Abstract
Objectives
Many patients with osteoarthritis have comorbid symptoms of FM, but it is unknown how these symptoms respond to surgical procedures that address nociceptive input in the periphery, such as total joint replacement. Here we explore differences in clinical characteristics between patients whose FM symptoms do and do not improve following total hip or knee replacement.
Methods
Participants were 150 patients undergoing knee or hip replacement who had a minimum FM survey score of 4 or greater prior to surgery. The top tertile of patients experiencing the most improvement in FM symptoms at month 6 were categorized as ‘Improve’ (n = 48) while the bottom two tertiles were categorized as ‘Worsen/Same’ (n = 102). Baseline symptom characteristics were compared between groups, as well as improvement in overall pain severity, surgical pain severity and physical function at 6 months.
Results
The Worsen/Same group had higher levels of fatigue, depression and surgical site pain at baseline (all P < 0.05). Additionally, they improved less on overall pain severity and physical functioning 6 months after surgery (both P < 0.05).
Conclusion
Most patients derive significant benefit in improvement of comorbid FM symptoms following total joint replacement, but a substantial proportion do not. Understanding the neurobiological basis for these different trajectories may help inform clinical judgment and improve patient care.
Keywords: osteoarthritis, fibromyalgia, total joint replacement, central sensitization
Rheumatology key messages
Most patients with osteoarthritis experience substantial improvement in comorbid fibromyalgia symptoms after total joint replacement.
A minority of patients with osteoarthritis regress to pre-surgical levels of fibromyalgia symptoms by month 6.
Osteoarthritis patients who show less benefit have higher levels of fatigue and depression before the procedure.
Introduction
It is now widely accepted that the symptoms used to define FM, such as widespread pain, fatigue and cognitive dysfunction, are present to some degree in all rheumatic disorders and chronic pain conditions [1]. The level of these symptoms, or the degree of FM symptoms an individual possesses are hypothesized to be associated with aberrant central nervous system mechanisms. This hypothesis is supported by imaging techniques designed to explore the brain’s structure, function and neurochemical features [2–4]. It was recently demonstrated that the degree of FM symptoms (measured on a continuum via the 2011 FM Survey Criteria [5]) in RA correlates with increased connectivity between the insula and Default Mode Network of the brain [6], replicating one of the most salient neurobiological findings in clinical FM populations [7]. These central differences may be why the degree of FM symptoms a patient has prior to surgery is a strong predictor of post-surgical outcomes such as the likelihood of improvement in pain and need for analgesics [8–11].
It has been hypothesized that comorbid FM symptoms in other rheumatological conditions may arise through two relatively distinct pathways: ‘bottom up’ and ‘top down’ mechanisms of central sensitization [12]. ‘Top down’ mechanisms of central sensitization refer to pain amplification that exists independent of peripheral inflammation and other nociceptive input. This concept is supported by the frequent disagreement between the degree of damage or inflammation in the joint and the severity of symptoms reported by the patient [13]. These ‘top down’ mechanisms are believed to be familial and to disproportionately affect females [14, 15]. In an OA patient whose comorbid FM symptoms are driven primarily by ‘top down’ mechanisms, surgery should have limited benefit for pain outside the surgical site.
Conversely, in some patients, peripheral sources of nociceptive input such as joint inflammation and damage sensitize the CNS to pain in a ‘bottom up’ fashion. According to this view, chronic injury and/or inflammation, such as that due to OA, drives central nervous system sensitization, resulting in comorbid FM symptoms. Woolf originally advanced the term ‘central sensitization’ to describe this process when spinal and brain mechanisms promote pain at sites distal from the primary injury, damage or inflammation, a phenomenon well established in animal models [13]. In a patient where comorbid FM symptoms are being driven by chronic nociceptive input in the knee or hip, arthroplasty should improve these symptoms by reducing the source of central sensitization. In fact, a reduction in global pain sensitivity does seem to occur in a subset of individuals receiving lower extremity arthroplasty [14].
Examining how comorbid FM symptoms change after lower extremity arthroplasty, where the primary source of nociceptive input is removed, allows for a natural experiment to identify phenotypes consistent with ‘bottom up’ vs ‘top down’ mechanisms. Using a cohort of OA patients undergoing hip and knee arthroplasty, we sought to identify different patterns of how FM symptoms change following the procedure, as well as the baseline characteristics associated with these patterns. We also sought to determine whether there were marked differences in the temporal pattern of these changes, and if these patterns were associated with different clinical outcomes following surgery.
Methods
Sample
The eligible pool of participants were 453 individuals undergoing either knee or hip replacement surgery at Michigan Medicine. These patients were recruited between April 2015 and November 2016. The current study was approved by the University of Michigan Institutional Review Board and all participants provided informed consent. Of these, 107 were using opioid medications prior to surgery, which may influence the comorbid symptoms of interest from the 2011 Fibromyalgia Survey Criteria, and were therefore excluded from further analyses. A comparison of symptoms between patients taking/not taking opioids at baseline is shown in Supplementary Table S1, available at Rheumatology online. Analyses were restricted to participants showing at least moderate levels of comorbid symptoms prior to surgery as indicated by a 4 or greater on the 2011 criteria; participants with scores below 4, the sample median of patients not taking opioids at baseline, were excluded. Of the remaining 201 participants, 51 did not have data available at the follow-up visit (month 6) and were also excluded. This resulted in a final sample of 150 participants. Demographic information is shown in Table 1.
Table 1.
Demographic and symptom information at baseline for Worsen/Same and Improve groups
| Worsen/Same (n = 48) | Improve (n = 102) | t | df | P | |||
|---|---|---|---|---|---|---|---|
| Mean (95% CI) | s.d. | Mean (95% CI) | s.d. | ||||
| Age, years | 61.75 (58.98, 64.52) | 9.54 | 63.61 (61.58, 65.63) | 10.31 | −1.054 | 148 | 0.294 |
| Widespread Pain Index (0–19) | 2.75 (2.18, 3.32) | 1.95 | 2.40 (2.06, 2.74) | 1.74 | 1.100 | 148 | 0.273 |
| Michigan Body Map non-surgical sites | 3.83 (2.89, 4.78) | 3.26 | 3.66 (3.08, 4.23) | 2.93 | 0.332 | 148 | 0.741 |
| Symptom Severity Index | 4.92 (4.32, 5.52) | 2.07 | 4.33 (3.94, 4.73) | 2.03 | 1.633 | 148 | 0.105 |
| FM survey score | 7.67 (6.82, 8.51) | 2.93 | 6.74 (6.25, 7.22) | 2.49 | 2.020 | 148 | 0.045 |
| Fatigue | 11.88 (10.97, 12.78) | 3.11 | 10.36 (9.65, 11.07) | 3.61 | 2.499 | 148 | 0.014 |
| Depression | 6.96 (6.07, 7.84) | 2.99 | 5.57 (5.11, 6.04) | 2.37 | 3.013 | 145 | 0.003 |
| Sleep disturbance | 26.91 (25.04, 28.79) | 6.39 | 26.45 (25.14, 27.76) | 6.57 | 0.399 | 144 | 0.690 |
| Physical function | 13.98 (13.04, 14.92) | 3.25 | 13.09 (12.45, 13.73) | 3.23 | 1.571 | 147 | 0.118 |
| Pain catastrophizing | 5.46 (3.62, 7.30) | 6.19 | 4.00 (3.07, 4.93) | 4.68 | 1.571 | 144 | 0.118 |
| Brief pain inventory pain severity (overall) | 4.85 (4.13, 5.58) | 2.49 | 4.72 (4.19, 5.25) | 2.70 | 0.290 | 148 | 0.772 |
| Pain severity (surgical site) | 6.90 (6.39, 7.40) | 1.75 | 6.23 (5.88, 6.58) | 1.76 | 2.162 | 148 | 0.032 |
| Frequency (%) | Frequency (%) | χ2 | P | ||||
| FM positive | 2 (4) | 3 (3) | 0.152 | 0.655a | |||
| Gender (female) | 33 (69) | 59 (58) | 1.637 | 1 | 0.201 | ||
| Surgical cohort (knee) | 25 (52) | 40 (39) | 2.20 | 1 | 0.138 | ||
Fisher’s exact test. DF: degrees of freedom.
Measures
The 2011 survey criteria for FM was used to assess widespread body pain and comorbid symptoms [5]. The 2011 criteria contain 19 areas that can be indicated as painful, and several questions about constitutional symptoms such as fatigue, cognitive difficulties and unrefreshing sleep. These aspects of the centralized pain spectrum can be analysed separately as the Widespread Pain Index (WPI) and Symptom Severity Index (SSI). The SSI serves as an index of somatic and constitutional symptoms that are frequently present in FM. A more detailed body map (the Michigan Body Map; MBM [15]) containing 35 sites was administered that includes all the WPI sites, allowing us to calculate the WPI. A non-surgical site pain index was created by removing the bilateral surgical sites from the respective cohorts (hips from the hip cohort, knees from the knee cohort)—this allows us to examine the impact of the procedure on pain outside the surgical site. Overall pain severity, and surgical site pain severity, was measured by the Brief Pain Inventory [16]. The Patient-Reported Outcomes Measurement Information System-Short Forms (PROMIS®-SF) were used to measure depression, anxiety, fatigue, sleep disturbance and physical function [17]. Six questions from the catastrophizing subscale from the Coping Strategies Questionnaire were used to measure catastrophic cognitive appraisal of pain [18]. Primary outcomes for baseline analyses were FM survey criteria (WPI and SSI), depression, fatigue and sleep disturbance. For longitudinal analyses the primary outcomes were change scores in physical function, overall pain severity and surgical site pain severity. Age, sex and surgical cohort were examined as potential confounding variables.
Group definitions
To identify groups that showed different patterns of comorbid symptom change after surgery, we first calculated a simple change score between baseline and month 6 for the 2011 FM survey criteria. We then regressed this change score on the baseline survey score to account for regression to the mean type effects. The resulting residuals were divided into three tertiles by rank values. We compared the proportion of each group who achieved at least a 30% reduction in FM symptoms by month 6 using the χ2 test, or Fisher’s exact test when expected counts are <5, with correction for multiple comparisons. We subsequently combined the top two tertiles into one group (n = 102), ‘Improve’, for comparison to the bottom tertile, ‘Worsen/Same’.
Statistical analyses
Baseline comparisons
Improve vs Worsen/Same comparisons were made on continuous outcomes by Student’s t-test and categorical outcomes by χ2 statistic. Primary outcomes for baseline analyses were FM survey criteria (overall, WPI and SSI), depression, fatigue and sleep disturbance. Additionally, anxiety, MBM non-surgical sites, non-surgical and surgical pain severity, and age were compared between groups. Surgical site (hip vs knee) and gender were categorical outcomes. We also compared those patients who were included in the study (with available 6-month data; n = 150) with those who were lost to follow-up (n = 48), on age, gender, surgical site and baseline levels of FM symptoms.
Longitudinal analyses
Trajectory of change
The trajectory of change in total 2011 FM survey scores, MBM non-surgical site pain (e.g. not surgical hip or knee) and SSI scores was examined in mixed-effect models, with random subject-specific intercept and time effects, using restricted estimation of maximum likelihood. Both linear and quadratic effects of time were modelled and the primary outcomes of interest were grouped by time interaction terms.
Improvement in non-surgical pain and physical function
Primary outcomes were change scores in Brief Pain Inventory overall pain severity and PROMIS physical function measures (pre-surgery—month 6 scores). These change scores were then compared between groups in general linear models controlling for baseline scores, participant age and surgical group.
Surgical-site pain after surgery
The primary outcome of surgical pain (yes/no) was compared between groups at each post-surgical time point, week 2, month 1, month 3 and month 6, by χ2 test.
Results
Group definitions
A very small proportion of those patients in the lowest tertile of FM improvement achieved a 30% reduction in FM symptoms (6.3%). Conversely, 89% of those in the middle tertile and 100% of those in the top tertile achieved 30% improvement. The difference between low and middle tertiles was significant (χ2 = 68.5; P < 0.001) as was the difference between low and high tertiles (χ2 = 85.7; P<0.001). However, there was no statistically significant difference in the proportion of those showing 30% improvement between the middle and high tertiles (Fisher’s exact test adjusted P = 0.08). These top two tertiles were subsequently combined, as mentioned above.
Baseline comparison
Age, gender and surgical cohort did not differ significantly between the groups (all P > 0.13; see Table 1). Similarly, there were no significant pre-surgical group differences on painful body sites measured by the WPI or non-surgical sites on the MBM, the SSI, sleep disturbance, physical function, pain catastrophizing or overall pain severity (all P>0.10). Conversely, total FM survey scores (P=0.045), fatigue (P=0.014), depression (P=0.003) and surgical site pain severity (P=0.032) were elevated in the Worsen/Same group compared with those who improved (see Table 1 for means and s.d. by group for each measure).
Those patients who did not have complete 6-month data were younger than those who did (mean loss to follow-up=56.02; s.d. =14.33; 95% CI: 51.86, 60.18; mean included=63.01; s.d. = 10.08; 95% CI: 61.39, 64.64; P<0.001). There were no significant differences in baseline FM symptoms (mean loss to follow-up=7.29; s.d. = 2.86; 95% CI: 6.46, 8.12; mean included = 7.03; s.d. = 2.66; 95% CI: 6.60, 7.46; P = 0.57). There were no differences in the proportion of each group having hip replacement (loss to follow-up=47.9%; included=56.7%; P=0.29) or in the proportion of female patients (loss to follow-up=56.3%; included=61.3%; P=0.53).
Longitudinal analyses
Trajectory of change
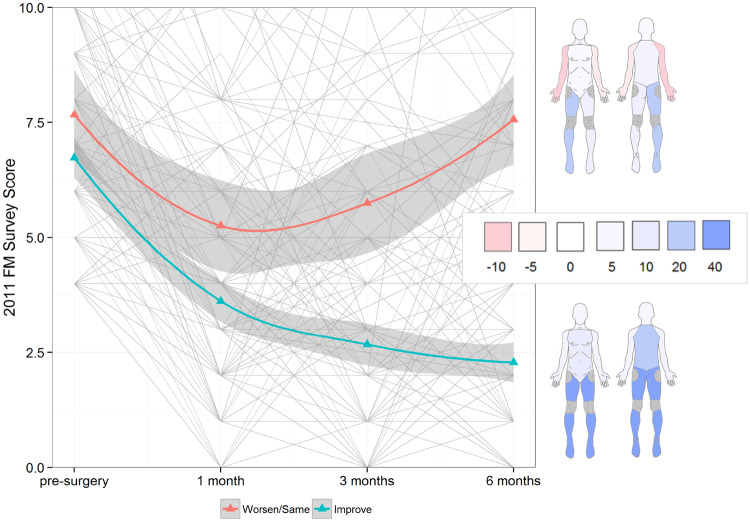
The effect of time on total FM scores differed substantially by group (time by group interaction, P<0.001). The change over time in MBM non-surgical pain sites (excluding the hip or knee) and SSI scores also differed by group (time by group interaction, both P<0.001) indicating that both comorbid pain and constitutional symptoms contribute to the different trajectories in total score improvement (see Fig. 1 for group trajectories and change in pain distribution over time). In both groups, improvement occurred at the 1-month time point, but by month 3 the Worsen/Same group showed substantial regression in their symptoms, such that by month 6 they had returned to pre-surgical levels of FM symptoms. Conversely, the Improve group continued to show benefits at month 3 and through month 6. These non-linear patterns are reflected in the greater deviation from linear change seen in the Worsen/Same group for total FM survey and SSI scores (quadratic effect of time by group interaction, both P<0.05). All model output is shown in Table 2. MBM non-surgical pain and SSI trajectories are shown in Supplementary Fig. S1, available at Rheumatology online.
Fig. 1.
Smoothed group trajectories of the 2011 FM survey score following surgery
Individual trajectories are shown in light grey. Colours on the body map correspond to percentage changes in each group showing improvement in each region. Regions are adapted from the regional pain definitions of the 2016 Fibromyalgia Survey criteria. Shaded regions show 95% CIs.
Table 2.
Fixed effects for models of change in FM total scores, non-surgical pain, and Symptom Severity Index
| Variable | Estimate | s.e. | df | t | P |
|---|---|---|---|---|---|
| FM total scores | |||||
| Intercept | 5.997 | 1.055 | 173.4 | 5.685 | <0.001 |
| Time | −0.016 | 0.141 | 369.5 | −0.114 | 0.910 |
| Time2 | 1.071 | 0.159 | 426.5 | 6.756 | <0.001 |
| Age | −0.011 | 0.016 | 147 | −0.675 | 0.501 |
| Surgical cohort (hip) | −0.250 | 0.317 | 148 | −0.787 | 0.432 |
| Group (improve) | −0.018 | 0.514 | 239 | −0.034 | 0.973 |
| Time×Group | −1.432 | 0.170 | 369.1 | −8.402 | <0.001 |
| Time2×Group | −0.394 | 0.192 | 426.1 | −2.059 | 0.040 |
| Non-surgical site pain | |||||
| Intercept | 3.283 | 0.993 | 179.1 | 3.306 | 0.001 |
| Time | −0.065 | 0.171 | 149.3 | −0.377 | 0.706 |
| Time2 | 0.547 | 0.159 | 280.96 | 3.442 | 0.001 |
| Age | −0.017 | 0.014 | 145.51 | −1.177 | 0.241 |
| Surgical cohort (hip) | 0.586 | 0.296 | 146.59 | 1.983 | 0.049 |
| Group (improve) | −0.195 | 0.513 | 225 | −0.381 | 0.704 |
| Time×Group | −0.810 | 0.207 | 149.02 | −3.908 | <0.001 |
| Time2×Group | −0.008 | 0.192 | 280.54 | −0.044 | 0.965 |
| Symptom Severity Index | |||||
| Intercept | 3.881 | 0.818 | 169.1 | 4.746 | <0.001 |
| Time | 0.149 | 0.101 | 385.4 | 1.468 | 0.143 |
| Time2 | 0.699 | 0.114 | 427.6 | 6.109 | <0.001 |
| Age | −0.004 | 0.012 | 146.4 | −0.328 | 0.743 |
| Surgical cohort (hip) | −0.571 | 0.248 | 147.3 | −2.307 | 0.022 |
| Group (Improve) | 0.284 | 0.383 | 234.5 | 0.743 | 0.458 |
| Time×Group | −1.035 | 0.123 | 385.1 | −8.441 | <0.001 |
| Time2×Group | −0.369 | 0.138 | 427.3 | −2.671 | 0.008 |
The quadratic time variable was centred. DF: degrees of freedom.
Improvement in non-surgical pain and physical function
Participants in the Worsen/Same group showed significantly lower levels of improvement in overall pain severity at month 6, after controlling for baseline symptom levels, age and surgical site (P = 0.004). The estimated improvement in overall pain severity for the Improve group was 2.25 points (95% CI: 1.84, 2.65; s.e. = 0.20) or 48% of pre-surgical levels, vs 1.21 points (95% CI: 0.63, 1.79; s.e.=0.29) or 25% or pre-surgical levels for the Worsen/Same group. Similarly, the Worsen/Same group showed significantly less improvement in physical function (P=0.021). The estimated improvement in physical function for the Improve group was 5.66 (95% CI: 2.95, 5.17; s.e. = 0.39) or 43% of pre-surgical levels, vs 4.06 (95% CI: 4.89, 6.43; s.e.=0.56) or 29% of pre-surgical levels for the Worsen/Same group. In analyses where patients still taking opioid medications after month 1 were removed [Worsen/Same n = 6 (12.5%), Improve n = 4 (3.9%)], the results were similar (data not shown).
Improvement in surgical-site pain
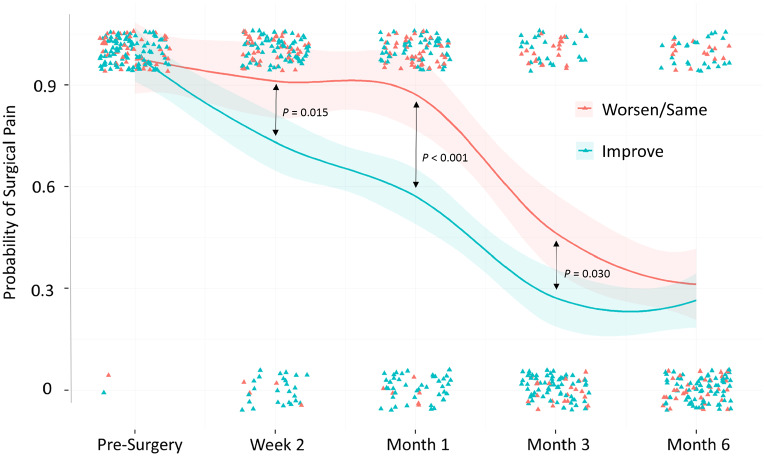
At month 6, there was no difference in the percentage of participants reporting no surgical-site pain (yes/no) between the two groups [Improve=74%, Worsen/Same=69%, χ2=0.37; degrees of freedom (df) = 1, P=0.54]. Conversely, at week 2 (Improve=27%, Worsen/Same=9%, χ2=5.92; df = 1, P=0.015), month 1 (Improve=43%, Worsen/Same=13%, χ2=12.99; df = 1, P<0.001), and month 3 (Improve=73%, Worsen/Same=54%, χ2=4.71; df = 1, P=0.030), a smaller proportion of participants in the Worsen/Same group reported being pain free at the surgical site (see Fig. 2).
Fig. 2.
Smoothed trajectories showing the probability of surgical pain from pre-surgery to month 6
Significant differences at week 2, month 1 and month 3 are shown with P values. Individual data points are shown as triangles. Shaded regions show 95% CIs.
Discussion
These analyses show that the trajectory of change in FM symptoms after joint replacement surgery follows distinct patterns. In most patients, significant improvement in FM symptoms, including multifocal pain and constitutional symptoms, occurs rapidly (i.e. 1 month) and is maintained through 6 months following the procedure. The observed improvement in both constitutional symptoms and widespread pain is important as it indicates that this phenomenon is not simply due to improved referred pain or biomechanics following the procedure. Conversely, approximately one-third of patients demonstrate a relatively modest improvement in these symptoms at 1 month, followed by a reversion to pre-surgical levels by month 6, despite improvement in pain at the surgical site. This means that for a subset of OA patients, no long-term benefit for comorbid highly burdensome symptoms like multifocal pain, fatigue and sleep disruption can be expected, as neither non-surgical site pain or constitutional symptoms improved for the vast majority of this subgroup. These different effects are not due to pre-surgical levels of comorbid FM symptoms, which were controlled for in our analyses. These phenotypes are consistent with divergent mechanisms in how comorbid symptoms are generated. In a recent series of reviews exploring the neurobiological evidence for central sensitization, Woolf and others advanced two hypothetical subtypes: ‘bottom-up’ and ‘top-down’ central sensitization [12, 19]. For most patients, with what can be called ‘bottom-up’ sensitization, the removal of the primary nociceptive generator reduces FM symptoms, perhaps by attenuating central sensitization. For a substantial minority of patients—the ‘top-down’ phenotype—the procedure has no long-term benefit for comorbid FM symptoms. Clearly, these novel distinctions need to be explored further, but in our view they provide a helpful framework for exploring the variability in how OA patients respond to total joint replacement.
There are several important implications of these findings both for the mechanistic understanding of central sensitization in patients undergoing joint replacement procedures and for their clinical care. These findings are foreshadowed to some degree by the neglected concepts of primary and secondary FM [20, 21]. In a majority of patients, comorbid FM symptoms respond favourably to surgery, suggesting that these symptoms were driven or maintained by nociceptive input in the affected joint. This finding is supported by a previous study indicating that successful OA surgery results in substantial improvement of responses to experimental pain measures outside the surgical site; patients whose surgery eliminated their pain showed a more normal inhibitory response to evoked pain than they did prior to surgery [14]. Animal models have demonstrated convincingly that regional and even widespread hyperalgesia follow localized chronic injury or inflammation such as sciatic constriction or repeated formalin injections—an analogue to ‘bottom-up’ sensitization [22]—and we have recently shown that peripheral inflammation is associated with profound changes in brain function in RA that promote core symptoms of FM [23]. Together these findings suggest that bottom-up mechanisms are important in rheumatic diseases.
Comparing the locations of pain improvement/non-improvement following surgery shows that in the Improve group, the largest effects are seen the lower body contiguous with or near the site of surgery, with some additional benefits for back pain. Conversely, the Worsen/Same group of patients do not show these effects. These patients showed modest improvement in the lower body, and an overall pattern of worsening in the upper extremities. This is consistent with some animal models of FM in which removal of nociceptive input in the periphery has no effect on global hyperalgesia once the animal becomes centrally sensitized [24].
Patients in the Worsen/Same group showed worsening of comorbid symptoms at the same time that surgical-site pain seemed to resolve, in contrast with those in the Improve group whose comorbid symptoms and surgical-site pain improved in tandem. Surgical site pain seemed to persist for longer in a larger proportion of patients in the Worsen/Same group. This suggests that the distinction between peripherally driven central-sensitization and centrally maintained central-sensitization is in fact an important one.
The Worsen/Same group of patients showed substantially less improvement in both overall pain severity and functional disability 6 months after the procedure. This may simply reflect the fact that their comorbid symptoms have not improved, despite comparable levels of surgical-site improvement to the Improve group. Nonetheless, these metrics represent important benchmarks for improvement following joint replacement. Because surgical site pain improved equally in the two groups, it could fairly be said that a good deal of the overall improvement observed in the Improve group is due to improvement in comorbid symptoms (e.g. fatigue, trouble thinking/remembering), rather than surgical site pain. Clinically, this means that monitoring improvement in symptoms outside the surgical site is important, and should be done in tandem with questions about the severity of pain in the surgical area. It is also critical to monitor these symptoms beyond the first month post-operatively, as both groups appeared to improve at 1 month, a time when substantial numbers of patients in both groups were still using narcotic analgesics. Furthermore, the difference between patient groups in those reporting surgical site pain was no longer apparent at 6 months, this suggests that longer (i.e. at least 6 months) follow-up may be necessary to identify patients who failed to respond to the procedure in terms of surgical site pain.
These findings raise the critical question of how patient phenotypes might be distinguished prior to surgery. Our analyses were designed to control for pre-surgical levels of FM symptoms, so that the trajectory of improvement could be isolated from regression to the mean type effects. Nonetheless, pre-surgical symptom levels revealed higher levels of fatigue, depression and surgical site pain severity in the Worsen/Same group. These differences were modest and do not appear to lend themselves to forming reliable cut-offs (effect sizes range from 0.35 to 0.51).
There are many ways in which these two different subsets of arthroplasty patients might be better differentiated prior to surgery that we did not measure in this study. For example, individuals with a ‘top-down’ form of FM that is most commonly studied in mechanistic studies have high rates of co-morbid chronic overlapping pain conditions that typically present earlier in life [25, 26]. Individuals with FM similarly have been repeatedly shown to have much higher rates of mood disorders [27], again supportive of a primary brain mechanism, rather than a peripherally driven phenomenon. We and others have also shown that individuals with the primary form of FM that occurs without obvious evidence of ongoing nociceptive input not only display evidence of pain sensitivity, but are also much more sensitive to the intensity and unpleasantness of other sensory modalities, such as light and sound, and that this is a critical feature that can correctly classify individuals with FM vs those without in functional neuroimaging studies [28–31].
These findings point to the further need for mechanistic studies that explore the neurobiological substrates of patients who show these different trajectories in improvement following conventional peripherally directed treatments. The clinical impact of better understanding these two subsets is obvious, since the bottom-up subset should benefit from aggressive use of peripherally directed therapies, including not only appropriate surgery or injections, but also the use of drugs such as the nerve growth factor antibodies that may soon become available and might be able to eliminate ongoing nociceptive input better than any currently available therapies [32, 33].
Limitations
We chose our cut-off for inclusion (minimum score of 4) using the distribution of the FM scores in the sample; this decision was still somewhat arbitrary as there are no subclinical cut-offs on the FM survey instrument to define moderate symptoms. Additionally, the loss of ∼25% of the patient sample at 6 months represents a potential source of bias. While included patients did not differ on baseline FM symptoms, they were older on average than those lost to follow-up, and therefore these patterns will need to be confirmed in younger patients. No information was collected about the duration of symptoms for either OA or FM, and consequently we cannot define secondary FM in the current sample. The group definitions used here were derived retrospectively and led to differences in subgroup sizes. Future studies may employ a matched design.
Patients undergoing knee and hip replacement show distinct trajectories in how FM symptoms improve, or do not improve, after the procedure. Improvement in comorbid FM symptoms appears to occur in a substantial number of patients undergoing arthroplasty. These trajectories are related to the overall impact of the procedure on measures of pain and disability after 6 months. These differences are not readily apparent in symptom phenotyping performed prior to the procedure, but may be revealed through mechanistic phenotyping using neuroimaging and quantitative sensory testing. Future studies will determine whether and how these patterns hold in other surgical cohorts with high levels of comorbid FM symptoms.
Supplementary Material
Acknowledgements
The authors thank the research staff from the Department of Anesthesiology and the surgical teams for their assistance in the success of this study. D.J.C., S.E.H. and C.M.B. designed the research. S.M. and E.K. performed the research and data collection. A.S. and A.T. analysed the data. All authors contributed to writing the manuscript. All authors read and approved the final manuscript. Additional support came from NIH/NIDA R01 DA038261 (D.J.C.—Contact/C.M.B.) and K12 DE023574 (D.J.C.—Contact/S. D. Kapila).
Funding: This work was supported by NIH/NIAMS P50 AR070600 and R01 AR060392 (D.J.C—Contact/C.M.B.).
Disclosure statement: D.J.C. reports personal fees from Abbott Pharmaceutical, grants and personal fees from Aptinyx, personal fees from Astellas Pharmaceutical, grants and personal fees from Cerephex, personal fees from Daiichi Sankyo, grants and personal fees from Pfizer, Inc., personal fees from Pierre Fabre, personal fees from Samumed, personal fees from Theravance, personal fees from Tonix, personal fees from Williams & Connolly LLP, and personal fees from Zynerba, outside the submitted work. S.E.H. reports grants from NIH during the conduct of the study; grants from NIH, VA, Cerephex, Eli Lilly, American Cancer Society, AAOGF; grants and personal fees from Aptinyx; personal fees from SUFU, Longitudinal Capital Management; personal fees and non-financial support from University of North Carolina, Chapel Hill; and an affiliation with Arbor Medical Innovations, outside the submitted work. In addition, S.E.H. has a patent, US 9307906, with royalties paid outside the submitted work. C.M.B. received consultancy fees from Biomet, Inc. and grants from American Society of Regional Anesthesia and Pain Medicine Chronic Pain Research, RO1 AR 060392, and the Michigan Genomics Initiative (unrelated to current work). The other authors have declared no conflicts of interest.
References
- 1. Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311:1547–55. [DOI] [PubMed] [Google Scholar]
- 2. Harris RE, Napadow V, Huggins JP. et al. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology 2013;119:1453–64. [DOI] [PubMed] [Google Scholar]
- 3. Napadow V, Harris RE.. What has functional connectivity and chemical neuroimaging in fibromyalgia taught us about the mechanisms and management of ‘centralized’ pain? Arthritis Res Ther 2014;16:5–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schrepf A, Harper DE, Harte SE. et al. Endogenous opioidergic dysregulation of pain in fibromyalgia: a PET and fMRI study. Pain 2016;157:2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolfe F, Clauw DJ, Fitzcharles M-A. et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol 2011;38:1113–22. [DOI] [PubMed] [Google Scholar]
- 6. Basu N, Kaplan CM, Ichesco E. et al. Neurobiological features of fibromyalgia are also present among rheumatoid arthritis patients. Arthritis Rheumatol 2018;70:1000. [DOI] [PubMed] [Google Scholar]
- 7. Napadow V, LaCount L, Park K. et al. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum 2010;62:2545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brummett CM, Janda AM, Schueller CM. et al. Survey criteria for fibromyalgia independently predict increased postoperative opioid consumption after lower-extremity joint arthroplasty: a prospective, observational cohort study. Anesthesiology 2013;119:1434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Janda AM, As-Sanie S, Rajala B. et al. Fibromyalgia survey criteria is associated with increased postoperative opioid consumption in women undergoing hysterectomy. Anesthesiology 2015;122:1103. [DOI] [PubMed] [Google Scholar]
- 10. Brummett CM, Urquhart AG, Hassett AL. et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol 2015;67:1386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dave AJ, Selzer F, Losina E. et al. The association of pre-operative body pain diagram scores with pain outcomes following total knee arthroplasty. Osteoarthritis Cartilage 2017;25:667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harte SE, Harris RE, Clauw DJ.. The neurobiology of central sensitization. J Appl Biobehav Res 2018;23:e12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152(3 Suppl):S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kosek E, Ordeberg G.. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain 2000;88:69–78. [DOI] [PubMed] [Google Scholar]
- 15. Brummett CM, Bakshi RR, Goesling J. et al. Preliminary validation of the Michigan Body Map (MBM). Pain 2016;157:1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keller S, Bann CM, Dodd SL. et al. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain 2004;20:309–18. [DOI] [PubMed] [Google Scholar]
- 17. Cella D,, Yount S,, Rothrock N. et al. The patient-reported outcomes measurement information system (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care 2007;45(5 Suppl 1):S3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosenstiel AK, Keefe FJ.. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain 1983;17:33–44. [DOI] [PubMed] [Google Scholar]
- 19. Woolf CJ. Pain amplification—a perspective on the how, why, when, and where of central sensitization. J Appl Biobehav Res 2018;23:e12124. [Google Scholar]
- 20. Clauw DJ, Katz P.. The overlap between fibromyalgia and inflammatory rheumatic disease: when and why does it occur? J Clin Rheumatol 1995;1:335–42. [DOI] [PubMed] [Google Scholar]
- 21. Hench PK. Secondary fibrositis. Am J Med 1986;81:60–2. [DOI] [PubMed] [Google Scholar]
- 22. Grace PM, Hutchinson MR, Maier SF. et al. Pathological pain and the neuroimmune interface. Nat Rev Immunol 2014;14:217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schrepf A, Kaplan CM, Ichesco E. et al. A multi-modal MRI study of the central response to inflammation in rheumatoid arthritis. Nat Commun 2018;9:2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DeSantana JM, da Cruz KM, Sluka KA.. Animal models of fibromyalgia. Arthritis Res Ther 2013;15:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aaron LA, Burke MM, Buchwald D.. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med 2000;160:221–7. [DOI] [PubMed] [Google Scholar]
- 26. Maixner W, Fillingim RB, Williams DA. et al. Overlapping chronic pain conditions: implications for diagnosis and classification. J Pain 2016;17:T93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arnold LM, Hudson JI, Hess EV. et al. Family study of fibromyalgia. Arthritis Rheum 2004;50:944–52. [DOI] [PubMed] [Google Scholar]
- 28. Geisser ME, Glass JM, Rajcevska LD. et al. A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. J Pain 2008;9:417–22. [DOI] [PubMed] [Google Scholar]
- 29. Geisser ME, Strader Donnell C, Petzke F. et al. Comorbid somatic symptoms and functional status in patients with fibromyalgia and chronic fatigue syndrome: sensory amplification as a common mechanism. Psychosomatics 2008;49:235–42. [DOI] [PubMed] [Google Scholar]
- 30. López-Solà M,, Woo CW,, Pujol J. et al. Towards a neurophysiological signature for fibromyalgia. Pain 2017;158:34–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. López-Solà M,, Pujol J,, Wager TD. et al. Altered functional magnetic resonance imaging responses to nonpainful sensory stimulation in fibromyalgia patients. Arthritis Rheumatol 2014;66:3200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tiseo PJ, Kivitz AJ, Ervin JE. et al. Fasinumab (REGN475), an antibody against nerve growth factor for the treatment of pain: results from a double-blind, placebo-controlled exploratory study in osteoarthritis of the knee. Pain 2014;155:1245–52. [DOI] [PubMed] [Google Scholar]
- 33. Cohen E, Lee YC.. A mechanism-based approach to the management of osteoarthritis pain. Curr Osteoporos Rep 2015;13:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.