratio: 0.30; 95% CI: 0.12 to 0.76; P=.01).
Conclusion
The in-patient treatment of COVID-19 pneumonia has rapidly evolved. The addition of dexamethasone has made a relevant improvement on survival. Other immunomodulators have yet to show an impact. Here we present the potential survival benefit of the Janus kinase–signal transducer and activator of transcription inhibitor tofacitinib on COVID-19 pneumonia. We found that adding tofacitinib-based anti-inflammatory therapy to a treatment regimen including dexamethasone in COVID-19 pneumonia seems to have potential benefit of improving survival when compared to dexamethasone alone.
Abbreviations and Acronyms: AOR, adjusted odds ratio; COVID-19, coronavirus disease 2019; CT, computed tomography; CRP, C-reactive protein; DRMC, Delta Regional Medical Center; IL, interleukin; JAK, Janus kinase; OR, odds ratio; PCR, polymerase chain reaction; STAT, Signal transducer and activator of transcription
The severe acute respiratory syndrome coronavirus 2 pandemic continues its ravaging toll on human life. Starting in Wuhan, China, inpatient coronavirus disease 2019 (COVID-19) mortality was at 28%.1 As therapies improved, mortality rates have decreased to approximately 20%.2, 3, 4, 5 Identified groups at higher risk of death include the African American race6,7and those with comorbidities as obesity,8, 9, 10 diabetes, and renal insufficiency.11,12
In our state of Mississippi, hospital mortality for COVID-19 index admissions available until June 30, 2020, is at 18.5%.13 Per the Centers for Disease Control and Prevention COVID-19 tracker, Mississippi holds nationally the fourth positions in deaths per 100K since the start of the pandemic.14
Delta Regional Medical Center (DRMC) services Washington County in the Mississippi Delta. The local demographics according to the United States Census Bureau in 2019 are comprised of 72.4% African Americans. The poverty rate is 32.6%.15 The Mississippi Department of Health data identifies 46.2% of the population with hypertension, and 42.6% suffer from obesity.16 Washington County’s severe acute respiratory distress syndrome positivity rate in mid-October 2020 stood at 5800 cases per 100K, one of the highest in the Mississippi counties larger than 30K residents.17
Initial identification of COVID-19 pneumonia in this community established the imperative for a committed, science-based, uniform approach to maximize survival of our patients. A COVID-19 committee comprised of physicians, pharmacists, nursing, and administrative staff was assembled. This committee was charged with ongoing implementation and adjustments of COVID-19 pneumonia therapies based on the most current experiences of colleagues around the United States, published COVID-19 pneumonia data, and guidelines coupled to ongoing monitoring of patients’ well-being and outcome.
This is a retrospective analysis of our experience treating COVID-19 pneumonia based on the usage of two anti-inflammatory therapies, tofacitinib and/or dexamethasone. Institutional Review Board approval was granted by WCGIRB 1-1365705-1.
Regimen Development
The first autopsy series of COVID-19 deaths by Fox et al18 documented three critical findings in lung parenchyma: viral cytopathic effect with intracellular small vesicles likely representing viral inclusions of unknown infectivity potential, florid lymphocytic inflammation, and diffuse capillary microthrombi. Coupled with the publication by Mehta et al19 that indicates control of the cytokine storm as key in dampening the rampant inflammation, these papers were instrumental in forming a treatment approach.
We concluded that an upfront comprehensive regimen based on the combination of antiviral therapy, anti-inflammatory, and anticoagulation therapy was needed to control the disastrous disease progression.
The use of steroids in April 2020 was strongly discouraged by the World Health Organization guidelines20 and published reviews.21 The four Janus kinase (JAK) receptors present on, among others, the surface of immune cells, will phosphorylate and activate signal transducers and activators of transcription (STATs), which in turn will modulate gene expression responsible for the production of numerous cytokines as interleukin (IL)–2, IL-4, IL-6, IL-7, IL-9, IL-10, IL-11, IL-15, IL-21, and IL-22.22 Tofacitinib, a nonspecific JAK-STAT inhibitor, is a potent immunomodulator approved as second-line therapy for several autoimmune diseases.23 By blocking this pathway, the production and serum levels of all these cytokines are significantly reduced. The production of other cytokines such as IL-1, IL-17, IL-18, transforming growth factor β and tumor necrosis factor is not affected by this pathway.22
The successful treatment outcome from Dr Najjar24 with the usage of tofacitinib in moderate-to-severe COVID-19 pneumonia among his patients was key in the adoption of anti-inflammatory therapy upfront. Tocilizumab (an IL-6 inhibitor) and tofacitinib were examined at the time. However, with more than 50 cytokines involved in the inflammatory pathway,25 it was believed early on, and later documented, that the cytokine-mediated inflammatory response is central to the pneumonitis pathophysiology.19,26,27 It was concluded that rather than targeting one chemokine versus another (tocilizumab for IL-6 or anakinra for IL-1), tofacitinib was a more reasonable choice due to its broad spectrum at cytokine production inhibition.
Regarding the black box warning of tofacitinib in relation to malignancies,23 hypercoagulability, and immunosuppression, it was concluded that 4 days of a US Food and Drug Administration–approved anti-inflammatory drug was unlikely to cause a malignancy risk. All patients were anticoagulated during hospital stay and at discharge; therefore, the clotting issue was of little concern. As to the infectious risk, all patients were screened for human immunodeficiency virus, viral hepatitis, and tuberculosis by QuantiFERON, and broad antibiotic coverage with azithromycin and ceftriaxone was instituted from day 1.
As the Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial2 was published in July 2020 proving a survival benefit for dexamethasone as an anti-inflammatory in COVID-19, we stopped using tofacitinib as the primary anti-inflammatory and adopted dexamethasone.
Patients and Methods
Participants and Eligibility
The patient cohort was a single-center experience comprised of patients from DRMC.
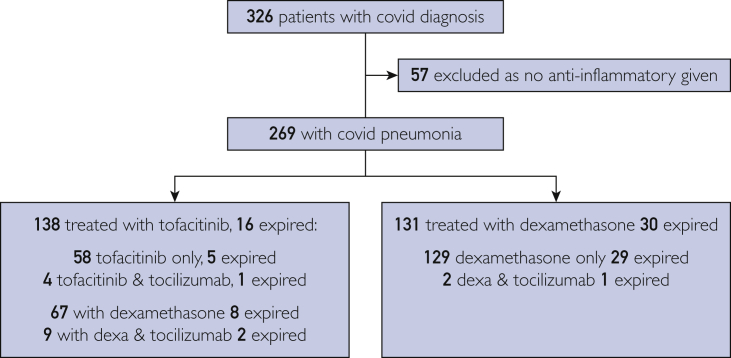
This is an intent-to-treat analysis. All patients admitted for COVID-19–related hypoxemia and discharged by September 30, 2020, were included, regardless of time of death. (Figure 1).
Figure 1.
Eligibility determination and group description. Covid, coronavirus disease 2019; Dexa, dexamethasone.
A total of 326 patients were eligible. Nine patients with repeat admissions were counted only once (if expired, then death was counted; if not, only one admission was counted). Fifty-seven patients who tested positive for COVID-19 had no hypoxemia and no evidence of inflammation; therefore, they were never treated with dexamethasone and/or tofacitinib and were not included in this study.
For the 269 remaining, most patients were admitted for COVID-19 pneumonia from the emergency room. Our admitting criteria were hypoxemia on room air with oxygen saturation less than 93% documented in the emergency department with bilateral chest infiltrates on x-ray or computed tomography (CT) scan. For patients who arrived by ambulance on oxygen, we did not record oxygen saturation on room air. Other admission criteria were prerenal azotemia from severe diarrhea or rhabdomyolysis that was occasionally present.
Polymerase chain reaction (PCR) results, at times, took several days to be reported. Per treatment protocol, based on the fundamental principle of early intervention, treatment was not delayed when the evaluating physician diagnosed presumptive COVID-19 pneumonia.
Sixty-five of these patients had their PCR testing performed at outside institutions. All but nine were traced and proven positive. Of the nine remaining, no PCR test could be traced. All were documented positive in the admitting notes, and had bilateral typical chest CT findings with hypoxemia, elevated C-reactive protein (CRP), and hyperferritinemia. Of these, five had follow-up tests that were positive for immunoglobulin G to severe acute respiratory syndrome coronavirus 2. Of the remaining four patients, one live patient in the tofacitinib group could not be reached, one in the dexamethasone group had expired, and two in tofacitinib group had expired. They were included in the analysis.
Protocol
In addition to appropriate supportive care, all admitted patients for COVID-19–induced hypoxemia or severe inflammation as noted by CRP greater than 50 mg/dL or ferritin greater than 500 ng/mL received the full regimen uniformly immediately on admission, whether patients required supplemental nasal oxygen, high-flow oxygen, or intubation. This decision was based on our conclusion that the need for hospital admission was, by itself, a significant severity factor, coupled with our incapacity to identify those patients whose conditions might deteriorate.
All admitted patients had their infectious aspect of the disease managed exclusively by Dr Singh and their coagulation and inflammatory aspects by Dr Hayek.
Antiviral therapy included the combination of hydroxychloroquine, azithromycin and zinc and subsequently changed to remdesivir as it became available. Many of our patients have stage 4 and 5 CKD in whom remdesivir usage is cautioned,28 in these patients, we opted to treat with 200 mg intravenously on arrival and 100 mg intravenously every 48 hours for 3 to 4 doses. The determination was made considering remdesivir and its active metabolite are approximately 74% renally eliminated; the available formulations contain relatively low concentrations of sulfobutylether-B-cyclodextrin as a carrier and the duration of treatment was 10 days or less.
Regarding anticoagulation, patients received a therapeutic dose of apixaban or enoxaparin. Unless contraindicated, patients were discharged on therapeutic apixaban for a total of 4 weeks from admission, to be followed by aspirin at 81 mg per day for 3 to 4 months aiming at reducing delayed vascular events from both the viral infection29 and the patient’s own risk factors.30
For anti-inflammatory therapy, we went through 3 separate phases.
Phase 1
From April 14 until the end of June 2020, we started tofacitinib 10 mg by mouth twice daily for 5 days. As we grew comfortable in its usage and noticed the need for extended therapy, we extended its usage up to 14 days. In intubated patients, when possible, a nasogastric tube was placed and tofacitinib was administered after being crushed by the pharmacy in compliance with regulatory requirement for handling this drug category.
As the medical news began reporting on the RECOVERY trial results,2 many of our patients started receiving, upon arrival to the emergency room, one or two doses of dexamethasone in addition to tofacitinib.
Phase 2
In July and August 2020, recognizing that a new standard-of-care had been established by RECOVERY,2 the COVID-19 hospital committee amended the regimen to uniformly cease the usage of tofacitinib and replace it with dexamethasone at 10 mg intravenous pyelography for 5 to 10 days.
Phase 3
In September 2020 (closure of the database), through data monitoring, we noticed that since stopping tofacitinib our mortality nearly doubled. However, we had a sense of a faster symptomatic improvement in the dexamethasone group. Therefore, we opted to revert to tofacitinib for 7 to 10 days with the addition of two dexamethasone doses of 10 mg, one on admission and one more 24 hours later.
Protocol Deviations
Starting August 2020, as the literature reported on tocilizumab, this monoclonal antibody was administered occasionally in patients with 100% oxygen requirement on arrival.
Outcomes
The primary outcome of interest in this analysis was the disposition (death or alive) of the patients at discharge.
Statistical Analysis
Data Source
After obtaining Institutional Review Board approval we retrospectively reviewed the records of 326 patients admitted for COVID-19. The analytical file for this study was synthetic derivative (de-identified, non–re-linkable copy) of the DRMC’s electronic medical records database.
Definition of Variables
Apart from sociodemographics (age, race, and sex), other variables of interest included: (1) Clinical parameters that were related to COVID-19 severity such as diabetes, hypertension, and obesity. Because of a critical nursing shortage and critical patient loads, other comorbidities could not be reliably identified in the electronic health record and where not manually retrieved; (2) Inflammatory severity and coagulation markers such as lymphocyte count, creatinine clearance, D-dimer, ferritin, CRP, and oxygen saturation were noted at the first day they were measured; (3) Intensive care unit patients refer to those requiring mechanical ventilation only. Because of our limited resources, all other patients on any other form of oxygen supplementation were treated on the floor; (4) The exposure of interest (treatment) was a binary categorical variable: dexamethasone only (referred to as the dexamethasone group) versus tofacitinib whether with dexamethasone or not (referred to as the tofacitinib group).
Analysis
Data management and analyses were performed using Statistical Analysis System, version 9.4 (SAS Institute, Cary, NC) software. The Pearson χ2 test homogeneity (or Fisher exact test where conditions for χ2 statistics were violated), assessed at the 5% level of significance, was used to explore potential differences in baseline distributions of the sociodemographic and clinical parameters between the two treatment groups.
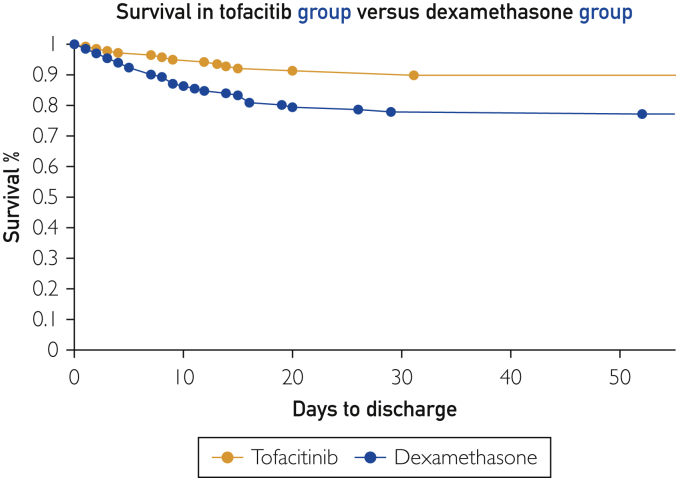
Binary logic regression model generating odds ratios (ORs) and corresponding 95% CIs were used to assess the likelihood that individuals diagnosed with COVID-19–related hypoxemia undergoing treatment with tofacitinib expired compared to those treated with dexamethasone only. Age and captured clinical parameters were adjusted for using a multiple logistic regression model, generating adjusted odds ratios (AORs) and corresponding CIs. The survival experience of the patients was modelled on a Kaplan-Meier survival plot, depicting the two treatment groups (Figure 2).
Figure 2.
Kaplan-Meier survival plot of the tofacitib group versus the dexamethasone group.
Results
The analytical sample comprised 269 patients. All patients met the endpoint of death or were discharged alive. Baseline characteristics between group comparisons are shown in Table 1. A total of 138 (51.3%) patients were uniformly exposed to tofacitinib, versus 131(48.7%) to dexamethasone without tofacitinib.
Table 1.
Sociodemographic and Clinical Characteristics Upon Admissions of Patients With COVID-19 Related Hypoxemiaa
| Characteristics | Total N=269 |
Dexamethasone n=131 | Tofacitinib n=138 | P |
|---|---|---|---|---|
| Mean age, years (SD) | 66.7 (14.6) | 61.3 (13.4) | .002 | |
| Years of age distribution, n (%) | ||||
| <60 | 34 (26.0) | 66 (47.8) | <.001 | |
| 60–70 | 47 (35.9) | 30 (21.7) | ||
| >70 | 50 (38.2) | 42 (30.4) | ||
| Race, n (%) | ||||
| African American | 108 (82.4) | 117 (84.8) | .87 | |
| Caucasian | 21 (16.0) | 19 (13.8) | ||
| Other | 2 (1.5) | 2 (1.5) | ||
| Sex, n (%) | ||||
| Female | 69 (52.7) | 83 (60.1) | .22 | |
| Male | 62 (47.3) | 55 (39.9) | ||
| Clinical Parameters, n (%) | ||||
| Diabetes | 18(13.7) | 32(23.2) | .047 | |
| Obesity | 64(48.9) | 88(63.8) | .01 | |
| Hypertension | 67(51.2) | 69(50.0) | .85 | |
| GFR < 45 mL/min | 40(30.5) | 34(24.6) | .28 | |
| Lymphocyte, per 103 μL | <1 | 68(51.9) | 65(47.1) | .43 |
| ≥1 | 63(48.1) | 73(52.9) | ||
| Ferritin, ng/mL | >1000 | 31(27.2) | 28(21.2) | .27 |
| ≤1000 | 83(72.8) | 104(78.8) | ||
| D-dimer, mg/mL | >1 | 99(76.7) | 74(54.4) | <.001 |
| ≤1 | 30(23.3) | 62(45.6) | ||
| C-reactive protein, mg/mL | >150 | 41(31.5) | 52(37.7) | .29 |
| ≤150 | 89(68.5) | 86(62.3) | ||
| % O2 saturation, mean (SD) | 85.4(14.3) | 86.2(12.2) | .21 | |
| Mechanical ventilation, n (%) | 31(23.7) | 32(23.2) | .93 | |
| Survival outcome | ||||
| Disposition, n (%) | Death | 30 (22.9) | 14(10.1) | .005 |
| LOS Median (IQR), days | 5.8 (5.2) | 6.8(7.9) | <.001 | |
COVID-19, coronavirus disease 2019; GFR, glomerular filtration rate; IQR, interquartile range; LOS, hospital length of stay.
A total of 44 patients expired; 14 (31.8%) were in the tofacitinib group and 30 (68.2%) were in the dexamethasone group. The proportions of death among the tofacitinib and dexamethasone groups were, respectively, 10.1% and 22.9% (Figure 2).
Among the 63 patients intubated in the intensive care unit, 32 (50.8%) died, with statistically significant high likelihood compared to those not intubated (OR: 31.20; 95% CI: 7.77 to 35.83; P<.001). However, there was no statistically significant difference in these mechanically ventilated patients based on the two treatment modalities (P=.93).
Exploring potential differences in baseline distributions of the variables between the two treatment groups, the data were indicative of a statistically significant difference for age, and for D-dimer being higher in the dexamethasone group (P<.001). However, the tofacitinib group had more diabetic (P=.047) and obese individuals (P=.01).
There were 62% reduced odds of dying among those treated with added tofacitinib compared to those treated without tofacitinib (OR: 0.38; 95% CI: 0.19 to 0.76; P=.01). Controlling the effects of age and the captured clinical parameters (pre-existing conditions, laboratory markers, and whether patients were mechanically ventilated) in relation to treatment modalities on outcome of dying, the result was ordered preserved and statistically significant, indicating 70% reduced odds of dying among the tofacitinib group compared to the dexamethasone group (AOR: 0.30; 95% CI: 0.12 to 0.76; P=.01) (Table 2).
Table 2.
Likelihood of Individuals Diagnosed With COVID-19–Related Hypoxemia Undergoing Treatment With Tofacitinib Expired Compared to Those Treated With Dexamethasone Only Controlling for Age and Captured Clinical Parameters (n=269)a
| Characteristics | AOR (95% CI)b | P | |
|---|---|---|---|
| Treatment | Tofacitinib vs dexamethasone | 0.30 (0.12 to 0.76) | .01 |
| Years of age distribution, n (%) | |||
| <60 | 1.79(0.60 to 5.39) | .30 | |
| 60–70 | 1.28 (0.45 to 3.65) | .65 | |
| >70 | — | — | |
| Diabetes | No vs yes | 0.60 (0.16 to 2.23) | .45 |
| Obesity | Yes vs no | 1.72 (0.68 to 4.36) | .25 |
| Hypertension | No vs yes | 0.86 (0.35 to 2.11) | .74 |
| GFR, mL/min | <45 vs ≥45 | 8.68 (3.07 to 24.56) | <.001 |
| Lymphocyte, per 103 μL | ≤1 vs >1 | 1.35 (0.56 to 3.30) | .50 |
| Ferritin, ng/mL | ≤1000 vs >1000 | 0.31 (0.10 to 0.95) | .04 |
| D-dimer, mg/mL | ≤1 vs >1 | 1.08 (0.40 to 2.95) | .88 |
| C-reactive protein, mg/mL | >150 vs ≤150 | 2.47 (1.03 to 5.90) | .04 |
AOR, adjusted odds ratio; COVID-19, coronavirus disease 2019; GFR, glomerular filtration rate.
The crude odds ratio for treatment is 0.38; 95% CI: 0.19 to 0.76; P=.01.
Toxicity
We used positive blood cultures as an objective criterion to document infections (Table 3). Positive blood cultures were low and similar in both groups. As stated, all our patients received broad-spectrum bacterial coverage from day 1 to protect from the immunosuppressive effect of tofacitinib.
Table 3.
Toxicities
| Tofacitinib | Dexamethasone | |
|---|---|---|
| Packed red blood cell transfusion | 20 | 20 |
| Positive blood cultures | 7 | 8 |
The use of full-dose anticoagulant was associated with a higher than the expected 2.5% bleeding rate reported in the non–COVID-19 population.31 We noted no difficulties in bleeding cessation when anticoagulation was stopped in bleeding patients and no relation to mortality.
All screening for human immunodeficiency virus and viral hepatitis were negative. One patient was positive for tuberculosis via QuantiFERON. His results were available 3 weeks after discharge (remained asymptomatic, treated per standard of care by Mississippi State Board of Health).
Hyperglycemia in our population was a common problem in the dexamethasone group often requiring insulin coverage. Tofacitinib does offer the added advantage of metabolic neutrality.
Discussion
At the hypoxemic state, autoimmune inflammation in COVID-19 pneumonia is a key mechanism of deterioration.32 Viral-induced autoimmunity is a well-described phenomenon whether with Epstein-Barr virus–associated systemic lupus erythematosus, hepatitis C virus–autoimmune hepatitis (HCV-AIH), or others.33 Similar aberrant immune response is key for inflammatory COVID-19 pneumonitis to develop as shown by Lucas et al34 in their longitudinal analyses of immunological response to COVID-19.
The publication by McGonagle et al35 supports the practice of intervening as early as possible for achieving good outcome. McGonagle et al35 confirm a direct relation between COVID-19 disease severity and rising cytokine levels.
These studies suggest a hyperinflammatory response that can rapidly progress and may increase the risk of death. Intervention aimed at modulating the inflammatory immune response by reducing toxic cytokine levels improves outcomes in the treatment of COVID-19 pneumonia. This approach is well established since the publication of the RECOVERY study2 that lead to the usage of dexamethasone in admitted patients. Unlike dexamethasone, no mortality benefit has been shown in treatments that target one cytokine36,37 hypothesized to be a central driver in inflammation.
Contrary to monoclonal antibodies, tofacitinib possesses, just like dexamethasone, a broad anti-inflammatory effect, yet target a different inflammatory pathway than dexamethasone.38
The outcomes data in the tofacitinib group lend credence to the concept that broadly reducing cytokine levels, whether with tofacitinib alone or in combination with dexamethasone, is associated with a greater mortality benefit. Moreover, this analysis indicates the safety of this approach as to the lack of vascular thrombosis or an increase in infectious complications. In addition, tofacitinib has the advantage of metabolic neutrality. Lack of hyperglycemia and inducing a catabolic state are significant benefits in an obese, critically ill, population.
The patient cohort included in this analysis has multiple demographic and comorbidity factors that have been associated with worse outcomes from COVID-19–related illness. The effect of the socioeconomic background for hospital COVID-19–related mortality variation was clarified by Wadhera et al39 showing an inpatient COVID-19 mortality twice as high in the New York City boroughs with a higher proportion of minorities and poverty when compared to more affluent ones (Bronx vs Manhattan). African Americans are known to be at highest6,7 risk of death from COVID-19. Other well-established risks of worse outcomes are age,6, 7, 8 obesity,9,10 and stages 4 and 5 chronic kidney disease.11,12 Admission oxygen saturation, degree of inflammation (CRP and ferritin), and hypercoagulability (D-dimers) are also established markers of severity of illness.40,41 All of these severity criteria were elevated in our presented population and may have translated into a higher mortality than the published 18% in our state. In fact, our dexamethasone only cohort mortality was 22.9%.
The decision to provide systemic dose anticoagulation upfront based on the New Orleans autopsy findings is consistent with other reports suggesting the efficacy of this approach.4 The higher-than-expected bleeding rate did not change outcomes in this patient population.
Caution in tofacitinib timing is warranted. The RECOVERY trial did show an increase in mortality when dexamethasone was administered early to patients who did not require supplemental oxygen. A similar deleterious impact may be present if tofacitinib is used early without antiviral coverage.
Study Limitations
As a retrospective analysis of hospitalized patients for COVID-19 pneumonia, our analysis has a few challenges especially in relation to quality. Although it focused on exploring the potential benefit of tofacitinib in hospitalized patients for COVID-19 pneumonia, a few patients received tocilizumab as noted. These few patients were not segregated in this analysis and may have affected the outcome. Another deficiency is a lack of rigorous documentation of comorbidities and sequential organ failure assessment scores. During these times, similar to many other hospitals, we were facing a critical shortage of nursing and medical staff with an overwhelming patient load. Consequently, documentation suffered enough to prevent a reliable analysis of factors such as chronic obstructive pulmonary disease and immunosuppression.
Future investigation that clearly delineates patients into those who receive these treatment regimens separately and in combination in a prospective factorial analysis may further elucidate the benefit of tofacitinib as a treatment modality for COVID-19 pneumonia.
Conclusion
The experience reported here builds on the works of previous studies of COVID-19 patients indicating a generalized severe inflammation with no one cytokine identifiable as a key driver. Rather than targeted against one cytokine, broad anti-inflammatory therapy is needed to stem the florid immune response associated with COVID-19 illness. Patient response to dexamethasone and now tofacitinib both support this theory. The RECOVERY trial group just published that tocilizumab therapy provided a significant survival benefit only when combined to dexamethasone42 and not as a single agent.
After considering this cohort’s unique demographics, the severity index, the uniformity in care, and low clinical toxicity, we found the substantial survival benefit experienced by the tofacitinib group to be a credible confirmation of this treatment. We conclude that a treatment protocol that includes anticoagulant therapy, and antiviral and anti-inflammatory therapy with tofacitinib, whether or not combined with a short course of dexamethasone, offers a solid model to move forward with further clinical research.
Acknowledgments
The authors thank Sessine Najjar, MD, for critical contribution in leading to successful usage of tofacitinib; and Carol Cashion Doolittle, CFNP, and Will Evans, PhD, MCHES, CWP, for their editorial assistance.
Footnotes
Potential Competing Interests: The authors report no potential competing interests.
References
- 1.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horby P., Lim W.S., Emberson J., Mafham M. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arshad S., Kilgore P., Chaudhry Z.S. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paranjpe I., Fuster V., Lala A. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76(1):122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joyner M.J., Senefeld J.W., Klassen S.A. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience. medRxiv. 2020 doi: 10.1101/2020.08.12.20169359. [DOI] [Google Scholar]
- 6.Golestaneh L., Neugarten J., Fisher M. The association of race and COVID-19 mortality. EClinicalMedicine. 2020;25:100455. doi: 10.1016/j.eclinm.2020.100455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.COVID-19 Hospitalization and Death by Race/Ethnicity Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html
- 8.Docherty A.B., Harrison E.M., Green C.A. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simonnet A., Chetboun M., Poissy J. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tartof S.Y., Qian L., Hong V. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med. 2020;173(10):773–781. doi: 10.7326/M20-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gansevoort R.T., Hilbrands L.B. CKD is a key risk factor for COVID-19 mortality. Nat Rev Nephrol. 2020;16(12):705–706. doi: 10.1038/s41581-020-00349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williamson E.J., Walker A.J., Bhaskaran K.J. Factors associated with COVID-19–related hospital death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staneva M., Byers P., Dobbs T. A first glance at COVID-19 hospitalizations in Mississippi: demographics, Comorbidities, and Outcome of Care. J Miss Med State Assoc. 2021;61(11/12):374–379. [Google Scholar]
- 14.https://covid.cdc.gov/covid-data-tracker/#cases_deathsper100k
- 15.United States Census Bureau Quick facts, Washington County, MS. https://www.census.gov/quickfacts/washingtoncountymississippi
- 16.Mississippi State Department of Health Washington County Health Profile. https://msdh.ms.gov/msdhsite/files/profiles/Washington.pdf
- 17.https://msdh.ms.gov/msdhsite/_static/resources/11199.pdf
- 18.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Brown J.Q., Heide R.S.V. Pulmonary and cardiac pathology in COVID-19: the first autopsy series from New Orleans. Lancet Respir Med. 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta P., Mcauley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical management of COVID-19 World Health Organization. https://www.who.int/publications/i/item/clinical-management-of-covid-19 Published May 27, 2020.
- 21.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 22.Hodge J., Kawabata T., Krishnaswami S. The mechanism of action of tofacitinib — an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34(2):318–328. [PubMed] [Google Scholar]
- 23.Tofacitinib. http://labeling.pfizer.com/ShowLabeling.aspx?id=959
- 24.Tofacitinib efficacy Personal communication by phone, S. Nejjar to M. Hayek. March. 2020;5 [Google Scholar]
- 25.Charo I.F., Ransohoff R.M. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354(6):610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 26.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. https://science.sciencemag.org/content/368/6490/473 Published May 1, 2020. [DOI] [PubMed]
- 27.Pedersen S.F., Ho Y.-C. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130(5):2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Remdesivir [package insert] https://www.gilead.com/-/media/files/pdfs/medicines/covid-19/veklury/veklury_pi.pdf
- 29.Smeeth L., Thomas S., Hall A., Hubbard R., Farrington P., Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351(25):2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 30.Recommendation: Aspirin Use to Prevent Cardiovascular Disease and Colorectal Cancer: Preventive Medication: United States Preventive Services Taskforce. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/aspirin-to-prevent-cardiovascular-disease-and-(6):cancer Published April 11, 2016.
- 31.Howe Z., Naville-Cook C., Cole D. Bleeding rates of Veterans taking apixaban or rivaroxaban for atrial fibrillation or venous thromboembolism. J Thromb Thrombolysis. 2019;47(2):280–286. doi: 10.1007/s11239-018-1770-7. [DOI] [PubMed] [Google Scholar]
- 32.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosupressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackay I., Leskovsek N., Rose N. Cell damage and autoimmunity: a critical appraisal. J Autoimmun. 2008;30(1-2):5–11. doi: 10.1016/j.jaut.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucas C., Wong P., Klein J. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584(7821):463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome–like disease. Autoimmun Rev. 2020;19(6):102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stone J., Frigault M., Serling-Boyd N. Efficacy of tocilizumab in patients hospitalized with COVID-19. N Engl J Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cavalli G., Luca G.D., Campochiaro C. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2(6):e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fajgenbaum D., June C. Cytokine storm. N Engl J Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wadhera R.K., Wadhera P., Gaba P. Variation in COVID-19 hospitalizations and deaths across New York City boroughs. JAMA. 2020;323(21):2192. doi: 10.1001/jama.2020.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrilli C.M., Jones S.A., Yang J. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suleyman G., Fadel R.A., Malette K.M. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in Metropolitan Detroit. JAMA Network Open. 2020;3(6):e2012270. doi: 10.1001/jamanetworkopen.2020.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horby P., Campbell M., Staplin N. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. Lancet. February 11, 2021 doi: 10.1101/2021.02.11.21249258. [DOI] [PMC free article] [PubMed] [Google Scholar]