Abstract
Purpose
Nasal irrigation or nebulizing aerosol of isotonic or hypertonic saline is a traditional method for respiratory or nasal care. A recent small study in outpatients with COVID-19 without acute respiratory distress syndrome suggests substantial symptom resolution. We therefore analyzed pharmacological/pharmacodynamic effects of isotonic or hypertonic saline, relevant to SARS-CoV-2 infection and respiratory care.
Methods
Mixed search method.
Results
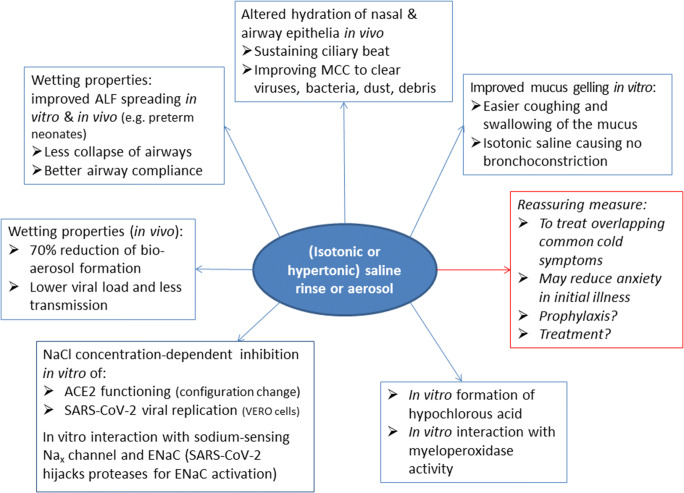
Due to its wetting properties, saline achieves an improved spreading of alveolar lining fluid and has been shown to reduce bio-aerosols and viral load. Saline provides moisture to respiratory epithelia and gels mucus, promotes ciliary beating, and improves mucociliary clearance. Coronaviruses and SARS-CoV-2 damage ciliated epithelium in the nose and airways. Saline inhibits SARS-CoV-2 replication in Vero cells; possible interactions involve the viral ACE2-entry mechanism (chloride-dependent ACE2 configuration), furin and 3CLpro (inhibition by NaCl), and the sodium channel ENaC. Saline shifts myeloperoxidase activity in epithelial or phagocytic cells to produce hypochlorous acid. Clinically, nasal or respiratory airway care with saline reduces symptoms of seasonal coronaviruses and other common cold viruses. Its use as aerosol reduces hospitalization rates for bronchiolitis in children. Preliminary data suggest symptom reduction in symptomatic COVID-19 patients if saline is initiated within 48 h of symptom onset.
Conclusions
Saline interacts at various levels relevant to nasal or respiratory hygiene (nasal irrigation, gargling or aerosol). If used from the onset of common cold symptoms, it may represent a useful add-on to first-line interventions for COVID-19. Formal evaluation in mild COVID-19 is desirable as to establish efficacy and optimal treatment regimens.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00228-021-03102-3.
Keywords: Saline, Sodium chloride, SARS-CoV-2, COVID-19, Mucociliary clearance, Acute respiratory distress syndrome
Introduction
The use of “Atemwegpflege” (care of the airways) or nasal care is a traditional German practice, as to provide moist to the airways and can be achieved by nasal sprays or inhalation of nebulized isotonic or hypertonic saline (Kochsalzlösung) [1–8]. This practice is being promoted by lung specialists, health care, and consumer organizations during COVID-19, whereby the use of nebulizing/aerosols with saline is recommended, either stating that, while it may not change the risk of infection, it helps to mitigate the first symptoms or claiming that it effectively can “dam” (thus reduce) virus infection (“Einfaches Inhalieren kann Tröpfcheninfektion effektiv eindämmern”) [2–6]. Recently, also the Deutsche Gesellschaft für Krankenhaushygiene (DGKH) has formulated gargling/rinse measures to contain SARS-CoV-2 transmission in household, nursing homes, and schools [9].
Whether nebulizing isotonic or hypertonic saline may help to alleviate shortage of breath is not known. Respiratory secretions due to COVID-19 infection may behave similarly as those of a severe bronchitis or bronchiolitis; cough can be dry, but secretions can be clear to mucopurulent, and thus need to be mobilized to be removed from the airways. Aerosol use was actively discouraged in Belgium, as it is believed to create more risks for viral transmission, according to the APB (official pharmacist organization in Belgium) and Sciensano reports [10–12]. Also the World Health Organization (WHO) discourages the use of aerosolizing procedures in general [13]. This risk was also raised at some stage in Germany, because of the fear that this technique would generate bio-aerosol drops, which could promote virus spread. This concept however was contradicted by a positioning statement of the German pneumologists [14]. A recent small study in outpatients with COVID-19 without acute respiratory distress syndrome (ARDS) suggests substantial symptom resolution with hypertonic saline [15, 16].
We therefore investigated the mechanisms by which saline nasal spray/irrigation or aerosol may limit SARS-CoV-2 infectivity and spread. In particular, we discuss the evidence from the literature about the effects of saline on bio-aerosol formation, mucus, alveolar lining fluid (ALF), ciliary beat and mucociliary clearance (MCC), the angiotensin-converting enzyme 2 (ACE2), the sodium channel (ENaC), viral replication (host protease furin, viral protein 3CLpro), and formation of hypochlorous acid (HOCl), for their interaction with SARS-CoV-2. The assessment is not meant to address a role of saline in severe COVID-19 ARDS. We also briefly reviewed relevant clinical data and formulate recommendations in support of isotonic or hypertonic saline as a simple rinse or aerosol for an early reassuring intervention for upper respiratory infection and COVID-19.
Methods
A mixed method approach was taken. At first, information on epidemiological data and treatments of COVID-19 was searched for on the local Internet, as available from the official national sites and as promoted by respectable health care-related organizations in German and Dutch to consumers. As this analysis revealed a common recommendation of use of saline in the frame of COVID-19 in Germany, first systematic searches on established relevant keywords related to saline were performed on PubMed. This was followed by broader searches on new aspects as research progressed and suggested potential other mechanisms of saline relevant to COVID-19. Publications that focused on chronic respiratory diseases were not retained, unless if relevant to discriminate effects from a pharmacodynamic, pathophysiological, or safety point of view. Similarly, the search for relevant clinical effects of oral, nasal, and respiratory hygiene with saline was limited to keywords related to acute upper respiratory tract infections, with or without COVID-19 (SARS-CoV-2). For the pathways followed and the keywords and restrictions used to handle the vast amount of publications on saline and NaCl on PubMed, see Supplement 1.
To note: in this paper, isotonic saline refers to 0.9% NaCl (also called physiologic serum), while hypertonic saline refers to concentrations of 2% and above (commonly 3–7% in experimental and clinical studies and formulation in the market). “Aerosol” refers to nebulizing iso- or hypertonic saline, using a mist-forming device for inhalation and humidification to clear the airways and to remove phlegm in viral respiratory infections. We use the term “bio-aerosol”, when referring to the micro-droplets, spontaneously produced during exhalation, such as during speaking, singing and coughing.
Results
The effects of isotonic and/or hypertonic saline are summarized in Fig. 1. They include the wetting/gelling properties, effect on MCC and hydration, SARS-CoV-2 viral replication and underlying mechanisms, as well as effect on the formation of hypochlorous acid (HOCl).
Fig. 1.
Proposed mechanisms of saline relevant to nasal or respiratory hygiene during COVID-19. Bio-aerosol refers to micro-droplets, spontaneously produced during exhalation, such as during speaking, singing, and coughing; aerosol refers to nebulizing and inhaling saline for inhalation in the respiratory airways, while saline rinse refers to nasal spraying/oral gargling of saline. ALF, alveolar lining fluid; isotonic, 0.9% NaCl (or 9 g/L); and hypertonic, varying concentrations above 0.9% NaCl (2%-7%, often 3%). ACE2 angiotensin-converting enzyme 2; MCC mucociliary clearance
Wetting effect on ALF spreading and bio-aerosol formation
Saline changes the physicochemical properties of ALF, mucus, and vesicles/bio-aerosols, thereby affecting the molecular behaviour of ionic and non-ionic surfactants, proteins, and phospholipids in particular. Collectively, these effects are referred to as “wetting properties” of NaCl [17, 18], underlying 2 relevant phenomena: (1) better ALF spreading and (2) suppression of bio-aerosol formation.
Surface tension is an important factor in alveolar wetting, the MCC and the phenomenon of capillarity. The alveolar cells produce surfactant that decreases the surface tension in the airways, so reducing the amount of energy required to expand the lungs. From a pathophysiological perspective, the role of pulmonary surfactant in wetting, re-spreading, and compressing the ALF to ultra-low surface tensions is a mechanism that is well-known from preterm children. Such infants might suffer from infant respiratory distress syndrome, characterized by a lack of lung surfactant, which is needed to reduce surface tension forces and is critical for normal lung inflation [19, 20]. Isotonic saline aerosol has been proven to remediate this problem by its wetting properties and to be lifesaving by improving airway compliance [19, 20]. Alveolar type II epithelial cells (AT2) promote the biosynthesis of lung surfactant. SARS-CoV-2 attacks the AT2 cells, causing defective functionality of these cells, which may cause exhaustion of pulmonary surfactant, raise the alveolar surface tension, and finally lead to alveolar collapse [21, 22]. Hence, the wetting properties of NaCl may provide a benefit in reducing surface tensions, thus improving airway compliance.
Airway surface liquid (ASL) or saliva droplets carrying the virus are believed to convert into a bio-aerosol infecting the environment and bystanders, and therefore, the use of aerosolizing procedures has been discouraged by several authorities [10–13]. Yet, one should not confound saline aerosol with viral bio-aerosols produced after harvesting from cell cultures, or with bio-aerosol-generating procedures in the hospital (for more information, see Supplement 2): to-date, independent reviews about bio-aerosol generating procedures did not find enhanced risks for transmission of SARS-CoV-2 with nebulizing saline aerosol. In the studies by Edwards [23–25] (Table 1), the delivery of isotonic saline aerosol, nebulized over 6 min, reduced the release of exhaled bio-aerosols from the lungs by an average of 72% (lasting up to 6 hours), an effect persisting over 6 h upon nebulizing 1.29% CaCl2 diluted in 0.9% NaCl. The highest effect was obtained in high bio-aerosol emitters [23, 26]. Aerosol administration of saline to the airways particularly diminished the exhalation of smaller particles that facial masks fail to filter out [23]. The effect of 6 min of isotonic saline nebulization on bio-aerosols was furthermore studied in a simulated cough model, producing under bursts of air a bio-aerosol of mucus mimetic. Saline reduced the fine mucin mimetic aerosol, while increased the droplet size of the mucus mimetic (volume-averaged median size 320 nm) instantaneously to 1 μm, and further to 65 μm at 30 min and 30 μM at 60 min [25]. Nebulizing aerosol in a swine model of influenza led to the inhibition of the viral airborne transmission [25]. The setup of another study by Simonds et al., simultaneously using non-invasive ventilation, did however not allow to draw firm conclusions [27]. The German positioning paper on COVID-19 by pneumologists acknowledges the relevance of this property of nebulized isotonic saline, concluding “Although nebulizers with nozzles increase the amount of aerosol in room air, they do not increase the risk of infection for medical staff. The inhalation of isotonic saline solution significantly reduces (bio)aerosol release from the lungs” [14].
Table 1.
Effects of nebulizing, rinsing and wetting with saline on bio-aerosol formation
| Report/study of | Method | Results and (proposed) mechanism |
|---|---|---|
| Nebulized (aerosol) in human | ||
| Saline (0.9%) [23, 26] | Assessment of exhaled bio-aerosol particles after saline or a surfactant formulation |
– Number of exhaled bio-aerosol particles was reduced by 72% with nebulized saline compared with surfactant, particularly in “high-producers” – Median droplet size containing surfactant was smaller compared with saline – Effect lasting up to 6 h |
| CaCl2 (1.29%) diluted in saline (0.9%) [24] | Assessment of exhaled bio-aerosol particles after saline or a surfactant formulation |
– Number of exhaled bio-aerosol particles was reduced as with nebulized saline – Effect lasting beyond 6 h |
| CaCl2 (1.29%-12.9%) diluted in saline (0.9%) [25] | Open-label trial in volunteers and influenza swine model |
– Fast production (within 15min) of less and finer bio-aerosol lasting (up to at least 6 h) – Suppression was most pronounced (99%) among those who exhale large numbers of particles – Aerosol administration of these salts to the airways diminishes the exhalation of the small particles that face masks fail to filter – In an influenza swine model, it completely blocked airborne transmission of disease – CaCl2 enhanced the effects of saline; the lowest CaCl2 concentration was already effective. The effect is ion-dependent ( (not observed by adding MgCl2) |
| Saline (0.9%) [27] | Nebulizer treatment while various aerosol-generating procedures |
– Small- and medium-size aerosol/droplet generation, no increase in large-size droplet count – Systematic error possible: not investigated which particles originated from patient or nebulizer, and whether viruses could be isolated from aerosol |
| Rinse effect in human | ||
| Saline nasal lavage (0.9%) [28] | Viral concentrations after single nasal lavage of infected volunteers | – Lower viral titres after saline nasal lavage in rhinovirus infections; after a single rinse, titres only returned to initial values after day 5 |
| Saline nasal irrigation & gargling (3%) [29] | Open-label, controlled trial in patients with common cold (coronaviruses other than SARS-CoV-2) |
– Hypertonic saline reduced transmission within household contacts by 35% (P = 0.006) as compared to controls – 30% more individuals had reduction in viral shedding by ≥0.5 log10 per day |
| Mechanistic (physicochemical) effects and in vitro studies | ||
| Saline bio-aerosol (0.9%) + mucins [30] | Mechanistic study/biophysical characterization in presence of mucins (confirmed in bull calves) | Charge shielding of mucin or mucin-like macromolecules that consequently undergo gelation, stabilizing ALF/air interface and reducing its breakup, resulting in a reduced tendency of the ALF to disintegrate into very small droplets |
| Saline droplets (emulsion) [31] | Mechanistic study of NaCl droplets ± surfactant | Added to nanoemulsions, NaCl makes finer micellar droplets “aggregate”, making the droplet size distribution to move to a bigger size range (so will lead to faster deposition), while surfactant in contrast breaks up the droplets to smaller sizes |
| Saline phospholipid droplets [32] | Mechanistic study of effect of NaCl on phospholipid vesicles and bilayers |
– Na+ and Cl− binds with the lipid head and induces strong hydrophobic repulsion on the lipid tail – This leads to enhanced hydrophobic repulsion on lipids and so forces lipids to attach firmly on the surface substrate Much larger external energy is needed for vesicle formation in salt solutions than in pure water |
| Sodium chloride aerosol [35] | Mechanistic study of NaCl and corn oil (bio)aerosol on filtration by face mask materials (FFP1, N95, P100, and elastomeric half-mask respirators) |
– NaCl bio-aerosol (even if consisting of finer droplets) penetrates less in face mask material, and thus is better filtered out, as compared with a corn oil bio-aerosol – The generally larger oily droplet aerosol was shown to penetrate deeper in the mask materials So, the ionic nature of NaCl aerosol leads to better capture of bio-aerosol by the mask material |
| Saline +/- surfactant bio-aerosol (+/- influenza virus particles) [38] | Mechanistic study in evaporated droplets |
– Salinity affects the structure of viral particles, whereas processes at the air-liquid interface drive virus inactivation in droplets, also depending on droplet composition and RH – Bio-aerosols of enveloped viruses would fail to undergo rapid rehydration upon entry of the nearly saturated humidity of the respiratory tract, as surfactant arrangement in the droplets would inhibit the reabsorption of water |
| Saline ± surfactant bio-aerosol (± influenza virus particles) [39] | Study of viral decay in droplets evaporated at different RH and concentrations of saline and protein |
– Viability depends on the RH (higher at RH <50% and at 100%) – Viability decreased in saline solutions, the extent dependent on the salt concentrations and presence of protein |
ALF alveolar lining fluid, NaCl saline, sodium chloride, RH relative humidity
Table 1 lists furthermore two studies that assessed the effect of nasal rinsing or gargling with saline on viral titres in nasal lavage. These show that saline may also reduce viral transmission via a direct rinse effect of the gargling or nasal rinse with saline [28, 29]: the data support that rinsing leads to considerable direct removal of virus from surface liquid.
From a mechanistic focus, it is proposed that isotonic saline changes the gelling properties of mucin and surface tension of the liquid film on the airway epithelium, leading to less droplet/phospholipid vesicle formation and, as such, to less release of exhaled bio-aerosols [14, 30]. While mucus droplets easily rehydrate after evaporation, their properties are changed by adding NaCl [30]. The mucus is gelled to less and stronger gelled particles, which leads to significantly less droplet formation upon nebulization [30]. This effect is unique to saline and is also observed in presence of other ions that have a more limited effect on aerosol reduction. The effect was also sustained in the presence of surfactants and polysaccharides (dextrans), although the particle count was slightly enhanced by the latter substances: it was moreover found that surface tension was not the determinant factor, but rather the conductivity and viscosity [30]. In the mono-, bi-, or multilayer vesicle technology, saline has been shown to supress the vesicle formation, to induce aggregation of vesicles, and to lead to faster vesicle deposition on surface substrates: the ionic nature makes it more easily to be captured by the substrate layer, while vice versa, as a result, droplets are more difficult to detach from the resulting salt-integrating surface substrate [31, 32]. Saline thus leads to much less bio-aerosol while remaining droplets may average larger sizes, as was shown by Edwards [23–25]: this will affect deposition indirectly in 2 ways: (1) faster deposition of droplets following coughing in the vicinity of the spreader (due to higher gravity and inertia of the larger droplets) [33]; (2) faster deposition of incidentally inhaled droplets in the nose and upper airways, as they are too large to penetrate into the bronchioles and lungs [34]. The bio-aerosol formation for airborne transport will thus become more difficult, as was evidenced by Edwards with saline aerosol in the swine model of influenza [25].
Moreover, not only the droplet size but also the enhanced ionic nature of the bio-aerosol, induced by NaCl wetting, may lead to easier capture /better filtration by filter material. It was shown that a fine NaCl bio-aerosol penetrates less deep in face mask material (FFP1 and N95) as compared with corn oil bio-aerosol [35]. CE-standard criteria for professional face masks ask filter capacities based on testing with plain paraffin aerosol: as surfactants highly change paraffin aerosol properties [36], this may not fully estimate the behaviour of viral-loaded bio-aerosols consisting of enveloped virus or surfactant (phospholipid) vesicles. The fact that the wetting effects of NaCl on bio-aerosol behaviour persist in presence of surfactant [30] may thus be relevant to filtering out viral-loaded bio-aerosol. Salt-covered masks have recently been developed as to destroy more quickly coronavirus droplets when landing on the mask material [37].
At last, saline will also lead to fast shrinkage of droplets upon evaporation in the ambient air (already ongoing at a relative humidity of 90–95%): loss of water induces phase separation, enhanced salinity, and decreased pH in the droplets/particles, as well as re-organization of the phospholipids at the outer air-droplet interface [38, 39]. The hydrophobic nature of the resulting phospholipid-coat was found to impair rehydration of virus-loaded particles [38]. Salt concentrations have been shown to adversely affect the survival of influenza virus in bio-aerosol [39], while increased hydrophobicity of the particles lead to faster and reduced deposition in the airways according to aerosol technology [34].
Role of saline in the MCC
The MCC is the major primary innate defence mechanism of the nose, airways, and lungs, continuously clearing these from dust, infectious, and other particles by the ciliary movements. Using different techniques, it has been shown that isotonic saline (aerosol or rinse) induces a positive effect on the ciliary beat frequency, reverses ciliostasis and promotes the MCC, both under physiological and damage-induced conditions [40–44]. Factors that play a role include the osmolality of the saline, the hydration of the ALF and the composition of the mucins.
Osmolality of saline. Pure water severely damages the normal human nasal epithelial cells, while isotonic saline (in contrast to hypo- and hypertonic saline) does not affect their morphology [45, 46]. Hypertonic saline has been shown to decrease the ciliary movement in human nasal epithelium [43, 47], while others report a faster MCC in healthy subjects, after-single dose nasal irrigation, or in the airways at 30 min, but not 4 h after inhalation (attributed to depletion of airway mucin) [44–50]. Hypertonic saline induces osmotic pressure, but has also been found to decrease the potential difference in nasal epithelia—a rapid, reversible, and dose-related effect indicating a direct effect of NaCl on ion transport across the human airway epithelium (not just attributable to a simultaneous change in osmolarity) [51]. Hypertonic saline has also been found to affect the nasal epithelial permeability [52, 53]. Its use may be associated with nasal burning/irritation [54], while both hypo- and hypertonic saline aerosols may induce bronchoconstriction or cough, as observed in patients with asthma or moderate to severe chronic obstructive pulmonary disease [55–58].
Hydration of the ALF. The MCC requires coordination between the periciliary liquid near the cell surface and the overlaying transported mucus layer [59, 60]. Therefore, these layers need to be appropriately hydrated in the lungs and airways, allowing the cilia to beat properly, to move the mucus and to transport the trapped pathogens and particles. The nasal and respiratory mucin forms a gel layer, serving as a liquid reservoir for the periciliary layer [61]. The height of the ALF or ASL and hydration of the periciliary liquid layer depend on opposing mechanisms in water transport: the outward chloride (Cl−) secretory transport through apical chloride channels (CFTR and CACC mediated), and the inward movement of water following active (re)absorption of Na+ through apical sodium channels ENaC. These ion transporting actions occur in concertation with the basolateral Na+/K+-ATPase, located in the ciliated cells [62–64]. Whereas several more ion transport processes are involved, Na+ and Cl− are the main drivers of the fluid movements. While the periciliary liquid layer normally contains NaCl in an amount below 50mM (< 0.29%), the upper layer of ASL would contain concentrations of these ions above 100mM: this gradient ensures effective and sustained transepithelial transport of ions and water, as well as effective ciliary beating [64]. Saline thus contributes to the hydration status of the nasal and airway mucosa, while it also regulates the height of the ALF.
Mucus properties. The properties of the mucins affect the MCC as well. Healthy mucus is a gel with low viscosity and elasticity, easily transported by ciliary action [61]. In contrast, pathologic mucus, such as produced during chronic pulmonary diseases or ARDS, has higher viscosity and elasticity: it is less easily cleared from the airways. Impaired mucus clearance induces cough, airway obstruction, and dyspnoea [61]. In elderly patients in particular, there is often already stasis of thick, dehydrated mucus within the nasal cavities and nasopharynx, leading to postnasal drip, cough, and globus (pharyngeus) sensation [65]. Decreased MCC may lead to persistent accumulation of mucus, so providing an environment for microbial growth of pathogens in the respiratory system and contributing to secondary infections and inflammation [66]. Saline equilibrates in the mucus, which leads to several actions of NaCl on the mucus: better diffusibility of pathogens into the mucin [67]; enhanced entrapment in the gelled mucus (gelling observed at 100 mM (0.6%) NaCl [30, 68]); reduced adhesion of mucins to the epithelium (observed at 0.9% NaCl) [69]; enhanced ciliary transportability and clearance (already observed from 0.5% (90 mM) saline onwards) [61, 70]; and so easier coughing up and swallowing of mucins, better cough clearance, and relief of (chronic) cough [69, 71]. As saline aerosol helps to fluidize the mucins in the deeper airway layers, also mucin-attached pathogens involved in secondary pulmonary infections are more easily cleared [66]. While most of these effects are obtained by isotonic or lower NaCl concentrations, highly dehydrated sputum and ASL, such as occurring in cystic fibrosis and chronic bronchopulmonary disease, require higher (hypertonic) saline concentrations, as to equilibrate the sputum for maximal transport by drawing additional water onto the ASL [71–73].
Saline thus contributes by different mechanisms to optimizing the MCC and to protect the airways against infection. This has been nicely illustrated in porcine ciliated airways: the viral yield of Influenza A virus in the ciliated epithelium was about two- to threefold higher 24 to 48-h post-infection in the case of ciliostasis, as compared to normal ciliary activity, while saline (up to 2%) led to full recovery of the ciliary activity and reversal of ciliostasis, thereby impeding the viral infection [42]; however, at NaCl concentration exceeding 2%, the recovery rate decreased the more the saline concentrations increased up to 11% NaCl hypertonicity [42]. This further underlines the modulatory role of the salt concentration.
Noteworthy, additional factors might affect the MCC. Adding bronchodilators to saline aerosols, such as salbutamol or terbutaline, may improve the nasal MCC [74, 75]. In contrast, many preservatives, excipients, antimicrobial agents, lidocaine/ anaesthetics, opioids, and mucolytics, such as acetyl cysteine, decrease the MCC [74, 76].
The ciliary activity and MCC are furthermore influenced by body and ambient temperature, pH, and moisture [62, 77]. These factors also modulate the dryness of the oronasal mucosa.
Role of saline in mucosal hydration
The nasal cavity gets moistened as warm breath condensate, originating from the lungs, moves over the cooler nasal epithelial surface during expiration. Drying of the respiratory mucus and excessive dryness in the nose are common problems that have been associated with reduced ciliary function and decreased MCC [41, 77]. For instance, passage of dry cold air current or chilling depresses the mucous membrane temperature and ciliary movement, which manifests to a greater extent in the nasopharynx than in postnasal spaces [77]. The speed of warming up of inhaled air depends on the respiratory frequency and volume of air inhaled, but hyperventilation leads to faster drying of the mucosa and reduced clearance from the lungs [41]. Elderly people may more frequently suffer from a dehydrated mucosa, while they also show altered cilia, slower ciliary beating, changed properties of mucus and slower MCC [65, 78]. Nasal dryness is also affected by the body temperature, the temperature of the nose fluctuating directly with the core body temperature [77]. Hence, also during high fever, there may be faster drying of the respiratory mucosa, making elderly patients with fever even more susceptible to viral aggression. Additionally, the prolonged use of facial masks is associated with dehydrated mucosa of the oronasal cavity [79]. This is not surprising, as the conditioning mechanism, consisting of mucosal cooling during inhalation and condensation of the saturated, warm, exhaled air [77, 80], is drastically reduced.
As a consequence, humidification with saline aerosol may be beneficial. It is not known whether the hydration by saline occurs via promoting or restoring the ciliary function and/or other factors. It has been shown that “a fringe of ciliary activity persists” as long as there is sufficient moisture [77]. However, if dryness of nasal epithelium lasted longer than 15–18 min in vitro, air humidification or water flushing of the epithelium could no longer restore the ciliary movement, while only isotonic saline or Ringer’s solutions did so [77]. In line with these observations on ciliary movement, the process of mucosal hydration in surface airway epithelia was found not to be dependent on the cilia itself, but in the first place on the presence of soluble mediators in the ASL [81]. The hydrating effects of saline (0.9–7.0%) have been well documented in chronic respiratory diseases that are associated with dehydrated mucins and reduced ASL in airways and/or lungs [72, 73, 82]. Yet, for a dry nose or larynx, isotonic saline is the concentration of choice to moisten the dry nasal mucosa [83, 84]. Health care professionals wearing well-fitting face masks report to suffer from less dryness in the nose and mouth if using isotonic saline nasal rinse/spray twice daily (morning/evening) [85]. Mucosal hydration by saline may also play a role in dry cough: nasal isotonic saline has been found to reduce dry cough associated with acute respiratory illness, such as during flu or common cold [86].
Interactions of saline with SARS-CoV 2]
NaCl can directly affect SARS-CoV-2 virus infectivity by interacting with its ionic or electrostatic charges. NaCl is listed as an antimicrobial against coronaviruses MHV-2, MHV-N (mouse hepatitis viruses), and CCV (canine coronavirus), as these viruses lose infectivity after exposure to NaCl 0.23% [87]. Machado et al. showed that the SARS-CoV-2 replication is dose-dependently inhibited by saline (0.8–1.7% NaCl) in Vero CL-81 cells [88]. Inhibition of viral replication started from 0.6% onwards, increasing to 50% inhibition at 0.9% (isotonic) saline and reaching 100% at 1.5% (so mildly hypertonic) saline. Saline, however, had no direct effect on the SARS-CoV-2 itself: saline-pretreatment of the virus could not inhibit subsequent viral replication in the host cells. The authors proposed different underlying mechanisms: (1) NaCl-induced hyperosmotic stress leading to the SARS-CoV-2 inhibition (yet, unlikely, as no direct effect on the virus was shown), (2) decreased expression of the phosphokinase C signalling pathway (yet, this would require time for down-regulation), and (3) depolarization of the host cells via ENaC and its sodium sensor, the Nax channel, thereby over-stimulating ENaC and leading to electrolyte movements stressing the mitochondria (unlikely as rather ENaC dysregulation occurs—see below).
We identified five interactions of saline, relevant to viral tropism, that have been documented and thus are reachable, already at concentrations with isotonic saline:
Chloride- and pH-sensitive conformation of ACE2. ACE2 is the entry receptor of SARS-CoV-2 and is present in the nose, oropharynx, and airways (particularly in ciliated cells) [89–91]: increasing saline concentrations have been shown to induce dose-dependently immediate steric hindrance in the ACE-2 receptor configuration for binding of angiotensin (Ang) II: the inhibition starts at 100 mM (0.58%) NaCl which is close to a minimal effective inhibitory concentration of saline on SARS-CoV-2 replication (0.8% NaCl) [88, 92, 93]. Also relevant pH-mediated effects on binding of the endosomal ACE2 receptor have been reported for the first SARS-CoV virus: these effects would occur through interaction at a terminal glycosylation site, thereby inducing less virus-receptor binding [94].
Inhibition of the viral protease 3CLpro. Human coronaviruses, such as SARS-CoV and SARS-CoV-2, typically harbour and make use of 3CLpro, a chymotrypsin-like cysteine protease that regulates the viral replication machinery. There is no significant blocking effect up to 50 mM of NaCl on 3CLpro. However, higher concentrations (100 mM (~0.6%)) of NaCl does exhibit an almost full 3CLpro block, while the enzyme activity is also strongly pH dependent (fully blocked at pH<6.0) [95–99]. Significant inhibition and disruptive effects on the enzyme’s dimerization occur as NaCl concentrations move to values of >100 mM [96, 100, 101].
Inhibition of the host protease furin. TMPRSS2 and furin are involved in the proteolytic activation (priming) of SARS-CoV-2 spike protein [102–104]. The proteolytic activity of furin was previously found to be inhibited by NaCl, starting at 75 mM (~0.4%) onwards, and achieving > 90% inhibition at 100 mM (0.6%), and complete inhibition at 200 mM (~1.2%). No inhibitory effect was observed with KCl or CaCl2 solutions that rather stimulated furin [105]. The inhibition is concordant with the finding of a furin insertion site by Anand et al that seems to be uniquely acquired by SARS-CoV-2 [112].
PH-shifts in nasal and airway environment. The pH of the incubation medium directly affects the unfolding of the SARS-CoV-2 spikes, which is hampered at pH 4.5 [106]. A role of mild acidification in impeding SARS-CoV-2 replication is also suggested by the outcomes of in vitro studies in Vero-cells: complete inhibition of viral replication was observed with pure (unbuffered) saline (pH 5.5) and with NH4Cl (pH range 4.6–6.0), but not with phosphate buffered saline (PBS pH 7.4) [88, 107]. Both the pH and Na-concentration might be relevant to viral replications, similarly as suggested by the volume effects of added unbuffered saline, various sodium-containing buffers, and different pH levels as transport medium, observed with external polymerase on the detection levels of extraction-free SARS-CoV-2 RT-PCR of saliva samples [108]. The pH of the nasal or airway surface liquid may be relevant to contracting the virus, the more as the healthy nose and airways, as well as sputum, have a slightly acidic pH (pH 5.5–6.5): this pH changes to more alkaline pH (pH 7.2–8.3) during common colds and chronic respiratory conditions [109]. The mechanisms whereby saline acts acidic in vivo are complex and involve various channels in the respiratory epithelium [109, 110].
Interaction with ENaC. As already discussed, ENaC is the main mechanism for maintaining the height of ASL and regulating the ALF by stimulating the sodium absorption, so also preventing immersion and immobilization of the ciliated epithelium [62–64, 111]. Based on protein sequencing, Anand et al. [112] identified a S1/S2 cleavage site in SARS-CoV-2 that can mimic the proteolytic activation of human ENaC: the virus can hijack several proteases that are involved in the activation and regulation of ENaC (TMPRRSS2, furin, prostasin, and matriptase [113–115]), furin in particular in view of a unique furin insertion site in its S protein [112]. Hence, this may lead to dysregulation of ENaC and the fluid balance in the lungs [116]. Yet, fluid homeostasis is also regulated by the cooperation between ENaC and the sodium sensing Nax channel, directly activating ENaC [117, 118]. This mobilization and activation of ENaC via the Nax sensing channel activating ENaC is likely to take place with saline because the threshold for Nax activation in vitro is 150 mM (0.88%) extracellular Na+ [117] and thus reachable with isotonic saline. In line with this mechanism, it was shown that iso- and hypertonic saline administered to an in vivo rat model was able to overcome pharmacological ENaC blockade of lung, leading to reabsorption of lung secretions [119].
Myeloperoxidase (MPO) activity
The interactions between NaCl and MPO are very complex, while those observed between SARS-CoV-2 and MPO so far have not been elucidated. The main actions of saline on MPO are discussed in Supplement 3. Basically, addition of NaCl to H2O2 producing cells in vitro will shift the MPO activity from peroxidation (to form H2O2) towards chlorination (to form HOCl). NaCl thus leads to the production of increased virucidal HOCl (bleach): this effect on MPO is already observed at 10 mM NaCl (0.058%), while the virucidal action of HOCl is observed at 0.09–1.7% NaCl [120, 121]. Moreover, whereas human MPO activity in neutrophils is active from 25 to 140 mM NaCl (0.14–0.82%) [122], the phagocytosis of pathogens requires continuous supply of chloride (Cl−) to sustain the HOCl generation in the phagosomes [123]. Alternatively, because Cl− also competes with thiocyanate as a natural substrate for MPO activity, saline may shift the substrate thiocyanate towards antioxidant pathways, while its concomitant presence with thiocyanate increases the yield of virucidal hypothiocyanite formation [124]. NaCl has also been shown to interact with reactive oxygen species production, as well as with extracellular neutrophil trap formation [125]. These complex interactions are beyond the scope of this review but have received substantial attention in the context of (severe) COVID-19 [see Supplement 3]. The effects of NaCl on MPO may be clinically relevant in less severe presentations as well, as a pilot study using aerosols of electrolyzed saline (so containing HOCl) proved to be highly effective in the treatment and viral clearance of SARS-CoV-2 in ambulatory patients with COVID-19 [126].
Translation of the mechanisms into clinical benefits
Supplement 4 reviews the most relevant sources supporting the use of isotonic and hypertonic saline aerosol in (non-COVID-19) ARDS and bronchiolitis, as well the scarce data obtained so far in COVID-19 positive patients. In ARDS or bronchiolitis by other respiratory agents, such as the syncytial respiratory virus infection, aerosols of both isotonic and hypertonic saline have been shown to exert beneficial effects, such as improving respiration and the clinical severity scores, while hospitalization rate and/or duration were reduced, in particular in children (Table 2A, Supplement 4). Yet, overall, better outcomes were seen with isotonic as compared to hypertonic saline, in part because the latter was associated with worse cough in infants. In order to treat and prevent common cold and/or flu, studies support both acute and preventive use of saline irrigation (Table 2B, Supplement 4). The benefit of saline irrigation to promote recovery from common cold is acknowledged by the WHO [127, 128]. With regard to treatment of COVID-19 patients, limited pilot studies are available thus far. Two studies (one in upper respiratory tract infection caused by seasonal coronaviruses or common cold virus [29], one in symptomatic COVID-19 [15]) use a control group and support the early use of nasal and oral rinse with hypertonic saline, started within 48 h of symptom onset [15, 29]. Two observational studies are relevant from a safety perspective. A survey of COVID-19 patients with anosmia mentioned the use of saline by 57% of the patients: anosmia resolved over time but the recovery was not documented by initial treatment; to note is that none was hospitalized or developed ARDS [129]. In a German study in patients with a more severe spectrum of illness, daily isotonic saline aerosol was used by 60 patients with COVID-19 ARDS. Administration was started prior to (bio-aerosol generating) non-invasive ventilation procedures: only 3 deteriorated and required intubation (all with severe comorbidity, of which one died); no health care workers got infected [130]. For more details, we refer to Supplement 4.
Table 2.
(A) Pharmacological effects of saline in the setting of COVID-19 and effective concentrations. (B) Reference values of Na+ and Cl- in nose, airway and lung fluids
| Parameter | Physiology/pathophysiology relevant to common cold/respiratory infection | Pathophysiology with regard to SARS-CoV-2 | Pharmacological effects of saline or reference concentrations |
|---|---|---|---|
| (A) Pharmacological effects of saline | |||
| Wetting—effect on ALF | |||
| Bio-aerosol/vesicle generation and ALF spreading |
Airborne transmission of COVID-19 by aerosol such as speaking Superspreading events in closed and non-ventilated areas |
SARS-CoV-2 attacks surfactant producing alveolar type II epithelial cells (AT2), raise the alveolar surface tension and so lead to alveolar collapse Infection of lower airways and lungs thought to occur via micro-aspiration of ultrafine droplets [144] |
NaCl (0.9%) leads to: - Wetting and fluid aggregation, leading to: - suppression of aerosol/vesicle formation: [72% reduction of bio-aerosol formation after 6 min aerosol, lasting up to 6 h] - Easier deposition/filtering of heavier /larger drops—so less easily deposition in the deeper airways and lungs - Reduction of viral shedding (shown for other viruses) - Improved ALF spreading and airway compliance (applied in premature newborns) |
| Viral shedding of rhinovirus/coronavirus |
Transmission of common cold viruses and COVID-19 occurs by apical shedding of viral particles and/or exosomes (secretion of virus containing vesicles) |
Viral shedding of SARS-CoV-2 may take place during infection |
NaCl (0.9%-3%) provides (shown for viruses other than SARS-CoV-2): - In vivo rinse effect, causing lower viral titres (5 days until back to initial values) - In vivo reduces viral shedding as demonstrated for other viruses (rhino- and other coronaviruses) |
| Mucociliary clearance (MCC) | |||
| MCC (ciliary beat) | Primary defence mechanism to expel pathogens, viral particles and debris |
SARS-CoV-2 preferentially targets ciliated cells in nose, nasopharynx, airways, olfactory bulb and reduces the MCC |
NaCl (0.9%) promotes ciliary beat and MCC in vitro and in vivo Dependent on osmolality/ionic strength - is reduced with hypotonic saline - variable effect with hypertonic saline in vitro: effects observed on the epithelial cell membrane (altered electrical conductance, permeability, cell deformation) |
| Mucins, containing sialic acids | Captures pathogens, viral particles and debris to remove these by MCC or upon coughing |
SARS-CoV-2 spike protein binds sialic acid (if not buffered at pH>7) and is found in sputum |
NaCl gels the mucus in vitro [shown at >90 mM—0.6%], so altering the MCC and cough clearance [> 0.9%] |
| Cough clearance |
Associated with MCC Stasis of thick, dehydrated mucus within the nasal cavities and nasopharynx lead to postnasal drip and cough |
SARS-CoV-2 is frequently associated with cough, while present in sputum [137] |
NaCl (0.6%) gels the sputum and (0.9%) reduces its adhesion, so promoting cough clearance |
| Mucosal hydration | |||
| Mucosal (de)hydration |
Periciliary fluid normally contains <50 mM NaCl (0.29%), while higher concentrations at the tips (>100 mM – 137 mM) as to maintain absorption gradient and ciliary movement. Ciliary beat and MCC is inhibited by dry mucosa. |
SARS-CoV-2 may lead to dysregulation of Na+-homeostasis in the airways/lung [116] More severe SARS-CoV-2 is frequently associated with conditions of dry mucosa, such as in the elderly, or during wearing face masks, fever, and infection |
In vitro and in vivo - Isotonic (0.9% NaCl): hydrating effect paralleling ciliary functioning - Hypertonic (usually 3% NaCl): hydrates and has strong rinsing effect (osmosis); |
| Viral replication | |||
| Viral replication | Replication of SARS-CoV-2 virus leads to overlapping common cold symptoms |
In vitro replication of the virus is inhibited by pure saline [88] |
NaCl inhibits SARS-CoV-2 replication in vitro in Vero-cells: pure saline • MIC: 0.6% • IC50: 0.9%—IC100: 1.5% [88] |
| (Na)Cl concentration at ACE2- expressing cells |
Strong Cl--dependency of ACE2-receptor : from a certain concentration onwards, NaCl induces steric hindrance of ACE2 receptor for its substrates [92] |
ACE2 is the entry receptor for SARS-COV-2 |
NaCl causing steric hindrance of ACE2 receptor in vitro: in HEPES • MIC: 0.5% • IC50: 1.4% |
| SARS-CoV-2 protease 3CLpro |
3CLpro is a protease shared by coronaviruses: its use upon proteolytic processing of their replicase polyprotein leads to production of infectious virus |
SARS-COV-2 uses its own protease 3CLpro for replication |
NaCl inhibits SARS-CoV-2 protease 3CLpro (deduced from graph): • MIC80: ~0.9% (150 mM) • IC90: ~1.8% (300 mM) |
| Host protease furin | Furin contains a sodium-sensor and is inhibited from a certain concentration by NaCl, but not by calcium or potassium chloride [105] |
SARS-COV-2 needs TMPRSS2 and has a furin-binding domain to prime the spike forACE2 entry |
NaCl inhibits furin (deduced from graph): • MIC: ~0.45% (75 mM) • IC~90%: ~0.9% (150 mM) • IC100: ~1.2% (200 mM) [105] |
| ENaC |
ENaC determines hydration and height of ALF and MCC, as well as drives reabsorption of diluted ALF hypersecretion; ENaC activity is regulated by various proteases and by the sodium sensor Nax |
SARS-CoV-2 possesses unique furin-insertion site, as well hijacks, so shares several proteases that inhibit/regulate ENaC, leading to less availability for ENaC and so to less fluid absorption in the lungs [112–116] |
Nax sensing of NaCl at 0.9%NaCl, stimulating ENaC and so sodium (re)absorption, contributing to the volume control and Na+-homeostasis, the control of ALF height and MCC [Threshold Nax: ~150 mM] [117] NaCl (0.9–1.5%) allows to overcome secretion in the lungs following pharmacologically induced blockade of ENaC [119] |
| Myeloperoxidase formation (MPO) | |||
| Hypochlorous acid (HOCl), myeloperoxidase (MPO) |
– Antiviral effects of NaCl are attributed to the production of HOCl from Cl- ions – HOCl is mainly produced by MPO : HOCl generation in the phagosomes requires a continuous supply of chloride |
SARS-CoV-2 is sensitive in vitro to HOCl |
NaCl 15-300 mM (= 0.09 to 1.7%) results in HOCl production MPO activity in neutrophil phagosomes increases with increasing NaCl concentrations from 25 mM (0.14%) NaCl onwards [112] |
| Relevance not found related to SARS-CoV-2 | |||
| CFTR | Apical Cl- secretion is mediated mainly by CFTR, relevant to dehydrated ALF (as in CF) |
Not reviewed – No specific interactions relevant to viral infection known so far |
(?) Rationale remains unclear besides the known effect of hypertonic saline in osmosis creation [so far not found to be relevant to SARS-CoV-2; relevant to conditions characterized by dehydrated ALF, thick mucus and impaired MCC.] |
| (B) Reference values in body fluids | |||
| Body fluid | Reference concentration of Na+ and Cl- | ||
| Saliva |
Low to highly variable :3–125 mM, average 5.6 mM [143] |
||
| Nose | |||
| Nasal fluid |
Averages: Na+: 128–150 mM; Cl−:>139 mM Highly variable -> range : 85–225 mM Na+ [139] |
||
| Inspiring via mouth versus via nose |
Na+:120 mM (mouth), 189 mM (nose) Cl−:134 mM (mouth), 205 mM (nose) [139] |
||
| Nasal mucus |
Na+:139–150 mM Cl−: 139 mM [139] |
||
| Common cold |
Na+: average 135 mM vs – 185 mM (spontaneous respiration healthy) – 128 mM on sneezing [139] |
||
| Airways | |||
| ASL | > 50 mM Na+ Cl- | ||
| Periciliary liquid |
≤ 50 mM NaCl [142] |
||
| Outer ASL |
>100; average 137 mM NaCl [142] + NaCl 0.9%: ALF remains isotonic during the 5 hours assessed ; only hypotonic solutions are not sustained] [142] |
||
| Distal airway fluid |
122 ± 2 mM Na+, 123 ± 4 mM Cl− [144] |
||
| Lung | |||
| ALF |
82-91 mM for Na+ and 82–108 mM for Cl− [141] |
||
| Sputum (mixture of saliva and mucus coughed up during infection/disease) |
0.74 % (123 mM) NaCl [140] |
||
ALF alveolar lining fluid, CFTR cystic fibrosis transmembrane conductance regulator, Cl− chloride− HOCl hypochlorous acid, IC50 inhibitory concentration to inhibit 50%, IC100 inhibitory concentration to inhibit 100%;MCC mucociliary clearance, MIC minimum inhibitory concentration, MPO myeloperoxidase, Nax sodium channel x, Na+ sodium
Discussion
In a majority of qPCR-positive persons, SARS-CoV-2 infection presents as asymptomatic or mild disease; yet, a minority of mainly older persons or patients with comorbidities or immune deficiency will develop severe ARDS. As COVID-19 ARDS is a serious complication, it requires early recognition and comprehensive management. Any preventive or acute treatment proposed for common cold—overlapping with COVID-19—should guarantee sufficient safety, if applied in an early stage. Since transmission may take place through bio-aerosol in the period prior to getting seriously symptomatic, effective hygiene measures, such as gargling or rinses, should be initiated as early as possible, as proposed by the DGKH. Treatment ideally should also be easy, safe, and at low cost. This analysis shows that saline rinse and aerosol are a safe and effective approach to treat and prevent upper respiratory tract infections and common colds, while the evidence presented in this manuscript also provides rational arguments for its application to contain and relieve mild COVID-19 infection, if started early within 48 h after the onset of symptoms. Although formal studies are welcome to address the optimal saline concentration(s) and frequency of applications, the data support the use of both isotonic and hypertonic saline, although for maintenance in the nose and/or lungs isotonic saline may be associated with less adverse events. Our recommendations based on this analysis are summarized in Table 3. These are in line with online recommendations to German consumers [1–7], and provide strong support for the gargling/rinse recommendations by the DGKH for use in the household, nursing homes, and school setting [9]. Our analysis thereby substantiates several mechanisms, relevant to SARS-CoV-2 infection.
Table 3.
Pharmacy practice recommendations for saline use, based on this literature analysis
| • Saline does not destroy SARS-CoV-2 and is thus only to be used as an add-on to basic hygiene measures | |
| • In case of acute common cold or upper respiratory symptoms in times of COVID-19 | |
| Nasal rinse |
From first symptoms of common cold or upper respiratory symptoms Rinse with pure “isotonic” saline (0.9%) 2–3 times/day* • This concentration combines a reliable positive effect on the MCC with desirable partial receptor block of the entry receptor and is well established for treatment or prevention of common cold and upper respiratory symptoms, and as nebulization/aerosol for treatment of bronchiolitis • Isotonic saline is devoid of the side effects that have occasionally been reported for hypertonic saline (effect on cell morphology, increased nasal epithelial permeability, nasal burning/irritation, and when nebulizing: induction of bronchoconstriction or cough) • [*Isotonic saline for nasal rinse unless if hypertonic saline is already used in the frame of other indications, in which case it can be continued] • Heating the saline is not needed; concentrations of salt reached upon inhaling sea salt solution are unknown • No special devices are needed |
| + Gargling |
In case of common cold symptoms with throat involvement: Or in case of COVID-19 positive testing -> Gargling can be done with self-made hypertonic saline 1 table spoon or 20g kitchen salt per litre, or as formulated by DGKH [9]: 1 flat-filled teaspoon in 100 mL: up to 12 times per day -> Don’t swallow; discard in sink |
| Respiratory care |
When using a saline aerosol with a nebulizing apparatus to reduce bio-aerosol formation and/or remove phlegm, hydrate the airways and/or reduce cough -> Continue with habitual strengths of sterile saline concentration (0.9%), unless otherwise indicated, or ask pharmacist -> Follow the cleaning instructions of the manufacturer for the cleaning of the device, to end with hand hygiene -> Preferentially to be performed in well-aerated place or outside |
| See your doctor if not getting better and/or if feeling short of breath or/and very sick with high temperature | |
| • Preventive use | |
|
Daily nasal and oral gargling may be useful in situations, such as also formulated by the DGKH [9]: e.g. • As nasal saline spray for hydration of the nasal mucosa (e.g. when feeling dehydrated due to carrying a mouth mask) • As gargling and nasal rinse, before seeing a frail or immune compromised person, before meals and qPCR-positive household, upon outbreak of respiratory illness in schools, or after visiting an unexpectedly crowded location, and thus if risk of contamination is believed to be higher Distancing and hygiene measures will prevail at any time |
|
| • Protective measures: always combine with protective measures | |
|
Nose and Mouth • Collect superfluous liquid with tissue paper and discard or in water with detergent and safely dispose in sink • Wash and/or safely collect the saline recipients, to end with hand hygiene Nebulization/aerosol • If there is no way to isolate or to aerate the room, use cotton sheets to cover your lap (plus head) to prevent aerosol dispersion • Ventilate the room and wash hands |
|
On one hand, saline provides advantages for preventive uses as a nasal spray or a rinse for the nose and oral cavity, because saliva has been implicated in transmission, while the unique physicochemical properties of saline lead to suppression of droplet formation [23–26]. If administered as a 0.9% saline aerosol for 6 min, the latter effect is the highest among superspreaders and leads to larger droplets that are more easily filtered by facial masks [23, 26]. This mechanism of action of saline was also shown to work experimentally in a swine model by preventing the airborne transmission of flu virus [25]. Its aerosol use, add-on to the strong protective hygiene measures in the hospital during non-invasive ventilation in COVID-19-ARDS patients, was not associated with enhanced transmission [130]. The findings are encouraging and invite to follow the recent recommendation of the DGKH to use gargling and nasal rinse with saline in a number of preventive situations, such as in care setting before meals or activities involving seniors or revalidating persons, family or professional reunions, church or religious gatherings (even if COVID-proof), or for kids in schools [9]. Infected bio-aerosols are thought to be formed during coughing, or also during speaking or singing when bronchial secretions move over the vocal cords [131, 132], while bio-aerosols are insufficiently blocked by cotton and medical masks (even surgical or N95 and if well-fitting) [133]. The unique wetting properties of NaCl identified in this study justify oronasal hygiene to be combined with the current face mask and distancing measures. In particular, maintenance of oronasal hygiene with saline (as aerosol, spray, or rinse) may achieve less penetration of the bio-aerosol through a mask [35]. The finding that upon evaporation the enhanced salinity may deform virus-containing droplets [38] deserves further study, as to find out more on SARS-CoV-2 decay in NaCl/virus-loaded bio-aerosols.
On the other hand, many mechanisms of saline were identified that strengthen the innate primary defence mechanisms, such as promoting the MCC and interaction with MPO. These are complemented by various mechanisms inhibiting SARS-CoV-2 replication—all presenting at the saline concentrations currently in use (Table 2). Multiple mechanisms of action justify early use from the first onset of symptoms of COVID-19. Firstly, these mechanisms of NaCl may prevent massive viral replication and destruction of the cilia in the oral and nasal cavities—possibly by inhibiting the protease activity of furin and 3CLpro: SARS-CoV-2 targets ciliated cells in the nose and airways, releasing virus or abundant secretory vesicles, while also impairing the MCC [134–136]. The SARS-COV-2 spike protein also contains glycosides that effectively bind sialic acids (the main constituents of mucus) [137]: as such, salt-induced gelling of mucus by the slightly acidic saline will improve viral clearance. As evidenced by Table 2, most in vitro pharmacological/pharmacodynamic effects of saline were achievable at concentrations reached by isotonic saline. This is reassuring from a pathophysiological point of view, since SARS-COV-2 mainly initiates infection in the nose and seeds by micro-aspiration to the lower airways, after which tracheal-produced virus would further seed via aspiration into the deep lung [136, 138]. Moreover, the reference values of Na+ and Cl− in the relevant fluids (saliva, nasal mucus and fluid (during common cold), periciliary liquid, ASL, ASL and sputum [139–144]) revealed to be generally lower than those in the mM NaCl concentration provided by isotonic saline (see Table 2B). As large volumes of saline can be safely repetitively used, this thus allows achieving relevant concentrations throughout the day to reach the described effects. Also safe long-term use is well documented in chronic lung disease. The analysis further suggests that due to its isotonic nature, saline may also help to reverse the bronchoconstriction in the case of hyper-secreted, thus potentially hypotonic ALF, as well as enhance cough clearance [17–20, 23, 30]. In view of the vast literature of saline, a limitation of this analysis though is that not all effects of saline or mechanisms relevant to SARS-CoV-2 may have been addressed.
To note, nasal and airway pH may be important in contracting COVID-19, in view of the observed pH-effects on protease and viral replication inhibition (Results Part 3). In addition, the pH affects the mucin binding by SARS-CoV-2, which was found to be absent if the mucins were buffered at pH 7.0 [145]. Our recommendation is therefore to use pure saline (pH 5.5) for respiratory hygiene rather than buffered (seawater) sprays, although these may already work to some extent through the simple rinsing effect [86, 146]. Although often lukewarm or heated saline solutions are recommended for rinsing or inhaling, there is no evidence that this leads to better action of salt than the then the use of saline at room temperature [147]. Other nasal spray compositions—with more or buffering ions, surfactants, emulsifiers, excipients, preservatives and/or active substances— may not necessarily lead to the same effects as identified in this analysis for pure saline, or may inhibit the ciliary beating [74–76]. Addition of certain surfactants to liquid formulations may enhance bio-aerosol formation [23, 30, 31] or be ciliotoxic (e.g. polyvidone-iodine [148]), while many (such as virucidal essential oils) may not respect the natural microbiome. Also the DGKH recommends saline formulations without additives, such as preservatives or decongestants [9]. Similarly, trypsin–containing sprays claiming protection against COVID-19 based on a simple trypsin digest [149] are to be avoided, because the protease trypsin has been shown to rather enhance viral invasion and syncytia formation in appropriately designed host models [107, 150]; such sprays sold OTC as medical devices in the European Union are prohibited for sale in Germany because of lack of proof of efficacy.
We propose isotonic rather than hypertonic saline unless for gargling, where hypertonic 2% NaCl is recommended (also by the DGKH). Isotonic saline is devoid of the side effects that we identified throughout this analysis, such as changes in cell morphology and increased nasal epithelial permeability, nasal burning/irritation, and induction of bronchoconstriction or cough. Home-made saline (prepared with simple kitchen salt) is often proposed in the Internet (DGKH: tea spoon flat filled with table/kitchen salt (=2g/100mL corresponding to 1 soup spoon (20 g) in one litre of water). In line with the DGKH, usual applications for airway care are to be carried out 2 to 3 times a day, while ongoing clinical trials in symptomatic COVID-19 patients propose oronasal irrigation up to 12 times per day. Sterile sprays and uni-doses may be safer for nebulizing aerosol. Whether COVID-19 infection only requires (oro)nasal irrigation, or also inhalation of nebulized iso- or hypertonic saline, or whether it can add to other treatment strategies to limit transmission or damage of SARS-CoV-2 to the lungs, deserves further evaluation.
At last, some myths can be debunked. (1) Administration of saline as rinse or aerosol should not be confounded with transmission-prone aerosolizing procedures—a common misconception in respiratory care. Use of oxygen flow or invasive procedures causes the formation of virus-loaded bio-aerosols, by providing air over the infected surfactant-containing surface of the respiratory tract. Saline aerosol in contrast leads to suppression and reduction of exhaled bio-aerosol, as reviewed under Results (point 1). Obviously, saline aerosol and irrigation should be combined with the basic hygiene measures for COVID-19 (see Table 3), with use of disposable or washable tissues to collect superfluous rinse and mucus, with hand washing and adequate room ventilation. (2) Neither is there evidence that nasal rinse would lead to worsening olfaction. Nasal saline irrigation has no effect on normal olfaction [151], and its use in nasal disease has not led to adverse outcome [152]. Its hydrating effect may possibly help to prevent and overcome dry nose during COVID-19 [153].
To summarize, despite the formal effects of saline should be studied in the future by use of (randomized controlled) trials, this review provides sufficient reasoning for the successful application and clinical relevance of saline in the context of mild and early COVID-19. Numerous actions of NaCl relevant to bio-aerosol reduction, primary defence mechanisms such as mucociliary clearance and HOCl production, and to containing SARS-CoV-2 infectivity were identified. These mechanisms are relevant as they are achievable at isotonic (or lower) and hypertonic saline concentrations, used for nasal rinse, respiratory hygiene (aerosol) and oral gargling. They may underlie the traditional use of saline for common cold.
Supplementary Information
(DOCX 29 kb)
(DOCX 27 kb)
(DOCX 37 kb)
(DOCX 32 kb)
Availability of data and material
Referenced material
Abbreviations
- ARDS
acute respiratory distress syndrome
- ALF
alveolar lining fluid
- ASL
airway surface liquid
- MCC
mucociliary clearance
- ACE
angiotensin converting enzyme
- Ang
angiotensin
- AT2
alveolar type 2 cells
Author contribution
Literature search: Huijghebaert Suzy and Hoste Levi
Data collection: Huijghebaert Suzy
Study design: Huijghebaert Suzy – Hoste Levi and Vanham Guido
Analysis of data: Huijghebaert Suzy – Hoste Levi and Vanham Guido
Manuscript preparation: Huijghebaert Suzy – Hoste Levi and Vanham Guido
Review of manuscript: Huijghebaert Suzy – Hoste Levi and Vanham Guido
Funding
Levi Hoste receives funding from the VIB Grand Challenges program.
Declarations
Competing interest
The authors declare no competing interests.
Footnotes
The original online version of this article was revised: The correct presentation of the Author names are shown in this paper.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
4/24/2021
A Correction to this paper has been published: 10.1007/s00228-021-03141-w
References
- 1.HNO-Ärzte im Netz (2020) Herausgegeben vom Deutschen Berufsverband der Hals-Nasen-Ohrenärzte e.V.) Tipps zur richtigen Nasenpflege [Tipps for adequate nasal care]. https://www.hno-aerzte-im-netz.de/unsere-sinne/hno-hygiene/tipps-zur-richtigen-nasenpflege.html. Accessed 19 June 2020
- 2.Lungenartze im Netz (Lung doctors in the Net) (2020) Einfaches Inhalieren kann Tröpfcheninfektion effektiv eindämmern. [Simple inhalation can limit efficiently droplet infection] https://www.lungenaerzte-im-netz.de/news-archiv/meldung/article/einfaches-inhalieren-kann-troepfcheninfektion-effektiv-eindaemmern/. Accessed 19 June 2020
- 3.Praxisvita (das Portal für Gesundheit & Medizin) (2020) Inhalieren bei Corona: Wie wirksam ist das Hausmittel? [Inhalation during Corona; How effective is this home remedy?] https://www.praxisvita.de/coronavirus-dieses-hausmittel-hilft-bei-leichten-symptomen-18411.html . Accessed 19 June 2020
- 4.Leichter Atmen bei Lungen- und bronchialerkrankungen (2020) Corona: Pflege der Atemwege vermindert Infektionsrisiko [Corona: Care of the airways reduces the risk of infection]. [24.03.2020] https://www.leichter-atmen.de/copd-news/atemwegspflege. Accessed 19 June 2020
- 5.PARI-Blog (2020) Treatment and nebuliser therapy for COVID-19 in hospital. Interview with the Prof. Dr Kamin, Medical Director of the Hamm Lutheran Hospital. https://www.pari.com/int/blog/treatment-and-nebuliser-therapy-for-covid-19-in-hospital-interview-with-the-prof-dr-kamin-medical-director-of-the-hamm-lutheran-hospital/. Accessed in English 27 July 2020. - Firstly accessed in German: Accessed 19 June 2020
- 6.Betreut.de (2020) Coronavirus: Was Senioren & ihre Betreuer wissen müssen. [Coronavirus: What seniors and care givers need to know] www.betreut.be. Accessed 14 July 2020
- 7.ETH Zurich (2020) Mit Atemwegspflege das Infektionsrisiko senken. [With airway care decrease the risk of infection.] https://ethz.ch/de/news-und-veranstaltungen/eth-news/news/2020/03/zukunftsblog-viola-vogel-mit-atemwegspflege-das-infektionsrisiko-senken.html. Accessed 14 July 2020
- 8.Bronchiectasis Toolbox (2020) Hydration and humidification. https://bronchiectasis.com.au/physiotherapy/principles-of-airway-clearance/hydration-and-humidification. Accessed 13 July 2020
- 9.Kramer A, Eggers M, Hübner N-O et al (2020) Empfehlung der DGKH. Viruzides Gurgeln und viruzider Nasenspray [Virucidal gargling and virucidal Nose sprays]. Deutsche Gesellschaft für Krankenhaushygiene e.V., 01.12.2020. Accessed 9 January 2021. https://www.krankenhaushygiene.de/pdfdata/2020_12_02_Empfehlung-viruzides-gurgeln-nasenspray.pdf
- 10.Sciensano (2020) Consensus over het rationeel en correct gebruik van mondmaskers tijdens de COVID-19-pandemie [Consensus on the rational and correct use of mouth masks during the COVID-19 pandemic]. https://covid-19.sciensano.be/sites/default/files/Covid19/consensus%20on%20the%20use%20of%20masks_RMG_NL.pdf. Accessed 13 July 2020
- 11.Sciensano (2020) Procedure voor huisartsen in geval van een mogelijk geval van COVID-19. Versie 08 juli 2020. [Procedure for doctors in the event of a possible case of COVID-19]. https://covid-19.sciensano.be/sites/default/files/Covid19/COVID-19_procedure_GP_NL.pdf . Accessed 13 July 2020
- 12.APB (2020) Aerosoltoestellen [Aerosol devices]. Information Update 20 March 2020. https://www.apb.be/APB%20Documents/NL/All%20partners/CORONAVIRUS_AEROSOL_VERHUUR_20_03_20.pdf. Accessed 19 June 2020
- 13.World Health Organization (2020) Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. Scientific Brief, 29 March 2020. https://www.who.int/publications-detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations. Accessed June 19, 2020.
- 14.Pfeifer M, Ewig S, Voshaar T, et al. Position paper for the state-of-the-art application of respiratory support in patients with COVID-19. Respiration. 2020;99:521–541. doi: 10.1159/000509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura KS, Freeman MH, Wessinger BC et al (2020) Interim analysis of an open-label randomized controlled trial evaluating nasal irrigations in non-hospitalized patients with COVID-19. Int Forum Allergy Rhinol Sep 11 [Epub ahead of print]. 10.1002/alr.22703 [DOI] [PMC free article] [PubMed]
- 16.ClinicalTrials.gov Identifier: NCT04347538. Impact of nasal saline irrigations on viral load in patients with COVID-19. https://clinicaltrials.gov/ct2/show/record/NCT04347538?term=saline&cond=covid-19&draw=2&rank=1
- 17.Santos FKG, Barros Neto EL, Moura TMCPA, et al. Molecular behavior of ionic and nonionic surfactants in saline medium. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2009;333:156–162. doi: 10.1016/j.colsurfa.2008.09.040. [DOI] [Google Scholar]
- 18.Staszak K, WieczorekD MK. Effect of sodium chloride on the surface and wetting properties of aqueous solutions of cocamidopropyl betaine. J Surfact Deterg. 2015;18:321–328. doi: 10.1007/s11743-014-1644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avery ME, Mead J (1959) Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Di Child 97(5_Part_I):517–5523. 10.1001/archpedi.1959.02070010519001 [DOI] [PubMed]
- 20.Ghadiali SN, Gaver DP (2008) Biomechanics of liquid-epithelium interactions in pulmonary airways. Respir Physiol Neurobiol 163(1-3):232-243. 10.1016/j.resp.2008.04.008 [DOI] [PMC free article] [PubMed]
- 21.Huang J, Hume AJ, Abo KM et al (2020) SARS-CoV-2 infection of pluripotent stem cell-derived human lung alveolar type 2 cells elicits a rapid epithelial-intrinsic inflammatory response. bioRxiv [Preprint]:175695. 10.1101/2020.06.30.175695 [DOI] [PMC free article] [PubMed]
- 22.Takano H. Pulmonary surfactant itself must be a strong defender against SARS-CoV-2. Medical Hypotheses. 2020;144:110020. doi: 10.1016/j.mehy.2020.110020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards DA, Man JC, Brand P, et al. Inhaling to mitigate exhaled bioaerosols. Proc Natl Acad Sci USA. 2004;101(50):17383–17388. doi: 10.1073/pnas.0408159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards DA, Fiegel J, DeHaan W, et al. Novel inhalants for control and protection against airborne infections. Resp Drug Delivery. 2006;1:41–48. [Google Scholar]
- 25.Edwards D, Hickey A, Batycky R, et al. A new natural defense against airborne pathogens. QRB Discovery. 2020;1:e5. doi: 10.1017/qrd.2020.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiegel J, Clarke R, Edwards DA. Airborne infectious disease and the suppression of pulmonary bioaerosols. Drug Discov Today. 2006;11(1-2):51–57. doi: 10.1016/S1359-6446(05)03687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonds A, Hanak A, Chatwin M, et al. Evaluation of droplet dispersion during non-invasive ventilation, oxygen therapy, nebuliser treatment and chest physiotherapy in clinical practice: implications for management of pandemic influenza and other airborne infections. Health Technol Assess. 2010;14:131–172. doi: 10.3310/hta14460-02. [DOI] [PubMed] [Google Scholar]
- 28.Hendley JO, Gwaltney JM. Viral titers in nasal lining fluid compared to viral titers in nasal washes during experimental rhinovirus infection. J Clin Virol. 2004;30(4):326–328. doi: 10.1016/j.jcv.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Ramalingam S, Graham C, Dove J, et al. A pilot, open labelled randomised controlled trial of hypertonic saline nasal irrigation and gargling for the common cold. Sci Rep. 2019;9:1015. doi: 10.1038/s41598-018-37703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe W, Thomas M, Clarke R, et al. Why inhaling salt water changes what we exhale. J Colloid Interface Sci. 2007;307:71–78. doi: 10.1016/j.jcis.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 31.Patel A, Longmore N, Mohanan A, Ghosh S. Salt and pH-induced attractive interactions on the rheology of food protein-stabilized nanoemulsions. CS Omega. 2019;4(7):11791–11800. doi: 10.1021/acsomega.8b03360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q, Li W, Hu N, et al. Ion concentration effect (Na+ and Cl-) on lipid vesicle formation. Colloids Surf B Biointerfaces. 2017;155:287–293. doi: 10.1016/j.colsurfb.2017.04.030. [DOI] [PubMed] [Google Scholar]
- 33.Liu S, Novoselac A. Transport of airborne particles from an unobstructed cough jet. Aerosol Sci Technol. 2014;48(11):1183–1194. doi: 10.1080/02786826.2014.968655. [DOI] [Google Scholar]
- 34.Heyder J. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. Proc Am Thorac Soc. 2004;1:315–320. doi: 10.1513/pats.200409-046TA. [DOI] [PubMed] [Google Scholar]
- 35.Rengasamy S, Zhuang Z, Niezgoda G, et al. A comparison of total inward leakage measured using sodium chloride (NaCl) and corn oil aerosol methods for air-purifying respirators. J Occup Environ Hyg. 2018;15(8):616–627. doi: 10.1080/15459624.2018.1479064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Negm N. Solubilization characteristics of paraffin oil in different types of surfactants. Egyptian J Chem. 2008;51(1):21–29. [Google Scholar]
- 37.Baimes C (2020) Alberta researcher wins award for salt-coated mask innovation. The Canadian Press, CBC. https://www.cbc.ca/news/canada/edmonton/alberta-researcher-award-salt-masks-covid-1.5813921. Accessed 10 January 2021
- 38.Vejerano EP, Marr LC. Physicochemical characteristics of evaporating respiratory fluid droplets. J R Soc Interface. 2018;15:20170939. doi: 10.1098/rsif.2017.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang W, Elankumaran S, Marr LC. Relationship between humidity and Influenza A viability in droplets and implications for influenza’s seasonality. PLoS ONE. 2012;7(10):e46789. doi: 10.1371/journal.pone.0046789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf G, Koidl B, Pelzmann B. [Zur Regeneration des Zilienschlages humaner Flimmerzellen] Regeneration of the ciliary beat of human ciliated cells. Laryngorhinootologie. 1991;70(10):552–555. doi: 10.1055/s-2007-998095. [DOI] [PubMed] [Google Scholar]
- 41.Daviskas E, Anderson SD, Gonda I, et al. Inhalation of hypertonic saline aerosol enhances mucociliary clearance in asthmatic and healthy subjects. Eur Respir J. 1996;9(4):725–732. doi: 10.1183/09031936.96.09040725. [DOI] [PubMed] [Google Scholar]
- 42.Fu Y, Tong J, Meng F, et al. Ciliostasis of airway epithelial cells facilitates Influenza A virus infection. Vet Res. 2018;49(1):65. doi: 10.1186/s13567-018-0568-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keojampa BK, Nguyen MH, Ryan MW. Effects of buffered saline solution on nasal mucociliary clearance and nasal airway patency. Otolaryngol Head Neck Surg. 2004;131(5):679–682. doi: 10.1016/j.otohns.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 44.Sood N, Bennett WD, Zeman K, et al. Increasing concentration of inhaled saline with or without amiloride: effect on mucociliary clearance in normal subjects. Am J Respir Crit Care Med. 2003;167(2):158–163. doi: 10.1164/rccm.200204-293OC. [DOI] [PubMed] [Google Scholar]
- 45.Kim C-H, Song MH, Ahn YE, et al. Effect of hypo-, iso- and hypertonic saline irrigation on secretory mucins and morphology of cultured human nasal epithelial cells. Acta Oto-Laryngologica. 2005;125:1296–1300. doi: 10.1080/00016480510012381. [DOI] [PubMed] [Google Scholar]
- 46.Sumaily I, Alarifi I, Alsuwaidan R, et al. Impact of nasal irrigation with iodized table salt solution on mucociliary clearance: proof-of-concept randomized control trial. Am J Rhinol Allergy. 2020;34(2):276–279. doi: 10.1177/1945892419892172. [DOI] [PubMed] [Google Scholar]
- 47.Min YG, Lee KS, Yun JB, et al. Hypertonic saline decreases ciliary movement in human nasal epithelium in vitro. Otolaryngol Head Neck Surg. 2001;124(3):313–316. doi: 10.1067/mhn.2001.113145. [DOI] [PubMed] [Google Scholar]
- 48.Bencova A, Vidan J, Rozborilova E, Kocan I. The impact of hypertonic saline inhalation on mucociliary clearance and nasal nitric oxide. J Physiol Pharmacol. 2012;63(3):309–313. [PubMed] [Google Scholar]
- 49.Talbot AR, Herr TM, Parsons DS. Mucociliary clearance and buffered hypertonic saline solution. Laryngoscope. 1997;107(4):500–503. doi: 10.1097/00005537-199704000-00013. [DOI] [PubMed] [Google Scholar]
- 50.Bennett WD, Wu J, Fuller F, et al. Duration of action of hypertonic saline on mucociliary clearance in the normal lung. J Appl Physiol. 2015;118(12):1483–1490. doi: 10.1152/japplphysiol.00404.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Middleton PG, Pollard KA, Wheatley JR. Hypertonic saline alters ion transport across the human airway epithelium. Eur Resp J. 2001;17:195–199. doi: 10.1183/09031936.01.17201950. [DOI] [PubMed] [Google Scholar]
- 52.Jiao J, Yang J, Li J, et al. Hypertonic saline and seawater solutions damage sinonasal epithelial cell air-liquid interface cultures. Int Forum Allergy Rhinol. 2020;10(1):59–68. doi: 10.1002/alr.22459. [DOI] [PubMed] [Google Scholar]
- 53.Miwa M, Matsunaga M, Nakajima N, et al. Hypertonic saline alters electrical barrier of the airway epithelium. Otolaryngol Head Neck Surg. 2007;136(1):62–66. doi: 10.1016/j.otohns.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 54.Hauptman G, Ryan MW. The effect of saline solutions on nasal patency and mucociliary clearance in rhinosinusitis patients. Otolaryngol Head Neck Surg. 2007;137(5):815–821. doi: 10.1016/j.otohns.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 55.Balmes JR, Fine JM, Christian D, et al. Acidity potentiates bronchoconstriction induced by hypoosmolar aerosols. Am Rev Respir Dis. 1988;138(1):35–39. doi: 10.1164/ajrccm/138.1.35. [DOI] [PubMed] [Google Scholar]
- 56.Makker HK, Holgate ST. The contribution of neurogenic reflexes to hypertonic saline-induced bronchoconstriction in asthma. J Allergy Clin Immunol. 1993;92:82–88. doi: 10.1016/0091-6749(93)90041-d. [DOI] [PubMed] [Google Scholar]
- 57.Taube C, Holz O, Mücke M, et al. Airway response to inhaled hypertonic saline in patients with moderate to severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1810–1815. doi: 10.1164/ajrccm.164.10.2104024. [DOI] [PubMed] [Google Scholar]
- 58.Lowry RH, Wood AM, Higenbottam TW. Effects of pH and osmolarity on aerosol-induced cough in normal volunteers. Clin Sci (Lond) 1988;74(4):373–376. doi: 10.1042/cs0740373. [DOI] [PubMed] [Google Scholar]
- 59.Mandelberg A, Amirav I. Hypertonic saline or high volume normal saline for viral bronchiolitis: mechanisms and rationale. Paed Pulmonol. 2010;45:36–40. doi: 10.1002/ppul.21185. [DOI] [PubMed] [Google Scholar]
- 60.Bartoszewski R, Matalon S, Collawn JF. Ion channels of the lung and their role in disease pathogenesis. Am J Physiol Lung Cell Mol Physiol. 2017;313(5):L859–L872. doi: 10.1152/ajplung.00285.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;2363(23):2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bustamante-Marin XM, Ostrowski LE. Cilia and mucociliary clearance. Cold Spring Harb Perspect Biol. 2017;9(4):a028241. doi: 10.1101/cshperspect.a028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hollenhorst MI, Richter K, Fronius M (2011) Ion transport by pulmonary epithelia. J Biomed Biotechnol Article ID 174306, 16pages. 10.1155/2011/174306 [DOI] [PMC free article] [PubMed]
- 64.Iwan IH, Dziembowska I, Słonina DA. Airways surface liquid and ion Transport - The mechanism maintained patency. Biom J Scie Techn Res. 2019;14(3):1–7. doi: 10.26717/BJSTR.2019.14.002543. [DOI] [Google Scholar]
- 65.Pinto JM, Jeswani S (2010) Rhinitis in the geriatric population. Allergy Asthma Clin Immunol 6(1):10. 10.1186/1710-1492-6-10 [DOI] [PMC free article] [PubMed]
- 66.Lillehoj EP, Kato K, Lu W, Kim KC. Cellular and molecular biology of airway mucins. Int Rev Cell Mol Biol. 2013;303:139–202. doi: 10.1016/B978-0-12-407697-6.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lieleg O, Vladescu I, Ribbeck K. Characterization of particle translocation through mucin hydrogels. Biophys J. 2010;98:1782–1789. doi: 10.1016/j.bpj.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCullagh CM, Jamieson AM, Blackwell J, Gupta R. Viscoelastic properties of human tracheobronchial mucin in aqueous solution. Biopolymers. 1995;35(2):149–159. doi: 10.1002/bip.360350203. [DOI] [PubMed] [Google Scholar]
- 69.Button B, Goodell HP, Atieh E, et al. Roles of mucus adhesion and cohesion in cough clearance. PNAS. 2018;115(49):12501–12506. doi: 10.1073/pnas.1811787115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wills PJ, Hall RL, Wm C, Cole PJ. Sodium chloride increases the ciliary transportability of cystic fibrosis and bronchiectasis sputum on the mucus-depleted bovine trachea. J Clin Inv. 1997;99(1):9–13. doi: 10.1172/JCI119138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin L, Chen Z, Cao Y, Sun G. Normal saline solution nasal-pharyngeal irrigation improves chronic cough associated with allergic rhinitis. Am J Rhinol Allergy. 2017;31(2):96–104. doi: 10.2500/ajra.2017.31.4418. [DOI] [PubMed] [Google Scholar]
- 72.Elkins MR, Bye PT. Mechanisms and applications of hypertonic saline. J R Soc Med. 2011;104(Suppl 1):S2–S5. doi: 10.1258/jrsm.2011.s11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goralski JL, Wu D, Thelin WR, et al. The in vitro effect of nebulised hypertonic saline on human bronchial epithelium. Eur Respir J. 2018;51(5):1702652. doi: 10.1183/13993003.02652-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boon M, Jorissen M, Jaspers M, et al. The influence of nebulized drugs on nasal ciliary activity. J Aerosol Med Pulm Drug Deliv. 2016;29(4):378–385. doi: 10.1089/jamp.2015.1229. [DOI] [PubMed] [Google Scholar]
- 75.Rusznak C, Devalia JL, Lozewicz S, Davies RJ. The assessment of nasal mucociliary clearance and the effect of drugs. Respir Med. 1994;88(2):89–101. doi: 10.1016/0954-6111(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 76.Workman AD, Cohen NA. The effect of drugs and other compounds on the ciliary beat frequency of human respiratory epithelium. Am J Rhinol Allergy. 2014;28(6):454–464. doi: 10.2500/ajra.2014.28.4092. [DOI] [PubMed] [Google Scholar]
- 77.Rivera JA (1962) Cilia, ciliated epithelium, and ciliary activity. International Series of Monographs and Applied Biology. 1st edn. Pergamon Press ltd, Oxfor-London-NewYork-Paris pp.50-58. ISBN 978008009623
- 78.Paul P, Johnson P, Ramaswamy P et al (2013) The effect of ageing on nasal mucociliary clearance in women: a pilot study. Pulmonol Article ID 598589:5 pages. 10.1155/2013/598589
- 79.Purushothaman PK, Priyangha E, Vaidhyswaran R (2020) Effects of prolonged use of facemask on healthcare workers in tertiary care hospital during COVID-19 pandemic. Indian J Otolaryngol Head Neck Surg:1–7. 10.1007/s12070-020-02124-0 [DOI] [PMC free article] [PubMed]
- 80.White DE, Bartley J, Nates RJ. Model demonstrates functional purpose of the nasal cycle. BioMed Eng OnLine. 2015;14:38. doi: 10.1186/s12938-015-0034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tarran R, Trout L, Donaldson SH, Boucher RC. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J Gen Physiol. 2006;127(5):591–604. doi: 10.1085/jgp.200509468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hildenbrand T, Weber RK, Brehmer D. Rhinitis sicca, dry nose and atrophic rhinitis: a review of the literature. Eur Arch Otorhinolaryngol. 2011;268(1):17–26. doi: 10.1007/s00405-010-1391-z. [DOI] [PubMed] [Google Scholar]
- 83.Harvey PR, Tarran R, Garoff S, Myerburg MM. Measurement of the airway surface liquid volume with simple light refraction microscopy. Am J Respir Cell Mol Biol. 2011;45(3):592–599. doi: 10.1165/rcmb.2010-0484OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tanner K, Roy N, Merrill RM, et al. Nebulized isotonic saline versus water following a laryngeal desiccation challenge in classically trained sopranos. J Speech Language Hearing Res. 2010;53(6):1555–1566. doi: 10.1044/1092-4388(2010/09-0249. [DOI] [PubMed] [Google Scholar]
- 85.Personal communications by pneumologists, dentists and paediatricians wearing daily well-fitting professional masks, July-October 2020
- 86.Slapak I, Skoupa J, Strnad P, Hornik P. Efficacy of isotonic nasal wash (seawater) in the treatment and prevention of rhinitis in children. Arch Otolaryngol Head Neck Surg. 2008;134:67–74. doi: 10.1001/archoto.2007.19. [DOI] [PubMed] [Google Scholar]
- 87.Newster (2020) Eco-sustainable technology for the processing of healthcare waste (HCW), on-site or in centralized treatment centers. Coronaviruses: SARS, MERS and Covid19. 28/02/2020 http://www.newstergroup.com/news/coronaviruses__sars_mers_and_covid19
- 88.Machado RRG, Glaser T, Araujo DB et al (2020) Hypertonic saline solution inhibits SARS-CoV-2 in vitro assay. bioRxiv 2020.08.04:235549. 10.1101/2020.08.04.235549
- 89.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hou Y, Zhao J, Martin W et al (2020) New insights into genetic susceptibility of COVID-19: an ACE2 and TMPRSS2 polymorphism analysis. BMC Med 18:art.No.216. 10.1186/s12916-020-01673-z [DOI] [PMC free article] [PubMed]
- 91.Sungnak W, Huang N, Bécavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rushworth CA, Guy JL, Turner AJ. Residues affecting the chloride regulation and substrate selectivity of the angiotensin-converting enzymes (ACE and ACE2) identified by site-directed mutagenesis. FEBS J. 2008;275(23):6033–6042. doi: 10.1111/j.1742-4658.2008.06733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guy JL, Jackson RM, Acharya KR, et al. Angiotensin-converting enzyme-2 (ACE2): comparative modeling of the active site, specificity requirements, and chloride dependence. Biochemistry. 2003;42(45):13185–13192. doi: 10.1021/bi035268s. [DOI] [PubMed] [Google Scholar]
- 94.Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chitranshi N, Gupta VK, Rajput R, et al. Evolving geographic diversity in SARS-CoV2 and in silico analysis of replicating enzyme 3CLpro targeting repurposed drug candidates. J Transl Med. 2020;18(1):278. doi: 10.1186/s12967-020-02448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Graziano V, McGrath WJ, DeGruccio AM, et al. Enzymatic activity of the SARS coronavirus main proteinase dimer. FEBS letters. 2006;580(11):2577–2583. doi: 10.1016/j.febslet.2006.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ferreira JC, Rabeh WM (2020) Biochemical and Biophysical characterization of the main protease, 3-chymotrypsin-like protease (3CLpro), from the novel coronavirus disease 19(COVID-19). Research Square. New York University Abu Dhabi, pp 1-17. https://assets.researchsquare.com/files/rs-40945/v1/e41c3648-96c7-4953-bb2b-a5c5d1a19e7f.pdf [DOI] [PMC free article] [PubMed]
- 98.Chang HP, Chou CY, Chang GG. Reversible unfolding of the severe acute respiratory syndrome coronavirus main protease in guanidinium chloride. Biophys J. 2007;92(4):1374–1383. doi: 10.1529/biophysj.106.091736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abian O, Ortega-Alarcon D, Jimenez-Alesanco A, et al. Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening. Int J Biol Macromol. 2020;164:1693–1703. doi: 10.1016/j.ijbiomac.2020.07.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grum-Tokars V, Ratia K, Begaye A, et al. Evaluating the 3C-like protease activity of SARS-Coronavirus: recommendations for standardized assays for drug discovery. Virus Res. 2008;133(1):63–73. doi: 10.1016/j.virusres.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shi J, Song J. The catalysis of the SARS 3C-like protease is under extensive regulation by its extra domain. The FEBS Journal. 2006;273(5):1035–1045. doi: 10.1111/j.1742-4658.2006.05130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bestle D, Heindl MR, Limburg H, et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci Alliance. 2020;3(9):e202000786. doi: 10.26508/lsa.202000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shang J, Wan Y, Luo C, et al. Cell entry mechanisms of SARS-CoV-2. PNAS. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hasan A, Paray BA, Hussain A et al (2020) A review on the cleavage priming of the spike protein on coronavirus by angiotensin-converting enzyme-2 and furin. J Biomol Struct Dyn:1–9. 10.1080/07391102.2020.1754293https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7189411/ [DOI] [PMC free article] [PubMed]
- 105.Izidoro MA, Gouvea IE, Santos JA, et al. Lindberg I, Juliano L (2009) A study of human furin specificity using synthetic peptides derived from natural substrates, and effects of potassium ions. Arch Biochem Biophys. 2009;487(2):105–114. doi: 10.1016/j.abb.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou T, Tsybovsky Y, Olia AS et al (2020) A pH-dependent switch mediates conformational masking of SARS-CoV-2 spike. bioRxiv [Preprint] 2020.07.04.187989 10.1101/2020.07.04.187989
- 107.Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smyrlaki I, Ekman M, Lentini A, et al. Massive and rapid COVID-19 testing is feasible by extraction-free SARS-CoV-2 RT-PCR. Nat Commun. 2020;11:4812. doi: 10.1038/s41467-020-18611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fischer H, Widdicombe JH. Mechanisms of acid and base secretion by the airway epithelium. J Membr Biol. 2006;211(3):139–150. doi: 10.1007/s00232-006-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reddi BA. Why is saline so acidic (and does it really matter?) Int J Med Sci. 2013;10(6):747–750. doi: 10.7150/ijms.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Enuka Y, Hanukoglu I, Edelheit O, et al. Epithelial sodium channels (ENaC) are uniformly distributed on motile cilia in the oviduct and the respiratory airways. Histochem Cell Biol. 2012;137(3):339–353. doi: 10.1007/s00418-011-0904-1. [DOI] [PubMed] [Google Scholar]
- 112.Anand P, Puranik A, Aravamudan M et al (2020) SARS-CoV-2 strategically mimics proteolytic activation of human ENaC. eLife 9:e58603. 10.7554/eLife.58603 [DOI] [PMC free article] [PubMed]
- 113.Jaimes JA, Millet JK, Whittaker GR (2020) Proteolytic cleavage of the SARS-CoV-2 spike protein and the role of the novel S1/S2 site. iScience 23:101212. 10.1016/j.isci.2020.101212 [DOI] [PMC free article] [PubMed]
- 114.Ji HL, Zhao R, Matalon S, Matthay MA. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol Rev. 2020;100(3):1065–1075. doi: 10.1152/physrev.00013.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem. 2009;284(31):20447–20451. doi: 10.1074/jbc.R800083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Szabó GT, Kiss A, Csanádi Z, Czuriga D (2020) Hypothetical dysfunction of the epithelial sodium channel may justify neurohumoral blockade in coronavirus disease 2019. ESC Heart Fail 17. 10.1002/ehf2.13078 [DOI] [PMC free article] [PubMed]
- 117.Noda M, Hiyama TY. The Nax Channel: What it is and what it does. The Neuroscientist. 2015;21(4):399–412. doi: 10.1177/1073858414541009. [DOI] [PubMed] [Google Scholar]
- 118.Marunaka Y, Marunaka R, Sun H et al (2016) Na+ homeostasis by epithelial Na+ channel (ENaC) and Nax channel (Nax): cooperation of ENaC and Nax. ATM 4(Suppl 1):S11. 10.21037/atm.2016.10.42 [DOI] [PMC free article] [PubMed]
- 119.Blé FX, Cannet C, Collingwood S, et al. ENaC-mediated effects assessed by MRI in a rat model of hypertonic saline-induced lung hydration. Br J Pharmacol. 2010;160(4):1008–1015. doi: 10.1111/j.1476-5381.2010.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ramalingam S, Cai B, Wong J, et al. Antiviral innate immune response in non-myeloid cells is augmented by chloride ions via an increase in intracellular hypochlorous acid levels. Sci Rep. 2018;8:13630. doi: 10.1038/s41598-018-31936-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang N, Francis KP, Prakash A, Ansaldi D. Enhanced detection of myeloperoxidase activity in deep tissues through luminescent excitation of near-infrared nanoparticles. Nat Med. 2013;19(4):500–505. doi: 10.1038/nm.3110. [DOI] [PubMed] [Google Scholar]
- 122.Suzuki K, Yamada M, Akashi K, Fujikura T. Similarity of kinetics of three types of myeloperoxidase from human leukocytes and four types from HL-60. Arch Biochem Biophysics. 1986;245(1):167–173. doi: 10.1016/0003-9861(86)90201-8. [DOI] [PubMed] [Google Scholar]
- 123.Wang G, Nauseef WM. Salt, chloride, bleach, and innate host defense. J Leukocyte Biol. 2015;98(2):163–172. doi: 10.1189/jlb.4RU0315-109R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chandler JD, Day BJ. Thiocyanate: a potentially useful therapeutic agent with host defense and antioxidant properties. Biochem Pharmacol. 2012;84(11):1381–1387. doi: 10.1016/j.bcp.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nadesalingam A, Chen JHK, Farahvash A, Khan MA. Hypertonic saline suppresses NADPH oxidase-dependent neutrophil extracellular trap formation and promotes apoptosis. Front Immunol. 2018;9:359. doi: 10.3389/fimmu.2018.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Delgado-Enciso I, Paz-Garcia J, Barajas-Saucedo CE, Mokay-Ramírez KA Meza-Robles C, Lopez-Flores R (2020) Patient-reported health outcomes after treatment of COVID-19 with nebulized and/or intravenous neutral electrolyzed saline combined with usual medical care versus usual medical care alone: a randomized, open-label, controlled trial. Res Sq [Preprint] 10:rs.3.rs-68403. 10.21203/rs.3.rs-68403/v1 [DOI] [PMC free article] [PubMed]
- 127.WHO (2020) Saline. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/myth-busters#saline
- 128.WHO (2020) Can rinsing your nose regularly with saline solution prevent Covid-19? https://www.who.int/docs/default-source/searo/thailand/12myths-final099bfbf976c54d5fa3407a65b6d9fa9d.pdf
- 129.Salmon Ceron D, Bartier S, Hautefort C, et al. APHP COVID-19 research collaboration. Self-reported loss of smell without nasal obstruction to identify COVID-19. The multicenter Coranosmia cohort study. J Infect. 2020;81(4):614–620. doi: 10.1016/j.jinf.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Voshaar T. COVID-19 Therapie aus Sicht eines Aerosol-Experten. PARI.de - Artzeportal 28 Juli 2020. https://www.pari.com/de/aerzteportal/news/covid-19-therapie-aus-sicht-eines-aerosol-experten Accessed 10 January 2021
- 131.Jayaweera M, Perera H, Gunawardana B, Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environ Res. 2020;188:109819. doi: 10.1016/j.envres.2020.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.WHO (2020) Transmission of SARS-CoV-2: implications for infection prevention precautions. https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions Accessed 10 January 2021
- 133.Ueki H, Furusawa Y, Iwatsuki-Horimoto K, et al. Effectiveness of face masks in preventing airborne transmission of SARS-CoV-2. mSphere. 2020;5(5):e00637–e00620. doi: 10.1128/mSphere.00637-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ehre C. SARS-CoV-2 infection of airway cells. N Engl J Med. 2020;383:969. doi: 10.1056/NEJMicm2023328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhu N, Wang W, Liu Z, et al. Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells. Nat Commun. 2020;11:3910. doi: 10.1038/s41467-020-17796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Robinot R, Hubert M, Dias de Mehlo G et al (2020) SARS-CoV-2 infection damages airway motile cilia and impairs mucociliary clearance. bioRxiv. 10.1101/2020.10.06.328369
- 137.Baker AN, Richards SJ, Guy CS, et al. The SARS-COV-2 spike protein binds sialic acids and enables rapid detection in a lateral flow point of care diagnostic device. ACS Cent Sci. 2020;6(11):2046–2052. doi: 10.1021/acscentsci.0c00855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hou YJ, Okuda K, Edwards CE, et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182(2):429–46.e14. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Burke W. The ionic composition of nasal fluid and its function. Health. 2014;06(08):720–728. doi: 10.4236/health.2014.68093. [DOI] [Google Scholar]
- 140.Grandjean Lapierre S, Phelippeau M, Hakimi C, et al. Cystic fibrosis respiratory tract salt concentration: an exploratory cohort study. Medicine. 2017;96(47):e8423. doi: 10.1097/MD.0000000000008423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kozlova I, Vanthanouvong V, Johannesson M, Roomans GM (2006) Composition of airway surface liquid determined by X-ray microanalysis. Ups J Med Sci 111(1):137-153. 10.3109/2000-1967-016https://www.tandfonline.com/doi/pdf/10.3109/2000-1967-016 [DOI] [PubMed]
- 142.Matsui H, Grubb BR, Tarran R, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95(7):1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 143.Wheatley CM, Cassuto NA, Foxx-Lupo WT, et al. Variability in measures of exhaled breath Na+, influence of pulmonary blood flow and salivary Na+ Clin Med Insights Circ Respir Pulm Med. 2010;4:25–34. doi: 10.4137/ccrpm.s4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Song Y, Thiagarajah J, Verkman AS. Sodium and chloride concentrations, pH, and depth of airway surface liquid in distal airways. J Gen Physiol. 2003;122(5):511–519. doi: 10.1085/jgp.200308866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hao W, Ma B, Li Z et al (2020) Binding of the SARS-CoV-2 spike protein to glycans. bioRxiv. 10.1101/2020.05.17.100537
- 146.Bastier PL, Lechot A, Bordenave L, et al. Nasal irrigation: from empiricism to evidence-based medicine. A review. Eur Ann Otorhinolaryngol Head Neck Dis. 2015;132(5):281–285. doi: 10.1016/j.anorl.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 147.Nimsakul S, Ruxrungtham S, Chusakul S, et al. Does heating up saline for nasal irrigation improve mucociliary function in chronic rhinosinusitis? Am J Rhinol Allergy. 2018;32(2):106–111. doi: 10.1177/1945892418762872. [DOI] [PubMed] [Google Scholar]
- 148.Niedner R. Cytotoxicity and sensitization of povidone-iodine and other frequently used anti-infective agents. Dermatology. 1997;195(Suppl2):89–92. doi: 10.1159/000246038. [DOI] [PubMed] [Google Scholar]
- 149.Gudmundsdottir Á, Scheving R, Lindberg F, Stefansson B (2020) Inactivation of SARS-CoV-2 and HCoV-229E in vitro by ColdZyme® a medical device mouth spray against the common cold. J Med Virol. 10.1002/jmv.26554.org/10.1002/jmv.26554 [DOI] [PMC free article] [PubMed]
- 150.Kido H. Influenza virus pathogenicity regulated by host cellular proteases, cytokines and metabolites, and its therapeutic options. Proc Jpn Acad Ser B Phys Biol Sci. 2015;91(8):351–368. doi: 10.2183/pjab.91.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu JJ, Chan GC, Hecht AS, et al. Nasal saline irrigation has no effect on normal olfaction: a prospective randomized trial. Int Forum Allergy Rhinol. 2014;4(1):39–42. doi: 10.1002/alr.21235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Piromchai P, Puvatanond C, Kirtsreesakul V, et al. Effectiveness of nasal irrigation devices: a Thai multicentre survey. PeerJ. 2019;27(7):e7000. doi: 10.7717/peerj.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Navarra J, Ruiz-Ceamanos A, Moreno JJ et al (2002) Acute nasal dryness in COVID-19. medRxiv 2020.11.18.20233874 [Preprint]. 10.1101/2020.11.18.20233874
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 29 kb)
(DOCX 27 kb)
(DOCX 37 kb)
(DOCX 32 kb)